Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2~67Q~9
GA E; SENSOR
FIELD OF THE INVENTION
This invention relates to gas sensors and more
particularly, to gas sensors in which interferants are removed
from the gas to be sensed.
BACKGROUND OF THE INVENTION
Active gas sensing elements, such as electrochemical
cells, gas responsive semiconductors, or catalytic combustion
elements, are conventionally contained in a housing having a
gas pervious wall that permits the gas being analyzed to reach
the sensing elements. In addition to the analyte, the gas
being analyzed may contain interferants that the sensing
elements respond to by giving a false indication of analyte
or that poison the sensing element so it does not properly
respond to the analyte. Conventionally interferants have been
removed by passing the gas through a bed of granular material
that absorbs or reacts with the interferant, such as, for
example, described in U.S. Pat. 5,633,704. An inherent 4
l~ 33/~
~ 6 problem with such filter beds is that a filter bed
sufficiently dense to efficiently remove interferants also
slows gas transfer through the filter to substantially reduce
the response of the sensor. Granular filter beds are also
. .
' ' ' , , ' , ' ' ~ ~ ~' ' , , :
.' ' ' ~. . ,~ . ' ' ' ' " ~ , . ', ' ' ' '
`` 2067~9
subject to channeling and may shed granules that contaminate
the sensor.
SUMMARY OF THE INVENTION
It is an object of this invention to provide a gas
sensor having a filter for removing interferant gas from the
gas being analyzed that exhibits both high removal efficiency
and high porosity. In accordance with this invention, a gas
sensor responsive to an analyte gas and an interferant gas
comprises a source of gas to be analyzed, a sensing element
and a filter means interposed between the source and the
sensing element, the filter means comprises a porous coherent
sinter of a fluorocarbon resin and finely divided filter
material that removes the interferant gas. On the preferred
filter, the sinter is supported and bonded to a porous
fluorocarbon membrane.
, ~
- . : .: , .- ., ,
-` 20~7~9
BRIEF DESCRIPTION OF TEIE DRAWINGS
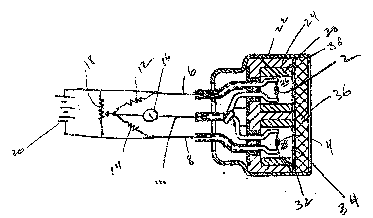
FIG. 1 is partly a section view of and partly a
schematic of a gas sensor in accordance with this invention
for measuring combustible gases.
FIG. 2 is an enlarged section of the interferant
filter means of FIG. 1.
DETAILED DESCRIPTION OF THE INVENTION
FIG. 1 shows a catalytic combustible gas detector
incorporating the new interferant filter in an otherwise
lQ conventional configuration. The gas detector comprises a
detecting element 2 and a compensating element 4 of the kind
described in U.S. Pat. 3,092,799. The detecting element is
a helical coil of platinum wire embedded in a pellet of
aluminum oxide and an oxidation catalyst, suitably palladium
or a palladium-platinum mixture. The compensating element
is a helical coil of wire embedded in a pellet that has no
catalytic activity.
The detecting and compensating elements are
electrically connected by leads 6, 8 and common lead 10 in a
bridge circuit comprising fixed resistors 12 and 14 and
21D~70~9
voltmeter 16. The meter zero can be adjusted by potentiometer
18. Power source 20 provides power for heating the detecting
and compensating elements as well as for the bridge. When the
heated detecting element is exposed to a combustible gas,
combustion increases the temperature of the element with a
consequent change of resistance. The gas does not burn on the
non-catalytic compensating element so the resistance of the
compensating element does not change. The resultant imbalance
of the bridge, indicated by meter 10, is a measure of the
combustible gas concentration. If an interfering gas reaches
the detecting element, an erroneous indication will result.
Metal housing 22 contains an inner polycarbonate
housing 24 with chambers 26 and 28 having Teflon cylindrical
liners 30 and 32. The interferant filter 34 is positioned
between the inner housing and sintered metal porous disc 36
to cover the chamber openings. The edge 38 o~ the housing 22
is formed over the edge of the disc making a tight assembly.
Referring to FIG. 2, the interferant filter includes
a gas diffusion membrane 40 having bonded thereto a filter 42
that reacts with or absorbs the interferant gas. The membrane
; 40 is preferably a Zitex or Goretex porous fluorocarbon
membrane. Other membrane materials may be used that have
- similar characteristics, includlng having a large number of
,
. .
` - 20~0~
pores, between about 40-70% porous, of small pore size (0.2
micron), and being thin enough to avoid significant increase
in the response time of the sensor to changes in the
concentration of the gas being measured. The filter 42 is
made up of a mixture of a finely divided powder filter
material mixed with a fluorocarbon resin dispersion (e.g.
Teflon dispersion). The mixture is painted onto the membrane,
dried-and sintered to form a good bond.
Catalytic elements of the type described for
measuring combustible gases are poisoned by hydrogen sulfide.
Exemplary of the invention is a sensor incorporating a filter
comprising silver that removes hydroyen sulfide thus
prolonging the life of the sensor. A Goretex expanded
polytetrafluoroethylene membrane having a O.lO" thickness, 0.7
g/cc density and 68% porosity is held between identical
toroidal flanges defining a central filter area of about 0.95
inches in diameter. A mixture of 500 mg of silver powder of
4 to 7 micron particle size with 250 microliters of Teflon 30,
an aqueous dispersion of TFE fluorocarbon resin, is spread
over the central area of the membrane. The assembly is heated
in an oven from about lOOoC to 2900C over a 45-minute period
to form a coherent, porous sinter of filter material and
fluorocarbon resin and securely bond the sinter to the
membrane. The cooled membrane is removed from the fixture and
., . .. , . . : , ,
.
2~7~
trimmed to remove the uncoated portion. The filters provide
intimate and effective contact of the filter material with gas
passing through the filter with substantially no flow
restriction that changes the response time of the element.
For example, sensors as shown in FIG.
incorporating the above described filter were exposed to (1)
air containing 50~ LEL methane to establish a sensor response
base, (2) air containing 23 ppm hydrogen sulfide for 14 hours
and then (3) air containing 50~ LEL methane to determine any
change in response. The loss of response was less than 6%.
In contrast, sensors without the interference filters, under
the same conditions, showed a loss of response from 40-60%.
The interferant filter does not restrict flow to interfere
with the normal operation of the sensor; typically the output
of the new sensor incorporating the interferant filter is 97-
99% of the output of the sensor without the interferant
filter.
It will be recognized by those skilled in the art
that this invention can be used with sensors other than the
exemplified catalytic combustible gas sensor, such as, for
example, electrochemical gas detecting elements and semi-
conductor gas det cting elements. The selection of filter
material will depend on the interferant gas to be removed and
may be an absorbent, such as activated carbon or silica ~el
,
:
, ~
~. . ' ` ' ~' ',
: .
,
2~704~
--7--
or a material that chemically reacts with and removes the
interferant. Illustrative reactants are manganese dioxide to
remove sulfur dioxide or potassium permanganate to remove
hydrogen sulfide when measuring carbon monoxide with an
electrochemical cell.
- .: , .
"
- - ~ - ~.