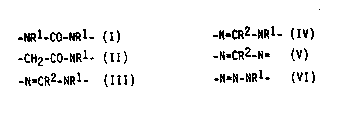Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
J~ ~ i
HAIR DY2ING ~oC~SS USI~G DI~CT DYE8
This invention rPlates to 3 proc~ss for dyeing hair
using substantive dyPs, ~-. x`~ p~^o_ess a y~~para~ion con-
taining a levelling agent to increase the uniformity of dye
absorption is applied to ~ha hair either be~ore or at the
same time as the substantive dye.
In addition to oxidation dyes which are formed from
oxidation dye precursors by oxidative coupling, substantive
hair dyes in particular play an important part in the dye-
ing of hair. Substantive hair dyes have the advantage that
they are used without oxidizing agents. The substantive
dyes used are, above all, nitrobenzene derivatives, for
example nitrophenylenediamines and nitroaminophenols, an-
thraquinone dyes, azo compounds and indophenols. On ac-
count of their poorer fastness properties, substantive dyes
are generally used only for temporary or se~ ermanent
hair coloring. However, an even greater d.isadvantag~ of
many substantive dyes is that they are unevenly absorbed by
the hair and, for example, color th~ ~o~~ seriously dama~d
hair ends much more intensively than the less damaged hair
roots. In addition, there ara of~ n dif~erences in color
tone between roots and ands, ~or e~am~le a shi,~ towards
blue in the case of a red dye. Accer~lngly, ~here ~as an
urgent need to improve the uniformity of hair dyeing and
the absorption of substantive dves onto the un~a~aged
region around the hair ~oots.
It has now been found that this objec~ can be achieved
by a hair dyeing process which is characterized in that a
preparation containing a 5- or 6-membered heterocyclic ring.
compound that contains in the ring a group of formulae I to
VI:
-NR1-CO-NRl- (I) -N=CR2-NRl- (IV)
-CH2-CO-NR1- (II) -N=CR2-N= (V)
-N=CR2-NRl_ (III) -N=N-NRl_ (VI)
in which R1 is hydrogen, a Cl_S alkyl group or a C2_~ hy-
droxyalkyl group and R2 is hydrogen or an NH2 group and, inaddition, the ring is closed by a difunctional hydrocarbon
radical containing 2 or 3 carbon atoms or by one of the
groups -NH-CO-, -N=CH-, -CH2-CO-, -CO-CO-, -CO-NH-CO- or
-C~I2-CO-CH2-,
as an auxiliary for improving the uniformity of dye absorp-
tion is applied to the hair before or during the applica-
tion of a substantive hair dye.
A difunctional hydrocarbon radical in the context of
the invention is preferably chosen from the ethylene, 1,2-
propylene, trimethylene, vinylene, propenylene, 1-ethanyl-
2-ylidene, 1-propanyl-3-ylidene and 1-propenyl-3-ylidene
group.
' r~
If the preparation containing the levelling agent to
be used in accordance with the invention is applied to hair
before the substantive hair dye, it is preferably applied
in the form of a water-based preparation, for example a
shampoo or a rinse, which contains the heterocyclic ring
compound, optionally together with formul~tion aids and
hair-cosmetic agents, in dissolved form. The l~velling
agent is preferably present in the preparation in a ~uan-
tity of 0.1 to 5% by weight. As further formulation aids,
the preparation may contain, in particular, water-soluble
surfactants in a quantity of 0.1 to 10~ by ~ei~ht, wat~-
soluble polymeric thickeners in a quantity of 0.1 to 2~ ~y
weight or solubilizers such as, for e~ample, lower alcohols
containing 2 to 4 carbon atoms, ethylene glycol, 1,2-pro-
pylene glycol, glycerol in ~uantities o~ 1 ~o ~G~ ~y
weight. The hair-cosmetic agents may be, for example,
quaternary ammonium compounds, cationic water-soluble pol-
ymers, water-soluble proteins, protein degradation products
or protein derivatives, pantothenic acid, vitamins, plant
extracts, saponins, or antiseborrhoeic agents and may be
present in small quantities of, in all, up to 5% by weight.
If the levelling agent to be used in accordance with
the invention is to be applied to the hair at the same time
as the substantive dye, a suitable heterocyclic ring com-
pound is directly incorporated in the hair dye.
Accordingly, the present invention also relates to a
hair-dyeing preparation containing substantive hair dyes
and dyeing auxiliaries in a cosmetic carrier which contains
as levelling agent a 5- or 6-membered heterocyclic ring
compound which bears groups of formulae I to VI in the ring
and in which the ring is closed by a difunctional hydro-
carbon radical containing 2 or 3 carbon atoms or by a group
-NH-CO-, -N=CH-, -CH2-C0-, -C0-CO-, -C0-NH-C0- or -CH2-CO-
CH2
The 5- or 6-membered heterocycles suitable as level-
ling agents in accordance with the invention are mostly
known from the literature and any derivatives thereof which
are not known from the literature may readily be synthe-
sized by standard methods for the production of such ring
systems. Heterocyclic ring compounds from the group con-
sisting of urazole, hexahydropyrimidin-2-one, 1,3-bis-(2-
hydroxyethyl)-imidazolidin-2-one, imidazol-2-one, hydan-
toin, parabanic acid, barbituric acid, pyrrolidin-2-onQ,
imidazole, 2-aminoimidazole, 1,2,4-triazole, 2-aminopyr-
imidine, 2-aminothiazoline and 1,2,3-triazole are partic-
ularly preferred levelling agents by virtue of their ready
accessibility.
Of the 5- or 6-membered heterocyclic ring compounds
mentioned above, those which contain a group of the formula
-NRl-C0-NRl-, in which Rl is hydrogen, a Cl_4 alkyl ~roup or
a C2_~ hydroxyalkyl group, are particularly preferred. The
~5 levelling agent is preferably present in the preparation in
a quantity of 0.1 to 5% by weight.
Suitable substantive hair dyes are any of the dyes
suitable for dyeing hair under physiologically acceptable
conditions, i.e. in particular dyes which are absorbed onto
hair keratin at temperatures below +40C. Such dyes are,
in particular, nitrobenzene dyes, for example nitrophenyl-
enediamines, nitroaminophenols, anthraquinone dyes, naph-
thoquinones and azo compounds. Particulars of suitable
direct dyes for dyeing hair can be found in the relevant
text books, for example in The Chemistry of Synthetic Dyes
(edited by K. Venkataraman, Academic Press, New York/Lon-
don, 1971), Vol. V, Chapter VII ("Hair Dyes"), pages 507
to 529. Examples of suitable hair dyes are 1-hydroxy-2-
amino-4,6-dinitrobenzene (picramic acid), l-hydroxyethyl-
amino-2-nitro-4-aminobenzene (H.C. Red No. 3), 1-hydroxy-
ethylamino-2-nitro-4-di-(2-hydroxyethyl)-aminobenzene(H.C.
Blue No. 2), 1-hydroxyethyl-2-nitro-4-(2-hydroxyethyl-eth-
yl)-aminobenzene (cardinal red), 1-(2'-methoxyphenylazo)-
2-hydroxy-7-trimethyl ammonium naphthalene (chloride, Basic
Red 76), 2-bromo-4,8-diamino-6-(3-trimethylammonium)-phen-
ylamino-1,5-naphthoquinone (chloride, Basic Blue 99) and 1-
(2'-sulfo-4'-methylphenyl)-amino-4-hydroxyanthraquinone,
etc.
The substantive hair dyes are typically present in
quantities of 0.05 to 5.0% by weiqht in the preparations
according to the invention for dyeing hair.
In addition to the substantive dyes, the hair dyes ac-
cording to the invention may also contain oxidation dye
precursors for toning and for producing natural color
tones. In this case, oxidative development of the oxida-
tion dye must be initiated before application to the hair
~0 by addition of a suitable oxidizing agent, for example
hydrogen pero~ide.
In addltion to the levelling agents mentioned and the
direct dyes, the substantive hair dyes according to the in-
vention may contain other auxiliaries, including in par-
ular:
- hair-cosmetic agents, for example water-soluble cat-
ionic polymers, glucose, D-panthenol, water-soluble
proteins, protein degradation products or protein
derivatives, cholesterol, vitamins, plant extracts,
saponins, or antiseborrhoeic agents; and
- pH regulators and buffers, for example triethanol-
amine, sodium citrate;
- complexing agents, for example l-hydroxyethane-l,l-
diphosphonic acid, nitrilotriacetic acid, ethylene-
diamine tetraacetic acid.
To produce the hair dyeing preparations according to
the invention, the direct dyes, the levelling agent and the
other dyeing auxiliaries according to the invention are in-
corporated in a suitable cosmetic carrier. In the most
simple case, the preparations according to the invention
ara a~ueous solutions of the substantive dyes, the level-
ling agent and othèr auxiliaries, if any. However, gels,
cream emulsions, shampoos and foam aerosols may also be
used as carriers. Preferred carriers for substantive dyes
are setting lotions, rinses and foaming aerosol prepara-
tions. .~part from water, typical constituents of such car-
riers are:
- surface-active agents, more particularly wetting ag-
ents and emulsifiers, in quantities of 1 to 20% by
weight,
- solubilizers, for example ethanol, isopropanol, 1,2-
propylene glycol, glycerol, diethylene glycol mono-
methyl ether, butyl diglycol, polyethylene glycols, in
quantities of 1 to 10% by weight,
- thic'o~ners, more particularly water-soluble polymers,
in quantities of 0.1 to 2~ by weight,
lo - preservatives, for example p-hydroxybenzoic acid
ester.
Through the ap~lication of the process according to
the inven~ion, the substantive dyes are absorbed better by
the undamaged oarts of the hair, more particularly the
lS r oo4s, ~he~_as th~ exc2ssive dyein~ of seriously damaged
parts or the hair, more particularly the ends, is consid-
erably reduced.
The following Examples are intended to demonstrate
this effect and to illustrate the invention without limit-
ing it in any way.
Bxamples
1. Product$on of tho dye preparations
Hair dyes according to the invention were prepared in
the form of aqueous solutions containing 1% by weight of
the substantive dye and 1% by weight of the levelling
heterocyclic ring compounds dissolved in water (Examples 1
and 3). For comparison, hair dyes were prepared without
addition of the levelling agent (Examples 2 and 4). Final-
ly, an aqueous solution of 1% by weight of a levelling
agent (Example 5) and a l~ solution of a substantive dye
tExample 6) were separately prepared.
2. ~air dyeing
The dye solutions of Examples l to 4 and 6 were then
applied to about 15 cm long hair strands weighing about 2
g which had been pretreated as follows:
The upper half of the hair strands (in the region of
the ends) were treated for 30 minutes with an aqueous solu-
~i;3~
tion of a cold-wave preparation based on ammonium thiogly-
colate. After fixing (10 minutes, potassium bromate solu-
tion), the same half of the hair strands was "ultra-
bleached" with an aqueous solution of hydrogen peroxide and
ammonium peroxydisulfate, followed by another treatment
w.ith the cold ~ave ~reparation, fixing and ultrableaching.
The lower half of the hair strands (root area) was ultra-
bleached only ~nce. In this way, the hair strands were
damaged to different extents in each half.
The dye ~olu~ions were then lef~ on ~he hair strands
for about 30 mlnut~s a~ 27 C ~nd subsequently washed out
with a standa~d sha~.r~oo. A~tQr ~i~.sing ~lth water, the
strands were then dried.
3. ~ r~ina' on o' ~ u~iCo.~y ol ~air dyeing by
~.aasur~i~23~ o~ a color ~ anca ~alues (DE values)
Each hair strand was measured at eight places (four in
the region of the root and four in the region of the end)
using a Datacolor color measuring system. To this end, the
sample to be measured was fixed in a clamp to a spectro-
photometer and the remission values were measured over the
visible light range of 390 to 700 nm at intervals of 10 nm
and evaluated by a computer (HP 2113 E minicomputer). The
computer program determined the standard color values under
the CIE system (Commission Internationale de l'Eclairage)
in accordance with DIN 5033 and converted them into color
difference figures in accordance with DIN 6174.
4. Hair dy~i~g in t~o st~ges
The levelling agent solution of Example 5 was applied
to a hair strand of the above-described and similarly pre-
treated type and, after loosely adhering solutions had beenstripped off and the hair dried, the dye solution of Exam-
ple 6 was applied (Examples 5 and 6). For comparison, a
hair strand was not pretreated with the solution of level-
ling agent, but instead was only treated with the dye solu-
tion of Example 6 (Example 6). The other tests were car-
ried out as in Examples 1 to 4.
~J .~ ''3'~
5. Results
The composition of the hair dye preparations and the
result of the dyeing tests are shown in Table 1. The
following compounds were used as levelling agent (hetero-
S cyclic ring compound):
Ll: urazole
L2: hexahydropyrimidin-2-one
L3: 1,3-bis-(2-hydroxyethyl)-imida~olidin-~-on~.
The following compounds were used as substantive dyes:
F1: 1-hydroxy-2-amino-~,6-dinitrob~n~er.e (picramic acid)
F2: 1-(2'-methoxyphenyla~o)-2-hyd~ox~-7-t~imethyl ammonium
naphthalene (Basic Red ~6)
F3: 1-hydroxyethylamino-2-ni~ro-~-~2-hydroxyethyl-ethyl)-
aminobenzene
The hair colors ob~ain~d ~ h dy~ scll~tions l and 3
according to the invention and by the t~o-stage process
according to the invention (5 + 6) show distinctly smaller
color differences between undamaged and damaged hair re-
gions than the hair colors obtained without levelling ag-
ents (Examples 2, 4 and 6) and no noticeable differences
in color tone.
~able 1
Composition _
(in g/100 g) 1 2 3 4 5 6 5+6
L1 1
lo L2 . 1
L3 _ _ _ 1 _ .
F1 1 1
F2 1 1 , _
~3 _ 1 -r-
l .
H20 98 99 98 99 99 99
_
Color Yel- Yel- Red Red / Red Red
- l~w low _
DE value 2.07 14.89 15.8 24.6 / 14.48 7.01
(color
difference)