Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2074909
Optical Fiber Including Acidic Coating System
Technical Field
This invention relates to an optical flber including an acidic
coating system which enhances the strength of the optical fiber.
5 Back~round of the Invention
Optical flber has taken off from its embryonic stage as a
communication medium about a dozen years ago to its present status as a
world class medium for reliable communications. Typically, optical flber
includes a fused silica portion comprising a core and a cladding and one or
10 more layers of coating material. The coating material protects the flber
which is drawn from a preform.
It has been conventional that the optical fiber coating materials
be acrylate-based ultraviolet curable materials which include
photoinitiators. These materials cure, i.e., are converted from a liquid to a
15 solid, by what is referred to as free radical cure. In a free radical cure
system, upon absorption of light, the photoinitiator component cleaves to
form a pair of free radicals. This free radical pair diffuses first from each
other and reacts with acrylate-terminated components to initiate a free
radical chain polymerization process. Acrylate-based coating systems are
20 known to be chemically neutral, that is neither acidic or basic by design.
Another group of coating materials are cationically curable. In
cationic cure, a cation or a proton is both the initiating and the propagating
species in the polymerization mechanism. See U.S. 4,956,198 and PCT
application publication No. WO90/03988. In the latter, cationic cure, which
25 may be used on flber coatings is such that polymerization continues even
after the exposure to curing energy has been discontinued, whereas in free
radical cure, the polymerization reaction may be incomplete inasmuch as
the reaction discontinues when exposure to curing energy is discontinued.
It has long been desired to increase the strength of the optical
30 flber from its present prooftest value of about 50,000 psi and to cause the
strength to be uniform throughout a length of flber. An optical flber which
has been provided with a hermetic coating has a prooftest value which may
reach 200,000 psi. However, difficulties experienced in the manufacture and
coloring of hermetic coated optical flber have hindered its widespread
35 acceptance.
.~
- 2- 2074go9
It is known that fused silica optical fiber strength is higher after
aging in acidic environments than it is after aging in neutral or passive
environments. See, for example, H.C. Chandan and D. Kalish "Strength
and Dynamic Fatigue of Optical Fibers Aged In Various pH Solutions"
5 which appeared at pp. 12-14 of the Proceedings of the Topical Meeting on
Optical Fiber Communication, Washington, D.C. 197~.
~ lth the quest for uniform strengths, there is a desire to provide
a mechanism for causing rlber strengths to be higher and more uniform. A
long-felt need for optical fibers has been to increase their strength and
10 resistance to stress cracking. Such properties would result in a more
reliable communication system. The resultant optical fiber could be used in
applications where higher strengths are required, such as in underwater
cable or in tethered vehicles. Seemingly, the art has not yet provided a
solution to the problem of increased fiber strengths which solution would
15 not detract from cure speed and which solution would be relatively easy to
implement .
Summary of the Invention
The foregoing problems of the prior art have been overcome by a
coated optical fiber set forth in claim 1.
20 Brief Description of the Drawin~
FIG. 1 is a schematic view of a manufacturing line on which
optical glass fiber is drawn from a preform and then provided with a coating
system;
FIG. 2 is an end view in cross section of optical fiber which
25 includes a coating system;
FIG. 3 is an end view in cross section of optical fiber which
comprises a coating system which includes a colorant material;
FIG. 4 is an end view in section of a buffered optical fiber which
includes a layer of a colorant material;
FIG. 5 is an end sectional view of an optical fiber which includes
a layer of buffering material which includes a colorant material; and
FIG. 6 is an end view in cross section of a plurality of optical
fibers held in an array by a matrix material.
- 207A~og
Detailed De~cription
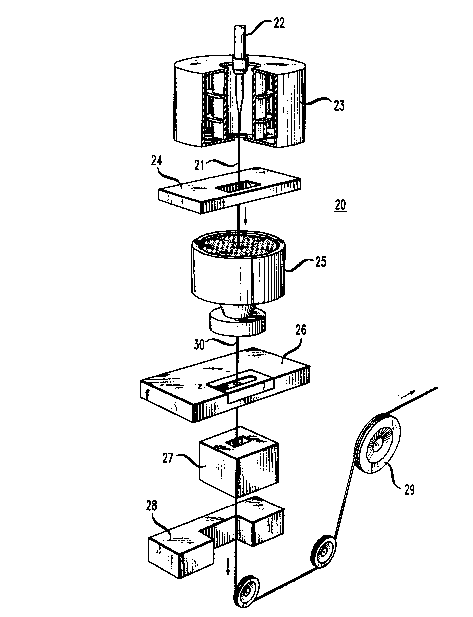
Referring now to FIG. 1, there is shown an apparatus which is
designated generally by the numeral 20 and which is used to draw glassy
optical fiber 21 from a specially prepared cylindrical preform 22 and then to
5 coat the optical fiber. The optical fiber 21 is formed first by locally and
symmetrically heating the preform 22 to a temperature of about 2000 C.
As the preform 22 is fed into and through a furnace 23, optical flber 21 is
drawn from the molten material.
As can be seen in FIG. 1, the draw system includes the furnace
10 23, wherein the preform 22 is drawn down to the optical fiber size, after
which the optical fiber 21 is pulled from the heat zone. The diameter of the
optical fiber 21 which is measured by a device 24 at a point below the
furnace 23 becomes an input into a control system. ~lthin the control
system, the measured diameter is compared to the desired value and an
15 output signal is generated to adjust the draw speed such that the fiber
diameter approaches the desired value.
After the diameter of the optical fiber 21 is measured, a
protective coating system is applied by an apparatus 25 to provide a coated
optical fiber 30. One method of applying dual layers of coating materials to
20 a moving optical fiber is disclosed in U.S. patent 4,474,830. Another system
for applying dual coatings on drawn optical fibers is disclosed in U.S. Patent
4,851,165.
Then, after the coated optical fiber 30 is passed through a
concentricity gauge 26, an ultraviolet light (W) device 27 for treating the
25 coating material to cure the coating material and a device 28 for measuring
the outer diameter of the coated fiber, it is moved through a capstan 29 and
is spooled for testing and storage prior to subsequent operations or sale.
The preservation of the intrinsically high strength of optical fibers is
important during their ribboning, jacketing, connectorization and cabling
30 and during their service lives.
A coating system 31 (see FIG. 2) which is applied to the optical
fiber after it has been drawn from the preform preferably comprises two
layers of radiation cured polymeric materials. An inner layer 32 which
contacts the glassy optical fiber 21 at a glass-coating interface 33 is referred35 to as the primary coating material and an outer layer 34 is referred to as the
secondary coating material. Typically, the primary and the secondary
2074909
coating layers each have a thickness of about 30 ~m.
The coated optical fiber 30 must meet desired performance
characteristics. For example, the coated fiber must have excellent
transmission characteristics. It must remain intact although subjected to
5 handling and the environment, it must be capable of being connected to
other coated optical fiber or to devices and it must be capable of being
tested.
More specifically, the interface between the primary coating
material and the glass flber must be characterized by suitable strength to
10 prevent delamination and must be such that the coating system can be
stripped from the optical fiber without tenacious residues being left on the
fiber surface. On the other hand, the surface of the secondary coating
material must be such that tacking does not occur between adjacent
convolutions of the fiber, resulting in a jerky payoff from a process spool.
15 Also, the outer surface of the secondary coating must be such that it is
compatible with the application of a relatively thick extruded overcoating
which may be referred to as buffering and/or colorant materials used for
identification in multifiber units. Importantly, the coated optical fiber must
have suitable dynamic and static fatigue resistance which are at least the
20 industry standards.
The optical fiber of the preferred embodiment of this invention
includes a cured primary coating material which maintains an ambient
environment having a pH of less than 7 about the glass fiber. The primary
coating material includes a component that upon curing generates an
25 environment which is sufficiently acidic in order to increase the strength of the coated optical fiber. Of course, the secondary coating material may
include a component which generates such an environment.
As mentioned hereinbefore, most optical fiber coatings used
today in the manufacture of coated optical i~lber are W curable acrylate-
30 based materials which are converted from a liquid to a solid by a free radicalpolymerization mechanism. For example, ultraviolet-curable coatings based
on acrylate-terminated polyurethanes are well known. These cure in the
presence of a free-radical polymerization catalyst initiated with ultraviolet
light. Acrylate-based coating systems, as was mentioned hereinbefore, are
35 known to be chemically neutral.
2074909
In order to provide an acidic environment around the drawn
optical fiber, an acidic terminated acrylate may be mixed into optical fiber
coating formulations to obtain a lower pH. Unfortunately with this
approach, the cure speed may be decreased which is an unacceptable
5 result. Also, it is not feasible simply to add an acid as a constituent to a
curable composition.
The coated optical fiber 30 of the preferred embodiment of this
invention is coated with a system which includes vinyl ether-terminated
oligomers, especially polyurethane oligomers. ~lnyl ether, in contrast to
10 acrylate-based coatings, is converted from a liquid to a solid by a cationic
polymerization mechanism.
~ lnyl ether-terminated polyurethanes are described in U.S. Pat.
No.4,472,01~ and also in U.S. Pat. No. 4,751,273. In each of these prior
disclosures, the vinyl ether-terminated polyurethane is formed by the
15 reaction of an aliphatic monohydric vinyl ether with a diisocyanate.
Cationically cured coating systems of this invention are used in
order to provide an environment in contact with the drawn glass fiber which
is sufficiently acidic to increase the dynamic and static fatigue resistance of
the coated optical fiber. Two basic chemistries may serve as examples of
20 such a coating system which provides such an acidic environment for fiber.
These are vinyl ether formulations or epoxy terminated materials. These two
are used in contrast to a free radical cure system. The cationically cured
formulations of this invention include a photoinitiator which, upon the
absorption of light, generates a strong acid. When they are cured, the
25 photoinitiator, not the vinyl ether or epoxy, absorbs light and undergoes a
reaction to generate the acid. Subsequently, the photogenerated acid reacts
with a vinyl ether or an epoxy terminated component to generate a cation
which can react with neutral epoxy or vinyl ether components to propagate
further the polymerization reaction. After irradiation, a latent acid is
30 present and continues to exist for a relatively long time. In contrast, in a
free radical cure system, there may be some free radicals present in which
the latency lasts for a relatively short period of time.
Cationic cure is one mechanism by which the coating material is
converted from a liquid to a solid upon exposure to light. Cationic cure may
35 be affected by ultraviolet or by visible light or by thermal means. The
difference between cationic cure and free radical cure is that in cationic
297~9
- 6 -
cure, a cation, R~, or a proton, H+, ;s both the initiating and the
propagating species in the polymerization mechanism. R+ is a cationic
chemical species that is derlcient by one electron. The photoinitiator
absorbs light and generates R+ or H+ . In free radical cure, the species
5 includes a free radical which is a reactive chemical species that has one
unpaired electron. The cation is a strong electron acceptor and as such has
the ability to accept electrons from a cationically curable end group thereby
initiating or propagating polymerization.
More particularly, a mechanism by which a cationically
10 polymerizable coating formulation is converted from a liquid to a solid is
shown in the following equations. In equation (a), a diaryliodonium
photoinitiator, Ar2I+ X-, generates a strong Bronsted acid, HX, such as
HBF4, HAsF6, HPF6, for example, upon photolysis caused by absorption of
light, hv, and subsequent reaction with a proton donor, SH.
Ar~X + SH ~ ArI + Ar- + S + HX (a)
Additional products, ArI, Ar- and S, are also formed but do not
participate in cati~nic polymerization. The photoinitiator in equation (a) is
;llustrative of a photoinitiator class which upon photolys;s generates a
Bronsted acid which is sufrlciently strong to affect the pH of the
20 environment which is adjacent the glassy portion of the optical flber and to
enhance the strength of the coated optical rlber. Triarylsulfonium salts also
generate strong Bronsted acids, for example, upon light absorption.
Typically a W curable composition includes a photoinitiator, an
oligomeric resin, one or more diluents and additives. The oligomeric resin
25 component is multifunctional in that it includes more than one end group
which can react with the photogenerated Bronsted acid to produce a
cationic species. Similarly, the diluent component or components also are
capable of reacting with the photogenerated Bronsted ac;d to produce a
cationic species. This is shown in equation (b) where species (~) is either an
30 oligomeric resin component or a difunctional diluent component. As shown
in equation (b), the Bronsted acid reacts with a cationically polymerizable
component, (~), of the coating material such as, for example, an oligomer or
a diluent to initiate polymerization.
a o~
- 7 ^
,0 ~ ,0
CH2 - CH ''~ R ~ CH - CH2 + HX
(b)
,~
+ ,0~
HOCH2CH ~ R ~--CH - CH2 + X
,~,
This initiation reaction generates a cationic species (ii) and the
corresponding conjugate base of the Bronsted acid, X~. The cationic
species (~i) is the reaction product of the photogenerated Bronsted acid and
10 the oligomeric component or of the photogenerated Bronsted acid and the
diluent component. The cationic species (~i) can react subsequently with a
monofunctional component (ili) as shown in equation (c) or with a
difunctional component (~) as shown in equation (d) to propagate the
cationic polymerization reaction as shown in equations (c) and (d),
15 respectively.
+ ' ,0~ ,0~
HOCH2 CH ~ R ~ CH - CH2 + R' CH - CH2
ii iii
rv r~J
(c)
+
HOCH2CH - OCH2 CH -
R
CH - CH2
`O '
iv
rv
_ -8- 207~9ng
HOClt2 CH R ~ CH2 + CH2 - CH --~ R' ^~ CH CH2 ----
rv
+ . ,0 ~
HOCH2CH - OCH2 CH ~ R'~-- CH - CH2 (d)
~ ~
R
CH - C~2
`O '
It is noteworthy that the propagation reaction shown in equation (d)
generates a crossl;nk between difunctional components (i) and (v), yielding
an intermediate crosslinked species (vi) Subsequently, the intermediate
crosslinked species, (vi), reacts further with a monofunctional type
15 component such as (iii), for example, or a difunctional component such as
(~), for example, to continue or propagate the cationic polymerization chain
reaction and form crosslinks between those components~ These crosslinks
are primarily responsible for the formation of the ultimate mechanical and
physical properties of the cured coating material~
The result of the initiation reaction (b) and propagating
reactions (c) and (d) is the conversion of the coating material from a liquid
to a solid which conversion also is referred to as cure~ -The epoxy terminated species shown in the foregoing equations
are illustrative of cat;onically polymerizable components only. Other
25 compositions including cyclic ether, lactone, vinyl ether, or styrenic groupsalso can be cationically polymerized, for example. A general reference for
cationic polymerization appears in Crivello, J V "Photoinitiated Cationic
Polymerization", in W Cur;ng: Science and Technology, S Peter Pappas,
Ed., Technology Marketing Corp~, 1980, 23-76.
As described above, the photoinitiator generates a strong
Bronsted acid upon photolysis. See equation (a). Only a portion of this
photogenerated acid reacts irreversibly with cationically polymerizable
coating constituents such as an oligomer resin or one or more diluents.
Consequently, consumption of the cationically polymerizable constituents
35 produces a cured coating formulation having Bronsted acid entangled in the
2074909
-
crosslinked coating material. Subsequently, the coating material in intimate
contact with the glassy optical fber cladding affords an acidic environment
surrounding and contacting the optical flber glass surface. It is also possible
that Bronsted acid entangled in the bulk of a crosslinked coating material
5 spaced from the glass interface may diffuse through to the optical fiber-
coating material interface, producing an environment disposed about the
optical fiber glass surface which is sufficiently acidic to enhance the
strength of the coated optical fiber.
The chemistry of cationic cure to optimize the pH of the cured
10 coating is by suitable choice of the photoinitiator, or the concentration of
the photoinitiator or by manipulating the concentration of the vinyl ether
and epoxy end groups of the coating formulation. Should a different
amount of acid be needed, it can be provided by varying the amount of
photoinitiator and/or vinyl ether or epoxy end groups, for example.
The typical proportion of cationic photoinit;ator which is
suggested for cationic polymerization is in the range of 1,7o to 4<~o, usually
3~O to 4,70, by weight. It has been found that a very rapid cure producing
superior cured properties is obtained by using smaller weight percent
amounts of a cationic photoinitiator.
In the coated optical fiber 30 of this invention, the acid which
has been generated persists for some time after processing. The lifetime of
the generated acid is much longer compared to free radicals which are
generated from corresponding photoinitiators. This is advantageous because
the fiber strength increases, without any diminution of the cure rate.
25 Optical fiber coatings continue to cure well after the initial exposure to
curing light on a draw tower. The advantage is the long lived, latent acidic
component in the cationically cured material which can affect a low pH
environment about the glass substrate and result in an enhanced strength
characteristic of the coated optical fiber. Enhanced glass strength is
30 obtained because static fatigue resistance of optical flber is pH dependent;
the lower the pH, the greater the static fatigue resistance. Further, it is
believed that the toxicology of cationically cured optical fiber coatings is
more benign than acrylate-based materials. Still further, their cure speed in
some cases is greater than acrylate-based materials because their cure is not
35 inhibited by oxygen.
207~909
- 10-
Apparently, the added ~lber strength benefit of cationically
cured coating materials has not yet been considered by the industry.
Although optimal pH values have been suggested in the literature by
immersing the optical fiber in different pH solutions, there appears to be
5 nothing in the prior art which suggests a coating which exposes the glass
fiber to an environment of a pH value which is sufficiently acidic to enhance
the strength of the coated optical fiber. To date, it appears that the effect
of the local environment on the strength of the coated glass fiber by
formulating a coating with a lower pH value has not been considered.
The optical fiber of the preferred embodiment of this invention
includes a layer of a coating material which is contiguous to the drawn glass
which includes resin which is terminated with cationically curable end
groups such as vinyl ether or epoxy, a diluent which is terminated with
cationically curable end groups, a photoinitiator, additives and, possibly,
15 colorant constituents. The additives are stabilizers which are included to
enhance the stability, such as oxidative, hydrolytic or shelf life, for example,of the fiber coating. The photoinitiator absorb lights and generates acid,
and as a result of the cationic cure, a latent acid species is present in the
polymer which reduces its pH at the glass-coating interface.
The cationically cured layer of coating material should be the
one, in the event that multiple coatings are used, contiguous to the glassy
portion of the optical fiber. However, if sùch a coating material is needed as
a secondary coating spaced from the glass flber by the primary coating,
there could be residual acid which diffuses through the primary coating to
25 the glassy portion.
It should be understood that the coating system which includes
the material which provides the acidic environment for the glassy portion of
the optical fiber may be other than that described thus far. For example, a
coating system for an optical fiber may include one or more layers of coating
30 materials which protect the glassy portion of the optical fiber and a layer 40
(see FIG. 3) of a colorant material for example. The colorant material such
as an ink, for example may be cationically curable, for example, such that it
provides an acidic component which diffuses through a layer or layers of
coating materials to provide a suitable acidic environment for the glass-
35 coating layer interface. Such a colorant material may be referred to as astrength enhancing colorant material.
207~909
1 1
Other embodiments are shown in FIGS. 4 and 5. In FIG. 4, a
coated optical rlber 30 is provided with a layer 42 of a buffering material
such as polyvinyl chloride (PVC), for example. About the layer 42 of
buffering material may be disposed a layer 44 of a colorant material. Either
5 the layer 42 or the layer 44 may be such as to provide an environment for
the glass-coating interface which is sufrlciently acidic to enhance the
strength of the coated optical fiber. In FIG. 5, a coated optical fiber 30 is
provided with a layer 46 of a buffering material which may include a
colorant material and which is such that it provides a suitable acidic
10 environment for the glass-coating interface.
Also, the coated optical rlbers may be included in a matrix
material 50 (see FIG. 6), several conrlgurations of which are disclosed in U.S.
patent 4,900,126. The coating system includes not only the layers of coating
material used to protect the ~lbers, but also the matrix material 50 which is
15 used to maintain the fibers in an array. In this embodiment, the matrix
material 50 may be cationically curable to provide the sufficiently acidic
environment for the glass-coating interface.