Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
HOECEIST A~CTIENGESELLSCH~FT HOE 89/F 282 K Dr . ZR/ je
I)e~criptïon
Aryl halophosphoramidites, aryl phosphonamidites, proces-
ses for their preparation and their use forthestabiliza-
tion of plastics
The present invention relates to new aryl halophosphor-
amidites, monoaryl phosphonamidites and bis~aryl] diphos-
phonamidites, processes for their preparation and their
use for the stabilization of plastics.
It is known that synthetic polymers have to be protected
by stabilizers or stabilizer systems against undesired
oxidative, ~hermal and photochemical damage during
preparation, processing and use. Such stabilizers are,
for example, composed of a phenolic antioxidant and one
or more costabilizers, some of which are also able to
reinforce the action of the phenolic ~omponents syner-
gistically.
The most used stabilizers include, for ex~mple, phosphor-
ous acid and phosphonous acid derivatives, the latter,
above all, being distinguished by a ~ery good stability
to hydrolysis.
The synthesis of phosphonamidites by stepwise reaction of
dihalophonites with alcohols or phenols and amines in the
presence of acid acceptors has been described by various
authors [Houben-Weyl, Methoden der organischen Chemie
(Methods of Organio Chemistry), phosphorus compounds El
p. 300 (1982)~. The yields obtained in these processes
were only about 60-75%.
In an analogous manner, suitable monophosphonamidites for
use as stabilizers for polymers are prepared by reaction
of alkyl or aryl dihalophosphonites, for example chloro-
phosphonites, with phenols and amines in the presence of
excess base, which is used to neutralize the hydrochloric
- 2 ~ t ~
acid formed, according to EP Offenlegunqsschrift 42,359.
Data relating to the purity of the products or ~he yields
achieved are not given there.
However, the process of EP Offenlegungsschrift 42,359 can
only be carried ou~ to a limited extent because of the
difficult preparation of the dihalophosphanes needed as
precursors which is, of cour~e/ disadvantageous if
industrial production is considered. Thus, for ex2~mple,
of the aromatic derivatives, only phenyldichlorophosphane
is an industrially available product through which solely
derivatives of benzenephosphonous acid are available.
This is confirmed in that in EP Offenlegungsschrift
42,359, except for one, all (no less than 17) ex2~mples
contain the unsubstituted phenyl radical.
In addition to the achievement of higher yields, it is
desirable for fulfilling the high requirements which are
placed on the stability, activity, low volatility and the
migration behavior of such stabilizers in practice to
have available directly derivatives of aryl phosphonous
acids substituted in the phenyl radical. However, their
availability by known methods founders on the fact that
appropriately substituted precursors are needed for this
purpose, which are hitherto unknown or cannot be prepared
economically~
It is therefore also an ob~ect of the invention to
prepare novel stabilizers for plastics having improved
properties, in particular with the aid of processes which
do not have such disadvantages.
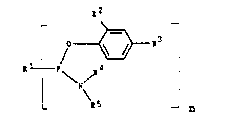
The present invention relates to aryl phosphon2~midites of
the formula ~I)
-- 3
¦ f ~ ~3 (I)
\
~ 5 tl
in which:
Rl, as a uniYalent radical, i5 a phenyl radical which
carries 1 to 3 substituent~, or a benzyl, ~-methylbenzyl
or ~ dLmethylbenzyl radical which in each case can
car~y 1 to 3 substituents on the nucleus, or a naphthyl
radical which can carry 1 to 5 ~ubstituent~, where at
least one of these substituents i~ an alkoxy radical or
alkylthio radical in each case havins 1 to 8, preferably
1 to 6, carbon atoms, or an aryl or aryloxy radical in
each case having 6 to 10, preferably 6 to 8, carbon atoms
or halogen having an atomic number of 9 to 35 and the
other substituents - also exclusively in the naphthyl
radical - are a non-aromatic hydrocarbon radical having
1 to 8 carbon a~om~, and R~, as a divalent radical, is a
phenylene radical which is unsubstituted or substituted
by up to 2 non-aroma~ic hydrocarbon radical~ having 1 to
8 carbon atom~, or a naphthylene or biphenylene radical
which is unsub~tituted ox can carry 1 to 4 non-aromatic
hydrocarbon radicals each having 1 to 8 carbon atoms a~
s~bstituents,
R2 i~ a non-~romatic hydrocarbon radical having 1 to 18
carbon atoms, an a~yl or an optionally substituted
arylmethyl radical, where the aryl radical in each case
contain~ 6 to 10 carbon atom~,
R3 i~ hydrogen, an alkoxy or alkylthio radical in each
ca e having 1 to 18 carbon atoms or a group mentioned
under R2, R' and R5 independently of one a~other in each
case are Cl-C22-alkyl, C2-C2l-oxaalkyl or -thiaalkyl,
C3-C~8-alkenyl or -alkynyl, C3-C24-alkoxycarbonylalkyl,
C5~Cl2~cycloalkyl, C6-Cl4-aryl, C7-Cl5-arylalkyl or an
optionally substituted C5-Cl7-piperidin-4-yl group, or R4
and R5, together with the ni~rogen atom, are also a ring
system containing 5 to 7 ring atoms which may additional-
ly further contain a heteroatom (O, N, S~ bonded via at
least one carbon atom and
n = 1 or 2.
If n = 1, the compounds are phosphonamidites and if n =
2 the compounds are bis-diphosphonamidites.
A preferred group of compounds are those in which Rl, as
a divalent radical, forms a biphenylene radical, R2 and
R3 in each case form a branched butyl radical and R4 and
R5 in each case form a butyl radical orl together with
the nitrogen atom, form ~he morpholide radical.
Other preferred compounds are those in which R1 is the
trimethylphenyl radical r R2 and R3 are the methyl radical
or a branched butyl radical and R4 and Rs, ~ogether with
the nitrogen, are either the piperidide or morpholide
radical or in which Rl is ~he tert.-butyl-l-phenyl radi-
cal, R2 is the tert.-butyl radical, R~ is the methoxy
radical or t~rt.-butyl radical and R4 and R5, together
with the nitrogen atom, are the morpholide radical.
Furthermore, those compounds in which R1 is unsubstituted
or substituted naphthyl are particularly preferred.
In R1, the substituents can in each case be identical or
different. The alXyl in the substitu0nts of the aromatic
radicals Rl is, for e~ample, one of the various hexyl or
octyl radicals, but preferably ha~ 1 to 4 carbon atoms
and is, for example, methyl, ethyl or one of the various
propyl or butyl radicals, which are optionally bonded to
the nucleus via O or S. Cs-C~-cycloalkyl may be mentioned
as a non-aromatic hydrocarbon radical, like the radicals
-- 5 --
mentioned further below under R2. In particul~r, for
example, the anisyl radicals or naphthyl radicals may be
mentioned, which, for example, further carry up to 2
substituents containing altogether up to 4 alkyl carbon
atoms, and also the various biphenyl radicals.
For R1 as a divalent radical, the various phenylene radi-
cals may, for example, be mentioned which are unsub-
stituted or carry 1 to 2 C1-C8-alkyl groups, in particular
Cl-C3-alkyl groups, or the various naphthylene radicals
and biphenylene radicals which are unsubstituted or
substituted by 1 to 4, preferably 1 to 3, Cl-C~-alkyl
groups, in particular C1-C3-alkyl groups.
Naturally, the substituents in Rl can only be combined in
such a way that no steric hindrance occurs. If R1 contains
3 substituents, no more than 5 carbon atoms should be
present in the two o-positions. In substituted naphthyl
radicals which c~ntain more than two s~bstituents, these
are advantageously distributed on both rings.
Suitable R2 and R3 radicals are, for example, non-aromatic
hydrocarbon radicals having 1 to 18 carbon atoms, such as
alkyl or cycloalkyl, and furthermore aromatic radicals
which have 6-18 carbon atoms including aliphatic groups,
where no more than 10 carbon atoms are part of an aroma-
tic ring system. Preferably, the radicals R2 and R3
contain 4 to 12 and, in particular, 6 to lO carbon atoms.
In particular, the following may be m~ntioned as non-
aromatic hydrocarbon radicals: alkyl, such as methyl,
ethyl, the various propyl, butyl, pentyl, hexyl, octyl,
decyl, dodecyl, hexadecyl and octadecyl radicals, and
cycloalkyl havin~ 5 to lO carbon atoms such as cyclopen-
~yl, cyclohexyl, cycloheptyl and cyclohexylmethyl (i.e.
both the hydrogenated ben~yl radical and the methyl-
cyclohexyl radical); C5 C10-aryl and arylmethyl may
further be mentioned, where the term aryl in each case
includes alkylaryl, carries at most three of the sub-
s~ituents mentioned under R2 and, including this, has at
6 ;~5~
most 14 carbon atoms.
If one of the radicals R2 or R3 is an alkyl r~dical,
tertiary alkyl groups containing 4-10 carbon atoms, such
as tert.-butyl, 2-methyl-2-butyl, 2-methyl-2-pentyl and
2-ethyl-2-butyl are particularly preferred. Other pre-
ferred compounds are those in which R2 or R3 is phenyl,
benzyl, ~-methylbenzyl and ~,~-dimethylbenzyl.
R4 and R5 are, for example, the radicals indicated for R2.
If they are alkyl, Cl-Cl2-alkyl groups are preferred.
R4 and R5, as C2-C21-oxa- or thiaalkyl, are, for example,
methoxymethyl, methylthiamethyl, ethoxymethyl, methyl-
thiaethyl or ethoxyethyl. Alkoxypropyl and slkylthia-
propyl groups are preferred, such as methoxypropyl,
ethylthiapropyl, butoxypropyl, octylthiapropyl, dodecyl-
lS oxypropyl, octadecylthiapropyl or octadecyloxypropyl.
R4 and R5 are, as C3-C18-alkenyl, for example allyl,
methallyl, n-hexen-3-yI, n-octen-4-yl, n-undecen-10-yl
or n-octadecen-17-yl. Allyl and methallyl are preferred.
R4 and R5, as C3-C1~-alkynyl are, for example, propargyl,
n-butyn-l-yl, n-butyn-2-yl or n-hexyn-l-yl. Alkynyl
groups having 3-6 carbon atoms are preferred, in par-
ticular propargyl.
If R4 and R5, together with the nitrogen atom to which
they are bonded, form a pyrrolidine, oxazolidine, piperi-
dine, morpholine, hexamethyleneimine or piperazine ring,these heterocycles can be substituted by 1 to 5, ad-
vantageously at most 2, methyl or ethyl groups. Ring
systems of this type are preferably unsubstituted.
If R4 and R5 are piperidin-4-yl groups, they may be
unsubstituted piperidin-4-yl, or the piperidine may be
substituted by up to 5 alkyl groups. The 2,2,6,6-tetra-
methylpiperidin-4-yl radical is particularly preferred.
- 7 -
The invention also relates to a process for the prepara-
tion of phosphonamidites of the formula ~I) in which Rl,
as a univalent radical, in addition to the abovementioned
meaning can also be phenyl and a phenyl substituted by 1
to 3 non-aromatic hydrocarbon radicals having 1 to 8
carbon atoms, and a non-aromatic hydrocarbon radical
having 1 to 18 carbon a~oms, preferably Cl-Cl8-alkyl, and
R2, R3, R4 and R5 have the abovemention d meaning, which
comprises initially reacting in a first step a halide
Rl(-X)n in which Rl has the abovementioned meaning,
n = 1 or 2 and X is a halogen having an atomic weight of
at least 35, preferably chlorine or bromine, under
Grignard conditions, i.e. advantageously with intimate
mixing, with finely particulate magnesium to give the
corresponding Grignard compounds R1(MgX)n and reacting
these in a second step with aryl halophosphoramidites of
the formula (II)
~2
O ~ ~3
X--
N
in which R2 R3, R4 and Rs have the abovementioned meaning
and X is chlori.ne or bromine, to give the phosphonamid-
ites (I). The process according to the invention is thus
also utilizable for the reaction of those compounds of
the formula R1(MgX)n in which Rl, for example, is the
tolyl, dimethylphenyl, trimethylphenyl or tert.-butyl-
phenyl radical.
With respect to economic accessibility~ those of thecompounds (II) where ~ = chlorine are particularly
preferred.
The first step of the process according to the invention
which can be carried out in any customary manner, i5
preferably carried out in an aprotic organic solvent,
such as an ether, for example diethyl, dipropyl or
diisopropyl ether, ethylene glycol dimethyl ether or
ethylene glycol diethyl ether, diethylene glycol dLmethyl
ether or diethylene glycol diethyl ether, methyl tert.~
butyl ether, dioxane or tetrahydrofuran.
As the Grignard compounds are sensitive to hydrolysis and
oxidation, it is advantageous to work under a protective
gas atmosphere, however, such a process is not compulsory
for the success of the reaction. Nitrogen and argon are
particularly suitable as protective gases.
The reaction temperature during the conversion into a
Grignard compound is in general between 20 and 125C,
preferably between 30 and 70C. The effect of ultrasound
during the conversion into a Grignard compound is some-
times advantageous.
In order to prepare the final product (I), the solution
ox suspension of the Grignard reagent in the second step
is metered into the halophosphoramidite (II) which is
advantageously diluted with an inert, aprotic solvent~
for example an aliphatic hydrocarkon fraction, hexane,
cyclohexane, toluene, xylene or one of the abovementioned
ethers, advantageously at a temperature below 0C. The
reaction temperature in this step is in general between
30C and +50C, but preferably between -20C and +20C.
As a rule, the reaction proceeds exothermically; accord-
ingly it may be expedient to control the course of the
reaction by cooling. The most favorable results are
achieved if the reaction components are employed accord-
ing to the reacti.on stoichiometry. However, it is also
possible to employ one reaction component in excess;
however, in general there are no particular advantages
associated with this. The mixture is expediently stirred
until the reaction i~ complete, and precipitated mag-
nesium halide is subssquently separated off. The solvents
;~?~
g
can be removed from the filtrate in a customary manner,
advantageously by distillation, in particular under
reduced pressure.
The synthesis of phosphonamidites with the aid of organo-
metallic compounds, for example by reaction of halo-
phosphoramidites with Grignard reagents, was hitherto
unknown. Ob~iously, owing to the known easy replaceabil-
ity of amino groups and also ester groups bonded to the
phosphorus by nucleophiles [Houben-Weyl, Methoden der
organischen Chemie (Methods of Organic Chemistry), volume
12/1, 44, (1963) and E 1,302 (1982)], a prejudice existed
in this respect in that in th~ reaction of halophosphor-
amidites with Grignard reagents, to a high extent side
reactions reducing the yield with its progress and a
still lower yield of desired product ca~sed as a result
may have to be taken into account.
It is therefore particularly surprising that, starting
from easily accessible halophosphoramidites, any sub-
stituted phosphonamidites can be obtained in such a high
2Q yield and purity according to the proces~ of the present
invention.
The products (I) can be separated from the crude products
~y any desired processess, but preferably by crystalliza-
tion.
The halophosphoramidites (II) required as starting
compounds, which are new except for that compound in
which X is chlorine, R2 is methyl, R3 is hydrogen and R4
and R5 are in each case ethyl [Izv. Akad. Nauk. SSSR, Ser.
Khim., (9), 2131~2133 (1968)], can be prepared in a
simple manner by a process to which the invention also
relates. However, this known compound has achieved no
industrial Lmportance. It is particularly unsuitable as
a starting material for stabilizers fox polymers.
The invention thus also relates to a process for the
lo- 2~5~
preparstion of these compound~ (II), which comprises
reactinq an aryl dihalopho~phite of the formula ~III)
X R2
p _ 0 ~ 3 (III)
with an amine of the formula XNR~R5 (IY) in the presence
S of at least an equimolar amount of an acid-binding agent,
where, in the formulae (III) and (IV), R2, R3, R~ and R5
have the meanings which are indicated for the process for
the preparation of the compounds (I) and X is a halogen
having an atomic weight of at least 35. The process thus
also extends to the preparation of the compound of the
formula (II) in which X is chlorine, R2 is methyl, R3 is
hydrogen and R4 and R5 are in each case ethyl, which has
hitherto been prepared in another manner.
This process can be carried out per se in any customary
lS manner. It is preferably carried out in an inert aprotic
solvent. Hydrocarbons such as pentane, hexane, heptane,
benzene, toluene, xylene, chlorobenzene and ethers such
as diethyl ether, dipropyl ether, diisopropyl ether,
ethylene glycol dimethyl ether or ethylene glycol diethyl
ether are particularly suitable for this purpose.
The compound~ of the formula (II) are expediently prepar-
ed by adding an equimolar amount of the amine (IV) and an
equimolar amount of a base suitable as an acid-binding
agent to the solution of the dihalide (III) with vigorous
mixing. In this case the reaction i~ in general carried
out between -30 and +30-C, preferably between -20 and
~lO-C. The mixing is carried out, for exampIe, by stir-
ring and, expediently, until the reaction i8 complete.
~he precipitated ammonium ~alt i~ then separated off. The
solvents can be removed from the filtrate in a customary
.. . .
~: ,' ' '- . ' ,
: . : ~ . .
:, ~ , . .
d ? :, s d
manner t preferably by distillation, the solvents ex-
pediently being distilled under reduced pressure.
Suitable bases, which are advantageously dil~l-ted with one
of the abovementioned solven~s, are, above all, tertiary
amines, such as trie~hylamine and pyridine. An excess of
amine HNR4R5 can also act as an acid-binding agent.
The products (II) obtained in this procedure are usually
obtained in a purity which is easily sufficient for
reaction with the Grignard compounds. A further purifica-
tion, for example by vacuum or thin-layer distillation,
is therefore as a rule unnecessary.
The invention finally relates to the use of the compounds
of the formula (I) alone or in combination with a pheno-
lic antioxidant for the stabilization of plastics, such
as polycarbonates, preferably polymerization plastics
such as polyolefins, in particul~r polypropylene. The
compounds of the formula (I) impart an Lmproved stability
against degradation by light, oxygen and heat to the
plastics in the molding compositions. However, the purity
of the crude reaction product obtained (85-93% according
to 31P-NMR) iS frequently sufficient for this use. Isola-
tion in a pure form is then unnecessary.
The present invention thus also relates to a plastic
molding composition, containing a thermoplastic or
thermoset material and an aryl phosphonamidite of the
formula (I) in the ratio of (90 to 99.99):(0.01 to 10).
The plastic molding composition according to the inven-
tion contains a thermoplastic or thermoset organic
polymer, for example one of the following:
1. Polymers of mono- and diolefins,forexample polyethyl-
ene of high, medium or low density (which, if desired,
can b~ crosslinked), pol~ypropylene, polyisobutylene,
poly-1-butene, polymethyl-1-pentene~ polyisoprene or
polybutadiene and polymers of cycloolefins, such as, for
example, cyclopentene or norbornene.
2. Mixtures of the polymers mentioned under l), for
example mixtures of polypropylene with polyethylene or
with polyisobutyl~ne.
3. Copolymers of mono- and diolefins with one another or
wi~hother vinyl monomers, such as, for example, ethylene-
propylene copolymers, propylene-l-butene copolymers,
propylene-isobutylene copolymers, ethylene-l-butene
copolymers, propylene-butadiene copolymers, isobutylene-
isoprene copolymers, ethylene-alkyl acryla~e copolymers,
ethylene-alkyl methacrylate copolymers, ethylene-vinyl
acetate copolymers or ethylene-acrylic acid copolymers
and salts ~hereof tionomers) and terpolymers of ethylene
with propylene and a diene, such as hexadiene, dicyclo-
pentadien~ or ethylidenenorbornene.
4. Polystyrene.
5. Copolymers of styrene or ~-methylstyrene with dienes
or acrylic derivatives, such as, for example, styrene-
butadiene, styrene-maleic anhydride, styrene-
acrylonitrile, styrene-ethyl methacrylate, styrene-
butadi~ne-ethyl acrylate, styrene-acrylonitrile-methyl
acrylate; mixtures of high impact strength and composed
of styrene copolymers and one other polymer, such as, for
example, a polyacrylate, a diene polymer or an ethylene-
propylene-diene terpolymer; and block copolymers of
styrene, ~uch a~, for example, styrene-butadiene-styrene,
styrene-isoprene-styrene, styrene-ethylene/butylene-
styrene or styrene-ethylene/propylene-styrene.
6. Graft copolymers of styrene, such as, for example,
styrene onto polybutadiene, styrene and acrylonitrile
onto polybutadiene, styrene and maleic anhydride onto
polybutadiene, styrene and alkyl acrylates or alkyl
methacrylates onto polybutadiene, styrene and acrylo-
- 13 - v~ qf~
nitrile on~o ethylene-propylene-diene terpolymers,
styrene and acryloni~rile onto poly(alkyl acrylates) or
poly(alkyl me~hacrylates), styrene and acrylonitrile onto
acrylate-butadiene copolymers, and mixtures thereof with
the copolymers mentioned under 5~, which are known, for
example, as so-called A~S, MBS, ASA or AES polymers.
7 Halogen-containing polymers, such as, for example,
polychloroprenP, chlorinated rubber, chlorinated or
chlorosulfonated polyethylene, epichlorohydrin homo- and
copolymers, in particular polymers of halogen-containing
vinyl compounds, such as, for example, polyvinyl
chloride, polyvinylidene chloride, polyvinyl fluoride,
polyvinylidene fluoride; and their copolymers such as
vinyl chloride-vinyliden~ chloride, vinyl chloride-vinyl
acetate or vinylidene chloride-vinyl acetate.
8. Polymers derived from ~ unsa~urated acids and
derivatives thereof, such as polyacrylates and polymeth-
acrylates, polyacrylamides and polyacrylonitriles.
9. Copolymers of the mcnomers mentioned under 8) with one
another or with other un~aturated monomers, such as, for
example, acrylonitrile-butadiene copolymers,
acrylonitrile-a:Lkyl acrylate copolymers, acrylonitrile-
alkoxyacrylate copoiymers, acrylonitrile-vinyl halide
copolymer~ or ac:rylonitrile-alkyl methacryla~e-butadiene
terpolymers.
10. Polymers derived from unsaturated alcohols and amines
or acyl derivatives or acetals thereof, such as polyvinyl
alcohol, polyvinyl acetate, stearate, benzoate, maleate,
poly~inylbutyral, polyallyl phthalate, polyallylmelamine.
11. Homo- and copolymers of cyclic ethers, such as
polyethylene glycols, polyethylene oxide, polypropylene
oxide or copolymers thereof with bisglycidyl ethex6.
12. Polyacetals, such a~ polyoxymethylene, and tho~e
J '~ f ~r3
polyoxymethylenes containing comonomers, such as, for
example, ethylene oxide.
13. Polyphenylene oxides and sulfides.
14. Polyurethanes derived on the one hand from poly-
ethers, polyesters and polybutadienes having terminalhydro~y groups and on ~he other hand from aliphatic or
aromatic polyisocyanates and precursors thereof
(polyisocyanates-polyols prepolymers).
15. Polyamides and copolyamides derived from diamines and
dicarboxylic acids and/or from aminocarboxylic acids or
the corr~sponding lactams, such as nylon-4, nylon-6,
nylon-6,6, nylon-6,10, nylon-11, nylon-12, poly-2,4,4-
trimethylhexamethyleneterephthalamide, poly-m-phenylene-
isophthalamide, and copolmers thereof with polyethers,
such as, for example, with polyethylene glycol, poly-
propylene glycol or polytetramethylene glycol.
15. Polyureas, polyLmides and polyamidoimides.
17. Polyesters derived from dicarboxylic acids and diols
and/or hydroxycarboxylic acids or the corresponding
lactones, such as polyethy~ene terephthalate,polybutylene
terephthalate, poly-1,4-dLmethylolcyclohexane terephtha-
late, poly-(2,2-bis(4-hydroxyphenyl)propane) tereph-
thalate, polyhydroxybenzoates, and block polyether esters
derived from polyethylene having terminal hydroxy groups,
dialcohols and dicarboxylic acids.
18. Polycarbonates.
19. Polysulfones and polye~her sulfones.
20. Crosslinked polymers derived on the one hand from
aldehydes and on the other hand frcm phenols, urea or
melamine, such as phenol-foxmaldehyde, urea-formaldehyde
and melamine-formaldehyde resins.
- 15 - 2~
21. Drying and non-drying alkyd resins.
22. Unsaturated polyester resins derived from copoly-
esters of saturated and unsaturated dicarboxylic acids
with polyhydric alcohols, and vinyl compounds as cross-
linking agents, as well as their halogen-containing non-
flammable modifications.
23. Crosslinkable acrylic resins derived from substituted
acrylic esters such as, for example, from epoxy
acrylates, urethane acrylates or polyester acrylates.
24. Alkyd resins, polyester resins and acrylate resins
crosslinked with melamine resins, urea resins, polyiso-
cyanates or epoxy resins.
25. Crosslinked epoxy resins derived from polyepoxides,
for example from bis(glycidyl) ethers or from cyclo-
aliphatic diepoxides.
26. Natural polymers, such as cellulose, natural rubber,
gelatin and polymer-homologous chemically modified
derivatives thereof, such as cellulose acetates, propio-
nates and butyrates, and the cellulose ethers, such as
methylcellulose.
27. Mixtures of the abovementioned polymers, such as, for
example, PP/EPDM, nylon-6/EPDM or ABS, PVC/EVA, PVC~ABS,
PVC/MBS, PC~ABS, PBTP/ABS, PC/ASA, PC/PBT, PVC/CPE,
PVD/acrylate, POM/thermoplastic PUR, POM/acrylate,
POM/MBS, PPE/HIPS, PPE/nylon-6,6 and copolymers, PA/HDPE,
PA/PP, PA/PPE.
28. Naturally occurring and synthetic organic materials
which are pure monomers or mixtures of monomers, such as,
for example, mineral oils, animal and vegetable fats,
oils and waxes, or oils, fats and waxes based on syn-
thetic esters or mixtures of these substances.
- 16 ~
29. Aqueous dispersions of natural or synthetic rubber.
The polymer is preferably a polyolefin, in particular
polypropylene. The amount of the polymer in the molding
composition according to the invention is 90 to 9g.99,
S preferably 98 to 99.98%, by weight.
The molding composition contains a~ stabilizer an aryl
phosphonamidite of the formula I and, if necessary, a
phenolic antioxidant.
The phenolic antioxidant is an ester of 3,3-bis(3~-t-
butyl-4'-hydroxyphenyl)butanoic acid of the formula (V)
OH
~ t-~4~;
CH3 - C - CH2 - C ~ - - R6 (V)
~\
t-C4Hg
OH
L m
in which R~ is a Cl-Cl2-alkyl radical or a Cl-Cl2-alkylene
radical and m i8 1 or 2. R5 is prefer~bly a C2-C~-alkylene
radical, in particular a C2-alkylene radical.
However, the phenolic antLoxidant can also be an eqter of
~-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid of
the formula (VI) t-C4Hg
HO- ~ -cH2-cH2-c-oH
t-C4Hg (VI)
. ~,
'' . , : , ~ ;
,
,
- 17 ;~I?~
in which the alcohol component is a mono- or polyhydric
alcohol having 1 to ..., preferably 1 to ..., carbons
atoms, such as, for example,
methanol diethylene glycol
octadecanol triethylene glycol
1,6-hexanediol pentaerythritol
neopentyl glycol tris(hydroxyethyl) isocyanurate
thiodiethylene glycol di-(hydroxyethyl)oxalamide.
The new stabilizers are incorporated in the organic
polymers by generally customary methods. They can be
incorporated, for example, by adding the stabilizers
before or during the polymerization, polycondensation or
polyaddition, or by admixing the compounds and, if
necessary, further additives to the melt before or during
the molding. They can also be incorporated by applying
the dissolved or dispersed compounds directly to the
polymer or admixing to a solution, suspension or emulsion
of the polymer, if appropriate then allowing the solvent
to evaporate. The amount to be added to the polymer is
0.01 to 10, preferably 0.025 to 5, in particular 0.05 to
1.0, % by weight, relative to the m~terial to be stabil-
ized.
The new compounds can also be added in the form of a
ma~ter batch containing these compounds, for example, in
a concentration of 1 to 50, preferably 2.5 to 20 %, by
weight, to the polymers which are to be stabilized.
In addition, the organic polymers to be stabilized can
also contain the following antioxidants, such as, for
example:
1. Alkylated monophenols, for example
2,6-di-t-butyl-4-methylphenol,
2-t-butyl-4,6-dimethylphenol,
2,6-di-t-butyl-4-ethylphenol,
2,6-di-t-butyl-4-n-butylphenol,
2,6-di-t-butyl-4-i-butylphenol,
2,6-di-cyclopentyl-4~methylphenol, ~
2-~-methylcyclohexyl)-4,6-dimethylphenol,
2,6-di-octadecyl-4-methylphenol,
2,4,6-tri-cyclohexylphenol,
2,6-di-t-butyl-4-methoxymethylphenol.
2. Alkylated hyd~oquinone~, for example
2 t 6-di-t-butyl-4-methoxyphenol,
2,5-di-t-butyl hydroquinone,
2,5-di-t-amyl hydroquinone,
2,6-diphenyl-4-octadecyloxyphenol.
3. Hydroxylated thiodiphenyl ethers, for example
2,2'-thio-bis(6-t-butyl-4-methylphenol),
2,2'-thio-bis(4-octylphenol),
4,4'-thio-bis(6-t-butyl-3-methylphenol),
4,4~-thio-bis~6-t-butyl-2 methylphenol).
4. Alkylidene bisphenols, for example
2,2'-methylene-bis(6-t-butyl-4-methylphenol),
2,2'-methylene-bis(6-t-butyl-4-ethylphenol),
2,2'-mathylene-bis[4~methyl-6-(~-methylcyclohexyl)-
phenol~,
2,2'-methylene-bis(4-methyl-6-cyclohexylphenol),
2,2'-methylene-bis~6-nonyl-4-methylphenol),
2,2'-methylene-bi~(4,6 di-t-butylphenol),
2,2'-ethylidene-bis(4,6-di-t-butylphenol),
2,2'-ethylidene-bis(6-t-butyl-4-isobutylphenol),
2,2'-methylene-bis[6-(~-methylbenzyl)-4~nonylphenol],
2,2'-methy:Lene-bis[6-(~ dimethylbenzyl)-4-
nonylphenol]~
4,4'-methylene-bis(2,6-di-t-butylphenol),
4,4~-methylene-bis(6-t-butyl-2-methylphensl),
1,1-bis(5-t-butyl-4-hydroxy-2-methylphenyl)-butane,
2,6-di-(3-t-butyl-5~methyl-2-hydroxybenzyl)-4-methyl-
phenol,
1,1,3-tris(5-t-butyl-4-hydroxy-2-methylphenyl)-butane,
1,1-bis(5-t-hutyl-4-hydroxy-2-methylphenyl)-3-n-
dodecylmercaptobutane,
- 19 - ~?65~
di-(3-l-butyl-4-hydroxy-5-methylphenyl)-dicyclo-
pentadiene,
di-~2-(3~-t-butyl-2~-hydroxy-5~-methyl-benzyl)-6-t-
butyl-4-methyl-phenyl] terephthalate.
5. ~enzyl compounds, for example
1,3,5-tri-(3,5-di-t-butyl-4-hydroxybenzyl)-2,4,6-
trimethylbenzene,
di-(3,S-di-t-butyl-4-hydroxybenzyl) sulfide,
isooctyl 3,5-di-t-butyl-4-hydroxybenzylmercapto-
acetate,
bis (4-t-butyl-3-hydroxy-2, 6-dimethylbenzyl)
dithioterephthalate,
1, 3, 5-tris ( 3, 5-di-t-butyl-4-hydroxybenzyl )
isocyanurate,
1,3,5-tris(4-t-butyl-3-hydroxy-2,6-dimethylbenzyl)
isocyanurate,
dioctadecyl 3,5-di-t-butyl-4-hydroxybenzylphosphonate,
calcium monoethyl 3,5-di-t-butyl-4-hydroxybenzyl-
phosphonate.
6. Acylaminophenols, for example
4-hydroxylauranilide,
4-hydroxystearanilide,
2,4-bis(octylmercapto)-6-(3,5-di-t-butyl-4-hydroxy-
anilino~-s-triazine,
octyl N-(3,5-di-t-butyl-4-hydroxyphenyl)-carbamate.
7. Esters of ~-~S-t-butyl-4-hydroy-3-methylphenyl)-
propionic acid with mono- or polyhydric alcohols, such
as, for example, with
methanol, diethylene glycol,
octadecanol, triethylene glycol,
1,6-hexanediol, pentaerythritol,
neopentylglycol, tris ( hydroxyethyl )
isocyanurate,
thiodiethylene glycol, di-(hydroxyethyl) oxalamide.
- . -
`
'. :~ . .
- ~
` - 20 - 2~
8. Amides of ~-(3,5-di-t-butyl-4-hydro~yphenyl)propionic
acid, such as, for example
N,N'-di-(3,5-di-t-butyl-4-hydroxyphenylpropionyl)-
hexamethylenediamine,
N,N'-di-(3,5-di-t-butyl-4-hydroxyphenylpropionyl)-
trimethylenediamine,
N,N'-di-(3,5-di-t-butyl-4-hydroxyphenylpropionyl)-
hydrazine.
Apart from these, the polymers to be stabilized can
additionally contain further additives, such as, for
example:
1. W absorbers and light stabilizers
1.1 2-(2'-Rydroxyphenyl)-benzotriazoles, such as, for
example, the
5'-methyl, 3',5'-di-t-butyl, 5'-t-butyl, 5'-(1,1,3,3-
tetramethylbutyl), 5-chloro-3',5'-di-t-butyl, 5-
chloro-3~-t-butyl-5~-methyl, 3~-sec.-butyl-5~-t-
butyl, 4'-octoxy, 3~,5~-di-t-amyl, 3',5'-bis-(~
dimethylbenzyl) derivative.
1.2 2-~ydro~ybenzophenones, for example the
4-hydroxy, 4-methoxy, 4-octoxy, 4-decyloxy, 4-dode-
cyloxy, 4-benzyloxy, 4,2',4'-trihydroxy, 2'-hydroxy-
4,4'-dimethoxy derivative.
1.3 ~sters of substituted or un~ubstituted benzoic acids,
for example
4-t-butylphenyl salicylate, phenyl salicylate, octyl-
phenyl salicyalate (sic), dibenzoylresorcinol, bis(4-
t-butylbenzoyl)resorcinol,benzoylresorcinol,2,4-di-
t-butylphenyl 3,5-di-t-butyl-4-hydroxybenzoate,
hexadecyl 3,5-di-t-butyl-4-hydroxybenzoate.
1.4 A!crylates, for example
ethyl or isooctyl ~-cyano-~,~-diphenylacrylate,
methyl ~-carbomethoxycinnamate, methyl or butyl
- 21 -
~-cyano-~-methyl-p-methoxycinnamate, methyl ~-carbo-
methoxy-p-methoxycinnamste, N-(~-carbomethoxy-~-
cyanovinyl)-2-methylindoline.
1.5 Nickel compounds, for example
nickel complexes of 2,2'-thio-bis[4-(1,1,3,3-tetra-
methylbutyl)phenol], such as the 1:1 or 1:2 complex,
if appropriate containing additional ligands, such as
n-butylamine, triethanolamine or N-cyclohexyl-
diethanolamine, nickel alkyl dithiocarbamates, nickel
salts of monoalkyl (methyl or ethyl) 4-hydroxy-3,5-
di-t-butylbenzylphosphonates, nickel complexes of
ketoximes, such as 2-hydroxy-4-methylphenyl undecyl
ketoxime, nickel complexes of 1-phenyl-4-lauroyl-5-
hydroxypyrazole, if appropriate with additional
ligands, nickel salts of 2-hydroxy-4-alkoxy-
benzophenones.
1.6 Sterically hindered amines, for example
1.6.1 bis(2,2,6,6-tetramethylpiperidyl) sebacate,
bis(l,2,2,6,6-pentamethylpiperidyl) sebacate,
bis(2,2,6,6-tetramethylpiperidyl) glutarate,
bis(l,2,2,6,6-pentamethylpiperidyl) glutarate,
bis(2,2,6,6-tetramethylpiperidyl) succinate,
bis(l,2,2,6,6-pentamethylpiperidyl) succinate,
4-stearyloxy-2,2,6,6-tetramethylpiperidine,
4-stearyloxy-1,2,2,6,6-pentamethylpiperidine,
4-stearoyloxy-2,2,6,6-tetramethylpiperidine,
4-stearoyloxy-1,2,2,6,6-pentamethylpiperidine,
2,2,6,6-tetramethylpiperidyl behenate,
1,2,2,6,6-pentamethylpiperidyl behenate,
2,2,4,4-tetramethyl-7-oxa-3,20-diazadispiro-
t5.1.11.2]-heneicosan-21-one,
2,2,3,4,4-pentamethyl-7-oxa-3,20-diazadispiro-
[5.1.11.2]-heneicosan-21-one,
2,2,4,4-tetramethyl-3-acetyl-7-oxa-3,20-diaza-
dispiro[5.1.11.2]-heneicosan-21-one,
2~2~4~4-tetramethyl-7-oxa-3~2o-diaza-2o-(~ aur
..
..
, . . .
;
- 22 ~?`~
oxyca~bonylethyl~-21-oxodispiro[5.1.11.2]-
heneicosane, 2,2,3,4,4-pentamethyl 7 oxa~3,20-
dia~a-20-(~-lauryloxycarbonylethyl)-21-oxodispiro-
[5.1.11.2]-heneicosane, 2,2,4,4-tetramethyl-3-
S acetyl-7-oxa-3,20-diaza-20-(~-laurylo~ycarbonyl-
ethyl)-21-oxodispiro-[5.1.11.2]-heneicosane,
1,1',3,3',5,5'-hexahydro-2,2',4,4',6,6'-hexaaza-
2,2',6,6'-bismethano-7,~-dioxo-4,4'-bis(1,2,2,6,6-
pentamethyl-4-piperidyl)-biphenyl, NN'N"N"'-
tetrakis{2,4-bis[N-(2,2,6,6-tetramethyl-4-
piperidyl~-butylamino]-1,3,5-triazin-6-yl}-4,7-
diazadecane-1,10-diamine, NN~N~N"'-tetrakis-{2,4-
bis E N-(1,2,2,6,6-pentamethyl-4-piperidyl)-
butylamino]-1,3,5-triazin-6-yl}-4,7-diazadecane-
l,10-diamine, NN'N"N"'-tetrakis-{2,4-bis[N-
(2~2,6,6-tetramethyl-4-piperidyl)-methoxypropyl-
amino]-1,3,5-triazin-6-yl}-4,7-diazadecane-1,10-
diamine, NN'N"N"~-tetrakis-{2,4-bis[N-(1,2,2,6,6-
pentamethyl-4-piperidyl)-methoxypropylamino]-1,3,5-
triazin-6-yl}-4,7-diazadecane-1,10-diamine, bis-
(1,2,2,6,6-pentamethylpiperidyl)-n-butyl-3,5-di-
tert.-kutyl-4-hydroxybenzylmalonate, tris(2,2,6,6-
tetramethyl-4-piperidyl) nitrilotriacetate, tetra-
kis-(2,2,6,6-tetramethyl-4 piperidyl)-1,2,3,4-
butanetetracarboxylic acid, 1,1'-(1,2-ethanediyl)-
bis-(3,3,5,5-tetramethylpiperazinone)~
1.6.2 Poly-N,N'-bis(2,2,6,6-tetramethyl-4-piperidyl)-1,8-
diazadecylene, condensation product from 1-(2-
hydroxyethyl)-2,2,6,6-totramethyl-4-hydroxypiperi-
dine and succinic acid, condensation product from
N,N'-bis(2,2,6,6-tetramethyl-4-piperidyl~hexa-
methylenediamine and 4-tert.-octylamino-2,6-
dichloro-1,3,5-triazine, condensation product from
N,N'-bis(2,2,6,6-tetramethyl-4-piperidyl)hexa-
methylenediamine and 4-morpholino-2,6-dichloro-
1,3,5-triazine.
In many cases, a combination of th~ compounds according
- 23 -
to the invention with the compounds mentioned und~r 1.6.1
is particularly advantageous.
1.7 Oxalamides, for example
4,4'-di-octyloxyoxan~lide, 2,2'-di-octyloxy-5,5'-di-
t-butyloxanilide, 2,2'-didodecyloxy-5,5'-di-t-butyl~
oxanilide, 2-ethoxy-2'-ethyloxanilide, N,N'-bis(3-
dLmethyl~minopropyl)oxalamide, 2-ethoxy-5-t-butyl-2~-
e~hyloxanilide and its mixture with 2-etho~y-2~-
ethyl-5,4-di-t-butyloxanilide, mixtures of ortho- and
para-methoxy- and of o~ and p-ethoxy-di-substituted
oxanilide~.
2. Metal deactivators, for example
N,N'-diphenyloxalamide, N-salicylyl-N'-salicyloyl-
hydra~.ine, N,N'-bis(salicyloyl)hydrazine, N,N' bis-
(3,5-di-t-butyl-4-hydroxyphenylpropionyl)hydrazine, 3-
salicyloylamino-1,2,3-triazole, bis(benzylidene)-
oxaldihydrazide.
3. Phosphites and phosphonites, for example
triphenyl phosphite, diphenyl alkyl phosphites, phenyl
dialhyl phosphites, tris~nonylphenyl) phosphite,
trilauryl phosphite, tri-(octadecyl) phosphite,
distearyl pentaerythrityl diphosphite, tris(2,4-di-t-
butylphenyl3 phosphite, di-(isodecyl) pentaerythrityl
diphosphite,bis(2,4-di-t-butylphenyl)pentaerythrityl
diphosphite~ tristearyl sorbityl triphosphite,
tetrakis(2,4--di-~-butylphenyl) 4,4'-diphenylenediphos-
phonite, 3,9-bis(2,4-di-t-butylphenoxy) 2,4,8,10-
tetraoxa-3,9-diphosphaspiro(5.5)undecane, trist2-t-
butyl-4-thio(2'-methenyl-4~-hydroxy-5'-t-butyl)phenyl-
5-methenyl)phenyl phosphite.
4. Pero~ide-de~troying compounds, for example
es~ers of ~-thiodipropionic acid, for example the
lauryl, stearyl, myristyl or tridecyl ester,
m~rcap~obenzimidazole, the zlnc salt of 2-mercapto-
b~nzLmidazole, zinc alkyldithiocarbamate~, dioctadecyl
s~
- 24 -
disulfide, dioctadecyl monosulfide, tetraXis(~-
dodecylmercapto)pentaerythritol propionate.
5. Basic costabilizers, for example
melamine, polyvinylpyrrolidone, dicyandiamide, tri-
allyl cyanurate, urea derivatives, hydrazine
derivatives, amines, polyamines, polyurethanes, alkali
metal and alkaline earth metal salts of higher fatty
acids or phenolates, for example Ca stearate, Zn
stearate, Mg stearate, Na ricinoleate, K palmitate,
antimony pyrocatecholate or tin pyrocatecholate,
hydroxides and oxides of alkaline earth metals or
aluminum, for example CaO, Mg~, ZnO.
6. Nucleating agents, for example
4-t-butylbenzoic acid, adipic acid, diphenylacetic
acid, dibenzylidenesorbitol.
7. Fillers and reinforcing agents, for example
calcium carbonate, silicates, glass fibers, asbestos,
talcum, kaolin, mica, barium sulfate, metal oxides and
hydroxides, carbon black, graphite.
0 8. Other additives, for example
plasticizers, lubricants, emulsifiers, pigments,
optical brighteners, fireproofing agents, anti~tatics,
blowing agents.
The various additional additives of the abovementioned
groups 1 to 6 are added to the polymers to be stabilized
in an amount of 0.01 to 10, preferably 0.01 to 5, % by
weight, relative to the total weight of the molding
composition. The relative amount of the additives of
groups 7 and 8 is 1 to 80, preferably 10 to 50, % by
weight, relative to the entire molding composition.
The organic polymers stabilized according to the inven-
tion can be used in a wide range of forms, for example as
sheets, fibers, ribbons, profiles or as binders for
- 25
paints, adhesives or cements.
Before the examples illustrating the invention, the
preparation of the starting materials (III), which are
known in some cases, is described. If these compounds are
still unknown, they can be prepared easily from PX3 (X=C1,
Br) and the phenol concerned in analogy to known com-
pounds (for example EP-PS 158,300). The phenols and
amines HNR4R5 (~V) used as starting materials are mo~tly
known compounds; otherwise they can be prepar~d by
analogous processes.
I) G~neral procedure for the preparation of the precur-
sors
A2yl dichlorophosphites tIII)
650 mmol (= 89.3 g) of phosphorus (III~ chloride, a
spatula tip-full (about 100 mg) of p-dimethylaminopyri-
dine and 500 mmol of the phenol concerned were added
together with the exclusion of air and moisture. The
solution cooled and evolution of HCl commenced. The
mixture was slowly heated to 90-100C with vigorous
stirring in the course of 60-90 min and was held at this
temperature for 2 hours in order to complete the reac-
tion. The low-boiling constituents were then stripped off
at 50C in the vacuum of the water ~e~ pump. The purity
f aryl dichlorophosphites was determined by means of 31p_
NMR spectroscopy and was in general between 70 and 92%.
A) 2,4-di-tert.-butylphenyl dichlorophosphiteO
Distillation of the crude product having a purity of
91.7% [31P-NMR: ~CDCl3 = 184.7 ppm] of the above compound
yielded 129 g of yellowish oil of b.p. 104-105C/0.05
mbar.
C14H21Cl2OP Calc.: 54.73 % C, 6.89 % H, 10.08 % P
(307.20) Found: 54.9 % C, 6.7 ~ H, 10.2 % P.
B) 2-~ert.-butylphenyl dichlorophosphite:
Distillation of the crude product having a purity of 84%
- 26 - 2~5~
[31P-NMR: ~CDCl3 = 185.1 ppm] of the above compound
yielded 94 g of colorless oil of b.p. 74-76~C/0.01 mbar.
CloHl3C12OP Calc.: 47.83 % C, 5.21 % H, 12.33 ~ P
(251.09) Found: 47.4 % C, 5.1 % H, 12.0 % P.
C) 2,4-dimethylphenyl dichlorophosphite:
Distillation of the crude product having a purity of 75%
t3lP-NMR: ~CDCl3 = 180.9 ppm] of the above compound
yielded 72 g of colorless oil of b.p. 58-60C/0.05 mbar.
C~HgCl2OP Calc.: 43.08 % C, 4.06 % H, 13.88 % P
(223.03) Found: 42.7 % C, 3.9 % H, 13.6 % P.
D) 2-tert.-butyl-4-metho~yphenyl dichlorophosphite:
Distillation of the crude product yield 110 g of color-
less oil of b.p. 108-110C/0.05 mbar.
C11Hl5Cl2O2P Calc.: 46.99 % C, 5.37 % H, 11.01 % P
(281.11) Found: 46.7 % C, 5.5 % H, 10.7 % P.
E) 2,4-di-sec.-butylphenyl dichlorophosphite:
Distillation of the crude product having a purity of 71%
[3lP-NMR: ~CDCl3 = 182.3 ppm] of the above compound
yielded 93 g of colorless oil of b.p. 108C/0.05 mbar.
C14H2lCl2OP Calc.: 54.73 % C, 6.89 % H, 10.08 % P
(307.20) Found: 54.5 % C, 6.7 % H, 10.0 % P.
F) 2-sec.-butylphenyl dichlorophosphite:
Distillation of the crude product having a purity of 78%
t31P-NNR: ~CDCl3 = 182.3 ppm] yielded 83 g of colorles~
oil of b.p. 92-93-C/0.1 mbar.
CloH13Cl2OP Calc.s 47.83 % C, 5.21 % H, 12.33 % P
(251.09) Found: 47.4 % C, 4.9 % H, 12.0 % P.
II) ~ample~ 1 to 12 - aryl chlorophosphoramidites (II)
General procedure for preparation
A solution of 500 mmol of the amine HNR4R5 and 500 mmol
(= 50.6 g) of triethylamine in 100 ml of toluene was
metered in the course of 30-40 minutes into a 601ution of
500 mmol of aryl dichlorophosphite in 400 ml of toluene
- 27 ~ S~ {j
which was stirxed 2t -10C under nitrogen in such a way
that the internal temperature did not exceed 0C. The
mixture was subsequently stirred at room temperatuxe for
a further 2 hours in order to complete the reaction.
After filtration and removal of the solven~ by distil-
lation in vacuo, the crude chloroamidites usually
remained as yellow oils. A further purification is in
general not necessary for further reaction with Grignard
reagents.
1) 2,4-di-tert.-butylphenyl chloropho6phite morpholide:
Starting from 153.6 g of 2,4-di-tert.-butylphenyl di-
chlorophosphite and 43.6 g of morpholine, 165 g of a
colorless solid of mOp. about 70C and a purity of 94%
[31P-NMR: ~CDCl3 = 156.9 ppm] of the above compound were
obtained.
C13H29ClNO2P Calc.: 60.41 % C, 8.17 % H, 8.65 % P
l357.86) Found: 6D.1 ~ C, 8.4 % H, 8.3 % P.
2) 2,4-di-tert.-butylphenyl ~hlorophosphite di-n-butyl-
amide: Starting from 153.6 g of 2,4-di-tert.-butylphenyl
dichlorophosphite and 64.62 g of di-n-butylamine, about
190 g of a yellow oil having a purity of 94% [31P-NMR:
~CDCl3 = 162.3 ppm~ of the above compound were obtained.
C2zH29ClNOP Calc.: 66.06 % Ct 9.82 ~ H, 7.74 % P
(399.99) Found: 66.4 % C, 9.6 % H, 7.7 ~ P.
3) 2-tert.-butylphe~yl chlorophosphite di-n-butylamide:
Starting from 125.54 g of 2-tert.-butylphenyl dichloro-
phosphite and 64.62 g of di-n-butylamine, about 165 g of
a yellow oil having a purity of 89% [3lP-NMR: ~CDCl3 =
161.8 ppm] of the above compound were obtained.
C1aH31ClNOP (343.87)
4) 2,4~di-tert.-butylphenyl ~hlorophosphite piperidide
Starting from 153.6 g of 2,4-di-tert.-butylphenyl di-
chlorophosphite and 42.57 g of piperidine, about 170 g of
a yellow oil having a purity of 88~ [31p_NMR: ~CDC13 =
157.7 ppm] of the above compound were obtained.
28 - Z ~
-
ClgH31ClNOP (355.89)
5) 2,4-di-tert.-butylphenyl chlorophosphite N-n-butylani-
lide: Starting from 153.6 g of 2,4-di-tert.-butylphenyl
dichlorophosphite and 74.6 g of N-n-butylaniline, about
200 g of a yellow oil having a purity of 89% t3lP-NMR:
~CDCl3 = 156.6 ppm] were obtained.
C24H3sClNP (419.97)
6)2,4-di-tert.-butylphenylN-(hesamethyleneimino)chloro-
phosphoramidite: Starting from 153.6 g of 2,4-di-tert.-
butylphenyl dichlorophosphite and 49.58 g of hexamethyl-
eneimine (also called homopiperidine)l about 176 g of a
yellow oil having a purity of 87% t3lP-NMR: ~CDC13 (sic) =
163.9 ppm] of the above compound were obtained.
C20H33ClNOP (369.91)
7) 2,-4-di-tert.-butylphenyl dicyclohexylchlorophosphor-
amidite: Starting from 153.6 g of 2,4-di-tert.-butyl-
phenyl dichlorophosphite and 90.65 g of dicyclohexyl-
amine, the reaction being completed at 60C, about 205 g
of a yellow resin having a purity of 84% t3lP-NMR: ~CDCl3
= 166.0 ppm] of the above compound were obtained.
C26H43ClNOP (452.05)
8) 2-tert.-butyl-4-aethosyphenyl chlorophosphite mor-
pholide: Starting from 140.56 g of 2-tert.-butyl-4-
methoxyphenyl dichloropho~phite and 43.6 g of morpholine,
about 152 g of colorless oil were obtained which
solidified at room temperature.
Cl5H23ClNO3P Calc.: 54.30 % C, 6.98 % H, 9.33 ~ P
(331.77) Found: 54.0 % C, 6.7 % H, 8.9 % P.
9) 2,4-di-sec.-butylphenyl chlorophosphite morpholide:
Starting from 153.6 g of di-sec.-butylphenyl dichloro-
phosphite and 43.6 g of morpholine, about 160 g of a
yellow oil having a purity of 80% t3lP-NMR: ~CDC13 = 159.8
ppm] of the above compound were obtained.
Cl8H29ClNO2P (357.86)
- ~9 -
10) 2,4-dLmethylphsnyl chlorophosphite morpholide: Start-
ing fxom 111.52 g of 2,4-dimethylphenyl dichlorophosphite
and 43.6 g of morpholine, about 120 g of a yellow oil
having a puri~y of 90% [31p_NMR: SCDC13 = 160.7 ppm] were
5 obtained. The distillation yielded a colorless oil of
b.p. = 148-150C/0.05 mbar
C1zHl7ClNO2P Calc: 52.66 % C, 6.26 % H, 11.31 % P
~273.70~ Found: 51.9 ~ C, 5.9 % H, 11.5 % P.
11) 2,4-dimethylphenyl chloropho6phite pip~ridide:
Starting from 111.52 g of 2,4-dimethylphenyl dichloro-
phosphite and 42.6 g of piperidine, 121 g of a yellow oil
having a purity of 89% [3lP-NMR: ~CDCl3 = 161.3 ppm] of
the above compound were obtained. The distillation
yielded a colorless oil of b.p. = 134-135C/0.05 mbar
Cl3HlgClNOP Calc: 57.46 ~ C, 7.04 % H, 11.39 % P
(271.72) Found: 57.1 ~ C, 6.9 % H, I1.2 % P.
12) 2,4-dimethylphenyl di-n-butylchlorophosphoramidite:
Starting from 111.52 g of 2,4-dimethylphenyldichlorophos-
phite and 64.6 g of di-n-butylamine, 143 g of a yellow
oil having a purity of 86% [3lP-NMR: ~CDC13 = 167.7 ppm]
of the above compound were obtained. The distillation
yielded a colorless oil of b.p. = 148C/0.05 mbar
Cl6H27ClNOP Calc: 60.84 ~ C, 8.61 ~ H, 9.80 % P
(315.82) Found: 60.5 ~ C, 8.4 % H, 9.9 % P.
III) ~amples 13 to 33 - aryl pho~phonamidites (I)
General procedure for preparation
The corresponding Grignard compound was prepared from
250 mmol of organobromine compound and 250 mmol (= 6.1 g)
of magnesium turnings in 170 ml of tetrahydrofuran under
a nitrogen atmosphere and with exclusion of moi~ture. The
resulting solution or suspension of the organometallic
compound was subsequently metered into a solution of
250 mmol of the chlorophosphoramidite (II) concerned in
120 ml of tetrahydrofuran with vigorous ~tirring at an
internal temperature of -20 to -lO~C in the course of 30
- 30 ~ ?~r~
- 40 minutes. The reaction mixture was then allowed to
come to room temperature and was stirred for a furthex
2.5 hours in order to complete the reaction. After
filtration of the precipitated magnesium salt, the
solvent was remo~ed by distillation first in the vacuum
of the water jet pump and then in a high vacuum and the
colorless or pale-beige residue was pulverized and dried
in a high vaouum.
The purity of desired product in the crude materials was
determined by 3lP-NMR spectroscopy. In the case of the
monophosphonamidites it was in general between 80 and 94%
(of the total P). In th cases indicated, the crude
mixture was crystallized from acetonitrile/acetone
mixtures in order to characterize the product.
13) 2',4'-di-tert.-butylphenyl ~2,4,6-trimethyl-1-
phenyl)phosphonite piperidide: Starting from 49.7 g of
bromomesitylene and 89.0 g of 2,4-di-tert.-butylphenyl
chlorophosphite piperidide, about 108 g of beige material
of softening point about 30C and a purity of 86~ of the
above compound were obtained [31P-NMR: ~CDCl3 = 132.7
ppm].
C2BH42NOP Calc: 76.49 ~ C, 9.63 % H, 7.04 ~ P
(439.62) Found:76.0 % C, 9.5 % H, 6.7 % P.
14~ 2',4'--cli-tert. butylphenyl (2,4,6-trimethyl-1-
phe~yl)phosphomite morpholides Starting from 49.7 g of
bromomesitylene and 8g.47 g of 2,4-di-tert.-butylphenyl
chlorophosphite: morpholide, about 110 g of coLorles~
material of m.p. 70-80C and a purity of 90% of the above
compound were obtained [31P-NMR: ~CDCl3 = 132.4 ppm~.
C27H40NOzP Calc: 73.43 % C, 9.13 ~ H, 7.01 % P
(441.60) Found: 73.1 % C, 9.5 ~ H, 6.5 % P.
15) 2',-4'-dLmethylphen~l (2,4,6-trimethyl-1-phenyl3-
pho~phonite morpholide Starting from 49.7 g of bromome-
sitylene and 68.43 g of 2,4-dimethylphenyl chlorophos-
phite morpholide, about 90 g of yellowish material of
- 31 -
softening point 90-95C and a purity of 91~ of the aboYe
compound were obtained [3lP-NMR: ~CDC13 = 135.1 ppm].
C21H23NO2P Calc: 70.56 % C, 7.89 % H, 8.66 % P
(357.44) Found: 69.9 % C, 8.1 % H, 8.2 % P.
16) 2',4'-di-secO-butylphenyl (2,4,6-trimethyl-1-
phenyl)phosphonite morpholide: Star~ing from 49.7 g of
bromomesitylene and ~9.46 g of 2,4~ sec.-butylphenyl
chlorophosphite morpholide, about 108 g of yellowish oil
having a purity of 86% of the above compound were ob-
tained [3lP-NMR: ~CDC13 = 134.3 ~nd 135 ppm (diastereo-
mers)].
C27H40NO~p (441.60~
17) 2~,4'-di-tert.-butylphenyl (2,4,5-trLmethyl-l-
phenyl)phosphonite morph~lide: Starting from 49.7 g of
lS 5-bromo-1,2,4-trimethylbenæene and 89.46 g of 2,4-di-
tert.-butylphenyl chlorophosphite moxpholide, about 100 g
of colorless resin having a purity of 93% of the above
compound were obtained [31P-NMR: ~CDC13 = 120.9 ppm].
Crystallization from acetonitrile yielded colorless
crystals of m.p. 130 - 132C.
C27H40NO2P Calc: 73.43 % C, 9.13 % H, 7.01 % P
(441.6) Found: 73.2 % C, 9.4 % H, 6.8 % P.
18) 2',4'-di-tert.-butylphenyl (4-~ert~-butyl-1-phenyl)-
phosphonite mo~pholide: Starting from 53.5 g of p-bromo-
tertO-butylbenzene and 89.46 y of 2,4-di-tert.-butyl-
phenyl phosphit~ (sic~ morpholide, about 110 g of color-
less resi.n having a purîty of 85% of the above compound
[31P-NMR: ~CDCl3 = 126.3 ppm] were obtained. Crystalliza-
tion from acetone/acetonitrile (1~1) yielded colorless
crystals of m.p. 106 - 108C.
19) 2'-tert.-butyl-4'-methoxyphenyl (4-tert.-butyl-l-
phenyl)pho~phonite morpholide: Staxting from 53.3 g of p-
bromo-tert~-butylbenzene and 82.95 g of 2-tert.-butyl-4-
methoxyphenyl chlorophosphite morpholide, about 10~ g of
beîge material of softening point about 70C and a purity
5 1~
- 32 -
of 82% of the above compound were obtained [31p_NNR:
~CDCl3 = 126.7 ppm].
C25H36NO3P t429.54).
20) 2~,4~-di-tert.-butylphenyl 4-biphenylphosphonite
morpholide: Starting from 58.3 g of 4-bromobiphenyl and
89.46 g of 2,4-di-tert.-butylphenyl chlorophosphite
morpholide, about 114 g of colorless material having a
purity of 88% of the above compound were obtained [31p_
NMR: 6CDC13 = 125.3 ppm]. CrystallizatiOn from acetone
yielded colorless crystals of m.p. 124 - 125C.
C30H3~NO2P Calc.: 75.76 % C, 8.05 % H, 6.51 % P
(475.61) Found: 75.9 % C, 8.35 % H, 6.3 % P.
21) 2',4'-di-tert.-butylphenyl (4-metho y-l-phenyl)-
phosphonite di-n-butyl~mide: Starting from 46.75 g of 4-
bromoanisole and 100 g of 2,4-di-tert.-butylphenyl
chlorophosphite di-n-butylamide, about 110 g of yellow
resin having a purity of 90% of the above compound were
obtained t3lP-NNR: ~CDCl3 = 127.9 ppm].
C29H46NO2P (471.67).
22) 2~,4~-di-tert.-butylphenyl l-naphthylphosphonite
morpholide: Starting from 51.8 g of l-bromonaphthalene
and 89.46 g of 2,4-di-tert.-butylphenyl chlorophosphite
morpholide, about 112 g of colorless material of soften-
ing point about 100-105C and a purity of 87% of the
above compound were obtained t3lP-NMRs CCDCl3 - 120.8
ppml. C2~H36NO2P Calc.: 74.8 % C, 8.07 % H, 6.88 % P
(449.58) Found: 74.2 % C, 8.1 % H, 6.4 % P.
23) 2',4'-di-tert.-butylphenyl 2-naphthylpho~phonite
morpholide: Starting from 51.8 g of 2-bromonaphthalene
and 89.46 g of 2,4-di-tert.-butylphenyl chlorophosphite
morpholide, about 110 g of colorless material of soften-
ing point about 100-C and a purity of 92% of the above
compound were obtained t3lP-NMR: 6CDCl3 = 125.0 ppm].
Colorless crystals of m.p. 120C were obtained from
acetonitrile.
- 33 -
2 ~ , A l$ >
Cz8H36NO2P Calc .: 74.80 % C, 8.07 ~ H, 6.88 ~ P
(449.58) Found: 74.5 % C, 8.3 % H, 6.9 % P.
24) 2~,4~-di-sec.-butylphenyl l-naphthylphosphonite
morpholide: Starting from 51.8 g of l-bromonaphthalene
and 89.46 g of 2,4-di-sec.-butylphenyl chlorophosphite
morpholide, about 116 g of ductile resin having a purity
of 80% of the above compound were obtained [3lP-NMR:
~CDCl3 = 121.2 and 121.6 ppm (diastereomers)~.
C28H36NO2P (449.58)-
25) 2',4'-dimethylphenyl l-naphthylphosphonite mor-
pholide: Starting from 51.8 g of 1-bromonaphthalene and
68.43 g of 2,4-dLmethylphenyl chlorophosphite morpholide,
about 91 g of colorless material of softening point 78 -
80C and having a purity of 89% of the above compound
[31P-NMR: 6CDCl3 = 121.6 ppm] were obtsined.
C22H24NO2P Calc.: 72.31 % C, 6.62 % H, 8.47 % P
(365.41) Pound: 71.9 % C, 6.4 % H, 8.1 % P.
26) 2',4'-di-tert.-butylphenyl (4-methyl-1-naphthyl)-
phosphonite morpholide: Starting from 55.27 g of 1-bromo-
4-methylnaphthalene and 89.46 g of 2,4-di-tert.-butyl-
phenyl chlorophosphite morpholide, about 113 g of beige
material of softening point about 90C and a purity of
88% of the above compound were obtsined t3lP-NMR:
~CDCl3 = 121.4 ppm~. A colorless powder of m.p. 110C was
obtained from acetonitrile.
C2~H38NO2P Calc.: 75.13 % C, 8.26 % H, 6.68 % P
(463.59) Found: 74.7 % C, 8.0 % H, 6.5 % P.
27) 2~,4'-di-tert.-butylphenyl (2-methyl-1-naphthyl)-
pho~phonite rpholide: Starting from 55.3 g of l-bromo-
2-methylnaphthalene and 89.46 g of 2,4-di-tert.-butyl-
phenyl chlorophosphite morpholide, about 108 g of nearly
colorless material of softening point about 70C and a
purity of 83% of the above compound were obtained t31P-
NMR: ~CDCl3 = 131.8 ppm]. Colorless needles of m.p. 115 -
118C were obtained from acetonitrile.
.
2~
-- 34 --
C25,H38NO2P Calc.: 75.13 9~i C, 8.26 % H, 6.68 % P
(463.59) Found: 75.6 9c C, 8.5 % H, 6.3 % P.
28) 2~,4~-di-tert.-butylphenyl l-napthylphosphonite
homopiperidide: Starting from 51.8 g of l-bromonaphthal-
ene, and 92.48 g of 2,4-di-tert.-butylphenyl chloro-
phosphite homopiperidide (see Example 6), about 110 g of
colorless, ductile resin having a purity of 88% t3lP-NMR:
6CDCl3 = 122.9 ppm] of the above compound were obtained.
Colorless crystals of m.p. 112 - 113-C were obtained from
acetonitrile/acetone (1:1).
C30H40NOP Calc.: 78.05 % C, 8.73 9~ H, 6.70 % P
(461.62) Found: 78.2 % C, 8.5 % H, 6.5 % P.
29) 2~,4'-di_tert.-butylphenyl ~6-metho~q-2-naphthyl)
phosphonite morpholides Starting from 59.3 g of 2-bromo-
6-methoxynaphthalene and 89.46 g of 2,4-di-tert.-butyl-
phenyl chlorophosphite morpholide, about 107 g of color-
less material of softening point about 45C and a purity
of 94% of the above compound were obtained [3lP-NMR:
~CDCl3 = 125-7 ppm]
C29H3,lNO3P Calc.: 72.62 96 C, 7.98 % H, 6.45 % P
(479.60) Found: 72.2 % C, 8.3 % H, 6.1 % P.
30) Bist2,4,-dimethylphenyl) 4,4~-biphenylenediphosphon-
ite morpholide]s Differing from the general procedure,
250 mmol (- .78 g) of 4,4'-dibromobiphenyl were converted
into a Grignard compound with 500 mmol (- 12.2 g) of
magnesium turnings in 500 ml of tetrahydrofuran under the
action of ultrasound (40 kHz) and reacted with 500 mmol
(= 136.9 g) of 2,4-dimethylphenyl chloropho~phite which
was dissolved in 200 ml of tetrahydrofuran. About lS0 g
of yellowish, ductile resin having a purity of 63% of the
above compound wereobtained ~31p_NMR: ~CDCl3 = 126.7 ppm].
C36H42N2O4P2 (628.68)
31) Bist(2,4,-di-tert.-butylphenyl) 4,4'-biphenylenedi-
phosphonite morpholide]: Differing from the general
procedure, 250 mmol (= 78 g) of 4,4'-dibromobiphenyl were
- 35 ~
converted into a ~rignard compound with 500 mmol
(= 12.2 g) of magnesium turnings in 500 ml of tetrahydro-
furan under the action of ultrasound (40 kHz) then and
reacted wi~h 178.9 g of 2,4-di-~art.-butylphenyl chloro-
phosphite morpholide in 200 ml of tetrahydrofuran. About200 g of yellowish powder of softening point 103 - 105C
and a purity of 70~ of the above compound were obtained
[31p_NMR: ~CDCl3 = 125.0 pp~.
C 4 8H66N204P2 (797-00)
32) Bis[(2,4,-di-tert.-butylphenyl) 4~4r-biphenylenedi-
phosphonite di-n-butylamide~: Differing from the general
procedure, 250 mmol (= 78 g) of 4,4'-dibromobiphenyl were
converted into a Grignard compound with 500 mmol
(= 12.2 g) of magnesium turnings in 500 ml of tetrahydro-
furan under the action of ultraso~tnd (40 kHz) and then
reacted with 500 mmol (= 200 g) of 2,4-di-tert.~butyl-
phenyl chlorophosphite di-n-butylamide in 200 ml of
tetrahydrofuran. About 210 g of a ductile, yellow resin
having a purity of 73% of the above compound were
obtained [3lP-NMR: ~CDCl3 = 126.g ppm].
Cs6Ha6N202P2 (881.27)
33)Bist(2'4'-di-tert.-butylphenyl)phenylene-1,4-diphos-
phonite morpholide]: Differing from the general proced-
ure, 250 mmol (= 58.97 g) of 1,4~dibromobenzene were
converted into a Grignard compound with 600 mmol (= 14.6
g) of magnesi~n turnings in 300 ml of tetrahydrofuran
under the action of ultrasound (40 kHz) and then re~cted
with 500 mmol (= 17809 g) of 2,4 di-tert.-butylphenyl
chlorophosphite morpholide in 200 ml of tetrahydrofuran.
About 170 g of an almost colorless resin were obtained.
Colorless crystals of softening point 230C were obtained
from acetone [31P-NMR ~CDCl3 = 124.6 ppm].
C42H62N204P2 Calc.: 69.97 ~ C, 8.66 ~ H, 8.59 % P
(720.90) Found: 69.5 % C~ 8.9 % H, 8.3 % P.
- 36 -
IV) ~xamples of use
The aryl phosphonamidites (I) according to the invention
listed below were employed for the experiments:
a) 2~,4~-di-tert.-butylphenyl (2~4~6-trimethyl-I-phenyl)-
phosphonite morpholide
b) 2',4'-di-tert.-butylphenyl (2,4,5-trimethyl-1-phenyl)-
phosphonite morpholide
c) 2',4'-di-tert.-butylphenyl 4-biphenylphosphonite
morpholide
0 d) 2~,4~-di-tert.-butylphenyl l-naphthylphosphonite
morpholide
e) 2',4'-di-tert.-butylphenyl 1-naphthylphosphonite
homopiperidide
f) 2',4'-di-tert.-butylphenyl ~S-methoxy-2-naphthyl)-
phosphonite morpholide
g) bis(2~4~-di-tert.-butylphenyl) phenylene-1,4-
diphosphonite morpholide
Example 34 and Comparative E~amples A to C
100.0 psrts by weight of unstabilized polypropylene
powder (density: 0.903 g/cm3; melt flow index MFI 230/5:
4 q/10 min) were mixed with 0.1 part by weight of Ca
stearate as acid acceptor and the amounts of phosphorus
compound listed in the tables and extruded repeatsdly by
means of a laboratory extruder (short-compression zone
screw, diameter of screw: 20 mm; length 20 D, length of
nozzle 30 mm, diameter 2 mm; number of revolutions:
125 rpm; temperature program: 200/230/230C). After the
1st, 5th and 10th pass, samples were taken from the
granules and used to measure the melt flow index
Z~6~
- 37 -
accordinq to DIN 53 735 and the yellowness as yellowness
index according to ASTM D 1925-70. In addition, the
granules of the 1st pass were used to produce extruded
sheets of the dimensions 60 x 60 x 1 mm, and the yellow-
ness was measured immediately and after hot storage (7days at 100C).
The results are listed in Tables 1, 2a and 2b.
Esample 35 and Comparative Examples D to F
100.0 parts by weight of unstabilized polypropylene
powder (density: 0.903 g/cm3; me}t flow index MFI 230/5:
4 g/10 min) were mixed with 0.1 part by weight of Ca
stearate as acid acceptor and 0.05 part by weight of
ethylene.glycol bis(3,3-bis(3't-butyl-4'-hydroxyphenyl)-
butyrate (sic) and the amounts of phosphorus compound
listed in the tables and extruded repeatedly by means of
a laboratory extruder (short-compression zone screw,
diameter of screw: 20 mm; length 20 D, length of nozzle
30 mm, diameter 2 mm; number of revolutions: 125 rpm;
temperature program: 200/230/230C). After the 1st, 5th
and 10th pass, samples were taken from the granules and
used to measure the melt flow index according to DIN 53
735 and the yellowness as yellowness index according to
ASTM D 1925-70. In addition, the granules of the l~t pass
were used to produce extruded sheets of the dimension~ 60
x 60 x 1 mm, and the yellowness was measured immediately
and after hot storage ~7 days at 100C).
The results are listed in Tables 3, 4a and 4b.
,. . : .
.. ,~, ~ .
. '
.
~ ~r~
-- 38 --
Table l: The effect of phosphorus compounds on the
proce~sing stability of polypropylene. Melt
flow index MFI 230~5 after multiple granula-
tion. (MFI in g/10 ~in)
~ ~ _ . _
Example Phosphorus compound MFI after
_ _ _ _ 1st _5th 10th qranulation
Comp
Ex. A none 10.5 19.0 34.0
Comp. 0.1 part by weight 6.4 7.0 10.5
Ex. h of tris(2,4-di-t~
butylphenyl)
phosphite
Comp. 0.1 part by weight 5.5 5.7 7.8
Ex. C of commercial
phosphonite )
34a 0.1 part by weight 5.6 6.1 7.5
of (phosphonite
according to the
invention)
34b ~ 1.0 1.0 1.3
34c " 6.1 6.3 11.3
34d ~ 5.4 5.5 6.0
34e ~ 1.0 1.3 2.0
34f " 1.1 1.1 1.7
34g " 1.~ 1.4 1.6
.
Tetrakis(2,4 di-t-butylphenyl) 4,4'-biphenylenediphos-
phonite
- 39 -
Table 2a: Coloration (yellowness index according to ASTM
D 1925-70) after multiple granulation of
polypropylene.
_ _
Example Phosphorus compound YI after
1st 5th10th aranulation
Comp.
Ex. A none 18.7 25.7 27.7
Comp. 0.1 part by weight 13.9 23.0 30.4
Ex. B of tris(2,4-di-t-
butylphenyl)
phosphite
Comp. 0.1 part by weight 12.8 21.0 26.1
Ex. C of commercial
phosphonite~)
34a 0.1 part by weight 8.2 15.5 22.8
of (phosphonite
according to the
invention)
34b " 2.5 2.6 7.7
34c ~' 16.3 20.4 33.2
34d " 14.5 15.0 18.8
34e ll 3.7 6.5 13.8
34f " 6.1 7.9 16.1
34g " 2.2 2.6 5.8
_ .
) Tetrakis(2,4-di-t-butylphenyl) 4,4'-biphenylenediphos-
phonite
Table 2b: Coloration of 1 mm extruded sheets immediately
after production and after heat treatment (7
days at 100C)
YI immediately YI after 7 days/100C
Comp. Ex. A 4.2 10.1
Comp. Ex. B 3.4 13.0
Comp. Ex. C 4.5 12.5
34a 2.5 11.1
34b 1.6 5.4
34c 4.7 9.3
34d 3.9 8.7
34e 1.5 5.5
34f 1.8 8.5
34g 1.0 4.5
t, J' !,
- 40 -
Table 3~ The effect of phosphorus compounds on the
processing stability of polypropylene. Melt
flow index MFI 230/5 after mul~ipl~ granula-
tion. (MFI in g/10 min)
Example Phosphorus compound MFI after
_ __ _ 1st 5th 10th qranulation
See D none 13.4 19.0 23.8
See E 0.01 part by weight7.1 7.8 8.8
of tris(2,4~di-t-
butylphenyl)
phosphite
See F 0.01 part by weight5.8 6.4 8.9
of commercial
phosphonite )
35a 0.01 part by weight5.0 4.8 5.4
of ~phosphonite
according to the
invention)
35b " 5.4 5.4 7.9
35c " 4.6 5.4 5.5
35d " 4.6 5.9 7.1
35e " 5.8 5.9 5.5
35f " 5.8 7A3 8.4
35g " 5.0 6.6 8.0
... . ~
) Tetrakis(2,4-di-t-butylphenyl) 4,4~-biphenylenediphos-
phonite
- 41 - 2~
Table 4a: Coloration (yellowness index according to ASTM
D 1925-70) after multiple granulation of
polypropylene.
5 Example Phosphorus compound YI after
1st 5th 10th aranulation
See D none 8.0 23.4 28.0
See E 0.05 part by weight 9.5 12.3 15.1
of tris(2~4-di-t-
butylphenyl)
phosphite
See F 0.05 part by weight 12.5 16.5 20.8
of commercial
phosphonite )
35a 0.05 part by weight 3.3 5.6 9.5
of (phosphonite
according to the
invention)
35b ~ 11.1 9.4 12.9
35c ~ 7.0 12.1 15.7
35d ~ 10.4 23.4 31.3
35e ~ 11.1 15.6 19.5
35f ~ 12.1 20.9 26.6
35g ~ 8.7 29.9 32.0
) Tetrakis(2,4-di-t-butylphenyl) 4,4~-biphenylenediphos-
phonite
Table 4b: Coloration of 1 mm extruded sheets immediately
after production and after heat treatment (7
days at 100C)
YI immediatelyYI after 7 days/lOO~C
See D 3.9 7.7
See E 2.3 5.0
See P 2.8 4.5
35a 1.8 11.2
35b 2.7 3.0
35c 2.3 2.8
35d 3.5 4.2
35e 2.9 4.5
35f 3.9 6.3
35g 2.7 2.9