Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~~~~a~~
75; DAM30
76/DAM31
- 1 - 1794oIB
TITLE OF THE INVENTION
(QUINOLIN-2-YLMETHOXY)INDOLES AS INHIBITORS OF THE
BIOSYNTHESIS OF LEUKOTRIENES.
20
BACKGROUND OF THE INVENTI DT
European Patent Applications 166,591 and
275,667 disclose a series of indole-based compounds
with activity as prostaglandin antagonists and
inhibitors of leukotriene biosynthesis respectively.
751L?Ari30 - 2 -- 17940IB
In EF 181,568 and EP 200,101 are disclosed a series
of compounds, containing two aromatic nuclei, which
are described as possessing activity as
lipoxygenaseinhibitors . In EP 279,263 is disclosed
a series of indoles, benzofurans and benzothiophenes
which are described as possessing activity as
lipoxygenase inhibitors. U.S. Patent 4,629,733
describes novel indolinones which are antithrombotic
and inhibit both phosphodiesterase and tumor
metastasis. The chemical preparation of
quinolylindoles is referred to by Sheinkman, ~t al.,
Chem. Ab., Vol. 67, 54017 (1967), without mentioning
any utility for such compounds. A number of N-acyl
derivatives of indole-3-acetic acid are described as
Potential anti-inflammatory agents by Biniecki, g
~1., Chem. Ab., Vol. 98, 197936 (1983), by Pakula,
~1., Chem. Ab., Vol. 105, 190835 (1986), and in
British Pat. Spec. 1,228,848.
~Ry OF THE INVENTIQN_
The present invention relates to compounds
having activity as leukotriene biosynthesis
inhibitors, to methods for their preparation, and to
methods and pharmaceutical formulations for using
these compounds in mammals (especially humans).
Because of their activity as leukotriene
biosynthesis inhibitors, the compounds of the present
invention are useful as anti-asthmatic,
anti-allergic, and anti-inflammatory agents and are
useful in treating allergic rhinitis and chronic
bronchitis and for amelioration of skin diseases like
psoriasis and atopic eczema. These compounds are
75iI?r'll~I3t~ - 3 - 17940TB
also useful to inhibit the pathologic actions of
leukotrienes on the cardiovascular and vascular
systems for example, actions such as result in angina
or endotoxin shock. The compounds of the present
invention are useful in the treatment of inflammatory
and allergic diseases of the eye, including allergic
conjunctivitis. The compounds are also useful as
cytoprotective agents and for the treatment of
migraine headache.
1o Thus, the compounds of the present invention
may also be used to treat or prevent mammalian
(especially, human) disease states such as erosive
gastritis; erosive esophagitis; inflammatory bowel
disease; ethanol-induced hemorrhagic erosions;
hepatic ischemia; noxious agent-induced damage ox
necrosis of hepatic, pancreatic, renal, or myocardial
tissue; liver parenchymal damage caused by hepatoxic
agents such as CC14 and D-galactosamine; ischemic
renal failure; disease-induced hepatic damage; bile
salt induced pancreatic or gastric damage; trauma- or
stress-induced cell damage; and glycerol-induced
renal failure.
The compounds of this invention are
inhibitors of the biosynthesis of 5-lipoxygenase
metabolites of arachidonic acid, such as 5-HPETE,
5-HETE and the leukotrienes. heukotrienes B4, C4, D4
and E4 are known to contribute to various disease
conditions such as asthma, psoriasis, pain, ulcers
and systemic anaphylaxis. Thus inhibition of the
3o synthesis of such compounds will alleviate these and
other leukotriene-related disease states.
75/DAri30 - 4 - 179401B
DETAILED DESCRIPTION OF THE INVENTI N
The present invention provides novel
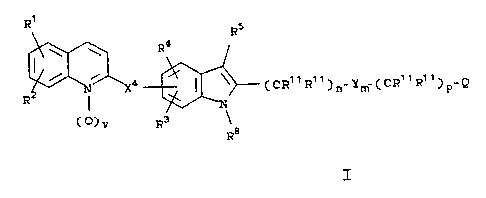
compounds of the formula I:
R1
R5
\ \ R4
N X4 I \ (CR»Ri~~ _Y (CR~tR~i~ _Q
n W p
R N
3
1~ (ply R
T
wherein:
R1~ R2~ R3~ R4 and R10 are independently hydrogen,
halogen, lower alkyl, lower alkenyl, lower alkynyl,
-CF3, -CN, -N02, -N3, -CCOH)R11R11, -C02R12, -SR14
-S(0)R14~ _S(0)2R14~ _S(0)2NR15R15~ -pRIS~ _NR15R15~
-C(0)R16 or -(CH2)tR2l;
R5 is hydrogen, -CH3, CF3, -C(0)H, X1-R6 or Xz-R7;
R6 and R9 are independently alkyl, alkenyl,
-(CH2)uPh(R10)2 or -(CH2)uTh(R10)2;
R7 is -CF3 or R6;
R8 is hydrogen or X3-R9;
3o each R11 is independently hydrogen or lower alkyl, or
two R11's on same carbon atom are joined to foam a
v\ en
a~~°)~:.r')~~
75/DArf30 - 5 - 1%940IB
cycloalkyl ring of 3 to 6 carbon atoms;
R12 is hydrogen, lower alkyl or -CH2R21;
R13 is lower alkyl or -(CH2)rR2l;
R14 is -CF3 or R13;
R15 is hydrogen, -C(0)R16, R13, or two R15 's on the
same nitrogen may be joined to form a monocyclic
heterocyclic ring of 4 to 6 atoms containing up to 2
heteroat oms chosen from 0, S or N;
R16 is hydrogen, -CF3, lower alkyl, lower alkenyl,
lower alkynyl or -(CH2)rRZl;
Rl~ is _(CH2)s-C(R18R18)-(CH2)s-R19 or
-CHZC(0)NR15R15;
R18 is hydrogen or lower alkyl;
R19 is a) a monocyclic or bicyclic heterocyclic ring
containing from 3 to 9 nuclear carbon atoms and 1 or
2 nuclear hetero-atoms selected from N, S or 0 and
with each ring in the heterocyclic radical being
formed of 5 ox 6 atoms, or b) the radical W-R20;
R20 is alkyl or -C(0)R23;
R21 is phenyl substituted with 1 or 2 R22 groups;
CA 02060557 2001-10-29
75/DAM30 - 6 - 17940IB
R22 is hydrogen, halogen, lower alkyl, lower alkoxy,
lower alkylthio, lower alkylsulfonyl, lower
alkylcarbonyl, -CF3, -CN, -N02 or -N3;
R23 is alkyl, cycloalkyl, or monocyclic
monoheterocyclic ring;
R24 is the residual structure of a standard amino
acid, or R18 and R24 attached to the same N can
to cyclize to form a proline residue;
m is 0 to
1;
n is 0 to
3;
p is 1 to when m is 1;
3
is 0 to when m is 0;
p 3
r is 0 to
2;
s is 0 to
3;
t is 0 to
2;
a is 0 to
3;
is 0 or
~ 1;
W is 0, S NR15;
or
X1 is 0, or NR15;
X2 is C(0), CR11R11, S, S(0) or S(0)2;
X3 is C(0), CR11R11, S(0)2 or a bond;
is CH=CH, CH2-Y1 or Y1-CH2;
X4
Y is X1 or 2;
X
Y1 is S, S(0)2 or CH2;
Q is -C02R12, -C(0)NHS(0)2R14~ _~S(0)2R14~
-S(0)2~R15 -C(0)NR15R15~ -C02R17~ -C(0)NR18R24~
-CH20H, or 1H- or 2H-tetrazol-5-yl;
CA 02060557 2001-10-29
75/DAM30 - 7 - 17940IB
and the pharmaceutically acceptable salts thereof.
A preferred embodiment of Formula I is that in which
X4 is CH2-Y1, Yl is 0 and the remaining sustituents
are as defined for Formula I.
A preferred embodiment of Formula I is that in which
R1, R2, R3 and R4 are hydrogen;
R5 is X2-R7 or -OR6;
R7 is R6;
R8 is R9;
R10 is hydrogen or halogen;
m is 0;
n is 1 to 3;
a is 0 in R6 and 1 in R9;
v is 0;
X2 is CR11R11 or S;
X4 is CH2-Y1;
25
Q is -C02R12; and the remaining substituents are as
def fined for Formula I ;
or the pharmaceutically acceptable salt thereof .
Def initions
The following abbreviations have the
indicated meanings:
Me = methyl
3o Bz = benzyl
Ph = phenyl
;.a 1
75!D.A~I30 - 8 - 17940IB
t-Bu = tert-butyl
i-Pr = isopropyl
c-C6H11 = cyclohexyl
c-Pr = cyclopropyl
c- - cyclo
Ac = acetyl
Tz = 1H- or 2H- tetrazol-5-y1
Th = 2- or 3- thienyl
c-C5H9 = cyclopentyl
1-Ad = 1-adamantyl.
Alkyl, alkenyl, and alkynyl are intended to
include linear, branched, and cyclic structures and
combinations thereof.
As used herein, the term '°alkyl" includes
"lower alkyl" and extends to cover carbon fragments
having up to 20 carbon atoms. Examples of alkyl
groups include octyl, nonyl, noxbornyl, undecyl,
dodecyl, tridecyl, tetradecyl, pentadecyl, eicosyl,
3,7-diethyl-2,2-dimethyl-4-propylnonyl, cyclododecyl,
adamantyl, and the like.
As used herein, the term "lower alkyl"
includes those alkyl groups of from 1 to 7 carbon
atoms. Examples of lower alkyl groups include
methyl, ethyl, propyl, isopropyl, butyl, sec- and
tert-butyl, pentyl, hexyl, heptyl, cyclopropyl,
cyclobutyl, cyclopentyl, cyciohexyl, cycloheptyl,
2-methylcyclopropyl, cyclopropylmethyl, and the like.
The term "cycloalkyl" refers to a
hydrocarbon ring having from 3 to 7 carbon atoms.
Examples of cycloalkyl groups are cyclopropyl,
cyclopentyl, cyclohegtyl and the like.
75 j LAS"130 - 9 - 17940IB
"Lower alkenyl" groups include those alkenyl
groups of 2 to 7 carbon atoms. Examples of lower
alkenyl groups include vinyl, allyl, isopropenyl,
pentenyl, hexenyl, heptenyl, cyclopropenyl,
cyclobutenyl, cyclopentenyl, cyclohexenyl,
1-propenyl, 2-butenyl, 2-methyl-2-butenyl and the
like.
"Lower alkynyl" groups include those alkynyl
groups of 2 to 7 carbon atoms. Examples of lower
alkynyl groups include ethynyl, propargyl,
3-methyl-1-pentynyl, 2-heptynyl and the like.
As used herein, the term "lower alkoxy'°
includes those alkoxy groups of from 1 to 7 carbon
atoms of a straight, branched, or cyclic
configuration. Examples of lower alkoxy groups
include methoxy, ethoxy, propoxy, isopropoxy,
cyclopropyloxy, cyclohexyloxy, and the like.
The term "monocyclic monoheterocyclic ring"
which defines R23 includes those monocyclie groups of
5 to 7 members containing only 1 heteroatom selected
from N, S or 0 in the sing. Examples include
tetrahydrofuran, tetrahydrothiophene, pyrrolidine,
piperidine, tetrahydropyran, and the like.
The term "monocyclic or bicyclic
heterocyclic ring" which defines R19 may be
2,5-dioxo-1-pyrrolidinyl, (3-pyridinylcarbonyl)
amino, 1,3-dihydro-1,3-dioxo-2H-isoindol-2-yl,
1,3-dihydro-2H-isoindol-2-yl,
2,4-imidazolinedion-1-yl, 2,6-piperidinedion-1-y1,
2-imidazolyl, 2-oxo-1,3-dioxolen-4-yl,
piperidin-1-yl, morpholin-1-yl, piperazin-1-yl and
the like.
75iDA~I30 - 10 - 17940IB
The point of attachment of any heterocyclic
ring may be at any free valence of the ring.
It is understood in the art that when the
variable v is 1, the nitrogen of the quinolinyl
N-oxide so formed is positively charged, and the
oxygen is negatively charged.
The term standard amino acid is employed to
include the following amino acids: alanine,
asparagine, aspartic acid, arginine, cysteine,
glutamic acid, glutamine, glycine, histidine,
isoleucine, leucine, lysine, methionine,
phenylalanine, proline, serine, threonine,
tryptophan, tyrosine and valine. (See F.H.C. Crick,
Symposium of the Society for Experimental Biology,
1958 (12) p. 140.)
It is understood that R1 and R~ may be
located at any of positions 3,4,5,6,7 or 8 of the
quinoline ring.
As used herein the term "lower alkylthio"
includes those alkylthio grougs of from 1 to 7 carbon
atoms of a straight, branched or cyclic
configuration. Examples of lower alkylthio groups
include methylthio, propylthio, isopropylthio,
cycloheptylthio, etc. By way of illustration, the
propylthio group signifies -SGH2CH2CH3.
The terms Ph(R10)2 and Th(R10)2 indicate a
phenyl or thienyl group substituted with two R10
substituents.
Halogen includes F, G1, Br, and I.
It is intended that the definitions of any
substituent (e.g., R1, R2, R15, Fh(R10)2, etc.) in a
particular molecule be independent of its definitions
elsewhere in the molecule. Thus, -NR15R15 represents
-NHH, -NHCH3, -NHC6H5, etc.
~d ;~.~ 4 ) .iA l.i ::7 .:
75/r~t30 - 11 - 1794oIB
The monocyclic heterocyclic rings formed
c~Then two R15 groups join through N include
pyrrolidine, piperidine, morpholine, thiamorpholine,
piperazine, and N-methylpiperazine.
The prodrug esters of Q (i.e., when Q =
C02R17) are intended to include the esters such as
are described by Saari ~t ate., J. Med. Chem., 21, No.
8, 746-753 (1978), Sakamoto ~ al., Chem. Pharm.
Bull., ~2, No. 6, 2241--2248 (1984) and Bundgaard et
l0 al., J. Med. Chem., 30, No. 3, 451-454 (1987).
Some of the compounds described herein contain
one or more asymmetric centers and may thus give rise
to diastereomers and optical isomers. The present
invention is meant to comprehend such possible
diastereomers as well as their racemic and resolved,
enantiomerically pure forms and pharmaceutically
acceptable salts thereof.
The pharmaceutical compositions of the
present invention comprise a compound of Formula I as
an active ingredient or a pharmaceutically acceptable
salt, thereof, and may also contain a
pharmaceutically acceptable carrier and optionally
other therapeutic ingredients. The term
"pharmaceutically acceptable salts" refers to salts
Prepared from pharmaceutically acceptable non-toxic
bases including inorganic bases and organic bases.
Salts derived from inorganic bases include aluminum,
ammonium, calcium, copper, ferric, ferrous, lithium,
magnesium, manganic salts, manganous, potassium,
sodium, zinc and the like. Particularly preferred
are the ammonium, calcium, magnesium, potassium
andsodium salts. Salts derived from pharmaceutically
acceptable organic non-toxic bases include salts of
~h .,a s.:~;-r r.
~'~~~~~~~'J
75i'D.~i30 - 12 - 17940IB
primary, secondary, and tertiary amines, substituted
amines including naturally occurring substituted
amines, cyclic amines and basic ion exchange resins,
such as arginine, betaine, caffeine, choline,
N,N1-dibenzylethylenediamine, diethylamine,
2-diethylaminoethanol, 2-dimethylaminoethanol,
ethanolamine, ethylenediamine, N-ethylmorpholine,
N-ethylpiperidine, glucamine, glucosamine, histidine,
hydrabamine, isopropylamine, lysine, methylglucamine,
l0 morpholine, piperazine, piperidine, polyamine resins,
procaine, purines, theobromine, triethylamine,
trimethylamine, tripropylamine, tromethamine and the
like.
When the compound of the present invention
is basic, salts may be prepared from pharmaceutically
acceptable non-toxic acids, including inorganic and
organic acids. Such acids include acetic,
benzenesulfonic, benzoic, camphorsulfanic, citric,
ethanesulfonic, fumaric, gluconic, glutamic,
2o hYdrobromic, hydrochloric, isethionic, lactic,
malefic, malic, mandelic, methanesulfonic, mucic,
nitric, pamoic, pantothenic, phosphoric, succinic,
sulfuric, tartaric, p-toluenesulfonic acid and the
like. Particularly preferred are citric,
hYdrobromic, hydrochloric, malefic, phosphoric,
sulfuric and tartaric acids.
It will be understood that in the discussion
of methods of treatment which follows, references to
the compounds of Formula I are meant to also include
the pharmaceutically acceptable salts.
The ability of the compounds of Formula I to
inhibit biosynthesis of the leukotrienes makes them
~~~~~:.j':3~4
75lPari3o - 13 - 17a4oz~
useful for inhibiting the symptoms induced by the
leukotrienes in a human subject. This inhibition of
the manunalian biosynthesis of leukotrienes indicates
that the compounds and pharmaceutical compositions
thereof are useful to treat, prevent or ameliorate in
mammals and especially in humans: 1) pulmonary
conditions including diseases such as asthma, 2)
allergies and allergic reactions such as allergic
rhinitis, contact dermatitis, allergic
conjunctivitis, and the liY,e, 3) inflammation such as
arthritis or inflammatory bowel disease, 4) pain, 5)
skin conditions such as psoriasis and the like, 6)
cardiovascular conditions such as angina, endotoxin
shock, and the like and 7) renal insufficiency
arising from ischaemia induced by immunological or
chemical (cyclosporin) etiology, and that the
compounds are cytoprotective agents.
The cytoprotective activity of a compound
may be observed in both animals and man by noting the
increased resistance of the gastrointestinal mucosa
to the noxious effects of strong irritants, for
example, the ulcerogenic effects of aspirin or
indomethacin. In addition to lessening the effect of
non-steroidal anti-inflammatory drugs on the
gastrointestinal tract, animal studies show that
cytoprotective compounds will prevent gastric lesions
induced by oral administration of strong acids,
strong bases, ethanol, hypertonic saline solutions
and the like.
Two assays can be used to measure
cytoprotective ability. These assays are; (A) an
ethanol-induced lesion assay and (~) an
~~~9~~~~~~~~c:~'~
75/D~PIaO - 14 - 17940zB
indemethacin-induced ulcer assay and are described in
EP 140,684.
The magnitude of prophylactic or therapeutic
dose of a compound of Formula I will, of course, vary
with the nature of the severity of the condition to
be treated and with the particular compound of
Formula I and its route of administration. It will
also vary according to the age, weight and response
of the individual patient. In general, the daily
1o dose range for anti-asthmatic, anti-allergic or
anti-inflammatory use and generally, uses other than
cytoprotection, lie within the range of from about
0.001 mg to about 100 mg per kg body weight of a
mammal, preferably 0.01 mg to about 10 mg per kg, and
most preferably 0.1 to 1 mg per kg, in single or
divided doses. On the other hand, it may be
necessary to use dosages outside these limits in some
cases.
For use where a composition for intravenous
administration is employed, a suitable dosage range
for anti-asthmatic, anti-inflammatory or
anti-allergic use is from about 0.001 mg to about 2~
mg (preferably from 0.01 mg to about 1 mg) of a
compound of Formula I per kg of body weight per day
and for cytoprotective use from about 0.1 mg to about
100 mg (preferably from about 1 mg to about 100 mg
and more preferably from about 1 mg to about 10 mg)
of a compound of Formula I per kg of body weight per
day.
3o In the case where an oral composition is
employed, a suitable dosage range for anti-asthmatic,
anti-inflammatory or anti-allergic use is, e.g. from
ewe ~3' E~ i~ .! I;~ '4
~/~Ariso - 1~ - 17q4ozB
about 0.01 mg to about 100 mg of a compound of
Formula I per kg of body weight per day, preferably
from about 0.1 mg to about 10 mg per kg and for
cytoprotective use from 0.1 mg to about 100 mg
(preferably from about 1 mg to about 100 mg and more
preferably from about 10 mg to about 100 mg) of a
compound of Formula I per kg of body weight per day.
For the treatment of diseases of the eye,
ophthalmic preparations for ocular administration
comprising 0.001-1% by weight solutions or
suspensions of the compounds of Formula I in an
acceptable ophthalmic formulation may be used.
The exact amount of a compound of the
Formula I to be used as a cytoprotective agent will
depend on, in r alia, whether it is being
administered to heal damaged cells or to avoid future
damage, on the nature of the damaged cells (e. g.,
gastrointestinal ulcerations vs. nephrotic necrosis),
and on the nature of the causative agent. An example
of the use of a compound of the Formula I in avoiding
future damage would be co-administration of a
compound of the Formula I with a nan-steroidal
anti-inflammatory drug (NSAID) that might otherwise
cause such damage (for example, indomethacin). For
such use, the compound of Formula I is administered
from 30 minutes prior up to 30 minutes after
administration of the NSAID. Preferably it is
administered prior to or simultaneously with the
NSAID, (for example, in a combination dosage form).
Any suitable mute of administration may be
employed for providing a mammal, especially a human
E~d i~ a'~ ;~ a
i t,
k~ ~., ~~ ;a .s
75,tD.AM30 - 16 - 17940IB
with an effective dosage of a compound of the present
invention. For example, oral, rectal, topical,
parenteral, ocular, pulmonary, nasal, and the like
may be employed. Dosage forms include tablets,
troches, dispersions, suspensions, solutions,
capsules, creams, ointments, aerosols, and the like.
The pharmaceutical compositions of the
present invention comprise a compound of Formula I as
an active ingredient or a pharmaceutically acceptable
salt thereof, and may also contain a pharmaceutically
acceptable carrier and optionally other therapeutic
ingredients. The term ~~pharmaceutically acceptable
saltsaa refers to salts prepared frum pharmaceutically
acceptable non-toxic bases or acids including
inorganic bases or acids and organic bases or acids.
The compositions include compositions
suitable for oral, rectal, topical, paxenteral
(including subcutaneous, intramuscular, and
intravenous), ocular (ophthalmic), pulmonary (nasal
or buccal inhalation), or nasal administration,
although the most suitable route in any given case
will depend on the nature and severity of the
conditions being treated and on the nature of the
active ingredient. They may be conveniently
Presented in unit dosage form and prepared by any of
the methods well-known in the art of pharmacy.
For administration by inhalation, the
compounds of the present invention are conveniently
delivered in the form of an aerosol spray
Presentation from pressurized packs or nebulisers.The
compounds may also be delivered as powders which may
s.~ , t.. ~ ,."; i1 ~~
~.~ ~) ~J ;~ ,.a -:t.
75/DAMdO - 17 - W9~~oIB
be formulated and the powder composition may be
inhaled with the aid of an insufflation powder
inhaler device. The preferred delivery system for
inhalation is a metered dose inhalation (MDI)
aerosol, which may be formulated as a suspension or
solution of Compound I in suitable propellants, such
as fluorocarbons or hydrocarbons.
Suitable topical formulations of Compound I
include transdermal devices, aerosols, creams,
lp ointments, lotions, dusting powders, and the like.
In practical use, the compounds of Formula I
can be combined as the active ingredient an intimate
admixture with a pharmaceutical carrier according to
conventional pharmaceutical compounding techniques.
The carrier may take a wide variety of forms
depending on the form of preparation desired for
administration, e.g., oral or parenteral (including
intravenous). In preparing the compositions for oral
dosage form, any of the usual pharmaceutical media
may be employed, such as, for example, water,
glycols, oils, alcohols, flavoring agents,
preservatives, coloring agents and the like in the
case of oral liquid preparations, such as, for
example, suspensions, elixirs and solutions; or
carriers such as starches, sugars, microcrystalline
cellulose, diluents, granulating agents, lubricants,
binders, disintegrating agents and the like in the
case of oral solid preparations such as, for example,
powders, capsules and tablets, with the solid oral
3o Preparations being preferred over the liquid
preparations. Because of their ease of
administration, tablets and capsules represent the
~~ °~'~ '~ '~
75 /~~I30 - is - 1794oIB
most advantageous oral dosage unit form in which case
solid pharmaceutical carriers are obviously
employed. If desired, tablets may be coated by
standard aqueous or nonaqueous techniques.
In addition to the common dosage forms set
out above, the compounds of Formula I may also be
administered by controlled release means and/or
delivery devices such as those described in U.S.
Patent Nos. 3,845,770; 3,916,899; 3,536,809;
3,598,123; 3,630,200 and 4,008,719.
Pharmaceutical compositions of the present
invention suitable for oral administration may be
presented as discrete units such as capsules, cachets
or tablets each containing a predetermined amount of
I5 the active ingredient, as a powder or granules or as
a solution or a suspension in an aqueous liquid, a
non-aqueous liquid, an oil-in-water emulsion or a
water-in-oil liquid emulsion. Such compositions may
be prepared by any of the methods of pharmacy but all
methods include the step of bringing into association
the active ingredient with the carrier which
constitutes one or more necessary ingredients. In
general, the compositions are prepared by uniformly
and intimately admixing the active ingredient with
z5 liquid carriers or finely divided solid carriers or
both, and then, if necessary, shaping the product
into the desired presentation. For example, a tablet
may be prepared by compression or molding, optionally
with one or more accessory ingredients. Compressed
3o tablets may be prepared by compressing in a
suitablemachine, the active ingredient in a
free-flowing form such as powder or granules,
~~ ~~ c$ ~~ a_i _'.~ '
75 ~'D:~rI30 -- 19 - 17940zB
optionally mixed with a binder, lubricant, inert
diluent, surface active or dispersing agent. Molded
tablets may be made by molding in a suitable machine,
a mixture of the powdered compound moistened with an
inert liquid diluent. Desirably, each tablet
contains from about 2.5 mg to about 500 mg of the
active ingredient and each cachet or capsule contains
from about 2.5 to about 500 mg of the active
ingredient.
la The following are examples of representative
pharmaceutical dosage forms for the compounds of
Formula I:
20
30
CA 02060557 2001-10-29
75/DAM30 - 20 - 17940IB
Iniectable Suspension (I.M.) m ml
Compound of Formula I 10
Methylcellulose 5.0
TweenM80 0.5
Benzyl alcohol 9.0
Benzalkonium chloride 1.0
Water for injection
to a total volume of 1 ml
to
Tablet mg/tablet
Compound of Formula I 25
Microcrystalline Cellulose 415
Providone 14.0
Pregelatinized Starch 43.5
Magnesium Stearate 255
500
Capsule mQ/capsule
Compound of Formula I 25
Lactose Powder 573.5
Magnesium Stearate 1.5
600
Aerosol Per canister
Compound of Formula I 24 mg
Lecithin, NF Liquid Concentrate 1.2 mg
Trichlorofluoromethane, NF 4.025 gm
Dichlorodifluoromethane, NF 12.15 gm
c', iYa3:.'b
7 5;'D:'1I'I30 - 21 - 17940IB
In addition to the compounds of Formula I,
the pharmaceutical compositions of the present
invention can also contain other active ingredients,
such as cyclooxygenase inhibitors, non-steroidal
anti-inflammatory drugs (NSAIDs), peripheral
analgesic agents such as zomepirac diflunisal and the
like. The weight ratio of the compound of the
Formula I to the second active ingredient may be
varied and will depend upon the effective dose of
each ingredient. Generally, an effective dose of
each will be used. Thus, for example, when a
compound of the Formula I is combined with an NSAID
the weight ratio of the compound of the Formula I to
the NSAID will generally range from about 1000:1 to
about 1:1000, preferably about 200:1 to about 1:200.
Combinations of a compound of the Formula I and other
active ingredients will generally also be within the
aforementioned range, but in each case, an effective
dose of each active ingredient should be used.
NSAIDs can be characterized into five groups:
(1) the propionic acid derivatives;
(2) the acetic acid derivatives;
(3) 'the fenamic acid derivatives;
(4) the biphenylcarboxylic acid derivatives;
and
(5) the oxicams
or a pharmaceutically acceptable salt thereof.
The propionic acid derivatives which may be
used comprise: alminoprofen, benoxaprofen, bucloxic
acid, carprofen, fenbufen, fenoprofen, fluprofen,
flurbiprofen, ibuprofen, indoprofen, ketoprofen,
ø~ "~i ,~f ~1~ 'r1
4 va
i5%D.=~I~Iu - ~z - 17940IB
miroprofen, naproxen, oxaprozin, pirprofen,
prano-profen, suprofen, tiaprofenic acid, and
tioxaprofen. Structurally related propionic acid
derivatives having similar analgesic and
anti-inflammatory properties are also intended to be
included in this group.
Thus, "propionic acid derivatives" as
defined herein are non-narcotic
analgesics/non-steroidal anti-inflammatory drugs
having a free -CH(CH3)COOH or -CH2CH2COOH group
(which optionally can be in the form of a
pharmaceutically acceptable salt group, e.g.,
-CH(CH3)C00-Na+ or -CH2CH2C00-Na'~'),
typically attached directly or via a carbonyl
function to a ring system, preferably to an aromatic
ring system.
The acetic acid derivatives which may be
used comprise: indomethacin, which is a preferred
NSAID, acemetacin, alclofenac, clidanac, diclofenac,
fenclofenac, fenclozic acid, fentiazac, furofenac,
ibufenac, isoxepac, oxpinac, sulindac, tiopinac,
tolmetin, zidometacin and zomepirac. Structually
related acetic acid derivatives having similar
analgesic and anti-inflammatory properties are also
intended to be encompassed by this group.
Thus, °'acetic acid derivative," as defined
herein are non-narcotic analgesics/non-steroidal
anti-inflammatory drugs having a free -CH~COOH group
(which optionally can be in the form of a
pharmaceutically acceptable salt group, e.g.
-CH2C00-Na+), typically attached directly to a ring
system, preferably to an aromatic or heteroaromatic
ring system.
., ,
a ~1 :, .;y :~-~ ~, l
S:,a 'l.. ~i~ zl , ~ : ~ $
7 ~!DAPT30 -- 23 - 17940TB
The fenamic acid derivatives which may be
used comprise: flufenamic acid, meclofenamic acid,
mefenamic acid, niflumic acid and tolfenamic acid.
Structurally related fenamic acid derivatives having
similar analgesic and anti-inflammatory properties
are also intended to be encompassed by this group.
Thus, "fenamic acid derivatives" as defined
herein are non-narcotic analgesics/non-steroidal
anti-inflammatory drugs which contain the basic
1o structure:
H
15 CO~ H
which can bear a variety of substituents and in which
the free -COOH group can be in the form of a
pharmaceutically acceptable salt group, e.g.,
-C00-Nay' .
2o The biphenylcarbo~ylic acid derivatives
which can be used comprise: diflunisal and
flufenisal. Structurally related biphenylcarboxylic
acid derivatives having similar analgesic and
anti-inflammatory properties axe also intended to be
25 encompassed by this group.
Thus, "biphenylcarboxylic acid derivatives"
as defined herein are non-narcotic
analgesics/non-steroidal anti-inflammatory drugs
which contain the basic structure:
a L lt' ~; ';~ ~y~~
75!D~1ri30 - 24 - 179+oIB
\ /
C02 H
which can bear a variety of substituents and in which
the free -COON group can be in the form of a
pharmaceutically acceptable salt group, e.g.,
-C00-Na+.
The oxicams which can be used in the present
invention comprise: isoxicam, piroxicam, sudoxicam
and tenoxican. Structurally related oxicams having
similar analgesic and anti-inflammatory properties
are also intended to be encompassed by this group.
~-5 Thus, "oxicams" as defined herein are non
narcotic analgesics/non-steroidal anti--inflammatory
drugs which have the general formula:
OH
2 o c~ o~ z~z-zR
i ~y
~ C H3
a a
wherein P, is an aryl or heteroaryl ring system.
The following NSA3Ds may also be used:
amfenac sodium, aminoprofen, anitrazafen,
antrafenine, auranofin, bendazac lysinate,
benzydanine, beprozin, broperamole, bufezolac,
cinmetacin, ciproquazone, cloximate, dazidamine,
~_b ' 't' ~J ~;') e:' ~ ~l
75/DArI30 - 25 - 17940IB
deboxamet, delmetacin, detomidine, dexindoprofen,
diacerein, di-fisalamine, difenpyramide, emorfazone,
enfenamic acid, enolicam, epirizole, etersalate,
etodolac, etofenamate, fanetizole cnesylate,
fenclorac, fendosal, fenflumizole, feprazone,
floctafenine, flunixin, flunoxaprofen, fluproquazone,
f opirtoline, fosfosal, furcloprofen, glucametacin,
guaimesal, ibuproxam, isofezolac, isonixim,
isoprofen, isoxicam, lefetamine HC1, leflunomide,
to lofemizole, lonazolac calcium, lotifazole,
loxoprof en, lysin clonixinate, meclofenamate sodium,
meseclazone, nabumetone, nictindole, nimesulide,
orpanoxin, oxametacin, oxapadol, perisoxal citrate,
pimeprofen, pimetacin, piproxen, pirazolac,
pirfenidone, proglumetacin maleate, proquazone,
pyridoxiprofen, sudoxicam, talmetacin, talniflumate,
tenoxicam, thiazolinobutazone, thielavin B, tiaramide
HC1, tiflamizole, timegadine, tolpadol, tryptamid and
ufenamate.
The following NSAIDs, designated by company
code number (see e.g., Pharmaprojects_), may also be
used>
4801565, AA861, AD1590, AFP802, AFP860, AI77B, AP504,
AU8001, BPPC, BW540C, CHINOIN 127, CN100, EB382,
EL508, F1044, GV3658, ITF182, KCNTEI6090, KME4,
LA2851, MR714, MR897, MY309, ON03144, PR823, PV102,
PV108, 8830, RS2131, SCR152, Sii440, SIR133, SPAS510,
SQ27239, ST281, SY6001, TA60, TAI -901 (4-benzoyl-1-
indancarboxylic acid), TVX2706, U60257, UR2301, and
WY41770.
Finally, NSAIDs which may also be used include
the salicylates, specifically acetyl salicylic acid
CA 02060557 2001-10-29
75/DAM30 - 26 - 17940IB
and the phenylbutazones, and pharmaceutically
acceptable salts thereof.
In addition to indomethacin, other Dreferred
NSAIDS are acetyl salicylic acid, diclofenac,
fenbufen, fenoprofen, flurbiprofen, ibuprofen,
ketoprofen, naproxen, phenylbutazone, piroxicam,
sulindac and tolmetin.
Pharmaceutical compositions comprising the
Formula I compounds may also contain inhibitors of
l0 the biosynthesis of the leukotrienes such as are
disclosed in EP 138,481 (April 24,1985), EP 115,394
(August 8, 1984), EP 136,893 (April 10, 1985), and EP
140,709 (May 8, 1985),
The compounds of the Formula I may also be
used in combination with leukotriene antagonists such
as those disclosed in EP 106,565 (April 25, 1984) and
EP 104,885 (April 4, 1984) and others known in the art
such as those disclosed in EP Application Nos. 56,172
(July 21, 1982) and 61,800 (June 10, 1982); and in U.K.
Patent Specification No. 2,058,785 (April 15, 1981).
Pharmaceutical compositions comprising the
Formula I compounds may also contain as the second
active ingredient, prostaglandin antagonists such as
those disclosed in EP 11,067 (May 28, 1980) or
thromboxane antagonists such as those disclosed in
U.S. Pat. 4,237,160. They may also contain histidine
decarboxylase inhibitors such as
a-fluoromethylhistidine, described in U.S. Pat.
4,325,961. The compounds of the Formula I may also
be advantageously combined with an H1 or H2-receptor
CA 02060557 2001-10-29
75/DAM30 - 27 - 17940IB
antagonist, such as for instance acetamazole,
aminothiadiazoles disclosed in EP 40,696 (December 2,
1981), benadryl, cimetidine, famotidine, framamine,
histadyl, phenergan, ranitidine, terfenadine and like
compounds, such as those disclosed in U.S. Patent
Nos. 4,283,408; 4,362,736; and 4,394,508. The
pharmaceutical compositions may also contain a K+/g+
ATPase inhibitor such as omeprazole, disclosed in
U.S. Pat. 4,255,431, and the like. Compounds of
l0 Formula I may also be usefully combined with most
cell stabilizing agents, such as
1,3-bis(2-carboxychromon-5-yloxy)-2-hydroxypropane
and related compounds described in British Patent
Specifications 1,144,905 and 1,144,906. Another
useful pharmaceutical composition comprises the
Formula I compounds in combination with serotonin
antagonists such as methysergide, the serotonin
antagonists described in Nature, Vol. 316, pages
126-131, 1985, and the like.
Other advantageous pharmaceutical
compositions comprise the Formula I compounds in
combination with anti-cholinergics such as
iPratropium bromide, bronchodilators such as the beta
agonist salbutamol, metaproterenol, terbutaline,
fenoterol and the like, and the anti-asthmatic drugs
theophylline, choline theophyllinate and
enprofylline, the calcium antagonists nifedipine,
diltiazem, nitrendipine, verapamil, nimodipine,
felodipine, etc. and the corticosteroids,
hydrocortisone, methylprednisolone, betamethasone,
dexamethasone, beclomethasone, and the like.
;~y :i~ 1V ,~J
7s!~Ar~3o - 2s ~- 1794ozB
Compounds of the present invention can be
prepared according to the following methods.
Temperatures are in degree Celsius.
The starting methoxy phenylhydxazines II are
either commercially available or axe described in the
chemical literature as are the acetamidophenols
XXVI. The benzyl phenylhydrazine starting materials
III axe prepared as described in EP 166,591 (17102
IA) and the ketones IV and _XXXI are prepared as
described in EP 166,591 and EP 275,667 (17496 IA).
The 2-(halomethyl)quinolines VII are available from
literature methods described in "Quinolines" Parts I
and II, G. Jones (ED.), John Wiley & Sons, Toronto,
1977 and 1982. The preparation of VII by
~5 halogenation of the corresponding 2-methylquinolines
is also described in the Jones' volumes. The benzyl
halides, (R10)2 PhCH2-Hal, are readily prepared and
many such compounds are described in the prior art,
such as U.S. Patent 4,808,608 (17323 IB). Hal in VII
and (R10)2 PhCH2-Hal represents Cl, Br or I.
Many syntheses of indoles are well-known in
the chemical literature: see for example,
"Heterocyclic compounds" Volume 25, Parts I, II, III,
W.J. Houlihan (Ed.), Interscience, J. Wiley & Sons,
N~Y~~ 1979, and "The Chemistry of Indoles" by R.J.
Sundberg, Academic Press, N.Y., 1970. One of the
most common syntheses is known as the Fischer Indole
Synthesis, and is abbreviated in the following
methods as "Fischer".
The -C02H and -C02R12 groups in the
intermediates and final products in the various
methods can be transformed to other representatives
of Q such as -CONHS(0)2R14, -NHS(0)2R14, -CONR15R15,
9 a~ sy
~:~ Y~ iY y._~ ~.:: a
75~'DAri30 - 29 - 17940I~3
-CH20H or tetrazol-5-y1 by the methodology described
in U.S. Patent 4,808,608 (17323IB). The preparation
of the gro-drug forms (Q is -C02R17) from the acids
may be effected by the methodology of EP 104,885
(16830 IA).
It will be apparent to one skilled in the
art that the various functional. groups (R1, R2, Y, Q,
etc.) must be chosen so as to be compatible with the
chemistry being carried out. Such compatibility can
often be achieved by protecting groups, or by
specific variations in the sequence of the reactions.
When R5 is S-R7, the corresponding
sulfoxides and sulfones can be prepared by oxidation
of the sulfides with one or two equivalents of an
oxidizing agent such as m-chloroperbenzoic acid or
monoperoxyphthalic acid or ozone (Trost, J. Org.
Chem., 1888, pg.532).
Many of the following methods involve a
basic hydrolysis of an ester function to obtain the
ccrresponding carboxylic acid. In all cases, the
free acid is obtained by acidification of the
reaction mixture with a suitable acid such as
hydrochloric, sulfuric, acetic, trifluoroacetic acid,
etc.
Compounds VIII, XI, XV, XTX, XXXVI, ~L,
XLIV, L_, ~I, LVIII, LIX and their precursor esters
are all examples of the Formula I compounds of the
present invention.
~.~ i~ t~ <~3 ~.~ ~sj
75!DAM30 - 30 - 179408
Method 1
a
R R
~ Rt o)~ phCH~-Tiel
Njao '
xEr~/eu4HHmct~cy ~~a
3 2
R R3
II t ~ III
CR )a ~ I
I V ~ ~ t)FIHCHfiR/IV
1 ~ ~O~Rta a) LSOH
o ~>P
R11 Att
R4
R 9 1)HOe-t-HU/HI~~A S
H° ~ I t ~ ocoar.~ axH,H, r~a° ~ ~ ~~P-cc~H
P 3
R3 R Rt9 R Rtt Rtt
CRto)a ~ I ~ CR,o~a \ I V
R .B-R
R, t)K~co3/n~/vII t)uch/taHH/cHacl,
a) LloH a)c
R' VI I
Rt r
Rs R~
o w R H
I i ~ i ~ C ~P-COaH ~ ~ ' C~ )P~~i~
R R3 Rt Rtt R3 Rit 'Rtt
CRto~a \ I VIII I~ CR,o~a \ 1 IX
t)R~COCl/AtCly
caH,cla
2 S a) H~or~nmoH
Rt
R° CORD R' CORD
t)x~co,/nrar/vxx
i . i ~ -CO H "~ ~ ~ I C ~ -COaMe
a
~~P a) L10H ~ P
R R3 Rft 'R9t R R Rt,
CRto~a \ I XICI~ CRto~' \ I X
b :s y, i ' ;!~ ;'' ... ...~
4.I ~.: ~: r ._~ ~'?
75/DArI30 - 32 - 17940IB
ri~hod 1_
Intermediate V is prepared by a Fischer
reaction between benzylphenylhydrazine III and ketone
IV, followed by hydrolysis with an aqueous solution
of an alkali hydroxide or other suitable hydroxide in
mixture with a suitable water miscible organic
solvent such as tetrahydrofuran (TTiF) or methanol
(MeOH). The methoxy acid V is demethylated by
heating with an alkali salt of an aliphatic thiol in
a suitable solvent such as hexamethylphosphorictri-
amide (HMPA) or N-methylpyrrolidone (NMP). The
reaction mixture is acidified and the crude acid so
obtained is converted to the methyl ester VI by
treatment with diazomethane. The phenol VI is
coupled to the 2-halomethylquinoline VII, by stirring
with a base (preferably an alkali hydride or
carbonate) in a suitable solvent such as dimethyl
formamide (DMF), NMP, acetone or the like. The
resulting ester is hydrolysed by base to yield VIII,
2p a compound of the present invention.
When intermediate V contains a sulfide group
attached to position 3, treatment with a Lewis acid,
such as A1C13, and an aliphatic thiol, simultaneously
effects demethylation and removes the sulfide group.
Suitable solvents for this reaction are methylene
chloride, 1,2-dichloroethane, etc. The resulting
acid is then converted to the methyl ester ~X_ with
diazomethane. A Friedel-Crafts reaction between IX
and an acid chloride, R~COC1, simultaneously
3o introduces the acyl substituent into the 3-position
of the indole ring and onto the phenolic hydroxyl
group. The acyl group is removed from the phenol by
:~~~L~~~~~~!~
75iDAI~I30 - 32 - 17940IB
treatment with sodium methoxide in MeU~i to yield
acylphenol ~. Phenol X is coupled with yTI as
described for the coupling of VI and VII above. In
these coupling reactions, it is at 'times advantageous
to add a catalyst such as potassium iodide or
tetraethylammonium bromide, especially when Hal is
chlorine. A final hydrolysis yields compound XI.
15
25
na l3' t.3 e~' .J
75; D,~q 30 - 33 - 17940IE
METHOD 2
Ra
FI9CHER ~ R5
ii + iv a~eo / ~ ~P,cozR'a
R3 ~ R~R~~
~)xHt~s/at~/-~s°c XI I
2) R°He1
R' Ra
s
Me0 / ~ ~ z -" ~_~~V/~~ .CO R~ a
.COzR Re=g_R'r / ,P a
P
R3 R R R» Ra R R Ri i
XIII
XVT_
1 5 1) LSOH 1) R~COC1/HEt3/THF
2) NeR-t-DU/tIME'A 3) R7COC1/DLLCla
3) CtI~NZ C'NyCiS
R4 9 ) NaCNHtiy/ZnI~ R4 COR7
5 CZHyCS~
HO \ ~ .CO Nk~ '-'~° R~COa \ ~ ,O~Rr2
/ , z 2) NAOtIO/t9soH /
P
2 O Ra ~ R R' ~ Ra R , R"
XI V R R
-- ~ ) ~cac03/nxF/vxi XVIII
z) L40H
t ) NaOMa/MoOFI
R a 5
R a) xaco~/Dtø/vIi
I \ \ \ 3) LiOH
.COaH
25 '~ )P
R Ra ~ y> >
a R R R
XV ( I) R R° COR'
I \ \ \
/ ~ / I , .COaH
~P
R R3 R Rm
R
XI X( I
'v 3t, t' ~%
~~ i9 ~~ u.7 ,:,,~ 3,1
75iBAM30 - S4 - 1~940IB
M~hod 2
Intermediate XII is prepared by a Fischer
reaction between methoxyphenyl hydrazine Tz and
ketone IV, followed by alkylation of the indole
nitrogen, after deprotonation using potassium
hexamethyldisilazane in an ether solvent such as
tetrahydrofuran (THF), with an alkyl or aralkyl
halide.
The methoxy group in XIII is removed using
l0 the conditions of Method 1. The corresponding phenol
XIV is now coupled with the 2-halomethylquinoline VII
by stirring with a base (preferably an alkali hydride
or carbonate) in a suitable solvent such as DMF, IMP
or the like. The resulting ester is hydrolysed using
15 base to yield XV a compound of the present invention.
When intermediate XIII contains a sulfide at
position 3, treatment with a Lewis acid such as A1C13
and an aliphatic thiol simultaneously effects
demethylation and removes the sulfide group.
20 Suitable solvents for this reaction are
dichloromethane or dichloroethane. In a variation of
Method 1, the phenolic hydroxyl in XVI is first
acylated with the reagent R7COC1 (XVII) in the
presence of a weak base such as triethylamine. A
25 Friedel-Crafts reaction. is then carried out on the
0-acylated intermediate, with an additional mole of
XVII and A1C13, to yield the intermediate VIII.
Acyl ester XVIII may 'then be reduced to a 3-alkyl
indole XIV using sodium cyanoborohydride in
30 dichloroethane using a zinc iodide catalyst.
Acyl ester XVTII is cleaved to the indole
phenol by hydrolysis with sodium methoxide in
methanol and is coupled to 2-halomethyl quinoline VIT
::~ ~ t '
i~.a 1 z ° ~ i ,; i ~. ~j
7s,~Darno - s5 - l~s~ozB
using a base such as an alkali hydride or carbonate in
a solvent such as DMF or NMP. Hydrolysis of the
resulting compound using base yields the compound of
the present invention XIX.
METHOD 3
Ra Ra Ra
(eN3)9ccocl ~\ a) ~1/NoNO~
HO ~ ~ t-BuCOz~t-BuCOz-~~y
1 ~~L~'~N_r~~ z) taa~ssoa / z
R3 XX ' R3 XXI R3 XXII
'
t~'°)$pnct~Nai
NSt a /HU,~ NHt /CFI~C ~.~
a a
R 5 msct~u/w R
~
t-BuCaz ~ I ~pzR~~ t-Bucoz ~
~p
XXIif R3 R~ R~ ~ Rs
XXIIT
tRro~z \ ~ ~R~a~z \ S
NaOP~/MeON
as pat trochod i
VI ' VIII I
s~ _ ~ g 3 i~ ~:; _w i
75l~ArI30 - 36 - 17940IB
Me~h_~3_
A suitably substituted aminophenol X~ is
protected on oxygen by the use of pivaloyl chloride
dissolved in CHZC12 using triethyl amine as base.
The pivaloate ester XXI is then diazotized using
hydrochloric acid and sodium nitrite in an aqueous
solvent and the transient diazonium species reduced
in si to the hydrazine XXII using sodium
hydrosulfite in water. Benzylation of the hydrazine
1o is effected as described in Method 1.
The 0-pivaloyl-N-benzylhydrazine XXITI is
subjected to a Fischer indolization using the
appropriate ketone IV to produce the indole XXIV.
Cleavage of the 0-pivaloyl group using sodium
methoxide in methanol transforms the product into the
phenolic indole VI which is converted to the products
of this invention as described in Method 1.
Method 4
R4
FI S ~iiER RS
XXI I a~ I_V > t - BuCOz
'COaI~~ z
~p
R~ XXV ~ ~~R, i
H
1 ) KHt~S/THF
2) Re-Hal
3) NaOMe/M~pH
ae par Method 2
XV~I) 4- XIV
~,:e~t~~r~ ~
75/D.~1rii30 - 37 - 17940TB
Method 4
The pivaloyloxyphenylhydrazine XXTI is used
directly in the Fischer indolization using ketone
IV. N-Alkylation of the indole XXV, as described in
Method 2, followed by removal of the pivaloyl group
as described, yields the phenolic indole XIV which is
converted as described in Method 2 to the products of
this invention.
ZO
Z5
25
75iDArt30 - 38 - 11940s~
Method 5
Ra R a
Ksco,/nr~wxx R
HO-~ O (
i a~' I , ~ '(
a '~y ~~~~
w W~~~~C
R ~ ~ ~ n1 R Rs
XXVI z XXVII
R _VII
KOH/oq. EtOH/hent
a
R'
R'
Ra Ra
9) Hci/HnNO,
1S w w
I , ~~ i ~ O-
i z) Nnagzo; i
R I,r-~z R ~ z
XXIX XXVIII R
2O f R'~)aPhCH;-Hal
f i- Pr ) aNEt /9ust7Er /CHaC1=
R'
Ra
9) Fiecher/xV
VIII I)
25 ~ ~z z) LiOH
R Rz
i XXX
~R~o~a \ I
~~~) 3~~~~'~''~
75;''B\'I30 - 39 - 17940IB
Pi~tho_~ S
A suitable N-acetylated aminophenol XXVI is
reacted with VIT using an alkali hydride or
carbonate, such as potassium carbonate as a base in a
polar solvent like DMF or NMP. The quinolinylmethoxy
acetanilide XXVII is then de-acetylated using
standard basic conditions, preferably using alcoholic
potassium hydroxide under reflux to produce the
quinolinylmethoxy aniline derivative XXVIII.
conversion of the quinolinylmethoxy aniline
derivative to the hydrazine analogue XXIX is effected
through reduction of the intermediate diazonium salt
using sodium hydrosulfite in an aqueous medium.
The hydrazine XXIX is then N-benzylated
using a benzyl halide in an organic solvent such as
methylene chloride containing an amine base such as
diisopropylethylamine and preferably
tetra-n-butylammonium bromide as catalyst.
The hydrazine XXX is then processed using a
Fischer indolization with ketone IV according to
Methods 1, 2, 3 and 4 to produce compounds of the
present invention.
30
'y~~~.~i~4i~~; ~~
Cu ~ c
75IDAri'y0 - 40 - 17940TB
ri~tn~t~
s
R'
O 4
1 ) F'IaCHER/XXSX R
R ~OaRt z _ I w w v w 5
n (
z) KHHDa/Ttig/RBNnl ~ ~ i CO R'a
t t ~ t t ~~~~~ ~n 2
R R R R3
R'~R"
XXXI XXXII R
LiAIH~/TtH' R" My Hr
I ~ ° R"
I
t R R R ~ R
w w ~ R" ~ XXxIII R
I i ~ a I .~ ~.iPsli~
N
n t7H
R ~a R, , Rt ~
XX3CCV R t) HeH~ixxxv
R77 2) LiOH
Hdl'~ ~ 'COzN.B
xxxv R,
Ra s
I w w a R77 Rt,
I I
2 5 '~ ' i
R 3 C~ ~O~ ~P~COa H
R Ra R" R"
XXXVI( I)
a~~~~,:.;j
75/1?!~i30 - 41 - 1~940IB
M e_'~~ 6
Hydrazine XXTX may also be transformed
directly to unsubstituted indoles by a Fischer
reaction with various ketones like XXXT.
N-Alkylation of the indoles is effected using the
conditions described in Method 2 to produce
quinolinylmetho~-yindole alkanoate esters XXXTT. Such
esters are transformed to ketones or carbinols via
Grignard conditions using alkyl magnesium halides in
ether solvents like diethyl ether or through the use
of lithium aluminum hydride in ether solvents like
THF. The carbinols XXXIV so produced may be further
transformed into ester compounds of the present
invention by reacting with a-halo esters XXXV using
sodium hydride as base in a suitable solvent like
THF. Subsequent hydrolysis of the esters using
Method 1 leads to acid compounds of the present
invention.
25
75;~'nari3o - 4~ - 179~oz~
rs~thod 7
R'~
A1C13/EtSH ~ FI
XII ---. HO ~ I ( ~)p OzRt z
( Rs=S-R~) R~ ~ Rft 'Rt t
H
XXXVII
VII
KzC03/DME
Rt
R4 H
XXIX + IV ~ ~ ~ ~ ~ ~O Rtz
)F 2
FISCHER R R3 H R~Rt t
XXXVIII
Rt Rs Rs
Rs-C1
( i ~ o I ( x)a OzRtz
A1C13
R R3 H Rt/1 \Rt s
XXXIX
IRe-Hal/basa
2 5 Xv I
75/DAI~i~0 - 43 - 17940IB
Method 7
Phenol XXXVII is obtained by treatment of XII
(R5 = S-R~) with a Lewis acid and a thiol, as in
Method 1 for the conversion of V to ~X. Compound
XXXVIII is then obtained by reaction of XXXVII with
VII in the presence of a base in a suitable solvent,
as described for the conversion of VI to VIII in
Method 1. The introduction of R5 in XXXIX is
conveniently effected by an electrophilic reaction
l0 between XXXVIII and R5-C1 (R5 not = Xl-R6). Such
reactions are frequently catalysed by Lewis acids or
proton acids such as A1C13, SnCl4, TiCl4, BBr3, HCI,
HBr and the like. They may be carrid out in a variety
of solvents, with a preference for non-protonic
solvents such as dichloromethane, 1,2-dichloroethane,
nitromethane, chlorobenzene and the like. It will be
obvious to one skilled in the art, that the chlorine
in R~-Cl, in this and the other Methods, may often be
replaced by another halogen or by a hydroxyl group, or
2o R5-C1 may be replaced by an acid anhydride (R~CO)20.
An alternative synthesis of XXXIX is to effect a
Fischer reaction between compounds IV and XXIX.
Introduction of R8 into XXXIX, is accomplished by
alkylation with R~-Hal and a base as described
Previously for Methods 2, 4 and S. Finally,
hydrolysis of the ester will yield XV. Alternatively,
the ester group in XXXIX can be hydrolysed, and the
corresponding free acid (R12 = H) alkylated on the
indole nitrogen with R8-Ha1 and an aqueous base, such
3o as NaOH, and a phase-transfer catalyst, such as
methyltrioctylammonium chloride. Alkylation of the
acid corresponding to XXXIX (R12 = H) can also be
'~~ f~ ~.' .;~ ;.,
~,~~5~;i;.?~:W
7 5ln~~i30 - 44 - 17940IB
effected using a strong base such as sodium hydride in
a solvent such as DME'. This latter procedure usually
gives the ester of XV in which the carboxyl group has
also been alkylated. The free acid XV can be obtained
by standard hydrolysis procedures. Tf R$ in XV or the
ester precurson of XV is alkenyl, it can be reduced to
alkyl using hydrogen gas, and a Pt or Pd catalyst in a
suitable solvent, at atmospheric pressure.
15
25
4~ E. ;5 1 <J .. N
75iDAri;~O - 45 - 1.7940~:~
M_~t ~ 8
Rt
Re -R~
( VI + VII) or ~ \ \ \ tz
r i ~ r B .C02R
(xxx + zV ~ C~)P
(R~-S_R~) R R3 R t Rtt
r
(Rto)z \ I
A1C13/CHZCIz
XL
Rt
R4
\ \
r ~ ( ) ~~R9 ~
~P
R Rt~Rrt
XLI (Rto)z
R'-COCl/L~~wls Acid/CHZClz
t 5
2 0 R Rm COR° R R4 R~
\ \ ~ \ \
r ~( )COzRtz+ ~ r r ~ )C03Rtz
x P R 3 N ~P
R R3 Rt/t \Rt t R Rm Rm
(Rt°)z \ ~ XLII (RtD)Z \ ~ XLIII
Hydrolysis ~ Hydrolyaia
XI ( I) R R'~ R~
\ \ \
r I ( ) ~OzH
P
R R3 R,~Rtt
(Rt°)z \ ~ XLIV (I)
75/DAri30 - 46 - 17940IB
Meths 8
Compound XL may be prepared either by the
coupling of VI to VII (Method 1) or by a Fischer
reaction between IV and XXX (Method 5). Compound
may be desulfurized by treatment with a Lewis acid
such as A1C13, or by reduction with Raney nickel, to
give compound XLI. A Friedel-Crafts reaction on XLI
with the reagent R7COC1 and a Lewis acid catalyst
such as A1C13 yields the 3-acyl derivative XLII,
Tp hydrolysis of which yields XI. In the Friedel-Crafts
reaction, carbon monoxide may be lost and compound
XLIII_ is formed; hydrolysis under standard conditions
then yields XLTV. The formation of XLIII_ occurs when
the cation R7+ is especially stable and when the
15 reagents R7COC1 and the Lewis acid are mixed before
adding XLI. If the Lewis acid is added last, the
main product is usually the acylated compound X~II.
If a milder Lewis acid such as TiCl4 is used, the
main product is also XLII.
It will be obvious to one skilled in the art
that the reagent R7COC1 can often be replaced by
R7C0-Hal (Hal = F, Br or I) or (R7C0)~0.
30
a~ ~~ ~i '~ <.i :i.j ,o
75/PAriSO - 47 - 179~~oIB
r~~2
Rg
rrto, pyr. ~ R nd(oac)r r co
----~ TF O ~ ~ 1 .COa I~ _
CF1'Cl~ 9[lp DlB0. MeOH, BCjH
R3 R tt ~Rtt t,t-ai°(diph.nyipho.-
phino)fertoc°ne
(RtD~z
XLV ~o_eo°c
~O R4 R' t. ueox ~ R~ 5
Z O ~ I 2. D28AL, THF \ I ,CO H
,COz2he ~ z
O R3 ~ptt o°C to r.C. R3 (~p~t
Rf 'R R R
(R,o~a ~ (R,o)a
XLVI ~ XLVII
Rt
Z5
Rg w w
s 1 ~ ~~~ph3
rs~o, ~ HCO ~ I ( >p.CpaH R XLIX
cts~clz R3 R" Rt t
~/J TtIY'
( Rt p' a~ -70°C to t. t.
Ra
..( ) 'COZH rte, ,o% adrc
/'7~\ p
R" Rt t Btoac
2~ (R~D~'~
L (x)
Rt
R4 5
.COQ H
~~p
3 0 R3 Rf, 'R"
(R,o~~ 1~
L= ( x
p~ ~ -, ..1 :_. .. ;~,
~, ~e~ iI ~...! , ~~
75IPt'~I30 - 48 - 1~940IB
Method
Indole phenol VI which may be prepared
according to Methods 1 or 2 is transformed to a
phenol triflate ~LV by treatment with trifluoro-
methyl sulfonic anhydride (Tf~O) in a solvent like
pyridine in dichloromethane. The phenol triflate may
be carboxymethylated to a compound like XLVI under
palladium acetate catalysis in an atmosphere of
carbon monoxide, a phosphine ligand like 1,1-bis(di-
1o phenylphosphinoferrocene) enhances this reaction.
Reduction of the carboxymethylated indole may be
effected with a variety of hydride reducing agents.
Conveniently diisobutylaluminumhydride is used in THF
on the hydrolysed ester. The reduced carbinol
Product XLVII is conveniently oxidized to a
formylated _derivative XLVIII with manganese dioxide
in methylene chloride as a typical solvent. Aldehyde
XLVIII can then be homologated under carbanion
conditions, typically using Wittig reagent ~LTX (see
2o UW ~ Pat. 4,851,409) as shown in 'the method, under
anydrous conditions in an etherial solvent like THF.
The temperature of this reaction is typically from
-70°C to room temperature. Indole styryl quinoline
analogues (trans) _L are thus formed. Further
transformation of the styryl system may be effected
by catalytic reduction using H2 and Pd/C in an
organic solvent like ethyl acetate to yield the
saturated compound LI.
75/DAri30 - 49 - ~7940IB
M~hocl 10
v
R~ R~
clceems, pp
Hp \ ~ m ~aN~ ~ ~ m
r
HakL DfH" /
Ra O~R, , ~ S R3 O~Rs r
R,t p_t R» p_t
LII LIII
-
1,5°C
Rs R~
w R" R" ,. rroNa, taon ~ ~9
.-. Ma t3 w
S ~ , I Ozt~ a, sock, toot! a / ~ "
R3 ~ tt p_t O
Yi R Z R O L~R~ ~
Lv ~ Rt > > p_,
- LI V
Pn,P
D1°%ana. l1,0
1
Ra Re R Rs Rs
2 0 ~ I Rt , R" dry y ~ w w r ~ ~ R" R, a
/ / ~ /
N~
R3 H R" P ~ ~ ~ H Rt! P''
L~ LVII
,) ~do),nnctt~cl
tans nrue
a) nyeroxy~ia
R~ CORD R° . H R'
R
w g~i~ R» a'ccclixlcl, ~ Rtt Rtt
QuCH=S ~ QUCHaS
/ O H / _'~"CO~H
R~ , t P-t ~ Otf,Cl,. -70°C R~ p_ y
R R
(R'°)a ~ ~ LIx (z) (R'°)a ~ ~ LvtII (I)
Rt
. .
Q~ _ ~
a '~ l
y f
isiDaMSO - 50 - l7g~olB
rz~ h~ ~d lo.
Indole thio analogues of I such as LIX are
conveniently prepared by the sequence shown in Method
10. The treatment of compound V with BBr3 in a
chlorinated solvent such as CH2C12 cleaves both the
methyl ether and the indole N-~benzyl group and
cyclizes _-the product to an indole lactam LII.
Derivatization of this compound as an N,N-dimethyl-
thiocarbamoyl indole LIII followed by thermal
rearrangement at >200°C gives rise to an N,N-dimethyl-
carbamoylthioindole derivative LIV. Depending on the
duration of heating, dethiolation (R5=-S-t-Bu ~ R~=~3)
may also take place. The hydrolysis of LIV may be
effected using strong base, typically sodium
methoxide in methanol is used. Spontaneous formation
of disulfide LV may occur in this reaction. The
reduction of LV can be achieved using triphenyl-
phosphine in aqeuous dioxane to produce ~VT_.
Coupling of LVI to an appropriately substituted
quinoline derivative VII takes place under organic
base catalysis; typically trimethylamine, in an
organic solvent such, as methylene chloride, is used.
Transformation of indole LVII to an N-benzylated
derivative LIX is achieved under standard conditions
described in Method 2 or by benzylation with an
appropriate benzyl halide using a base such as sodium
hydride in a non-protic solvent such as dimethylforma-
mide.
~1 J ~, ~~.i ~;J
v~; ~~rszo - 5i - i~9~.ozB
Method 11
10
-CHzOH
L i A1 I~
s(o)cl2 R'~SCo)a~z
-COzR~2 Ri2-H -COC1 - . _CONHS(O)ZR'~
R~ 7~1 i ~
bas a R OH R~ sRl 5NH
R' 2= H
bas a
- COzR, ~ - CONR~ 5R7 s
dek~ydrate/I?z05
R~ 5 = R~ 5= H
0
-CN
Na N~
~N~
N
N~N
1 H- or 2H-tetrazol-5-yl
f~3i~.:::~a i
75/DAPi30 - 52 - 179~~OIB
riethod 11
The preparation of the various definitions of
Q is outline in Method 11, starting from the readily
available carboxylic acid derivative -C02R1z.
It will be obvious to one skilled in the art that many
of the reactions indicated are reversible. Thus,
by way of illustration, the -CN group can serve as the
starting material to prepare the amide and carboxylic
acid functional groups. The reactions depicted in
Method 11 as well as methods for synthesis
of the sulfonamide group (-S(0)2NHR15) are well-known
in the art. See, for instance the following text books:
1. J. March, Advanced Organic Chemis~r , 3rd ed., J.
Wiley and Sons, Toronto, 1985.
2. S.R. Sandier and W. Karo, Qrganic Functional Grout/
Preparations, I & II, Academic Press, Toronto, 1983
and 1986.
25
s~ ~1 ~ '~ ~~> ;~~ ~a
75iDAri30 - 53 -- 179408
Piethod 12
R~
a H~ C
QuCH20 ,C02R~ 2
a ~~2 '
R~ O p LX
XXX R~ ~
cR~o~z
l0
R4
C H3
\ .C02R~ 2
QuC Hz O ~X~~1
~' s~ //~~~p ~ J Pz O~ /CH3 S 03 H
R3 R~~ R1t Ce~lvent,
C R~ off' / I
2 0 LXI
R4
R~
H
QuCHZO ~,, ~ ~.C02R~ ~ \ \
2 5 p3 P Qu = ~ ~ ~ .
R,~ Ri1 R
~R~o~2
XLI
CA 02060557 2001-10-29
75/DAM30 - 54 - 17940IB
Method 12
3-Unsubstituted indole analog XLI, described
in Method 8, may be more conveniently prepared by the
process illustrated in Method 12.
Thus, the suitably substituted hydrazine
XXX is reacted with the suitably substituted methyl
ketone LX to provide the hydrazone LXI. The
hydrazone LXI is treated with a combination of
phosphorous pentoxide and methane sulfonic acid,
°Ptionally in the presence of a suitable co-solvent,
such as sulfolane, dichloromethane and the like, to
provide the 3-unsubstituted indole XLI.
Representative Compounds
Table I, and Table II and Table III
illustrate compounds having the formulae Ia, Ib and
2o Ie respectively representative of the present
invention. "Attach point" is the position on the
indole nucleus where the quinolylmethoxy moiety is
attached.
30
3 , .a ." '. j ; ''!
il ~i.1 'eJ '~.~ :.D ..
75:'pAM:O - 55 - 1794oIB
TABLE I
R~
R5
-CHZp ~~--CHZ-Y-(CRttRtt~P CCZH
R
Rs
RB Ia
Ex R1 R3 ATTACHRB R5 Y- (CR11R11)
R2
, P
No. POINT
1 H,H H 5 -CH2Ph-4-C1-S-t-Bu C(Me)2
2 H,H H 5 -CH2Ph-4-C1Me C(Me)2
3 H,H rl 5 -CH2Ph-4-S-t-Bu-S-t-Bu C(Me)2
4 H,H FI 5 -CH2Ph-4-C1-SPh C(Me)2
6 H,H H 5 -CH2Ph-4-Cl-S(0)2Ph C(Me)2
7 H,H H 5 -CH2Ph-4-C1-S(0)Ph C(Me)2
8 H,H H 5 -CH2Ph-4-C1H C(Me)2
9 H,H FI 5 -CH2Ph-4-C1-C(0)Ph C(Me)2
10 H,H H 5 -CH2Ph-4-C1-CH2Ph C(Me)2
11 H,H H 5 -CH2Ph-4-C1-C(0)CH2-t-BuC(Me)2
12 H,H H 5 -CH2Ph-4-C1-S-t-Bu CH20CH2
13 H,H H 5 -CH2Ph-4-Cl-CH2CH2-t-BuC(Me)2
14 H,H H 5 -CH2Ph-4-C1-S-t-Bu CH(Me)
15 6-C1, H 5 -CH2Ph-4-C1Me C(Me)2
7-C1
16 H, 7-C1 H 5 -CH2Ph-4-C1Me C(Me)2
17 H,H 4-allyl5 -CH2Ph-4-CI-S-t-Bu C(Me)2
18 H,H A-allyl5 -CFl2Ph-4-C1H ~(Me)2
~.a t9 a) ~,~ ~~:~ '.:1 r,'i
75; PAri30 - 56 - 17940IB
TABT~E II
R5
\ \
I / ~ CH O / ~ \ CHZ-Y-(CR~'R~i~P_COZH
\ ri
Ra I b
Ex ATTACH R8 R5 Y-(CRiIRII)P
No. POINT
19 6 -CH2Ph-4-C1 -S-t-Bu C(Me)2
4 -CH2Ph-4-C1 -S-t-Bu C(Me)2
21 7 -CH2Ph-4-C1 -S-t-Bu C(Me)2
22 5 -CH2Ph-4-C1 -S-t-Bu CH20CH(Me)
15 23 4 -CH2Ph-4-C1 H C(Me)2
24 6 Me -C(0)Ph-4-G1 C(Me)2
6 Me -CH2Ph-4-C1 C(Me)2
26 5 -CH2Ph-4-Cl -0-i-Ps C(Me)2
27 5 -CH2Ph-4-C1 -S-t-Bu CH(Et)
20 28 5 -CH2Ph-4-C1 ~-C(0)-CF3 C(Me)2
29 5 -CH2Ph-4-C1 -C(0)CH2-t-BuCH(Me)
5 H -C<0)CH2-t-BuC(Me)2
31 5 -CH2Ph-4-CF3 .-C(0)CH2-t-BuC(Me)2
32 5 -CH2Ph -C(0)CH2-t-BuC(Me)2
25 33 5 -CH2Ph-3-OMe -C(0)CH2-t-BuC(Me)2
34 5 -CH2CHCH2 -C(0)CH2-t-BuC(Me)2
5 -CH2Ph-4-0Me -C(0)CH2-t-BuC(Me)2
36 5 Me -C(0)CH2-t-BuC(Me)2
37 6 H -CH2Ph-4-C:L C(Me)2
30 38 6 -S(0)2Ph -CH2Ph-4-C1 C(Me)2
39 6 -CH2Ph -CH2Ph-4-C1 C(Me)2
5 -CH2Ph-4-Cl -S(0)2-t-Bu C(Me)2
41 5 -CH2Ph-4-C1 -S(0)-t-Bu C(Me)2
fir,! '7~ is ~'a e~.:~ y,) J
75iDAM30 - 57 - 17940IB
TABLE II (cont.)
Ex ATTACH R8 R5 Y-(CIt11Ft11)P
No. POINT
42 6 -CH2CHCH2 -CH2Ph-4-CI C(Me)2
43 6 -CCH2)2CH3 -CH2Ph-4-C1 C(Me)2
44 6 -CH2CH3 -CH2Ph-4-CZ C(Me)2
45 5 -GH2Ph-4-C1 -C(0)Ph-4-t-Bu C(Me)2
46 5 -CH2Ph-4-C1 -C(0)Ph-4-C1 C(Me)2
47 5 -CH2Ph-4-C1 -t-Bu C(Me)2
48 5 -CH2Ph-4-C1 -C(0)Me C(Me)2
49 5 -CH2Ph-4-C1 -C(0)-c-Pr C(Me)2
50 5 -CH2Ph-4-C1 -C(0)CH2CH2-c-G5H9C(Me)2
51 5 -CH2Ph-4-C1 -C(0)CH2CH<rIe)2 C(Me)2
52 5 -CH2Ph-4-C1 -C(a)Et C(Me)2
53 5 -(;H2Ph-4-C1 -C(0)CH(Me)2 C(Me)2
54 5 -GH2Ph-4-C1 -C(0)C(Me)3 C(Me)2
55 5 -CH2Ph-4-C1 -C(0)CH2Ph C(Me)2
56 5 -CH2Ph-4-~' -G(p)CH2-t-Bu C(Me)2
57 5 -CH2Ph-4-Br -C(0)CH2-t-Bu C(Me)2
58 5 -GH2Ph-4-I -C(0)CH2-t-Bu C(Me)2
59 5 -CH2Ph-4-C1 -C(Me)2Pr C(Me)2
60 5 -CH2Ph-4-C1 -C(Me)2Et C(Me)2
61 5 -CH2Ph-3-F -t-Bu C(Me)2
62 5 -CH2Ph-4-C1 -CH(Me)2 C(Me)2
63 5 -CH2Ph-4-C1 -c-Pr C(Me)2
64 5 -CH2Ph-4-C1 -(1-Me)-c-Pr C(Me)2
65 5 -CH2Ph-4-C1 -c-C5H9 C(Me)2
66 5 -CH2Ph-4-C1 -c-C6H11 C(Me)2
67 5 -CH2Ph-4-C1 -C(Me)2Ph C(Me)2
68 5 -CH2Ph-4-C1 -C(Me)2Ph-4-C1 C(Me)2
~J ~.~ ~ '4: ~_,~ ~
:~ s~
75/DAM30 - 58 - 17940IB
TABLE II (coat.)
Ex ATTACH R$ R5 ~-(CR11R11)p
No. POTNT
J
69 5 -CH2Ph-4-C1 -1-Ad C(Me)2
70 5 -CH2Ph--4-C1 -CH2-1-Ad C(Me)2
71 6 -t-Bu -CH2Ph-4-C1 C(Me)2
72 6 -C(Me)2Et -CH2Ph-4-C1 C(Me)2
5 -CH2Ph-4-CI -C(0)CH2-t-BuC(Et)2
73
20
30
Fa ~~ ~3 ~ _S ',~ 'J
75!~art3o - 59 - 1794oz~
TABLE III
~ ~ ~ ~ S-tBu
~~~2y~ CR11 R11 ~ pQ
N
/ ~ Ie
-..
C1
Ex. No. x4 "~-(C~11R1~)p Q
76 CH20 C(Me)2 -C(0)NHS(0)2Me
77 CH20 C(Me)2 -NHS(0)2Hh-4-Me
78 CH20 C(Me)z -C(0)NH-t-:8u
79 CH20 OCH2CH<Me) -C02H
8~ CH20 CH2CH2 Tz
81 CH~O OCH(Me) Tz
82 CH20 C(Me)2 -S(0)2NH-Et
83 CH20 C(Me)2 -C02CH2C(0)~lMez
84 CH20 C(Me)2 -C(0)NHCH~COZH
g5 CH20 C(Me)~ -CH20H
86 (E)-CH=CH C(Me)2 -C02H
87 CHZCH~ C(Me)2 -C02H
88 CHZS C(Me)z -C02H
89 CHZS(0)2 C(Me)2 -C02H
'., ,.\
~1?t~i,~.:..~.. .
75iD.AM30 - 60 - 17~40IB
A~s~ys for Determining Biological ACtivi
Compounds of Formula I can be tested using the
following assays to determine their mammalian
leukotriene biosynthesis inhibiting activity.
Ra Peritoneal Pol~morphonuclear (PMN) L~a~COC_.~
Assay
Rats under ether anesthesia are injected
(i.p.) with 8 mL of a suspension of sodium caseinate
(6 grams in Via. 50 mL water). After l5-24 hr. the
rats are sacrificed (C02) and the cells from the
peritoneal cavity are recovered by lavage with 20 mL
of buffer (Eagles MEM containing 30 mM HEPES adjusted
to pH 7.4 with NaOH). The cells are pelleted (350 ~
g, 5 min.), resuspended in buffer with vigorous
shaking, filtered through lens paper, recentrifuged
and finally suspended in buffer at a concentration of
10 cells/mL. A 500 mL aliquot of PMN suspension and
test compound are preincubated for 2 minutes at 37°C,
2o followed by the addition of l0 mM A-23187. The
suspension is stirred for an additional 4 minutes
then bioassayed for LTB~~ content by adding an aliquot
to a second 500 mL portion of the PMN at 37°C. The
LTB4 produced in the first incubation causes
~5 aggregation of the second PMN, which is measured as a
change in light transmission. The size of the assay
aliquot is chosen to give a submaximal transmission
change (usually -70%) for the untreated cowtrol. The
percentage inhibition of LTB4 formation is calcuated
30 form the ratio of transmission change in the sample
to the transmission change in the compound-free
control.
CA 02060557 2001-10-29
75/DAM30 - 61 - 17940IB
Human PolYmorphonuclear (PMN) Leukocyte LTB4 Assay
A. Preparation of Human PMN. Human blood was
obtained by antecubital venepuncture from consenting
volunteers who had not taken medication within the
previous 7 days. The blood was immediately added to
10% (v/v) trisodium citrate (0.13 M) or 5% (v/v)
sodium heparin (1000 IU/mL). PMNs were isolated from
anticoagulated blood by dextran sedimentation of
erythrocytes followed by centrifugation through
Ficoll-HypaqueM(specific gravity 1.077), as described
by Boyum (Scand. J. Clin. Lab. Invest., 21 (Supp.
9~, 77(1968)). Contaminating erythrocytes were
removed by lysis following exposure to ammonium
chloride (0.16 M) in Tris buffer <pH 7.65), and the
PMNs resuspended at 5 x 105 cells/mL in HEPES (15
mM)-buffered Hanks balanced salt solution containing
Ca2+ (1.4 mM) and Mg2+ (0.7 mM), pH 7.4. Viability
was assessed by Trypan blue exclusion and was
typically greater than 98%.
B- Generation and Radioimmunoassay of LTB4.
PMNs (0.5 mL; 2.5 x 105 cells) were placed in plastic
tubes and incubated (37°C, 2 min) with test compounds
at the desired concentration or vehicle (DMSO, final
concentration 0.2%) as control. The synthesis of
LTB4 was initiated by the addition of calcium
ionophore A23187 (final concentration 10 mM) or
vehicle in control samples and allowed to proceed for
5 minutes at 37°C. The reactions were then
terminated by the addition of cold methanol (0.25 mL)
3o and samples of the entire PMN reaction mixture were
removed f or radioimmunoassay of LTB4.
Samples (50 mL) of authentic LTB4 of known
CA 02060557 2001-10-29
75/DAM30 - 62 - 17940IB
concentration in radioimmunoassay buffer <RIA) buffer
(potassium phosphate 1 mM; disodium EDTA 0.1 mM;
ThimerosalTM0.025 mM; gelatin 0.1%, pH 7.3) or PMN
reaction mixture diluted 1:1 with RIA buffer were
added to reaction tubes. Thereafter [3H]-LTB4 (10
nCi in 100 mL RIA buffer) and LTB4-antiserum (100 mL
of a 1:3000 dilution in RIA buffer) were added and
the tubes vortexed. Reactants were allowed to
equilibrate by incubation overnight at 4°C. To
l0 separate antibody-bound from free LTB4, aliquots (50
mL) of activated charcoal <3% activated charcoal in
RIA buffer containing 0.25% DextranMT-70) were added,
the tubes vortexed, and allowed to stand at room
temperature f or 10 minutes prior to centrifugation
01500 x g; 10 min; 4°C). The supernatants containing
antibody-bound LTB4 were decanted into vials and
TM
Aquasol 2 (4 mL) was added. Radioactivity was
quantified by liquid scintillation spectrometry.
Preliminary studies established that the amount of
methanol carried into the radioimmunoassay did not
influence the results. The specificity of the
antiserum and the sensitivity of the procedure have
been described by Rokach g~ ~1. (Prostaglandins
Leukotrienes and Medicine 1984, ~, 21.) The amount
of LTB4 produced in test and control (approx. 20
ng/106 cells) samples were calculated. Inhibitory
dose-response curves were constructed using a
four-parameter algorithm and from these the IC50
values were determined.
Asthmatic Rat Assay
Rats are obtained from an inbred line of
asthmatic rats. Both female (190-250 g) and male
CA 02060557 2001-10-29
75/DAM30 - 63 - 17940IB
(260-400 g) rats are used.
Egg albumin <EA), grade V, crystallized and
lyophilized, is obtained from Sigma Chemical Co., St.
Louis. Aluminum hydroxide is obtained from the Regis
Chemical Company, Chicago. Methysergide bimaleate
was supplied by Sandoz Ltd., Basel.
The challenge and subsequent respiratory
recordings are carried out in a clear plastic box
with internal dimensions 10 x 6 x 4 inches. The top
of the box is removable; in use, it is held firmly in
place by four clamps and an airtight seal is
maintained by a soft rubber gasket. Through the
center of each end of the chamber a Devilbiss~M
nebulizer (No. 40) is inserted via an airtight seal
and each end of the box also has an outlet. A
Fleisch No. 0000 pneumotachograph is inserted into
one end of the box and coupled to a Grass volumetric
pressure transducer (PTS-A) which is then connected
to a Beckman Type R Dynowraph through appropriate
2o couplers. While aerosolizing the antigen, the
outlets are open and the pneumotachograph is isolated
from the chamber. The outlets are closed and the
pneumotachograph and the chamber are connected during
the recording of the respiratory patterns. For
challenge, 2 mL of a 3% solution of antigen in saline
is placed into each nebulizer and the aerosol is
generated with air from a small Potter diaphragm pump
operating at 10 psi and a flow of 8 liters/minute.
Rats are sensitized by injecting
3o (subcutaneously) 1 mL of a suspension containing 1 mg
EA and 200 mg aluminum hydroxide in saline. They are
CA 02060557 2001-10-29
75/DAM30 - 64 - 17940IB
used between days 12 and 24 postsensitization. In
order to eliminate the serotonin component of the
response, rats are pretreated intravenously 5 minutes
prior to aerosol challenge with 3.0 mgm/kg of
methysergide. Rats are then exposed to an aerosol of
3% EA in saline for exactly 1 minute, then their
respiratory profiles are recorded for a further 30
minutes. The duration of continuous dyspnea is
measured from the respiratory recordings.
1o Compounds are generally administered either
orally 1-4 hours prior to challenge or intravenously
2 minutes prior to challenge. They are either
dissolved in saline or 1% Methocel'or suspended in 1%
methocel. The volume injected is 1 mL/kg
(intravenously) or 10 mL/kg (orally). Prior to oral
treatment rats are starved overnight. Their activity
is determined in terms of their ability to decrease
the duration of symptoms of dyspnea in comparison
with a group of vehicle-treated controls. Usually, a
compound is evaluated at a series of doses and an
ED50 is determined. This is defined as the dose
(mg/kg) which would inhibit the duration of symptoms
by 50%.
The invention is further defined by
reference to the following examples, which are
intended to be illustrative and not limiting. All
temperatures are in degrees Celsius.
Example 1_
3-(N-(p-Chlorobenzyl)-3-(t-butylthio)-5-
(quinolin-2-ylmethoxy)indol-2-yl]-2,
2-dimethylvropanoic acid
~i ~~ '~ ~~: ~ ~ F
75/l~ArI30 - 65 - 17940TB
Step A: 3-[N-p-Chlorobenzyl-3-(t-butylthio)-5-
methoxyindol-2-yl]-2,2-dimethylpropanoic
acid methyl ester
To a solution of 39 g of methyl
5-(t-butylthio)-2,2-dimethyl-4-oxopentanoate in a
mixture of 300 mL of toluene and 150 mL of glacial
acetic acid was added 15 g of NaOAc and 50 g of
1-(4-methoxyphenyl)-1-(p-chlorobenzyl)hydrazine
hydrochloride. The reaction was maintained with
stirring at room temperature for 3 days under argon
in the dark. The mixture was poured into 3 L of H20
and extracted with 3 x 500 mL of EtOAc. The ethyl
acetate was washed with 3 x 500 mL of water then
solid NaHC03 was added. The mixture was filtered and
the filtrate washed twice with water. The organic
phase was dried over MgS04 arid evaporated to dryness
to provide the title compound. m.p. 102-103° C.
3-[N-(p-Chlorobenzyl)-3-(t-butylthio)-5-
methoxyindol-2-yl]-2,2-dimethylpropanoic
The compound from Step A was hydrolysed
using 325 mL of THE, 600 mL of MeOH and 325 mL of
1.OM LiOH. The solution was heated to 80° C for 3
h. The solution was acidified with 1N HC1 and
extracted with. 3 x 200 mL of EtOAc. The organic
phase was washed with Oater (2 x 150 mL) and dried
over MgS04. The solution was evaporated to dryness
to provide the title compound. m.p. 190-191° C.
"l 0 ~9
i o
75lDArI30 - 66 - 17940IB
Anal C, H, N; Calc. C 65.27; H 6.57; N 3.04,
Found C 65.28; H 6.58; N 3.04
tep~: Methyl 3-[N-(p-chlorobenzyl)-5-hydroxy-3-
(t -butylthio)indol-2-yl]-2,2-dimethyl-
propanoate
A solution of 61 mL of t-butylthiol in 650
mL of dry HMPA at 0° C was treated portionwise with
26 g of 50% NaH in mineral oil after removal of oil
with hexane. The reaction was stirred at RT for 30
mins and 46 g of the compound from Step B was added.
The reaction was then heated under N2 at
175° C for 5 hours. The solution was cooled, and
Poured onto crushed ice, after which it was treated
with 2 N HCl to pH 5 and extracted with EtOAc (3 x
500 mL). The organic phase was washed with H20 (3 x
200 mL) dried (MgS04) and evaporated. The residue
was dissolved in 300 mL of ether and ethereal
2o diazomethane was added until all acid was consumed.
The excess solvent was removed and the oily residue
triturated with hexane to leave a crystalline mass
which was recrystallized from EtOAc/hexane to provide
the title compound as a white crystalline solid, m.p.
170-171° C. From the mother liquors was isolated
methyl 3-[N-(p-t-butylthiobenzyl)-5-hydroxy-3-
(t-butylthio)indol-2-yl~-2,2-dimethyl propanoate
which was used as such in Example 3.
h, 'al ~.l ' l ;.~~a ;~ ~~
75,'DAM30 - 67 - 17940IB
Step D: Methyl 3-[N-(p-chlorobenzyl)-3-(t-butylthia)-
5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-
dimethylpropanoate
Methyl 3-[N-(p-chlorobenzyl)-5-hydroxy-3-
(t-butylthio)indol-2-yl]-2,2-dimethylpropanoate (33.6
g) from Step C was dissolved in 500 mL of dry DMF and
the solution was charged with 2.4 g of KI, 30.3 g of
K2C03, 4.77 g of Cs2C03 and 23.5 g of
2-(chloromethyl)quinoline hydrochloride. The
reaction was stirred at RT, under N2, for 72 hours
then it was poured into water (1.5 L), acidified with
1N HCl and extracted (3 x 200 mL) with GH2C12. The
organic phase was washed with H20 (3 x 150 mL), dried
and evaporated. The residue was dissolved in hot
EtOAc and upon cooling crystallized to deposit 22.0 g
of the title compound, m.p. 166-167° C..
Step E: 3-[N-(p-Chlorobenzyl)-3-(t-butylthio)-5--
(quinolin-2-ylmethoxy)indol-2-yl]-2,
2-dimethv7~ropanoic~.id
Using the hydrolytic procedure of Step B but
substituting the ester of Step D for the ester of
Step A provided the title compound, which was
recrystallized from 1:1 EtOAc/hexane. m.p. 208°C.
Anal G, H, N: Calc. C 69.55; H 6.01; N 4.77,
Found C 69.77; H 6.05; N 4.70
a; ~2 ~A
~~ k)
75iDArI30 - 68 - 17940IB
Example lA
3-[N-(p-Chlorobenzyl)-3-(t-butylthio)-5-
(quinolin-2-ylmethoxy)indol-2-y17-2,2-
dimethylpro~anoic acid
a A: N-Acetyl-4-(quinolin-2-~lmethoxy)aniline
A mixture containing 2-(chloromethyl)-
quinoline hydrochloride (100.0 g), 4-acetamido-
Phenol (70.69 g) and milled anhydzous potassium
carbonate (194 g) was stirred in DMF (1.2 L) using a
mechanical stirrer for 48 hours. The mixture was
carefully poured onto icelwater (3 L) with vigourous
stirring. After the ice had melted, the solid was
filtered and rinsed thoroughly with water. It was
recrystallized from 95% ethanol and filtered to give
the title co~r~pound in three crops.
25
76/L~r'1b131 -69-~ 179401B
Step B: 4-Lpuinolin-2-ylmethox~~anili~
A suspension of N-acetyl-4-(quinolin-2-yl-
methoxy)aniline (Step A, 108.9 g) in 1 L of 950
ethanol containing 10 M KOH (120 mL) was heated at
reflux under nitrogen in a heating mantle. When the
hydrolysis was complete (approx. 36 h), the reaction
mixture was cooled and ethanol was partially remaved
under vacuum. The mixture was then diluted with
water (200 mL) and the fine off-white crystals were
collected and thoroughly rinsed with water. The
material, after air-drying, yielded the title
compound which was used as such in the next step.
~ ~Ouinolin-2 ylmethoxy~,l~henylhydr zin
A quantitiy of 84 g of 4-(quinolin-2-
ylmethoxy)aniline from Step B was suspended in 300 mL
of deionized H20 and 84 mL of 12 ~1 HC1. The
suspension was stirred vigourously to obtain a fine
particle suspension. Then a precooled solution (5°C)
of 23.88 g of sodium nitrite dissolved in 75 mL of
deionized H20 was added dropwise to the suspension at
5°C over 25 minutes. The solution was stirred at 5°C
for 60 min to obtain the diazonium salt as a clear
brown solution, The presence of excess HN02 was
confirmed by KI-starch paper, and the pI-I of the
solution was about 3Ø If a white suspension
persisted after 1 h, the mixture was filtered through
a glass wool plug, to give the diazonium salt in the
filtrate.
~e~'lmr~'' a
7h.'PAh131 -70- 1?940IB
In the meantime a sodium hydrosulfite
solution was prepared by dissolving 321 g of sodium
hydrosulfite (approx. 85°s purity) in 2 L of deionized
water, and cooled at 0° to 5°C. To this solution
caere added 15 mL of 2N NaOH and 2 L of ether. The
biphasic solution was kept near 0°C by additon of
crushed ice and was stirred vigorously. To this
solution was added dropwise the diazonium salt
solution with stirring maintained throughout. At the
end of the addition an orange solid was formed and
600 mL of NaOH (2N) was added over 30 minutes. The
reaction was finally stirred for 60 minutes at 25°C.
The solid was collected, suspended in ether (1 L) and
filtered. The process was repeated with 2 L of water
to yield the title compound as a pale yellow solid
after freeze-drying overnight. m.p. 73-85°C (dec).
Step D: 1-(p-Chlorobenzyl)-1-[4-(quinolin-2-yl-
methoxv Lnhenvllhvdrazine
A quantity of 10 g of 4-(quinolin-2-
ylmethoxy)phenylhydrazine from Step C was added to a
solution of 10.5 mL of diisopropylethylamine and 150
mL of CH2C12. To the yellow suspension was added
9~11 g of p-chlorobenzyl chloride followed by 3.64 g
of Bu4NBr and 50 mL of CH2C12. The reaction was
stirred for approximately 24 hours. When no starting
material remained, the reaction was diluted with H20
and extracted 3 times with CH2C12. The combined
organic phase was washed once with water and dried
(MgS04), filtered and evagorated to dryness. The
6 s 's, J ~ ~ ~ ~, o ' s
~~~arnl -~1-- 1~~9o1B
solid residue was dried under vacuum overnight prior
to being swished in ether/methanol 90/10 to give the
title compound as a pale yellow solid. m.p. 130°C.
Step E: 3-[N-(p-Chlorobenzyl)-3--(t-butylthio)-5-
(quinolin-2-ylmethoxy)indol-2-yl]-2,2-
dimethylorooanoic acid
The methyl ester of the title compound was
l0 prepared according to the method described in Step A
of Example 1 but using the phenylhydrazine from Step
D of Example lA as starting material.
The title compound was prepared under the
conditions described in Step B of Example 1.
Example 2
3-[N-(p-Chlorobenzyl)--3-methyl-5-(quinolin-2-yl-
methox~)indol-2-yll-2.2-dimethylpro,~anoic acid
The title compound was prepared according to
the method of Example 1, but using methyl 2,2-
dimethyl-4-oxohexanoate as.starting material in Step
A in place of methyl 5-t-butylthio-2,2-dimethyl-
4-oxopentanoate. m.p. 215-217° C.
Example
3-[N-(p-t-Butylthiobenzyl)-3-(t-butylthio)-5-
(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl-
pro_panoic acid
~~ ~ '~ a ''~~y ~y~
? 6/P~M31 -72-- 17940IB
The methyl ester byproduct from Step C of
Example 1 was reacted 2-(chloromethyl)quinoline
according to the conditions of Steps D & E of Example
1 to provide the title compound. m.p. 172-173° C.
xam le 4
3-[N-(p-Chlorobenzyl)-3-(phenylthio)-5-(quinolin-
2lvlmethoxx)indol-2-vll-2,2-dimethylpropanoic acid
The title compound was prepared according to
the method described for Example 1, but substituting
methyl-5-phenylthio-2,2-dimethyl-4-oxopentanoate for
methyl 5-t-butylthio-2,2-dimethyl-4-oxopentonoate in
Example 1 (Step A).
Anal. C, H, N for sodium salt 2 H20:
CaIC. C 64.91; H 5.30; N 4.20
Found C 64.94; H 5.04; N 4.15
example 5
3-[N-(p-Chlorobenzyl)-3-(phenylsulfonyl)-5-
(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl-
p~ opanoic acid, N-oxide
Methyl 3-[N-(p-chlorobenzyl)-3-
(phenylthio)-5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-
dimethyl propanoate (430 mg) from Step D of Example 4
was dissolved in 5 mh cold CH2C12 and treated with a
solution of 448 mg of 80% m-chloroperbenzoic acid
(MCPBA) in CH2C12. After 24 hours, the solution was
poured onto 10 mL of sat. aqueous NaHC03 solution,
a~ Ci 1~ ~ .,v 'iff
75;'DF~b131 -73- 1794DIB
extracted with 3 x 10 mL of CH2C12, washed with 2 x
mL of H.~O, dried with magnesium sulfate and
evaporated to dryness. The residue was crystallised
from 2:1 CH2C12/EtOAc to yield 280 mg of the title
5 compound as its methyl ester. Hydrolysis using the
conditions described in Example 1 (Step 8) provided.
the title compound, m.p. 197° C (dec.)
Anal. C~ H, N: Calc. C 66.0; H 4.77; N 4.28
10 Found C 66.06: H 4.77; N 4.19.
examples 6 n 7
3-(N-(p-Chlorobenzyl)-3-(phenylsulfonyl)-5-(quino-
lin-2-ylmethoxy)indol-2-yl]-2,2-dimethylpropanoic
acid an,~
3-(N-(p-Chlorobenzyl)-3-(phenylsulfinyl)-5-(quino-
lin-2-ylmethoxy)indol-2-yl]-2,2-dimethylpropanoic
Methyl 3-(N-p-chlorobenzyl-3-(phenyl
thio)-5-(quinolin-2-methoxy)indol-2-yl]-2,2-
dimethyl propanoate (430 mg) from Example 4 (Step D)
was dissolved in S mL of cold methylene chloride and
a solution of 150 mg of SO% (MCPBA) in methylene
chloride was added. After 24 hours, the reaction
solution was poured onto 10 mL of saturated aqueous
sodium bicarbonate solution and this mixture was
extracted 3 times with 10 mL of methylene chloride.
The combined organic phases were washed twice with 10
mL of water, dried with magnesium sulfate and
evaporated under vacuum.
~? ~ ~ t~) ~ .~j ,;
7E/DAhi31 -74- 17940IB
Chromatography over silica gel (2 hexane: 1
ethyl acetate) provided two compounds which were
separately hydrolyzed using the procedure described
in Example 1 (Step B).
3-[N-(p-Chlorobenzyl)-3-(phenylsulfonyl)-5-(quinolin-
2-ylmethoxy)indol-2-yl]-2,2-dimethylpropanoic acid:
Anal. C, H, N for sodium salt H20:
Calc. C 63.57; H 4.89; N 4.12
Found C 63.28; H 9.77; N 3.90
3-(N-(p-Chlorobenzyl)-3-(phenylsulfinyl)-5-(quino-
lin-2-ylmethoxy)indol-2-yl]-2,2-dimethylpropanoic
acid:
Anal. C, H, N for sodium salt' H20:
I5 Calc. C 63.38; H 5.17; N 4.11
Found C 63.28; H 4.89; N 3.97
~amole 8
3-(N-(p-Chlorobenzyl)-5-(quinolin-2-ylmethoxy)
~~2-.Yl] -2 ~ 2-dimeth~l~~~anoic aClf~
Stew A: Methyl 3-(N-(p-chlorobenzyl)-5-hydroxyindol-
2~y11-2,L2--dimethylpropanoate
A suspension of 1.0 g of 3-LN-(P-chloro-
benzyl)-3-(t-butylthio)-5-methoxyindol-2-yl]-2,2-
dimethylpropanoic acid (from Example 1 Step B) in 50
mL of CH2C12 was treated with 1.3 mL of ethanethiol
and 3.47 g of A1C13 at 0°C under argon. After 90 min
the mixture was poured onto 50 mL 1N HC1, extracted
with 3 x 50 mL of CH2C12 washed with 2 x 50 mL of
H20, dried with MgS04 and the solvent removed. The
'i'' a ~~' ~'' ~'1 t ~~
ixt 'Yi '~~ ~D~ ,.~ ~~ n
76!DAi4131 -75- 17940IB
residue was dissolved in 10 mL ether and ethereal
diazomethane added until all the acid was r_onsumed.
The excess solvent was removed and the residue
chromatographed on silica gel to afford the title
compound.
Step g: 3-[N-(p-Chlorobenzyl)-5-(quinolin-2-yl-
methoxy)indol-2-yl]-2,2-dimethylpzopanoic
The title compound was prepared by treating
the ester from Step A with 2-(choromethyl)quinoline
hydrochloride under the conditions of Step D and
effecting hydrolysis under the conditions of Example
1 {Step B), m.p. 193--194°C.
Example 9
3-(N-(p-Ghlorobenzyl)-3-benzoyl-5~-(quinolin-2-yl-
methQx_y)in$ 1~-2-y~l-2.2- im_ethy pr~anoic acid
step A: Methyl 3-(N-{p-chlorobenzyl)-3-benzoyl-5-
benzoyloxyindol-2-vll-2 2-dimethylprooan
Methyl 3-(N-(p-chlorobenzyl)-5-hydroxy
indol-2-yl]-2,2-dimethylpropanoate (609 mg) from
Example 8 (Step A) was dissolved in 10 mL of
1,2-dichloroethane and the solution charged with 0.5
mL of benzoyl chloride and 680 mg of A1C13. The
reaction was heated to 80° C under argon for 1.5 h,
then quenched with 20 mL of 0.5N Na, K ta.rtrate
solution, extracted with 3 x 20 mL of ether, washed
with 10 mL of H20 and dried (MgS04). Removal of
j ~.9 'ii ~~ ~: ~ ' ~"s
76i DAhi31 -76- 17940IB
solvent provided an oily residue which was
chromatographed on silica gel to give the title
compound.
Step B: Methyl 3-(N-(p-chlorobenzyl)-3-benzoyl-5-
hvdroxyindol-2-yll-2.2 dim hvipropanoat~
The compound from Step A (300 mg) was
dissolved in 4 mL of MeOH and treated with 1 mL of a
1.4 M solution of NaOMe in MeOH under argon for 3
hrs. The mixture was poured onto 20 mL of NH40Ac
(25o solution), extracted with 3 x 15 mh of ether,
washed with 10 m1, of H20, dried over MgS04 and the
solvent removed under vacuum. The resulting oil was
Purified by chromatography on silica gel to afford
the title compound.
Step C: 3-(N-(p-Chlorobenzyl)-3-benzoyl-5-(quinolin-
2-yl-methoxy)indol-2-yl]-2,2-dimethyl-
propanoic acid
The title compound was prepared using the
conditions described in Step D and Step E of Example
l, but substituting the ester from Step B for the
ester of Example 1 , Step C; m.p. 165-166°C.
x m a 10
3-(N-(p-Chlorobenzyl)-3-benzyl-5-(quinolin-
2-y1-methoxy)indol-2-yl]-2,2-dimethylpropanoic
acid
i ~ ~ ~ ~~,.' F
vas ~!W',..j ~4y' c..:
76/DADi31 -77- 17940IB
Diethyl 3-[N-(p-Chlorobenzyl)-3-benzyl-5-
(benzoyloxy)indol-2-yl]-2,2-dimethyl-
prop_anoate
Methyl 3-[N-(p-chlorobenzyl)-3-
benzoyl-5-(benzoyloxy)indol-2-yl]-2,2-dimethyl-
propanoate (360 mg) (prepared in Step A of Example
9), 800 mg of znI2, and 500 mg of sodium
cyanoborohydride were stirred in 5 mL of
dichloroethane at RT under argon for 30 min. The
temperature was then raised to 65° C for 3 hr. After
the solution had cooled. it was poured onto 10 mL of
NH40Ac (25% solution), extracted with 3 x 15 mL of
ether, washed with 10 mL of H20 and dried (MgSOq).
The solution was evaporated to dryness and the
residue was chromatographed on silica gel to yield
the title compound as a white foam.
Step ~: 3-(N-(p-Chlorobenzyl)-3-benzyl-5-(quinolin-
2-yl.-methoxy)indol-2-yl]-2,2-dimethyl-
prczp~noic ac~Lid _
The title compound was prepared under the
conditions described in Step B and Step C of Example
9 but substituting the ester from Example 10 (Step A)
for the ester of Example 9 (Step A), m.p. 178°C.
Exam ~e.ll
3-[N-(p-Chlorobenzyl)-3-(3,3-dirnethyl-1-oxo-1
butyl)-5-(quinolin-2-ylmethoxy)indol-2-yl]-2,
2- 'methv~propenoic acid
Ly ;;1 yq ,i~.. i
>; 1~ 5~ '~: c_. .
76i PArI31 -78- 179401B
The title compound was prepared according to
the method described in Example 9, but using
t-butylacetylchloride in place of benzayl chloride in
Step A, m.p. 183-184°C.
Example 12
2-[N-(p-Chlorobenzyl)-3-(t-butylthio)-5-(quinolin-
2-ylmethoxv)indol-2-yliethoxvethanoic acid
~te~ A: Methyl 2-[N-(p-Chorobenzyl)-3-(t-butylthio)-
5-(quinolin-2-ylmethoxy)indol-2-yl]
ethanoate
The title compound was prepared according to
the method outlined in Steps A-D of Example 1, but
using methyl 4-t-butylthio-3-oxo-butaxioate in Step A
instead of methyl 5-t-butylthio-2,2-dimethyl-
4-oxopentanoate.
~tgp B: 2-[N-(p-Chlorobenzyl)-3-(t-butylthio)-5-
~.guinalin-2 ylmethoxy) ind,~l-2-yll ethanol.
The compound from~Step A (192 mg) was
dissolved in 3 mL of THF at RT under an argon
atmosphere and treated with 30 mg of lithium aluminum
hydride. After 1 hr, the reaction was poured onto 30
mL of 0.5 N Na,K tartrate solution and extracted with
3 x 10 mL of EtCAc. The organic layer was washed
with 10 mh of H20, dried (MgS04) and evaporated to
dryness to yield the title compound.
~6 'll iY ".> c. ~' S:~~~
76/DAhS31 -79- 1794DIB
Step ~: 2-[N-(p-Chlorobenzyl)-3-(t-butylthio)-5-
(quinolin-2-ylmethoxy)indol~-2-yl]ethoxy-
ethanoic acid
To 91 mg of 2-(N-(p-chlorobenzyl)-3-(t-
butylthio)-5-(quinolin-2-ylmethoxy)indol-2-
yl]ethanol from Step B in 2 mL THF at 0° C under an
argon atmosphere was added ~0 mg of 80% sodium
hydride over 30 min. Ethyl bromoacetate (0.3 ml) was
added to the solution and the reaction stirred at RZ'
overnight. The reaction was poured onto 14 mL of
NHqOAc (25% solution), extracted with 3 x 10 mL of
EtOAc, washed with 20 mL of H20 and dried over
MgSO~. Removal of the solvent followed by column
chromatography on silica gel afforded the ethyl ester
of title compound. Hydrolysis of this ester under
the conditions described in Step B of Example 1
provided the title compound, m.p. 185°C (dec.).
Example 13
3-(N-(g-Chlorobenzyl)-3-(3,3-dimethyl-1-
butyl)-5-(quinolin-2-ylmethoxy)indol-2-yl]-
2 2-dimethvlprQpanoic acid
The title compound was prepared according to
the method described in Example 10 but using methyl
3-(N-(p-chlorobenzyl)-3-(3,3-dimethy-1-oxo-
1-butyl)-5-(t-butylacetyloxy)-indol-2-yl]-2,z-
dimethylpropanoate (obtained as an intermediate from
Example 11) as starting material, m.p. 188°C (dec.).
"J ~1<~ i~ ;~ '~j
(.,e ~.iv i' c..i' ,...'': o
76; L1.~931 -80- 1'7940IB
Example 14
3-[N-(p-Chlorober~zyl)-3-(t-butylthio)-5-(quinolin-
2 ylmethoxy indol-2-yl~-2-methylpropanoic acid
The title compound was prepared according to the
method of Example 1 using methyl 5-t-butyl-
thio-2-methyl-4-oxopentanoate as starting material in
Step A in place of methyl 5-t-butylthio-2,2-
dimethyl-4-oxopentanoate.
1H NMR (250 MHz, acetone-d6) $ 1.05 (3H, d, ,I = 6Hz),
1.15 (9H, s), 2.7 (1H, m), 3.2 (2H, d, J = 7Hz), 5.4
(2H, s), 5.6 (2H, s), 6.9 (IH, dd), 7.0 (2H, d). 7.3
(4H, m), 7.6 (1H, td), 7.7 (1H, d), 7.8 (1H, td), 7.9
(1H, d), 8.1 (1H, d), 8.3 ppm (IH, d).
20
Example 15
3-[N-(p-Chlorobenzyl)-3-methyl-5-(6,7-dichloro-
quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl-
propanoic acid
The title compound was prepared according to
the method described in Example 1 but using methyl
2,2-dimethyl-4-oxohexanoate as starting material in
Step A and 2-(bromomethyl)-6,7-diehloroquinoline in
Step D.
Anal. C, H, N: Calc. C 63.21; H 4.74; N 4.91
Found C 63.47; H 4.94; N 4.67
al c'''
m
76iDAM31 -81- 17940IH
Example 16
3-[N-{p-Chlorobenzyl)-3-methyl-5-(7-chloroquino-
lin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl-
prQpanoic acid
The title compound was prepared according to
the method described in Example 15 but using
2-(bromomethyl)-7-chloroquinoline instead of
2-(bromomethyl)-6,?-dichloroquinoline. m.p.
105-107°C.
Anal. C. H, N: Calc. C 67.41; H 5.24; N 5.24
Found C 67.82; H 5.12; N 4.32
xamp a 17
3-[N-(p-Chlorobenzyl)-4-allyl-5-(quinolin-2-yl-
methoxy)-3-(t-butylthio)indol-2-yl]-2,2-dimethyl-
p~_ps~noic acid
~te~ p A: Methyl 3-dN-(p-chlorobenzyl)-5-allyloxy
3-(t-butylthio)indol-2-yl]-2,2-dimethyl
p~o_p_anoic acid
500 mg. of methyl 3-[N-(p-chlorobenzyl)-5-
hydroxy-3-(t-butylthio)indol-2-yl]-2,2-dimethyl-
Propanoate .from Step C of Example 1 was dissolved in
S mL of DMF and 20 mg of K2C03 and 150 mg of a11y1
bromide were added. The reaction was stirred for 16
hrs. Water was added and the organic phase extracted
with EtOAc (3 x 5mL). The organic phase was dried
with MgS04 and evaporated to yield, after
chromatography on silica gel (EtOAc:hexane 1:5), the
title compound.
w ~~ ~~ ~'~ a 'f
7oi'D~231 -82- 17990IB
~t~p B: Methyl 3-(N-(p-chlorobenzyl)-3-(t-butylthio)--
9-allyl-5-hydroxyindol-2-yl]-2.2-dimethyl-
proQanoa~e
500 mg of the ester of Step A was converted
to the title compound by heating to 180° in m-xylene
for 4 hours.
Step C: 3-[N-(p-Chlorobenzyl)-9-allyl-5-(quinolin-2-
ylmethoxy)-3-(t-butylthio)indol-2-y1]-2,2-
dimeth~lpropanoic acid
The title compound was prepared from the
compound of Step B using the methodology of Example
1~ (Steps D and E), m.p. 103-105°C.
Anal. C, H, N: Calc. C 69.09; H 6.11; N 4.35
Found C 70.55; H 6.31; N 9.29
Example 18
3-~N-(p-Chlorobenzyl)-4-allyl-5-(quinolin-2-yl-
methoxv) indol-2 ~l ] -2 , 2-dimethy~_propanoa.c acid
The methyl ester of the title compound was
Prepazed according to the method of Example 17 but
substituting methyl 3-~N-(p-chlorobenzyl)-5-
hydroxyindol-2-yl]-2.2-dimethylpropanoate as starting
material (obtained in Step A Example 8) for the ester
in Example 17 (Step A). Hydrolysis was then effected
according to the conditions of Step B of Example 1 to
provide the title compound, m.p. 196-197°C (dec.).
'' ~
S? ~ e1 ~~ a
7 6/DAD931 -83- 1 7940TB
Example 19
3-[N-(p-Chlorobenzyl)-6-(quinolin-2-ylmethoxy)-3-
(t-butylthio)indol-2-yl]-2,2-dimethylpropanoic
The title compound was prepared according to the
conditions of Example 1, Steps A to E, but
substituting 1-(3-methoxyphenyl)-1-(p-chloro-
benzyl)hydrazine hydrochloride for the starting
material in Example 1 (Step A). Chromatographic
separation of the desired regioisomer was achieved at
Step A by isolating the most polar product, methyl
3-[N-(p-chlorobenzyl)-3-(t-butylthio)-6-methoxy-
indol-2-yl]-2,2-dimethylpropanoate. The properties
of the title compound were as follows: m.p.
165-167°C.
Arial C, H, N: Calc. C 69,54; H 6.01; N 4.77
Found C 69.46; H 6.18; N 4.96
25
Example 20
3-[N-(p-Chlorobenzyl)-4-(quinolin-2-ylmethoxy)-3-
(t-butylthio)indol-2-yl]-2,2-dimethylpropanoic
Methyl 3-[N-(p-chlorobenzyl)-3-(t-butylthio)-
4-methoxyindol-2-yl]-2,2-dimethylpropanoate was
obtained as a by-product from Step A of Example 19
and isolated by chromatography as the less polar
Product. The compound was used as starting material
for the preparation of the title product using the
methodology of Steps H to E of Example 1.
' '7
~D~~.,~~'3
76/DAri31 -84- 17940IB
Anal C, H, N: Calc. C 69.54; H 6.01; N 4.77;
Found C 69.80; H 6.24; N 4.86
Example 21
3-[N-(p-Chlorobenzyl)-3-(t-butylthio)-7-(quinolin-
2-ylmethoxy)indol-2-yl]-2,2-dimethylpropanoic
The title product was prepared according to
Steps A to E of Example 1 but substituting
1-(2-methoxyphenyl)-1-(p-chlorobenzyl)hydrazine
hydrochloride for 1-(4-methoxyphenyl)-1-(p-
chlorobenzyl hydrazine hydrochloride in Example 1
(Step A), m.p. 206°C.
Anal. C, H, N: Calc. C 69.54; H 6.01; N 4.77,
Found C 69.40; H 5.00; N 4.65
Example 22
2-C2-CN-{p°-Chlorobenzyl)--3-(t-butylthio)-5-{quino-
lin-2-ylmethoxy)indol-2-yl]ethoxy]propanoic acid
~odiWm salt dih.~r rate
Step A: Methyl 2-[2-[N-(p-chlorobenzyl)-3-{t-butyl-
thio)-5-quinolin-2-ylmethoxy)indol-2-yl]-
.Pthox~~prQpanoate
The title compound was prepared from 251 mg
of 2-CN-(p-chlorobenzyl)-3-{t-butylthio)-5-
{quinolin-2-ylmethoxy)indol-2-yl]ethanol (Step B of
Example 12) under the conditions described in Step C
of Example 32 using methyl ~,~.-2-bromopropanoate
instead of ethyl bromoacetate.
h»' UJ r, ~ (~
7 6fL~Ai°~331 -85- 17940IB
B: 2-[2-[N-(p-Chlorobenzyl)-3-(t-butylthio)-5-
(quinolin-2-ylmethoxy)indol-2-yl]ethoxy)-
,prQp~noic acid sodium salt dihvdrate
The acid corresponding to the title compound
of Example 22 was prepared from the ester of Step A,
of Example 22 under the conditions described in Step
B of Example 1. A quantity of 204 mg of the acid was
suspended in 1.5 mL of EtOH and treated with 1 eguiv.
of 1N aq. NaOH and freezed dried for 2 days to afford
the title compound.
Anal. C, H, N: Calc. C 61.25; H 5.61; N 9.33,
Found C 61.75; H 5.7D; N 3.97
Exam lp ~ 23
3-[N-(p-Chlorobenzyl)-4-(quinolin-2-ylmethoxy}-
inaol-2-Y~2,2-dim~hvJ.pr_2panoic aczd
2D ~tP~ ~: Methyl 3-[N-(p-chlorobenzyl)-4-
hvdrox~yindol-2-yll-2.2-dimethylprQp~n
The title compound was prepared using
methodology from Step A of Example 8 but substituting
3-[N-(p-chlorobenzyl)-3-(t-butylthio)-4-methoxy
indol-2-yl]-2,2-dimethylpropanoic acid (Step B of
Example 20} for the propanoic acid in Example 8 (Step
A).
S' .9 ._~
~r ~ ,~~ ? y.
76IDAr131 -86- 17940IH
Step B: 3-[N-(p-Chlorobenzyl)-4-(guinolin-2-yl-
methoxy)indol-2-yl]-2,2-dimethylpropanoic
The title product was prepared according to
conditions described in Steps D and E of Example 1
substituting methyl 3-[N-(p-chlorobenzyl)-4-hydroxy
indol-2-yl]-2,2-dimethylpropanoate for the propanoate
in Example 1 (Step D), m.p. 158-i60°C.
Anal. C, H, N: Calc. C 72.20; H 5.45; N 5.61
Found C 72.25; H 5.60; N 5.75
Example 24
3-[N-Methyl-3-(p-chlorobenzoyl)-6-(quinolin-2-yl-
methoxv)ind~l-2-v11~2.2-dimet~yl~ropanoic acid
Step A: Methyl 3-[6-methoxy-3-(t-butylthio)
~ndol-2-vll-2,2- im '~~hvlprop~noate
A mixture of 4.2 g of 3-methoxyphenyl-
hydrazine hydrochloride and 4.9 g of methyl
5-(t-butylthio)-2,2-dimethyl-4-oxopentanoate in 100
mL of t-butanol was refluxed for 18 hours. The
mixture was cooled to R.T., and evaporated to
dryness. The residue was suspended in ether (150 ml)
and stirred for 30 min. The salts were filtered and
the filtrate evaporated to dryness to give a residue
which was chromatographed on flash silica gel using
as eluant ethyl acetate:toluene (1:99) to isolate the
title compound as the most polar product; m.p. 133°C.
76.~'DAM31 -57~ 17940IB
SteQ-f3: Methyl 3-[N-methyl-3-(t-butylthio)-6-
methox~indgl-2-~1 i -2. , 2-dimethyl~ro~anoate
A solution of 1.75 g of the indole from Step
A in 30 mL of THF and 3 mL HMPA was cooled to -78°C
and to this solution was slowly added a solution of
0.54M KHMDS in toluene (10.2 mL). The mixture was
stirred at this temperature for 15 min. and treated
with 0.34 mL of iodomethane. The mixture was stirred
at -75°C for 5 h, quenched with 1N HC1 (100 mL),
extracted with ethyl acetate, and the organic layer
washed with H20, dried over Na2S04 and evaporated to
dryness. The residue was chromatographed on flash
silica gel using ethyl acetate:hexane (20:50) as
eluant to afford the title compound as a solid; m.p.
97-98°C.
Step C: Methyl 3-[N-methyl-6-hydroxyindol-2-yl]-
,~ 2-dimethylpropanoate
To a cold solution of 940 mg of the indole
ester from Step B and 1.6 mL of ethanethiol in CH2C12
(50 mL) was added portion-wise 4.3 g of AlCl3. After
complete addition, the mixture was stirred at R.T.
for 2 h. The mixture was then cooled to 0°C and
carefully quenched with a solution of 0.5 M Na,K
tartrate (200 mL) and extracted with CH2C12. The
organic layer was dried aver Na2S04 and evaporated to
dryness to give a solid which was chromatographed on
flash silica gel using ethyl acetate: hexane (30:7U)
as eluant to afford the title compound; m.p.
125-126°C.
~y r~: ~ '3 .1
~~ '~a ~;J f ::~ ' .
76; L?AD131 -88- 179~O1B
Step D: Methyl 3-(N-methyl-6-(p-chlorobenzoyloxy)-
3-(p-chlorobenzoyl)indol-2-yl]-2,2-di-
methylpropan~ate _
To a cold solution of 393 mg of hydroxy
indole from Step C in 5 mL of THF were added 0.31 mL
of Et3N followed by 0.21 mL of p-chlorobenzoyl
chloride. The mixture was stirred at R.T for 15 min
and quenched with H20. The mixture was extracted
with ethyl acetate which was dried over Na2S04 and
evaporated to dryness to give a solid which was
dissolved in 10 mL of 1,2-dichloroethane. To this
mixture were added successively at R.T. 0.38 mL of
p-chlorobenzoyl chloride and 803 mg of A1C13. The
mixture was heated at 80°C for 3 h, cooled to R.T.
and quenched with 50 mL of 0.5 N HC1. The mixture
was extracted with CH2Cl2, washed with H20, dried
over Na2S04 and evaporated to dryness. The residue
was chromatographed on flash silica gel using ethyl
acetate: hexane (20:80) as eluant to afford the title
compound as a white solid. m.p. 138°C.
StP~p E: Methyl 3-(N-methyl-3-(p-chlorobenzoyl)-6-
hwdrox~rindol-2-vll-2 , 2-dimethylp~~anoate
To a suspension of 270 mg of the p-chlorobenzoate
from Step D in 3 mL of MeOH was added 1.2 mL of a
solution of 1.3M NaOMe in MeOH and the mixture was
stirred at R.T. for 2 hr. The reaction mixture was
poured onto 25% aq. NHqOAc and extracted with ethyl
acetate. The organic extract was dried over Na2SOq,
y I,
.1 ~~ , v
'~..~ 'i> i~ '.
76/DAP231 -89- 179401B
evaporated to dryness and the residue chromatographed
on flash silica geT using ethyl acetate: hexane
(40:60) as eluant to afford the title compound as a
yellow foam.
StP~p F: Methyl 3-[N-methyl-3-(p-chlorobenzoyl)-6--
(quinolin-2-ylmethoxy)indol-2-yl]-
2.2-dimethylpropanoate
To a solution of 180 mg of the phenol from
Step E in 5 mL of DMF were added 124 mg of milled
K2C03 followed by 150 mg of 2-(bromomethyl)
quinoline. The mixture was stirred at R.T. for I8 h,
poured onto 25fl aq. NH40Ac and extracted with ethyl
acetate. The extract was dried over Na2S04 Grad
evaporated to dryness to give an oil which was
chromatographed on flash silica gel using ethyl
acetate: hexane (30:70) as eluant to give the title
compound as a foam.
StP~p G: 3-[N-Methyl-3-(p-chlorobenzoyl)-6-(quinolin-
2-ylmethoxy)indol-2-y1)-2,2-dimethyl-
proganoic acid
To a solution of 23D mg of ester from Step F'
in 1.5 mL of THF and 3 mL of MeOH was added 1M aq.
LiOH and the mixture stirred at 80°C for 4 h. The
mixture was cooled to 2t. T. and evaporated to dryness
in vacuo. The residue was dissolved in a mixture of
2D mL of 25% aq. NH40Ac and 20 mL of ethyl acetate
using vigourous stirring. The organic layer was
~~ ~~.y ; ~ ~3
76/DAI~I31 -90- 17940IB
separated, dried over Na2S04 and evaporated to
dryness to give a yellow solid (216 mg). This solid
was swished for 2 h in 5 mL of a mixture of
Et20:hexane (1:1). The solid was filtered and rinsed
with a 1:2 mixture of Et20:hexane to give the title
product as a yellow solid, m.p. 203-205°C.
Anal. C, H, N: Calc. C 70.65; H 5.16; N 5.32;
Found C 70.42; H 5.25; N 5.40
15
Example 25
3-[N-Methyl-3-(p-chlorobenzyl)-6-(quinolin-2-yl-
methoxy)indol-2-yl]-2,2-dimethylpropanoic acid,
~n3ium salt hemih~rdrate
StP~p A: Methyl 3-[N-methyl-3-(p-chlorobenzyl)-6-
(p-chlorobenzoyloxy)indol-2-yl]-2,2-dimethyl-
pro anoate _
To a solution of 500 mg of the benzoyl
derivative from Step D of Example 24 in 10 mL of
1,2-dichloroethane were added 1.19 g of Z,nI2 and 700
mg of NaBH3CN. The mixture was heated at 65°C for 5
hours and cooled to R.T. The mixture was quenched
with 1N aq. HC1 and extracted with CH2C12. The
extracts were washed with brine, dried over Na2S04
and evaporated to dryness to give an oil which was
chromatographed on flash silica gel using ethyl
acetate: hexane (15:85) as eluant to isolate the title
compound as a white foam.
~~ ~, .,' ~,. , ; .,
F.J i~ iJ '~Y '.J t'
76lDAri31 -91- 179901B
~tP~ $: Methyl 3-IN-methyl-3-(p-chlorobenzyl)-
6 ~ydzoxyindol-2-vl l -2 , 2-dimP~thvl~~r~~noate
To a suspension of 425 mg of
p-chlorobenzoate from Step A in 3 mL of MeOH was
added 1.9 mL of a solution of 1.3M NaOMe in MeOH.
The mixture was stirred at R.T. for 1 h, poured into
20 mL of 25% aq. NH40Ac, and extracted with ethyl
acetate. The organic extract was dried over Na2S04
and evaporated to dryness to give an oiI which was
chromatographed on flash silica gel using ethyl
acetate: hexane (30:70) as eluant to give the title
compound as a white foam.
Methyl 3-(N-methyl-3-(p-chlorobenzyl)-
6-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-
~3imethyl~ropanoate
To a solution of 315 mg of the ester from
Step B in 3 mL of OMf' were added 225 mg of milled
K2C03 and 272 mg of 2-(bromomethyl)quinoline. The
mixture was stirred at R.T. for 18 h, poured into 25%
aq. NH~OAc, and extracted with ethyl acetate. The
organic extract was dried over Na2S0~ and evaporated
to dryness to give an oil which was chromatographed
on flash silica gel using ethyl acetate: hexane
(30:70) as eluant to give the title compound as a
foam.
6,. ~i ~' '~,'..,~ ;,i ;:'
76;p.~I31 -92- 179401B
p: 3-[N-Methyl-3-(p-chlorobenzyl)-6-(quinolin-
2-ylmethoxy)indol-2-yl)-2,2-dimethyl
propanoic acid sodium halt hemihydrate
To a solution of 367 mg of the ester from
Step C in 3 mL of THF and 6 mL of MeOH was added 1 M
aq. LiOH and the mixture was heated at 80°C for 2 h.
The mixtuze was cooled to R.T. and evaporated to
dryness. The residue was dissolved in a mixture of
20 mL of 25o aq. NH40Ac and 20 mL of ethyl acetate
(vigourous stirring required). The organic layer was
separated, dried over Na2S04 and evaporated to
dryness to give a white solid (346 mg). The solid
was swished at R.T. for 2 h with 10 mL of a mixture
of Et20:hexane (1:1), filtered, rinsed with a mixture
of (1:2) Et20:hexane and the solid collected to give
the title compound as its free acid, a white solid:
m.p. 185°C.
The title compound was prepared by
dissolving the above acid in 1 mL of EtOH to which
0.63 mL of 1N aq. NaOH was added. The mixture was
freeze dried for 2 days to give the title product as
a white solid.
Anal. C, H, N: Calf. C 67.32; I-I 5.29; N 5.07;
Found C 67,15; H 5.35; N 5.17
EXAMFLE 26
3-(N-(4-Chlorobenzyl)-3-i-propoxy-5-(quinolin-2-
ylmethoxy)indol-2-yl]-2,2-dimethylpropanoic
~i 3 f ~v~ ._' ''j ~'
'6iDAr131 -93- 17940IB
The title compound is prepared according to the
method of Example 1, but using methyl 5-i-propoxy-
2,2-dimethyl-4-oxogentanoate as starting material in
Step A in place of methyl 5-t-butylthio-2,2-dimethyl-
9-oxopentanoate.
~PLE 7
3-(N-(4-Chlorobenzyl)-3-(t-butylthia)-5-(quinolin-2--
ylmethoxv)indol-211-2-eth~~~ropanoic acid
Step A: Met~l 4-chloro-2-ethyl-9-pentenoat~
A 2L 3-necked flask equipped with a
mechanical stirrer, pressure equalizing addition
funnel, and nitrogen inlet was charged with
diisopropylamine (28 mL, 200 mmol) and dry THF (400
mL). The mixture was Gaoled to 0°C and a 1.6 1~2
solution of butyl lithium in hexane (125 mL, 200
mmol) was then added over a 15 minute period and
stirring was continued for an additional ~5 minutes.
The resultant solution of lithium
diisopropylamide (200 mmol) was cooled to -78°C and
then butyric acid (9.1 mL, 100 mmol) was added over a
15 minute period. The reaction was allowed to warm
to room temperature (1 hour) and then heated at 55°C
for 3.5 hours. The resultant gel was cooled to -78°C
and then treated 2,3-dichloro-1-propene (10.1 mL, 110
mmolj over a 15 minute period. The mixture was then
allowed to warm to room temperature and stirred for
1B hours.
~~~~rv'~~
76/Dt'~M31 --94- 17940IB
The reaction mixture was diluted with Et20
(400 mL), extracted with H20 (400 mL) and with NaOH
IN (300 mL). The aqueous layers were combined,
acidified with HC1 (2N, until pH 1-2) and the product
was extracted with EtOAc (2 x 3D0 mL). The organic
layers were combined, washed with brine (200 mL) and
dried over MgS04. Filtration and concentration gave
a yellow oil which was dissolved in dry MeOH (150 mL)
and acetyl chloride (1 mL) was added dropwise. The
resultant solution was gently refluxed for 20 hours.
The reaction was allowed to cool to room temperature
and it was concentrated. The resultant residue was
diluted with Et20 (600 mL), washed with NaHC03 sat.
(200 mL), washed with brine (200 mL), and dried over
M9S04. Filtration and concentration gave a yellow
oil which was purified by Kugelrohr distilation (bp
110°C at 0.2 mm Hg) to give pure (250 MHz NMR) methyl
4-chloro-2-ethyl-4-pentenoate.
B: Methyl 5-~r~o-2-ethyl-4-oxopentanQa a
To a cold {0°C) solution of methyl
4-chloro-2-ethyl-4-pentenoate from Step 2 (1.67 g,
9.5 mmol) in MeOH (31 ml) and H20 (16 ml) was added
Br2 dropwise (0.60 mL, 11.6 mmol). The resulting
Yellow solution was stirred at room temperature fo.r 1
hour. EtOAc (300 mL) and H20 (200 mL) were added.
The organic layer was separated, washed with H20, 1N
NaOH, H20, brine and dried over MgS04. Filtration
and concentration gave a yellow liquid which was
purified by flash chromatography (EtOAc/Hexane (1:9))
to give pure (NMR 250 MHz) methyl 5-bromo-2-ethyl-
4-oxopentanoate.
~5 ~~ v :~~ '~ 'f
76/DAM31 -95- 17940IB
Step C: Methyl 5-(t-butylthio)-2-ethyl-4-
Qxo~entanoate
To a cold (0°C) stirred solution of the
bromoketone from Step 2 (490 mg, 2.07 mmol), in 10 mL
of dry THF, were sequentially added 2-methyl--2-propyl
thiol (0.30 mL) and triethylamine (0.40 mL, 2.9
mmol). The reaction mixture was then allowed to warm
to room temperature. After 18 hours the white solid
was removed by filtration and the filtrate was
concentrated. The resulting yellow residue was
purified by flash chromatography (Et20/Hexane (9:50))
to give the pure title compound {250 MHz NMR).
Step D: 3-(N-(4-Chlorobenzyl)-3-(t-butylthio)-
5-(quinolin-2-yl-methoxy)indol-2-yl~-
2-ethyl~ropanoic acid.
The title compound was prepared according to
the method of Example 1, but using methyl
5-t-butylthio-2-ethyl-4-oxopentanoate as starting
material in Step A in place of methyl
5-t-butylthio-2,2-dimethyl-4-oxopentanoate.
Anal. C, H, N. for sodium salt ° 2H20:
Calc. C 63.30; H 5.94; N 4.34
Found. C 63.29; H 5.87; N 4.37
The sodium salt of the title compound in
this and other Examples was prepared by the method of
Example 25.
~, ~ ~~~1~ 's~llv~_'i , ) d
76iI).W31 -96- 17940IB
EXAMPLE 28
3-[N-(4-Chlorobenzyl)-3-trifluoroacetyl-5-(quinolin-2-
ylmethoxy)andol-2-1~17-2.2-dimethvlpropanoic amid
S~.p A: Methyl 3-[N-(9-chlorobenzyl)-3-
trifluoroacetyl-5-hydroxyindol-2-yl]-
2.2-dimethyl~ropanoate
Methyl 3-[N-(4-chlorobenzyl)-5-hydroxy
indol-2-yl]-2,2-dimethylpropanoate (310 mg) from
Example 8 (Step A) was dissolved in 3 ml of
1,2-dichloroethane and the solution charged with 0.6
ml of trifluoroacetic anhydride and 500 mg of AICI3.
The reaction was stirred at RT, under argon for 4h,
then quenched with 20 mL of 0.5 N Na, K tartrate
solution, extracted with 3x20 ml of Et20, washed with
10 mL of H20 and dried over MgS04. Removal of
solvent provided an oily residue which was
chromatographed on silica geI to give the title
compound.
Step B: 3-[N-(4-Chlorobenzyl)-3-trifluoroacetyl-
5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl-
propanoic acid.
The title compound was prepared using the
conditions described in Step D and Step E of Example
1, but substituting the ester from Step A for the
ester of Example 1. Step C.
Anal. C, H, N. for sodium salt ~ 7H20
Calc. C 51.72; H 5.29; N 3.76
Found. C 51.81; H 5.19; N 3.73
Ed vy '~! Vi :l ~.~ ~Y
7s/DA.hI31 -97- 17940IB
EXAMMFLE 29
3-[N-(4-Chlorobenzyl)-3-(3,3-dimethyl-1-oxo-1-butyl)-
5-(quinolin-2-ylmethoxy)indol-2-yl]-2-methylpropanoic
Step A: Methyl 5-(t-butylthio)-2-methyl-4-
oxopentanoate
The title compound was prepared according to
the method described in Example 27, but using
propionic acid as starting material in Step A in
place of butyric acid.
StP~p B: 3-(N-(4-Chlorobenzyl)-3-(3,3-dimethyl-
1-oxo-1-butyl)-5-(quinolin-2-ylmethoxy)indol-2-
~1-2-methy~propanoic acid
The title compound was prepared according to
the method described in Example 11, but substituting
5-t-butylthio-2-methyl-4-oxopentanoate as starting
material in Example 1 Step A in place of methyl
2p 5-t-butylthio-2,2-dimethyl-4-oxopentanoate.
Anal. C, H, N, for sodium salt ° 1.5 H20
Calf. C 66.50; H 5.907 N 4.43
Found. C 66.58; H 5.87; N 9.40
ExAMFLE 30
3-[3-(3,3-Dimethyl-1-oxo-1-butyl-5-(quinolin-2-
methoxy~indol-2-yll-2,2-dimethvluropanoic acid
Step A: Methyl 3-[3-(t-butylthio)-5-methoxy
indol-2-yll-2 ~2~lim~ h~lpropanoa~e
76IDAM31 -98- 17940TB
A mixture containing 4-methoxyphenyl
hydrazine~HCl (70.66 g, 0.405 mol) and methyl
5-(t-butylthio)--2,2-dimethyl-4-oxopentanoate (99.54
g, 0.405 mol) in tBuOH (400 mI) was heated at a
gentle reflux for 48 hours. The mixture was allowed
to cool to RT and the precipitated NH4C1 was removed
by filtration. The residue was concentrated and
fractionated on a plug of silica using EtOAc/hexane
(1:3) as eluent. Evaporation of the appropriate
fraction gave an orange-brown solid which was
crystallized from EtOH (100 ml). Yield from two
crops afforded 55.68 of the title compound.
1H NMR (CD3COCD3): 8 1.20(s, 6H); 1.25 (s. 9H) 3.33
(s, 2H); 3.62 (s, 3H); 3.81 ppm (s, 3H); in addition
to aromatic protons.
Step B: Methyl 3-(5-hydroxyindol-2-yl)-
2.2-dimet~l_pro~anoate
To a solution of the compound from step A
(25.50 g, 73 mmol) in CH2C12 (250 ml) at 0°C was
added A1C13 (87.70 g, 9 mol eq.) portion-wise. When
the addition was complete, the ice-bath was removed
and the mixture was stirred at RT for 3 hours. EtSH
(27 ml, 5 mol eq.) was added and the resulting
mixture was stirred for a further 5 hours. It was
then slowly poured onto an ice-cold 1M solution of
Na, K tartrate. The product was extracted into
CH2C12 (x2) and the organic phase was washed with aq.
NaCl (x3). Conventional work-up followed
chromatography on silica gel using EtOAc/hexane 1:5
~~ 1 J $_" r;;J c~ zr ,of
76/DADI31 -99- 17940IB
to 3:2 afforded 13.60 g of the title compound, 750
yield.
1H NMR (CDC13): 8 1.26 (s, 6H); 2.95 (s, 2H); 3.72
(s, 3H); 6.11 (s, 1H); 6.71 (bd, 1H); 6.95 (s. 1H);
7.16 (d, 1H); 7.26 ppm (s, 1H)
Step C: Methyl 3-[5-(quinolin-2-ylmethoxy)-
indol-2 yl~ -2 , 2-dimethyl~ro~anoate
A mixture of the phenol from step B (13.62
g, 55.14 mmol) and 2-bromomethylquinoline (12.85 g,
1.05 mol eq.) and anhydrous ~2C03 (15.22 g, 2 mol.
eq.) in DMF (40 ml) was stirred at RT for 48 hours.
The mixture was then poured onto ice/water and after
all the ice had melted, the brown solid was collected
and air-dried. The dried material was passed through
a plug of silica (using EtOAc/hexane (1:3) as eluent)
to remove the color; yield: 19 g, 88p.
Recrystallization from EtOH afforded 14.178 of pure
title compound, m.p. 131-132°C.
Step D: Methyl 3-(3-(3,3-dimethyl-1-oxo-1-butyl)-
5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl-p
r2panoate
To a suspension of A1C13 (5.7 g, 42 mmol) in
CH2C12 (30 mL) at 0°C was added 3,3-dimethylbutanoyl
chloride (2.4 mL. 17 mmol). After 15 minutes at 0°C,
a solution of the ester from step C (3.0 g, 7.7 mmol)
in CH2C12 (10 mL) was added by double-tipped needle-
The mixture was stirred a further 20 minutes, at
which point it was poured into a mixture of 0.5 M
_;'. st (-a ;u ;.. ..
s' ..~ :'y ~f
.. .. .. -y ..: G
76!DAM31 -100- 17940IH
Na, K tartrate (150 mL) and ice (100 g). The product
was extracted with EtOAc, and the organic layer was
washed successively with 0.5 M NaK tartrate, H20, and
brine. The solvent was then removed and
yellow/orange oil (3.5 g) was used without
purification in the following step.
Ste~_E: 3-~3-(3,3-Dimethyl-1-oxo-1-butyl)-
5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-
imethylpropanoic acid
The crude ester from step D was dissolved in
a mixture of MeOH (20 mL), THF (20 mL), and H20 (5
mL). To this was added :~0 M NaOH (2..3 mL, 23 mmol).
After stirring for 3 hours, the solution was cooled
to 0°C, and HOAc (1.5 mL) was added dropwise. The
solution was partly concentrated to remove the THF
and MeOH, and the product was then extracted into
EtOAc. The organic layer was washed with H20 and
brine. After drying (MgS04), the solution was
filtered and evaporated to give a pale orange solid.
The product was stirred vigourously with a mixture of
isopropanol (30 mL) and H20 (3 mL) to give the title
compound as an off-white amourphous solid (2.6 g).
mp= 193-196°C (dec)
EXAMPLE 31
3-[N-(9-Triflouromethylbenzyl)-3-(3,3-dimethyl-1-
oxo-1-butyl)-5-(quinolin-2-yl-methoxy)indol-2-yl]-2,2-
dimethylpro~anoic acid
The product from Example 30 (100 mg, 0.21
~~q .; ka ~~ ",
_.
,<~ ~.° ~7 ~ ,
76,~DAD331 -101- 179401B
mmol) and 4-trifluoromethylbenzyl bromide (98 mg,
0.41 mmol), and methyltrioctylammonium chloride (83
mg, 0.21 mmol) were dissolved in a mixture of 50%
NaOH (2 mL) and benzene (0.5 mL). After vigourous
stirring for 3.5 hours, the reaction mixture was
cooled to 0°C and was acidified with HOAc (2 mL).
The product was extracted with EtOAc, and the organic
layer was washed with H20 and brine. Following
evaporation of the solvent, the residue was purified
bY flash chromatography on silica gel, eluting with
I:5 EtOAc/hexane containing 1% HOAc. The resulting
yellow foam was triturated with 1:4 Et20/hexane to
give the title compound as a pale yellow solid (37
mg, 28%).
I5 1H NMR (CDC13) 8 8.22 (1H, d, J=8.5 Hz), 8.I3 (1H, d,
J=8.5 Hz), 7.87-7.70 (3H, m), 7.62-7.45 (4H, m),
7.00-6.85 (4H, m), 5.45 (4H, s), 3.58 (2H, brs), 2.87
(2H, s), I.30 (6H, s), I.03 ppm (9H, s).
~PLE ~2
3-[N-Benzyl-3-(3,3-dimethyl-1-oxo-I-butyl)-5-
(quinolin-2-ylmethoxy)indol-2-yl)-2,2-dimethyl-
propanoic acid
Following the method of Example 31, with
benzyl bromide as the alkylating agent, the title
compound was obtained as an off-white solid
(mp=180-183 °C (dec)).
S 1. d ' t %~ - ,~t
~-'~ ~:° ~J ;..~ ,_ y
7E; DaI~i31 -102- 179401B
EX~aMPbE 3 3
3-(N-(3-Methoxybenzyl)-3-(3,3-dimethyl-1-oxo-1-
butyl)-5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-
ime~hy ropanoic acid
Following the method of example 31, with
3-methoxybenzyl bromide as the alkylating agent, the
title compound was obtained as an off-white solid.
(mp=173-175 °C (dec)).
EXAMPLE ~4
3-(N-Allyl-3-(3,3-dimethyl-1-oxo-1-butyl)-5-(quinolin
2-~lmethoxy,indol-2-yll-2,2-dimethyl~ropanoic acid
~: Allyl 3-(N-allyl-3-(3,3-dimethyl-
1-oxo-1-butyl)-5-(quinolin-2-ylmethoxy)indol-2-yl]-
2,2-dlimethvlvropanaate _
To a solution of the product from Example 30
(100 mg, 0.21 mmol) in dry DMF (2 mL) was added 80%
NaH (14 mg, 0.47 mmol), followed 15 minutes later by
allyl bromide (0.35 mL, 4 mmol). After 2 1/2 hours,
saturated NH4C1 solution was added, and the product
was extracted with EtoAC. The organic layer was
washed with H20 and brine, dried over MgS04, filtered
and was then evaporated to give the title compound as
a yellow oil which was used as such in the next step.
SteQ B: 3-(N-Allyl-3-(3,3-dimethyl-1-oxo-
1-butyl)-5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-
dimethylpropanoic acid
The crude ester from step 1 was treated as
G~ ~t~ t1 is ,., ,
i
n:; aJ' 'y.~ "sY c,.:
?h;'L~.AI~S31 -103- 179401H
in the method of Example 30, Step 2 to give the title
compound as an off-c.hite solid (mp=146-148°C (dec)).
EX_AMpLE 3 5
3-(N-(4-Methoxybenzyl)-3-(3,3-dimethyl-1-oxo-1-butyl)-
5-(quinolin-2-ylmethoxy)indol-2-yl)-2,2-dimethyl-
~ropanoic acid
Following the method of Example 34, with 4
methoxybenzyl chloride as the alkylating agent, the
title compound was obtained as a white solid.
1H NHIR (250 MHz, acetone-d6) $ 3.05 (9H, s), 1.27 (6H,
s) 2.38 (2H, s), 3.73(3H, s), 3.78 (2H, s) 5.46(2H,
s). 5.48(2H, s), 6.70-8.40 ppm (13H, aromatics).
EXAMPLE 36
3-[N-Methyl-3-(3,3-dimethyl-1-oxo-1-butyl)-5-
(quinolin-2-ylmethoxy)indol-2-yl)-2,2-dimethyl-
prop._anoic said -
Following the method of Example 34, with
methyl iodide as the alkylating agent, the title
compound was obtained as a white solid.
Anal. C, H, N for sodium salt . 1. H20
Calc. C 68.42; H 6.70: N 5.32
Found. C 68.36; H 6.81; N 5.44
7b:!DAM31 -104- 179g0IB
EXAMPLE 37
3-[3-(4-Chlorobenzyl)-6-(quinolin-2-ylmethoxy)indol-2-
yll-2,2-dimethylpropanpir acid
Step A: ~-(Ouinolin-2 ylmethoxy)phenvl~drazine
The title compound was prepared using the
conditions described in Step A, Step B and Step C of
Example lA, but replacing the phenol in Step A with
3-acetamidophenol; m.p. 55-70°C (dec).
step B: Ethyl f~4-chlorobenzene)prapanoa a
A solution of 4-chlorobenzaldehyde (28 g) and
(carboethoxymethylene)triphenylphosphorane (73 g) in
toluene (500 mL) was refluxed for 2 hours. The
reaction was cooled to room temperature and
concentrated under vacuum to a total volume of 150
mL. Then pure hexane (500 mL) was added and the
mixture was left for 18 hours at room temperature.
The solid (triphexylphosphine oxide) was filtered,
rinsed with hexane and the filtrate evaporated to give
crude product which was distilled at 0.5 mm Hg. and
the fraction boiling at 130°C was collected to give
ethyl 4-chlorocinnamate, which was reduced as
follows: the cinnamate (21 g) was hydrogen aced in
EtoAc (300 mL) in the presence of 5% Pd on C (2 g) for
3 hours at atmospheric pressure. After completion,
the reaction mixture was filtered through a celite
pad, rinsed with EtOAc and the filtrate evaporated to
dryness to give the title product as an oil.
Step C: Methyl 6-(9-chlorophenyl)-2,2-
dimethyl-4-ox~hexanoate
76/DAhI31 -105- 1794UIB
To a solution of ethyl (4-chlorobenLene)-
propanoate (Step B, 10 g) in dry THF (500 mL) at -78°C
was added 0.58M potassium hexamethyldisilazane in
toluene (243 mL). The mixture was stirred at -60°C
for one hour. Then a solution of 2,2-dimethylsuccinic
anhydride (6 g) in THF {100 mL) was added slowly and
the mixture was slowly warmed to room temperature and
finally stirred for 18 hours. Water (1000 mL) was
added and the organic layer separated. The aqueous
layer was washed with EtOAc (3 x 250 ml) and acidified
with 1N HCl. The aqueous layer was extracted with
EtOAc, the extract was dried (Na2S04) and evaporated
to give a residue which was dissolved in THF (100 mL)
and MeOH (200 mL) and treated at reflux caith 1N LiOH
(100 mL) for 4 hours. The mixture was cooled to room
temperature and concentrated under vacuum until H20
distilled off. Water (500 mL) was added, the mixture
acidified with 1N aq. HCl, extracted with EtOAc, the
extract was dried (Na2S04) and evaporated to give the
title product as its carboxylic acid. The compound
was treated with diazomethane in ether, evaporated to
dryness and chromatographed over silica gel, eluting
with EtOAc-hexane (10:90) to give the title product as
a white solid; m.p. 52-54°C.
Step D: Methyl 3-[3-(4-chlorobenzyl)-6-(quinolin-
2-ylmethox~)indol-2 yl7-2.2-dime~vlpropanoate
To a solution of methyl 6-(4-Chlorophenyl)
-2,2-dimethyl-4-oxohexanoate (Step C, 2.8 g) in
toluene (30 mL) and glacial HOAc (15 mL) was added
portion-wise solid 3-(quinolin-2-ylmethoxy)-
phenylhydrazine (Step A, 3.2 g) and stirred at room
~i~~JJa~~:'1~~~
76/DAM31 -106- 1'79401B
temperature for 2 hours. The reaction mixture was
diluted with Et20 (200 mL), washed with 1N NaOH, H20,
dried (Na2S04) and evaporated to give crude hydrazone
which was immediately treated as follows: the crude
hydrazone was dissolved in a mixture of PPE(15 mL) and
1,2-dichloroethane (30 mL) and stirred at 90°C for 18
hours. The reaction mixture was cooled to 0°C and
carefully treated with 1N NaOH to bring to pH 9. The
mixture was then extracted with ether, the extract was
washed with H20, dried (Na2S04) and evaporated to give
a residue, which was chromatographed in a column of
flash silica gel (eluting with EtOAc-hexane 25:75) and
isolating the most polar component as the title
product as a foam.
Ste,~~ E: 3-[3-(9-Chlorobenzyl)-6-(quinolin-
2-ylmethoxy)indol-2-yl]-2,2-dimethylpropanoic
The title compound was prepared using the
2p conditions described in Step B of Example l, but
substituting the ester from Step D for the ester of
Example 1, Steg A.
Anal. C, H, N for sodium salt ~ 1 1/2 H20
Calc. C 65.75; H 5.33; N 5.11
Found. C 66.08; H 5.31; N 5.08
EXAMPLE 38
3-[N-(Phenylsulfonyl)-3-(4-chlorobenzyl)-6-(quinolin-
2-ylmethoxy)indol-2-vll-2,2-dim~thylnropanoic acid
~~ ~ ~~ ~ °>
76/DAD131 -107- 17990IB
Step A: Methyl 3-[N-(phenylsulfonyl)-3-
(4-chlorobenzyl)-6-(quinolin-2-ylmethoxy)indol-2
yl~-2.2-dimethylpropanoate
To a solution of methyl 3-[3-(4-
chlorobenzyl)-6-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-d
imethylpropanoate (Step D, Example 37) (208 mg) in dry
THF (5 mL) and HMPA (0.5 mL) at -78°C was added 0.58M
potassium hexamethyldisilazane in toluene (0.77 mL)
and the solution then stirred at -78°C for 15
minutes. Then freshly distilled benzenesulfonyl
chloride (0.062 mL) was added and stirred at -78°C for
2.5 hours. The reaction mixture was quenched with 25%
aq. NH40Ac, extracted with EtOAc, dried over Na2S04
and evaporated to give crude product. Chromatography
of the residue in a column of flash silica gel
(eluting with EtOAc-hexane 25:75) afforded the title
product as an oil.
3-[N-Phenylsulfonyl)-(3-(4-chlorobenzyl)-
6-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl
.~~czpanoic acid
'fhe title compound was prepared under the
conditions described in Step B of Example l, but
substituting the ester from Step A for the ester of
Fxample 1, Step A.
Anal. C, H, N for sodium salt.
Calc. C 67.65; H 4.89; N 9.38
Found. C 68.07; H 5.18; N 4.32
~~ tY ~7~ C 1 ~ j '~,~
76~'DA1~131 -108- 17940TB
EXAMPLE
3-(N-Benzyl-3-(4-chlorobenzyl)-6-(quinolin-2-
Ylmethoxv)indol-2-yll-2.2-dimeth~l~ropanoic acid
The title compound was prepared under tire
conditions described in Step A and Step B of Example
38, but substituting benzyl chloride for the
benzenesulfonyl chloride from Example 38 (Step A).
Anal. C, H, N for sodium salt ~ 1/2 H20
Calf. C 71.66; H 5.36; N 4.52
lp Found. C 71.65; H 5.49; N 4.44
XAMPLE 40 AND
3-(N-(4-Chlorobenzyl)-3-(t-butylsulfonyl)-5-(quinolin-_
15 2-ylmethoxy indol-2 yll-2,2-dimethyl~ropanoic acid ansl
3-[N-(4-chlorobenzyl)-3-(t-butylsulfinyl)-5-(quinolin-
2-ylmethox~~indol-2-vll-2,2-dimethvlpronan~c acid
The title compounds were prepared using the
conditions described for Examples 6 and 7, but
20 substituting the ester of Example 4 (Step D) for the
ester of Example 1 (Step D).
3-(N-(4-chlorobenzyl)-3-(t-butylsulfonyl)-5-(quinolin-2
-ylmethoxy)indol-2-yl]-2,2-dimethylpropanoic; acid:
Anal. C, H. N for sodium salt ~ 1 1/2 H20
Calc. C 61.12; H 5.58; N 4:19
Found C 61.31; H 5.39; N 9.19
3-[N-(4-chlorbenzyl)-3-(t-butylsulfinyl)-5-(quinolin-
2-ylmethoxy)indol--2-yl]-2,2-dimethylpropanoic acid:
~w9i.~~l~I.'~.:,;t
J 1..' ::' 6
7 ti,'DAb131 -109- 17940IB
Anal. C, H, N for sodium salt ° 2 H20
Calc. C 61.76; H 5.79; N 4.24
Found C 61.94; H 5.68; N 4. i9
EXAMPLE 42
3-jN-Allyl-3-(4-chlozobenzyl)-6-(quinolin-2-ylmethoxy)
indol-2 yll-2,2-dimethvl~rQpanoic acid
The title compound was prepared under the
conditions described in Step A and Step B of Example
38, but substituting allyl bromide for the
benzenesulfonyl chloride from Example 38 (Step A).
Anal. C, H, N for sodium salt ~ 2 H20
Calc. C 66.38; H 5.74; N 4.69
Found C 66.57; H 5.75; N 4.73
EXAMPLE
3-jN-(n-Propyl)-3-(4-chlorobenzyl)-6-(quinoline-2-
y~methoxy~~ndol-2 ;y~l-2~ 2-dimethylgropanoic acid
A solution of methyl 3-jN-allyl-3-
(4-chlorobenzyl)-~-(quinolin-2-ylmethoxy)indol-z-yl)-
2,2-dimethylpropanoate {Example 42, methyl ester) {190
mg) was hydrogenated in EtOAc {4 mL) in the presence
of 5% Pd on charcoal at atmospheric pressure for 1
hour. Filtration on Celite pad and evaporation of
liquors afforded the methyl ester of 'the title
product. Hydrolysis of this ester under the
conditions described in Step B of Example 1 provided
the title compound.
"°° ~1 i ~ it c_,~ _~''~ ,,'
?6~'DAIYI31 -110- 17940TB
Anal. C, H, N for sodium salt ~ 1 1/2 H20
Calc. C 67.17; H 5.98; N 4.75
Found C 67.38; H 5.44; N 4.85
EXAL4PLE 4 4
3-[N-Ethyl-3-(4-chlorobenzyl)-6-(quinolin-2-ylmethoxy)
indQl-2-.T11-2,2-dimethyl~ropanpic acid
The title product was prepared under the
conditions described in Step A arid Step B of Example
38, but substituting iodoethane for the
benzenesulfonyl chloride from Example 38 (Step A).
Anal. C, H, N for sodium salt . 2 H20
Calc. C 65.69; H 5.86 N 4.79
Found C 65.81; H 5.21; N 4.77
EXAN~PLE~ S
3-[N°(4-Chlorobenzyl)-3-(4-t-butylbenzoyl)-5-
(quinolin-2-yl-methoxy)indol-2-yl)-2,2-
dimethvlpro,~anoic amid
The title compound was prepared according to
the method described in Example 9, but using
4-t-butylbenzoyl chloride in place of benzoyl chloride
in Step A.
Anal. C, H, N, for sodium salt . 2 1/2 H2o
Calc. C 67.81; H 5.97; N 3.86
Found. C 67.91; H 6.01; N 3.69
i; .yl .~j y- f~1 ;If
76iDAM31 -111- 17990IB
EXAMPLE 4~
3-[N-(4-chlorobenzyl)-3-(4-chlorobenzoyl)-5-(quinolin-
2-ylmethox~ indol-2-vll-2,2-dimethy_l~~_panoic acid
The title compound was prepared according to
the method described in Example 9, but using
4-chlorobenzoyl chloride in place of benzoyl chloride
in Step A.
Anal. C, H, N for sodium salt . 2 H20
Calc. C 63.89; H 4.78; N 4.03
Found C 64.20: H 4.61; N 3.99
EXAMPLE 47
3-[N-(4-Chlorobenzyl)-3-(l,l-dimethylethyl)-5-
(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl-
grQpanoic acid
Methyl 3-[N-(4-chlorobenzyl)-5-(quinolin-
2-ylmethoxy)indol-2-y1~-2,2-dimethylpro,~anoate
Methyl 3-[N-(4-chloxobenzyl)-3-
(t-butylthio)-5-(quinolin-2-ylmethoxy)indol-2-yl]-
2,2-dimethylpropanoate (3.77 g, 6.27 mmol) from Step D
of Example 1, was dissolved in 75 mL of dry CH2C12 and
the solution was charged with 6.27 g (47.0 mmol) of
A1C13 and the mixture was stirred at RT, under Ar, for
1.75 hours. The reaction was then quenched by the
addition of 0.5N Na, K tartrate (150 mL) and the
resulting mixture was extracted with EtOAc (3x). The
organic extracts were washed with 0.5N Na, K tartrate
(lx) and with brine (lx), and dried (MgS04).
'~e ~ ~ ':.~ iJ ;.~ (..~ 'il,
'.TO~'i~Ai,931 -112- 17940IH
Filtration and removal of solvents provided a brown
oily residue which was chromatographed on silica gel
using EtOAc-hexane (1:3) to give the title compound.
Step H: Methyl 3-[N-(4-chlorobenzyl)-3-
(l,l-dimethylethyl)-5-(quinolin-2-ylmethoxy)-
in ol-2-yll-2 2-dimethylpropanoate
Trimethyiacetyl chloride (4.23 g, 4.32 mL,
35.09 mmol) was added to a cold suspension (0°C) of
to A1C13 (11.7 g, 87.7 mmol) in dry CH2C12 (6o mL) unaer
Ar. The yellow mixture was stirred at 0°C for 15
minutes, and a solution of 9.00 g (17.54 mmol) of
methyl 3-[N-(4-chlor0benxyl)-5-(quinolin-2-
ylmethoxy)indol-2-yl]-2,2-dimethylpropanoate (prepared
in Step A) in CH2C12 {40 mL) was added dropwise (over
10 minutes) at 0°C. The reaction mixture was stirred
for 10 minutes and slowly poured onto an ice-cold and
vigourously stirred mixture of 0.5M aqueous Na, K
tartrate (500 mL) and EtOAc (400 mL). After 20
minutes. the aqueous layer was extracted with EtOAc
(2x) and the combined organic extracts were washed
with H20 (2x), with 1N aqueous NaOH (2x), with H20
(Zx) arid dried (NigS04). Filtration and removal of
solvents provided a yellow oily residue which was
chromatographed on silica gel using EtOAc-hexane {1:4)
to give the title compound.
Step C: 3-[N-(4-chlorobenzyl)-3-(l,l-dimethyl-
ethyl)-5-(quinolin-2-ylmethoxy)indol-2-
Yl~-2,2-dimethy~ropanoic acid
The title compound was prepared according to
l~ .~ 5~ t ~_t .~ c
76.'DAbI31 -113- 17940IB
the conditions described in Step B of Example 1, but
substituting the ester from Step B for the ester of
Example 1. The title compound was recrystallized from
EtOAc-EtOH; m.p. 201-202°C.
Anal. Calc. C 73.57 H 6.36 N 5.05
Found C 73.75 H 6.37 N 5.03
EXAMPLE 48
3-(N-(4-Chlorobenzyl)-3-acetyl-5-(quinolin-2-
ylmethox~) indol-2 x~11-2, 2--dimethylprQpanoiE, acid
The title compound was prepared according to
the conditions described in Step B and Step C of
Example 47, from methyl 3-(N-(4-chlorobenzyl)-5-
(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl-
propanoate (prepared in Step A of Example 47) but
using acetyl chloride in place of trimethylacetyl
chloride in Step B.
25
1H NMR (CD3COCD3) S 1.20 (6H, s), 2.64 (3H,s), 3.62
(2H, br s), 5.47 (2H, s) 5.57 (2H, s), 6.90-6.99 (3H,
m), 7.28-7.37 (3H, m), 7.56-7.83 (4H, m), 7.97 (1H, d)
B.05 (1H, d), 8.39 ppm (lH,d).
EXAMPLE 49
3-(N-(4-Chlorobenzyl)-3-cyclopropanecarbonyl-5-
(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl-
propanoic acid
The title compound was prepared according to
( > a~ ~ o~ ~ rs ~~.. r,. ,~~ ~
l, ~;3 i,3 ~i ;,~ .. r
76~'L'Ah131 -114- 17940IB
the conditions described in Step B and Step C of
Example 47, from methyl 3-[N-(4-chlorobenzyl)-5-
(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl-
propanoate (prepared in Step A of Example 47), but
using cyclopropanecarbonyl chloride in place of
trimethylacetyl chloride in Step B.
Anal. C, H, N for sodium salt ~ 1 H20:
Calc. C 67.27; H 5.31; N 4.61
Found C 67.27; H 5.16; N. 4.58
EXAMPLE 50
3-[N-(4-Chlorobenzyl)-3-(3-cyciopentylpropanoyl)-5-
(quinolin-2-ylmethoxy)indol-2-yl]-2,2-
dimethvlpropanoac~~d
The title compound was prepared according to
the conditions described in Step B and Step C of
Example 47, from methyl 3-[N-(4-chlorobenzyl)-5-
(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl-
propanoate (prepared in Step A of Example 47), but
using 3--cyclopentylpropanoyl chloride in place of
trimethylacetyl chloride in Step B.
1H NMF2 (CD3COCD3) S 1.09 (2H, m) 1.22 (6H, s),
1.40-1.91 (9H, m), 2.94 (2H, t), 3,68 (2H, br s),
5.46(2H, s), 5.58 (2H, s), 6.91-6.99 (3H, m), 7.30(3H,
m), 7.53-7.63 (2H, m) 7.72-7.82 (2H, m), 7.96 (1H, d),
8.06 (1H, d), 8.34 ppm, l,lh, d).
3D
,,
. , ,.
i~ v ;i u,.; ...
76; P~I31 -115- 17940zB
~~AMPLE 51
3-[N-(4-Chlorobenzyl)-3-(3-methylbutanoyl)-5--
(quinolin-2-yl-methoxy)indol-2-yl]-2,2-dimethyl-
~ropanoic acid
The title compound was prepared according to
the conditions described in Step B arid Step C of
Example 47, from methyl 3-[N-{4-chlorobenzyl)-5-
(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl
propanoate (prepared in Step A of Example 47), but
using 3-methylbutanoyl chloride in place of
trimethylacetyl chloride in Step B.
1H NMR {CD3COCD3): cS 0.98 (6H, d). 1.24 (6H. s), 2.30
(1H, m), 2.85 {2H, d), 3.70 (2H, br s), 5.46 (2H, s),
5.58 (2H, s), 6.96 (3H, m), 7.30 (3H, m), 7.55-7.63
(2H, m), 7.73-7.82 (2H, m), 7.95 {1H, d), 8.07 {1H,
d), 8.36 ppm {1H, d).
EXAMPLE 52
3-[N-(4-Chlorobenzyl)-3-prapanoyl-5-(quinolin-2-
ylmethoxy)indol-2-v11-2,2-dimethvl~~~anoic acid _
The title compound was prepared according to
the conditions described in Step B and Step C of
Example 47, fram methyl 3-[N-(4-chlorabenzyl)-
5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl-
propanoate (prepared in Step A of Example 47), but
using propanayl chloride in place of trimethylacetyl
chloride in Step B.
Anal. C, H, N for sodium salt . 1H20
Calc. C 66.61; H 5.42; N 4.71
Found C 66.87; H 5.45; N 4.69
y r. -, . i
F~ ~l ~.i ~~ ,,,r ~~
76/DAP231 -116- 17940aB
~._X?~M~,E 53
3-(N-(4-Chlorobenzyl)-3-(2-methylpropanoyl)-5- ,
(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl- '
propanoic acid
The title compound was prepared according to
the conditions described in Step B and Step C of
Example 47, from methyl 3-(N-(4-chlorobenzyl)-5-
(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethylpropanoate
(Prepared in Step A of Example 47), but using
2-methylpropanoyl chloride in place of trimethylacetyl
chloride in Step B.
1H-NM3Z (CD3COCD3): $ 1.07 (6H, d), 1.16 (6H, s), 3.34
(1H, m). 3.64 (2H. br s), 5.46 (2H, s), 5.57 (2H, s),
6.95 (3H, m), 7.32 (3H, m), 7.45 (1H, br s), 7.60 (1H,
br t), 7.71-7.83 (2H, m), 7.97 (1H, d), 8.07 (1H, d),
8.36 ppm (1H, d).
EXAI4PLE 54
3-(N-(4-Chlorobenzyl)-3-trimethylacetyl-5-(quinolin-2-
ylmethoxy)indol-2-yl]-2,2-dimethylpropanoic acid,
sodium salt
Step A: Methyl 3-(N-(4-chlorobenzyl)-3-trimethylacetyl-
5-(quinolin-2-ylmethoxy)indol-2-y1]-2,2-di-
meth~lpropanoate
I~gth~,3 A: The indole from Example 47, Step A
(2.00 g, 3.9 mmol) and trimethylacetyl chloride (0.86
9~ 7.1 mmol) were dissolved in sieve-dried CH2C1? (15
mL). The mixture was cooled to -25°C and A1C13 (1.64
Sy ~~ r~~ ' ~'I
s.r ~ i
76iD.~131 -117- 17940IB
g, 12.3 mmol) was added in two portions 5 minutes
apart. After 10 minutes at -20 to -25°C, 7 mL of 2.5 M
aqueous HOAc was added to the mixture such that the
temperature stayed below -20°C. The mixtuxe was then
warmed to RT and the layers were separated. The
organic layer was washed with H20, Saturated aqueous
NaHC03, and H2O, and then evaporated to dryness. The
resulting oil was crystalized from MeOH (20 mL) to give
1.4 g (600) of the title compound.
Method B: A solution of TiCl4 (6 mL of a 1.0
M solution in CH2C12, 6.0 mmol) and trimethylacetyl
chloride (0.491 g, 4.1 mmol) was cooled to -5°C. To
the cooled solution was added a solution of the indole
from Example 47, Step A (1.025 g, 2.0 mmol) in 2 mL of
CH2C12 over a 5 minute period. After 30 minutes, the
reaction was quenched by the addition of 3 mL of 2.5 M
aqueous HOAc. The mixture was warmed to RT and the
layers separated. The organic layer was washed with
H2O, saturated aqeuous NaHC03, and H20, and then
evaporated to dryness. The residual oil was
crystallized from ZO mL of MeOH to give 625 mg (530) of
the title compound.
1H-NMR (CDC13) c~ 1.22 (6H, s), 1.30 (9H, s), 3.29 (2H,
s), 3.61 (3H, S), 5.28 (2H, s), 5.47 (2H, s), 6.7-8.2
ppm (13H, m)
IR(Nujol mull) 1636, 1730 cm-1
r~~i)~~~~1~
7b,~'D~'1h131 -118- 17940IH
3-[N-(4-Chlorobenzyl)-3-trimethylacetyl-5-
(quinolin-2-ylmethoxy)indol-2-y1]-2,2-dimethyl-
p~panoic acid. sodium halt
The acylated methyl ester from Step A (401 mg,
0.67 mmol), absolute EtOH (1.62 g), and NaOH (64.2 mg
of a 50.9% aq. solution, 0.82 mmol) were refluxed 42
hours. During the latter stages of the reaction,
product crystallized. At the end of the reflux, the
product was filtered and washed with EtOH to give 288
m9 (74%) orange solid. The material was slurried in 3
mL EtOH for 8 hours at RT to give 190 mg of the title
compound as a pale orange solid.
1H-NMR (CD30D) 8 1.08 (6H, s), 1.15 (9H, s), 3.23.(2H,
s). 5.40 (2H, s), 5.55 (2H, s), 6.7-8.2 ppm (13H, m)
IR (Nujol mull) 1680, 1575 Cm-1.
EXAMPLE 55
3-[N-(4-Chlorobenzyl)-3-phenylacetyl-5-(quinolin-2-yl
methoxv)indol-2-v17-2.2-~i~m~h~ r~panoic ac~.d
The title compound was prepared according to
the conditions described in Step B and Step C of
Example 47, from methyl 3-[N-(4-chlorobenzyl)-5-
(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethylpro-
panoate (prepared in Step A of Example 97), but using
phenylacetyl chloride in place of trimethylacetyl
chloride in Step s.
Anal. C, H, N for sodium salt . 1H20
Calf. C 69.46; H 5.22; N 4.26
Found C 69.714 H 5.25; N 4.11
~'~ ~I j ~i ;,,; .',~~
76iD~131 -119- 17940IB
EXAMPLE
3-[N-(4-Fluorobenzyl)-3-{3,3-dimethyl-1-oxo-1-butyl)-
5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl-
propanoic acid
Using the procedure of Example 31, but
replacing 4-trifluoromethylbenzyl bromide with
4-fluorobenzyl bromide, the title compound is obtained.
EXAMPLE 57
3-[N-(4-Bromobenzyl)-3-{3,3-dimethyl-1-oRO-1-butyl)--5-
(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl-
propanoic acid
Using the procedure of Example 31, but
replacing 4-trifluoromethylbenzyl bromide with
4-bromobenzyl bromide, the title compound is obtained.
_E_XAMPLE 58
3-[N-(4-rodobenzyl)-3-(3,3-dimethyl-1-oxo-1-butyl)--
5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-dimethyl-
propanoic acid
Using the procedure of Example 31, but
replacing 4-trifluoromethylbenzyl bromide with 4-iodo-
benzyl bromide, the title compound is obtained.
'y
76i DADS31 -120- 17940IB
EXAMPLES 59-72
Operating as described in the previous
example s the following compounds are prepared:
R5
i i
~ ~ CHzO ~ ~ \~H2_y_CCR~1Rii~P_C02H
N
Ra
Ex ATTACH Rg R5 Y-(CR11R11)p
No. POINT
59 5 -CH2Ph-4-C1 -C(Me)2Pr C(Me)2
60 5 -CH2Ph-~-C1 -C(Me)2Et C(Me)2
61 5 -CH2Ph-3-F' -C(Me)3 C(Me)2
62 5 -CH2Ph-4-C1 -CH(~1e)2 C(Me)2
63 5 -CH2Ph-4-C1 -c-Pr C(Me)2
64 5 -CH2Ph-4-Cl -(1-Me)-c-Pr C(Me)2
65 5 -CH2Ph-4-C1 -c-C5H9 C(Me)2
66 5 -CH2Ph-4-C1 -c-C6H11 C(Me)2
67 5 -CH2Ph-9-C1 -C(Me)2Ph C(Me)2
68 5 -CH2Ph-4-C1 -C(Me)2Ph-4-C1 C(B1e)2
69 5 -CH2Ph-4-C1 -1-Ad C(Me)2
70 5 -CH2Ph-4-C1 -CH2-1-Ad C(Me)2
71 6 -t-Bu -CH2Ph-4-C1 C(Me)2
72 6 -C(Me)2Et -CH2Ph-4-C1 C(Me)2
/~:~ 'r.; w:~ 'i ..' ...
7o1P.~131 -121- 179401B
~X MA PLE 73_
3-(N-{4-Chlorobenzyl)-3-(3,3-dimethyl-1-oxo-1-butyl)-
5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-diethyl-
propanoi~ acid
S~~p A: Methyl 4-chloro-2,2-diethyl-4-pentenoate
To a cold solution (0°C) of diisopropylamine
(0.80 mL, 5.7 mmol) in THF (5 mL) was added a 1.6 M
solution of butyllithium in hexane (3.4 mL, 5.4 mmol)
over a 5 minute period and stirring was continued for
an additional 45 min. Then a solution of methyl
4-chloro-2-ethyl-4-pentenoate (800 mg, 5 mmol) from
Example 27, Step A. in THF (2 mL) was added and the
reaction was stirred at 0°C for another 30 minutes.
Then ethyl iodide (440 ~L, 5.5 mmol) was added and
the reaction was allowed to proceed at room
temperature for 2 hours. The reaction was quenched
with NH40Ac buffer (50 mL of 25°s w/v) and extracted
with EtOAc. The organic layer was separated, dried
over rIgS04, filtered and concentrated. The crude
product was purified by Kugelrohr distillation (b. p.
120°C at 0.1 mm Hg) to give the title compound.
Step-B: Methyl 5-(t-butylthio)-2,2-diethyl--4-
oxczpent anoate
The title compound was prepared according to
the method of Example 27, Step B and Step C, but
using methyl 4-chloro-2,2-diethyl-4-pentenoate as
starting material in Step B in place of methyl
4-chloro-2-ethyl-4-pentenoate.
t ~ a
~~ ' a
ml ~.1~ ~~V ~~ v.~ ., v
76iDAM31 -122- 1794o1B
~gp~: Methyl 3-[N-(4-chlorobenzyl)-3-(t-buøylthio)-
5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-
s~i~~vlprQpanoate _ _
To a mixture of l-(4-chlorobenzyl)-1-[4-
(quinolin-2-ylmethoxy)phenyl]hydrazine from Example
lA, 5tep D, (660 mg, 1.7 mmol) and anhydrous NaOAc
(160 mg, 1.95 mmol) in toluene (3 mh) was added
glacial HOAc (1.5 mL). After 30 minutes, a solution
containing methyl 5-(t-butylthio)-2,2-diethyl-4-oxo-
Pentanoate from Step B (402 mg, 1.47 mmol) in toluene
(1 mL) was added and the reaction mixture stirred for
24 hours at room temperature and for 48 hours at 65°C.
The reaction was then diluted with EtOAc, washed with
NH40Ac buffer (25% w/v) and dried over MgS04. Filtra-
tion and concentration gave a viscous oil which was
purifed by flash chromatography on silica gel (eluant:
EtOAc-hexane 15:85) to give the title compound.
1H NMR (250 MHz acetone-d6); 8 0.85 (6H, t), 1.1 (9H,
2p S), 1.7 (4H, q), 3.2 (2H, S), 3.6 (3H, S), 5.4 (2H,
s). 5.5 (2H. S) 6.9 (3H, m), 7.3 (4H, m), 7.6 (1H,
dd). 7.7 (11-1, d), 7.8 (1H, td), 7.9 (1H, d), 8.1 (1H,
d), 8.3 ppm (1H, d).
$teo B: Methyl 3-[N-(4-chlorobenzyl)-5-(quinolin-2-
ylmethoxy indol-2-y11-2,2-diethylpro~ano~te
The title compound was prepared according to
the method of Example 47, Step A, but using methyl
3-[N-(4-chlorobenzyl)-3-(t-butylthio)-5-(quinolin-2-
3p ylmethoxy)indol-2-yl]-2,2-diethylpropanoate from Step
C in place of methyl 3-(N-(4-chlorobenzyl)-3-(t-butyl-
thio)-5-(quinolin-2-ylmethoxy)indol-2-yl]-2,2-
dimethylpropanoate.
fN ii 'u LY c J ,.1
76!DAD231 -123- 17940TB
Step E: Methyl 3-[N-(4-chlorobenzyl)-3-(3,3-dimethyl-
1-oxo-1-butyl)-S-(quinolin-2-ylmethoxy)indol-
2-yll-2.2-diethylgropanoate
To a cold solution (0°C) of methyl 3-[N-(4-
chlorobenzyl)-5-(quinolin-2-ylmetho~cy)indol-2-yl]-2,2
diethylpropanoate (from Step D) (177 mg, 0.33 mmol)
in CH2C12 (3 mL) was added A1C13 4220 mg, 1.65 mmol)
followed by t-butylacetyl chloride (82 ~L, 0.66 mmol).
The reaction was stirred at 0°C for 20 minutes and
then quenched with 30 mL of 0.5 N Na, K tartrate
solution, and extracted with 3 x 30 mL of EtOAc. The
organic layers were combined and dried over MgS04.
Filtration and concentration gave an oily residue
which was purified by flash chromatography (eluant:
I5 EtOAc-hexane 17:83) to give the title compound.
Step E: 3-[N-(4-Chlorobenzyl)-3-(3,3-dimethyl-1-oxo-
1-butyl)-5-(quinolin-2-ylmethoxy)-indol-2-
~11-2.2-dieth~propanoic aEid
The compound from Step E was hydrolysed
using THF (2.5 mL), MeOH (0.6 mL) and NaOH (1N, 1.5
mL). The solution was heated at 70°C for 2 weeks.
The reaction was neutralized by addition of Na40Ac
buffer (20 mL of 25~ w/v) and extracted with EtOAc (3
x 30 mL). The organic layers were combined, dried
over MgS04, filtered and concentrated. The resulting
residue was purified by flash chromatography (eluant:
EtOAc-hexane-HOAc (250:750:1)) to give the title
compound.
Anal. C, H, N for sodium salt~l 1/2 H20;
Calf. Ce 67.70; H, 6.43; N, 4.15
Found C, 67.67; H, 6.3I; N, 4.06
~ ~ ~ ,~ ~~ ~ '
76!DAM31 -124- 17940IB
~~E 74
Methyl 3-[N-(4-chlorobenzyl)-3,6-bis(acetyl)-5-(quino-
lin-2-ylmetho::y_)ind~1-2-X11-2 2-dimethylpropanoate
The title compound was isolated in Step B of
Example 48 from a chromatography on silica gel (EtOAc-
Hexane 2:3j.
1H NMR (CD3COCD3): $ 1.22 (6H, s), 2.63 (3H, sj,
2.66 (3H, sj, 3.58 (3H, s), 3.65 (2H, s), 5.61 (2H,
s). 5.65 (2H, s), 6.95 (2H, d), 7.31 (2H, d), 7.62
(1H, br t), 7.71-7.85 (4H, m), 7.97 (1H, dj, 8.08
(1H, d) 8.40 ppm (1H, d).
EXAMPLE 75
Methyl 3-[N-(4-chlorobenzyl)-3,6-bis(cyclopropane-
carbonyl)-5-(guinolin-2-ylmethoxy)indol-2-yl]-2,2-
dimethylpropanoate
The title compound was isolated in Step g of
Example 49 from a chromatography on silica gel (EtOAc-
ap hexane 3:7) and was recrystallized from EtOAc-EtOH;
m, p. 166--167°C.
30
76/DA~~231 -125- 17940IB
EXAMPLES 76-8~
Using the techniques of Methods 1 through 12 as
required, the following compounds are prepared:
ID#1066
(
5-tHu
~~H~Y( CR~~Rp pQ
Ie
C
Ex. No. X4 Y-(CR11R11)p Q
76 CH20 C(Me)2 -C(O)NHS(O)2Me
77 CH20 C(Me)2 -NHS(O)2Ph-4-Me
78 CH20 C(Me)2 -C(O)NH-t-Bu
79 CH20 OCH2CH(Me) -C02H
80 CH20 CH2CH2 Tz
81 CH20 OCH(Me) Tz
82 CH20 C(Me)2 -S(O)2NH-Et
83 CH20 C(Me)2 -C02CH2C(0)NMe2
84 CH2O C(Me)2 -C(0)NHCH2C02H
85 CH20 C(Me)2 -CH20H
86 (E)-CH=CH C(Me)2 -C02H
87 CH2CH2 C(Me)z -C02H
gg CH2S C(Me)2 -C02H
89 CH2S(O)2 C(Me)2 -COZH