Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2062~
MET~OD AND APPARArUS FOR ~ P~ING GAS USED IN CARBON
DIOXIDE LABER GEw~RATOR
BA~K~Ku~Nv OF THE lNv~ ON
The present invention concerns a method of
regenerating mixed gas used in a cArbo~ dioxide laser
generator with the aid of a catalyst as well as an apparatus
10 for working the method. The invention further concerns a
method of reactivating the catalyst by Lecov~L-ing it from
poisoning by NOX and a method of pretreatment the catalst for
r~ ci n~ the poiconi n~ .
A~ the laser gas for c~rb~n dioxide laser generator of
15 high output and high pulse there has been u~ed a mixed gas
consisting of He, N2 and CO2 at a mixing ratio of 8:1:1
~volume). Due to diacharging a portion of C02 is
decrn~cee~, and if the decompsition products remain in the
gas, output of the laser generator decreaRes and arcing
20 ~ h~rge will occur.
Under constant ~upply of fresh gas the high output can
be maintained. ~'.J~l, He-gas which shares a major part of
the mixed gas for laser is ~yrencive~ and supply of the fre~h
mixed gas as dem~nded make~ the runnin~ cost~ very high.
25 Thus, efforts have been made to ~ 'ine CO and C02 formed
in the carbon ~i~Y;~ laser ~eneL~Lo~ to form C02 and reuse
20~2708
the thus regenerated gas. It i8 known that precious metal
cataly~t are useful for practicing the reaction.
The in~entors intended to provide an industrially
practicable method of regenerating the mixed gas used in the
carbon dioxide la~er generator, and estab1;~he~ the method
which enables reuse of the mixed gas cont~in;ng CO and C02 by
recombining them, and disclose~ it (J~p~n~se Patent
Di~closure Hei 3-84980).
The method compri~e~ preheating the mixed gas used in
10 the carbon dioxide ~aser generator and contacting it with a
catalyst to react CO and ~2 in the mixed gas, while u~ ng
the reactio~ heat for preheating the mixed gas to be treated,
and then, cooling the reacted gas to the temperature 3uitab1e
for reu~e in the laser oscillation and, after removing dust
15 therefrom, recycling it to the laser genera-or. The
reaction conditions preferable for this regeneration are:
reaction temperature 80 - 200 ~C, ~pace velocity of the mixed
ga8 in the catalytic reactor 4,000 - 14,000 /Hr. r- i n~
under pressure makes it pos~ible to use a lower reaction
20 te~pe~ture and reduce the size of the apparatus, and
th~ ~fole, i8 ~ '. Practically, if practiced under
a pre~ure of 5 kg/cm2 G or higher, efficient reac~ion can be
made even at a preheating temeprature as low as near the
normal temperature ( about 40~C~, and the loads of the
25 preheater and the cooler may be much re~l~ced.
The method proved to be succe~s~ul to 30me extent.
~c.~e~ in aase where the operation continued for a long
- CA 02062708 1998-04-29
period, particularly, operated under the conditions where the
laser output is high, the activity of the catalyst decreases
and the regeneration of the gas becomes insufficient.
SUMMARY OF THE INVENTION
The object of the present invention is to provide an industrial
method and apparatus for regenerating the mixed gas used in a carbon dioxide
laser generator so that the gas may be used repeatedly, and so that the catalyst
remains active for an extended period of time.
In one aspect of the invention there is provided a method of
regenerating He-N2-CO2 mixed gas for reuse in a carbon dioxide gas laser
generator comprising contacting the mixed gas used in the laser generator
with a precious metal catalyst at a temperature exceeding 200~C and up to
15 350~C to react CO and ~2 with NOX in the used gas; utilizing the reaction heat
for preheating the unreacted mixed gas; cooling the reacted mixed gas to a
temperature usable in the laser oscillation; removing dust from the mixed
gas; and recycling the mixed gas to the laser generator.
An apparatus according to the invention comprises a gas preheater
for preheating the mixed gas used in the laser generator; a reactor which has a
heating means and is packed with a precious metal catalyst for reacting CO
and ~2 with NOX in the preheated mixed gas; a gas cooler for cooling the gas
' CA 02062708 1998-04-29
after the reaction; a filter for removing dust from the gas after cooling; a
means for recycling the regenerated gas to the laser apparatus; and a means for
supplying a He-CO-02 mixed gas to the reactor packed with the catalyst for
reactivating the catalyst.
BRIEF EXPLANATION OF THE DRAWINGS
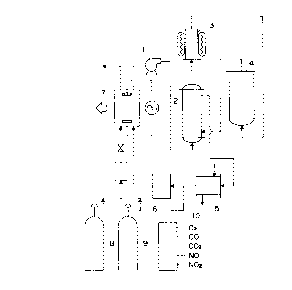
Fig.l is a flow chart illustrating the structure of
the apparatus for regenerating the gas for laser generator;
Fig.2 is a flow chart illustrating the experimental
10 apparatus with which the present invention was established;
Fig.3 concerns experimental data for the present
method, or the graph showing that the Pt-A1203 catalyst used
in the laser gas regenerating apparatus recovers its activity
at a high temperature after being poisoned by NO;
Fig.4 also concerns experimental data showing that the
gas regeneration carried out subsequent to the experiments of
3A
2062708
Fig.5 also concerns experimental date showing the
results of regeneration of a gas having a composition
simulating to the composition after u~e of the la~er gas
according to the present invention;
Fig.6 is practical data of the working example
according to the preqent method of mixed ga~ regeneration
showing the chAn~es of C0-reactivity and N0-reactivity
~pq~jng on the temperature in case of Le~ene.a~ing the
laser gas cont~; n; ng NO;
Fig~7 is pracitcal data of the wc ' ing example
according to the present method showing the chAn~es with the
passage of time of C0-reactivity and N0-reactivity in case
where the laser gas cont~;nlng N0 was regenerated by use of a
reactivated c~talyst;
Fig.8 is comparative data of the working example using
the cataly~t which is pretre~ted according to the present
invention and thQ catalyst which is not pretreated by showing
the ~h~n~6 with the pA~~~gq of time of C0-rea~tivity;
Fig.9 is data o~ a working example in which the la~er
20 gas regeneration according to the present invention i~
combined with laser o~cillation showing the ~h~nges in ~2-
c~c~Lration in the laser gas by comparing the ca~es where
the Ley~ La~Or i8 used or not used;
Fig.lO is data of the working example, to which the
25 data of Fig.9 i~ related, Yhowing the Gh~ngss with the
passage of time in the laser output by comparing the cases
where the L~ne-a~or is used or not used.
20627~8
DET~TT~n D~C~ ON OF THE lhv~luN
The method of r~geneiating the gas for carbon dioxide
laser ge~.eL~to~ is a method of r~gen~ldling He-N2-C02 mixed
gas used in carbon dioxide gas laser gene~aLo~ for reuse,
which method coIprises contacting CO, ~2 and NOX in the ~ixed
ga6 used in the laser g~e~dLor with a precious metal
catalyst at a tempe~d~ure P~n~;nq 200~C and up to 350~C;
while ut;l;7;n~ the heat of reaction y~elaLed by the
catalytic reaction for prQh~tjn~ the un~eac~ed mixed gas;
10 cool m g the m1xed gas after the reaction to a teD~ld~f~ at
which the gas may be used for gen~ration of the laser,o
removing du~t in the gas and recycl m g the gas to the laser
g~n~ r. Suitable space velo~ity for pasBing the mixed
gas in the catalyst bed is Ln the range of 4,000 - 15,000
15 /~r.
The ~ethod of rea~tivating the catalyst according to
the present invention is to rQactivate the catalyst with
lowered activity due to ri~ning by NOX C~ntAin~e~ in the
mixed ga8 after laser di3charge m practice of the above
2~ described method of ~g~n~ ing the mi~ed gas, and co~p~is~s
passins a ~ge,rF,~tin~ gas consisting of 0.2 - 0.8 % of CO,
0.1 - 0.4 ~ Of ~2 and the balance of He through the catalyst
layer at a temp~alu~ of 400 - ~00 oc.
The ~ethod of ~eLl~rt;ng the cataly~t according to
25 the preoent i~ n ccmpri~es, prios to pr~cticing the
above d~QC- ;h~ mothod o~ ting mixed gas, pas~ing a
2~62708
.
He-N2-CO2 mixed gMs cont~;n;ng CO of 1,000 - 3,000 ppm or He
gas cont~n~nq CO of 1,000 - 3,000 ppm in the catal~yt layer
at a temp~rature of 60 - 150 ~C . Suitable space veloclty
i~ 100 - 500 /Hr.
~he apparatu~ ~or regenerAting the gae for carbon
dioxide gas laser according to the pre~ent invention, with
which the above described method of L~gen~Ling the mixed
~as, the method o~ reactivating the catalyst and the method
of ~ Laa~ing the catalyst are carried out, comprises, as
lO illustrated in Fig.1, a gas~gas heat PY~hAng~r 2 for
preheatlng the mixed gas used in the cArhe~ ~iQyi~e gas la~er
yeneL~G~ 7; a ga~ heater 3; a reac~o 4 having a heatiny
mean~, in which a catalyst for reacting CO and ~2 with NOX in
the mixed gas i8 p~ck~; a ga~ cooler 5 for cooling the mixed
15 ga~ after the reaction; a filter 6 for removing dust from the
mixed ga~ after cool;n~; all the devices being connected in
the above mentioned order; a means for recycling the
~ ¢nCL~ gas to the laser ~~ne~d~or such as a blower; and
a means for ~upplying a He-CO-D2 mixed gas 9 for reactivating
20 the catalyst. In ~ig.l, n~eral reference 10 indicates a
gas analy~er.
The rePc~G~ to be employed in the present invention
i8, pl~La~bly, a type of self heat ~Y~h~n~;ng or a reactor
in which the ga~ before the reaction is heated by the gas
2~ after the reaction. The app~ratus must be durable to the
temp~a~ of 400 - 500 ~C h~ e the reactivation of the
c~taly~t i5 carried out at this level of temperature.
- .
- . - .
"; ~ ~
~ .. .. .. .
2062708
Pre~erable cataly~ts to be used in this in~ention are
tho e of precious metals such as Pt, Rh and Pd supported on a
carrier such as alumina or silica, which are known a~ the CO-
oxidation catalyst. Particularly, Pt-A1203 catalyst is the
most u~eful. The content of the precious metal in the
catalyst i8 usually in the range of 0.3 - 5 %.
The gas cooler and the filter may be chosen from the
known apparatus on the basis of the reaction conditions and
the quantity of the gas to be treated. The filter preferbly
10 has the perf~- ~n~e of removing 99.9 % or more of the dust of
particle ~izes of 0.1 micrometer or more.
In the practical use of the gas regeneration apparatus
of the pre~ent invention, it is of course possible to recyle
all of the mixed gas. In case where it is preferable to
15 reduce the load of the regeneration apparatus 80 as to avoid
accumulation of CO and ~2 gas in the ~cy~ne~a~ed gas, i~ is
also possible to ch~o~e as an alternative, as shown in Fig.1,
to r~leA~e a portiong of the used gas and to rerl~n;~h fresh
mlxed ga~ of the equivalent quantity from a suitable source
2~ such as a bomb 8. It is convenient to supply the gases for
reactivation and pretreatment of the catalyst through the
la~er generator.
In the He-N2-C02 mixad gas for the cArho~ dioxide gas
laser yeneLator CO and ~2 occur due to the discharge therein
25 a~ the result of the following reactions:
C~2 + e ~ CO + O + e (1)
O + O = ~2 (2)
2062708
It h_s been practiced to ~es~..aLate CO2 by c~a~ g the
reaction bc~ n the CO and the ~2 with tho aid of a
cataly~t~
CO I 0-5 ~2 ' CO2 ~3)
The inventors investigated the reason of relatively
rapid sl~-'~ of the activity of the catalyst for this
reaction and found that NO and NO2 ~hereinafter represented
by HNOx") occur due to the ~1~chArge in accordance with the
folcwing reaction~: -
N2 + e = N I N I e (4)
N + O = NO (5)
NO + O = NO2 (6)
and that the NOX i~ strongly A~ at the active points to
pnj~o~ the catalyst. Quantity of NOX y~e~ed in the gas
15 i8 very slight under the conditions where the la~er output is
low, _nd the NOX doe~ not cause a serious problem. The
quantity, }~ -~1, increases i~ the la~er output is ;-
hiY1~nO~. PO;~Qn1~ by NOX iB significant at a lower
reaction tem~6 ~tu~ (80 - 200 oc) chssen in the previous
2 0 in:vent i Qn .
In the p~e-e~t invention, the catalyst activity is
maintained for much longer period by ch~o~in~ a higher
tem~e~LuLa (200 - 300 ~C~.
When the activity d~cLaaD~d si~nifi~antly, the NOX
25 ad~orbed by th~ cataly~t i~ decompo~ed in accordance with the
following reaction-:
NO ~ CO = 0.5 N2 + C~2 (7)
.
: ;, . ~ ~ . .:
,. ,. , ~ - ,. ... , ~
- . . . I
.. . . . . .
2062708
N02 = 0.5 N2 + ~2 (8)
so that the cataly~t may be recovered from poisoning and
reactivated. Thus, it i8 possibLe to repeat the p.ocedu~e~
of ~agene aLing the mixed gas for cAr~on ~i~Y;~e gas laer
go~LaLo~.
The techno1ogy of L~ene aLing the mixed gas according
to the present invention ensures enjoying the merit~ of the
previously pL~p~sad invention that enables repeated use of
the used gas for la~er in the carbon ~;oYi~ gas laser
10 ~er.e~ator and reduction of the running costs for the laser
operation.for a long period
The m~thod of pretreating the catalyst according to
the present invention AnhA~e~ the above merits by reducing
cataly~t poi~oning by NOx~ The method of reactivating the
15 catalyst makes it r~s;hle to reactivate the cataly3t of
~ l activity due to the po;~oning and to use it
- repeatedly. The investment for the apparatus for
LC~ ating the laser gas is low and the space necess~ry for
the npparatus i5 also umall.
EXAMPLES
;E~n~Al ~~mple
An experimental apparatus as illustrated in Fig.2 was
composed and ~3~d to determine the acti~ity, extent of
25 p~;~o~ing and effect of reactivation. In the figure numeral
~efe ~I.ce 11 ;n~ic~tes a gas mixer; 12, a gas heater; 13, a
reactor; 14, a pressure maintAin;ng valve; 15, a gae
,
.
~ :, .. : . . -
: :, .. .
2062708
analyser; and 16, a gas meter~ The cataly~t u~ed was of the
following specification:
Pt-Al203 ~supported Pt: 4.3 g/liter)
Particles (particle sizes 3 + 0.3 mm)
Bulk density 0.36 kg/liter
6 Pore volume ~BET) 1.4 cm3/g
There were surplie~ N2 gas and two kind~ of mixed
gases (N2 ~ NO/N02 and He + C0 + ~2) from bombs to the mixer
11 at a determined rates, and thus obtained mixed gases were,
10 after being heated in a heater 12 to a determined
temperature, intro~nce~ into the re~c~o~ 13. The gases from
the reactor pa~s the pressure maint~;ning valve 14 and were
~ubjected to volume measurement, and then, treated by an
exhau~t system for release~ Analysis of the gases were
15 carried out with the apparatus 15 e~irpe~ at ~he downstream
of the pressure maintAin~ng valve.
Fir~tly, N2 gas contAin;ng N0 o~ 100 ppm was ~ i
at a rate of SV = 5000 /Hr, and the tempe~a~ure was ch~nged
from 20~C to 50~C and 150~C, to observe chAng~ in adsorption
ZO . At 20~C and 50~C, a breakthrough, i.e~, detection of N0
at the outlet of the re~c~o~ was appreciated 5 minute~ after
the introduction of the gas. At 150~C, h~ eL, it was
detenmined that it takes about 10 minutes until the
breakthrough be~ins. Thus, it was found that the time until
25 the breakthrough i8 lonqer at a higher tempela~ure.
Then, after having the active point~ poisoned with N0,
He-C0-02 (C0/02 = 2/1) mixed ga~ was pa~sed to deter~i n9
ln
.
-
2062708
whether the activity ~ec~v6~. When an N2 ga~ contyalintn~ NOof 58.1 ppm wa~ passed at 88~C for 140 minutes, the catalytic
activity for the reaction of CO + ~2 became extre~ely low.
~hen, paQ~ing pure N2 gas at 100~C for 7 minute~ resulted in
a temporary ~ec~vaLy of the activity, but the activity
decreased in a short period, and the Lacove~y of the activity
was 50 % or so.
In order to investigate the influence of the reaction
tempeL~re, experiments under the conditions ~hown in Fig.3
lO were c_rried out. At first, the catalyst pretreated with
He-CO gas wa~ subjected to NO-poiQon;n~ ~with the above
mentioned N2 gas contAin;n~ 58.1 ppm NO) to the saturation,
and then He-CO-O2 mixed gas ~CO/O2 = 2~1) was pA~seA at 80~C.
In the fir~t 2 hour~ activity was appreciated, but it
15 d~croaJed rapidly. After ~ hours N2 gas was passed at 330~C
for 6 hour~, and no ~cuv~ of the acitvity waq appreciated.
The ro~ction tempe ~uLe was elevated to 200~C, and
He- CO-02 mi~ed gas ~CO: 0.60 ~, ~2: 0.30 ~, the hAl~nte:He) - -
was pAs~ed at a rate of SY = 5000 /Hr. The activity
20 l~cu._L~d to the initial level. In the graph of Fig.3, a
temporary decrease of the activity i~ t~c~l~ed after 19
hours. This might have ~een cfl~e~ by rerun of the
exp~rim~mt~ after stan~ing without flowing gas fo~ a whole
day, and the reason i8 cQn~i~tred to be that the adsorbed CO
25 and ~2 moved around on the c~atalyst surface to inactivate the
active point~ ~h~.~of.
.. ..
: , . , :
-
- , ~ ,
:,. : - .
. .
. . ~ .
2~62~08
The catalyst was subjected to NO-poinsoning under the
same conditions as mentioned above, and then, to reactivation
with the mixed gas of the same composition a~ above and under
the conditioDs of temperature and flow rate also mentioned
above. The results are shown in Fig.4. From the graph of
the figure it is understood that the catalyst, even after
lap~e o$ 30 hour~, retains sufficient activity to promote the
reaction of CO + ~2-
The gases of three different composition~ similar to
10 those after u~e in the carbon ~io~ gas laser generatorwsre sllrpli~ , and the content~ of CO and NO at the outlet
of the r~actor were determined. The results are as in
Fig.5. The catalyst activity remained high without
remarkable ch~ng~. The reason why the CO-content is
15 relatively high i~ aonsidered to be the preferential
reactions of:
~0 + 0.5 ~2 = N~2
N~2 + 2 Co - 2 C02 + 0~5 N2
to the reaction~ of:
NO + CO = 0.5 N~ + C02
CO + 0.5 ~2 ~ C~2
On the basis of the fact that the content of NO is ~u~essed
low, it is ~ec~ed that, even though NOx occurs in the laser
gas after use, the catalyst will not be significantly
25 d~teriorated and can be used for a long time.
Wor~in~ le 1
2062708
In the apparatus of the structure shown in Fig.l, a
cylindrical r~aator was u3ed and a mesh was placed in the
bottom thereof, on which 12 liters of 0.5% Pt-Al2O3 catalyst
was packed with pack;n~ of Raschig rings thereon.
S Temperature sensors were put in the cataly~t layer for
m~a~uring the ch~nges of the tem~eraLuL~s at the upper part
~gas inlet side), intermediate part, and the lower part ~gas
outlet side) of the catalyst layer ~o a~ to det~ i n~ how the
activities change at the respective parts of the catalyst
lO layer.
The reac~ol was combined with a carbon dioxide gas
laser generator, to which a He-N2-CO2 mixed gas was supplied
through a mass-flow control valve. A portion of the gas was
~ampled and transferred to the above regeneration apparatus
15 with a blower. The transferred gas was, after being
eubjected to heat recovery at the heat ~Y~hAn9er~ heated at
the heater and int~ud~\ce~ into the reactor at a temperature
of 250 - 300 ~C for recambination reaction of the side
products at the catalyst layer. The gas, by way of the heat
20 aY~hAn~er~ moves to the cooler and is cooled to a t - a~re
lower than that of the laser chamber. The coole~ ga~ i~
recycled to the laser generator after beinq filtered for
removal of the dust.therein.
Qantitative analysis of NOX wa~ done at the inlet and
25 the outlet of the reactor under keeping the temperature of
the catalyst layer constant at 300~C with varying quantities
2062708
of the recycled gas, and the gas conversion and O2-re~ctivity
were calculated. The results are ~hown in Table 1.
~ hen, He-N2-C02 mixed ga~ contA; n; ng C02 of O . 6 % ~ ~2
of 0.3 % and NO of 26 ppm or 162 ppm were su~rl;sd to the
reactor at variou~ temperatures. The space velocity was 500
~Hr. The xeactivity of CO and the reactivity of NO showed
the ~h~n~8 ~p9n~;ng on the temp~Latule as illu~trated in
Fig.6. From the data it is evident that the reactivities
incr~a~e drastically at the tempeL L~e above 200~C. This
10 effect saturatQs at thQ temperature around 240 - 250 ~C, and
therefore, a temp~rature higher than this limit provides no
further merit. The upper limit of the reaction temperature,
350~C, was ~9ci~ from the practical vi~w points such as
easines~ in control.
TABLE 1
Quantity Co~c~enLrations C~ n~rations Conv~l~ions 02
of Recy- of Ga~e~ at o~ Ga~e~ at of Ga~es RQacti-
cled Gas na~-~-ol Inlet R~C~G~ Outlet vity
(N liter ~ppm) (ppm) (%) (~)
/min.) NO N02 NO~NO N02 NOx NO N~2 NOx
____________ ___ ___ ____ ____ ____ ______
400 2971100 9 28 3~ 6g.0 60.6 63.0 g9.~
400 316192 8 25 33 74.2 59.0 64.1 99.4
500 4093133 10 31 41 75.0 66.7 69.2 98.8
600 501060 5 nil 5 90.0 100 91.7 99.8
700 315485 5 13 18 83.8 75.9 78.8- 99.2
1000 1407021020 nil 20 85.7 100 90.5 99.8
. . '
,
20627~8
Work; n~ ~YAm~le ~
After having the catalyst poisoned by contacting N2-
gas contA;ning NO of S8.1 ppm at 88~C as described in the
above working example, a ~e-CO-O2 gas cont~; n; ng CO of 0.6 %
and ~2 Of 0.30 % was passed through the catalyst layer under
the conditions of t~mperature 400~C, and space velocity of
2,000 /Hr for 3 hours to reactivate the catalyst.
~ hen, a He-CO-O2 mixed qas cont~inlng NO of 66.4 ppm
in addition to CO of 0.6 ~ and ~2 of 0.3 % (simulated to the
10 state of the laser gas aftex use) was passed through the
apparatus at 270~C and space velocity of S,000 /~r for
~g~neL~-ion of the ~as.
The r~activity of CO and the reactivity of NO ~re as
shown in Pig.7. It was ascertained that, in view of the
lS fact that th~ activities are kept at the lev~l as high as 100
% or near, the above reactivation of the catalyst was
effective.
Workin~ Exam~le 3
A new cataly~t was p~9~9~ in the reactor, to which a
20 He-gas contAin;n~ CO of 1,000 ppm was passed at 150~C and
space velocity of 300 /Hr for 1 hour to pretreat the
catalyst.
Then, a He-CO-O2 mixed ga~ cont~; n i ng NO of 6 3 . 6 ppm
in addition to CO of 0.6 % and ~2 of 0.3 ~ (also, simulated
25 to the ~tate after use of the laser ga~) wa3 passed through
the apparatus at 240 - 250 ~C for reactivation of the
reactor. The reactivity of CO was maintained at 100 ~ for a
2062708
long period of time as seon in Fig.8. The activity of the
catalyst which was not subjected to the above reactivation
treatment i8 also shown in Fig.8. Fig.8 show~ decrease of
the acitvity after 3 - 4 hours of running.
Working ~Y~mDlo 4
O~c;~ation of the carh~n dioxide ga~ la~er was carried
out under the following conditions;
Mixêd gas composition C02/N2/He = 1/1/5
Ropeating rate 100 pps
Laser output 620 W
Chamber volume 1000 liters
Volume in the ~;~ch~rging zone 1 liter
Pres~ure in the la~er gas 1 atm.
The ,~ ~Gl u~d in Example 1 was used as the
15 l~a ~a~G~ of the gas, with which the mixed ga~ was
resen~.a~ under the conditions below:
Te~,a~ure of ~ ne-ation 200~C
P,~a~u,. in L~3f~--ator 1 atm.
Ratê in gas recycling 1 m3/min.
Space velocity 5,000 ~Hr
The O2-~c~nc~rations in the Ch '- were prio~icAlly
measured. The re~ults are shown in Fig.9 in comparison with
the case of uSlng no gas Le~a.~e~aLo~. As seen in Fig.9, if
the ~ rator is not used, arcing occurs only 5 minutes
after initiation of the laser s~cilation, and after 20
minute~ ~2 co~c~ ra*ion r~t~-' to 20,000 ppm, at which
level no further operation can be continued. On the other
,, ~ ' , ,
'' ' ,' ' ,: ' ' ~
,
~ ' ' ' ' ': ' ' ' ' '
2062708
hand, in case of using the regenerator, ~2 concentraion does
not ~-c~ 2,000 ppm and operation of the laser generator for
a long period i3 poBs~hle.
The ~hAn~ in the la~er output with passage of the
time was observed al50 in comparison of use and non-use of
the gas regenerator. The results are as shown in Fig.10.
From this figure it is unde~tood that, lf the regenerator is
not u~ed, the la~er output ~ fic~ntly decreases, and that,
contrarily to this, if used, the laser output can be
maintained at al~ost a constant level.
...
,."~. ~ . ~ .
.
. ~
- , . . ~. - - .- .
- .
- . , .