Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
f
Case 970-9793
PI~k~MJf.II~1 OQ~'T~~.S
The present invention relates to pleuromutilin derivatives. In
particular it provides c~lexes of a pleuromutilin derivative and
cyclodextrin and pharniaceutical preparations containing theses complexes
Pleuromutilin derivatives are antibiotics with excellent
micro-biological activity against a series of pathogenic microorganisms.
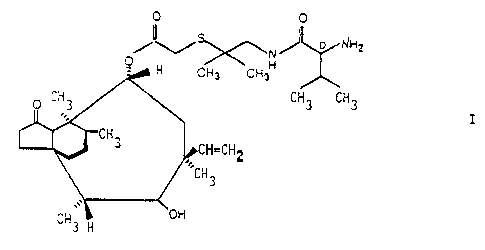
In particular, the pleur~nutilin derivative of fozrmi7.a I
0 0
0 ~NZ
0 " N
CHgH
CH3 CH3
I
H2
""3 H
namely 14-O-[1-((D)-2-amino-3-methylbutyrylamino)-2-methylgropan-2-ylthio-
acetyljmutiliny in free base or in acid addition or quaternary salt form,
is highly effective against many gram-positive and gram-negative bacteria.
Due to these properties the above compound may be employed as an
antibiotics for the treatment of conditions induced by these pathogens,
predaninantly in veterinary medicine, for example on poultry, pigs, cattle,
-2- Case 970-9793
sheep and goats, especially in the treatment of respiratory tract disorders
and dysentery.
Cyclodextrins are cyclic oligosaccharides made up of a-(1->4)-
linked D-glucopyranose units, e.g., for a-, p and y-cyclodextrin,
respectively of 6, 7 or 8 units, which are described in detail in the
literature, e.g. in J. Szejtli, "Cycl.odextrin Technology", Kluwer Academic
Publishers (1988) and in D. Duchene "Cyclodextrins and their Industrial
Uses", Editions de Sante (1987). In the preparations according to the
invention any cyclodextrin, e.g. a-, (3- or y -cyclodextrin or a derivative
thereof may be used. It is preferable to use p- or y-cyclodextrin or a
derivative thereof, especially (i- or y -cyclodextrin. a-, p- and
y -cyclodextrins are non-toxic compounds which may be used without risk as
pharmaceutical excipients for oral preparations. Moreover, in many
countries ~i-cyclodextrin is also permissible as a food additive for human
use.
Derivatives of cyclodextrin are for example ethers with lower
alcohols, such as methylcyclodextrin or hydroxypropylcyclodextrin.
It is knawn fr~n the literature that cyclodextrins can form
inclusion compounds (inclusion complexes) with appropriate guest molecules,
while the consequences on the effects obtained with the resultant c~nplexes
are difficult to predict. Based on the properties of the preparations
according to the invention, it can be concluded that inclusion complexes
similarly exist here. The obsezved desirable properties cannot be obtained
solely fr~n mere physical mixture.
It is observed that, surprisingly, with the cce~ound of formula I
in free base or in acid addition or quaternary salt form the formation of
complexes with cyclodextrins results in a marked improvement of the
properties of the resultant pharmaceutical preparations, particularly as
regards stability in feed mixtures upon storage.
T'he c~rg~ound of fortmila I is known from e. g. Sandoz USP 4' 675' 330
as Example 12 therein.
-3- Case 970-9793
The preferred form of application of antibiotics to animals for
therapeutic and prophylactic use is administration in the drinking water or
feed. Y~lhilst the compound of forniula I in the form of its water-soluble
hydrochloride may be administered easily and efficiently via the drinking
water, application via the feed is difficult, because the substance is
broken down very rapidly by constituents contained in conventional complete
feed mixtures. However, the stability of ready feed/medicament mixtures is
an essential requirement of practical application, since after stocks of
such mixtures are produced it must be possible to stockpile them for at
least several months.
A form of preparation is therefore desirable which has adequate
stability in ready feed mixtures and can be administered orally to animals.
Apart from the stability requirement of the feed, other essential
requirements for application are good absorption, inexpensive production
costs, a simple production process and acceptance by the treated animal.
Tt has now been found that preparations containing complexes of
the compound of formula I in free base or in acid addition or quaternary
salt forth with a cyclodextrin fulfill these requirements to a large extent.
The invention thus concerns c~lexes of the pleuramutilin
derivative of formula I as defined above in free base or in acid addition
or quaternary salt form and a cyclodextrin, hereinafter briefly named "the
complexes of the invention".
The invention further concerns pharmaceutical preparations
comprising a coa~lex of the pleur~utilin derivative of formula I as
defined above in free base or in pharn~aceutically acceptable acid addition
or quaternary salt form and a cyclodextrin, together with at least one
pharmaceutically acceptable carrier or diluent, hereinafter briefly named
"the preparations of the invention".
The c~lexes of the invention can be prepared by a process which
comprises complexing the c~npound of formula I in free base or in acid
addition or quaternazy salt form with an appropriate cyclodextrin.
-4- Case 970-9793
The process of the invention is effected in conventional manner,
preferably by contacting with an appropriate cyclodextrin under conditions
suitable for complex formation. Various conventional method variants may
be used thereby. It is possible to simply bring to dryness an aqueous
solution of the compound and a cyclodextrin. To that effect lyoph_ili°
zation, spray-drying and/or evaporation can be employed. The complexes may
further be obtained by moistening a physical mixture with water and
kneading intensively. The complexes may also be produced by trituration of
a such physical mixture of the two cc~rponents, e.g. in a ball mill.
E~rthezmore, it is possible to obtain the complexes by crystallization from
a solution or a solvent mixture.
The ratio of compound of formula I to cyclodextrin may vary within
a wide range. The molar ratio of components can e.g. be in the range of
from about 1 : 0.25 to about 1 : 2, preferably a ratio of from about
1 : 0.75 to about 1 : 1.25 is employed, especially a ratio of about 1 : 1.
It is demonstrated by e.g. 1H-NMR spectroscopy (see under 3.
below) that a chemical bond comprising at least hydrogen bonding or Van der
Waals forces is formed between the compound of formula I and the
cyclodextrin.
The preparations of the invention are manufactured in conventional
manner, e.g. by a process which comprises mixing a complex of the
pleuromutilin derivative of formula I as defined above in free base or in
pharmaceutically acceptable acid addition or quaternary salt form and an
appropriate cyclodextrin together with at least one pharmaceutically
acceptable carrier or diluent.
The preparations of the invention can be manufactured with the
compound of formula I in free base form or in water-soluble or
water-insoluble salt form. Preferably the hydrochloride is employed.
-5- Case 970-9793
The resultant inclusion complexes are described in more detail in
the following experiments:
1. Phase-solubility
Based on the phase-solubility diagram (see "Methods of
investigating and preparing inclusion compounds and their industrial uses"
in D. Duchene, "~clodextrins and their Industrial Uses", Editions
de Sante [1987]), the following types of inclusion compounds are
obtained with the compound of formula I in the form of the base and
- S-cyclodextrin: type AL
- y-cyclodextrin: type BS.
For the compound of formula I the complex binding constant is
1050 M-i with ~-cyclodextrin and 1400 M-1 with y-cyclodextrin.
2. Calorimetry
In micro-calorimetric examination of the complex of the compound
of formula I in the form of the hydrochloride and ~-cyclodextrin
(L. E. Briggner et al. Microcalorimetric titration of ~-cyclodextrine with
adamantane-1-carboxylate, Thermochimica Acta 109 [1986] 139-143), a complex
binding constant of 1004 M'1 is obtained.
3, ig_~_g~pectrum:
In the 1H-NMZt-spectrum of a solution of the compound of formula I
in the form of the hydrochloride and ~-cyclodextrin in D20, marked changes
in the signal position appear when compared with a pure solution of the
compound of formula I, and these are attributable to the following
functional groups:
~~~~~~b
-6- Case 970-9793
Compound of Complex of
Proton no. formula I ~-cyclodextrin and compound
(hydrochloride) of formula I (Example 3)
(PPm) (Fpm)
16 d 0.73 s broad 0.69
I7 d 0.97 s broad 0.96
18 s 1.45 s 1.49
8e d 1.99 d 1.72
4 s 2.56 s broad 2.58
20.20 dd 5.24 dd broad 5.35
19 dd 6.37 s broad ~6.5
4. Differential scanning calorimetry:
A preparation of 7-cyclodextrin and the compound of formula I in
hydrochloride form corresponding to Example 1 shows in differential
scanning calorimetry an endothermic signal of 10°K/min at 193°C.
5. Complex analysis
Determination of the content of a preparation corresponding to
Example 1 resulted in
- compound of formula I (hydrochloride) 28.6 %
- H20 5.9 %
This content corresponds to a molar ratio of 1 : Z of compound of
formula I (HC1) to cyclodextrin. .
~n~ ~~~
-7- Case 970-9793
In the following non-limitative Examples all temperatures are in
degrees Centigrade:
Example 1
5.76 g y-cyclodextrin are dissolved in 18 ml of water under
heating and 2.5 g compound of formula I (hydrochloride) are added. The
solution is slowly cooled to 5° whilst stirring. The resultant white
crystalline precipitate is decanted and dried in a vacuum drying chamber.
Example 2:
7.8 g ~-cyclodextrin are dissolved in 60 ml of water under heating
and 3.87 g compound of formula I (hydrochloride) are added. The solution
is evaporated to dryness under reduced pressure. The dry residue is
crushed through a 1 mm sieve.
Example 3:
The procedure is effected in a manner analogous to Example 2, with
the exception that the solution is spray-dried at an air temperature of
180°.
Eaa~mple 4:
The procedure is effected in a manner analogous to Example 2, with
the exception that the solution is frozen to -40° during 3 hours and
lyophilized.
Eaamiple 5:
2.9 g 7-cyclodextrin are dissolved in 10 ml of water under heating
and 2.5 g of compound of formula I (hydrochloride) are added. The solution
is spray-dried at an air temperature of 180°.
''~.~v~~~;...
-8- Case 970-9793
Example 6:
7.7 g compound of formula I (hydrochloride) are mixed with 15.5 g
~-cyclodextrin and 15 ml of water are added in a kneading machine. The
moist mass is kneaded intensively for 3 hours. It is subsequently dried in
a vacuum drying chamber and crushed through a sieve.
Example 7:
3.5 g compound of formula I (hydrochloride) are mixed with 14.1 g
~-cyclodextrin and ground in a ball mill for 6 hours.
Example 8: Pre~mi=
The preparations corresponding to Examples 1 to 7 are mixed with a
meal feed mixture to a 2 % pre-mix. The pre-mix is diluted to a
concentration of 200 ppm of compound of formula I by mixing in a freefall
mixer with further meal feed mixture.
Example 9: Feed pellets
A feed mixture according to Example 8 is pressed into feed pellets
using a appropriate pellet press and steam.
~~'~~~~
-9- Case 970-9793
Some user-relevant properties of the preparations according to the
invention are described in the following tests:
1. Feed stability
As model feed a ready feed for piglets of the following
composition is used:
oats 10.0
corn 29.0
barley 26.7
wheat 8.0
Soya scraps 23.0 ~ ,
calcium carbonate1.05 ~
dicalcium phosphate1.5 ~
iodized cattle 0.25 ~
salt
pre-mi~ure
(mineral-vitamin)0.5 ~
Various forms of the preparation are mixed with the ready feed in
a concentration of 200 p~xn of compound of formula I and stored for several
weeks at 30°. Both the starting value of the fresh mixtures and the
content values after storage for 2, 4 and 8 weeks are analysed by high
pressure liquid chromatography (I~7~C).
Table 1 shows the improvement in storage stability when using the
complexes of the invention in a feed mixture, as compared with a
corresponding preparation containing no cyclodextrin:
Table 1
Content (in ~)
Feed mixture
with initial after after after
value 2 weeks 4 weeks 8 weeks
Compound of formula I 100 15 8 6
(hydrochloride) alone
Complex of Example 3 100 89 87 90
Complex of Example 6 100 91 92 88
-10- Case 970-9793
For determination of the optimum ratio of compound of formula I to
cyclodextrin, investigations are carried out using various concentrations
of cyclodextrin. Results as regards stability in feed mixtures are
sunanarized in Table 2 [i:n the first column "ratio" means the ratio of
compound of formula I (hydrochloride) to ~-cyclodextrin]:
Table 2
Content (in %)
Initial
Ratio value after after after
1 month 2 months 3 months
1 : 0 100 9 9 7
1 : 2 100 86 89 91
1 : 1 100 90 89 96
1 : 0.75100 49 45 38
1 : 0.5 100 18 9 8
1 : 0.25100 14 8 9
2. Oral absorntion:
Absorption following administration to rats and pigs of 25 mg/kg
body weight of a preparation according to Exa~le 3 is tested and compared
with pure compound of foamila I (hydrochloride) in the drinking water.
Blood samples are taken after a period of 24 hours and the concentration of
active substance in plasma determined by microbiological analysis. The
results in Table 3 show the resorption of the ccsnpound of formula T when
administered in the form of a complex of the invention:
-11- Case 970-9793
Table 3
Cm$x (ug/ml) AUC (pg/ml.h)
rat pig rat pig
Compound of formula I 8.6 1.2 139 21.4
(hydrochloride) alone
Complex according to 11.7 1.6 294 24.3
Example 3
Cmmx = maximum plasma concentration
AUC = area under curve
~~°~ 3F
-12- 970-9793
The complexes of the pleuromutilin derivative of formula I as
defined above in free base or in pharmaceutically acceptable acid addition
or quaternary salt form are therefore indicated for use as pharmaceuticals,
particularly as antibiotics in the treatment of conditions induced by
gram-positive and gram-negative bacteria, such as respiratory tract
disorders and dysentery.
For this use the dosage to be employed will vary, of course,
depending on the particular complex used, the mode of administration, the
subject to be treated and the treatment desired. However, in general,
satisfactory results are obtained when the complexes are administered at a
daily dosage in e.g. pigs of from about 1 mg/kg to about 100 mg/kg body
weight, conveniently from about 5 mg/kg to about 10 mg/kg, if desired given
in divided dosages 2 to 4 times daily.
The complexes of the invention rcray be administered in similar
manner to known standards for use in such indications. The c~nplexes may
be admixed with conventional pharmaceutically acceptable carriers and
diluents and, optionally, further excipients, and administered e.g. orally
in such forms as feed pellets and premixes.