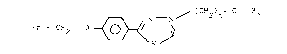Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
207306~
ves~ of ~-(hetero)aryl~ethvloxy phenyl diazole. a
method of preparinq them and use thereof in thera~y
The invention relates to new deriva.ives of 4-(hetero)
arylmethyloxy phenyl diazole, a method of preparing them
and use thereof in therapy.
More precisely, the derivatives according to the invention
have the formula:
~.--N / ( CH2)n- ~ ~ l
Ar - Cit2 - O ~W /~ (I)
in which R1 denotes C1-C4 alkyl and Ar is an aryl or
heteroaryl group chosen from among the following:
(i) R2 where R2 represents a hydrogen atom,
one or two halogen atoms, a CN, NO2 or CF3 group,
one, two or three C1-C4 alkyl or C1-Cb alkoxy groups
or an amino group substituted by two C1-C4 alkyl
groups, in which case the -W-V- chain represents
-N = N- or -O-C-
o
and n = 2-6;
(ii) pyridyl, in which case the -W-V- chain represents
-N=N- and n = 1-6.
Hereinbefore and hereinafter, the expression "C1-C4 alkyl"
denotes straight-chain or branched hydrocarbon groups
containing 1 to 4 carbon atoms, i.e. methyl, ethyl,
2~7~0~ ~
n-propyl, i-propyl, n-butyl, i-butyl, sec.-butyl or tert-
butyl; the expression "C1-C4 alkoxy" has the formula
O-(Cl-C~ alkyl) ; the term halogen denotes fluorine, chlorine
bromine or iodine~ and the term pyri~yl denotes 4-pyridyl,
3-pyridyl or 2-pyridyl.
The invention also relates to addition salts of a mineral
acid (e.g. hydrochloric or sulphuric acid) or an organic
acid (e.g. acetic, oxalic, maleic or tartric) of those
derivatives (I) which are salt-forming.
The invention also covers a method of preparing the
derivatives (I), the method comprising:
(a) condensation of compounds having the formula:
X - ( CH2 ) n ~ ORl ( I I )
where n = 2-6, R1 = Cl-C4 alkyl and X denotes a good
leaving group such as a halogen atom (chlorine,
bromine or iodine) or a mesyloxy or tosyloxy group, on
to respective compounds having the formula:
~ CH2- o ~ ~v (III)
where -W-V- denotes -N=N- or -O-g- and R2 has the same
meaning as in formula (I), in the presence of a base
such as a metal hydride, inter alia sodium hydride, in
an aprotic anhydrous solvent such as dimethyl
formamide;
(b) condensation of compounds having the formula:
2~73~
Ar - CH2 - X (IV)
where Ar has the same meaning as in formula (I) and X
has the same meaning as in formula (II), on to
respective compounds having the formula:
N N / 2 n
~0 ~ \ (V)
where R1 and n have the same meanings as in formula (I)
and -W-V- represents either -N=N- or -O-C- when Ar in
formula (IV) is a phenyl ring substituted by R2, the
latter having the same meaning as in formula (I), or
-N=N- when Ar in formula (IV) represents pyridyl,in the
presence of a base such as K2C03 or a metal hydride such
as sodium hydride, in an anhydrous solvent such as DMF
or acetonitrile;
(c) condensation of compounds having the formula:
2 CH - ~ ~ c-NH-NH-(cH2)n ORl (VI)
where n, R1 and R2 have the same meaning as in formula
(I), with phosgene in an aprotic solvent such as
dioxane, chloroform or toluene, for the purpose of
cyclisation of the -I_NH_NH- (CH2)n-OR1 chain
2 o 7 ~ ~ ~ L~
compounds (VI~ into
N - N'( 2 n
\0
The formula (V) compounds can be obtained by debenzylation
in the presence of hydrogen and a catalyst such as
palladium on carbon, of the respective formula (I)
compounds having the special structure:
~ 2 ~ /(CH2)n-ORl (Ia)
where -W-V-, R1 and n have the same meaning as in formula
(I), preferably in an alcoholic solvent such as ethanol or
methanol.
The formula (III) compounds having the special structure:
Rz 2 ~ NN~\ (IIIa)
where R2 has the same meaning as in formula (I), can be
obtained by action of sodium azide on compounds having
the formula:
~ CH2 - ~ CN (VII)
. . . ~
~ ~ ~ o ~
where R2 has the same meaning as in formula (I), preferably
by the method of R. M. HERBS~ described in J. Org. Chem.
22, 1142, 1957.
The formula (VII) compounds are obtained by treating the
compound having the formula:
H0 ~ - C~ (VIII)
with a metal hydride such as sodium hydride in an aprotic
solvent such as DMF and by reacting the resulting compound
with formula (IV) compounds in which Ar = ~ ~
R2
The formula (III) compounds having the special structure
~ CH2- 0 ~ / ~ (IIIb)
where Rz has the same meaning as in formula (I), can be
obtained by the method of FREUND and GOLDS~ITH described
in Ber. 21, 1240, 1882, by using hydrazides having the
formula:
z 2 ~ C - NH - NH2 (IX)
where R2 has the same meaning as in formula (I).
The formula (IX) compounds can be obtained by action of
hydrazine respectively on the ethyl or methyl ester of
acids having the formula:
,, ~
2~73~
2 C!~2-- {~} C - OH (X)
where R2 has the same meaning as in formula (I), in an
alcoholic medium, preferably ethanol.
The formula (VI) compounds are obtained by action of the
methyl or ethyl ester of acids of formula (X) i.n an
alcoholic medium such as ethanol or methanol, on the
respective compounds having the formula:
NH2 ~ NH - (CH2)n - ORl (XI)
where n and R1 have the same meaning as in formula (I), the
last-mentioned compounds being obtained preferably by the
method described in CA 63 : 9962.
The acid addition salts of the salt-forming derivatives of
formula (I) can be obtained in conventional manner by
action of an inorganic or organic acid on the salt-forming
derivatives of formula (I), the acids and derivatives
preferably being used in the form of miscible solutions.
The following preparations are given by way of example in
order to illustrate the invention:
Exam~le 1:
5-(4-benzyloxy phenyl) 2-methoxyethyl 2H tetrazole -
[(I) ; n = 2, R1 = CH3, -W-V- : - N=N-, Ar = phenyl]
Code No: MD 230300
2~7~
1st ste~: 4-benzyloxy benzonitrile (VII)
5 10-2 mol of NaH was added little by little to a solution
of 5 10-2 mol of 4-hydroxy benzonitrile in 100 ml DMF so as
to obtain a temperature o~ 25C. The solution was then
heated to 50C until no more hydrogen was liberated.
After cooling to 0C, 5.10-2 benzyl chloride was added.
The reaction mixture was heated to 40C for 1 hour, then
poured into 300 ml iced water. The resulting solid was
separated by filtration.
2nd step: 5-(4-benzyloxy phenyl) tetrazole [(IIIa); R2= H]
6.6 x 10-2 mol of sodium azide and 6.6 x 10-2 mol of
acetic acid were added to a solution of 5 x 10-2 mol of 4-
benzyloxy benzonitrile (VIII) in 20 ml butanol. The
reaction medium was then reflux-heated for 4 hours. 1 g
sodium azide, 2 g acetic acid and 10 ml butanol were
added and the reaction medium was again reflux-heated for
2 days. After concentration by evaporation of the solvent,
the residue was dissolved in 20 ml of 10% aqueous NaOH.
After filtration, the aqueous phase was extracted with
ether. The alkaline solution was acidified with 2 N HCl
to obtain the expected compound, which was isolated with
an 85~ yield (M.P. = 228C).
3rd step: 5-(4-benzyloxy phenyl) 2-methoxyethyl
2H tetrazole (I)
2x102 mol of the compound obtained in the second step was
added to a solution of 2x10-2 mol of sodium hydride in 50
ml D~F and the reaction medium was heated to 60 - 80C
until no more gas was evolved. After cooling, 2x10-2 mol
of 2-chloro-1-methoxyethane was added little by little.
The reaction medium was heated to 80C for 12 hours, then
2~73~6~
concentrated to two-thirds and poured on to iced water.
After extraction with ether, the expec:ted product was
obtained with a 48% yield.
M.P. = 96C
I~ (KBr, ~ cm~1) : 1610, 1450
H NMR (~ ppm, DMSOd6) : 3.2 (3H) ; 3.9 (2H) ; 5-3 (2H) ;
7.2 (2H) ; 7.4 (5H) ; 8 (2H).
The other formula (I) derivatives in which Ar =
can be obtained in the same manner, inter alia the
following:
5-[4-(4-chlorobenzyloxy) phenyl] 2-methoxyethyl
2H-tetrazole
[(I) ; -W-V- : -N=N-, R1 = CH3, n = 2, Ar = 4-chloro
phenyl]
Code No: MD 230305
Yield = 44%
M.P. = 90C
IR (KBr, ~ cm1); 1620, 1420, 1410, 1210
lH NMR (~ ppm, DMSOd6) : 3.2 (3H) ; 3.9 (2H) ; 4.9 (2H);
5.2 (2H) ; 7.2 (2H) ; 7.5 (4H) ; 8 (2H);
5-[4-(3-chlorobenzyloxy) phenyl] 2-methoxyethyl 2H
tetrazole
[(I) : -W-V-:-N=N-, R1 = CH3, n = 2, Ar = 3-chloro pheny~
Code No: MD 230324
M.P. = 92C
1H NMR (~ ppm, DMSOd6) : 3.4 (3H) ; 4 ~2H) ; 4.8 (2H);
5.l (2H) ; 7 (2H) ; 7.3 (4H) ; 8 (2H)-
207~
Exam~le 2:
5-[4-(4-chlorobenzyloxy)phenyl] 3-methoxyethyl 3H-1,3,4
oxadiazol 2-one
[(I) : -W-V-:-o-C-, R1 = CH3, n = 2, Ar = 4-chloro phenyl]
Code No: MD 230306
1st step: 1-[4-(4-chlorobenzyloxy) benzoyl] hydrazine (IX)
2.75 mol of hydrazine hydrate was added to a solution of
0.275 mol of ethyl ester of 4-(4 chloro benzyloxy) benzoic
acid (X) in 300 ml ethanol. The reaction medium was
reflux-heated for 48 hours. After cooling, the expected
product was separated by filtration and recrystallised
from ethanol.
. Yield = 63%
. M.P. = 168C
nd step: 5-[4-(4-chlorobenzyloxy) phenyl~ 3H 1,3,4-
oxadiazol 2-one (IIIb)
A solution of 0.0723 mol of phosgene in 5 ml toluene was
added to a solution of 0.0723 mol of the compound obtained
in the preceding step in 200 ml toluene. The mixture was
agitated at ambient temperature for 18 hours. After
filtration, the substance obtained was recrystallised from
butanol.
. Yield = 78%
. M.P. = 236C
rd step: 5-[4-(4-chlorobenzyloxy) phenyl] 3-methoxyethyl
3H 1,3,4-oxadiazol-2-one (I)
0.016 mol of NaH was added to a solution of 0.016 mol of
2~3~
the compound obtained in the preceding step in 50 ml DMF, followed
by heating to 60 - 80C until no more hydrogen was
evolved. After cooling, 0.016 mol of 2-chloro 1-methoxy
ethane was added dropwise, and then the reaction medium
was heated to 80C for 12 hours, then concentrated to
two-thirds and poured on to iced water.
The expected product was obtained by extraction with
ether, with a 36% yield.
. M.P. = 140~C
. IR (KBr, ~ ~m~1) : 1780, 1610, 1245, 1115, 1000.
. 1H NMR (DMSOd6) ~ ppm : 3.2 (3H) ; 3.8 (4H) ;
5.2 (2H); 7.2 (2H) ; 7.5 (4H) ; 7.7 (2H).
Example 3:
2-methoxyethyl 5-[4-(4-pyridyl methyloxy)phenyl] 2H
tetrazole hydrochloride [(I); -W-V-:-N=N-, n = 2, R1 =
CH3 , Ar = 4-pyridyl]
Code No: MD 230307
1st step: 5-(4-hydroxyphenyl) 2-methoxyethyl 2H tetrazole
[(V) ; -W-V-:-N=N-, n=2, R1 = CH3]
0.04 g of 10% palladium on carbon moistened to 50% was
added to a solution of 0.6x103 mol of compound MD 230300
in 20 ml of a mixture of methanol and methylene chloride
(50-50) and a stream of hydrogen was supplied at normal
pressure for 3 hours. After filtration and concentration,
the resulting substance was recystallised from a mixture
of ethyl ether and petroleum ether.
. Yield = 75%
. M.P. = 110C
2~3~
nd step: 2-methoxyethyl 5-[4-(4-pyridyl methyloxy)
phenyl] 2H tetrazole hydrochloride ~I)
0.0567 mol of K2CO3 and 0.0227 mol of 4-chloromethyl
pyridine were added to a solution of 0.0227 mol of the
compound obtained in the preceding step in 100 ml
acetonitrile. The reaction medium was reflux-heated for
48 hours, then concentrated and dissolved in a 0.1 N
solution of NaOH. After extraction with methylene
chloride, the organic phase was dried on sodium sulphate
and concentrated. The resulting base was dissolved in
ethanol, followed by addition of hydrochloric ethanol.
The expected hydrochloride precipitated and was
recrystallised from ethanol.
. Yield = 15%
. M.P. = 190C
. IR (KBr, ~ cm~l) : 2400, 2100, 2000, 1640, 1615, 1470,
1260, 1250, 1120
. 1H NMR (~ ppm, DMSOd6) : 3.2 (3H) ; 3.9 (2H) ; 4.9 (2H) ;
5.6 (2H) ; 7.2 (2H) ; 8 (4H) ; 8.9 (2H) ; 7.7 (lH
exchangeable).
The other derivatives (I) are obtained in the same manner,
inter alia 2-(methoxyethyl) 5-[4-(3-pyridyl
methyloxy)phenyl] 2H tetrazole [(I) ; -W-V-:-N=N-, n ~ 2,
Rl = CH3, Ar = 3-pyridyl]. Code No: MD 230308.
. M.P. = 160C
. IR (KBr, ~ cm~1) : 2550, 2100, 1615, 1550, 1420, 1405,
1260
. 1H NMR (~ ppm, DMSOd6) : 3.3 (3H) ; 4 (2H) ; 4.95 (2H) ;
5.5 (2H) ; 7.3 (2H) ; 8 (3H) ; 8.5-8.9 (3H) ; 9.1 (lH
exch).
The formula ~I) derivatives and their pharmaceutically
2 ~ 7.~
acceptable acid addition salts have been s~udied in
laboratory animals and have shown pharmacological
activity, inter alia selective activity for inhibiting
type B monoamine oxidase (MAO-B).
The activity of the compounds in inhibiting monoamine
oxidase was shown by measurements of ~AO in vitro.
The MAO activity was determined, using a mitochondrial
suspension of rat brain as a source of enzyme. The
standard method of determination consists in
pre-incubating the enzyme for 20 minutes, first in the
absence and then in the presence of the inhibitors. The
activities were determined by using serotonin (5 HT) and
phenethylamine (PEA) as substrates of MAO-A and MAO-B, the
reaction time being 40 minutes with 5 HT and 10 minutes
with PEA. The method of operation used was that of P.C.
Baker: Dev. Biol. 14, 267, 1966.
The activities of a number of compounds according to the
invention as against MA0-B and MA0-A are given via their
inhibition constants Ki(MAo-B) and Ki(MAo-A) and are shown
in the following Table.
TABLE
.
Compound testedKi (MAO-B) Ki (MAO-A)
Code Number
_
MD 2303001.56 x 10-8 M/L inactive
MD 2303051.84 x 10-7 M/L inactive
MD 2303065 x 10-9 M/L inactive
MD 2303243.5 x 10-8 M/L inactive
With regard to the toxicity of the formula (I) derivatives
and their pharmaceutically acceptable acid addition salts,
~7 ?~
after oral administration to the mouse, no toxicity was
observed for 24 hours, up to a dose of 1500 mg/kg.
The formula (I) derivatives and their pharmaceutically
acceptable acid addition salts can be used for preparation
of drugs for inhibiting type B monoamine oxidase. These
drugs are of use in therapy, inter alia for treatment of
neurological disturbances connected with pathological
ageing, disturbances of memory, mood, schizophrenia,
psychasthenia or psychic slowing-down due to ageing,
certain forms of depression and Parkinson's disease.
The drugs can be administered to man or any warm-blooded
animal in various pharmaceutical forms well known in the
art, inter alia in the form of compounds formulated for
oral, parenteral or rectal administration.
For oral administration, the compositions can be in the
form of pills, dragees or capsules, prepared by
conventional methods using known supports and excipients
such as binders, fillers, lubricants and disintegrating
agents; they may also take the form of solutions, syrup or
suspensions.
For parenteral administration, the compositions according
to the invention can be in the form of injectable
solutions, suspensions or emulsions comprising a
parenterally acceptable,oily or agueous liqui~ vehicle.
For rectal administration, the compositions can be in the
form of suppositories containing conventional bases for
suppositories.
The amount of the active principles, i.e. derivatives (I)
and their pharmaceutically acceptable acid addition salts,
2~73a~l~
1~
which can be administered depends inter alia on the way
of administration, the body weight of the patient and the
therapeutic power of the compounds used. The amount
orally administered may generally be up to 50 mg/kg of
active principle per day (in one or two doses); the
amounts for parenteral administration may be up to 5 mg/kg
of active principle per day (in one or more doses);and the
amounts for rectal administration may be up to 10 mg/kg of
active principle per day (in one or two suppositories).