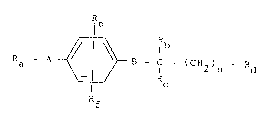Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
So12-~58/000.5~9 2 ~ 73 ~ ~ ~
Phenyla.lykyyl clerivatives
The present invention re].ates to phenylalkyl
derivatives, processes for their preparation and
pharmaceutical compositions containing them.
We have now found that certain new phenylalkyl
dexivatives have valuable and interesting
pharmacological properties in particular as angiotensin-
antagonists.
Viewed from one aspect of the invention thus
provides compounds of formula I
R - A ~ B - C - ( CH2 ) - Rd
Rf
(wherein
n represents the number O or l;
A represents a straight-chained or branched alkylene
group; -:
B represents an oxy~en atom, a carbonyl, hydroxy-
methylene, suIphenyl,~sulphinyl or sulphonyl group, a
straight-chained or branched~alkylene group, a C24-
alkylidene group, a l,l-cycloalkylene:group or an imino
group optionally substituted by an alkyl group or by a
C14-alkanoyl group;
Ra represents~a chlorine or bromine atom, a hydroxy, :
a~lkylsulphonyloxy,~phenylsulphonyloxy or phenylalkyl- . :
sulphonyloxy`~group~:or~a~group of the formula ;~
:
,~
- 2 - 2~73~1
R2
~---É~
N
1~ N ~,, E~-R
R 2
,. , ~ _
R3
N E-R~
R 2~
~ N~ :
3 X
N - N
3 ~ ~E-R
`
` 3 \ ~ :
X--<~ ~ E-R~
N
R3 : :
; : : : :
~: ~
-- 3 - 2~73~1
R ;~
f~3 t~
~j/ \
o
R ~3~ N E- R ~
D6~D or
7 IN
D -
~: N
D 3
':
~wherein on~ of the groups D1, D2 and D3 represents a
methylene or imino:group and the remaining groups of D1,
D2 and:D3 are methine groups, whilst additionally a
methine group:may be~replaced by a nitrogen atom and one
of the methine groups may be~substituted by a group~:Rs
and, optionally, another of the:methine groups may be
substltuted by a~group R4;
:
2~73~3
none, one or two of the groups D," D5, D6 an~ D7 represent
a nitrogen atom and the remaining groups of D4, Ds~ D6
and D7 each represent methine groups, whilst additionally
a methine group may be substit~lted by a group R~ and
another methine group may be substituted by a group Rs;
E represents a carbon-carbon bond, an oxygen or sulphur
atom, a hydroxymethylene or carbonyl group or an imino
group optionally substituted by a C16-alkyl group, by a
cycloalkyl group, by a C~5-alkanoyl group or by an
allyl, phenyl or benzyl group;
X represents an oxygen or sulphur atom or an imino group
optionally substituted by an alkyl, phenyl or
phenylallcyl group;
R1 represents a straight-chained or branched C19-alkyl,
C26-alkenyl or C26-alkynyl group optionally substituted
by a cycloalkyl group, by a fluorine, chlorine or
bromine atom, or by a hydroxy, amino, alkylamino,
dialkylamino or ~,~-difluoroethane group, or R1 may
represent a C14-perfluoroalkyl group or a cycloalkyl
group, optionally mono- or disubstituted by a
trifluoromethyl group or by an alkyl group;
R2 represents a hydrogen, fluorine, chlorine or bromine
atom, a C15-alkyl or -perfluoroalkyl group or a cyano or
nitro group;
R3 represents a hydrogen atom, a cyano group, a C16-alkyl
group optionally substituted by a hydroxy or alkoxy
group, a Cl6-per~luoroalkyl group, a C36-alkenyl group
optionally substituted by fluorine atoms, a phenylalkyl
or phenyl(C24)alkenyl group, or a C1s-alkyl group which
is terminally substituted by an imidazol-l-yl,
tetrazolyl, phthalimido, R6COO-, R7S-, R7SO-, R7CO-,
R NHCOO-, R7NHCO-, R7NHCONR7-, R8CONR7- or R8S02NR7 group
,: : ' :
::
2~73~
-- 5
by a triazolyl group itself optionaly mono- or
disubs~itu-ted by an acetoxy or alkyl group;
R6 represen-ts a Cl~-alkyl or -perfluoroalkyl g~oup, or a
cycloalkyl, phenyl, benzyl, phenylethyl, adamantyl,
naphthyl, naphthylmethyl or naphthylethyl group;
R7 represents a hydrogen atom or a group R6;
R8 represents a group R7 or a pi.perazino group optionally
substituted by an alkyl or phenyl group in the 4-
position, or R~ represents a py:rrolidino, piperidino,
hexamethyleneimino, morpholino, R70- or (R7)2N-group;
R4 represents a hydrogen, fluorine, chlorine or bromine
atom, a trifluoromethyl group, a cycloalkyl group, or a
C16-a].kyl group optionally substituted by a cycloalkyl,
hydroxy, alkoxy, alkylamino, dialkylamino,
alkoxycarbonyl, alkylaminocarbonyl or dialkylamino-
carbonyl. group; and
R5 represents a hydrogen, ~luorine, chlorine or bromine
atom, a straight-chained or branched C16-alkyl or C16
-per~luoroalkyl group, a C26-alkenyl or C26-alkynyl
-~. group, in which the above-mentioned alkyl and alkenyl
moieties may each be mono- or disubstituted by a
heteroaryl, hydroxy, alkoxy, amino, alkylamino,
dialkylamino, alkylcarbonylamino, N-alkyl-
alkylcarbonylamino, carboxy, alkoxycarbonyl,
alkylcarbonyloxy, piperidinocarbonyl,
morpholinocarbonyl, aminocarbonyl, alkylaminocarbonyl,
dialkylaminocarbonyl, tetrazol-5-yl, tetrazol-5-yl-
aminocarbonyl, alkylsulphenyl, alkylsulphinyl,
alkylsulphonyl, aminosulphonyl, alkylaminosulphonyl,
dialkylamirlosulphonyl, alkylsulphonylaminocarbonyl,
heteroarylaminosulphonyl or alkylcarbonylaminosulphonyl
group, or
. ' ~` ' . :
,
' '~: ' . ' ' : ' '
. .
2~73g~
-- 6
Rs represents a C17-alkoxy yroup substi-tuted in the 2-,
3-, ~-, 5-, 6- or 7-position by an imidazolyl,
tetrazolyl, benzimldazolyl or -tetrahydrobenzimidazolyl
group, or a phenylalkoxy group optionally substituted in
the alkoxy moiety by a l~l-tetrazol-5-yl or 1-
triphenylmethyl-tetrazol-5-yl yroup, or
a carboxy group or a group which is metabolically
converted into a carboxy group ln vivo, or
a C14-alkylsulphony].oxy group, a benzenesulphonyloxy or
phenylalkanesulphonyloxy group, or
\
an acylam.ino group optionally substituted at the
nitrogen atom by a Cl6-alkyl group or by a phenyl,
cycloalkyl, phenylalkyl, cycloalkylalkyl, bicyclohexyl
or biphenyl group, wherein the acyl moiety is a C17-
alkanoyl group, an alkoxycarbonyl group with a total of
2 to 4 carbon atoms, a C16-alkylsulphonyl group, a
benzoyl, benzenesulphonyl, phenylalkanesulphonyl,
naphthylenesulphonyl, cycloalkylcarbonyl, phenylalkanoyl
or cycloalkylalkanoyl group, in which the above-
mentioned phenyl nuclei may each be mono- or
disubstituted by a fluorine, chlorine or bromine atom or
-- by a methyl or methoxy group and the substituents may be
identical or di~ferent, or
a phthalimino, homophthalimino, 2-carboxyphenylcarbonyl-
amino or ~-carboxyphenylmethylamino group, in which a
carbonyl group in a phthalimino group may be replaced by
a methylene, alkyl-methylene or dialkyl-methylene group
and a methylene group in a homophthalimino group may be
substituted by one or two alkyl groups, wherein the
phenyl nuclei in any of said groups may be additionally
mono- or disubstituted by alkyl or alkoxy groups
optionally wholly or partially hydrogenated, in which
the substituents may be the same or different, or
;, : .: ' ,' ' . : , . . .
`~ ! , ' . . ., : . : .,
.,; ~. . . . . .
2~3~
a 5-, 6- or 7-membered alkyleneimino or alkenyleneimino
group optionally substituted by one or two alkyl groups
or by a tetramethylene pentamethylene group, wherein a
methylene yroup may be replaced by a carbonyl or
sulphonyl group, or
a bicycloalkane-2,3-dicarboxylic acid imino or
bicycloalkene-2,3-dicarboxylic acid imino group, wherein
the bicycloalkane and bicycloalkene moieties each
contain 9 or 10 carbon atoms, may be substituted by 1, 2
or 3 methyl groups and an endomethylene group may be
replaced by an oxygen atom, or
a glutaric acid imino group in which the n-propylene
group may be per~luorinated, may be substituted by one
or two alkyl groups or by a tetramethylene or
pent~methylene group, or
a maleic acid imino or succinimido group optionally
mono- or disubstituted by an alkyl or phenyl group,
wherein the substituents may be identical or difEerent,
or
a 5-membered heteroaromatic ring bound via a carbon atom
--- or via an imino group, which contains an imino group, an
oxygen or sulphur atom, or which contains an imino group
and an oxygen, sulphur or nitrogen atom, or R5 represents
a 6-membered heteroaromatic ring bound via a carbon atom
which contains 1 or 2 nitrogen atoms, which 5- or 6-
membered heteroaromatic ring is attached via two
adjacent carbon atoms to an n-propylene, n-butylene or
1,3-butadienyl group or via an imino group and an
adjacent carbon atom an n--propylene, n-butylene or 1,3-
butadienyl gxoup and, in an anellated pyridine ring thus
formed, a methine group may be replaced by a nitrogen
atom and a vinylene group in the 3-, 4-position relative
to the nitrogen atom of the pyridine ring formed may be
,., ~ , . . .
.
, . ~ . . . . .
. ~
.
,
.~ , ,
~33~
replaced by a su:Lphur atom or, in an anellated phenyl
riny thus formed, one or two methine yroups may be
replaced by nitrogen a~oms, whils-t addl.tionally the
above-mentioned fused aromatic or heteroaromatic rinys
may be monosubstikuted in the carbon s-tructure by a
~luorine, chlorine or bromine atom, or by an alkyl,
alkoxy, hydroxy, phenyl, nitro, amino, alkylamino,
dialkylamino, alkanoylamino, cyano, carboxy,
alkoxycarbonyl, aminocarbonyl, alkylaminocarbonyl,
dialkylaminocarbonyl, trifluoromethyl, alkanoyl,
aminosulphonyl, alkylamino sulphonyl or
dialkylaminosulphonyl group or may be disubstituted by
fluorine or chlorine atoms or by methyl, methoxy or
hydroxy yroups, and any -NH- yroup present in an
imidazole ring may be substituted by a C16-alkyl group
or by a cycloalkyl group, or
a pyrrolidine, piperidine or pyridine ring bound via a
carbon atom, wherein a phenyl group may be fused onto
the pyridine ring via two adjacent carbon atoms and a
methylene gr~up adjacent to the nitrogen atom in a
pyrrolidine or piperidine ring may be replaced by a
carbonyl group, or
an imidazolidinedione group optionally substituted by an
alkyl, phenylalkyl, tetramethylene, pentamethylene or
hexamethylene group, or
a pyridazin-3-one or dihydro-pyridazin-3-one group
optionally substituted in the 2-position by an
optionally phenyl-substituted alkyl group, and ~ith the
carbon structure additionally optionally substituted by
1 or 2 alkyl groups, or
an R~1-NR~o-CO-NR9 group
(wherein
.
2~P~3~
_ 9 _
R9 represents a hydro~en atom, a Cl~-alkyl group or a
phenylalkyl group,
R~o represents a hydrogen a~om, a C18-alkyl group, a C3 5-
alkenyl group, a phenylalkyl group or a C57-cycloalkyl
yroup, and
R11 represents a hy~rogen atom or a C16-alkyl group, an~
one of the yroups R9, R10 and R1l may also represent a
bicyclohexyl or hiphenylyl group, and
R~o and Rl1 together with the nitro~en atom between them
may represent a straight-chained C46-alkyleneimino group
or a morpholino group, and
R9 and R11 together may represent a C24-alkylene group));
Rb represents a cyano, trifluoromethylcarbonylamino,
trifluoromethylcarbonylaminomethyl, trifluoromethyl-
sulphonylamino, trifluoromethylsulphonylaminomethyl,
alkylsulphonylamino, alkylsulphonylaminomethyl,
arylsulphonylamino, arylsulphonylaminomethyl,
aralkylsulphonylamino, aralkylsulphonylaminomethyl,
arylsulphonylaminocarbonyl, benzylsulphonylamino-
carbonyl, sulpho, aminosulphonyl, alkylaminosulphonyl,
aralkylaminosulphonyl, arylaminosulphonyl,
alkylcarbonylaminosulphonyl, aralkylcarbonylamino-
sulphonyl, arylcarbonylaminosulphonyl, sulphomethyl,
aminosulphonylmethyl, alkylaminosulphonylmethyl,
aralkylaminosulphonylaminomethyl, arylaminosulphonyl-
methyl, alkylcarbonylaminosulphonylmethyl, aralkyl-
carbonylaminosulphonylmethyl, arylcarbonylamino-
sulphonylmethyl, phosphino, O-alkyl-phosphino, O-
aralkyl-phosphino, O-aryl-phosphino, phosphono, O-alkyl-
phosphono, O-aralkyl-phosphono, O-aryl-phosphono, 0,0
dialkylphosphono, phosphono-methyl, O-alkyl-phosphono-
.~., . . ~ ~ . , - . . . . . .
- : - ~ : .
, , ,
,:
1. : ~ : , . . . .
2~3~
-- 10 --
me-thyl, O-aralkyl--phosphono-methy:l, O-aryl-phosphono-
methyl, O,O-dialkylphosphono-methyl, phosphato, O-alkyl-
phosphato, O-aralkyl-phosphato, O-aryl-phosphato or O,o-
dialkoxy-phosphoryl group, a lH-tetrazolyl, lH-
tetrazolylalkyl, lH-tetrazolylaminocarbonyl or triazolyl
group optionally substituted by an alkyl,
trifluoromethyl, phenylallcyl or triphenylmethyl group,
or an alkylsulphonylaminocarbonyl or perfluoro-
(C16alkyl-sulphonylaminocarbonyl group, a carboxy group,
a group which is metabolically converted into a carboxy
group ln vivo, or an aralkoxycarbonyl group;
Rc represents a hydrogen atom, an alkyl, aralkyl, aryl,
carboxy or alkoxycarbonyl ~roup;
Rd represents a strai~ht-chained or branched C110-alkyl
chain, a straight-chained or branched C210-alkenyl or
C210-alkynyl group, a cycloalkyl or cycloalkylalkyl
group, a phenyl group optionally mono- or disubstituted
by ~luorine, chlorine or bromine atoms or by methyl or
methoxy groups, or a biphenyl, naphthyl or heteroaryl
group;
Re and Rf represent hydrogen atoms and if Rs represents a
--- phthalimino, homophthalimino, 2-carboxyphenylcarbonyl-
amino or 2-carboxyphenylmethylamino group, whilst a
carbonyl group in a phthalimino group may be replaced by
a methylene, alkyl-methylene or dialkyl-methylene group
and a methylene group in a homophthalimino group may be
substituted by one or two alkyl groups, wherein the
phenyl nuclei in any of said groups may be additionally
mono- or disubstituted by alkyl or alkoxy groups
optionally wholly or partially hydro~enated, in which
the substituents may be the same or different, or
a carboxy group or a group which is metabolically
converted into a carboxy group in vivo, or
: . ~ ': .' ', . ..
- ~ , :
, : , : ~ '" " ' " ` ."' ~'
~: :
2~73~
a 5-, 6- or 7-membered alkyleneimino or alkenyleneimino
group op-tionally substituted by one or two alkyl ~roups
or by a tetramethylene or pentamethylene group, wherein
a methylene group of the alkyleneimino or
alkenyleneimino group may be replaced by a carbonyl or
sulphonyl group, or
a bicycloalkane-2,3-dicarboxylic acid imino or
bicycloalkene-2,3-dicarboxylic acid imino group, wherein
the bicycloalkane and bicycloalkene moieties may each
contain 9 or 10 carbon atoms and may be substituted by
1, 2 or 3 methyl groups and an endomethylene group may
be replaced by an oxygen atom, or
a glutaric acid imino group wherein the n-propylene
group may be perfluorinated or substituted by one or two
alkyl groups or by a tetramethylene or pentamethylene
group, or
a maleic acid imido or succinimido group optionally
mono- or disubst.ituted by an alkyl or phenyl group, in
which the substituents may be identical or different, or
a 5-membered heteroaromatic ring bound via a carbon atom
or via an imino group and containing an imino group, an
oxygen or sulphur atom or containing an imino group and
an oxygen, sulphur or nitrogen atom, or a 6-membered
heteroaromatic ring bound via a carbon atom and
containing 1 or 2 nitrogen atoms, wherein the 5-or 6-
membered heteroaromatic ring is attached via two
adjacent carbon atoms to an n-propylene, n-butylene or
1,3-butadienyl group or via an imino group and an
adjacent carbon atom to an n-propylene, n-butylene or
1,3-butadienyl group, and in the anellated pyridine ring
thus formed, a methine group may be replaced by a
nitrogen atom and a vinylene ring in the 3~ position
relative to th~e nitrogen atom of the pyridine ring
- ,
2~3~.
- 12 -
~ormed may be replaced by a sulphur atc)m or, in ~n
anellated phenyl ring -thus formed, one or two methine
groups may be replace~ by nitroyen atoms, whils-t
ad~itionally the above-mentioned fused aromatic or
heteroaromatic rings may be monosubstituted in the
carbon skeleton by a fluorine, chlorine or bromine atom
or by an alkyl, alkoxy, hydroxy, phenyl, nitro, amino,
alkylamino, dialkylamino, alkanoylamino, cyano, carbo~y,
alkoxycarbonyl, aminocarbonyl, alkylaminocarbonyl,
dialkylaminocarbonyl, trifl.uoromethyl, alkanoyl,
aminosulphonyl, alkylaminosulphonyl or dialkylamino-
sulphonyl group or may be disubstituted by fluor.ine or
chlorine atoms or by methyl, methoxy or hydroxy groups
and any -NH- group present in an imidazole ring may be
substituted by a C16-alkyl group or by a cycloalkyl
group, or
a pyrrolidine, piperidine or pyridine ring bound via a
carbon atom, in which a phenyl group may be condensed
onto the pyridine ring via two adjacent carbon atoms and
a methylene group adjacent to the nitrogen atom in a
pyrrolidine or piperidine ring may be replaced by a
carbonyl group, or
an imidazolidinedione group optionally substituted by an
alkyl, phenylalkyl, tetramethylene, pentamethylene or
hexamethylene group, or
a pyridazin-3-one or dihydro-pyridazin-3-one group
optionally substituted in the 2-position by an
optionally phenyl-substituted alkyl group and
additionally optionally substituted in the carbon
skeleton by 1 or 2 alkyl groups, or
an R11-NR10-CO-NR9 group (wherein R9, R10 and R11 are
defined as hereinbefore),
: ~
.
2 ~
- 13 ~
then Re may also represent a hydrogen~ fluorine, chlorine
or bromine atom or an alkyl or alkoxy group, and
Rf may also represent a f]uorine, chlorine or bromine
atom or an alkoxy group;
wherein, unless otherwise stated, any alkyl, alkylene or
alkoxy moiety contains 1 to 4 carbon atoms and any
cycloalkyl moiety contains 3 to 7 carbon atoms'
any aryl group, un].ess otherwise specified is a phenyl
group optionally mono- or di-substituted by a fluorine,
chlorine or bromine atom or by a hydroxy, alkyl, alkoxy,
phenylalkoxy, phenyl, nitro, amino, alkylamino,
dialkylamino, alkanoylamino, cyano, carboxy,
alkoxycarbonyl, aminocarbonyl, alkylaminocarbonyl,
dialkylaminocarbonyl, trifluoromethyl, alkanoyl,
aminosulphonyl, alkylaminosulphonyl or dialkylamino-
sulphonyl group, wherein each alkyl moiety may contain 1
to 4 carbon atoms, or a naphthyl group,
any heteroaryl group, unless otherwise specified is a 5-
membered heteroaromatic ring which contains an imino
group, an oxygen or sulphur atom, or contains an imino
group and an oxygen, sulphur or nitrogen atom, or
contains an imino group and 2 or 3 nitrogen atoms, and a
6-membered heteroaromatic ring which contains 1, 2 or 3
nitrogen atoms, whilst tha above-mentioned rings may
additionally be mono- or di-substituted by a fluorine,
chlorine or bromine atom or by an alkyl, alkoxy,
hydroxy, phenyl, nitro, amino, alkylamino, dialkylamino,
alkanoylamino, cyano, carboxy, alkoxycarbonyl,
aminocarbonyl, alkylaminocarbonyl, dialkylaminocarbonyl,
triflu~romethy:l, alkanoyl, aminosulphonyl,
alkylaminos~lphonyl or dialkylamino-sulphonyl group)
and the isomer mixtures, tautomers, enantiomers and
.
, , ' ! . , ' ~ '
2~3~1
salts thereof, more part:icularly for pharmaceutical use
the physiologically acceptable salts thereof with
inorganic or organ.ic acids or bases.
The new compounds of formula I above wherein Ra
represents a chlori.ne or bromine atom, a hydroxy,
alkylsulphonyloxy, phenylsulphonyloxy or
phenylalkylsulphonyloxy group, are valuable intermediate
products and the other compounds of formula I above and
the physiologically acceptable salts thereof have
va.luable pharmacological properties, being angiotensin-
antagonists, especially angiotensin-II-antagonists.
The present invention thus relates to the new
above-mentioned phenylalkyl derivatives, whilst the
corresponding O-substituted phosphono or phosphato
compounds and the corresponding cyano, alkoxycarbonyl or
triphenylmethyl compounds are also valuable intermediate
products.
The present invention thus also relates to new
pharmaceutical compositions containiny the above-
mentioned pharmacologically active compounds of formula
I or corresponding physiologically acceptable salts
thereof which are particularly suitable for treating
h~pertension and cardiac insufficiency, also for
treating ischaemic peripheral circulatory disorders,
myocardial ischaemia (angina), for preventing the
progression of cardiac insuf~iciency after myocardial
infarction, for treating diabetic nepthropath~,
glaucoma, gastrointestinal disorders and diseases of the
bladder.
Particularly important compounds according to the
invention include those of formula I wherein Ra~ Rbj Rc,
Rd, R~, Rf, A, B and n are as hereinbefore defined with
the proviso that
D4, Ds~ D6 and D7 represent methine groups with the
additional provisos (a) to (j), or
. ~
- : - ,: ,
.
,
- 15 - 2~73~
D7 represents a nitrogen atom ancl the yroups D~" D5 and D6
represent methine groups wi-th the ad~itional provisos
(a) to (g) and (k) to (m) or (a) to (g) and (n) to (p),
or
one of the groups D4, D5 and D6 represents a nitrogen
atom and the remaining groups of groups D4, Ds~ D6 and D7
represent methine groups, or
two of the groups D4, Ds~ D6 and D7 represent nitroyen
atoms and the remaining groups of groups D4, Ds~ D~ and D7
represent methine groups,
with the additional provisos that either
(a) n represents the number 1, or
(b) Æ has the meanings given for E he.reinbefore with the
exception of the carbon-carbon bond, or
(c) A has the meanings given for A hereinbefore with the
exception of the methylene group, or
~d) B has the meanings for B given hereinbefore with the
exception of the oxygen atom, or
(e) Rb has the meanings given for Rb hereinbefore with
the exception of the carboxyl group, or
(f) Rc has the meanings given for Rc hereinbefore with
the exception of the hydrogen atom, or
(g) Rd has the meani.ngs given for Rd hereinbefore with
the exception of the phenyl group, or
(h) Rl has the meanings given for R1 hereinbefore with
the exception of the n-butyl group, or
'
~ ~ :
.; . ' : : .
2~3g~1
- 16 -
(i) R, has -the meanings given for R4 hereinbefore with
the except.ion of the methy~ group in position 7, or
(j) R5 has the meanings given for Rs hereinbefore wi.th
the exception of the hydroyen atom, or
(k) R1 has the meanings given for R1 hereinbefore with
the exception of the ethyl group, or
(1) R4 has the meanings given for R4 hereinbefore with
the exception of the methyl group in position 7, or
(m) Rs has the meanings given for Rs hereinbefore with
the exception of the methyl group in position 5, or
(n) R1 has the meanings g.iven for R1 hereinbefore with
the exception of the n-propyl group, or
(o) R4 has the meanings given for R4 hereinbefore with
the exception of the hydrogen atom, or
(p) R5 has the meanings given for R5 hereinbefore with
the exception of the hydrogen atom, and
the remaining groups have the meanings given
hereinbefore,
whilst additionally a methine group mentioned in the
above definitions of groups D4, D5, D6 and D7 may be
substituted by a group R4 and a further methine group
ment~oned in the above definitions of groups D4, D5, D6
and D7 may be substituted by a group Rs;
and the isomers and salt thereof.
Examples of the heteroaromatic groups mentioned
hereinbefore in the definitions of groups D4, Ds/ D6 and
- , . . .
:~ .: . . . .. :, -
.. , , ~ - . : , ,
,
,
, .:
:
.
2 ~
- 17 -
D7 include the pyri~o, pyrlmido, pyrazino or pyridazino
groups which may be suhstituted in the carbon skeleton
by the groups R4 and R5.
Examples of groups which are metabolically
converted into a carboxy group ln vivo, in the compounds
of the invention include, for example, the esters of
formulae
-CO-OR',
-CO-O-(HCR'')-O-CO-R''I and
-CO-O-(HCR")-O-CO-OR"'
(wherein
R' represents a straight-chained or branched C16-alkyl
group, a Cs7-cycloalky]. group, a benzyl, 1 phenylethyl,
2-phenylethyl, 3-phenylpropyl, pivaloyloxymethyl,
phthalidylme~hyl, (1,3-dioxa-2-oxo-4-methyl-cyclopenten-
5-yl)-methyl, methoxymethyl or cinnamyl group,
R" represents a hydrogen atom or a methyl group and
R"' represents a straight-chained or branched C16-alkyl
group, a Cs7-cycloalkyl group, a phenyl, benzyl, 1-
phenylethyl, 2-phenylethyl or 3-phenylpropyl group).
-- Preferred compounds according to the in~ention
include those of formula I wherein
n represents the number 0 or 1;
A represents a straight-chained or branched alkylene
group;
B represents an oxygen atom, a straight-chained or
branched C13-alkylene group or an imino group optionally
substituted by an alkyl group or by a C14-alkanoyl
group;
Ra represents a group of the formula
.
2~7~
-- 18 --
?
2 ~ ~
.~ _
R3
E - R
3 X
~"
o\ or
4 ~r
D7 N
:
. ,.... . ~ . . : ~
. ~ .
2~3~
-- 19 --
(whereirl
none, one or two o~ the groups D,,, Ds~ D6 ~nd D7
represents a nitro~en atom and the remaining groups of
D" Ds~ D6 ~nd D7 each represent methine groups, whilst
additionally a methine group may be substituted by the
group R4 and a fur-ther methine group may be substituted
by the group Rs;
E represents a carbon-carbon bond, an oxygen or sulphur
atom or an imino group optionally substituted by a C1~,~
alkyl group;
.
X represents an oxygen or sulphur atom:
Rl represents a straight-chained or branched C16-alkyl
group, a straight-chained or branched C26-alkenyl or
C26-alkynyl group, whilst the above-mentioned saturated
and unsaturated alkyl moieties may each be substituted
by a fluorine, chlorine or bromine atom or by a hydroxy
or amino group, or a C36-cycloalkyl group;
R2 represents a hydrogen, fluorine, chlorine or bromine
ato~, a C1s-alkyl or C15-perfluoroalkyl group or a cyano
or nitro group;
.
R3 represents a hydrogen atom, a cyano group, a C13-alkyl
group optionally substituted by a hydro~y or alkoxy
group, a C13-perfluoroalk~l group, a C36-alkenyl group
optionally substituted by fluorine atoms, a Cl3-alkyl
group which is substituted in the terminal position by
an R6COO-, R7S-, R7SO-, R7S02-, R7CO-, R7NHCOO-, R7NHCO-,
R7NHCONR7-, R8CONR7- or R8SO2NR7-group
(wherein
R6 represents a C14-alkyl or C14-perfluoroalkyl group or
a cycloalkyl, phenyl, benzyl or phenylethyl group;
-,,
: .,
- .-'
' : ` .. ~' ' ' '
:~:'" : ~ '. ' : ,.
: ' . :
2~3~ 1
- 20
R7 represents a hydroyen atonl or a group R6; and
R8 represents a group R7);
R4 represents a hydrogen, fluorine, chlorine or bromine
atom, a trifluoromethyl group, a cycloalkyl ~roup, a
C16-alkyl group optionally substituted by a hydroxy,
alkoxy or alkoxycarbonyl group; and
represents a hydrogen, ~luorine, chlorine or bromine
atom, or
a straight-chained or branched C16-alkyl or C16-
perfluoroalkyl group, a C~6-alkenyl or C26-alkynyl group,
whilst the above-mentioned alkyl and alkenyl moieties
may each be mono- or di-substituted by a het~roaryl,
hydroxy, alkoxy, amino, alkylamino, dialkylamino,
alkylcarbonylamino, N-alkyl-alkylcarbonylamino, carboxy,
alkoxycarbonyl, alkylcarbonyloxy, aminocarbonyl,
alkylaminocarbonyl, dialkylaminocarbonyl or tetrazol-5-
yl group, or
a C1s-alkoxy group or an ~-(lH-tetrazol-5-yl)-benzyloxy
group, or
,- .
a carboxy group or a group which is metabolically
converted into a carboxy group in vivo, or
a C14-alkylsulphonyloxy group or a benzenesulphonyloxy
or phenylalkylsulphonyloxy group, or
an acylamino group optionally substituted at the
nitrogen atom by a C16-alkyl group or by a phenyl,
cycloalkyl, phenylalkyl or cycloalkylalkyl group, in
~: which the acyl group is a C1s-alkanoyl group, an
alkoxyca:rbonyl group with a total of 2 to 4 carbon
atoms, a C16-alkylsulphonyl group, a benzoyl,
:: ~
.
.
:
:
2~73$~1
- 21 -
benzenesulphony], cycloalkylcarbonyl, phenylalkanoyl or
cycloalkylalkanoyl group, wh.ilst the above-mentioned
phenyl nuclei may each be ~ono- or di-substituted by a
fluorine, chlorine or bromine atom or by a methyl or
methoxy group and the substituents may be identical or
different, or
a phthalimino or homophthalimino group, wherein a
carbonyl group in a phthalimino group may be replaced by
a methylene, alkyl-methylene or dialkyl-methylene group,
and a methylene group in a homophthalimino group may be
substituted by one or two alkyl groups wherein the
phenyl nucle.i in any Oe said groups may be additionally
mono or disubstituted by alkyl or alkoxy groups
optionally wholly or partially hydrogenated, in which
the substituents may be the same or different, or
a 5-, 6- or 7-membered alkyleneimino group optionally
substituted by one or two alkyl groups or by a
tetramethylene or pentamethylene group, wherein a
methylene group o~ the alkyleneimino group may be
replaced by a carbonyl or sulphonyl group, or
a maleic acid imido or succinimido group optionally
mono- or disubstituted by an alkyl or phenyl group,
wherein the substituents may be identical or dif~erent,
or
a 5-membered heteroaromatic ring bound via a carbon atom
or via an imino group, which contains an imino group, an
oxygen or sulphur atom or which contains an imino group
and an oxygen, sulphur or nitrogen atom, or a 6-membered
heteroaromatic ring bound via a carbon atom which
contains 1 or 2 nitrogen atoms, which 5- or 6-memb~red
he~eroaromatic ring is connected via two adjacent carbon
atoms to an n--propylene, n~butylene or 1,3-butadienyl
group or via an imino group and an adjacent carbon atom
-- - , :
.
2~3~1
- 22 -
to an n-propylene, n-hutylene or 1,3-butadienyl group
and, in an anellate~ pyridine ring thus formed, a
methine group may be replaced by a nitroyen atom and a
vinylene group in the 3-, 4-po6ition relative to the
nitrogen atom of -the pyridine ring ~`ormed may be
replaced by a sulphur atom or, in an anellated phenyl
ring thus formed, one or two methine groups may be
replaced by nitrogen atoms, whilst additionally the
above-mentioned fused aromatic or heteroaromatic rings
may be monosubstituted in ~he carbon structure by a
fluorine, chlorine or bromine atom or by an alkyl,
alkoxy, hydroxy, phenyl, nitro, amino, alkylamino,
dialkylamino, alkanoylamino, cyano, carboxy,
alkoxycarbonyl, aminocarbonyl, alkylaminocarbonyl,
dialkylami.nocarhonyl, trifluoromethyl, alkanoyl,
aminosulphonyl, alkylamino-sulphonyl or
dialkylaminosulphonyl group or may be disubstituted by
~luorine or chlorine atoms or by methyl, methoxy or
hydroxy groups, and any NH- group present in an
imidazole ring may be substituted by a C16-alkyl group,
or
an R11-NRlo-CO-NR~ group (wherein
R9 represents a hydrogen atom, a C16-alkyl group or a
phenylalkyl group,
R1o represents a hydrogen atom, a C16-alkyl group, a
phenylalkyl group or a C57-cycloalkyl group,
R-1 represents a hydrogen atom or a C16-alkyl group, or
R10 and R11 together with the nitrogen atom between them
represent a straight-chained C46-alkyleneimino group or
a morpholino group, or
~ and R11 together represent a C24-alkylene group));
.
,:.. .
.
2~ ~3~ ~
-- 23 -
Rb represents a cyano, carboxy, arylsulphonylamino-
carbonyl, benzylsulphonylaminocarbonyl, sulpho,
alkylcarbonylaminosu]phonyl, a:ralkylcarbonylamino-
sulphonyl, arylcarbonylaminosulphonyl, alkylcarbonyl-
aminosulphonylmethyl, aralkylcarbonylaminosulphonyl-
methyl, arylcarbonylaminosulphonylmethyl, phosphino, o-
alkyl-phosphino, phosphono, O-alkyl-phosphono, 0,0-
dialkylphosphono, phosphono-methyl, O-alkyl phosphono-
methyl, O,O-dialkylphosphono-methyl, phosphato or 0-
alkyl-phosphato group, a lH-tetrazolyl or lH-tetrazolyl-
alkyl group optionally subst.ituted by a phenylalkyl or
triphenylmethyl group, an (C16alkyl)sulphonylamino-
carbonyl or perfluoro(C16alkyl)sulphonylaminocarbonyl, a
group which is metabolically converted into a carboxy
group ln vivo, or an aralkoxycarbonyl group;
Rc represents a hydrogen atom, a methyl, phenyl, benzyl,
carboxy or alkoxycarbonyl group;
R~ represents a straight-chained or branched C16-alkyl
group, a straight-chained or branched C26-alkenyl or
C26alkynyl group, a cycloalkyl or cycloalkylalkyl group,
a phenyl group optionally mono- or disubstituted by
fluorine, chlorine or bromine atoms or by methyl or
-- methoxy groups, or a biphenyl, naphthyl or heteroaryl
group;
Re and Rf represent hydrogen atoms or, if
R5 represents a phthalimino or homophthalimino group,
wherein a carbonyl group in a phthalimino group may be
replaced by a methylene, alkylmethylene or
dialkylmethyl~ne group and a methylene group in a
homophthalimino group may be substituted by one or two
alkyl groups, wherein the phenyl nuclei in any of said
groups may be additionally mono- or disubstituted by
alkyl or alkoxy groups optionally wholly or partially
' ' ' " ' '' ' ~ ' '' '" ' `;
.. : : '
.
~73~ L
- 2~ -
hydrogenated, in which the sub~ti-tuents may be the same
or di~ferent, or
a carboxy group or a group which is metabolically
converted into a carboxy group ln VlVo, or
a 5-, 6- or 7-membered alkyleneimino group optionally
substituted by one or two alky:L groups or by a
tetramethylene or pentamethylene group, wherein a
methylene group of the alkyleneimino group may be
replaced by a carbonyl or sulphonyl group, or
, ~ .
a maleic acid imido or succinimido group optionally
mono- or di-substituted by an alkyl or phenyl group, the
substituents being identical or different, or
a 5-membered heteroaromatic ring bound via a carbon atom
or via an imino group, which contains an imino group, an
oxygen or sulphur atom or which contains an imino group
and an oxygen, sulphur or nitrogen atom, or a 6-membered
heteroaromatic ring bound via a car~on atom which
contains 1 or 2 nitrogen atoms, which 5- or 6-m~mbered
heteroaromatic ring is attached via two adjacent carbon
atoms to an n-propylene, n-butylene or 1,3-butadienyl
group or via an imino group and an adjacent carbon atom
to an n-propylene, n-butylene or 1,3-butadienyl group
and, in an anellated pyridine ring thus formed, a
methine group may be replaced by a nitrogen atom and a
vinylene group in the 3-, 4-position relative to the
nitrogen atom of the pyridine ring formed may be
replaced by a sulphur atom or, in an anellated phenyl
ring thus formed, one or two methine groups may be
replaced by nitrogen atoms, whilst additionally ~he
above-mentioned fused aromatic or heteroaromatic rings
may be monosubstituted in the carbon structure by a
fluorine, chlorine or bromine atom or by an alkyl,
alko~y, hydroxy, phenyl, nitro, amino, alkylamino,
~ ,
: , : , , . . :
; ' . ~
,: ' ~ ' . ' , ,
-~ ' ' , : ,.
. .
.
.
2~3~1
- 25 -
diallcylamino, alkanoylamino, cyano, carboxy,
alkoxycarbonyl, am:inocarbonyl, alkylami.nocarbonyl,
dialkylaminocarbonyl, tri~luoromethyl, alkanoyl,
aminosulphonyl, alkylamino-sulphonyl or
dialkylaminosulphonyl group or may be disubstituted by
fluorine or chlorine atoms or by methyl, methoxy or
hydroxy groups, and any -NH- group present in an
imidazole ring may be substituted by a C16~alkyl group,
or
an R11-NR10-CO-NR~ group wherein R9 to R11 are defined as
hereinbefore,
then Re may also represent a hydrogen, ~luorine, chlor:ine
or bromine atom or an alkyl or alkoxy group and
Rf may also represent a fluorine, chlorine or bromine
atom or an alkoxy group;
whilst unless otherwise specified groups which are
converted metabolically into a carboxy group in vivo are
as defined hereinbefore,
any alkyl, alkylene or alkoxy moiety contains 1 to 4
carbon atoms and any cycloalkyl moiety contains 3 to 7
carbon atoms,
any aryl group, unless otherwise specified is a phenyl
group optionally mono- or disubstituted by a fluorine,
chlorine or bromine atom or by a hydroxy, alkyl, alkoxy,
phenylalkoxy, phenyl or nitro group, wherein each alkyl
moiety may contain ~rom 1 to 4 carbon atoms, or a
naphthyl group, and
any heteroaryl group, unless otherwise specified is a 5-
membered heteroaromatic ring which contains an imino
group, an o~ygen or sulphur atom or which contains an
- . , .
!. ~ ~ ` ' ! . .
.~: `. ' ' ` ': , ` . ' '
. .
' .: ' ' :. '
,
2~3~
imino group and an oxygen, sulphur or nitrogen atom or
which contains an imino group and 2 or 3 nitrogen atoms,
and a 6-membered heteroaromatic ring which contains 1, 2
or 3 nitrogen atoms, whilst the above-mentioned rings
may additionally be mono- or d:isubstituted by a
fluorine, chlorine or bromine atom or by an alkyl,
alkoxy, hydroxy, phenyl or nitro group]
the isomer mixtures, the tautorners, enantiomers and
salts thereof, with the exclusion of the following
compounds:
(i) 2-n-butyl-1-[4-[(~-carboxy)benzyloxy]benzyl]-
benzimidazole,
(ii) 2-n-propyl-7-methyl-3-[4-[(~-carboxy)benzyloxy]-
benzyl]imidazo[4,5-b]pyridine and
(iii) 5,7-dimethyl-2-ethyl-3-[4-~(~-carboxy)benzyloxy]-
benzyl]imidazo[4,5-b]pyridine and their salts.
Particularly preferred compounds according to the
invention include those of formula I wherein
` n represents the number O;
A denotes a methylene, ethylene or ethylidene group;
B denotes an oxygen atom, a methylene, imino,
methylimino or acetylimino group;
Ra denotes a group of the formula
3 ~,"~ E - R
N
,: . - ,:, . . :
. .
:: , , ~ : . - ,
`, '' ' ~
~73~
N ~ E- 1
N~
o
or
15 ~ \~E-R
D7 N
(wherein
none, one or two of the groups D4, D5, D6 and D7
represents a nitrogen atom and the remaining groups of
D4, Ds~ D6 and D7 each represent methine groups, whilst
additionally a methine group may be substituted by a
group R4 and another methine group may be substituted by
a group R5;
E represents an oxygen atom or a carbon-carbon bond;
R1 represents a straight-chained or branched C16-alkyl
group; :
-
R2 represents a hydrogen, fluorine, chlorine or bromine
atom;
~ '
: ~: ' ;' ' ' '.' ' . ' . ~ ~ ' ' . '
2~73~
R3 represents a hydroxyalkyl group h~vi.ng l or 2 carbon
atoms;
R4 represents a hydroyen atom or a C13-alkyl group; and
R5 represents a hydrogen, fluorine, chlorine or bromine
atom, or
a straight-chained or branched C13-alkyl group or an ~-
(lH-tetrazol-5-yl)-benzyloxy group, or
an amino or nitro group, or
a carboxy group or a (C13-alkoxy)carbonyl group, or
an acylamino group optionally substituted at the
nitrogen atom by a C15-alkyl group, wherein the acyl
yroup is a Cl5-alkanoyl group or a benzenesulphonyl
group, the above-mentioned phenyl nucleus optionally
being mono- or di-substi-tuted by a methyl or mekhoxy
group and the substituents b~ing identical or different,
or
a 5-, 6- or 7-membered alkyleneimino group optionally
substituted by one or two methyl groups, wherein a
methylene group of the alkyleneimino group may be
replaced by a carbonyl or sulphonyl group, or a 2,3-
dimethylsuccinimido, phthalimino, homophthalimino or
isoindolin-1-on-yl group, or
a benzimidazol-2-yl group optionally substituted in the
1-position by a C13-alkyl group, or
an R~1-NR10-CO-NR9 group (wherein
R9 represents a hydrogen atom, a C1s-alkyl group or a
phenylalkyl group having 1 to 3 carhon atoms in the
alkyl moiety,
2~7~
- 29 -
R10 represents a hydrogen a-tom, a C1s-alkyl group or a
cyclohexyl group,
R11 represen-ts a hydrogen atom, a benzyl group or a C1s-
alkyl yroup or
R9 and R1.l together represent a C23-alkylene group));
Rb represents a carboxy, lH-tetrazolyl or O-alkyl-
phosphono or alkylsulphonylaminocarbonyl group haviny 1
to 3 carbon atoms in the alkyl moiety;
Rc represents a hydrogen atom or a phenyl group;
Rd represents a straight-chained or branched C14-alkyl
group, a cyclohexyl, cyclohexylmethyl, phenyl, biphenyl,
methoxyphenyl, chlorophenyl, pyridyl or naphthyl group;
Re and Rf represent hydrogen atoms or, if
R5 represents a benzimidazol-Z-yl group optionally
substituted in the 1-position by a C13-alkyl group, or
a 2,3-dimethylsuccinimino group, or
, ~ .
a 5 , 6- or 7-membered alkyleneimino group optionally
substituted by one or two methyl groups, wherein a
methylene group may be replaced by a carbonyl or
sulphonyl group, or
an R11-NR10-CO-NR~ group, wherein R9 to R11 are defined as
hereinbefore,
then Re may also represent a hydrogen, chlorine or
bromine atom or a methoxy group and
Rf:may also represent a chlorine or bromine atom or a
- , ~ :
:
,,
: ~ :
~7~3-~ 1
- 30 -
methoxy group,
the isomer mixtures, the tautomers, en~ntiomers ~nd
sal~s thereof, with -the exclusi.on of -the following
compound~:
(i) 2-n-butyl-1-[4-[(~~carboxy)benzyloxy]benzyl]-
benzimidazole,
(ii) 2-n-propyl-7-methyl-3-[~-[(~-carboxy)benzyloxy]-
benzyl]imidazo[4,5-b]pyridine and
(iii) 5,7-dimethyl-2~ethyl-3-[4-[(~-carboxy)benzyloxy]-
benzyl]imidazo[4,5-b]pyridine, and thelr salts.
The present invention particularly relates to the
following compounds of formula I:
(a) 2-n-propyl-4-methyl-1-[4-[(~-carboxy)benzyloxy]-
benzyl]benzimidazole;
(b) 2-n-butyl-1-[4-[(~-carboxy)benzyloxy]benzyl]-6-
dimethylaminocarbonylamino-benzimidazole;
(c) 2-n-propyl-6-(1-methyl-benzimidazol-2-yl)-4-methyl-
[4-~(~-carboxy)benzyloxy]benzyl]benzimidazole;
(d) 2-methyl-4-[4'-[(~-carboxy)benzyloxy]benzyloxy]-
quinoline;
(e) 2-n-butyl-8-methyl-3-[4-[(~-carboxy)benzyloxy]-
benzyl]quinazolin-4-one semihydrate;
(f) 2-n-propyl-4-methyl-6-(1-methyl-benzimidazol-2-yl)-
1-[4-[~-(lH-tetrazol-5-yl)benzyloxy]benzyl]-
benzimidazole;
(~) 2-n-butyl-6-(N-propionyl-methylamino)-1-[4-[(1-
.
.
; ~
, . :
.~ .
., ~ .
2~73~
3l
carboxy-3-methyl)butyloxylbenzyl]benzimidazole;
(h) 2-n-butyl-5-methyl-6-(3-benzyl-3,4,5,6-tetrahydro-
2(1~l)-pyrimidinon-1-yl)-3-[~-[~-(lH-tetrazol-5~yl)-
benzyloxy]benzyl]imidazol[4,5-b]pyridine; and
(i) 2-n-butyl-1-[4-[~ -ethyl--phosphono)benzylamino]-
benzyl]benzimidazole;
and the isomers, isomer mixtures, the tautomers,
enantiomers and salts thereof.
Viewed from a further aspect the invention also
provides a process for the preparation of compounds of
the invention, said process comprising at ]east one of
the following steps:
a) reacting a compound of formula II
Ra H (II)
(wherein
Ra is as hereinbefore defined) with a compound of formula
III
Z1 ~ A ~ C-(C12) -Rd (III)
Rf
:::
(wherein
A, B, n, Rb, Rc, Rd, Re and Rf are as hereinbefore defined
and
Z1 represents a nucleophilic leaving group such as a
halogen atom, e.g. a chlorine, bromine or iodine atom,
. ~. . . .
-
:~' ': ~ ` , :
~3~
- 32 -
or a substituted sulphonyloxy group, e.g. a
methanesulphonyloxy, phenylsulphonyloxy or p-toluene-
sulphonyloxy group) optionally followed by hydrolysis;
b) reacting a compound of formula IV
~ ~ / (IV)
`., Rf
(wherein
R~, Re~ Rf and A are as hereinbefore defined,
B' represents a skraight-chained or branched Cl4-
alkylene group and
Z2 represents a nucleophilic leaving group such as a
halogen atom, e.g. a chlorine, bromine or iodine atom,
or a substituted sulphonyloxy group, e.g. a
methanesulphonyloxy, phenylsulphonyloxy or p-
toluenesulphonyloxy group) with a compound of formula V
Rb
I
CH -- ( CH2 ) n ~ Rd ( V )
Rc
(wherein
Rb, Rd and n are as hereinbefore defined and
Rc' represents an alkoxycarbonyl group) if necessary with
subsequent hydrolysis and/or decarboxylation;
c) (to prepare compounds of formula I wherein B
represents an oxygen or sulphur atom or an optionally
alkyl-substituted imino group) reacting a compound of
formula VI
:
-
~ .
2 ~
~ (VI)
Rf
(whereinRa~ Re~ R~ and A are as hereinbefore defined and
B" represents an oxygen or sulphur atom or an optionally
alkyl-substituted, eg. C16 alkyl substituted, imino
group) with a compound of formula VII
El~b
Z3 - C - (CH2) n ~ Rd (VII)
Rc
(wherein
Rb, Rc, Rd and n are as hereinbefore defined and
Z3 represents a nucleophilic leaving group such as a
halogen atom, e.g. a chlorine, bromine or iodine atom,
or a substituted sulphonyloxy group, e.g. a
methanesulphonyloxy, phenylsulphonyloxy or p-toluene-
sulphonyloxy group) if necessary with subsequent
hydrolysis;
d) (to prepare a compound of formula I wherein Rb
represents a carboxy group) converting a compound of
formula VIII
R - A ~ s - c (cH~ - Rd
(VIII)
:: :
~ :
2 ~1 ~ 3 ~
- 3~ -
(wherein
~, Rc, Rd, Re, Rf, A, ~ and n are as hereinbefore defined
and
Rb' represents ~ group which may be converted into a
carboxy group by hydrolysis, thermolysis or
hydrogenolysis) into a compound of formula I by
hydrolysis, thermolysis or hydrogenolysis;
e) (to prepare a compound of formula I wherein Rb
represents a lH-tetrazolyl group) cleaving a protective
group -from a compound of formula IX
e
K - A ~ ~ B - C ( C H 2 ) ~ ( I X )
R
Rf c
(wherein
Ral Rc~ Rd, Rel Rf, A, B and n are as hereinbefore defined
and
Rb" represents a lH-tetrazolyl group protected in the 1-
or 2-position by a protecting group);
f) (to prepare a compound of formula I wherein Rb
represents a lH-tetrazolyl group) reacting a compound of
formula X
Re
~ CN
2a ~ A ~ `~ B - C (CH ) 2 (X)
R~
(wherein
Ra~ Rc~ Rd, Rel Rf, A, B and n are as hereinbefore
defined) with hydrazoic acid or a salt thereof;
.. . . . .
. :': . '~ ' ' . ' ' ' ' :
,
2~3~ ~
- 35 -
g) (to prepare compoun~s of formula I wherein Ra
represents a group of -the forrnula
~D9 N
D ~ ~ ~ E~
7 N
cyclisi.ng a compound of formula XI
~D X1
D5 ~ (XI)
i)
(wherein
D1, D2, D3, D4, Ds/ D6 and D7 are as hereinbefore defined,
one of the groups X1 or Y1 represents a group of formula
e
- 11r/ - A -~3 B - C ~CHz ~ - Rd
R
Rf
and the other group X1 or Y1 represents a group of
formula
Z4~ ~Zs
- NH - C - E - R
(wherein
R1, A, B, E, n, Rb, Rc, Rd, Re and Rf are as hereinbefore
defined, Z4 and Zs~ which may be identical or different,
represent optionally substituted amino groups or hydroxy
or mercapto groups optionally substituted by lower alkyl
(eg. C1~6aIkyl) group_ or
... ..
, . . .
.,
2 ~ 7 ~3 ~
- 36 -
Z4 and Zs toge-ther represent an oxygen or sulph~r atom,
an imino group optionally substituted by a C1~-alkyl
group, or an alkylenedioxy or alkylenedithio group each
having 2 or 3 carbon atoms));
h) (to prepare compounds of formula I wherein Rs
represents an R11-NR10-CO-NR9 group) reacting a compound
of formula XII
a A ~ 1 2 n Rd (XII)
r Rc
R~
(wherein
Rb, Rc, Rd, Re~ Rf, A, B and n are as hereinbefore defined
and
Ral represents a group Ra as defined hereinbefore wherein
Rs is an amino group, a C1~-alkylamino group or a
phenyl(C14alkyl)amino group) with a compound of formula
XIII
R10
N - CO ~ Z6 (XIII)
R11
(wherein
R~o represents a hydrogen atom, a C18-alkyl group, a C3 5-
alkenyl group, a phenylalkyl group or a Cs7-cycloalkyl
group,
R11 represents a hydrogen atom or a C16-alkyl group, or
one of the groups R10 or R11 may also represent a
bicyclohexyl or biphenylyl group, or
R10 and R11 together with the nitrogen atom between them
,
. ' :', : ~
2~73~
- 37 -
represent a s-traight-chained C46-alkyleneimino group or
a morpholino group, an~
Z6 represents a nucleophilic leaving group such as a
chlorine or bromine atom or, if one of the groups R1o or
R11 represents a hydrogen atom,
Z6 together with ~10 or R11 represents a nitrogen-carbon
bond);
i) (to prepare compounds of formula I wherein Rs
represents an acylamino group optionally substitu~ed at
the nitrogen atom by a C16-alkyl group or by a phenyl,
cycloalkyl, phenylalkyl, cycloalkylalkyl, bicyclohexyl
or biphenyl group, wherein the acyl group is a C~ 7-
alkanoyl group, a C24-~alkoxycarbonyl) group, a C1-6-
alkylsulphonyl group, a benzoyl, benzenesulphonyl.,
phenylalkanesulphonyl, naphthalenesulphonyl,
cycloalkylcarbonyl, phenylalkanoyl or cycloalkylalkanoyl
group, whilst the above-mentioned phenyl nuclei may each
be mono- or di-substituted by a fluorine, chlorine or
bromine atom, by a methyl or methoxy group and the
substituents may be identical or different, a
phthalimino ~r homophthalimino group wherein the phenyl
nucleus in each case may be mono- or di-substituted by
C13-alkyl or C13-alkoxy groups, or a maleic acid imido or
succinimido group optionally mono- or di-substituted by
an alkyl or phenyl group, wherein the substituents may
be identical or different) acylating a compound of
formula XIV
Ra" - A ~ B - c - (CH2~n d (XIV)
Rf
(wherein
,.. :
~ ` "'' '" '"
.
2 0 r~ 3 ~ ~ l
-- 3~ --
Rb, RC~ RCl, Rc~ Rf, A, B and n ar.e as he:reinbefore defined
and
R~" represents a group R~ as he:re:inbefore defined wherein
Rs represents an amino group optionally substituted by a
C16-alkyl group or by a phenyl, cycloalkyl, phenylalkyl,
cycloalkyalkyl, bicyclohexyl or biphenyl yroup) with a
compound of formula XV
HO - U - R12 (XV)
(wherein
U represents a carbonyl or sulphonyl group and
R12 represents a C16-alkyl group, a C13-alkoxy group, a
phenyl, phenylalkyl, naphthyl or cycloalkyl group,
wherein the above-mentioned phenyl nuclei may each be
mono- or disubstituted by a fluorine, chlorine or
bromine atom or by a methyl or methoxy group and the
substituents may be idPntical or different, or, if U
represents a carbonyl group, R12 may also represent a
hydrogen atom, a phenyl group which is substituted in
the o-position by a carboxy or carboxymethyl group and
wherein the phenyl nucleus may be mono- or di-
substituted by C~3-alkyl or C13-alkoxy groups and may
additionally be wholly or partially hydrogenated, a 2-
carboxy-ethyl or 2-carboxy-ethenyl group in which the
ethyl and ethenyl moiety may each be mono- or di-
substituted by an alkyl or phenyl group)
or with a reactive derivative thereof such as an acid
halide, acid ester or acid anhydride thereof;
j) to prepare compounds of formula I wherein Rb
represents a phosphono or O-alkyl-phosphono group)
hydrolysing a compound of formula XVI
Re
~ (XVI)
:: ' , : ~ :;
.
' . ~
2~ ~3~
- 39 -
(wherein
A, B, Ra~ Rc, Rd, R~, Rf and n are as hereinbefore define~
and
Rb"' represents an O-alkyl- or O,O-dia:l.kyl-phosphono
group in whlch each alkyl moiety may ~ontain 1 to 5
carbon atoms);
k) resolving a compound of formula I by isomer
separation into the isomers or enantiomers thereof;
1) converting a compound of formula I into a sal.t
thereof, more particularly for pharmaceutical use into a
physiologically acceptable salt thereof with an
inorganic or organic acid or base, or converting a salt
of a compound of formula I into the free compound; and
m) performing a process as defined in any one of steps
(a) to (1) above on a corresponding protected compound
and subsequently removing the protecting group used.
The reaction of step (a) is conveniently carried
out in a solvent or mixture of solvenks such as
methylene chloride, diethylether, tetrahydrofuran,
dioxane, dimethylsulphoxide, dimethylformamide or
benzene, optionally in the presence of an acid binding
agent such as sodium carbonate, potassium carbonate,
sodium hydroxide, potassium tert.-butoxide,
triethylamine or pyridine, whilst the latter two may
simultaneously also be used as solvent, preferably at
temperatures between O and 100C, e.g. at temperatures
between ambient temperature and 50C.
The subsequent hydrolysis is appropriately carried
out either in the presence of an acid such as
hydrochloric acid, sulphuric acid, phosphoric acid,
trichloroacetic acid or trifluoroacetic acid or in the
presence of a base such as sodium hydroxide or potassium
hydroxide in a suitable solvent such as water,
, . , ~ . .
, : : ~ : : . .
:
: ~ :
2~3~
-- ~o --
water/meth~nol, ethanol, wa-ter/ethanol,
water/isopropanol or water/dioxane a~ ternperatures
between -10C and 120C, e.g. at -temperatures between
ambient temperature and the boiliny te~perature of the
reaction mixture.
In the reaction, a mixture of possible isomers is
preferably obtained which may subsequelltly, if desired,
be resolved into the corresponcling isomers, preferably
by chromatography, using a carrier such as silica gel or
aluminium oxide.
The reaction o~ step (b) is conveniently carried
out in a solvent or mixture o~ solvents such as
methylene chloride, diethylether, tetrahydrofuran,
dioxane, dimethylsulphoxide, dimethylformamide or
benzene, optionally in the presence of an acid binding
agent such as sodium carbonate, potassium carbonate,
sodium hydroxide, potassium tert.-butoxide,
triethylamine or pyridine, whilst the latter two may
simultaneously also be used as solvent, preferably at
temperatures between 0 and 100C, e.g. at temperatures
between ambient t0mperature and 50C.
The optional subsequent hydrolysis is appropriately
carried out either in the presence of an acid such as
hydrochloric acid, sulphuric acid, phosphoric acid,
-~ trichloroacetic acid or trifluoroacetic acid or in the
presence of a base such as sodium hydroxide or potassium
hydroxide in a suitable solvent such as water,
water/methanol, ethanol, water/ethanol,
water/isopropanol or water/dioxane at temperatures
between -10C and 120C, e.g. at temperatures between
ambient temperature and the boiling temperature of the
reaction mixture.
The optional subsequent decarboxylation is
conveniently carried out in the presence of an acid such
as hydrochloric acid, sulphuric acid, phosphoric acid,
acetic acid, t:richloroacetic acid or trifluoroacetic
acid in a suitable solvent such as water,
, : ' . '
i . . .
2~73~1
water/methanol, ethanol, water/ethanol,
water/isopropanol or water/dioxane ~t te~peratures
between 50~C and 120C, e.g. at temperatures between
60~c and the boiling tempe~ature of the reaction
mixture.
The reaction o~ step (c) is conveniently carried
out in a solvent or mixture of solvents such as
methylene chloride, diethylether, tetrahydrofuran,
dioxane, dimethylsulphoxide, dimethylformamide or
benzene, optionally in the pres~ence of an acid binding
agent such as sodium acetate, sodium carbonate,
potassium carbonate, sodium hydroxide, potassium tert.-
butoxide, triethylamine or pyridine, whilst the latter
two may simultaneously also be used as solvent,
preferably at temperatures between 0 and lOO~C, e.g. at
temperatures between ambient temperature and the boiling
temperature of the reaction mixture.
The optional subsequent hydrolysis is appropriately
carried out either in the presence of an acid such as
hydrochloric acid, sulphuric acid, phosphoric acid,
trichloroacetic acid or trifluoroacetic acid or in the
presence of a base such as sodium hydroxide or potassium
hydroxide in a suitable solvent such as water,
water/methanol, ethanol, water/ethanol,
water/isopropanol or water/dioxane at temperatures
between -10C and 120C, e.g. at temperatures bPtween
ambient temperature and the boiling temperature o~ the
reaction mixture.
In step (d) functional derivatives of the carboxy
group such as the optionally substituted amides, esters,
thiolesters, orthoesters, iminoethers, amidines or
anhydrides, the nitrile group or the tetrazolyl group
may be converted by hydrolysis into a carboxy group,
esters with tertiary alcohols, e.g. the tert.butylester
group, may be converted by thermolysis into a carboxy
group and esters with aralkanols, e.g. the benzylester,
may be converted by hydrogenol.ysis into a carboxy group.
- : .
~ ' : ': '
2~73~ 1
- ~2 -
The hy~rolysis of step (d) is appropriately carried
out either :in the presence of an acid such as
hydrochloric aci~, sulphuric aci~, phosphoric acid,
trichloroacetic acid or trifluoroacetic acid or in the
presence of a base such as sodium hydroxide or potassium
hydroxide in a suitable solvent such as water, water/
methanol, ethanol, water/ethanol, water/isopropanol or
water/dioxane at temperatures between -10C and 120C,
preferably at temperatures between ambient temperature
and the boiling temperature of the reaction mixture.
When hy~rolysis is carried out in the presence of an
organic aci~ such as trichloroacetic acid or
trifluoroacetic acid, any alcoholic hydroxy yroups
present may simultaneously be converted into a
corresponding acyloxy group such as the trifluoroacetoxy
group.
If Rb' in a compound of formula VIII represents a
cyano or aminocarbonyl group, these groups may also be
converted into the carboxy group with a nitrite, e.g.
sodium nitrite, in the presence of an acid such as
sulphuric acid, which may simultaneously also be used as
solvent, at temperatures between 0 and 50C.
If Rb' in a compound of formula VIII represents for
example a tert.-butyloxycarbonyl group, the tert.-butyl
group may also be thermally cleaved, optionally in an
inert solvent such as methylene chloride, chloroform,
benzene, toluene, tetrahydrofuran or dioxane and
preferably in the presence of a catalytic amount of an
acid such as trifluoroacetic acid, p-toluenesulphonic
acid, sulphuric acid, phosphoric acid or polyphosphoric
acid, preferably at the boiling temperature of the
solvent used, e.g. at temperatures between 40C and
100 C .
If Rb' in a compound of formula VIII represents a
benzyloxycarbonyl group, for example, the benzyl group
may also be hydrogenolytically cleaved in the presence
of a hydrogenation catalyst such as palladium/charcoal
,
,
- ' ~
.:
2~73~
- ~3 -
in a suitable solvent such as methanol, eth~nol,
ethanol/water, glacial ace-tic acid, ethyl acetate,
dioxane or dimethylformamide, preferably at temperatures
between 0 and 50C, e.g. at ambient temperature, under a
hydrogen pressure of 1 to 5 bar. During hydrogenolysis,
other ~roups may be reduced at the same time, e.g. a
nitro group may be reduced to the amino group, a
benzyloxy group to the hydroxy group, a vinylidena group
to the correspondiny alkylidene group or a cinnamic acid
group to the corresponding phenyl-propionic acid group,
or they may be replaced by hydrogen atoms, e.g. a
halogen may be replaced by a hydrogen atom.
Suitable protecting groups for use in step (e)
include, for example, the triphenylmethyl, tributyl tin
or triphenyl tin groups.
The cleaving o-f a protecting group used in step (e)
is preferably carried out in the presence of a
hydrohalic acid, preferably in the presence of
hydrochloric acid, in the presence of a base such as
sodium hydroxide or alcoholic ammonia in a suitable
solvent such as methylene chloride, methanol,
methanol/ammonia, ethanol or isopropanol at temperatures
between 0 and 100C, but preferably at ambient
temperature or, if the reaction is carried out in the
presence of alcoholic ammonia, at elevated temperatures,
e.g. at temperatures between 100 and 150C, preferably
at temperatures between 120 and 140C.
The reaction of step (f) is preferably carried out
in a solvent such as benzene, toluene or
dimethylformamide at temperatures between 80 and 150C,
preferably at 125C. Appropriately, either the
hydrazoic acid is liberated during the reaction from an
alkali metal azide, e.g. sodium azide, in the presence
o~ a weak acid such as ammonium chloride or the
tetrazolide salt obtained in the reaction mixture during
the reaction with a salt of hydrazoic acid, preferably
with aluminium azide or tributyl tin azide, which is
:
2 ~ L
also preferably produced in the reaction mixture by
reactiny aluminium chlori~e or tributyl tin chlori~e
with an alkali metal azide such as sodium azide, is
subsequently libera-ted by acidification with a dilute
acid such as 2N hydrochloric or 2N sulphuric acid.
The cyclisation of step (g) is conveniently carried
out in a solvent or mixture of solvents such as ethanol,
isopropanol, glacial acetic acid, benzene,
chlorobenzene, toluene, xylene, glycol, glycol-
monomethylether, diethyleneglycoldimethylether,
sulpholane, dimethylformamic~e, tetraline or in an excess
of the acylating agent used to prepare the compound of
formula XI, e.g. in the corresponding nitrile,
anhydride, acid halide, ester or amide, e.g. at
temperatures between 0 and 250C, but preferably at the
boiling temperature of the reaction mixture, optionally
in the presence of a condensing agent such as
phosphorusoxychloride, thionylchloride,
sulphurylchloride, sulphuric acid, p-toluenesulphonic
acid, methanesulphonic acid, hydrochloric acid,
phosphoric acid, polyphosphoric acid, acetic anhydride
or optionally in the presence of a base such as
potassium ethoxide or potassium tert.-butoxide.
However, cyclisation may also be earried out without a
- solvent and/or condensing agent.
However, it is partieularly advantageous to carry
out the reaction by preparing a eompound of formula XI
in the reaction mixture by reducing a corresponding o-
nitro-amino compound, optionally in the presence of a
carboxylic acid of formula Ra-E-COOH, or by acylation of
a corresponding o-diamino compound. When the reduction
of the nitro group is broken off at the hydroxylamine
stage, the N-oxide of a compound of formula I is
obtained in the subsequent cyclisation. The resulting
N-oxide is then converted by reduction into a
corresponding compound of formula I.
The subsequent reduction of the N-oxide of formula
' - ': '. '............... ; - .
- . . ~
2 ~ 7 ~
- ~5 -
-L obtained is preferably carried out in a solvent such
as water, water/ethanol., methanol, glacial acetic acid,
ethyl acetate or dimethylformamide with hydrogen in the
presence of a hydrogenation catalyst such as Raney
nic]cel, platinum or palla~ium/c:harcoal, with metals such
as iron, tin or zinc in the presence of an acid such as
acetic acid, hydrochloric acid or sulphuric acid, with
salts such as iron(II)sulphate, tin(II)chloride or
sodium dithionite, or with hydrazine in the presence of
~aney nickel at temperatures between 0 and 50C, but
preferab y at ambient temperature.
The reaction of step (h) is preferably carried out
in a solvent such as tetrahydrofuran, dioxane, ethylene
chloride or benzene, optionally in the presence of an
acid-~inding agent such as triethylamine or pyridine,
expediently at temperatures between 0 and 100C,
preferably at temperatures between 20 and ~0C.
Examples of reactive derivatives of a compound of
formula XV usd in step (i) include the esters thereof
such as the methyl, ethyl or benzylester, the thioesters
thereof such as the methylthio or ethylthioester, the
halides thereof such as the acid chloride and the
anhydrides or imidazolides thereof.
The reaction of step (i) is conveniently carried
out in a solvent or mixture of solvents such as water,
methylene chloride, chloroform, ether, tetrahydrofuran,
dioxane or dimethylformamide with a corresponding
carboxylic acid in the presence o* an acid activating or
dehydrating agent such as thionylchloride, with the
anhydrides thereof such as acetic acid anhydride, with
the esters thereof such as ethyl acetate, with the
halides thereof such as acetylchloride or
methanesulphonyl chloride, optionally in the presence of
an inorganic or tertiary organic base such as sodium
hydroxide, potassium carbonate, triethylamine or
pyridine, whilst the latter two may simultaneously also
be used as solvent, at temperatures between -25 and
~ ' ' , .
- .: . : .
.
: : :
2~73~
- ~6 -
lOO'C, but preferably at temperatures between 10 ~nd
800C.
The reaction of step (j) is pre~erably carried out
in a solvent such as methanol, methanol/water, ethanol
or isopropanol in order to prepare a corresponding 0-
alkylphosphono compound of formula I in the presence of
a base such as potassium hydroxide or potassium
methoxide or in order to prepare a corresponding
phosphono compound of ~ormula I in the presence of an
acid such as hydrobromic acid at the boiling temperature
of the reaction mixture.
It is particularly advantageous to prepare a
phosphono compound of formu].a I by reacting a
corresponding O-alkyl or O,O-dialkyl compound of formula
XVI with bromine/t.rimethylchlorosilane in acetonitrile
with subsequent hydrolysis using water at ambient
temperature.
If according to the invention a compownd of formula
I is obtained wherein R2 or ~5 represents a nitro group,
this may be converted by reduction into a corresponding
compound of formula I wherein R2 or Rs represents an
amlno group, or
if according to the invention a compound o~ formula I is
obtained wherein B represents an imino group, this may
be c~nverted by alkanoylation into a corresponding
compound of formula I wherein B represents an imino
group substituted by a C14-alkanoyl group, or
if according to the invention a compound of formula I is
obtained wherein B represents an imino group, this may
be converted by alkylation into a corresponding compound
of formula I wherein B represents an imino group
substituted by a Cl3-alkyl group, or
i~ according to the invention a compound of formula I is
obtained wherein Rb or Rb and Rs each represent a carboxy
- ~ . , , , , :
2~17~
- ~7 -
group, this may be conver-ted by esterifica-tion into a
corresponding compound of formula I wherein Rb or Rb and
Rs represent a group which is me-tabolically converted 1n
v o into a carboxy yroup, or
if according to the invention a compound of formula I is
obtained wherein Rb represents a carboxy group, thi~ may,
after conversion into a corresponding acid halide, be
converted by reaction with a corresponding sulphonamide
into a compound of formul.a I wherein Rb represents an
alkanesulphonylaminocarbonyl, perfluoroalkanesulphonyl-
aminocarbonyl, arylsulphonylami.nocarbonyl or benzyl-
sulphonylaminocarbonyl group.
The subsequent reduction of the nitro group is
preferably carried out in a solvent such as water,
water/ethanol, methanol, glacial acetic acid, ethyl
acetate or dimethylformamide, suitably with hydrogen in
the presence of a hydrogenation catalyst such as Raney
nic~el, platinum or palladium/charcoal, with metals such
as iron, tin or zinc in the presence of an acid, with
salts such as iron(II)sulphate, tin(II)chloride, sodium
sulphide, sodium hydrogen sulphite or sodium dithionite,
or with hydra~ine in the presence of Raney nickel at
temperatures between 0 and 80C, but preferably at
temperatures between 20 and 40C.
The s~bsequent alkanoylation is suitably carried
out in a solvent or mixture of solvents such as water,
methylene chloride, chloroform, ether, tetrahydrofuran,
dioxane or dimethylformamide with a corresponding
carboxyli.c acid in the presence of an acid-activating or
dehydrating agent such as thionylchloride, with the
anhydrides thereof such as acetic anhydride, with the
esters thereof such as ethyl acetate, with the halides
thereof such as acetylchloride, optionally in the
presence of an inorganic or tertiary organic base, such
as sodium hydroxide, potassium carbonate, triethylamine
' '' ~ ". ' ' ' .: '
- ~8 -
or pyrid:ine, wh.ilst -the latter two may simultaneously
also be used as solvent, a-t temperatures bet~leen -25 and
100C, but preferably at temperatures between -lo and
80~C.
The subsequent alkylation is suitably carried out
in a solvent or mixture of solvents such as methylene
chloride, diethylether, tetrahydrofuran, dioxane,
dimethylsulphoxide, dimethylformamide or benzene,
optionally in the presence of an acid binding agent such
as sodium carbonate, potassium carbonate, sodium
hydroxide, potassium tert.butoxide, triethylamine or
pyridine, whilst the latter two may simultaneously also
be used as solvent, with an alkylatin~ agent such as
methyliodide, ethyliodide, isopropylbromide, dimethyl-
sulphate, diethylsulphate or methyl p-toluenesulphonate,
pre~erably at temperatures between 0 and 100C, e.g. at
temperatures between ambient temperature and 50~C.
The conversion of a carboxyl group into a group
which is metabolically converted ln vivo into a carboxy
group, is conveniently carried out by esterification
with a corresponding alcohol or with a corresponding
reactive acyl derivative, suitably in a solvent or
mixture of solvents such as water, methylene chloride,
chloroform, ether, tetrahydrofuran, dioxane or
dimethylformamide or in an excess of the acylating agent
as solvent, optionally in the presence of an acid-
activating or dehydrating agent such as thionylchloride,
with the anhydrides, esters or halides thereof,
optionally in the presence of an inorganic or tertiary
organic base, such as sodium hydroxide, potassium
carbonate, triethylamine or pyridine, whilst the latter
two may simultaneously also be used as solvent, at
temperatures between -25 and 100C, but preferably at
temperatures between -10 and 80C. :~
The subsequent reaction of an acid halide with a
corresponding sulphonamide is suitably carried out in a
solvent such clS acetone, tetrahydrofuran or dioxane in
.: ., . . ,... :
~, . . . . . .
_ ~9 ~ 3~1
-the presence of a base such as triethylamine at
temperatures between 0 and 50C, preferably at
temperatures between 10 and 30~c.
In the reaction steps (a) to (j) described
hereinbefore, any reactive ~roups present such as
hydroxy, amino or alkylamino groups ~ay optionally be
protected during the reaction by conventional protecting
groups which are split off again after the reaction.
Examples of protecting (~rol1ps for a hydroxy yroup
inclwde trimethylsilyl, acetyl, benzoyl, methyl, ethyl,
tert.-butyl, benzyl and tetrahydropyranyl groups and
prot~cting yroups for an amino, alkylamino or imino
group include acetyl, benzoyl, ethoxycarbonyl and benzyl
groups.
The optional subsequent cleaving of a protecting
group is preferably carried out by hydrolysis in an
aqueous solvent, e.g. in water, isopropanol/water,
tetrahydrofuran/water or dioxane/water, in the presence
of an acid such as hydrochloric acid or sulphuric acid
or in the presence of an alkali metal base such as
sodium hydroxide or potassium hydroxide at temperatures
between 0 and 100C, preferably at the boiling
temperature of the reaction mixtureO However, a benzyl
group is preferably cleaved by hydrogenolysis, e.g. with
hydrogen in the presence of a catalyst such as
palladium/charcoal in a solvent such as methanol,
ethanol, ethyl acetate or glacial acetic acid,
optionally with the addition of an acid such as
hydrochloric acid at temperatures between 0 and 50C,
but preferably at ambient temperature, under a hydrogen
pressure of 1 to 7 bar, preferably of 3 to 5 bar~
An isomer mixture of a compound of formula I thus
obtained may if desired be resolved by chromatography
using a substrate such as silica gel or aluminium oxide.
Moreover, the compounds of formula I obtained may
be converted into the acid addition salts thereof, more
particularly for pharmaceutical use the physiologically
.
- . ,
' ~, .
-
`
,~
: :
2~73~41
- 50 -
accept~ble sal-ts thereof wit~ lnoryanic or oryanic
acids. Sultable acids for -this purpose include
hydrochloric acid, hydrobromic acid, sulph-lric ~cid,
phosphoric acid, fumaric acid, succinic acid, lactic
acid, citric acid, -tartaric acid or maleic acid.
Furthermore, the new compounds of formula I thus
obtained, if they contain a carboxy or lH-tetrazolyl
group, may if desired subsequently be converted into the
addition salts thereof with inorganic or organic bases,
more particularly for pharmaceutical use into the
physioloyically acceptable addition salts thereof
Suitable bases include for example sod:ium hydroxide,
potassium hydroxide, cyclohexylamine, ethanolamine,
diethanolamine and triethanolamine.
The compounds of formulae II to XVI used as
starting materials are known from the literature in some
cases or may be obtained by methods known from the
literature.
Thus, for example, substituted pyrimidines of
formula II are described in EP-A-424317, substituted
pyrimidinones are described in EP-A-407342 and
EP-~-419048, substituted triazoles are described in
EP-A-409332, substituted triazolinones, triazolthiones
and triazolimines are described in EP-A-412594,
substituted pyrazoles in EP-A-411507, substituted
quinazolinones in EP-A-411766, substituted quinolines in
EP-A-412848, 5-membered condensed imidazo derivatives in
EP-A-407102, benzimidazoles in EP-A-392317,
imidazopyridines and purines in EP-A-400974 and
EP-A-399731 and substituted imidazoles in EP-A-253310.
The compounds of formulae III and IV used as
starting materials may be obtained by reacting a
eorresponding alcohol with hydrohalic acid, with
tetrabromomethane/triphenylphosphine or wi-th a
eorresponding sulphonic acid halide.
The eompounds o~ ~ormulae VIII, IX, X, XII, XIV and
XVI used as starting materials may be obtained by
- : .
,
.
'
:
: : .
2~73~
- 51 -
alkylating a corresponding compound w:ith a compound
analogous to a compound o~ formula II:[.
The new compounds of formula I and the
physiologically acceptable adclition salt~ thereof have
valuable pharmacological properties. They are
angiotensin antagonists, particularly angiotensin-II-
anta~onists.
By way of example, the ~ollowing compounds of
formula I
A = 2-n-propyl-4-methyl-1-[4-[(~-carboxy)benzyloxy]-
benzyl]benzimidazole;
B -- 2-n-butyl-1-[4-[(~-carboxy)benzyloxy~benzyl]-6-
dimethylaminocarbonylamino-benzimidazole;
C = 2-n-propyl-6-(1-methyl-benzimidazol 2-yl)~4-methyl-
1-[4-[(~-carboxy)benzyloxy]benzyl]benzimidazole;
D = 2-methyl-4-[4'-[(~-carboxy)benzyloxy]benzyloxy]-
quinoline;
E = 2-n-butyl-8-methyl-3~[4-[(~-carboxy)benzyloxy]-
- benzyl]quinazolin-4-one-semihydrate;
F = 2-n-propyl-4-methyl-6-(1-methyl-benzimidazol-2-yl)-
1-[4-[~-(lH-tetrazol-5-yl)benzyloxy]benzyl]-
benzimidazole;
G = 2-n-butyl-6-(N-propionyl-methylamino)-1-[4-[(1-
carboxy-3-methyl)butyloxy]benzyl]benzimidazole;
H = 2-n-butyl-5-methyl-6-(3-benzyl-3,4,5,6-tetrahydro-
2(lH)-pyrimidinon-l-yl)-3-[4-[~-(lH-tetrazol-5-yl)-
benzyloxy]benzyl]imidazol[4,5-b]pyridine; and
'' : ' '
: ,
2~73~1
-- 52 -
I = 2-n-butyl-1-[4-[[~-[~-ethyl-phosphono~benzylamino]-
benzyl]benzimidazole
were investigated for their biological effects as
follows:
Description of method: Angiotensin II-receptor bondin~
The tissue (rats lung) is homogenised in Tris-
buffer (50 mMol Tris, 150 mMol NaC]., 5 mMol EDTA, pH
7.40) and centrifuged twice fo:r 20 minutes at
20,000 x g. The finished pellets are resuspended in
incubating buffer (50 mMo]. Tris, 5 mMol MgCl2, 0.2% BSA,
pH 7.40) 1:75, based on the mo:ist weight of the tissue.
Each 0.1 ml of homogenate is incubated for 60 minutes at
37C with 50 pM [12sI]-angiotensin II (NEN, Dreieich,
FRG) with increasing concentrations of the test
substance in a total volume of 0.25 ml. Incubation is
ended by rapid filtration through glass fibre filter
mats. The filters are each washed with 4 ml of ice cold
buffer (25 mMol Tris, 2.5 mMol MgCl2, 0.1% BSA, pH 7.40).
The bound radioactivity is measured using a gamma-
counter. The corresponding ICso value is obtained from
the dose-activity curve.
In the test described, substances A to I show the
following ICso values:
. ~
Substance ICso [nM]
A 76
B 8
C
D 2000
E 2000
F 12
G 830
H 27
560
,, ., ~ . , ,: . , , :
:: :
2~3~
- 53 -
Moreover, when the above-mentioned compoun~s were
administered in a dose of 30 mg/kg i.v. no toxic si~e
effects, e.g. negative inotropic effects or disorders in
heart rhythm, were observed. 'rhe compounds are
therefore well tolerated.
In view of their pharmacological properties, the
new compounds and the physiologically acceptable
addition salts thereof are suit:able for the treatment of
hypertension and cardiac insufiiciency and also for
treating ischaemic peripheral circulatory disorders,
myocardial ischaemia (angina), for the prevention of the
progression of cardiac insufficiency after myocardial
infarction and for treating diabetic nephropathy,
glaucoma, gastrointestinal diseases and bladder
diseases.
The new compounds and the physiologically
acceptable addition salts thereof are also suitab]e for
treating pulmonary diseases, e.g. lung oedema and
chronic bronchitis, for preventiny arterial re-stenosis
after angioplasty, for preventing thickening of blood
vessel walls after vascular operations, and for
preventing arteriosclerosis and diabetic angiopathy. In
view of the effects of angiotensin on the release of
acetylcholine and dopamine in the brain, the new
angiotensin antagonists are also suitable for
alleviating central nervous system disorders, e.g.
depression, Alzheimer's disease, Parkinson syndrome,
bulimia and disorders of cognitive function.
Thus viewed from a further aspect the invention
provides a pharmaceutical composition comprising a
compound of formula I or a physiologically acc~ptable
salt thereof together with at least one physiologically
acceptable carrier or excipient.
V:iewed from a still further aspect the present
invention provides the use of a compound of formula I or
a physiologically acceptable salt thereof for the
manufacture of a therapeutic agent with an angiotensin
~73~
- 5~ -
antagon:istic activi-ty.
In particular, the present invention provides the
use of a compound of formula I or a physiologically
acceptable salt thereof for the n\anufacture of a
therapeutic agent to treat hyper-tension and cardiac
insufficiency and also for treating ischaemic peripheral
circulatory disorders, myocardial ischaemia (angina),
for -the prevention of the progression of cardiac
insufficiency after myocardial inearction and for
treating diabetie nephropathy, glaueoma,
gastrointestinal diseases and bladder diseases.
Additiona]ly, the present invention provides the
use of a eompound of formula I or a physiologieally
aeceptable salt thereof for the manufacture of a
therapeutic agent to treat lung oedema and chronic
bronchitis, for preventing arterial re~stenosis after
angioplasty, for preventing thickening of blood vessel
walls after vaseular operations, and for preventing
arterioselerosis and diabetic angiopathy as well as
other pulmonary diseases.
Yet further, the present invention provides the use
of a eompound of formula I or a physiologieally
aeeeptable salt thereof for the manufacture of a
therapeutie agent to treat depression, Alzheimer's
disease, Parkinson syndrome, bulimia, disorders of
eognitive funetion and other nervous system disorders.
Viewed from a yet still further aspeet the present
invention provides a method of treatment of the human or
non-human animal body to eombat hypertension and eardiac
insuffieiency and also for treating ischaemie peripheral
eireulatory disorders, myoeardial isehaemia (angina),
for the prevention of the progression of eardiae
insuffieiency after myoeardial infaretion and for
treating diabetie nephropathy, glaueoma,
gastrointestinal diseases and bladder diseases, said
method eomprising administering to said body a eompound
of formula I or a physiologieally aeeeptable salt
,
- . . .
,~ , - : ' - : : ,
2 ~ ~ 3 ~
thereof.
In particular, the present invention provides a
method of treatment of the human or non-human animal
body to combat lung oedema and chronic bronchitis, for
preventing arterial re-stenosis after angioplasty, for
preventing thickening of blood vessel walls after
vascular operations, and for preventing arteriosclerosis
and diabetic angiopathy as wel:L as other pulmonary
diseases, said method comprising administering to said
body a compound of formula I or a physiologically
acceptable salt thereof.
Additionally, the present invention provides a
method of treatment of the human or non-human animal
body to combat depression, ~lzheimer's disease,
Parkinson syndrome, bulimia, disorders of cognitive
function and other nervous system disorders, said method
eomprising administering to said body a compound of
formula I or a physiologically acceptable salt thereof.
The dosage required to achieve these effects in
adults is appropriately, when administered
intravenously, 20 to 100 mg, preferably 30 to 70 mg,
and, when administered orally, 50 to 200 mg, preferably
75 to 150 mg, 1 to 3 times a day. For this purpose, the
eompounds of general formula I prepared according to the
-^~ invention, optionally in conjunction with other active
substances, such as hypotensives, diuretics and/or
ealcium antagonisks, may be incorporated together with
one or more inert eonventional carriers and/or diluents,
e.g. with corn stareh, lactose, glueose,
mierocrystalline cellulose, magnesium stearate,
polyvinylpyrrolidone, citric acid, tartaric acid, water,
water/ethanol, water/glycerol, water/sorbitol,
water/polyethylene-glycol, propylene-glycol,
eetylstearyl alcohol, carboxymethylcellulose or fatty
substances such as hard fat or suitable mixtures
thereof, in conventional galenic preparations such as
plain or coated tablets, capsules, powders, suspensions
.
,, : ,
2~73~
- 56 -
or suppositories.
Additional active substances which may be included
in the combinations mentioned above might be, for
example, bendroflumethiazide, chlorothiazide,
hydrochlorothiazide, spironolactone, benzothiazide,
cyclothiazide, ethacrinic acid, furosemide, metoprolol,
prazosine, atenolol, propranolol, (di)hydralazine
hydrochloride, diltiazem, felodipin, nicardipin,
nifedipin, nisoldipin and nitrendipin. The dosage for
these active substances is appropriately one fifth of
the lowest recommended dose Up to 1/1 of the normally
recommended dose, i.e., for example, 15 to 200 mg of
hydrochlorothiazide, 125 to 2000 mg of chlorothiazide,
15 to 200 mg of ethacrinic acid, 5 to 80 mg of
furosemide, 20 to 480 mg of propranolol, 5 to 60 mg of
felodipine, 5 to 60 mg of nifedipin or 5 to 60 mg of
nitrendipin.
The following non~limiting Examples are provided to
illustrate the invention. All percentages and ratios
given are by weight other than eluant or solvent ratios
which are by volume.
.~.~
:
.
. 2~73~ ~
. ~"
Example A
4-(~-Ethoxycarbonyl-benzyloxy)benzylbromide
a) EthYl 2-bromo-phenylacetate
43.0 g (0.2 mol) of DL-2-bromo-phenylacetic acid are
dissolved in 400 ml of ethanol at ambient temperature
with stirring. At 5-10C 11.8 g (0.2 mol) of
thionylchloride are slowly added dropwise whilst cooling
with ice. A_ter 12 hours at ambient temperature the
solvent is distilled off and the residue is taken up in
ethyl acetate. After extraction with saturated sodium
bicarbonate solution and saturated saline solution the
product is dried over sodium sulphate and evaporated
down.
Yield: 41,85 g (86% of theory),
Rf value: 0.60 (silica gel; eluant: ethyl
acetate/petroleum ether = 1:19)
b) ~ Ethoxycarbonyl-benzyloxy)benzyl alcohol
41.8 g (0.172 mol) of ethyl 2-bromo-phenylacetate and
21.3 g (0.172 mol) of 4-hydroxybenzyl alcohol are
dissolved in 850 ml of acetone and 24.8 g (0.18 mol) of
potassium carbonate and 5.0 g ~0.03 mol) of potassium
iodide are added thereto. The reaction mixture is
refluxed for 60 hours with stirring. Then the inorganic
salts are filtered off and the residue is washed with
hot acetone. The filtrate is evaporated down and the
residue is purified ovsr a silica gel column (particle
si~e: 0.063-0.02 mm), initially using pstroleum ether as
eluant and then using mixtures of petroleum ether and
ethyl acetate of incrsasing polarity ~9:1, 8:2 and 7:3),
The unified fractions are evaporated down ln _cuo.
Yield: 30.65 g (62.5% of theory),
Rf vaIue: 0.40 (silica gel; eluant: ethyl
acetate/pstroleum ether = 3:7)
,: . . . . . . .
.. .
,
~ ' '
, .
2~ 13~ ~
- 58 -
c) 4~ h'thox~r o~l-henzyloxy)benzylbro_ide
28.6 g (0.l mol) of 4-(~-ethoxycarbonyl-benzyloxy)benzyl
alcohol are dissolved in 300 ml of dichloromethane and
mixed with 31.6 g (0.12 mol) of triphenylphosphine.
Whilst cooling with ice, a solution of 40.0 g (0.12 mol)
of carbon tetrabromide in 100 ml of dichloromethane is
added dropwise. The mixture is stirred for 30 minutes
at ambient temperature and then concentrated by
evaporation. The residue is purified over a silica yel
column ~particle size: 0.063-0.02 mm), using as eluant
first petroleum ether and then mixtures of petroleum
ether and ethyl acetate of increasing polarity (9:1 and
8:2). The unified fractions are evaporated down 1n
vacuo.
Yield: 20.45 g (59% of theory),
Oil, Rf value: 0.70 (silica gel; eluant: ethyl acetate/
petroleum ether = 1:4)
C17H16BrO3 (349.24)
Calculated:C 58.20 H 4.95 Br 22.80
Found:58.23 4.84 23.12
.,_ .
, . ~ , . . .
~ ,
. ' ' ~ ..
2~3~
- 59 -
Example 1
2-n-Propyl-4-methyl-1-[~-[(Q-carboxy)benzyloxy]benzyl]-
benzimidazole
a) 2-n-Propyl-4-methyl-1-[4-[(~-ethoxycarbonyl)-
benzyloxy]benzylLbenzimidazc)le _ __ _
350 mg (2.0 mMol) of 2-n-propyl-4-methyl-benzimidazole
(prepared analogously to Chemistry of ~Ieterocyclic
Compounds, Vol. 40, Part 1, ~-12) are adde~ to a
solution of 220 mg o~ potassium tert.butoxide in 30 ml
of dimethylsulphoxide. After 30 minutes at ambienk
temperature a solution of 700 mg (2.0 m~ol) of 4-(Q-
ethoxycarbonyl-benzyloxy)benzy:Lbromide in 5 ml oP
dimethylsulphoxide is added dropwise. Af-ter 12 hours at
ambient temperature the reaction solution is stirred
into ice water and extracted 3 times with 100 ml of
ethyl acetate. The combined organic phases are washed
with 100 ml of saline solution and dried over sodium
sulphate. After the solvent has been eliminated, the
residue is purified over a silica gel column (particle
size 0.063-0.02 mm), using as eluant methylene chloride
to start with, then mixtures of methylene chloride and
ethanol of increasing polarity (50:1 and 25:1). The
uniform fractions are concentrated by evaporatio~ in
vacuo.
Yield: 500 m~ (55% of theory),
Oil, Rf value: 0.55 (silica gel; eluant: methylene
chloride/methanol = 13:1)
b) 2-n-Propyl-4-methyl-1-[4-[(Q-carboxy)benzyloxy]-
benz~l]benzimidazole
500 mg (1.1 mMol) of 2-n-propyl-4-methyl-1-[4-[(Q-
ethoxycarbonyl)benzyloxy]benzyl]benzimidazole are
dissolved in 10 ml of ethanol and mixed with 10 ml of
1 N sodium hydroxide solution. The mixture is stirred
at ambient temperature for 30 minutes. Then the mixture
is concentrated by evaporation and taken up in water.
.
~ .
.
`.
2~3~
- 60 -
The solu-tion i.s acidified by the addition of glacial
acetic acid. The precipitate formed i6 guction
filtered, washed with water until neutral and dried.
The crude product is taken up in methylene chloride and
purified over a silica gel column (particle size
0.063--0.02 mm), using as eluant a mixture of methylene
chloride and methanol (19:1). The uniform fractions are
concentrated by evaporation ln vacuo and the residue is
triturated with hexane/ether and suction filtered.
Yield: 60 mg (13% of theory),
Melting point: amorphous
C26Hz6N2O3 (414.51)
Mass spectrum: (M + H)t = 415
Example 2
2-n-Butyl-1-[4-[(~-carboxy)benzyloxy]benzyl]-6-
propionylamino-benzimidazole-hydrochloride
a) 2-n-butyl-1-[4-[(~-ethoxycarbonyl)benzyloxy]benzyl]-
6 nitro-benzimidazole and
2-n-butyl-1-[4-[(~-ethoxycarbonyl)benzyloxy]benzyl]-
5-nitro-banzimidazole _ _
Prepared analogously to Example la from 2-n-butyl-5-
nitro-benzimidazole (prepared analogous].y~o Chemistry
of Heterocyclic Compounds, Vol. 40, Part 1, 6-12) and 4-
[(~-ethoxycarbonyl)-benzyloxy]benzylbromide.
Yield: 70.5% of theory,
Oil, Rf value: 0.25 (silica gel; eluant: methylene
chloride/ethanol = 50:1)
b) 2-n-Butyl-6-amino-1-[4-[(~-ethoxycarbonyl)benzyloxy]-
benzyl]-benzimidazole
Prepared by catalytic hydrogenation of 2-n-butyl-1-[4-
[(~-ethoxycarbonyl)benzyloxy]benzyl]-5/6-nitro-
benzimidazole in the presence of Raney nickel in ethanol
at 50~C under 5 bars of hydrogen pressure, followed by
chromatographic separation of the 5-amino-2-n-butyl-l-
~, . . .
,
.
:
2~3~
- 61 -
[4-[(~-ethoxycarbonyl)~benzyloxy]benzyl]herlæimidazole.
Yield: 21.8% of theory,
Oil, Rf value: 0.30 (silica gel; eluant: petroleum
ether/methylene chloride/ethanol =
7:2:1)
c) 2-n-Butyl-6-propionylamino-1-[4-[(~-ethoxycarbonyl)-
benzyloxy]benzyllbenzimi~ ole
Prepared from 2-n-butyl-6-amino-1-[4-[(~-ethoxy-
carbonyl)benzyloxy]benzyl~benzimidazole and propionyl
chloride/pyridine.
Yield: 70.2% of theory,
Melting point: 207~209C
d) 2 n-Butyl-1-[4-[(~-carboxy)benzyloxy]benzyl]-6-
propionylamino-benzimidazole-hydrochloride
Prepared analogously to Example lb from 2-n-butyl-6-
propionylamino-1-[4-[(a-ethoxycarbonyl)benzyloxy]-
benzyl]benzimidazole and 1 N sodium hydroxide solution
in ethanol.
Yield: 43.5% of theory,
Melting point: sinters from 93C
C29H31N34 x HCl (522.67)
Calculated: C 66.50 H 6.12 N 8.08
Found: 66.73 6.34 7.81
Example 3
2-n-Butyl-1-[4-[(~-carboxy~benzyloxy~benzyl]-6-
dimethylaminocarbonylamino-benzimidazole
_ .
Prepared analogously to Example lb from 2-n-butyl-1-[4-
[(a-ethoxycarbonyl)benzyloxy]benzyl]-6-dimethylamino-
carbonylamino-benzimidazole and 2 N sodium hydroxide
solution in ethanol.
Yield: 80% of theory,
Melting point: 183-185C
C29H32N4O4 (500.60)
:
.: .
~73~3
- 62 -
Calculated:C 69.59 }I 6.~ N 11.19
Eound:69.5~ 6.~3 10.66
Example 4
2-n-Butyl-1-[4-[(~-carboxy)benzyloxy]benzyl] 6-
cyclohexylaminocarbonylamino-benzimidazole-hydrochloride
a) 2-n-Butyl-1-[4-[(~-ethoxycarbonyl)benzyloxy]benzyl]-
6-cyclohexylaminocarbonylamino-be-nzimidazole
Prepared from 2-n-butyl-1-[4-[(~-ethoxycar~onyl)benzyl-
oxy]benzyl]-6-amino-benzimidazole and cyclohexyl-~
~' isocyanate/triethylamine in te-trahydrofuran.
Yield: 65.4% of theory,
Oil, Rf value: 0.40 ~silica gel; eluan-t: methylene
chloride/ethanol = 19:1)
b) 2-n~Butyl-1-[4-[(~-carboxy)benzyloxy]benzyl]-6-
cyclohexylaminocarbonylamino-benzimidazole-
hydrochloride
Prepared analogously to Example lb from 2-n-butyl-1-[4-
[(~-ethoxycarbonyl)benzyloxy]benzyl]-6-cyclohexylamino-
carbonylamino-benzimidazole and 1 N sodium hydroxide
solution in ethanol.
Yield: 40.7% of theory,
Melting point: sinters from 184C
C33H3sN4~4 x HCl (591.17)
Calculated: C 67.00 H 6.60 N 9.45
Found: 67.15 6.71 9.30
Example 5
2-n-Butyl-1-[4-[(~-carboxy)benzyloxy]benzyl]-5-
cyclohexylaminocarbonylamino-benzimidazole-hydrochloride
Prepared analogously to Example lb from 2-n-butyl-1-[4-
[(a-ethoxycarbonyl)benzyloxy]benzyl]-5-cyclohexylamino-
carbonylamino-benzimidazole and l N sodium hydroxide
: ~ -
,.: :
. .
,
2~73~
- 6~ -
solu-tio~ in ethanol.
Yield: 46.1% of theory,
Melting point: sinters from 196C
C33H3sN44 x HCl (591.17)
Calculated: C 67.00 H 6.60 N 9.45
Found: 67.22 6.74 9.49
Example_6
2-n-Butyl-1-[4-[(~-carboxy)benz:yloxy]benzyl]-6-(3,4,5,6-
tetrahydro-2(lH)-pyrimidinon-l--yl)-benzimidazole
. ~
Prepared analogously to Example lb from 2-n-butyl-1-[4-
[(~-ethoxycarbonyl)benzyloxy]benzyl]-6-(3,4,5,6
tetrahyclro-2(lH)-pyrimidinon-l-yl)-benzimidazole and 1 N
sodium hydroxide solution in ethanol.
Yield: 8.8% of theory,
Melting point: 260-262C
C30H32N4O4 (512-61)
Mass spectrum: (M + H)+ = 513
Example 7
2-n-Butyl-1-[4-[(~-carboxy)benzyloxy]benzyl]-6-(3-
benzyl-3,4,5,6-tetrahydro-2(1~)-pyrlmidinon-1-yl)-
benzimidazole
Prepared analogously to Example lb from 2-n-butyl~ 4-
[(~-ethoxycarbonyl)benzyloxy]benzyl]-6-(3-benzyl-
3,4,5,6-tetrahydro-2(lH)-pyrimidinon-l-yl)-benzimidazole
and 1 N sodium hydroxide solution in ethanol.
Yield: 80.4% of theory,
Melting point: 124-126C
C37H38N404 (602.74)
Calculated: C 73.73 H 6.35 N 9.30
Found:73.47 6.50 9.05
: - . . . .
:, , : ., , ': ,
,,
2~73,~1
- 6~ -
_ample 8
2-n-Butyl-1-[4-[(~-carboxy)benzyloxy]benzyl]-6-(N-cyclo-
hexylaminocarbonyl-methylamlno~-benzimidazole
a) 2-n-Bu-tyl-1-[4-[(~-ethoxycarbonyl)benzyloxy]benzyl]-
6-(N-cyclohexylaminocarbonyl-methylamino)-
_ nzimidazole
Prepared analogously to Example la from 2-n-butyl-6-(N-
cyclohexylaminocarbonyl-methylamino)benzimidazole and 4-
[(~-ethoxycarbonyl)-benzyloxy]benzylbromide and
subsequent chromatographic separation of the 2-n-~utyl-
1-[4-[(~-ethoxycarbonyl)benzyloxy]benzyl]-5-(N-
cyclohexylaminocarbonyl-methylamino)-benzimidazole.
Yield: 50.0% of theory,
Oil, Rf value: 0.50 (silica gel; eluant: methylethyl
ketone/xylene = 1:1)
b) 2-n-Butyl-1-[4-[(~-carboxy)benzyloxy]benzyl]-6-(N-
cvclohexylaminocarbonyl-methylamino)benzimidazole
Prepared analogously to Example lb from 2-n-butyl-1-[4-
[(~-ethoxycarbonyl)benzyloxy]benzyl]-6-(N-cyclohexyl-
aminocarbonylmethylamino)benzimidazole and 1 N sodium
hydroxide solution in ethanol.
Yield: 93.0% of theory,
Melting point: 168-169C
C34H40N4O4 (568.72)
Calculated: C 71.81 H 7.09 N 9.85
Found: 71.78 7.02 9.71
Example g
2-n-Propyl-5-(2-methyl-propionylamino)-3-[4-[~-
carboxy)benzyloxy]benzyl]imidazo[4,5-b~pyridine
a) 2-n-Propyl-5-(2-methyl-propionylamino)-
imidazo r 4,5-b]pyridine
2.62 g (15 mMol) of 2-n-propyl-5-amino-
..
: , " ' '
, . .
2 ~
- 65 -
imidazo[4,5-b]pyri~ine are suspended in 100 ml of
absolute methylene ~h]oride an~ 3.16 g (30 mMol) o~
isobutyric acid chloride are a~ded, with stirring an~
cooling with ice. Then, at -5 DC/ a solution of 3.03 g
(30 mMol) of -triethylamine in 5 ml of methylene chlori~e
is added dropwise. ~fter one hour at ambient
temperature the reaction mixture is combined with loO ml
o~ 2 N hydrochloric aci~ and the mixture is stirred for
a further hour. ~hen the solut;ion is poured onto ice
water and mixed with saturated sodium hydrogen carbonate
solution. The aqueous phase is extracted 3 times with
100 ml of ethyl acetate, the combined organic phases are
then washed with saline solution, dried over sodium
sulphate and concentrated by evaporation. The crude
product is taken up in methylene chloride and purified
over a silica gel column (particle size 0.063-0.2 mm),
using as eluant methylene chloride to begin with,
followed by mixtures of methylene chloride and ethanol
of increasin~ polarity (25:1 and 19:1). The unified
fractions are concentrated by evaporation and the
residue is triturated with petroleum ether/ether = 1:1
and suction filtered.
Yield: 2.05 g (56% of theory),
Melting point: 206C
Cl3H18N40 (246.30)
Calculated: C 63.39 H 7.37 N 22~.75
Found: 63.64 7~57 22.93
b) 2-n-Propyl-5-(2-methyl-propionylamino)-3-[4-[~-
ethoxycarbonyl)benæyloxy]benzyl]imidazo[4,5-b]-
pyridine
Prepared analogously to Example la from 2-n-propyl-5-(2-
methyl-propionylamino)imidazo[4,5-b]pyridine and 4-[(~-
ethoxycarbonyl)benzyloxy]benzylbromide.
Yield: 33.7% of theory,
Meltin~ point: 100-101C
: . ~ ~ ' , , " . :, ;
2 ~ 7 3 ,~
- 66 -
c) 2-n-Propyl-5-(2-methyl-propionylamino) 3-[~-[(~-
carboxy)benz~ oxy1benzyll1midazo L4,5-~,~rldine_
Prep~red analogously to Example lb from 2-n-propyl-5-(2-
methylpropionylamino)-3-[4-[(~-ethoxycarbonyl)-
benzyloxy]benzyl~imidazo[4,5-b]pyridine ~nd l N sodium
hydroxide solution in ethanol.
Yield: 52.0% of theory,
Melting point: 134C
C28H30N4o4 (486-58)
Calculated: C 69.12 H 6.21 N 11.51
Found: 69.03 6.11 11.36
Example 10
2-n-Butyl-5-valeroylamino-3-[4-[(~-carboxy)benzyloxy]-
benzyl~imidazo[4,5-b]pyridine-semihydrate
Prepared analogously to Example lb from 2-n-butyl-5-
valeroylamino-3-[4-[(~-ethoxycarbonyl)benzyloxy]benzyl]-
imidazo[4,5-b]pyridine and l N sodium hydroxide solution
in ethanol.
Yield: 57.0% of theory,
Melting point: sinters from 96C
C30H34N4o4 x 0-5 H20 (532.65)
Calculated: C 68.82 H 6.74 N 10.70
Found: 68.99 6.67 10.58
Example 11
2-n-Propyl-5 (l-methyl benzimidazol-2-yl)-7-methyl-3-[4-
[(~-carboxy)benzyloxy]benzyl]imidazo[4,5-b]pyridine
Prepared analogously to Example lb from 2-n-propyl-5-(1-
methyl-benzimidazol-2-yl)-7~methyl-3-[4-[(~-
ethoxycarbonyl)benzyloxy]benzyl-imidazo~4,5-b]pyridine
and 1 N sodium hydroxide solution in ethanol.
Yield: 41.4% of theory,
Melting point: 242-244C
.
'
' : `
.
.
2~73~3~ 1.
- 67 -
C33~l3~N5O3 (545-65)
Calculated: C 72.6~ ~l 5.73 N 12.83
Found: 72.51 5.75 12.97
Example 12
2-n-Propyl-6~ methyl-benzimiclazol-2-yl)-4-methyl-1-[4-
[(~-carboxy)benzyloxy]benzyl]benzimidazole
Prepared analogously to Example! lb from 2-n-propyl-6-(1-
methyl~benzimidazol-2-yl)-4-methyl-1-[4-[(~-
ethoxycarbonyl)benzyloxy]benzyl]benzimidazole and 1 N
~`~ sodium hydroxide solution in ethanol.
Yield: 41.0% of theory,
Melting point: amorphous
C34H32N4O3 (544-66)
Calculated: C 74.98 H 5.92 N 10.28
Found: 74.55 6.06 10.08
Mass spectrum: (M ~ H)~ = 545
Example 13
2-Methyl-4-[4'-[(~-carboxy)benzyloxy]benzyloxy]quinoline
a) 2~Methyl-4-[4'-[(~-ethoxycarbonyl)benzyloxy]-
^~ benzyloxy]quinoline
Prepared analogously to Example la ~rom 4-hydroxy-
quinaldine and 4-[(~-ethoxycarbonyl)benzyloxy]benzyl-
bromide.
Yield: 19.8~ of theory,
Oil, Rf value: 0.55 (silica gel; eluant: methylene
chloride/ethanol = 19:1)
b) 2-Methyl-4-[4'-[(~-carboxy)benzyloxy]benzyloxy]-
quinoline
Prepared analogously to Example lb from 2-methyl-4-[4'- .
[(~-ethoxycarbonyl)benzyloxy]benzyloxy-quinoline and 1 N
sodium hydroxide solution in ethanol.
.,
:', . ~ , :
- ~ ' . ' ' ' . . .
2~3~
~ 6~ -
Yield: 66.6% of theory,
Melting point: 142 143JC
C25H2lNO4 (399 50)
Calculated: C 73.17 H 5.30 N 3.51
Found: 73.56 5.25 3.34
Mass spectrum: (M -~ H)~ = 400
_xample 14
2-n-Propyl-5-n-butyrylamino-3-[4-[(l-carboxy-3-methyl)-
butyloxy]benzyl]imidaæo[4,5-b]E)yridine
a) 2-n-Propyl-5-n-butyrylamino-3-[4-[(1-methoxycarbonyl-
3-methyl)butyloxy]benzyl]imidazo[4,5-blpyridine _ _
Prepared analogously to Example la from 2-n-propyl-5-n-
butyrylamino-imidazo[4,5-b]pyridine and 4-[(1-
methoxycarbonyl-3-methyl)butyloxy]benzylbromide, with
subsequent chromatographic separation of the 2-n-propyl-
5-n-butyrylamino-1-[4-[(1-methoxycarbonyl-3-methyl)-
butyloxy]benzyl]imidazo[4,5-b]pyridine.
Yield: 47.0% of theory,
Oil, Rf value~ 0.30 (silica gel; eluant: methylene
chloride/ethanol = 19:1)
b) 2-n-Propyl-5-n-butyrylamino-3-[4~ carboxy-3-
methyl)-butyloxy]benzyl~imidazo[4c5-b]pyridine
Prepared analogously to Example lb from 2-n-propyl-5-n-
butyrylamino-3-[4-(1-methoxycarbonyl-3-methyl)-
butyloxy]benzyl]imidazo[4,5-b]pyridine and 1 N sodium
hydroxide solution in ethanol.
Yield: 59~0% of theory,
Melting point: 147-148C
C26H34N404 (466.59)
Calculated:C 66.93H 7.35 N 12.01
Found: 66.69 7.48 11.85
... .. . . . .
-~ '. ' . .
'
2~73~
- 69 -
Example 15
2-n-Butyl~1-[4-[(~-carboxy)cyclohexylmethyloxy]-
benzyl]benzimidazole
Prepared analogously to Example lb from 2-n-butyl-1-[4-
[(~-methoxycarbonyl)cyclohexylmethyloxy]benzyl]-
benzimidazole and 1 N sodium hydroxide solution in
ethanol.
Yield: 92.0% of theory,
Melting point: 208C (decomp.)
C26H32~2O3 (420-56)
Calculated: C 74.25 H 7 . 66 N 6.66
Found: 73.98 7.52 6.41
Example 16
2-n--Butyl-4-chloro-5-hydroxymethyl-1-[4-[(~-carboxy)-
benzyloxy]benzyl]imidazole
. . . _ . .
a) 2-n-Butyl-4-chloro-5-hydroxymethyl-1-[4-[(~-
methoxvcarbonyl)benzyloxylbenzyl]imidazole
Prepared analogously to Example la from 2-n-butyl-4-
chloro-5-hydroxymethyl-imidazole and 4-[(~-
methoxycarbonyl)benzyloxy]benzylbromide, with subsequent
chromatographic separation of the 2 n-butyl-5-chloro-4-
hydroxymethyl-1-[4-[(~-methoxycarbonyl)benzyloxy]-
benzyl]imidazole.
Yield: 21.0% of theory,
Oil, Rf value: 0.70 (silica gel; eluant: methylene
chloride/methanol = 6:1)
b) 2-n-Butyl-4-chloro-5-hydroxymethyl-1-[4-[(~-carboxy)-
benzyloxy]benzyllimidazole-h,vdrochloride
Prepared analogously to Example lb from 2-n-butyl-4-
chloro-5-hydroxymethyl-1-[4-[(Q-methoxycarbonyl)-
benzyloxy]benzyl]imidazole and 1 N sodium hydroxide
soIution in ethanol.
. : :
' . ' : . . ~
:
' ~ ,
2~3~
- 70 -
Yield: 34.0% of theory,
Melting point: 176~C
C23H2sClN20~, HCl (465.38)
Calculated: C 59.36 H 5.63 N 6.02
Found: 59.53 5.43 5.97
Exam~le 17
2-n--Butyl-5-chloro-4-hydroxymelhyl-1-[4-[(1-carboxy-3-
methyl)butyloxy]benzyl]imidazo:Le
. . _
Prepared analogously to Example lb from 2-n-butyl=5-
chloro-4-hydroxymethyl-1-[4-[(1-methoxycarbonyl-3
methyl)butyloxy]benzyl]imidazole and 1 N sodium
hydroxide solution in ethanol.
Yield: 66.0% of theory,
Melting point: 175-176'C
C2lH2sClN204 (408-93)
Calculated: C 61.68 H 7.14 N 6.84
Found: 61.34 7.11 6.58
Example 18
2-n-Butyl-5-chloro-4-hydroxymethyl-1-[4-[(1-carboxy)-n-
propyloxy]benzyl]imidazole
_
Prepared analogously to Example lb from 2-n-butyl-5-
chloro-4-hydroxymethyl-1-[4-[(1-methoxycarbonyl~-n-
propyloxy]benzyl]imidazole and 1 N sodium hydroxide
solution in ethanol.
Yield: 70.0% of theory,
Melting point: 182-184C
C19H25ClN204(380-88)
Calculated:C 59.91 H 6.61 N 7.35
Found:59v67 6.53 7.22
:
.
: ,:
2~73(~
- 7L -
Example 19
2-n-Butyl-4-chloro-5-hydroxymethyl-1-[4-[(1-carboxy)-n-
propyloxy]benzyl]imidazole
Prepared analogously to Example lb from 2-n-butyl-4-
chloro-5-hydroxymethyl-1-[4-[(1-methoxycarbonyl)-n-
propyloxy]benzyl]imidazole and 1 N sodium hydroxide
solution in ethanol.
Yield: 66.0% of theory,
Melting point: 125-127C
C19H2sClN2O,~ (380.88)
Calculated: C 59.91 H 6.61 N 7.35
Found: 59.74 6.60 6.90
Example 20
2-n-Butyl-4-chloro-5-methoxymethyl-l-[4-[(~-carboxy)~
benzyloxy]benzyl]imidazole
.
Prepared analogously to Example lb from 2-n-butyl-4-
chIoro-5-methoxymethyl-1-[4-C(~-methoxycarbonyl)-
benzyloxy]benzyl]imidazole and 1 N sodium hydroxide
solution in ethanol.
Yield: 45.0% of theory,
Melting point: 156-158C
C24H27ClN2o4 (4~2.95)
Calculated: C 65.08 H 6.14 N 6.32
Found: 64.70 6.38 6.03
Example 21
2-n-Butyl-4-chloro 5-hydroxymethyl-1-[4-[(1-carboxy-3-
methyl)butyloxy]benzyl]imidazole
Prepared analogously to Example lb from 2-n-butyl-4-
chloro-5-hydroxy-methyl-1-[4-[(1-methoxycarbonyl-3-
methyl)butyloxy]benzyl]imidazole and 1 N sodium
. - . .
- : ,
- :
- : ~ :. ..
-~ ~
.
2~7~
- 72 -
hydroxicle solution in ethanol.
Yield: 57.0% of theory,
Melting point: 16~-165~C
C2lH2~ClN20~, (408.93)
Calculated: C 61.68 H 7.14 N 6.84
Found: 61.63 7.09 6.71
Example 22
2-n-Butyl-4-hydroxymethyl-5-chloro-1-[4-[(~-carboxy)-
benzyloxy]benzyl]imidazole
.. . . . _ ..
Prepared analogously to Example lh from 2-n-butyl~4-
hydroxymethyl-5-chloro-1-[4-[(~-me-thoxycarbollyl)-
benzyloxy]benzyl]imidazole and 1 N sodium hydroxide
solution in ethanol.
Yield: 18.0% of theory,
Melting point: 193C
C23H2sClN20~ (428.92)
Rf value: 0.10 (silica gel; eluanto methylene
chloride/methanol = 9:1)
Mass spectrum: ~M + H)+ = 394/396 (Cl)
Example 23
2-n-Butyl-5-chloro-4-hydroxymethyl-1-[4-[(1-carboxy)-n-
~utyloxy]benzyl]imidazole
.... _ .
Prepared analogously to Example lb from 2-n-butyl-5-
chloro-4-hydroxymethyl-1-[4-[(1-methoxycarbonyl)-n-
butyloxy]benzyl]imidazole and 1 N sodium hydroxide
solution in ethanol.
Yield: 27.0% of theory,
Melting point: 154C
CzoHz7ClN204 (394 90)
Rf value: 0.20-0.30 (silica gel; eluant: methylene
chlori~e/methanol = 9:1)
Mass spectrum: (M + H)~ = 394/396 (Cl)
, ' : '
~73~
- 73 -
Example 24
2-n-Butyl-4-hydroxymethyl-5-chloro-1-[4-[(~-carboxy)-p-
phenyl-benzyloxy]benzyl]imidazole
Prepared analogously to Example lb from 2-n-bu-tyl-4-
hydroxymethyl-5-chloro-1-[4-[(~-methoxycarbonyl)-p-
phenyl-benzyloxy]benzyl]imidazole and 1 N sodiu~
hydroxide solution in ethanol.
Yield: 72.0% of theory,
Melting point: 182~C
C29H29ClN20~, (505.02)
Calculated: C 68.97 H 5.78 N 5.54
Found: 68.50 5.83 5.46
Example 25
2-n--Butyl-4-chloro-5-hydroxyme-thyl-1-[4-[(a-carboxy)-p-
phenyl-benzyloxy]benzyl]imidazole
.
Prepared analogously to Example lb from 2-n-butyl-4-
chloro-5-hydroxymethyl-1-[4-[(~-methoxycarbonyl)-p-
phenyl-benzyloxy3benzyl]imidazole and 1 N sodium
hydroxide solution in ethanol.
Yield: 75.0% of theory,
Melting point: 185-186C (decomp.)
C29H29ClN2O4 (505.02)
Calculated: C 68.97 H 5.78 N 5.54
Found: 68.87 5.80 5.42
Example 26
2-n-Butyl-4-hydroxymethyl-5-chloro-1-[4-[(~-carboxy)-2-
naphthylmethyloxy]benzyl]imidazole-semihydrate
. . _
Prepared analogously to Example lb from 2-n-butyl-4-
hydroxymethyl-5-chloro-1-[4-[(~-methoxycarbonyl)-2-
naphthylmethyloxy]benzyl]imidazole and 1 N sodium
.,: . .
: '. , - '~ ' ' '
2 ~ 7 .~
- 74 -
hydroxide sollltion in ethanol.
Yield: 69.0% of theory,
Melting point: from 188UC (decomp.)
C27H27clN2o4 x 0-5 H20 (487
Calculated: C 66.~5 H 5.78 ~ 5.74
Found: 66.63 5.66 5.75
Example 27
2-n-Butyl-4-chloro-5-hydroxymet:hyl-1-[4-[(~-carboxy)-2-
naphthylmethyloxy]benzyl]imidazole-semihydrate
Prepared analogously to Example lb from 2-n-butyl-4-
chloro-5-hydroxymethyl-1-[~-[(~-methoxycarbonyl)-2-
naphthylmethyloxy]benzyl]imidazole and 1 N sodium
hydroxide solution in ethanol.
Yield: 82.0% of theory,
Melting point: from 142C (decomp.)
C27H27ClN204 x 0.5 H20 (487.99)
Calculated: C 66.45 H 5.78 N 5.74
Found: 66.62 5.66 6.12
Example 28
2-n-Butyl-4 hydroxymethyl-5-chloro-1-[4-[(~-carboxy)-1-
naphthylmethyloxy]benzyl]imidazole-hydrate
Prepared analogously to Example lb from 2-n-butyl-4-
hydroxymethyl-5-chloro-1-[4-[(~-methoxycarbonyl)-1-
naphthylmethyloxy]benzyl]imidazole and 1 N sodium
hydroxide solution in ethanol.
Yield: 91.0% of theory,
Melting point: from 110C (decomp.)
C27H27ClN204 x H20 (497-00)
Calculated: C 65.25 H 5.88 N 5.63
Found: 65.58 5.89 5.83
.,. . : . .. .
2~3g~
- 75 -
Example 29
2-n-Butyl-4-chloro-5-hydroxymethyl-1-~4-[(~-carboxy)-1-
naphthylmethyloxy]benzyl]imidazole-hydrate
. . _ . . _ . . .
Prepared analogously to Example lb from 2-n-butyl-4-
chloro-5-hydroxymethyl-1-[4-[(~-methoxycarbonyl)-1-
naphthylmethyloxy]benzyl~imidazole and 1 N sodium
hydroxide solution in ethanol.
Yield: 42.0% of theory,
Melting point: rrom 100C (decomp.)
C27H27ClN2o4 x H2O (497.00)
Calculated: C 65.25 H 5.88 N 5.63
Found: 65.21 5.70 6.10
Example 30
2-n~Butyl-4-chloro-5-hydroxymethyl-1- L 4-[(~-carboxy)-p-
methoxy-benzyloxy]benzyl]imidazole
Prepared analogously to Example lb from 2-n-butyl-4-
chloro-5-hydroxymethyl-1-[4-[(~-methoxycarbonyl)-p-
methoxy-benzyloxy]benzyl]imidazole and 1 N sodium
hydroxide solution in ethanol.
Yield: 58.0% of theory,
Melting point: 165C
C24H27ClN2Os (458.95)
Calculated: C 62.81 H 5.93 N 6.10
Found: 62.69 6.02 5.g3
Example 31
2-n-Butyl~4-chloro-5-methoxymethyl-1-[4-[(1-carboxy)-n-
pentyloxy]benzyl]imidazole
_ _
Prepared analogously to Example lb from 2-n-butyl-4-
chloro-5-methoxymethyl-1-[4~ methoxycarbonyl~-n-
pentyloxy]benzyl]imidazole and 1 N sodium hydroxide
-:
, .
~3~3~
- 76 -
solution in ethanol.
Yield: 84.0% of theory,
Melting point: ~6~C
Cz2H3lClN2O4 (422-96)
Calculated: C 62.45 H 7.38 N 6.62
Found: 62.23 7.47 6.79
Example 32
2-n-Butyl-4-chloro-5-hydroxymethyl-1-[4-[(1-carboxy)-n-
butyloxy]benzyl]imidazole
Prepared analogously to Example lb from 2-n-butyl-4-
chloro-5-hydroxymethyl--1-[4-[(1-methoxycarbonyl)-n-
butyloxy]benzyl]imidazole and 1 N sodium hydroxide
solution in ethanol.
Yield: 50.0% of theory,
Melting point: 137-138C
C20H27ClN24 (394 90)
Calculated: C 60.83 H 6.89 N 7.09
Found: 60.67 6.94 6.67
Example 33
2 n-Butyl-4-chloro-5-hydroxymethyl-1-[4-[(1-carboxy)-n-
pentyloxy]benzyl]imida~ole
. .
Prepared analogously to Example lb from 2-n-butyl-4-
chloro-5-hydroxymethyl-1-[4-[(1-methoxycarbonyl)-n-
pentyloxy]benzyl]imidazole and 1 N sodium hydroxide
solution in ethanol.
Yield: 61.0% of theory,
Melting point: 150C
C21H29ClN2O4 t408-93)
Calculated: C 61.65 H 7.14 N 6.85
Found: 62.31 6.85 7.21
.:
. ~ ,~' :
.
2~73~
Example
2-n-sutyl-4-hydroxymethyl-5-chloro-l-[4-[(l-carboxy)-n
pentyloxy]benzyl]imidazole
Prepared analogously to Example lb from 2-n-butyl-4-
hydroxymethyl-5-chloro-1-[4-[(1-methoxycarbonyl)-n-
pentyloxy]benzyl]imidazole and 1 N sodium hydroxide
solution in ethanol.
Yield: 57.0% of theory,
Melting point: 168C
C21Hz9ClN2O4 (408.93)
Calculated: C 61.65 H 7.14 N 6.85
Found: 61.59 7.11 6.91
Example 35
2-n-Bu-tyl-8-methyl-3-[4-[~-carboxy)benzyloxy]benzyl]-
quinazolin-4-one-semihydrate
a) 2-n-Butyl-8-methyl-3-[4-[(~-ethoxycarbonyl)-
benzyloxy]benzyl]quinazolin-4-one
Prepared analogously to Example la from 2-n-butyl-8-
methyl-quinazolin-4(lH)-one and 4-(~-ethoxycarbonyl-
benzyloxy)benzylbromide.
Yield: 37% of theory,
Oil~ Rf value: 0.45 (silica gel; eluant: petroleum
ether/ethyl acetate = 4:1)
b) 2-n-Butyl-8-methyl-3-[4-[(~-carboxy)benzyloxy]-
benzyl]quinazolin-4-one-semihydrate
. _ _
Prepared analogously to Example lb from 2-n-butyl-8-
methyl-3-[(~-ethoxycarbonyl)benzyloxy]benzyl]-
quinazolin-4-one and lN sodium hydroxide solution in
ethanol.
Yield: 84% of theory,
Melting point: 201-204C
... . . . .
.
: : :
~ ,
,
~73~3~
- 78 -
C2s~2~N2O~ x 0-5 H2O (~65.56)
Calculated: C 72.2~ H 6.28 N 6.02
Found: 72.4~ 6.33 5.75
Example 36
2-n-Butyl-4-chloro-5-hydroxymethyl~1-[~[(1-carboxy-2-
cyclohexyl)ethoxy]benzyl]imidazole
Prepared analogously to Example lb from 2-n-butyl-4-
chloro-5-hydroxymethyl-1-[4-[(1-methoxycarbonyl-2-
cyclohexyl)ethoxy]benzyl]imidazole and lN sodium ~
hydroxide solution in ethanol.
Yield: 67.1% of theory,
Melting point: 145-150C
C24H33clN2O4 (448.96)
Calculated: C 64.20 H 7.40 N 6.24
Found: 63.97 7.38 6.01
Example 37
2-n-Butyl~4-hydroxymethyl-5-chloro-1-[4-[(1-carboxy-2-
cyclohexyl)ethoxy]benzyl]imidazole
Prepared analogously to Example lb from 2-n-butyl-4-
hydroxymethyl-5-chloro-1-[4~[(1-methoxycarbonyl-2-
cyclohexyl)ethoxy]benzyl]imidazole and lN sodium
hydroxide solution in ethanol.
Yield: 59.1% of theory,
Melting point: 180-183C
C24H33ClN2O4 (448.96)
Calculated: C 64.20 H 7.40 N 6.24
Found: 63.99 7.20 6.27
Mass spectrum: m/e = 448/450 (Cl)
: - .
. .
:
- 79 - 2~3~
Example 3~
2-n-Butyl-l-[~-[(l-c~rboxy-3-methyl)butyloxy]benæyl]-
benzimidazole
Prepared analogously to Example lb from 2-n-butyl-1-[4-
[(l-methoxycarbonyl-3-methyl)butyloxy]benzyl]-
benzimidazole and 1 N sodium hydroxide solution in
ethanol. ~
Yield: 82.3% of theory,
Melting point: 150-152JC
C24~3~N2O3 (394.52)
Calculated: C 73.07 H 7.66 N 7.10
Found: 72.83 7.37 7.14
Mass spectrum: m/e = 394
Example 39
2-n-Butyl-1-[4-[(1-carboxy-2-cyclohexyl)ethoxy]benzyl]-
benzimidazole
Prepared analogously to Example lb from 2-n-butyl-1~[4-
[(1-methoxycarbonyl-2-cyclohexyl)ethoxy]benzyl]-
benzimidazole and 1 N sodium hydroxide solution in
ethanol.
Yield: 82.0% of theory,
Melting point: 165-170C
C27EI34N2O3 (434.58)
Calculated: C 74.63 H 7.88 N 6.44
Found~ 74.46 7.77 6.30
Mass spectrum: m/e = 434
Example 40
.
2-n-Butyl-6-propionylamino-1-[4-[(1-carboxy-3-methyl)-
butyloxy]benzyl]benzimidazole
Prepared analogously to Example lb from 2-n-butyl-6-
:;
.
: :
2~7~
-- ~o --
propionylamino-1-[4 [(1-methoxycarbonyl-3-methyl)-
butyloxy]benzyl]benzim:idazole and L N sodium hydroY.ide
solution in e-thanol.
Yield: 80.3% of theory,
Melting point: 120-130C
C27~3sN304 (465.59)
Calculated: C 69.65 H 7.58 N 9.02
Found: 69.30 7.8~ 8.83
Mass spectrum: m/e = 465
Example 41
2-n-Butyl-5-propionylamino-1-[4-[(1-carboxy-3-methyl)-
butyloxy]benzyl]benzimidazole-semihydrate
. _ _ _ _
Prepared analogously to Example lb from 2-n-butyl-5-
propionylamino-1-[4-[(1-methoxycarbonyl-3-methyl)~
butyloxy]benzyl]benzimidazole and 1 N sodium hydroxide
solution in ethanol.
Yield: 82.9% of theory,
Melting point: 165-170C
C27H35N3O4 x 0.5 H2O (474
Calculated: C 68.32 H 7.64 N 8.85
Found: 68.63 7.72 8.90
- ~
Example 42
2-n-Butyl-6-cyclohexylaminocarbonylamino-1-[4-[(1-
carboxy-3-methyl)butyloxy]benzyl]benzimidazole
_
Prepared analogously to Example lb from 2-n-butyl-6-
cyclohexylaminocarbonylamino-1-[4-[11-methoxycarbonyl-3-
methyl)butyloxy]benzyl]benzimidazole and 1 N sodium
hydroxide solution in ethanol.
Yield: 90.8% of theory,
Melting point: 155-160C
C3lH42N4o4 (534-71)
Calculated: C 69.64 H 7.9~ N 10.48
. ~
~ 0 73
Found: 69.58 ~.03 10.36
Mass spectrum: (M + H)+ - 535
Example 43
2-n-Butyl-5-cyclohexylaminocarbonylamino-1-[4-[(1-
carboxy-3-methyl)butyloxy]benzyl]benzimidazole
-
Prepared analogously to Example lb from 2-n-bu~yl-5-
cyclohexylaminocarbonylamino-1-[4-[tl-methoxycarbonyl-3-
methyl)butyloxy]benzyl~benzimiclazole and 1 N sodium
hydro~ide solution in ethanol.
Yield: 95.7% o~ theory,
Melting point: 220-225C
c31H42N44 (534-71)
Calculated: C 69.64 H 7.92 N 10.48
Found: 69.49 7.99 10.52
Mass spectrum: (M-tH)+ = 535
Example 44
2-n-Butyl-6-(N-propionyl-methylamino)-1-[4-[tl-carboxy-
3-methyl)butyloxy]benzyl]benzimidazole
Prepared analogously to Example lb from 2-n-butyl-6-(N-
propionylmethylamino)-1-[~-[(1-methoxycarbonyl-3-
methyl)butyloxy]benzyl]benzimidazole and 1 N sodium
hydroxide solution in ethanol.
Yield: 66.8% of theory,
Melting point: 170-175C
C28H37N304 (47g.63)
Calculated: C 70.12 H 7.78 N 8.76
Found: 70.35 7.85 8.91
;
. ~ . - . ~ ,
2 ~
-~ 82 -
Examp~e fi5
2-n-Butyl-5-(N-propionyl-methylamino)-1-[4-[(]-carboxy-
3-methyl)butyloxy]benzyl]benzimidazole
. _ _ .. . .
Prepared analogously to Example lb from 2-n-butyl-5-(N
propionylmethylamino)-l-[4-[(1--methoxycarbonyl-3-
methyl)butyloxy]benzyl]benzimidazole and 1 N sodium
hydroxide solution in ethanol.
Yield: 62.0% of theory,
Melting point: 172-175~C
C2sH37N3O4 (479.63)
Calculated: C 70.12 H 7.78 M 8.76
Found: -~0.11 7.80 8.63
Mass spectrum: m/e = 479
Example 46
2-n-Butyl-6-(N-cyclohexylaminocarbonyl-methylamino)-l-
[4-[(1-carboxy-3-methyl)butyloxy]benzyl]benzimidazole
Prepared analogously to Example lb from 2-n-butyl-6-(N-
cyclohexylaminocarbonyl-methylamino)-l-[4-[(1-
methoxycarbonyl-3-methyl)butyloxy]benzyl]benzimidazole
and 1 N sodium hydroxide solution in ethanol.
Yield: 76.1% of theory,
Melting point: 170-175C
C32H44N4O4 (548.73)
Calculated: C 70.04 H 8.08 N 10.21
Found: 69.95 8.10 10.22
Example 47
2-n-Butyl-5-(N-cyclohexylaminocarbonyl-methylamino)-l-
[4-[(1-carboxy-3-methyl)butyloxy]benzyl]benzimidazole
. _
Prepared analogously to Example lb from 2-n-butyl-5-(N-
cyclohexylaminocarbonyl-methylamino)-l-[4-[(1-
.
' . : .
:~
, : , . :`
2 ~
- ~3 -
methoxycarbonyl-3-methyl)butyloxy]benzyl]benzimidazole
and 1 N sodium hydroxide solution in ethanol.
Yield: 72.9% of theory,
Melting point: 178-1~2C
C32H44N4O4 (548.73)
Calculated: C 70.04 H 8.08 N 10.21
Found: 69.77 7.97 10.04
Mass spectrum: m/e = 548
Example 48
2-n-Butyl-3-[4-[(~-carboxy)benzyloxy]benzyl]-5-methyl-6-
dimethylaminocarbonylamino-imidazo[4,5-b]pyridine
Prepared analogously to Example lb from 2-n-butyl-3-[4-
[(~-ethoxycarbonyl)benzyloxy]benzyl]-5-methyl-6-
dimethylaminocarbonylamino-imidazo[4,5-b3pyridine and
2 N sodium hydroxide solution in ethanol.
Yield: 80.0% of theory,
Melting point: 191-193C
C29H33N5O4 (515.62)
Calculated: C 67.55 H 6.45 N 13.58
Found: 67.49 6.62 13.57
Example 49
2-n-Butyl-3-~4-[(~~carboxy)benzyloxy]benzyl] 5-methyl-6-
~3-benzyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinon-l-yl)-
imidazo[4,5-b]pyridine
Prepared analogously to Example lb from 2-n-butyl-3-[4-
[(~-ethoxycarbonyl)benzyloxy]henzyl]-5-methyl-6-(3-
ben7yl-3,4,5,6-tetrahydro-2(lH) pyrimidinon~l-yl)-
imidazo[4,5-b]pyridine and 2 N sodium hydroxide solution
in ethanol.
Yield 87.5% of theory,
Melting point: 157-160C
C37H3gN504 (617.75)
. - ,. . . . ..
' ' ., '
. ...
~73~
- n4 -
Calculated: C 71.9~ H 6.36 N 11.3~
Found: 71.71 6.3610.96
Example 50
2-n-Butyl-6-(N-methylaminocarbonyl-n-pentylamino)-1-[4-
[(~-carboxy)benzyloxy]benzyl]benzimidazole
-
Prepared analogously to Example lb from 2-n-butyl-6-(N-
methylaminocarbonyl-n-pentylamino)-1-[4-[((x-
methoxycarbonyl)benzyloxy]benzyl~benzimidazole and 1 N
sodium hydroxide solution in ethanol.
Yie]d: 76.0% of theory,
Melting point: 170-172C
C33H40N~O4 t556-71)
Calculated: C 71.20 H 7.2a N10.07
Found: 70.80 7.34 9.96
Example 51
2-n-Butyl-6 (N-cyclohexylaminocarbonyl-n-pentylamino)-l-
[4-[(~-carboxy)benzyloxy]benzyl]benzimidazole
.
Prepared analogously to Example lb from 2-n-butyl-6-(N-
cyclohexylaminocarbonyl-n-pentylamino)-1-[4-[(~-
methoxycarbonyl)benzyloxy]benzyl]benzimidazole and 1 N
sodium hydroxide solution in ethanol.
Yield: 65.0% of theory,
Melting point: 83C
C38H48N4o4 (624-83)
Calculated: C 73.05 H 7.74 N 8.97
Found:72.80 7.51 8.59
: . . . ~ .
:
.
- ~5 -
Example 52
2-n-Butyl-4-hydroxymethy],-5-ch'Loro-1-[4-[(~-carboxy-~-
phenyl)benzyloxy]benzyl]benzim:idaæole
Prepared analogously to Example lb from 2-n-butyl-4-
hydroxymethyl-5-chloro-l-[4-[(c~-methoxycarbonyl-~-
phenyl)benzyloxy]benzyl]benzimidazole and 7 N sodium
hydroxide solution in ethanol.
Yield: 50.0% of theory,
Melting point: 165~C (decomp.)
Cz9H29ClN204 (505-02)
Calculated: C 68.97 H 5.79 N 5.55
Found: 68.50 5.72 5.51
Example 53
2-n-Butyl-6-(p-methyl--phenylsulphonylamino)-1-[4-[(~-
carboxy)benzyloxy]benzyl]benzimidazole
Prepared analogously to Example lb from 2-n-butyl-6-(p-
methyl-phenylsulphonylamino)-1-[4-[(~-methoxycarbonyl)-
benzyloxy]benzyl]benzimidazole and 1 N sodium hydroxide
solution in ethanol.
Yield: 81.0% of theory,
Melting point: 150C
C33H33N30sS (583.71~
Calculated: C 67.90 H 5.70 N ~.20
Found: 67.18 5.70 6.96
Example 54
2-n-Butyl-6-~N-methyl-p-methyl-phenylsulphonylamino)-1-
[4-[(Q-carboxy)benzyloxy]benzyl]benzimidazole
_ _
Prepared analogously to Example lb from 2-n-butyl-6-(N-
methyl-p-methyl-phenylsulphonylamino)-1-[4-[(~-
methoxycarbonyl)-benzyloxy]benzyl]benzimidazole and 1 N
2~3~ ~
- ~6 -
sodium hydroxide solution in ethanol.
Yield: 96.0% of -theory,
Melting point: 218C
C34H3sN3sS (597.71)
Calculated: c 68.32 H 5.90 N 7.03
Found: 67.82 5.84 6.85
Example 55
2-n-Butyl-6-(N-n-pentyl-p-methyl-phenylsulphonylamino)-
1-[4-[(~-carboxy)henzyloxy]benzyl]benzimidazole
Prepared analogously to Example lb from 2-n-butyl-6-(N-
n-pentyl-p-methyl-phenylsulphonylamino)-l-[4-[(~-
methoxycarbonyl)-benzyloxy]benzyl]benzimidazole and 1 N
sodium hydroxide solution in ethanol.
Yield: 94.0% of theory,
Melting point: 202~C
C3sH43N3sS (653.81)
Calculated: C 69.80 H 6.63 N 6.43
Found: 69.47 6.57 6.64
Example 56
2-n-Butyl-1-[4-[(~-carboxy)benzyloxy]-(~-methyl)-
.~,
benzyl]-benzimidazole
a) 4-[(~-Ethoxycarbonyl~benzyloxylacetophenone
Prepared analogously to Example la from ethyl 2-bromo-
phenylacetate and 4-hydroxyacetophenone.
Yield: 78.0% of theory,
Rf value: 0.58 (silica gel; eluant: toluene/ethyl
acetate = 85:15)
b) 1-[4-[(~-Ethoxycarbonyl)benzyloxylphen~llethanol
2.3 g (7.7 mMol) of 4-[(~-ethoxycarbonyl)benzyloxy]-
acetophenone are dissolved in 50 ml of ethanol and at
ambient temperature 0.28 g (7.7 mMol) of sodium
,
~ ~ .
, '.. ' : ' ' '~
. ~ : - . .
.' .
. .
2 ~ r~
- 87 -
borohydride are ad~ed thereto in batches. The react;on
mixture is stirred at ~0~C for 2 hours. After cooling
to ambient temperature it is concentrated by evaporation
and the residue is mixed with water arld dilute
hydrochloric acid. After extraction with ethyl acetate
the organic phase is drie~ with sodium sulphate. The
solution is evaporated down and the residue is
chromatographed over a silica gel column (particle size:
0.063-0.02 mm) using toluene/ethyl acetate (7:3). The
uniform fractions are combined and evaporated down.
Yield: 1.0 g (~3% of theory),
Oil, Rf value: 0.50 (silica gel; eluant: toluene/ethyl
acetate = 7:3)
c) l-[~-[(~-Ethoxycarbonyl)benzyloxy]phenyl]-1-
ethanesulphonyloxyethane _ _
0.5 g (1.7 mMol) of l-[~-[(~-ethoxycarbonyl)benzyloxyJ-
phenyl]ethanol and 0.22 g (2.2 mMol) of triethylamine
are dissolved in 10 ml of methylene chloride. At 5C,
0.23 g (2.0 mMol) of mesyl chloride are added dropwise
with stirring. After one hour at ambient temperature
the reaction mixture is poured onto ice water, extracted
with methylene chloride and dried over sodium sulphate.
The crude product obtained is reacted directly without
any further purification.
d) 2-n-Butyl-1-[4-[(~-ethoxycarbonyl)benzyloxy]-(~
methyl)benzyl]benzimidazole
A mixture of 0.26 g ~1.5 mMol) of 2-n-butyl-
benzimidazolel 3 ml (1.7 mMol) of triethylamine and 1-
[4-[(~-ethoxycarbonyl)benzyloxy]phenyl]-1-
methanesulphonyloxyethane (crude) is heated to 80C for
30 minutes. After cooling, water is added and the
mixture is extracted twice with ethyl acetate. The
organic phases are combined, dried and evaporated down.
The crude product is chromatographed over a silica gel
column (particle size: 0.063-0.02 mm) with toluene/ethyl
,
: ' ' ' ' ~ '
. ~ :
;i - , '
`~ :
2073~ 1
acetate (~:2). The uniform fractions are combi.ned and
evaporated down.
Yield: O.lo g (15% of theory),
Rf value: 0.35 (silica gel; eluant: to:Luene/ethyl
acetate - 8:2)
e) 2-n-Butyl-1-[4-[(~-carboxy)benzyloxy]-(~-methyl)-
benzylJbenzimidazole
Prepared analogously to Example lb from 2-n-butyl-1-[4-
[(~-ethoxycarbonyl~benzyloxy]-(~-methyl)benzy].]-
benzimidazole and l N sodium hydroxide solution in
ethanol.
Yield: 82% of theory,
Melting point: 109C
C27H28N2o3 (4~8.51)
Calculated: C 75.67 H 6.59 N 6.54
Found: 75.93 6.76 6.32
Example 57
2-n-Butyl-1- E 4-[~ (lH-tetrazol-5-yl)benzyloxy]benzyl]-6-
(3-benzyl-3,4,5,6-tetrahydro-2(lH)pyrimidinon-l-yl)-
benzimidazole
a) ~
Prepared from benzylcyanide and sodium azide/ammonium
chloride in dimethylformamide.
Yield: 70% of theory,
Melting point: 130-132C
b) N-Triphenylmethyl-5-benzyl-tetrazole
Prepared from 5-benzyl-lH-tetrazole and triphenylmethyl-
chloride.
Yield: 84% of theory,
Melting point: 158-160C
c) N-TriPhenylmethyl-5-f -bromo)benzyl-tetrazole
2.0 g (5 mMol) of N-triphenylmethyl-5-benæyl-tetrazole,
: :
~, ; : ~ :
, -
. . . ,, .... :
:: :
2~73~ 1
_ ~9 _
0.89 g (5 mMol) of N-bromosuccinimide and 30 mg of azo-
bisisobutyronitrile are dissolved in 25 ml of carbon
tetrachloride and heated to boiling for one hour under
UV-irradiation. After cooling~ -the succinimide formed
is filtered off and the filtrate is evaporated down.
Yield: 74.6% of theory,
Melting point: 160-162C
d) 2-n-Butyl-1-[(4-benzyloxy)benzyl]-6-(3-benzyl-
3,~,5,6-tetrahydro-2(lH)pyrimidinon-1-yl)-
benzimi__zole
_
Prepared analogously to Example la from 2-n-butyl-6-(3-
benzyl-3,~,5,6-tetrahydro-2(lH)pyrimidinon~1-yl)-
benzimidazole and ~-benzyloxybenzyl chloride.
Yield: 65.5% of theory,
Rf value: 0.45 (silica gel; eluant: methylene
chloride/ethanol = 19:1)
e) 2-n-Butyl-1-[(4-hydroxy)benzyl]-6-(3-benzyl-3,4,5,6-
tetrahydro-2(1H)pyrimidinon-l-yl)-benzimidazole
2.4 g of 2-n-butyl-1-[(4-benzyloxy)benzyl]-6-(3-benzyl-
3,4,5,6-tetrahydro-2(1H)pyrimidinon-1-yl)-benzimidazole
are dissolved in 100 ml of ethanol and 30 ml of
dimethylformamide, mixed with 2.4 g of (10%) palladium
on activat~d charcoal and hydrogenated with 5 bars of
hydrogen at ambient temperature. The catalyst is
filtered off, the filtrate is evaporated down and the
residue is chromatographed over a silica gel column
(particle size: 0.063-0.02 mm) with methylene
chloride/ethanol. The uniform fractions are combined
and evaporated down and the residue is triturated with
ether and suction filtered.
Yield: 1.1 g (55% of theory),
Melting point: 190-191C
. . .
.. ..
-
'
2073~
- 90 -
f) ~-n-Butyl-l-[~-[(~-(N-triphenylmethyl~tetrazol-5-yl)
benzyloxy]benzyl]-6-(3-benzyl-3,~,5,6-tetrahydro-
2(1~l)pyrimidinon-1-yl)-ben~lmidazole _ __
O.5 g (1.1 mMol) of 2-n-butyl-1-[(4-hydroxy)benzyl]-6-
(3-benzyl-3,4,5,6-tetrahydro-2(1ll)pyrimidinon-1-yl)-
benzimidazole, 0.74 g (5.4 mMol) of potassium carbonate
and 0.93 g (1.1 mMol) of N-triphenylmethyl-5-[(~-bromo)-
benzyl]tetrazole are dissolved in 10 ml of
dimethylsulphoxide and stirred for 2 hours at ambient
temp~rature. The product is precipitated by the
addition of saline solution, suction filtered, washed
with water and dried.
Yield: 0.9 g (97~ of theory),
Melting point: from 150C (decomp.)
g) 2-n-Butyl-1-[4-[~-(lH-tetrazol-5-yl)benzyloxy]-
benzyl]-6-(3-benzyl-3,4,5,6-tetrahydro-2(lH)-
~yrimidinon-1-yl)-benzimidazole
O.9 g (1.0 mMol) of 2-n-butyl-1-[4-[(~-N-triphenyl-
methyl)tetrazol-5-yl)benzyloxy]benzyl]-6-(3-benzyl-
3,4,5,6-tetrahydro-2(lH)pyrimidinon-1-yl)-benzimidazole
are dissolved in 40 ml of ethanol, 10 ml of methylene
chloride and 12 ml of 4N hydrochloric acid and stirred
for 2 hours at ambient temperature. After the addition
of 30 ml of water, the mixture is extracted with
methylene chloride. The organic phase is washed with lN
sodium hydroxide solution, acidified with dilute acetic
acid and dried over sodium sulphate. The crude product
is chromatographed over a silica gel column (particle
size: 0.063-0.02 mm) with methylene chloride/ethanol
(50:1 and 19:1). The unified fractions are combined and
evaporated down.
Yield: 0.22 g (34~ of theory),
Melting point: from 134C (decomp.)
C37H38N8o2 (626-76)
Calculated:C 70.90 H 6.11 N 17.88
Found:70.72 6.00 17.68
, . .. . . .
:
,~ ' .
2~73~ ~
- 91 -
Example 58
2-n-Propyl-4-methyl-1-[4-[~-(lH-tetrazol-5-
yl)benzyloxy]benzyl]benzimidazole
a) 2-n-Propyl-~methyl-1-[4-[~-((N-triphenylmethyl)-
tetrazol-5-yl)benzy]oxy]benzY11benzimidazole
Prepared analogously to Example 57~ from 2-n-propyl-4-
methyl-l-[t4-hydroxy)benzyl]benzimidazole and N~
triphenylmethyl-5-[(~-bromo)benzyl]tetrazole.
Yield: 99% of theory,
Rf value: 0.68 (silica gel; eluant: methylene
chloride/methanol = 19:1)
b) 2-n-Propyl-4-methyl-1-[4-[~-(lH-tetrazol-5-yl)-
benzyloxy]benzyl] enzimidazole
Prepared analogously to Example 57g from 2-n-propyl-4~
methyl-l-[4-[~-((N-triphenylmethyl)tetrazol-5-yl)-
benzyloxy]benzyl]benzimidazole and methanolic
hydrochloric acid.
Yield: 63% of theory,
Melting point: amorphous
C26H26N6 (438.54)
Calculated: C 71.21 H 5.98 N 19.17
Found: 70.99 5.98 18.96
Example 59
2-n-Propyl-4-methyl-6-(1-methyl-benzimidazol-2-yl)-1-[4-
[~-~lH-tetrazol-5-yl)benzyloxy]benzyl]benzimidazole
_ .
a) 2-n-Propyl-4-methyl-6-(1-methyl-benzimidazol-2-yl) 1-
[4-[~-((N-triphenylmethyl)tetrazol-5-yl3benzyloxy]-
benzyl]benzimidazole
Prepared analogously to Example 57f from 2-n-propyl-4-
methyl-6-(1-methyl-benzimidazol-2-yl)-1-[(4-hydroxy)-
benzyl]benzimidazole (synthesised analogously to
Examples 58d and 58e) and N-triphenylmethyl-5-(~ bromo)-
.' ` ~
! . '
,
`` ' ' `~ ' " , ' .', '. '
2~73~
- 92 -
benzyl--tetrazole.
Yield: 94% of theory,f value: 0.70 (silica gel; eluant: methylene
chloride/~ethanol = 9:1)
b) 2-n-Propyl 4-methyl-6-(1-methyl-benzimidazol-2-yl)-l-
[4-[~-(lH-tetrazol-5-yl)benzyloxy]benzyl]-
benzimidazole
. . .
Prepared analogously to Example 57g from 2-n-propyl-~-
methyl-6-(1-methyl~benzimidazol-2-yl)~ 4-[~-((N-
triphenylmethyl)-tetrazol-5-yl)benzyloxy]benzyl]-
benzimidazole and methanolic hydrochloric acid.
Yield: 62% of theory,
Melting point: 165-166'C
C3~H32Ns (568.69)
Calculated: C 71.81 H 5.67 N 19.71
Found: 71.68 5.60 19.55
Example 60
2-n-Propyl-5~n-butyrylamino-3-[4-~(1-(lH-tetrazol-5-yl)-
3-methyl)butyloxy]benzyl]imidazo[4,5-b]pyridine
_ .
a) 2-n-Propyl-5-n-butyrylamino-3-[4-[(1-cyano-3~methyl)-
butyloxy]benzyllimidazo[4 5-blpyridine
Prepared analogously to Example 57f from 2-n-propyl-5-n-
butyrylamino-3-[(4-hydroxy)benzyl]imidazo[4,5-b]-
pyridine and 2-bromo-4-methyl-valeronitrile.
Yield: 85% of theory,
Rf value: 0.40 (silica gel; eluant: ethyl
acetate/petroleum ether = 1:1)
b) 2-n-Propyl-5-n-butyrylamino-3-[4-[(1-(lH-tetrazol-5-
yl)-3-methyl)butyloxy]benzyllimidazor4.5-blpyridine
A mixture of 800 mg (1.78 mMol) of 2-n-propyl-5-n-
butyrylamino-3-[4-[(l-cyano-3-methyl)butyloxy)benzyl]-
imidazo[4,5-b]pyridi.ne, 2.2 g (3.3 mMol) of sodium azide
and 1.8 g (3.3 mMol) of ammonium chloride in 8 ml of
- -
:.
2~3~
-- 93 --
dimethylformamide is s-tirred for 5 hours at î30c with
stirring. It is then stirrecl into ice water, the
product precipitated is suction filtered, washed with
water, dried and purified over a silica yel column
(methylene chloride/ethanol = 19:1). The fractions
containing the desired substance are evaporated down,
the residue obtained is triturated with ether, suction
filtered and dried. When the purification step
described above is repeated, 370 mg (42% of theory) of
the product are obtained, m.p. 175-177C.
C26H34N~o2 (490-61)
Calculated: C 63O65 l-l 6.99 N 22.84
Found: 63.61 7.03 22.83
Example 61
2-n-Butyl-4-hydroxymethyl-5-chloro-1-[4-[1-(lH-tetrazol-
5-yl)propyloxy]benzyl]benzimidazole sesqui-hydrochloride
Prepared analogously to Example 60b from 2-n-butyl-4-
hydroxymethyl-5-chloro-1-[4-(1-cyano-propyloxy)benzyl]-
imidazole and sodium azide/ammonium chloride in
dimethylformamide.
Yield: 78% of theory,
Oil, C~9H2sClN602 x 1.5 HCl (459.59)
Calculated: C 49.65 H 5.81 N 18.28
Found: 49.41 6.03 18.52
Example 62
2-n-Butyl -5-hydroxymethyl-4-chloro-1-[4-[1-(lH-tetrazol-
5-yl)propyloxy]benzyl]benzimidazole sesqui-hydrochloride
Prepared analogously to Example 60b from 2-n-butyl-5-
hydroxymethyl-4-chloro-1-[4-(1-cyano-propyloxy)benzyl]-
imidazole and sodium azide/ammonium chloride in
dimethylformamide.
Yield: 70% of theory,
, . . . .
,
.
2~73~1
9~ -
Oil, C~9H2sClN6O2 x 1.5 HC~ (459.59)
Calculated: C 49.65 H 5.81 N L8.28
Found: 49.86 5.76 18.26
Example 63
2-n-Butyl-1-[4 [(~-carboxy)benzylamino]benzyl]-
benzimidazole semihydrate
a) 2-n-Butyl-1-[4-[(Q-ethoxycarbonyl)benzylamino]-
benzvl~benzimidazole
1.0 g (3.6 mMol) of 2-n-butyl-1-[(4-amino)benzyl]-
benzimidazole, 0.87 g (3.6 mMol) of ethyl 2-bromo-
phe.nylacetate and 0.5 g of sodium acetate-trihydrate are
dissolved in 20 ml of ethanol and stirred for 18 hours
at ambient temperature. Then the reaction mixture is
refluxed for a further 4 hours. The solvent is
evaporated down, water is added to the residue which is
made alkaline with dilute ammonia solution and extracted
with ethyl acetate. The combined organic phases are
washed with water, dried over magnesium sulphate and
concentrated by evaporation. The residue is purified
over a silica gel column (particle size: 0.063-0.02 mm),
using petroleum ether with 10-15~ ethyl acetate as
eluant. The uniform fractions are combined and
evaporated down.
Yield: 1.0 g (63% of theory),
Rf value. 0.53 (silica gel; eluant: petroleum
ether/ethyl acetate = 3:1)
b) 2-n-Butyl-l- E 4 - E (Q-carboxy)benzylamino]benzyl]-
benzimidazole semihydrate
Prepared analogously to Example lb from 2-n-butyl-1-[4-
[(Q-ethoxycarbonyl)henzylamino]benzyl]benzimidazole and
lN sodium hydroxide solution in ethanol.
Yield: 99% of theory,
C26H27N3O2 x 0-5 HzO (422.54)
Calculated: C 73.91 H 6.68 N 9.95
:
2~3c~
- 95 -
Eound: 7~.05 6.55 9.91
Exam~le 6~
2-n-Butyl-1-[4-[(~-carboxy)-N-acetyl-benzylaminoJ
benzyl]benzimidazole
a) 2-n-Butyl-1-[4-[(~-ethoxycarbonyl)-N-acetyl-
benzylamino~benzylJbenzimidazole
O.5 g (1.1 mMol) of 2-n-butyl-:l-[4-[(~-ethoxycarbonyl)-
benzylamino]benzyl]benzimidazo:Le are dissolved in 5 ml
of ace-ticanhydride and stirred a-t 120C :for 3 hours.
The solvent is eliminated, the residue is purified over
a silica gel column (particle size: 0.063-0.02 mm),
using ethyl acetate as eluant. The uniEorm fractions
are combined and evaporated down.
Yield: 0.35 g (64% of theory),
Rf value: 0.50 (silica gel; eluant: methylene
chloride/ethanol = 95:5)
b) 2~n-Butyl-1-[4-[(~-carboxy) N-acetyl benzylamino]-
benzyl]benzimidazole
Prepared analogously to Example lb from 2-n-butyl-1-[4-
[(~-ethoxycarbonyl)-N-acetyl-benzylamino]benzyl]-
benzimidazole and lN sodium hydroxide solution in
ethanol.
Yield: 74% of theory,
Melting point: 212-214C
Cz8H29N3O3 (455.56)
Calculated: C 73.82 H 6.42 N 9.12
Found: 73.50 6.5~ 9.28
Example 65
... , ~ , , .
~ ,
2g~3~
- 96 -
2-n-Butyl-1-[4-[(2-carboxy-3-phenyl)propyllbenzyl]
benzimidazole
a) 2-n-Butyl-1-[(4-hydroxymethyl)benzylJbenzimidazole
1.2 g (30 mMol) of lithium aluminium hydride are
suspended in 200 ml of absolute tetrahydrofuran. At
ambient temperature a solution of 10.6 y (30 mMol) of 2-
n-butyl-1-[(4-ethoxycarbonyl)benzyl]benzimidazole in
lO0 ml of absolute tetrahydrofuran is added dropwise.
The mixture is stirred for 2 hGurs at ambient
temperature and then slowly hydrolysed, with cooling,
with aqueous sodium hydroxide solution. The
crystallised salts are suction filtered and the filtrate
is concentrated by evaporation. The residue is mixed
with water and ex-tracted wi-th ethyl acetate. The
combined organic phases are washed with water, dried and
concentrated by evaporation. The residue is purified
over a silica gel column (particle size: 0.063-0.02 mm),
using methylene chloride/methanol (30:1) as eluant. The
uniform frac-tions are combined and evaporated down.
Yield: 6.5 g (74% of theory),
Rf value: 0.60 (silica gel; eluant: methylene
chloride/methanol = 19:1)
b) 2-n-Butyl-1-[(4-chloromethyl)benzyl]benzimidazole
6.2 g (21 mMol) of 2-n-butyl-l-[(4-hydroxymethyl)-
benzyl]benzimidazole are dissolved in 10 ml of
thionylchloride and refluxed for 10 minutes. Then the
excess thionylchloride is distilled off and the residue
is mixed with ice water. After neutralisation with
saturated sodium hydrogen carbonate solution, the
mixture is extracted with ethyl acetate. The combined
organic phases are washed with water, dried and
evaporated down.
Yield: 6.0 g (91% of theory),
Rf value: 0.65 (silica gel; eluant: methylene
chloride/methanol = 19:1)
i : , .
2~3~ ~
- 97 -
c) 2-n-Butyl-1-[~-[(2,2-bls-ethoxycarbonyl-3~phenyl)-
propyllbenzyllbenzimidazole
2.4 g (9.6 mMol) of diethyl benzylmalonate are dissolved
in 25 ml of dimethylsulphoxide, mixed with 1.1 y
(9.6 mMol) of potassium -tert.butoxide and stirred for 30
minutes at ambient temperature~ Then a solution of
3.0 g (9.6 mMol) of 2-n-butyl-:L--[(4-chloromethyl)
benzyl]ben~imidazole in 25 ml of dimethyl~ulphoxide is
added dropwise. After one hour at ambient temperature
the mixture is heated to 100C for 15 minutes. The
reaction mixture is combined with ice water. 'rhe
product which crystallises out is suction fi.ltered and
extracted with ethyl acetate. The combined organic
phases are washed with water, dried and evaporated down.
Yield: 4.2 g (83% of theory),
Rf value: 0.15 (silica gel; eluant: methylene
chloride/methanol = 30:1)
d) 2-n-Butyl-1-[4-[(2-carboxy~3-phenyl)propyl]benzyl]-
benzimidazole
0.44 g (0.8 mMol) of 2-n~butyl-1-[4-[(2,2-bis~
ethoxycarbonyl-3-phenyl)propyl]benzyl]benzimidazole and
0.12 g of sodium hydroxlde are taken up in 30 ml of
water and refluxed for 8 hours. The mixture is then
acidified with glacial acetic acid. The product which
crystallises out is suction filtered and purified over a
silica gel column (particle size: 0.063-0.02 mm), using
methylene chloride/methanol (19:1) as eluant~ The
uniform fractions are combined, evaporated down,
triturated with ethe~, suction filtered and dried.
Yield: 0.1 g (39% of theory),
Melting point: 139-140C
C28H30N22 (426-56)
Calculated: C 78.84 H 7.09 N 6.57
Found: 78.72 6.96 6.56
Example 66
- - :
;
;, .
~ .
. :; : ' :
. i .. ..
2~73~
9~
2-n-Butyl-1-[4-[(2-carboxy-2-phenyl)ethyl]benzy]]-
benzimidazole
.
a) 2-n-Butyl-1-[4-[(2,2-bis-ethoxycarbonyl-2-phenyl)-
ethyllbenzyl]benzimidazole
Prepared analogously to Example 65c from 2-n-butyl-1-
[(4-chloromethyl)benzyl]benzimidazole and diethyl-
phenylmalonate/potassium tert.butoxide.
Yield: 82% of theory,
R~ value: 0.20 (silica gel; el~lant: methylene
chloride/methanol = 40:1)
b) 2-n-Butyl-1-[4-[(2-carboxy-2-phenyl)ethyl]benzyl]-
benzimidazole
.
Prepared analogously to Example 65d from 2-n-butyl-1-[4-
[(2,2-bis-ethoxycarbonyl-2-phenyl)ethyl]benzyl]-
benzimidazole and aqueous sodium hydroxide solution in
ethanol.
Yield: 37% of theory,
Melting point: 171-172C
C27H28N2o2 (412-54)
Calculated: C 78.44 H 6.66 N 6.88
Found: 78.61 6.84 6.79
Example 67
2-n-Propyl-5-(N-ethyl-cyclohexylcarbonylamino)-3-[4-[(~-
carboxy)benzyloxy]benzyl~imidazo[4,5-b]pyridine
. .
Prepared analogously to Example lb from 2-n-propyl-5-(N-
ethyl-cyclohexylcarbonylamino)-3-[4-[(~-ethoxycarbonyl)-
benzyloxy]benzyl]imidazo[4,5-b]pyridine and lN sodium
hydroxide solution in ethanol.
Yield: 87% of theory,
Melting point: 113-115C
C33H3gN44 (554.70)
Calculated: C 71.46 H 6.91 N 10.10
Found- 71.60 6.99 lO.00
.
. .
-
,
, : . ' .. ' :
:- . ; . :
.. .
: . , ' ' ,
.
.
2 ~ J
_ 99 _
ExampLe 6~
2-n-Propyl-5-(N-cyclohexylaminocarbony:L-ethylamino)-3-
[4-[(~-carboxy)b~nzyloxy]benzyl]imidazo[4,5-b]pyridine
Prepared analogously to Example lb from 2-n-propyl-5-(N-
cyclohexylaminocarbonyl-ethylamino)-3-[4-[(~-
ethoxycarbonyl)benzyloxy]benzyl]benzimidazole and lN
sodium hydroxide solution in ethanol.
~'ield: 20~ of theory,
Meltiny point: 183 D C
C33E~39Ns 4 ( 569.72)
Calculated: C 69.57 H 6.90 N 12.29
Found: 69.80 6.69 11.97
Example 69
2-n-Butyl-4-methyl-7-[ Q- ( lH-tetrazol-5-yl)benzyloxy]-1-
[4-[~-(lH-te-trazol-5-yl)benzyloxy]benzyl]benzimidazole
.
Prepared analogously to Example 57g from 2-n-butyl-4-
methyl-7-[ Q- ( 1-triphenylmethyl-tetrazol-5-yl)benzyloxy]-
1-[4-[~-((1-triphenylmethyl)tetrazol-5-yl)benzyloxy]-
benzyl]benzimidazole and 4N hydrochloric acid.
Yield: 52% of theory,
Melting point~ from 166C (decomp.)
C3sH34N1 02 ( 626.72)
Calculated: C 67.68 H 5.46 N 22.35
Found: 67.70 5.52 22.66
. ~
: , . :, , ~ .
: ~ .
~7.~g(~
-- 100 --
Example 70
2-n-Butyl-5-methyl-6-(3-benzyl-3,4,5,6-tetrahydro-2(lH)-
pyrimidinon-1-yl)-3-[4-[~-(lH-tetrazol-5-yl)benzyloxy]-
benzyl]imidazo~4,5-b]pyridine
Prepared analogously to Example 57g from 2-n-butyl-5-
methyl-6-(3-benzyl-3,4,5,6-tetrahydro-2(lH)pyrimidinon-
l-yl)-3-[4-[~-((1-triphenylmethyl)tetrazol-5-
yl)benzyloxy]benzyl]imidazo[4,5-b]pyridine and 4N
hydrochloric acid.
Yield: 75% of theory,
~elting point: 185-187C
C37H39N902 (661-79)
Calculated: C 69.24 H 6.12 N 19.64
Found: 68.97 6.17 19.91
Example 71
2-n-Butyl-5-dimethylaminocarbonylamino-1 [4-[~-(lH-
tetrazol-5-yl)benzyloxy]benzyl]benzimidazole
Prepared analogously to Example 57g from 2-n-butyl-5-
dimethylaminocarbonylamino-1-[4-[~-((1-triphenylmethyl)-
tstrazol-5-yl)benzyloxy~benzyl]benzimidazole and 4N
hydrochloric acid.
Yield: 66% of theory,
Melting point: 207-209~C
C29H32N802 (524-68)
Calculated: C 64.19 H 6.31 N 20.65
Found: 63.~5 6.33 2C.44
Example 72
2-Ethyl-5,7-dimethyl-3-[4-[~-(lH-tetrazol-5-yl)-
benzyloxy]benzyl]imidazo[4,5-b]pyridine semihydrate
. _
Prepared analogously to Example 57g from 2-ethyl-5,7-
.:
.
.
~:
2 0 l 3 ~
-- .I O :L
dimethyl-3-[~-L~ -triphenylmethyl)tetrazol-5-y])-
benzyloxy]benzyl]imidazo[4,5-b]pyridine-~emihydrate and
4N hydrochloric acid.
Yield: 83% of theory,
Melting point: 104-105C
C25H2sN7o x 0 5 H20 (448.53)
Calculated: C 66.95 H 5.89 N 21.83
Found: 67.05 5.94 22.36
Exam~_e 73
2~n-Propyl-4-methyl-1-[4-[~-(0-ethyl-phosphono)-
benzylamino~benzyl]benzimiclazole hydrate
a) 2-n-Propyl-4-methyl-1-[~-[~-(0,0-diethyl-phosphono)-
benzylamino]benzyllbenzimidazole
6.5 g (23.3 mMol) of 2-n-propyl-4-methyl-1-[(4-amino)-
benzyl]benzimidazole are stirred into 4.2 g (40 mMol) of
benzaldehyde. The mixture is stirred at ambient
temperature for 2 hours. Then 10.72 g (77.6 mMol) of
diethylphosphite are added and the mixture is heated to
100C for 45 minutes. The precipitate formed after
cooling to ambient temperature is triturated with 250 ml
of ether, suction filtered and dried in air. It is then
recrystallised from 150 ml of petroleum
ether/isopropanol (2:1).
Yield: 8.7 g (74% of theory),
Melting point: 110-115C
b) 2-n-Propyl-4-methyl-1-[4-[~-(0-ethyl-phosphono)-
benzylaminolbenzyllbenzimidazole hYdrate
0.5 g (1 mMol) of 2-n-propyl-4-methyl-1-[4-[~-(0,0-
diethylphosphono)benzylamino]benzyl]benzimidazole and
1.0 g of pulverised potassium hydroxide ar~ dissolved in
~0 ml of methanol and refluxed for 9 hours. After
cooling to ambient temperature, 25 ml of ice water are
added. The pH is adjusted to 6.5 by the addition of
glacial acetic acid and conc. ammonia solution. After
'
: ~
2~1~3~ ~
- 102 -
the addition of solid sodium chloride, extraction is
carried out six times, each time using lOo ml of ethyl
acetate. The combined organic phases are washed with
saturated saline solution and dried over magnesium
sulphate. After evaporation of the solvent i vacuo the
residue is triturated wi-th ether and dried over
potassium hydroxide.
Yield: 0.35 g t73% of theory),
Melting point: from 158C (decomp.)
C27H32N3O3P x H2O (495.57)
Calculated: C 65.44 H 6.92 N 8.48
Found: 65.66 6.52 8.49
Example 74
2-n-Butyl-1-[4-[~-(O-ethyl-phosphono)benzylamino]-
benzyl]benzimidazole
a) 2-n-Butyl-1-[4-[~-(O,O-diethyl-phosphono)-
benzylamino]benzyl]benzimidazole
Prepared analogously to Example 73a from 2-n-butyl-1-
~(4-amino)benzyl]benzimidazole, benzaldehyde and
diethylphosphite.
Yield: 51% of theory,
Melting point: 149-153C
. ~,
b) 2-n-Butyl-1-[4-[~-(O~ethyl-phosphono)benzylamino]-
benzyl]benzimidazole
Prepared analogously to Example 73b from 2-n-butyl-1-[4-
[~-(O,O-diethyl-phosphono)benzylamino]benzyl]
benzimidazole and methanolic potassium hydroxide
solution.
Yield: 67% of theory,
Melting point: 118-123C (decomp.)
Cz7H32N3O3P (477-55)
Calculated: C' 67.91 H 6.76 N 8.80
Found: 67.74 7.02 8.64
''
2~J3,~
- 103 -
Exam~l~ /5
2-n-Butyl-l-[4-[(~-carboxy)benzyloxy]benzyl]-
benzimidazole
a) 2-n-Butyl-1-[4-[(~-ethoxycarbonyl)benzyloxy]-
benzyl]benzimidazole
Prepared analogously to Example la from 2-n-butyl-
benzimidazole and 4-[(~-ethoxycarbonylbenzyloxy)-
benæyl]bromide.
Yield: 22.0% of theory,
Oil, Rf value: 0.65 (silica yel; eluant: ethyl
acetate/ethanol = 9~1)
C2B~30N23 ( 442.60)
Calculated: C 75.99 H 6.83 N 6.33
Found: 75.97 6.79 5.99
b) 2-n-Butyl-1-[4-[(~-carboxy)benzyloxy]benzyl]-
benzimidazole
Prepared analogously to Example lb from 2-n-butyl-1-[4-
[(a-ethoxycarbonyl)benzyloxy]benzyl]benzimidazole and
1 N sodium hydroxide solution in ethanol.
Yield: 38.0% of theory,
Melting point: 223-225C
C26H26N23 ( 414.51)
Calculated: C 75.34 H 6.32 N 6.76
Found: 75.03 6.34 6.73
Example 76
2-n~Propyl-7-methyl-3-[4-[(~-carboxy)benzyloxy]benzyl]-
imidazo[4,5-b]pyridine
_ _
a) 2-n-Propyl-7-methyl-3-E4-[(~-ethoxycarbonyl)-
benzyloxy]benzyl]imidazo~4 5-blpyridine
Prepared analogously to Example la from 2-n-propyl~
methyl-imidazo[4,5-b]pyridine and 4 [(~-ethoxycarbonyl)-
benzyloxy]benzylbromide.
, . . .
. . ~ ,
.-, . ~ ;,, ,
20r~3~
-- 10~ --
Yield: 34.0% of thPory,
Oil, Rf value: 0.40 (silica gel; eluant: methylene
chloride/ethanol - 25:1)
b) 2-n-Propyl-7-me-thyl-3-[4-[(cY-carboxy)benzyloxy]-
benzyl]imidazo~4 5~b]~yridine _ _ _ _
Prepared analogously to Example lb from 2-n-propyl-7-
methyl-3-[4-[(~-ethoxycarbonyl)benzyloxy]benzyl]-
imidazo[4,5-b]pyridine and 1 N sodium hydroxidë solution
in ethanol.
Yield: 66.4% of theory,
Melting point: 108C
C2sH2sN3O3 (415.50)
Calculated: C 72.27 ~l 6.06 N 10.11
Found: 72.25 6.03 9.87
Example 77
5,7-Dimethyl-2-ethyl-3-~4-[(c~-carboxy)benzyloxy]benzyl]-
imidazo[4,5-b]pyridine semihydrate
.
Prepared analogously to Example lb from 5,7-dimethyl-2-
ethyl-3-[4-[(~-ethoxycarbonyl)benzyloxy]benzyl]~
imidazo[4,5-b]pyridine and lN sodium hydroxide solution
in ethanol.
Yield: 89.3% of theory,
Mèlting point: 251 253C
C2sH25N3O3 x 0-5 ~2 (424
Calculated: C 70.73 H 6.17 N 9.B9
Found: 71.00 5.97 9.79
Example 78
2-n-Butyl-1-[4-[(~-carboxy)benzyloxy]-3,5-dichloro-
benzyl]-6-cyclohexylaminocarbonylamino-benzimidazole
Prepared analogously to Example lb from 2-n-butyl-1-[4-
[(cY-ethoxycarbonyl)benzyloxy]-3,5-dichlorobenzyl]-6-
. . : . ,
, ~ - , , -
.
,
~: . . : . ,
21~73~
- 105 -
cyclohexylaminoca~bonylamino-benzimidaZole and lN sodium
hydroxicle sol~ltlon in ethanol.
Yield: 95.6% of theory,
Melting poin-t: 251-253C
C33H36Cl~N4O4 (623-53)
Calculated: C 63.56 H 5.82 ~~.98 Cl 11.37
Found: 63.39 5.88 8.8711.85
Example 79
2-n-Butyl-1-[4-[(~-carboxy)benzyloxy]-3-methoxybenzyl]-
6-dimethylaminocarbonylamino-benzimidazole
Prepared analogously to Example lb from 2-n-butyl-1-[4-
[(~-ethoxycarbonyl)benzyloxyl-3-methoxybenzyl]-6-
dimethylaminocarbonylamino-benzimidazole and 2N sodium
hydroxide solution in ethanol.
Yield: 87.5% of theory,
Melting point: 170-172C
C30H34N4s (530-62)
Calculated: C 67.90 H 6.46 N 10 . 56
Found: 67.64 6.25 10.33
Example 80
2-n-Butyl-1-[4-[(~-carboxy)benzyloxy]-3-methoxybenzyl]-
5-dimethylaminocarbonylamino-benzimidaæole
. _ _ _ . . . _ . . . ~ . .
Prepared analogously to Example lb from 2-n-butyl-1 t4-
~ ethoxycarbonyl)benzyloxy]-3-methoxybenzyl]-5-
dimethylaminocarbonylamino-benzimidazole and 2N sodium
hydroxide solution in ethanol.
YieldO 78.6% of theory,
Melting point: 154-156C
C30H34N4Os (530-62)
Calculated:C 67.90 H 6.46 N 10.56
Found:67.74 6.37 10.24
'' ' : ' ,' ' ' ' ~' '~ ' :
.. ~ . ' ' . . . . , ,, ~ .
. , . ~ .:
21~3~
- ~06 -
E ~lmple 8:L
2-n-Propyl-1-[4-[(~-carboxy)benzyloxy] 3,5-
dimethoxybenzyl]-6-(1-methyl-benzimidazol-2-yl)-4-
methyl-benzimidazole hydrate
_ _ . _ _
Prepared analogously to Example lb from 2-n-propyl~l-[4-
[(~-ethoxycarbonyl)benzyloxy]-3,5-dimethoxybenzyl]-6-(l-
methyl~benzimidazol-2-yl)-4-methyl-benzimidazole and 2N
sodium hydroxide solution in ethanol.
Yield: 91.4% of theory,
Melting point: 148-150C
C36H36N40s x HzO (622.72)
Calculated: C 69.44 H 6.25 N 9.00
Found: 69.77 6.09 8.92
Example 82
2-n-Propyl-l-[4-[(~-carboxy)benzyloxy]-3,5-
dibromobenzyl]-6-(l-methyl-benzimidazol-2-yl)-4-methyl-
benzimidazole hydrate
_
Prepared analogously to Example lb from 2-n-propyl-l-[4-
[(~-ethoxycarbonyl)benzyloxy]-3,5-dibromobenzyl]-6-(1-
methyl-benzimidazol-2-yl)-4-methyl-benzimidazole and 2N
sodium hydroxide solution in ethanol.
Yield: 58.4~ of theory,
Melting point: 229-230~C
C34H30Br2N4o3 x H20 (720.46)
Calculated:C 56.68 H 4.47 N 7.77
Found:56.78 4.64 7.75
, . : .
' ~ ~
: . , :
,
.
2~7~
~07 -
Example 83
2-n-Propyl-6~(2,3-dimethylsuccinimino)-1-[4-[(~-
carboxy)benzyloxy]-3,s-dimetho~ybenæyl]-benzimid~zole
semihydrate
Prepared analogously to Example lb from 2-n-propyl-6-
(2,3-dimethylsuccinimino)-1-[4--[(~-ethoxycarbonyl)-
benzyloxy]-3,5-dimethoxybenzyl]-benzimidazole and 2N
sodium hydroxide solution in et:hanol.
Yield: 13.8% of theory,
Melting point: 129-13:L~C
C34H37N307 x 0-5 H20 (608.69)
Calculated: C 67.09 H 6.29 N 6.90
Found: 67.29 6.37 6.86
Example 84
2-n-Butyl-6-(1-cyclohexen-1,2-dicarbonylimino)-1-[4-[(~-
carboxy)benzyloxy]benzyl]benzimidazole semihydrate
Prepared analogously to Example lb from 2-n-butyl-6-(1-
cyclohexen-1,2-dicarbonylimino)~ 4-[(~-
ethoxycarbonyl)benzyloxy]benzyl~benzimidazole and lN
sodium hydroxide solution in ethanol.
Yield: 23.3% of theory,
Melting point: 247-249C
C34H33N305 x 0-5 H20 (572.66)
Calculated: C 71.30 H 5.98 N 7.33
Found: 71.47 6.05 7.15
Example 85
2-n-Propyl-4-methyl-1-[~-[(~-carboxy)-2-chloro-
benzyloxy]benzyl]benzimidazole
: .
Prepared analogously to Example lb from 2-n-propyl-4-
methyl-l-[4-[(~-ethoxycarbonyl)-2-chlorobenzyloxy]-
: ~, :: `~ ',' ' '` , . : . : ,
. , .
.:
2~73~
-- 10~ --
benzyl]ben~imidazole and 2N sodium hydroxide solution in
ethanol.
Yield: 40.7% of theory,
Melting point: sinters from 105~C
C26H2sClN2O3 (448.97)
Calculated: C 70.00 H 5.62 N 6.28 Cl 7.91
Found: 69.94 5.72 6.29 7.92
Example 86
2-n-Propyl-4-chloro-6-(1-oxo-2-isoindolin-2~yl)-l-[4-
[(~-carboxy)benzyloxy]benzyl~benzimidazole
_
Prepared analogously to Example lb from 2-n-propyl-4-
chloro-6-(1-oxo-2-isoindolin-2-yl)-1-[4-[(~-
ethoxycarbonyl)benzyloxy]benzyl]benzimidazole and 2N
sodium hydroxide solution in ethanol.
Yield: 61.0% of theory,
Melting point: 252-254C
C33H28ClN3O4 (566.06)
Calculated: C 70.00 H 5.02 N 7.42Cl 6.25
Found: 69.81 5.22 7.876.54
Example 87
2-n-Butyl-6-(1-cyclohexen-1,2-dicarbonylimino)-1-[4-[~-
(lH-tetrazol-5-yl)benzyloxy]benzyl]benzimidazole
.
Prepared analogously to Example 57g from 2-n-butyl-6-(1-
cyclohexen-1,2-dicarbonylimino)-l-[4-[(~-(N-
triphenylmethyl)tetrazol-5-yl)benzyloxy]benzyl]-
benzimidazole and 85% formic acid in methylene chloride.
Yield: 43.7% of theory,
Melting point: 149-151C
C34H33N7O3 ~587.68)
Calculated. C 69.49 H 5.66 N 1~.68
Found: 69.40 5.83 16.31
. , ~ . . .
- . . .
.
': :
2~3~1
-- 109 -
xample 88
2-n-Butyl-5-methyl-6-dimethylaminocarbonylamino-3-[4-[c~-
(lH-tetrazol-5-yl)benzyloxy]benzyl]imidazolL4,5-b]-
pyridine
Prepared analogously to Example 57g from 2-n-butyl-5-
methyl-6-dimethylaminocarbonylamino-3-[4-[(~-(N-
triphenylmethyl)tetrazol-5-yl)benzyloxy]benzyl]imidazo-
[4,5-b]pyridine and 4N hydrochloric acid in ethanol.
Yield: 75% oE theory,
Melting point: 198-200UC
C29H33N902 (539-64)
Calculated: C 64.54 H 6.16 N 23.36
Founcl: 64.37 6.24 23.57
Example 89
2-n-Butyl-6-(cyclohexylaminocarbonyl-N-methylamino)-l-
[4-[~-(lH-tetrazol-5-yl)benzyloxy]benzyl]benzimidazole
Prepared analogously to Example 57g from 2-n-butyl-6-
(cyclohexylaminocarbonyl-N-methylamino)-1-[4-[(~-(N-
triphenylmethyl)-tetrazol--5-yl)benzyloxy]benzyl]-
benzimidazole and 4N hydrochloric acid in ethanol~
Yield: 85.-l~6 of theory,
Melting point: 195-197C
C34H40N8o2 (592-74)
Calculated: C 68.89 H 6.80 N 18.90
Found: 68.82 6.91 18.77
Example 90
5,7-Dimethyl-2 ethyl-3-[4-[~-(lH-tetrazol-5-yl)-
benzyloxy]-3,5-dichlorobenzyl]imidazo[4,5-b]pyridine
hydrate
Prepared analogously to Example 57g from 5,7 dimethyl-2-
~ . . ,
,:
~ :
:'
- ~ , -
2~3~
-- 110 --
ethyl-3-[4-[(~-(N-triphenylmethy])tetrazol-5-
yl)benzyloxyl-3,5-dichlorobenzyl]imidazo[4,5-b]pyridine
and 85~ formic acid in methylene chloride.
Yield: 77~2% of theory,
Melting point: 143-145C
C2sH23cl2N7o x H2O (526.42)
Calculated: C 57.04 H 4.78 N18.62 Cl 13.47
Found: 57~51 4.82 19.03 13.38
Examp1e 91
2-n-Butyl-6-cyclohexylaminocarbonylamino-1-[4-[~--(lH-
tetrazol-5-yl)benzyloxy]-3,5-dichlorobenzyl]-
benzimidazole semihydrate
___
Prepared analogously to Example 57g from 2-n-butyl-6-
cyclohexylaminocarbonylamino-1-[4~[t~-(N-triphenyl-
methyl)tetrazol-5-yl)benzyloxy]-3,5-dichlorobenzyl]~
benzimidazole and 85% formic acid in methylene chloride.
Yield: 83% of theory,
Melting point: 129-131C
C33H36cl2Nso2 x 0-5 H2O (655.62)
Calculated: C 60.36 H 5.68 N 17.07
Found: 60.40 5.78 17.19
Example 92
2-n-Butyl-6-dimethylaminocarbonylamino-1-[4-[~-(lH-
tetrazol-5-yl)benzyloxy]-3-methoxybenzyl]benzimidazole
Prepared analogously to Example 57g from 2-n-butyl-6-
dimethylaminocarbonylamino-1-[4-[(~-(N-triphenylmethyl)-
tetrazol-5-yl)benzyloxy~-3-methoxybenzyl]benzimidazole
and 4N hydrochloric acid in ethanol. -
Yield: 75~ of theory,
Melting point: 158-162C
C30H34N8O3 (554.65)
Calculated: C 64.97 H 6.18 N 20.20
.
,
'
:
~73~
-- 11.1 --
Found. 6~.71 6.20 19.91
Example 93
2-n-Propyl-4-methyl-6-(1-methyl-benzimidazol-2-yl)-1-[4-
[~-(lH-tetrazol-5-yl)benzyloxy]-3,5-dimethoxybenzyl]~
benzimidazole hydrate
Prepared analogously to Example 57g from 2-n-propyl--4-
methyl-6 (1-methyl-benzimidazo:L-2~yl)-1-[4 [(~-(N-
triphenylmethyl)tetrazol-5-yl)benzyloxy]-3,5-
dimethoxybenzyl]benzimidazole and 85% formic acid~in
methylene chloride.
Yield: 83% of theory,
Melting point: 152-154C
C36H36N~03 x ~I20 (646.75)
Calculated: C 66.86 H 5.97 N 17.33
Found: 67.08 5.9~ 16.97
Example 94
2-n-Propyl-4-methyl-6-(2,3-dimethylsuccinimino) 1-[4-[a-
(lH-tetrazol-5-yl)benzyloxy~-3,5-dimethoxybenzyl]-
benzimidazole semihydFate
Prepared analogously to Example 57g from 2-n-propyl-4-
methyl-6-(2,3-dimethylsuccinimino) 1-t4-[(~-(N-
triphenylmethyl)tetrazol-5-yl)benzyloxy~-3,5-
dimethoxybenzyl]benzimidazole and 85% formic acid in
methylene chloride.
Yield: 74.6% of theory,
Melting point: 192-144C
C34H37N705 x 0-5 H20 (632.71~
Calculated: C 64.54 H 6.05 N 15.50
~ound: 64.46 6.10 15.25
:
'
. -- ~ . .
- : : :. , .:
:, ~
. ;, . ~ ~ :: ; . - - .. . . . . . .
:
2~73~
~ 112 -
Example 95
2-n-Propyl-4~methyl-6-(1-methyl-berlzimidazol-2-yl)-1-[4-
[~ LH-tetrazol-5~yl)benzyloxy]-3,5-dibromobenzyll-
benzimidazole
___ _ .
Prepared analogously to Example 57g from 2-n~propyl-4-
methyl-6-(1-methyl-benzimidazol-2-yl)-1-[4-[(~-(N-
triphenylmethyl)tetrazol-5-yl)benzyloxy]-3,5- ~
dibromobenzyl]benzimidazole and 8S~ formic acid in
methylene chloride.
Yield: 21.6% of theory,
Melting point: 132-134C
C34H30Br2N~0 (726.47)
Calculated: C 56.21 H 4.16 N 15.42Br 21.99
Found: 56.53 4.50 15.42 22.03
Example g6
2-n-Butyl-5-dimethylaminocarbonylamino-1-[4-[Q-(lH-
tetrazol-5-yl)benzyloxy]-3-methoxybenzyl]-benzimidazole
Prepared analogously to Example 57g from 2-n-butyl-5-
dimethylaminocarbonylamino-1-[4-[(~-(N-triphenylmethyl)-
tetrazol~5-yl)benzyloxy]-3-methoxybenzyl]benzimidazole
and 4N hydrochloric acid in ethanol.
Yield: 88.2% of theory,
Melting point: 181-185C
C30H34N8O3 (554-66)
Calculated: C 64.96 H 6.18 N 20.20
Found: 65.18 5.92 19.97
:
' : ~: '. , :, .: . ' .
2 ~
~ 113
Exan)ple 97
5,7-Dimethyl-2-ethyl-3-[4-[~-(0-ethyl-phosphono)-
benzyloxy]benzyl]imidazo[4,5-b]pyridine sodium salt-
semihydrate
_ _
Prepared analogously to Example 73b from 5,7-dimethyl~2-
ethyl-3-[4-[~-(0,0-diethylphosphono)benzyloxy]benzyl~-
imidazo[4,5-b]pyridine and potassium hydroxide in
methanol.
Yield: 52% of theory,
Melting point: from 150C (decomp.)
H30N4NaO3p x 0-5 H20 (509-
Calculated: C 61.29 H 6.33 N 11.00
Found: 60.95 6.34 lO.9B
Example 98
2-n-Propyl-4-methyl-1-[4-[~-(0-ethyl-phosphono)-N-
methyl-benzylamino]benzyl]benzimidazole semihydrate
. _
a) 2-n-Propyl-4-methyl-1-[4-[~-(0,0-diethylphosphono)-N-
methyl-benzylaminolbenzyl]benzimidazole
1.1 g ~2 mMol) of 2-n-propyl-4~methyl-1-[4-[~-(0,0-
diethylphosphono)-benzylamino]benzyl]benzimidazole are
dissolved in 1 ml of formic acid and mixed with 2 ml of
a 37~ formalin solution. The mixture is stirred for 2
hours at 100C, cooled and poured onto ice. After the
addition of conc. ammonia the mixture is extracted twice
with ethyl acetate. The combined organic phases are
washed with saturated saline solution and dried over
magnesium sulphate. The organic phase is evaporated
down ln vacuo and the residue obtained is purified over
a silica gel column (particle size: 0.063-0.02 mm, ethyl
acetate~petroleum ether = 1:1 to 1:2). The uniform
fractions are combined and evaporated down in vacuo.
. ~
.
2~73~ 1
Yield: 0. 60 g (58% of theory),
Rf value: 0.45 (silic~ y~l; eluan-t: petroleum
ether/ethyl acet~te = 1:1)
b) 2-n-Propyl-4-methyl-1-[~-[~ (0-ethyl-phosphono)-N-
methyl-henzylamino]benzyl~enzlmldazole semih~drate
Prepared analoyously to Example 73b from 2-n-propyl-4-
methyl-l-[4-[~-(0,0-diethylphosphono)-N-methyl-
benzylamino]benzyl]benzimidazole and potassium hydroxide
in methanol.
Yield: 77% of theory,
Melting point: from 106C (decomp.)
C28H34N303P x 0-5 H20 (500-
Calculated: C 67.18 H 7.05 N 8.40
Found: 67.25 7.02 8.42
Example 99
2-n-Propyl-4-methyl-1-[4-[~-(0-ethyl-phosphono)benzyl-
amino]benzyl]benzimidazole hydrobromide
. _ _
0.5 g (1.0 mMol) of 2-n-propyl-4-methyl-1-[4-t~-(0,0-
diethylphosphono)benzylamino]benzyl]benzimidazole are
dissolved in 10 ml of 48% hydrobromic acid and refluxed
for 5 hours. After cooling at ambient température, the
pH is adjusted to 6 by the addition of concentrated
ammonia and glacial acetic acid. ~he precipitate formed
is suction filtered and dried over potassium hydroxide
at 40C.
Yield: 47% of theory,
Melting point: from 140C (decomp.)
sH2sN33P x HBr (530.42)
Calculated: C 56.61 H 5.51 N 7.92
Found: 56.48 5.80 8 05
.
. . : -
. ::.: , . ~ :.
2~73(~
- 115 -
Example 100
2-n-Propyl-~-methyl-1-[4-[(~-methanesulphonylamino-
carbonyl)benzyloxy]benzyl]benzimidazole
. _
410 mg (1 mMol) of 2-n-propyl-4-methyl-1 [4-[(~-
carboxy)benzyloxy]benzyl]benzimidazole are dissolved in
100 ml of thionylchloride, mixed with 1 drop of
dimethylformamide and refluxed for 1 hour. Then, after
the addition of -toluene, the mixture is evaporated to
dryness and taken up in 30 ml of acetone. After the
addition of 1 ml of triethylamine and 150 mg (1.58 mMol)
of methanesulphonic acid amide the mixture is stirred
for 2 hours at ambient temperature. The reaction
mixture is then evaporated down and the residue is
purified over a silica gel column (particle size:
0.063-0.02 mm; methylene chloride/ethanol = 50:1, 19:1,
9:1 and 4:1). The uniform fractions are combined,
evaporated down and the residue obtained is triturated
with ether and dried.
Yield: 15.3% of theory,
Melting point: 139-141C
Cz7N29N3O4S (491.60)
Calculated: C 65.g5 H 5.94 N 8.54
Found: 65.67 6.27 8.89
Example 101
2-Ethyl-4-methyl-1-[4-[(~-carboxy)benzyloxy]benzyl]-6
(n-butanesultam-l-yl)benzimidazole
Prepared analogously to Example lb ~rom 2-ethyl-4-
methyl 1-[4-~[(~-ethoxycarbonyl)benzyloxy]benzyl]-6-(n-
butanesultam-l-yl)-benzimidazole and 2N sodium hydroxide
solution in ethanol.
.,. ~ ~ ~ - . .
.: ~ , :,
,
. ,;
, ' , . '
.
2~3~
- 116 -
Example 102
2-Ethyl-4-methyl-1-[~-[~ H-tetrazol-5-yl)benzyloxy]-
benzyl]-6-(n-butanesultam-1-yl)benzimidazole
. ~
Prepared analogously to Example 57g from 2-ethyl-4-
methyl-1-[4 [(~-(N-triphenylmethyl)tetrazol-5~yl)-
benzyloxy]benzyl]-6-(n-butanesultam-1-yl)-benzimidazole
and 4N hydrochloric acid in ethanol.
Example 103
2-n-Butyl-1-[4-[~-(ethoxycarbonyl)-~-(2-pyridyl)-
methoxy]benzyl]-6-(N-cyclohexylaminocarbonyl-
methylamino)-benzimidazole
Prepared analogously to Example la from 2-n-butyl-1-(4-
hydroxybenzyl)-6-(N-cyclohexylaminocarbonyl-
methylamino)-benzimidazole and ethyl ~-bromo-pyridyl-2-
acetate.
Yield: 90.0% of theory,
R~ value: 0.70 (silica gel; ethyl acetate/ethanol/
ammonia = 90:10:1)
C33H44N504 (574.76)
Calculated: C 70.20 H 7040 N 11.69
Found: 69.97 7.26 11.21
Example 104
2~n-Butyl-3-[4-[(~-carboxy)benzyloxy]-3-methoxybenzyl]-
5-methyl-6-dimethylaminocarbonylamino-imidazo[4,5-b]-
pyridine
Prepared analogously to Example lb from 2-n-butyl-3-[4-
[t~-ethoxycarbonyl)benzyloxy]-3-methoxybenzyl~-5-methyl-
6-dimethylaminocarbonylamino-imidazo[4,5-b]pyridine and
2N sodium hydroxide solution in ethanol.
Yield: 91.8% of theory,
- . .. . .
, i : , . :
.
: ' :
:~
- 117 -
Melting point: 174-176C
Rf value: 0.60 (sili~a gel; ethyl acetate/ethanol/
ammonia -- 50:45:5)
C30H3sNsOs (545.65)
Calculated: C 66.0~ H 6.~7 N 12.8~
Found: 66.25 6.39 12.95
Example 105
2-n-Butyl-1-[4-[~-(ethoxycarbonyl)-~-(2-pyridyl~meth-
oxy]benzyl]-6 dimethylaminocarbonylamino-benzimidazole
.. . . _ _
Prepared analogously to Example la ~rom 2-n-butyl-1-(4-
hydroxybenzyl)-6-dimethylaminocarbonylamino-
benzimidazole and ethyl ~-bromo-pyridyl-2-acetate.
Yield: 87.5% of theory,
Rf value: 0.50 (silica gel; ethyl acetate/ethanol/
ammonia = 90:10:1)
C30H3sNsO4 (529-65)
Calculated: C 68.03 H 6.66 N 13.22
Found: 68.09 6.83 13.06
Example 106
2-n-Butyl-l-[4-[~-(ethoxycarbonyl)-Q- ( 2-pyridyl)meth-
oxy]benzyl]-6-cyclohexylcarbonylamino-benzim.idazole
Prepared analogously to Example la from 2-n-butyl-1-(4-
hydroxybenzyl)-6-cyclohexylcarbonylamino-benzimidazole
and ethyl ~-bromopyridyl-2-acetate.
Yield: 75.3% of theory,
Rf value: 0.50 (silica gel; ethyl acetate/ethanol/
ammonia = 90:10:1)
C34H40N404 (568-73~
Calculated:C 71.80 ~ 7.09 N 9.35
Found:71.82 7.21 9.83
:~ '
:
: ,
2~3~
Exa~le_l07
2-n-Butyl-3-[~-[~ l-tetrazol-5-yl)benzyloxy]-3-
methoxybenzyl]-5-methyl-6-dimethylaminocarbonylamino-
imidazo[4,5-b]pyridine
Prepared analogously to Example 57g from 2-n-butyl-3 [4-
[~-((N-triphenylmethyl)-tetrazol-5-yl)benzyloxy]-3-
methoxybenzyl]-5-methyl-6-dimethylaminocarbonyIamino-
imidazo[4,5-b]pyridine and 4 N hydrochloric acid in
ethanol.
Yield: 60.0% of theory,
Melting point: 197-199C
Rf value: 0.60 (silica gel; ethyl acetate/ethanol/
ammonia = 50:45:5)
C30H35N903 (569.68)
Calculatedo C 63.25 H 6.19 N 22.13
Found: 62.94 6.13 21.87
Example 108
2-n-Butyl-1-[4-[~-(lH-tetrazol-5-yl)-~-(2-pyridyl)-
methoxy]benzyl]-6-dimethylaminocarbonylamino-
benzimidazole
.. .. . _ , .
Prepared analogously to Example 57g from 2-n-butyl-1-[4-
[~-((N-triphenylmethyl)-tetrazol-5-yl~-~-(2-
pyridyl)methoxy]benzyl]-6-dimethylaminocarbonylamino-
benzimidazole and 4 N hydrochloric acid in ethanol.
Yield: 25.0% of theory,
Melting point: 197-203C
Rf value: 0.50 (silica gel; ethyl acetate/ethanol/
ammonia = 50:45:5)
C28H31N902 (525.63)
Mass spectrum: (M+H) = 526
.- , ~ . . , ,:
'' ' ' '
,
2~3~
- 119 -
Example 109
2-Ethyl-3-[4-[~-(lH-tetrazol-5-yl)benzyloxy]benzyl] 5-
methyl-6-dimethylaminocarbonylamino-imidazo[4,5-b~-
pyridine
Prepared analogously to Example 57g from 2-ethyl-3-[4-
[~-((N-triphenylmethyl)-tetrazol-5-yl)benzyloxy]benzyl]-
5 methyl-6-dimethylaminocarbonylamino imidazo[4,5-b]-
pyridine and 4 N hydrochloric acid in ethanol.
Yield: 50.0% of theory,
Melting point: 234-236C
C27H29N902 (511-60)
Calculated- C 63.39 H 5.71 N 2~.64
Found: 63.36 5.87 24.52
Example 110
2-n-Butyl-4-methyl-1-[4-[~-(lH-tetrazol-5-yl)benzyloxy]-
benzyl]-6-(2,3-dimethylsuccinimino)benzimidazole
Prepared analogously to Example 57g from 2-n-butyl-4-
methyl-1-[4-[~-t(N-triphenylmethyl)-tetrazol-5-yl)-
benzyloxy]benzyl]-6-(2,3-dimethylsuccinimino)-
benzimidazole and 4 N hydrochloric acid in ethanol.
~ .~
Yield: 80.0~ of theory,
Melting point: 175-177C
C33H3sN73 (577 703
Calculated: C 68.61 H 6.11 N 16.97
Found: 68.45 6.24 17.00
Example 111
2-n-Butyl-4-methyl-1-[4-~(~-carboxy)benzyloxy]benzyl]-6-
cyclohexylcarbonylamino-benzimidazole
. . _ _
Prepared analogously to Example lb from 2-n-butyl-~-
methyl-1-[4-[(~-ethoxycarbonyl)benzyloxy]benzyl]-6-
,, . . ~
`:
2 ~ rl~ 3 ~
- 120 -
cyclohexylcarhonyLamino-benzimldazole and 2 N sodium
hydroxide solution in ethanol.
Yield: 81.8% of theory,
Melting point: 191-193 D C
C34H39N30,, (553.70)
Calculated: c 73.75 H 7.10 N 7.58
Found: 73.70 6.94 7.48
Example 112
2-n Butyl-4-methyl-1-[4-[~-(lH-tetrazol-5-yl)benz~loxy]-
benzyl]-6-cyclohexylcarbonylamino-benzimidazole
-
Prepared analogously to Example 57y from 2-n-butyl-4-
methyl-1-[4-[~-((N-triphenylmethyl)-tetrazol-5-yl)-
benzyloxy]benzyl]-6-cyclohexylcarhonylamino-
benzimidazole and 4 N hydrochloric acid in ethanol.
Yield: 85.0% of theory,
Melting point: 195-198C
C34H39N702 (577-72)
Calculated: C 70.68 H 6.80 N 16.97
Found: 70.81 6.98 16.97
Example 113
Z-n-Butyl-4-methyl-1-[4-[~-(carboxy)benzyloxy]benzyl]-6-
tert.butylcarbonylamino-benzimidazole
Prepared analogously to Example lb from 2-n-butyl-4-
methyl-1-[4-[(~-ethoxycarbonyl)benzyloxy]benzyl]-6-
tert.butylcarbonylamino-benzimidazole and 2 N sodium
hydroxide solution in ethanol.
Yield: 78.6% of theory,
Melting point: 179-181~C
C32H37N304 (527-67)
Calculated: C 72.34 H 7.07 N 7.96
Found: 72.51 6.95 7.65
,
.
' ' `' ~ ~ ' " "' , ~ .
~73$~
- ~21 -
Exam~æ~ 114
2-n-Butyl~4-~ethyl-1-[4-[~-(tetrazol-5-yl)benzyloxy]-
benzyl]-6-tert.butylcarbonylamino-benzimidazole
. _ .. . . _
Prepared analogously to Example 57g from 2-n-butyl-4-
methyl-1-[4 [-((N--triphenylmethyl)-tetrazol-5-
yl)benzyloxy]benzyl]-6-tert.butylcarbonylamino-
benzimidazole and 4 N hydrochloric acid in ethano].
Yield: 75.0% of theory,
Melting point: 191-193C
C32H37N7O2 (551.70)
Calculated: C 69.67 H 7.76 N 17.77
Found: 69.42 7.80 17.68
Example 115
2-Ethoxy-7-ethoxycarbonyl-1-[4-[(~-ethoxycarbonyl)-
benzyloxy]benzyl~benzimidazole
_
Prepared analogously to Example 63a from 2-ethoxy-7-
ethoxycarbonyl-l-(4-hydroxy)benzyl-benzimidazole and
ethyl 2-bromo-phenylacetate.
Yield: 45.5% of theory,
Oil, Rf value: 0.30 (silica gel; ethyl acetate/petroleum
` ether = 30:70)
Example 116
2-Ethoxy-7-carboxy-1-[4-(~-carboxy~benzyloxy]benzyl]-
benzimidazole semihydrate
Prepared analogously to Example lb from 2-ethoxy-7-
ethoxycarbonyl-1-[4-[(~-ethoxycarbonyl)benzyloxy]-
benzyl]benzimidazole and lN sodium hydroxide in ethanol
at 80C.
Yield: 90.9% of theory,
Melting point: 180-182C
:: '
....
2~ ~33~.~
- 122 -
C2sH22N206 x 0.5 H20 (~55- )
Calcula-ted: C 65.92 H 5.09 N 6.15
Found: 65.8~ 4.97 6.17
Examp~e 117
2-Ethoxy-7-ethoxycarboxy~1-[4-(~-carboxy)benzyloxy]-
benzyl]-benzimidazole
Prepared analogously to Exampl~e lb from 2-ethoxy-7-
ethoxycarbonyl-1-[4-[(~-ethoxycarbonyl)benzyloxy~=
benzyl]benzimidazole and lN sodium hydroxide in ethanol
at room temperature.
Yield: 59.6% of theory,
Melting point: 185-187C
C27H26N206 (474.50)
Calculated: C 68.34 H 5.52N 5.90
Found: 67.84 5.64 5.90
Example 118
2-Ethoxy-7-carboxy-1-[4-[~-(lH-tetrazol-5-yl)-
benzyloxy]benzyl]benzimidazole-semihydrate
. . .
Prepared analogously to Example lb from 2-ekhoxy-7-
ethoxycarbonyl-1-[4-[~-((N-triphenylmethyl)tetrazol-5-
yl)benzyloxy]benzyl]benzimidazole and lN sodium
hydroxide in ethanol.
Yield: 78.3~ of theory,
Melting point: 154-156C (decomp.)
C2sH22N6O4 x 0.5 H20 (47
Calculated: C 62.62 H 4.83 N 17.52
Found:62.76 4.92 17.24
. ~
- . : : , - .. ::~ , , ~
:
: ',, , ' ' .:, . ., .: .~: ~ ', , . ~
. .
2~73~
- I.23 -
Example_119
2-Ethoxy-1-[4-[~-carboxy)benzyloxy]benzylJ-lH-
benzimidazol-4-carboxylic acid
. ~
Prepared analogously to Example lb from 2-ethoxy-1-[4-
[(~-ethoxycarbonylbenzyloxy]benzyl]-4-ethoxycarbonyl~lH-
benzimidazole and lN sodium hydroxide in ethanol.
Yield: 34.9~ of theory,
Melting point: 131-133C
Rf value: 0.25 (silica gel; ethyl acetate/ethanol/
ammonia = 80:~0:2)
- . ~
: ' ' ,-
. . ' :
.~ ' .
~3,5~4 L
- 12~ -
In the Examples of Pharmaceutlcal Formulations which
Eollow, any suitable compound of formula I, particularly
compounds A to I o~ the pharmacological test report, m~y
be used as the active substance:
Exam~le I
Ampoules containing 50 mg of active substance per 5 ml
Composition:
Active substance 50 my
KH2P04 2 mg
Na2HP04 x 2H20 50 mg
NaCl 12 mg
Water ~or injections ad 5 ml
Preparation:
The buffer suhstances and isotonic substance are
dissolved in some of the water. The active substance is
added and, once it has been completely dissolved, water
is added to make up the required volume.
Example II
Ampoules containing 100 mg of active substance per 5 ml
.. ... .. _ _
.
Composition:
Active substance 100 mg
Methyl glucamine 35 mg
Glycofurol 1000 mg
Polyethyleneglycol-polypropylene-
glycol block polymer 250 mg
Water for injections ad 5 ml
2 ~ 7 ,~
- 125 -
Pre~aration:
Methyl glucamine is dissolved ln some of the water and
the active substance is dissolved with stirring and
heating. After addition of the solvents, water is added
to make up the desired volume.
Example III
Tablets containing 50 mg of active substance
.
Composition:
Active substance 50.0 mg
Calcium pho~phate 70.0 mg
Lactose 40.0 mg
Corn starch 35.0 mg
Polyvinylpyrrolidone 3.5 mg
Magnesium stearate 1.5 mg
200.0 mg
Preparation:
The active substance, CaHPO4, lactose and corn starch are
uniformly moistened with an aqueous PVP solution. The
mass is passed through a 2 mm screen, dried at 50~C in a
circulating air dryer and screened again.
After the lubricant has been added, the granules are
compressed in a tablet making machine.
.
. :, ' ' '. ' . . '
.' . ; .
.
~3~1
- 126 -
Example IV
Coated table-ts containing 50 mg of active substance
Composition:
Active substance 50.0 my
Lysine 25.0 mg
Lactose 60.0 mg
Corn s-tarch 34.0 mg
Gelatin 10.0 mg
Magnesium stearate
180.0 mg
Preparation:
The active substance is mixed with the excipienks and
moistened with an a~ueous gelatin solution. After
screening and drying the granules are mixed with
magnesium stearate and compressed to form tablet cores.
The cores thus produced are covered with a coating by
known methods. A colouring may be added to the coating
suspension or solution.
- Example V
~-
Coated tablets containing 100 m~ of active substance
... . . _ _
Composition:
Active substance 100.0 mg
Lysine 50.0 mg
Lactose 86.0 mg
Corn starch 50.0 mg
Polyvinylpyrrolidone 2.8 mg
Microcrystalline cellulose 60.0 mg
Magnesium stearate 1.2 mg
350~0 mg
. ~:
.
' . ' ' ' `:' ` ~', ; :
~' : .
2~3~
- 127 -
_eparat]on:
The active substance is mixed with the excipients and
moistened with an aqueous PVP solution. The moist mass
is passed through a 1.5 mm screen and dried at ~5~C.
After drying, it is screened agairl and the magnesium
stearate is added. This mixture is compressed into
cores.
I'he cores thus produced are covered with a coating by
known methods. Colourings may be added to the coating
suspension or solution.
Example VI
Capsules containing 250 mg of active substance
Composition:
Active substance 250.0 mg
Corn starch 68.5 mg
Magnesium stearate 1.5 mg
320.0 mg
Preparation:
The active substance and corn starch are mixed together
and moistened with water. The moist mass is screened
and dried. The dry granules are screened and mixed with
magnesium stearate. The final mixture is packed into
size ~ hard gelatin capsules.
.
- ' . :
.
3 ~ ~
- 12~ -
Example VII
Oral suspension containing 50 mg O e active substance per
5 ml
Composition:
Active substance 50.0 mg
Hydroxyethylcellulose 50.0 my
Sorbic acid 5.0 mg
70% sorbitol 600.0 mg
Glycerol 200.0 mg
Flavouring 15.0 mg
Water ad 5.0 ml
Preparation:
Distilled water is heated to 70C. Hydroxyethyl-
cellulose is dissolved therein with stirring. By the
addition of sorbitol solution and glycerol the mixture
is cooled to ambient temperature. At ambient
temperature, sorbic acid, flavouring and active
substance are added. The suspension is evacuated with
stirring to remove any air. One dose of 50 mg is
contained in 5.0 ml.
Example VIII
Suppositories containing l00 mg of active substance
-
Composition:
Active substance l00.0 mg
Solid fat (adeps solidus) 1600.0 mq
1700.0 mg
~ ~ ' . ':
:
.
`
2 ~ c ~
- 129 -
Pre~ar~atlon:
The hard fat is melted. At 40C the ground active
substance is homogeneously dispersed in the melt. It is
cooled to 38C and poured into slightly chilled
suppository moulds.
. .
.
:
:, :