Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ W091/17162 2 0 7 8 V 0 7 PCT/EP91/00737
IMIDAZOPYRIDINE PAF ANTAGONISTS
.
lhis invention rC1atcC eo imidazopyridines spcci~ic.~ to
ccrtain 4-sul)stitutcd-1-(2-met~ liolida.o~4,J-~lp~rid-l-y~ 7cn%et)c
derivatives. ~he compoun~s are potenc and sYl~c~iu~ ~nta~onists
o~ platelct activa~inl, [ac~or havi[lg clinical u.i~i~y itl t~lC
treacment of al]ergic and infla~macory conditions in numans and
animals.
Platelet activatin~ factor (P~;F, i-0-al~ 1-^-ace.yl-sn-
glyceryl-3-phosphorylcholine) is an ether phoDholiDid whose
structure was first elucidated in 197~. It is ?roduced by,
released fro~ and interacts with many pro-lnfl2m~atory cells~
platelets and the kidney. In addition to porent piatelet
;
aggregating activity, PAF exhibits a wide spectru~ of biological
activities elicited either directly or via the release of other
powerful mediators such as thro~boxane A2 or ehe leukotrienes,
which make PAF inhiHitors of potential value in the treat~ent of a
variety of conditions including allergic, inflammatory and
~ hypersecretory conditions such as asth~a, arthritis, rhinitis.
',! bronchitis and urticaria, the treatment of circulatory shock,
gastric ulceration, psoriasis and cardiovascular conditions.
including an~ina, thrombosis and s~ro'~e.
In our European patent applicaeicn no 0258033 we disclose a
; series of 2-substituted 1,4-dihydropyridine dcrivatives as PAF
antagonists. In our later European patent application no 0310386
we disclose a ~urther series of dihvd~opvridinc P~F antagonisc
wherein the 2-posi~ion substituent in~ludes in par;icular a
2-methyl-i~idazo[4,5-c]pyrid-1-yl-?henpl group. ~he present
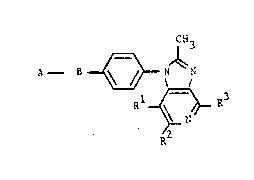
invention provides further PAF antagonists having ~he formula:
SUBS~I~U~E S~EET
W O 91/1716~ 0 7 8 ~ ~ ~ PCT/EP91/00737 j,l
A - B ~ `; ~ ~
Rl ~ R3 .... (I)
R-
and phfi~aceu~ically acceptable sales tbereof,
wherein A is a C~-C~ alkyl, perfluoro(Cl-C~)alkyl, C3-C8
cycloai'~yl, arvi or het~rocyclic group, wherein said aryl or
heterocyclic group may be unsubstituted or substituted with from
one to three substituents each independently chosen from Cl-C4
alkyl, halo, oxo, Co2R4, CoNR5R6, OH, Cl-C4 alkoxy, NH2, N02, CN
and (CH3)3SiCH2;
B is a Cl-C5 alkylene or C2-C5 alXenylene chain which may
opeionally be substituted by one or more Cl-C4 alkyl, Cl-C4
alkoxy, perfluoro(Cl-C4)alkyl, C3-C7 cycloalkyl, phenyl, oxo, OH,
CN, CoNR5R6 or CO2R groups and wherein up to two carbon atoms in
said-chain can intependently be replaced by O, StO)m, -N= or NR ,
and wherein said chain or part of said chain, may form, or may
form part of, a 5-7 membered saturated or mono-unsaturated ring
which may contain a nitrogen ato~ or NR7 group, a nitrogen and
oxygen atom, or one or two oxygen ato2s, said ring being
optionally substicuted with any o the foregoing chain
substituents, and, in the case where the group A is an aryl or
heterocyclic group, the ring may optionally be fused to said aryl
or heterocyclic group;
each of Rl, R2 and R3 is independently H or CH3;
SUBSTITUTE SHEET
:. : ` , ... . .
. .
., 207soo7
W O 91/17162 PCT/EPg1/00737
R is H, Cl-C4 alkyl or ary](Cl-C4~alkyl;
RS and R6 are each independently H or Cl-C4 alkyl, or F~5 is ~1 and
R is C3-C& cycloalkyl or aryl, or the two ~roups R5 and R
together with the nitrogen atom to which they are attached, form a
piperidino, 4-ketopiperidino, morpholino or piperazinyl group;
R is H, Cl-C4 alkyl, CO2(Cl-C4)alkyl, aryl(Cl-C4?alkyl or
heteroaryl(Cl-C4)alkyl;
and m is û, l or 2;
with the proviso that A-B is not C~H5OCOCH2CO- or CH~COCH~CO~CH~-.
In the above definitions, the term aryl includes phenyl,
naphthyl, tetrahydronaphthyl and indanyl, each of said groups
being optionally substituted as defined in A above; alkyl groups
having 3 or more carbon atoms may be straight or branched-chain;
and halo means fluoro, chloro, bromo or iodo. In the definition
of A, the term heterocyclic group means a 5 or 6 membered ring
containing up to four nitrogen atoms, or one or two nitrogen atoms
together with a further oxygen or sulphur atom, or up to two
oxygen atoms or a sulphur atom, as heteroatom, and said ring may
' be saturated or unsaturated and substituted with one or more
subsitutuents as defined in A above. and may optionally be fused
to a phenyl or further 5 or 6 membered heterocyclic ring.
Examples of particular heterocyclic groups include pyridyl,
quinolyl, benzimidazolyl, benzthiazolyl, benzdioxolanyl,
benzothienyl, triazolyl, imidazolvl, indazolyl, indolinyl,
piperidyl and morpholinyl.
The term heteroaryl used in relation to R7 means a 5 or 6
membered aromatic heterocyclic group including, for example,
pyridyl, thienyl and imidazolyl.
SUBSTITUTE SHEET
2~7~
W O 91/17162 PCT/EP9t/00737 ~A
As defined ove a variety of linking groups B, are po~sible
and as well as simple straight-chain or br~nched alkylcne and
alkenylene groups, the invention includes groups cont~ining an
ether, thioether, amine or amide group and various cyclic
variations thereof:
a) Thus, i~ one particular aspec~ ~f t:~e invencion the linking
group, B, is an ether group havin~ an oxygen atom and up to four
carbon atoms in the chain lin~ing .;~e gro~3 A t~ p;~enjl r~ ng.
The linking group may optionally have a further oxygen atom in the
chain and said chain may optionail~ be substi.uted by hyd~cxy, o~o
(to give an ester group), Cl-C4 alkoxy, Cl-C4 alkyl or phenyl. In
this embodiment the linking group may also form part of a 5 to 7
membered cyclic ether group containing one or two oxygen atoms in
the ring which may optionally be substituted by Cl-C4 alkyl
hydroxy, oxo, or Ci C4 alkoxy and which may optionally be fused to
a phenyl or tetrahydronaphthalene ring. Thus the ring may be for
example, a tetrahydropyranyl, dioxolanvl, dioxanyl or dioxepanyl
ring.
In this aspect the group, A, is preferably a phenyl group
which may optionally be substituted as defined in A above. Thus
parcicular and preferred examples of chis type include compounds
of the following formulae-:
~
O~X
~ O ~~O
X ~CO N ~.~ e ¢~f o l x
SUBSTITUTE ~IEET
..
. .
~`~` W O 91/17162 2 ~ 7 8 ~ 0 7 PCT/EP91/00737
wherein X is:
\¢~ r~1 e
p3
R
R , R and R being preferably H.
Coupounds of this type may be pre?areà Dy â variesv Of
methods as will be known to those skilled in the art. In one
process the ethers are prepared by reac~ion of ~he corresponding
hydroxyalkyl derivative of formula:
HO - D - X
wherein X is as previously defined and D is Cl-C4 alkylene group
which may optionally be substituted as defined in B above; by
reacting with an appropriate phenol or heteroaryl terivative of
formula Ar-OH wherein Ar is an aryl or heteroaryl group which may
optionally be substituted as defined for A above. The reaction is
performed in an inert organic solvent e.g. tetrahydrofuran, in tha
presence of triphenylphosphine~and diethylazodicarboxylate
(Mitsunobu reaction). Certain transfor~ation reactions are
possible on the products, thus for example when the phenol is
substituted by CO2R , wherein R is Cl-C, alkyl, hydrolysis will
give the corresponting carboxylic ac~d (wherein R4 is H~. This in
eurn may be reacted with a variety of amines of formula R R NH to
give the corresponding carboxamide derivatives where the
substituent is of formula CoNR5R and R5 and R6 are as previously
defined. SUBSTITUTE SHEE~r
~ . ,
20780~7
W O 91/17162 ~ PCT/EP91/00737 ''
In an alternative process, an oxirane of formula:
~ ~ or ~ CH3
wherein X is as previously defined, may be reacted with a phenolic
anion e.g. by heating in dimethylformamide, to give the
corresponding compounds of formula(I) wherein the linking group B
is
OH OH
-O-~H2-CH- or 2 Cl
CH3
respectively.
~ Cyclic diethers are readily prepared by reaction of a diol
; with the appropriate aldehyde or ketone. Thus for example
reaction of
. .
CH
~ ~ HC ~
yields the corresponding 2,4-benzodioxepine derivative. l`his
reaction may also be performed bv using the trimethylsilyl
derivative of the diol, following the procedure of T. Tsunoda. M.
Suzuki and R. Noyori, Tetrahedron Letters, 1980, 21, 1357.
SUBSTITUTE SHEET
. `
.
.
' ;.~
20780~7
" W O 91/17162 PCT/EP91/00737
Other variants are possible, thus for example reaction of a
compound of the formula:
; OH
H(CH2)2CH-X ... (IV)
with butyl lithium and chlorotrimethylsilane, followed by reaction
with an aldehyd~, gives a 2-aryl-dioxane derivative.
Finallv ester-linked derivatives may be prepared by reaction
of the carboxylic acid ot formula H02C-Dl-X with an alkanol of
formula A-Dl-OH or by reaction of an alkanol of formula HO-Dl-X
with an acid of formula A-Dl-CO2H wherein A, and X are as
previously defined and Dl is as previously defined for D or it may
be a direct bond.
Appropriate reagents and conditions for all of the above
reactions are given in standard text books and by reference to the
experimental examples given hereafter.
b) In a further aspect of the invention, the linking group a
contains an a~ide group together with up to three further carbon
atoms in the chain linking A to the phenyl ring. The nitrogen
atom may optionally be substituted by Cl-C4 alkyl and the chain
may optionally be substituted by Cl-C4 alkyl or phenyl, or include
a further oxo substituent. In this embodiment the group A may be
phenyl, optionally substituted as defined in A above or it may be
naphthyl, indanyl, or a heterocyclic group, for example a pyridyl,
quinolyl, indazolyl, benzimidazolyl or benzthiazolyl group.
SUBSTITUTE SHE~T
. . ..
W O 91/17162 2 0 7 8 0 ~ 7 PCT/EP91/00737 -~
;
Thus particular and preferred examples of this type include:
~uNHOC 1 X ~ ~ N ~ X Cl ~ ~ X
- Compounds of this tvpe are ~enerally orepared bv
reaction of an amine of formula A-D -NHR with an acid o. rormuia
H02C-D -~, wherein R is H or Cl-C4 alkyl and A, Dl and ~ are as
previously defined. The reaction may conveniently be achieved via
the acid chloride which may be prepared by reaction of the acid
with, for example, oxalyl chloride in accordance with normal
practice. Alternat1vely ~n amine of for~ula
Rl9NH_Dl_x ... (VI)
may be reacted with a carboxylic acid of for~ula A-D -C02H in an
analgous manner.
c) In another aspect of the invention, the linking group
contains NR or -N~, together with up to four carbon ato~s in the
chain, which may optionally be substituted by oxo or
C02(Cl-C4)alkyl. The amino substituent R7 is preferably H, Cl-C4
alkyl, C02tCl-C~)alkyl or aryl(Cl-C )alkyl. The linking group in
thls case may optionally be cyclised to form a pyrrolidinyl group
or plperidino group, which may option211y be fused to a benzene
ring, or it may be an oxazoline ring.
In this aspect the group A is prererably phenyl, optionally
substituted as previously defined.
SUBSTITUTE SHEET
. ,` . . . ,: , :.. ,
..
, , ~'- ' ' '`- '' ' ' .
~780a7
^ W O 91/17162 PCT/EP91/00737
Thus particular and preferred examples include:
¢~N~X ~ ~) X
~'
Compounds of this ty?e maJ be pr2?a ed b: .educ~i:e alk;la.ion of
an arylamine or aryi(Cl-C,)alkylamine with an aldehyde of the
formula
HC0-D -X ... (VII)
wherein X and D~are as previously defined.
The reaction is readily achieved via reduction of the
Schiff's base using, for example, sodium borohydride or sodium
cyanoborohydride. A number of further transformation reactions
are possible on the product, as previously described thus, for
example, an aryl-nitro group may be reduced to the corresponding
amino compound, for example using stannous chloride and, in the
case of the 2-aminoanilinomethyl derivative, this may be cyclised
by reaction with triethylorthoformate to the corresponding
benzlmidazolylmethyl derivative. Further reactions include, for
example, reaction of the amine products with n-butyl lithium
followed by reaction with a Cl-C4 alkylchloroformate to give the
N-alkoxycarbonyl derivatives, or aiky a~ion ~o give the products
where R7 is Cl-C4 alkyl, aryl(Cl-C. alkyl) or heteroaryl(Cl-C4)-
alkyl.
d) In a further aspect of the ir.~eneion, tne linking group B is
a 7 membered saturated or mono-unsaturated ring containing -NR -
wherein R7 is as previously defineà. The ring may optionally be
SU~STITUTE SHEET
20~8~7
W O 91/17162 ~ PCT/EP91/00737 ~`
substituted as previously defined under B; preferred substituents
include oxo and C02R , particularly when R i8 ~ethyl. R iB
preferably H, Cl-C4 alkyl, aryl(Cl-C4)alkyl or heteroaryl(Cl-C4)-
alkyl. In this embodiment A is preferably phenyl or substituted
phenyl and said phenyl ring is benzofused to the 7-me~bered ring
B. Thus particular and preferred compounds of this type include-:
H 0 ~l
Cl ~ ~ C02CH3 ~ R-
Cl `~' ~ d~l~ ~X
(VIII) (IX)
wherein R21 is H, methyl, 4-chlorobenzyl or 2-pyridylmethyl and X
is as previously defined.
The above compounds are prepared by cycllsation of a compound
of the formula:
22) ~ 2 C2CH3
<~ C02CH3
X
wherein each R2 is independently H or an aryl group substituent
as definet in A above, to give the 3-methoxycarbonyl-tetrahydro-
benzazepin-2-one tVIII). Subsequent treatment by heating in
pyridine with lithium iodide gives tne tetrahydrobenzazepin-2-one
(IX) (R2l~H), whicb may subsequently be alkylated on nitrogen by
reaction with a strong base, e~g. socium hydride, followed by
reaction with the appropriate alkyl or substituted-alkyl halide to
give the l-substituted-tetrahydrobenzazepin-2-ones.
SUBSTITUTE SHEET
. .
: ,
..: ..
, . : .
2078~7
;`1`_~ W O 9t/17162 PCT/EP91/00737
11
Further particular and preferred compounds of this type
include-:
O O
H \\
)3 ~ ~ O IR ~3 ~
X H X
wherein X is as previously defined and R is preferably H.
These compounds are prepared by ring closure of the
corresponding open chain compound of formula:
O
22) ~ ~ (R )
;
respectively by heating under acidic conditions.
e) In a further aspect of the invention the llnXlng group B
contains a S(0) group together with up to four carbon atoms where
m is 0-2 . Thus the sulphur atom may be present as a thioether,
sulphone or sulphoxide group. The chain may optionally be
substltuted by Cl-C4 alkyl or hydroxy. The group A is preferably
phenyl optionally substltuted as previously defined in A above.
Particular and preferred examples of this type include:
CONMe~ Cl ~ ,5~ X
SUBSTITUTE S~-IFET
:
:, , `
; :
2~78~7
W O 91/17162 P~/EP91/00737 '~`
Compounds of this type may be prepared by reaction of an aryl or
aralkyl thiol of formula A-D -SH with an alcohol of formula
HO-Dl-X in the presence of triphenylphosphine and
diethylazodicarbo~:ylate to give compounds where the linking group
B is -Dl-S-Dl-, where-ln each Dl is as previously defined with the
proviso that the number of atoms in the chain linking A to the
phenyl ring does not exceed 5.
As be'ore conv2~tiooal ;rans o~_at-o? reac~ions cen be perrormed
on the products, for example to give the aryl-carboxamide
derivatives ~la nydrolysis OL- ti~e corresponding esters and
reaction of the resulting carboxylic acid with an amine.
The sulphones and sulphoxide derivatives (m = l or 2) can be
prepared from the thioethers by conventional oxidation, for
example using meta-chloroperbenzoic acid.
f) Finally, in a further aspect of the invention, the linking
group B is a Cl-C4 alkylene or alkenylene group which may
optionally be substituted by one or more OH, oxo, C02R or
perfluoroalkyl groups. The group A is preferably phenyl
optionally substituted as defined in A above or is
heptafluoropropyl. Thus examples of preferred compounds include:
Cl
Cl ~ C02Et
~ Cl
; Compounds or this type mav be prepare_ by a number or different
methods. In one procedure the aldehyde of formula (VII) may be
reacted with dimethylmalonate followed by reaction with the anion
derived from 4,5-dichloro-2-nitrotoluene, to provide the compound
SU8S'rlTl)TE SHEEl'
.' ' '.
- WO 91/17162 Q~r/EP91/OOm
of formula (I) wherein the linking group B ls a dimethyl
ethylmalonate group of formula:
IH(C~7CH3)2
Alternatively reaction of an arvl aldehyde with a ketoester of
formula (~):
O
wherein R4 is C~-C~ alkyl and X is as previously defined, Yields
the 3-aryi-2-alko~ycarDonylprop-2-ene-1-one derivative. Reduction
gives the 3-aryl-2-alkoxycarbonyl-1-hvdroxypropyl derivative where
the linking group is-:
R 02C IH
--CH2-CH-CH-
Further possibilities include the reaction of aldehyde (~II) with
a benzyl triphenylphosphonium bromide or chloride to give l-aryl
ethene derivatives. Finally reac~ion of an ester of formula:
R 02C ~ X
wherein R is Cl-C4 alkyl and ~ and X are as previously defined,
by reaction with, for example, a perfluoroalkyl magnesium iodide;
gives the corresponding perfluoroalkyl-carbonyl derivative. The
ketone may be further reacted, for example with a further Grignard
addition to give further disubstituted-methanol derivatives.
All of the reactions described in a) to f~ above are entirely
conveneional and alternative methods and procedures to all of the
compounds within the scope of claim ~ will be evident to those
skilled in the art. Appropriate reagents and conditions for their
performance may be readily established by reference to standard
SUBSTITUTE S~EET
,
, ` . ~ .
" : ` `' :
20~8~
W 0~91/17162 PCT/EP91/00737
text books and to the examples provided hereafter.
The compounds may be purified using conventional methods such
as recrystallisation or column chromatography as appropriate, and
compounds having acidic or basic centres may be isolated as the
free acid or base or in salt form. Compounds having asymmetric
centres may be isolated as the racemic mixtures or resolved to
give the individual enantiomers. The invention includes all
enantiomers whether resolved or not.
The pharmaceutically acceptable acid addition salts of the
compounds o~ the formula (~) which form such salts are those
formed from acids which form non-toxic acid addition salts, for
example the hydrochloride, hydrobromide, sulphate or bisulphate,
phosphate or acid phosphate, acetate, citrate, fumarate,
gluconate, lactate, maleate, succinate, tartrate, metbane-
sulphonate, benzenesulphonate and p-toluenesulphonate salts.
; The actlvity of the compounds of the invention is shown by
their ability to inhibit the platelet aggregating activity of PAF
in vitro. Testing is performed as follows:
Blood samples are taken from either rabblt or man into 0.1
vol disodium ethylenedlamine tetraacetic acid buffer and the
samples centrifuged for 15 minutes to obtain platelet rich plasma.
~he plasma is further centrifuged to give a platelet pellet which
is washed with a buffer solution (4 m~ RH2P04, 6mM Na2HP04, 100 mM
NaCl, O.l~ glucose and 0.1% bovine serum albumin, pH 7.25) and
finally resuspended in buffer solution to a concentration of
2 x 108 platelets/ml. A sample (0.5 ml) is pre-incubated with
stirring for two minutes at 37C in a Paton aggregometer, either
with vehicle alone, or with vehicle containing the particular
SUBSTITUTE S~tEET
. .
., ` ; .~'
207~0~7
` ` W O 91/17162 PCT/EP9t/00737
compound under test. PAF ls added at a sufflclent concentration
to give a maximum aggregating response ln the abser.ce o~ test
compound (lO 8 to lO 9 molar), and the platelet aggregation i~
measured by following the increase in light transmission of ti1e
; solution. The experiment is repeated in the presence of test
compound at a range of concentrations and the concentration of
compound required to reduce the response to 50~, of its maximur.
value is recorded as the IC50 value.
The activity of the compounds of formula (I) is also
demonstrated in vivo by their abilitv to protect mice from the
lethal effect of an injection of PAF. A mixture of PAF (50 ~glkg~
and DL-propranolol (5 mg/kg) in 0~9/o w/v sodium chloride is
injected (0.2 ml) via a tail vein into mice. The compounds under
test are either injected into the tail vein immediately prior to
the PAF/propranolol injection or administered orally by gavage two
hours earlier. The compounds are tested at several doses in
groups of 5 mice and the dose which reduces mortality to 50X is
recorded as the PD50 value.
SUBSTITUTE StlEET
`~` ' ~ ' .'. . `
,
2a~sao7
W O 91/17162 PCT/EP91/00737
16
For therapeutic use the compounds of the formula tI) wlll
generally be administered in admixture with a pharmaceutical
carrier selected with regard to the intended route of
administration and standard pharmaceutical practice. For example,
they may be adminis~ered orally ir. the fon~ of tablets containing
such e~cipients as starch or lactose, or in capsules or ovules
either alone or in ad~ turP with excipients, or in the form of
eli.; rs o. suspens~c..s cGn.alnihO ~ avou.ing or colouring agents.
They may be injected parenterally, for example, intravenously,
intramuscularly or suDcutanoousl,. For pa.enteral administration,
they are best used in the form of a sterile aqueous solution which
may contain other substances, for example, enough salts or glucose
to make the solution isotonic with blood.
For administration to man in the curative or prophylactic
treatment of allergic bronchial conditions and arthritis, oral
dosages of the compounds will generally be in the range of from
2-lO00 mg daily for an average atult patient (70 kg). Thus for a
typical adult patient, individual tablets or capsules contain from
i to 500 mg of active compound, in a suitable pharmaceutically
acceptable vehicle or carrier. ~osages for intravenous
atministration would typically ~e within the range l to lO mg per
single dose as required. For the treatment of allergic and
bronchial hyper-reactive conditions, inhalation via a nebuliser or
aerosol may be the preferred rouee or drug administration. Dose
levels by this route would be within the range O.l to 50 mg per
single dose as required. In prac~ice the physician will determine
the actual dosage which will be most suitable for an individual
patient and it will vary with the age, weight and response of the
SUBSTITUTE SHEET
,`.
~, . . . . .
2078007
... . .
W O 91/17162 PCT/EP91/00737
particular patient. The above dosages are ex.emplsry of the
average case but there can, of course, be individual instances
where higher or lower dosage ranges are merited, and such sre
within the scope of this invention.
Thus in a further aspect the invencion provides a
pharmaceutical composition comprising a compound of the formula
(I), without proviso, or a pharmaceuticallv acceptable salt
thereor, together with a pharmaceuticallv acceptable diluent or
carrier.
The invention aiso inciudes a compound of the formula (I),
without proviso, or a pharmaceutically acceptable salt thereof.
for use in medicine, in particular in the treatment of allergic,
inflammatory and hypersecretory condltions in a human being.
The preparation of the compounds of the invention is further
illustrated by the following Examples.
SUBSTITUTE SltEET
' ' ''
.
: .
2 ~ ~ ~ P,Q.62 ! ' PCT/EP91/00737
EXAMPLE 1
Methyl 2-~4-(2-me~hylimidazo~4,5-c]pyrid-l-yl~benzyloxylben%oa~e
A mixture of 4-(2-methylimidazo[4,5-c]pyrid-1-yl)benz~l
alcohol (2.39 g, 10 m~ol), methyl salicylate (1.67 g, 11 m~ol~>
- triphenylphosphine (2.88 g, 11 ~mol) and dry tetrahydrofuran (50
ml) was stirred at room temperature under nitroge~.
Diethylazodicarboxylate (2.09 g, 12 mmol) was added dropwise over
five minutes. The resulting solution was stirred at roo~
temperature for 1 hour and the solvent then removed under reduced
pressure. The residue was purified by chromatography os silica
eluting with a mixture of dichloromethane and methanol (97:3).
The product containing fractions were combined and evaporated to
dryness. The resitue was crystallised from diethyl ether to yield
the title product (3.42 g, 92%), m.p. 126-128C.
Found: C,70.92; H,S.ll; N,11.17. C22HlgN303 requires C,70.78;
H,5.09; N,11.26X.
i EXAMPLES 2-8
The following compounds were made following the procedure of
Example 1 using the appropriate phenol as starting material.
RlO
~8 ~ ~ _ C8
R9
SUBSTITUTE S~EET
.
.
~ ' ' ' ' , ` ., . ~ . . ` .. . .
~u7sao7
- "~WO 91/17162 PCI/EP91/00737
~ _ _ ~ U~ ~ ~ C`l ~
,_ Z ~ o~ ~ X ~ ~, ~ ~_
O O O O O G C O
~: `D~ u~~ ~ 1_~
~ 5 ~_ ~ u~ ~ ~ ~r ~
111 U~, L~ Ul ~ ~ ~ ~
O ~ ~ ~1 ~ ~J ~ ~ I
__ ~
O O . O r O
: . . ___
_......................... .g _ :Z ~ ,
SUBSTITUTE SHEE
20i80~7
WO-91/17162 PCr/EP91/00737 '
.. _. ... ~
o ~`, oo ~' ~ ,_
,_ Z ~ ~ ~ _i
~C ~_, _,~ ~
~: ~ ~ ._ ~ o~ ~ ,_
_ = ~:~ L^, U~ ~D ~ ~
C) r~ U) ~ 0 0
r 0~ r~ 1~ 0 0
,~ ~n ~ ~ ~
~ ~_~
I~Oy = _ ._
E o
r~ c
SUBSTITUTE SHEE~
,
2078007
': W O 91/17162 PCT/EP91/00737
21
EXAMPLE 9
2-[4-(2-MethYlimidazo[4,5-clpvrid-1-yl)benævloxv~benzoic acid
Meth~l 2-[4-(2-methyli~idazo[4,5-c]p~.~rld-1-yl)benzyloxy]-
be~zoate (3.73 g, 10 m~ol) was dissolved in ethanol (100 ml), 2N
sodium hydro~ide (20 ml) added and the solution stirred at room
temperature for 2 hours. The solvent volu~e was reduced to 30 ml
under reduced pressure and the residue poured into water (100 ml).
The aqueous pnase was washed wi.h d~chloromethane (2 x 50 ml) and
acidified with glacial acetic acid. The ecid mixture was then
e~tracted witll dichloromethanP (3 ~ 75 ml) and the combined acid
extracts dried over Na2S04, filtered and the solvent evaporated
under reduced pressure to yield a white solid. Trituration with
diethyl ether gave the pure title product (2.03 g 57%). m.p.
217-219C. Found: C,68.48; H,4.89; N,11.41. C21H17N3O3 0.5 H20
requires C,68.65; H,4.73; N,11.21%.
EXAMPLE 10-13
The followlng compounds were prepared in a si~ilar ~anner
from the appropriate benzoate of Examples 2-5.
C01H
R8~_ 0CH
R9
SUBSTITUTE SHEET
"
,
w~ a~ 7 i PCl tEP91/00737
22
.
Z X O~ C _ .
. ~ .
v r,o ~ ~ r- c~J r~ r~ r~
a~o ~a = ~ ~ _ ~ C ~ CJ _ ;~
~ ~rl Irl U~ 1\1 . U~ ~:1 ~ ~-- I
~ O --~ E ~J r~ E rr~ ~ ~ ~
I I ~
~ o I~ _ . U _
:',
__ _._ ... _._ _
.
CO~ :~ _~ _ ~ .
~1 _
SUBSTITUTE SHEET
.
' :.,
,
2~78~07;
. ~ ~
W O 91/17162 PCT/EP91/00737
; 23
EXAMPLE I4
N,N-Dimethyl 2-[4-(2-methvlimidazo[4,5-c]pyrid-1-Yl)benzYlO~Y
benzamide
2-~4-(2-Methylimidazo[4,5-c]pyrid-1-yl)benzylo~y]benzoic acid
(359 mg, 1 mmol) was stirred in dichloromethane (15 ml) and 3
drops of N,N-dimethylformamide were added. Oxalyl chloride (25
mg, 2 mmol) was added dropwise over 1 minute. The resulting
solution was stirred at room temperature for 30 ~inutes tnen
evaporated to dryness. The residue was redissolved in drv
dichloromethane (5 ml) and added dropwise to an ice-cold solution
of dimethylamine (1 ml) in ethanol (9 ml) over a five minute
perlod. The mixture was stirred at 0C for 30 minutes and the
solvent tben evaporatet to dryness. The residue was stirred in
ethyl acetate (100 ml), the solution washed with water (2 x 50
i ml), driet over Na2S04, filtered and evaporated to dryness. The
residue was further purified by chromatography on silica eluting
with a mixture of dichloromethane and ~ethanol (96:4) and the
` product containing fraction were combined and evaporated to
dryness. The crude product was crystallised from diethyl ether
(258 mg, 67%) m.p. 148-I50C. Found: C,71.12; H,5.78; N,14.29.
C23H22N402 requires C,71.50; H,5.70; N,14.51%.
EXAMPLES 15-21
The ~ollowing compounds were prepared from the corresponding
benzoic acid following the above procedure.
coRll
R8~0CH~ ~ ~K
SUBSTITUTE SHEET
, . .
~ 20~o`~ ~ -
WO 91/17162 PCI'/EP91/00737
_._ ~ jvv; ~
~ `JU~ ~;r `J`~ ~t~
u~ :: r- ~ O Cl~ ~ _ ~ O O o~
. - ~ ~ - ~ ~ U~ ~
~ r ~ ~1 ~ D ~ ;~ ~ L^~ ~ Lr~ C~
~ ' `D~OO ~0~0~ ~_~ `D`D
__
_l _~ Z æ
E o "~
SUBSTITUTE SHEET
;, 2~780~7
.~.. WO 91/17162 PCI'/EP91/00737
. ~. _ _ .._ ~ ~ ~
û. Z I` o ~ ~D O Cl:1C~ O
~ ~o C ~ ~J ~ ~ ~ ~ ~`J ~
V~ ~ ~ X - ~ ~ O' ~ X
. X ~ ~ ~ ~o o~ _~ o
.C o ~J ~ ~ ,,~ _
G _ C "~ _ ~ . ~ ~J ~ _ ~S ,~, .
~ O ~ C ~`5 ~ _ ~O C
_ __ ._
.; e ~ ~ ~ o _,
N Cl~ O ~
.~ ~ _, .
~ _~ Z Z
_ t~ .
. __ . .. __
... ... _._ .. -
e Z c~ . _
-
SUBSTlTlJT6 SI~EEF
....... ..
,
" 20780~ l
W O 91/17162 PCT/EP91/00737 ~3
26
EXAMPLES 22-29
The following compounds were prepared using the procedure of
Example 1, starting with 2-methyl-2-[4-(2-methylimidazo~4,5-c~-
pyrid-l-yl) phenyl]propan-l-ol and reacting with the appropriate
phenol, followed by conversion to the corresponding acid or a~.ide
following the procedures of Examples 9 and 14 as appropriate.
E~8~ 2 CH ~3-- Cr.
SUBSTITUTE SHEET
~78~7
~W O 91/17162 P~r/EP91/00737
I T~-
U~ _ G. C' .-
a~ O _ ,~ ~ O ~ C G ~ -
~ ~ --. G 1` G
,~ ~ r
r ~n -I
. 0,~,~, .
..
SUBSTITUTE SHEET
2078a'37
WO 91/17162 PCI/EP91/00737 ,,".~
U c ~ ~ A ~ A
_1 X c~
__.
SUBSTITUTE S~IEEl-
'207~D~7
Wo 91/17162 PCI/EP91 737
, ~, .
EXAMPLE 3_
1-(4-~2-chlorophenoxvethvlDheny-~])-2-methylimidazo[4~5-c]pyridine
2-Chlorophenol (0.87 mmol, 112 mg), 1-~4-(2-hydroxyethyl-
phenyl)]-2-methylimidazo[4,5-c]pyridine (0.87 mmol, 220 mg) and
triphenylphosphine (0.87 mmol, 228 mg) were dissolved in dry
tetrahydrofuran (10 ml). Diethylazodicarboxylate (0.87 mmol, 151
mg) was added dropwise and the solution stirred for 24 hours, then
eYaporated to drynesc~ The residue was purified by column
chro~atographq on si]i~ el~tir.~, w'~.. ' chloL~e~nane an~ methanol
(97:3), and the 2roduct-containing fractions evaporated to dryness
to give an oil (0.23 g, 73~) which crystallised on standing. M.p.
111-113C. Found: C,68.78; H,4.96; N,11.47. C21H18ClN30 0.25 H20
requlres C,68.48; H,5.06; N,11.41%.
SUBSTIT.UT~!::~EET
7 ~ ~ PCT/EP9l/00737 _
EXf~PLE 31
1_(2_Chlorophenoxy)-2-[4-(2-methylimidazo~4,5-clpyrid-1-yl~-
phenyl]propan-2-ol
A mixture of 2-[4-(2-methylimidazo[4,5-c]pyrid-1-yl)phenyl~-
2-methyloxirane (133 mg, 0.5 mmol), 2-chlorophenol (129 mg, 1
mmol) and potassium carbonate (138 mg, 1 mmol) ~as stir ed in
N,N-dimethylformamide (5 ml) at 90C under nitrogen for 10 hours.
The cooled mi~ture was poured into brine (;0 ml) ,her e~.~~ac~e~
with dichloromethane (3 x ~n ml), Thc com~ neu ~rganic extracts
were dried over Na2S04, filtered and evaporated to drynPss. The
residue was further purified by column chromatography on silica
' eluting with dichloromethane/methanol (97:3). The product-
containing fractions were evaporated to dryness and the crude
~ product crystallised from diethyl ether. (119 mg, 61X). m.p.
; 225-227C. Fount: C,66.95; H,S.24; N,10.36. C22~20ClN302
requires C,67.09; H,5.08; N.10.67X.
EXAMPLES 32-36
The following compounts were prepared as described above
using the appropriate oxirane and phenol as starting materials:
R8 ~ OCH? I ~ CH3
~'
SUBSrITUTE S~EET
2~7~007
`: WO 91/17162 PCI/EP91/00737
~ ~ 1 1
~ ~ ~ '~
_ . _
SUBSTITUTE SHEET
.
W O 91/17162 PCT/EP91/00737 ,.
EXAMPLE 37
2 - r 4 - ( 2 -~l2 t hv-Li~ida~ c ~ ~ ~ 5-c i p~ rid~ v ~ 3-benzo [ e ]
dio~eDane
p-Toluenesulphonic acid hydrate (0.23 g, 1.2 mmol) was added
in small portions to a boilillg solution of 4-(2-methyimidazo~4.5-
c]pyrid l-yl)-benzaldehyde (0.23 g, 1 ~mol) and 1,2-bis-hydroxy-
methyl-benzene (0.16 g, 1.2 mmol) in dichloromethane (6 ml). This
mixture W25 refluxed through a scxl~let thimble containing 4
Angstrom ~olecular sieves for 2 h~llrs, then pa.. ;iol-~d be~ween
dichloromethane and satz~rat2d aqueous sodium bicarbonate. The
organic layer was separated, dried over magnesium sulphate and the
solvent evaporated to yield a foam which was crystallised from
et~yl acetate/diethyl ether (0.3 g, 822). m.p. 142-144C. Found:
C,73.29; H,5.38; N,11.32. C22H19N302 requires C,73.93; H.5.36;
N,11.76Z.
EXAMPLES 38 - 47
The Examples in tbe follouing Tables were prepared following
the above proceture reacting 4-(2-methylimidazo[4,5-c]pyrid~l-yl)-
benzaldehyde with the appropriate diol or with butanol.
A ~
SUBSTITUTE SHEET
.''
,~
20780~7
W O 91tl7162 PCT/EP91/00737
33
. _ _._ .. _
Example A-B- m.p, Analysis %
C(Theoretical in brackets)
C H N
33 _
0 foam72.33 5.36 11.25
. ~ol (72.51 4.98 11.53)
; ¦ (0.25 mole CH3C02C2H5)
~ _ ._
- I 39 ~lass70.40 5.66 10.26
! ~ H2 ~ 0 ~ (70.39 5.55 10.63)
(0.25 ~ole CH3C02C2H5)
~- _ .
61
73.22 5.62 11.18
(72.82 5.58 11.07)
(0.5 ~ole CH3C02C2H
_ . _ _ _ _ _
41 1 0 ~51-53 73.68 5.59 11.33
(73.93 5.36 11.76)
__; ~ .
42 ~ ~88-8 76.60 5.47 9.34
(76.40 5.53 9.22)
~" 0 (0.25 mole CH3CO~C,H5)
_ . . . -
43 ~ foam ! 73.02 5.72 10.36
(73.05 5.89 9.86)
(0-5 mole CH3CO2c2H5)
. _
SUBSTITUTE StlE~T-
- - - . ~ . . - .
~. :
'`,,
.. , s
~: ~v~(~uu ~
W O 91/17162 . ~ . PCl/EP91/00737 ~.
34
Examplel A-B-¦ ~.p.l Analysis /,
No I ¦ c ~ eorecical in br~7ckets)
¦ I ~ H 1
, I ! - - __ I
44 CH3(CH2)30 oil 70.9~ 8.02 10.79
3(CH2)30 (70.61 7.96 10.95
. 0.25 moie CH3CO~C H )
i 45 ~ _ . ~
. /~ 0 ~ 1;4-o 1 /4.02 ~.7010.95
; ~ I (74.37 5.7011.31)
~ ._ - , - .
46 ~== \ r 0 148-501 74.37 5.8011.40
) ~ (74,37 5.7011.31)
47 157-9 ' 72.26 5.3911.41
~ ~72 11 5 5011.47)
SUEIST~TUTE SHEET
'. ' : ` ,
2~730~7
W O 91/17162 PCT/EP91/00737
EXAMPLE 48
2-~4-(2-Methylimidazo~4,5-c]pvrid-1-yl)phenyl]-2-me~hyl-4 phenYl-
1,3-dioxolane
Neat 1,2-bis(trimethylsilyloxy)-1-phenylethane (0.17 g, 0.6
mmol) was added to a cold (-78C), stirred solution of
trimethylsilyl trifluoromethanesulphonate (0.1 ml) in
dichloromethane (1 ml). After 5 minutes, a solution of
4-(2-methylimidazo[4,5-c]pyrid-1-yl)acP~ophenone ~0.13 g, 0.5
mmol) in dichloromethan~ fl ml) 'as aducu. lh~ resultant solution
was war~ed to 20C during 2 hours, then stirred for 72 hours
before partitiouing between dichloromethane and saturated sodium
blcarbonate solution. The organic layer was washed with brine,
dried over magnesium sulphate and evaporaeed to a yellow oil.
Silica gel chro~atography uslDg 10~ methanol iD ethyl acetate as
eluant afforded a colourless foam (0.1 g, 48%). The product was a
1:3 mixture of cis/trans isomers. m.p. less than 40C. Found:
C,72.56; H,5.85; N,10.53. C23H21N302.~H20 requires C,72.61;
H,5.ô3; N,11.04Z.
EXAMPLE 49
2-(2-Chloro~henyl)-4 14-~2-~ethYlimidazo~4,5-c]p~rid-1-~l)phen l~-
1,3-dioxane
~ utyllithium (1.6M in hexane; 0.91 ml, 1.46 mmol) was added
in trops to a stirred suspension of l-[~-tl,3-dihydroxypropyl)-
phenyl]-2-methylimidazo~4,5-c]pyridine (0~2 g, 0.7 mmol) in
anhydrous tetrahydrofuran. The ~ixture was heated to re~lux ~o-
30 minutes, then cooled ant treated with chlorotrimethylsilane
SUBS~ E SH~E~
2~780~7
W O 91/17162 PCrJEP91/00~37
36
(0.22 ml, 1.7 mmol). After stirring for 16 hours, the golvent was
evaporated and the residue partitioned between dichloromethane and
saturated aqueous sodlum bicarbonate. The organic laver was drled
over magnesium sulphate and concentrated to an oil. To a solution
of this oil in anhydrous dichloromethane (5 ml) at 0C were added
sequentially a solution of trimethylsilyl trifluoromethane-
sulphonate (0.18 ml, 0.92 mmol) in dichloromethane (1 ml) followed
by 2-chlorobenzaldehyde (0.1 g, 0.7 mmol). The mixture was
stirred at a~bi2nt temperatllr~ for 24 h^u.s, and .hel~ par~irioned
between dichlorsm2than2 and saturated, aqueous sodium bicarbonate.
The organic layer was dried over magnesium sulphate and evaporated
to an oil. Silica-gel chromatography eluting with 10% methanol in
etbyl acetate and trlturation with diethyl ether afforded a white
solid (0.15 g, 52Z), m.p. 161-164C. Found: C,67.81; H,5.02;
N~10-14. C23H20ClN302 requlres C,68.06; H,4.97; N,10.35X
AMP~E 50
4-~4-(2-Methylimidazo~4,5-c3p2rid-1-yl)~henyl]-2-(3,4,5-
tr methox~phenvl)-1,3-dioxane
The procedure of Example 49 was followed but replacing
2-chlorobenzaldehyde with 3,4,5-trimethoxybenzaldehye in the
second stage of the process to yield the title product as a white
solid in 28~ yield. M.p. 135C. Found: C,66.95; H,5.97; N,8.8~.
C2~H27N305. 0.25 H~0 requires C,67.01; H,5.95; ~,9.02X.
SU8STITUTE SHEET
, W O 91/17162 2 0 7 8 0 Q 7 PCT/EP91/00737
37
EXAMPLE 5 1
2-~4-(2-Methvlimidazo~4,5-c]pyrid-~ )phenyl]-4-pheny~ 3
dioxolane
_
1,2-Bistrimethylsilyloxy-l-phenylethane (from Preparation 6)
(0.34 g, 1.2 mmol) was added to a cold (-70C), stirred solution
of trimethylsilyl trifluoromethane-sulphonate (0.23 ml, 1.2 mmol)
in dry dichloromeehane (2 ml). After 5 minutes, a solution of
4-(2-methylimidazo~4,5-c]-pyrid-1-yl)benzaldehyde (0.24 g, 1 mmol)
in 2 mi or dichloromethane w~s added. The m 2;ur~ was alloweb to
warm to 22-24C then stirred at this temperature for 22 hours.
The reaction mixture was partitioned between saturated aqueous
sodium bicarbonate and dichloromethane, the organic layer was
dried (MgSO4) and evaporated to a residue which was purifled by
sllica-gel chromatography (5X methanol in ethyl acetate as eluant)
to afford the title compound as a foam (0.031g, 8%). Found:
C,71.37; H,5.39; N,10.83. C22~19N302. 0.75 H20 requires C,71.24;
H,5.57; N,11.27~.
SUBSTITUTE S~IEET
.
,
20780~7
W O 91/17162 ' PCT/EP91/00737 -
EXAMPLE 52
2-Chlorobenzvl 4-(2-methylimidaæo~4,5-c]pyrid-1-Yl)benzoa~e
Oxalyl chloride (0.7 ml, 8 mmol) and dime~hylfor~a~'c'e (1
drop) were added to a stirred suspension of 4-(2-methylimidazo-
[4,5-c]pyrid-1-yl)benzoic acid (0.51 g, 2 mmol~ in dichlorometnane
(10 ml) at 0~ under nitrogen. After 1 hour, the solvenr ~as
evaporated and the residue dissolved in dichloro~ethane (lu ml~ tG
which was added 2-chlorobenzyl alcohol (0.86 g, 6 ~mol) and
4-dimethylamino-pyridine ('7~3 c rr-s~als~ ~irer 1~ hours, he
mixture was diluted with dichloromethane and ~ashed with aqueo~s
sodium carbonate. The organic layer was dried (MgS04) and
evaporated to an oil. Flash chromatography, eluting with 15%
methanol ~n ethyl acetate left a residue which solidified on
trituration with diethyl ether (0.255g, 33%). M.p. 147-149C.
Found: C~66.86; H,4.32; N,11.15. C21H16C12N302 requires C,66.76;
H,4.27; N,11.12;;.
EXA~fPLE 53
~4-(2-Methylimidazo~4,5-c]pyrid-1-yl)benzvlo~ldi henylmethane
hydrochloride
4-(2-Methylimidazo[4,5-c]pyrid-1-yl)benzyl alcohol ~1 mmoi,
253 mg), and benzhydrol (1 mmol, 184 mg) were dissolved ir.
dichloromethane (5 ml). ~'rifluoroacecic acid (0.5 ~1~ was added
dropwise and the mixture stirred ror 15 minutes. The solution ~as
poured into 2~; sodium hYdroxide and che aaueous phase e~racted
with dichloromethane (3 x 4~ ml). The co~bined organic extractC
were dried over MgS~,, filtered and evaporated to dryness. The
residue was further purified by column chromatography on silica
SUBSTITUTE SHEET
. .
..
W O 91/17162 2 0 7 ~ ~ ~ 7 PCT/EP91/00737
39
gel eluting with dichloromethane/methanol (95:5). The product
containing fractions were evaporated to drvness, dissolved ln
ether and treated with ethanolic hvdroges chloride. ~he resulti
solid was recrystallised from eth~l ace~a~e/methanol, (6A~ mg,
14%). M.p.235-237C. Found: C,7~.,3; H,5.5~ 5~
C27H23N3O.HCl. 0.25 H20 requires C,72.65; H,5.49; `~,C.42,'
~ A.~PLE ~4
~4-(2-Methylimidazor4.~-e!nvrid~ bc".vlo-~ -chloro~ne~
methane hvdrochloride
4-(2-Methylimidazo[4,5-c]pyrid-1-yl)benzyl alcohol (1 mmol,
253 mg) was dissolved in dimethylformamide (10 ml) and sodium
hydride (60X in oil, 1.15 mmol, 46 mg) was added and the mixture
stirred at room temperature for 1 hour. Freshly distilled
chlorobenzyl chloride (1.1 mmol, 177 mg) was added and the mixture
stirred for 4 hours at room temperature. The reaction was
quenched with lN hydrochloric acid (1 ml) then basified with 5X
sodium carbonate solution and the aqueous phase extracted with
dichloromethane (3 x 50 ml). The organic extracts were dried over
MgS04, filtered and evaporated to dryness. The residue was
purified by column chromatography on silica elucing with
dichloromethane/methanol (97:3) and the produce containing
fractions evaporated to dryness. The oil was redissolved in
diethyl ether and ~reated with ethereal hvdrogen chloride. lhe
solid precipitate was recrvstallised tro- eth~l aceta;e/methanol
to give the title product, (67 mg, 177,). ~.p. 218-A~20C. Found:
C,62.44; H,..75; N,10.34. C21H18Cl,;~O.H~i. 0.25 H~0 requires
C,62.31; H,4.86; 10.387
SUBSTITUTE S~IEET
. .
...
, . . .
, . ~ . .
20780~7
W O 91/17162 PCT/EP91/00737
; 40
EXAMPLE 55
5~(2-Chlorophenvl~-2-[4-(2-methylimidazo~4~5-c~pvrid-l-vl~phen
delta~-oxazoline
2-t2-chlorophenyl)-2-hydroxyethyl]-4-(2-methylimidazo[4~5-c]
pvrid-l-vl) benzamide (0.5 mmol, 200 mg) and triphenylphosphine
(0.6 ~ol, 157 mg) were dissolved in tetrahydrofuran (5 ml) at
room te~perature. Diethvlazodicarboxylate (0.6 mmol, 104 mg) was
ad~d d opwis2 and the mi~ure stlrred for 12 hours then poured
into ether 150 ml~ and ex~-acted ~ith 0.5 N :,vurochioric aci~ ~ x
25 ml). The combined aqueous eY.tracts where basified with 2~
sodium hydroxide then re-extracted with dichloromethane (3 x 50
ml), dried over NaS04, filtered and evaporated to dryness. The
residue was partially purified by column chromatography eluting
with ethyl acetate/diethylamine (97:3), the product-containing
fractions were evaporated and the residue further purified by
preparative thin-layer chromatography in dichloromethane/methanol
(95:5) to give the title product (25 mg, 13%), m.p. 127-130C.
Found: C,67.68; H,4.67; N,13.79. C22H17ClN40 requires C.67-95;
H,4.38; N,14.41Z.
SUBSTITUTE SHEET
.
. .
~ . WO 91/17162 2 0 7 8 0 ~ 7 PCT/EP91/00737
EXAMPLE 56
~,N-Diethvl-~4-(q-methylimidazo[4,S-clpyrid-l-yl)phenYl~-
propionamide
a) Methyl 3-[4-(2-methvlimidazo]4,5-c1pyrid-l-yl)phenyl]-
propanoate (3.56 mmol, 1.05 g) was dissolved in ethanol t25 ml)
and 2.~ sodium hvdro.:ide (10 ml) added. The solution was stirred
at roo~ temperature for 1 hour then poured into water and
acid- fied with glac al ac-tic acid. TAe soiution was extracted
with dichioromethane t3 x 50 ml! i an.d ~ hc cO~L-llêd eX~L~LS were
dried o~er ~gS04, rilterêd and evaporated to ~ield 3-[4-(2-methyl-
imidazo[4,5-c]pyrid-1-yl)phenyl]propanoic acid.
b) ~4-(2-Methylimidazo[4,5-c] pyrid-l-yl)phenyl]propanoic acid
(1 mmol, 281 mg) was stirred in dichloromethane (10 ml) and one
drop of dimethylformamide adted. Oxalyl chloride (2 mmol, 254 mg)
was added dropwise and the resulting solution stirred at room
temperature for 30 minutes then evaporated to dryness. The
residue was redissolved in dichloro~ethane tlO ml) and
diethylamine (3 mmol, 219 mg) were added and the solution stirred
for 30 minutes at room temperature. The reaction mixture was
poured into water and extracted with dichloromethane (3 x 25
The combined extracts were dried over ~a2S04, filtered and
evaporated to dr~ness and the residue was purified b~ column
chromatography on silica eluting with cichloromethane/methanol
(9;:3). ~he product containing fracticns were evaporated to
dryness and the oil crystallised from dieth~l ether/he~ane to give
the title compound ~194 mg, 58%), m.D. lq3-lq5C. Found: C,71.1q;
H,7.22; ~;,16.84. C20H2 ~ 0 requires C, 1.43; H,7.1~; ~;,16.67,~.
SUE~STITUTE S! IEET
,
-, ` ... ..
. .
. , ,~, . . .. ; . ~. 1.".. ,. ; ., ,' ... ` . .
' .. . ' ::. ' ` '
207~0Q7
W O 91/17162 ~ ` PCT/EP91/00737 ,.
,
EXAMPLES 57-60
The following compounds were prepared as described in Example
56(b) above using t~e appropriate a~ine instead Oc dieth:'a~irc.
; C~13
R CO(CH~
Exa~ple R13 Im.p. Analysis %
(Theorecic~l in brackets)
57 . 237-67.35 5.13 18.59
239(67.37 5.15 18.49)
NH- (0-25 mole CH2C12)
58 ~ 1171-70.93 7.25 16.33
(CH3)3cNH- ¦173 (70.8Q 7.26 16.52)
II (0.17 ~ole a~o)
. ~
59 ~190-65.21 5.95 13.01
CH~H- 194~65.02 5.8 13.19
(HCl, H~0)
. . . _ .
~ 155- 68.~4 6.5~ 3
~ 157 (68.57 6.~9 16.00)
SUBSTITUTE SHEET
, .. ~ . . . .: . .
. ~ .' . ....
~,. ..
.O W O 91/17162 2 o 7 8 ~ ~ 7 PCT/EP91/00737
43
EXAMPLES 6l-64
Ethyl 2-[4-(2-methylimidazo~4,5-c]pvrid-1-yl)phenyl]propanoate
(Preparation ~T) was hydrolysed following the procedure of E;:am~lc
56 (a) and reacted with appropriate amines following the procedure
of Example 56(b~ to give the following co~po~nd~s:
Ch3 ICr~
\~
Example R13 m.p. Analysis %
C (Theorec~ical in brack
61 (C~3)3cNH- 165- 71.45 7.22 16.95
167 (71.43 7.14 16.67)
62 ~ ~ 100- 1 70.34 5.50 20.25
NH- 104 (70.59 5.32 19.61)
. . , _ __ . _
(C2H5)~N- !151- 71.14 7.26 16.33
161 (71.~3 7.1~ 16.67!
T
64 ~ 1123- 7~.10 ,.03 16.5
~ ¦1 5 t7_.41 6.90 16.0
. . _ .
SUBSTITUTE StlEET
~ . . ~. , -. , .
, . . . .
- . ~, ` ,
- : , . . ..
wo 91il7162 2 0 7 8 ~ 0 7 PCT/EP91/00737,~
EXAMPLE 65
~ -(2,4-Difluorobenzvlamin~methYl)Dhenyl~-2-methylimldazo~4,5-
c]pvridine
a) A suspension o} 4-(2-methylimldazo[4,5-c]pYrid-l-yl)-
benzaldehyde (750 mg, 3.16 mmol). 2,4-difluorobenzyl-
amine (500 mg, 3.5 mmol) and silica gel (230-400 mesh) (1 g) in
anhydrous dichlorome~hane (20 ml) was stirred for 16 hours at
embient temperature. The suspension was f.ltered, the filtrate
evaporat~d to dryness and th~ resid-_al gum ;ri-u~ea wi~n
anhydrous diethyl ether to af~ord the Schiff's base as white solid
(700 mg, 61%).
b) Sodium borohydride (57 mg, 1.5 mmol) was added to a stirred
solution of the above product (500 mg, 1.4 mmol) in anbydrous
methanol (10 ml) at 0C. The stirred solution was warmed to 20C
over 1 hour, then the solution concentrated, the residue acitified
to pH 1 with 2M hydrochloric acid, water (20 ml) added ant sodium
bicarbonate added to pH 8. The solution was extracted with
ethyl acetate (50 ml). The ethyl acetate extract was wasbed with
water, dried over ma~nesium sulphate and concentrated to dryness.
The residual gum was triturated with anhydrous diethyl ether to
give the title co~pound as a white solid. tO.l g, 20~), m,p.
108-110C. Found: C,69.06, H,5.00; ~,15.48. C~IHl8F2Nl requires
C,69.22; H,4.98; N,15.37~.
SUBSTITUTE SHEET
-, .
. , :
.. , ", .
. ' .
~ ; ~
W O 91/17162 2 0 i8 0 ~ 7 PCT/EP91/00737
' 45
EXAMPLES 66-69
lhe abo~e procedure was followed using the appropriate amlne
in step (a) to yield the following products
CH3
~ -CH~ ~ t~ t~
E~a~ple R14 m.p. I Analysis
No ¦ (Theoreeical in brackets)
66 ~ CH2NH- 88-69.73 5.28 15.58
1 90(69.51 5.28 15.44)
67 131- ! 77.51 5.97 16.20
t~' - 1321 (77.62 5.92 16.40)
¦ tO.25 mole H20)
, 1 _ 104-69.26 5.76 1~.79
I I ~ ( 2)2NH 106(69.28 5.68 14.69)
. _ . _ .
64 ~ ~ roa~74.5,6.43 14.10
t/4.5~ 6.78 l~.jO)
¦ (hydrate!
.. . .
SUBSTITUTE SHEET
'
W O 91/17162 2 0 7 8 0 0 7 PCT/EP9t/00737 ~
46
EXAMPLE 70
1-[4-(1,2,3,4-Tetrahydroisoquinolir.-1-vl-~eth~l)phenyll-2-~ethvl-
imidazo[4,5-c]pyridine
Glacial acetic acid was added ~o a stirred solution o.
1,2,3,4-tetrahydroisoquinoline (3.5~ g, ~6.~ mmol) in anhyd.ous
methanol (15 ml) until the pH was ,. 4-(2-Methyl midazo[4,~c7-
pyrid-1-yl)benzaldehyde (1.56 g, 6.6 mmoi) was then added and the
reactants stirred at ambient ;emDP~a~ure ,~r ~0 ~ nutes Derore
adding sodium cyanoborohydride (0.63 b~ 10 ~vl)~ lh~ reaC~all~C
were stirred at ambient tempera~u~e ~or ~0 minu~es, then ~a~er (sC
ml) added. 2M Hydrochloric acid was added to pH 2, followed b~
sodium bicarbonate to pH 8 and the aqueous solution was ehen
extracted with dichloromethane (100 ml). The organic exeract was
washed with water (50 ml), drled over magnesium sulphate and
concentrated under vacuum.
The residual gum was chromatographed on silica (230-400
mesh), eluting with 5% diethylamine in ethyl acetate. Appropriate
fractions were combined and concentrated and the residual gum
triturated with diethyl ether to give the title compound as a
white solid (450 mg, 20~). tl.p. 110 -112C. Found: C,77.73;
H,6-21; N,15,76- ~13H22N4 requires C,7/.94; H,6.26; N,lj.817O.
SUBSTITUTE SHEET
'
^; W O 91/17162 2 0 7 8 ~ 0 7 PCT/EP91/00737
EXAMPLES 71-73
1he following co~pounds were prepared by the above procedure
usin~ the appropriaee amine ins~ead cf t~rr2~ droiso~uinol.irc
CH3
~, -C~
'~
_
Example Rl~ m.~. ! Analysis /~
No. ~ IC ¦ (Theoretical in brackets)
__ _ j C H
71 1 ~ 140- 77.83 6.22 16.11
l I _ 142 (77.94 6.26 15.81)
7~ ¦ 102CH3 gu~ I characterised b~ N~P~( )
¦ ~ CH2-CH-NH- l l
2 _ 269- 1 66.67 4.8~ 19.14
~H- l273 1 (66.84 4.7/ 19.49
.
(1) ~ (C~C13): 2.58(3H,s!, 3.01(1H,dd -11.6Hz~ 3.10(1H,dd J=li,
6Hz), 3.60(1H,~ J~6Hz), 3.74(3H,s), 3.,8 and 3.98(each lH,c
J=15Hz), 7.08(1H,d J=4.5Hz)t 7.~-. .4( H,~, 7.~7¢3'r.,d J=c~
8.41(1H,d J=4.5Hz) and 9.08(1H,s!.
SUBSTITUTE S~IEET
;' ' , . ., " . ' . .
~. ' . . ,~
W O 91/17162 2 0 7 8 0 a 7 PCT/EP91/00737
48
EXAMPLE 74
1-(4-~-Phthalimidomethylphenyl)-2-methvlimidazo[4,5-c]pvridlne
To a stirred suspension of 4-(2-methylimidazo~4,5-c]pyrid-1-
yl)benzyl alcohol (431 mg, 1.8 mmol) in anhydrous teerahydrofuran
(10 ml) under nitrogen were added in turn, phthalimide (26~ mg,
1.8 mmol), triphenylphosphine (471 mg, 1.8 mmol) and
diethylazodicarboxylate (6.283 ml, 1.8 mmol). The solution was
stirred ae ambient tempera~ure for 16 hours then evaporated to
~rvnP99 anA ~hc rcs-Jual ~uul cilLu~L~rapned on silica (~30-4uu
mesh), eluting with 10% - 20% methanol in ethyl acetate.
Appropriate fractions were combined, concentrated and the residual
gum triturated with ethyl acetate to ~ive the title compound as a
white solid (200 mg, 30X), m.p. 222-224C. Found: C,71.44;
H~4-42; N~15-22- C22H16N402 requires C,71.73; H,4.38; N,ls.21%.
EXAMPLE 75
1-~4-~N-(2-Amino~henyl~sminomethAYl]ohen-1~-2-methVlimidazo~4.5-C ! -
pvridine
Stannous chloride dihydrate (280 2g, 1.25 mmol~ was added to
a s~irred solution of 2-methyl-1~4-~N-(2-nitrophen)~l)amino-
methyl]phenyl~-2-methylimidazol'4,5-c]p~ridine (from Example 73)
(90 mg, 0.25 mmol) in 2M hydrochloric acid (0.5 ml), ethanol (1
ml) and wacer (1 ml). The solution was stirred under reflu~: for
hours then cooled to ambient temperature and partitioned between
ethyl acetate and saturated aqueous sodium bicarbona e The
or~anic e~tract was washed with water~ dried over magnesium
sulphate and concentrated to drvness. The residual gum was
chromatographed on silica (230-400 mesh), eluting with 2Z-10,.
SUBSTITUTE StlEET
wo 9l/17162 2 0 7 8 0 G 7 PCT/EP9l/00737
'
diethylamine in ethyl acetate. Appropriate fractions were
combined, concentrated and the residual ~um triturated with
dieth~l ether to give the title compound as an o~-white solid (~7
mg, 27%), m.p. 190-195C. Found: C,71.99; H,5.88; ~,20.28.
C20HI9~5. 0.l25 CH3C02C2H5 requires C,72.33; H,5-92; ~20.57~o-
EXAMP~E 76l-r4-Benzi~idazol-l-ylmeth,~l)phenyl~-2-~ethvlimidazo[4~5-c]
nV r ~ ' n O
A solution of 1-~4-[N-(2-aminophen~l)a~inomethyl]phenY~ -2-
methyl-imidazo[4,5-c]pyridine (300 mg, 0.91 mmol) and triethyl-
orthoformate (5 ml, 30 mmol) in formic acid (0.5 ml) was stirred
under reflux for 1 hour, then cooled to ambient temperature,
diluted with water (S0 ml) aod stirred at ambient temperature for
16 hours. The solution was concentrated and the residue
partitioned between ethyl acetate and saturated aqueous sodium
bicarbonate (pH 8). The organic extract was separated, washed
with water, dried over magnesium sulphate and concentrated to
drvness. The residual gum was chromatographed on silica (230-400
mesh), eluting with 20Z methanol in eth~l acetate. Appropriate
fractions were combined and concentrated under high vacuum to give
the title compound as a foa~ (150 mg, ~9Z). Found: C,73.57;
H,5-06; ~,20-16- C21H17~5. 1/4 H~0 recuires C,73.34; H,5.13;
0.36%.
SUBSTITUTE SHEET
' ': ' ' `' ', '' . ' : . ',
~ ` ' -. , ' ' , . ', `,
2~78~
W O 91~17162 PCT/EP91/00737,~.
EXAMPLE 7 7
1-~4-(N-(2-Ch_.~robenzyl)-N-ethoxvcarbonylamino)phenYll-~-methYI-
imidazo~ 4, 5-c]pvridine
n-~utyl lithium (1.6M in hexane) (1.19 ml, 1.9 mmol) was
added dropwise to a stirred solution o~ -(2-chiorobenz~
; aminomethyl)phenyl~-2-methylimidazo~,5-clp~ridine (0.61 ~, 1.7
mmol) in anhydrous tetrahydrofuran1 under ~t.ogen, at -30~.
After stirrin~ for 20 minutes at _30C~, et:~l chlororor~ace (C.2~
ml. 2.? ~m^'~ as ad ~ ' ald .~ irred rea_.lon ~;~;ur~ wa~ed tc
ambient temperature over 16 hours. Saturated aqueous sodium
bicarbonate solution was added to the stirred reaction mixture and
the product extracted into ethyl acetate. The organic extract was
washed with water, dried over magnesium sulphate and concentrated
to an oil (700 mg). Chromatography on silica elut~ng with 5Z
methanol in ethyl acetate gave the title co~pound as a solid (90
mg, llX), m.p. 118-122C. Found: C,65.32; H,5.30; N,12~51.
C24H?3C1~40?.~H2O requires C,64 93; H,5.45; ~ 12 62,'
EXAMPLE 78
1-~4-~N-Benz~ e~hoxv~rbonvlamino)ehenvl]-~-meth~l-imidazo~4,5-
-c]pvridine
This compound was prepared as described in the previous
Example starting with the corresponding ~-ben~.laminometh~l
derivative to give the title ~-benz~l compound as a solid (37~).
~I.p. 1~9-132C. Found: C~71~7~J; H,j.~2-; `:.13.S5.
requires C,71.98; H,6.04; ~13.9g~o.
SUBSTITUTE StlEET
~e W O 91/17162 2 0 7 8 0 ~ 7 PCT/EP91/00737
E,YAMPLE 79
N-(2-Chlorophenvlacetvl)-4-(2-methylimidazo~4,5-c]pYrid-l-
vl)benzvlamine
. .
a) Raney nickel (0.25 g) was added to a solution of
4-(2-methylimidazo~4,5-c]pyrid-1-yl)benzonitrile (2.3~ g, 0.G7
mmol) in acetic anhydride (15 ml) and the reaction m :ture
hydrogenated at 50C and 50 p.s.i. (3.45 bar) ror 1 nour. The
reaction mixture was filtered, the filtrate dilu~ea ~ith water (5
ml~ dnU aonc~n~ra~ed hyd-ochlorlc 2C' ~ ) ar.d t~e sol~tio~.
stirred at 100~C for 16 hours. On cooling to ambient temperature.
2~1 sodium hydroxide was added to pH 9 and the product extracted
with ethyl acetate. The organic extract was washed with water~
dried over magnesium sulphate and concentrated under reduced
pressure. Chromatography on sllica, eluting with ethylacetate:
methanol:diethylamine (90:5:5) gave 4-(2-methylimidazo~4,5-c]-
pyrid-l-yl)benzylamine. (0.6g, 252).
b) Oxalyl chloride (0.7 ml, 8 mmol) was added to a stirred
solution of 2-chlorophenylacetic acid (340 mg, 2 mmol) in
anhydrous dichloromethane (6 ml) under nitrogen. Anhydrous
; dimethylformamide (2 drops) was added and the solution stirred aè
ambient temperature for 2 hours. The solution was concentrated
under high vacuum, then re-dissolved in dichloromethane (10 ml)
and a solution of 4-(2-methylimidazo[~,5-c~-pyrià-1-yl)benzylamine
(0.6 g, 2.5 mmol) in anhydrous dichloromethane (10 ml) added over
5 ninutes. lhe solucion was stirred a~ ambien~ temperature for 16
hours, then washed with saturated aqueous sodium carbonate (pH 9),
dried over magnesium sulphate and concentrated under vacuum
SUBSTITU~E StlEET
. . ' '`'
W O 91/17162 2 0 7 8 0 a 7 PCT/EP91/00737, r-
Chromatograph on silica, eluting with 10% methanol in ethyl
acetate ~ve the title compound as a foam (350 mg, 45%). Found
~,66.08; H.4.95; N,13.75. C22HIgClN O.~H~0 requires C.66.08:
~,5.04; N,14.01%.
EXAMP~E 80
~-(3',4'-DichlorobenzoYl~amino-4-(2-methvlimidazo~4,5-c~pvrid-1-
yl acetophenone
..~Eeh~ 2~ e;hyii-uiu;~ pyrid-i-vl~-2-oximlnoben.o~,laceta.e
A solution ol sodium nitrite (3.3 g, 47 mmol) in water (40
ml) was added in drops to a solution of ethyl 4'-(2-methyl-
imidazo[4,5-c]pyrid-1-yl~benzoylacetate (12.6 g, 39 mmol) in
glacial acetic acid (45 ml) at 5C. After 1.5 hours the ~ixture
was partitioned between tichloromethane and brine. The organic
layer was washed again with brine and then with saturated aqueous
sodium bicarbonate, dried (MgSO4) and evaporated to an oil which
rapidly crystallised on addition of ether (9.61 g, 707~), (2:1
mixture of syn/anti isomers). M.p. 168-170C.
b) Ethyl 2-acetamido-4'-(2-methYlimidazo~4,5-c]p~rid-1-ylbenzoyl-
acetate
A solution of ehe product from al above (6 g, 17 mmol) in
acetic acid (33 ml) and acetic anhydride (9 ml~ was hydrogenated
over 5~ palladium on carbon (1 g) at 50 p.s.i. (3.45 bar! at 30C
ror 2 hours. The mi~tute was filtered ehrough a filter pad,
washing the ca~e with methanol and the filtrate was evaporated.
The residue was chromatographed eluting with methanol and then 10,
methanol in ethyl acetate to afford 2 colourless foam (6.1 g,
94X). M.p. 71-73C.
SUBSTITUTE SHEET
.`
'
~ wo gl/17162 2 0 7 8 0 ~ 7 PCT/EP91/00737
c) 2-Amino-4'-(2-methvlimidazo~4,5~clpYrid
dihvdrochloride
A solution of the product from b) above (1.2 g, 3.2 mmol~ in
2M hydrochloric acid (30 ml) was heated at reflux for 3 hours.
The solution was evaporated to dryness to vield the amine
hydrochloride salt as a colourless foam (1.35 g), which was stored
under vacuum.
d) N-(3',4'-Dichlorobenzoyl)amino-4-(2-methylimidazo~4,5-c]p~rid-
i-yi ace~oinenone
N-~ethylmorpholine (1.6 ml, 16 mmol) was added to a stirred
suspension of the product from c) above (0.7 g, 1.8 mmol),
l-hydroxybenzotriazole (0.34 g, 2.4 mmol), 1-(3-dimethyl-
aminopropyl)-3-ethylcarbodiimide hydrochloride (0.89 g, 4.7 mmol)
and 3,4-dichlorobenzoic acid (0.42 g, 2.2 mmol) in dichloromethane
(25 ml). After 26 hours, the mixture was evaporated and
partitioned between water and ethyl acetate. The organic layer
was dried (MgS04) and evaporated to an orange gum t0.69 g~-
Chromatography, eluting with 15X methanol in ethyl acetate,
afforded a foam which solidified upon tri~uration with dieth~l
ether, (0.3 g, 38%), m.p. 108-110C. Found: C,58.78; H,3.76;
~'~12-20%- C22H16C12N402.~H20 requires C,58-93; H,3-83; ~12-50~-
SUBSTITUTE SHEET
.
.
.
WO 91/17162 2 0 ( 8 a o 7 PCI'/EP91/00737 _
54
EXAMPLES 8l-83
. _
lhe following compounds were pre~ared as described in the
previous E~ample using the a~propri~te acid in S~eD d):
oCH3
R 15 -COl;iH-CH ., -C
~xample I pl5 I ~-?~ ~ ^nalysis '~
No C l(Theoretical in brackees)¦
¦ C H
81 148- ; 67.82 4.88 17.57
150 (67.78 4.98 17.96)
H3C (0.25 mole H20)
._
82 ~ 2 Z q - charac~erised b~ :~MR ( 1)
83 ', ~ CH2Si(CH3)3 197- characterised by NMR (2j
(1) ,S (DMS0-d6) 2.56(3H,s), 5.16(2H.d J=SHz) 7.28(1H,d J=5.;H7),
7.38(1H,t J-8Hz), 7.80t3H,m), 7.91(1H,d J=7Hz~, 8.31(3H,m)~ 8.5j
and 8.94(each lH,s), 10.43 (lH.t J=5Hz)~ and 13.0~3 (lH,s).
(2) (CDC13) 0.05(9H,s), 2.56(2H,s), 2.nl(3n,5~ 5.04(2H,d,
J=5Hz), 6.90 br(lH,t), 7.15(3H,~), ,.38(1H,t, J=7Hz), 7.54(1H,d,
J~7Hz), 7.60(2H,dJ-9Hz), 8.32(2H,d J-9Hz!, 8.45(1H,d J=5H~ and
9.12(1H,s). SUBSTITUTE SHEET
.
,
.
.: -. , - ' . . - `: ` ' , `' ,
-` ~ . .
.
wo gl/~7162 2 0 7 8 ~ ~ 7 Pcr/EP9l/0073~
EXAMPLE 84
~-(2-Meth~l~ benzovl)amino-4-(2-methylimidazo~4,5-c]pvrid-1-vl)-
aceto~henone
Sodium methoxide (284 mg, 5.26 mmol) was added to a stirred
solution of N-(2-trimethylsilylmethYlbenzoyl)-4-(2-meehYl-
imidazo~4,5-c]pyrid- l-yl)aminoacetophenone (from Ex2mple 83
above) (1.2 g, 2.63 ~mol) in anhydrous c_~etnyl~orm22ide (10 ml)
under nitro~en. The solution was seirred a. lOO~C for 30 minutes.
tn~n concen~rated to dryness. The rasidue was dlssolved in ethyl
acetate (50 ml), washed with water (3 x 50 ml), dried over
magnesium sulphate and concentrated under reduced pressure.
Chromatography on silica (230-400 mesh), eluting with 15% methanol
in ethyl acetate, and trlturation with diethyl ether gave the
title compound as a hygroscopic white solid (200 mg, 20Z). lH NMR
(CDC13): 2.56(3H,s); 2.64(3H,s); 5.04(2H,d, J-5Hz);
6.90(1H,broad); 7.17tlH,d,J=5Hz); 7.28(3H,m); 7.58(1H,d,J=7Hz);
7.60(2H,d,J=9Hz); 8.32(2H,d,J=9Hz); 8.4;(1H,d,J=5Hz); 9.12(1P,s).
EXAMPLE 85
4-(2-Methylimidazo[ ~ -~l)-N-~3-quinolin~l)benzamide
Oxalyl chloride (508 mg, 40 mmol) was added dropwise to a
stirred suspension of 4-(2-methylimidazo~4,5-c]pyrid-1-yl~benzoic
acid (253 mg, 1.0 mmol) in dry dichloro-ethane (4 ~.1) at 0c unde~
nitrogen. After the addltion was complere, the suspension was
sonicaced at room temperature for 1 ho_- ~he solvent wzs ~he~
evaporated under reduced pressure ~o ~i~e an o r-white solid,
which was resuspended in dry dichlorome:hane (4 ml) and a solution
SUBSTITUTE SHEET
" ' ` .
-` .. . ~.`: . .
` -
, : "''` . `'
, : `: ` , . ,.. ~`
WO 91/17162 PCI/EP91/00737- ...
2078~7
56
of 3-amino-quinoline (288 mg, 2.0 mmol) and triethylamine (505 mg,
5.0 mmol) in dr~ dichloromethane (7 ml) was added dropwlse. The
resulting ~i~ture was sonicated for 1 hour at roo~ temperature,
and then treated with saturated aqueous sodium bicarbonate (20
mi). The mixture was extracted with dichloromethane (3 ~ 30 ml)
and the combined extracts were dried (Na2S04) and concentrated
under reduced pressure. The residue was purified b~ flash
chromatographv eluting with dichloromethane/methanol. Fractions
CUllL~ining produc~ were comblned, concentrated and triturated with
a little ether to give the title compound, as a white solid, m.p.
259-261C. Found: C,72.56; H,5.45; N,18.13. C23H17N50 requires
C,72.80; H,4.52; N,18.46%.
The following compounds shown in the Table were prepared by a
similar method substituting the corresponding amine for
3-aminoquinoline.
c~3
NH-CO ~IN ~\'
SUBSTITUTE SHEET
,
~ .
- `~.WO 91/17162 PCI-/EP91/00737
2078i~o7
57
_,
¦Example R16 m.p.Analy8is ~
No C(Theoretical in brackets'
C H
I
86 1 ~ l221_223 1 68.86 4.58 21.01
(69.28 4.59 21.27)
7 1 ~ ,i~ 9 1 74.99 5.57 15.40 I
~74.98 5.47 15.21) .
88 ~ 218-220 75.02 5.36 15.12
(74.98 5.47 15.21)
89 5C202C ~ 193-195 68.92 5.07 13.80
(68.98 5.03 13.99)
... ._
~ C2CH3 ~220-222 ¦68.35 4.72 14.40 ¦
(68.38 4.70 14.50)
. _. _ I
91 ¦ ¦309-31~ 1 63.47 4.04 17.80 !
' '(63.45 4.14 17.62)
S tO.67 mole 820)
_
92 1 269-271 6l.63 4.3, ~2.83
(67.64 4.46 ~.54)
H (0.25 mole H20)
,
SUBSTITUTE SHEET
.
.
. ; ,, ` . ..
- .
' ~ . ~ .. . .
W O 91/17162 2 0 7 8 0 0 7 PCT/EP91/00737,-J
. ~
j. , .
.~
... . _ .
~xample R16 I m.p. ~ Analysls
No ~ (Theoretical in bracke~s)
. I
I C H ~:
93 / l280-~83 ~ 60,86 4.33 21.96
h I (66.33 4.54 ~.27)
H , (0.5 mole H?0)
94 ~ C02CY.3 19 -196 characterised bv h~lR (1)
~ 213-215 ~ 76.02 5.36 13.07
C~- I (76.39 5.38 13.20)
I ~ ! ~ 33 mole H20)
t
96 1 ~ Cl 1 94-q/ 66.55 4.88 14.88
I ~ CH~- ' (66.93 4.55 14.87)
-
97 ' 153-15566.21 4.58 1~38
C0 (66.30 4.77 18.41~ -
(0.5 mole H20)
(1) ~ (300MHz,CUC13) 2.64(3H,s), 4.11(3r.,s), 7.20(1H,d~J=5Hz),
7.48(1H,e,J~7Hz), 7.60(2H,d,J=8Hz). .65(1H,t,J=7Hz),
7.92(1H,d,J-8Hz), 7.95(1H,d,i=8Hz), 8.38(2H,d,J=8Hz)~
8.46(1H,d,J=5Hz), 8.79(1H,s), 9.13(1H,s), 9.44(1H,s).
SUBSTITUTE SHEET
~ W O 91/17162 PCT/EP91/00737
2l3780V7 '
59
EXAMPIE 98
4-(2-Methylimida~o[4,5-c]p~rid-1-vl.)benzovlbenzo[c]pYrrollne
Sodium hvdride (60~ oil dispersion, 0.~ ~, 5 rmol? wes added
to a stirred solution of 4-t2-methylimidazo[4,5-c]pvrid-l-Yl)-
benzamide (0.5 g, 2 mmol) in tetrahvdrofuran (5 mlj and
dimethylformamide (5 ml). After warr,ing a~ aO~C for 20 minu~es,
1,2-bis-bromomethylbenzene (0.53 g, 2 mmol) was added. After ~
hours at 25C, the solvents were evaporated and the residue was
par;L;iu~ be~ween aqueous sodlu~ b ca-~ona~e and ethyl ace~ate.
The organic layer was dried (~gS04) and evaporated to a residue
which was purified by flash chromatography, eluting with 5,~
diethylamioe in ethyl acetate, to afford a white solid (0.038 g,
7%), m.p. 226-229C. Found: C,72.79; H,5.14; N.15.12.
C22H18N4O.~H2O requires C,72.71; H,5.27; N,15.41%.
EXAMP~E 99
N,N-~is(~-Chlorobenzyl)-4-(2-meth~limidazo~4,5-c]pyrid-1-~1)-
benzamide
Sodium hydride (60% dispersion in ~ineral oil), (44 mg,
1.1 mmol) was added to a stirred solution of ~-(2-methylimida2O-
[4,5-c]-pyrid-1-yl)-benzamide (252 mg, 1 mmol), in anhvdrous
tetrahydrofuran (1 ml) and anhydrous dimethyl~ormamide (1 ml).
The solution was stirred at 50C for an additional 20 minutes
before addlng a solution of ~-chloroben~ylchloride (1l, ~g,
1.1 mmol) in anhvdrous tetrah~drofu a-. 1 rl~. The reactio..
mixture was stirred ae ambient te~pera~ure for 16 hours~ then
diluted with water, 2~ hydrochloric ac~c added to pH 1, followec
by sodium bicarbonate to give pR 8 and ~he product was extractec
SUBSTITUTE SHEET
.
. .
W O 91/t7162 PCT/EP91/00737.~, 20780jO7
;; i~ .
with ethvl acetate. The organic extract was washed wlth water,
dried over magnesium sulphate and concentrated under reduced
pressure. The residual gum was chromatographed on silica elutin
with 15% methanol in ethyl acetate. The faster running (less
polar) of the two ensuing fractions was concentrated and the
residual gum crYstallised with ether in an ultrasonic bath to give
the title compound as a white solid (8 mg, 2%), m.p. 176-180C.
Found C~66-36; H,4-42; N,ll.10. C28H22C12N 0. 0 33 H 0
recuire~ ~ ~s ~a; U,4./.G; ~ ,9~,o
EXAMPLE 100
Methyl-2-[4-(2-methylimidazo~4,5-c]pyrid-1-yl)benzylthio]benzoate
4-(2-Methylimidazol4,5-c3pyrid-1-yl)benzyl alcohol (7.78
mmol, 1.86 g), methyl thiosalicylate (8.56 ~mol, 1.44 g~ and
triphenylphosphine (18.56 mmol, 2.24 g) were dissolved in dry
tetrahydrofuran (30 ml). Diethylazodicarboxylate (9.34 mmol, 1.95
g) was added dropwise and the mixture stirred for 2 hours at roo~
temperature then evaporated to dryness. The residue was purified
by column chromatography on silica eluting with tichloro~ethane/
methanol (97:3). l`he oily product was dissolved in
dichloromethane and treated with anhvdrous HCl. The hydrochloride
crystallised on standing (1.66 g, 507,) M.p. 249-253C. Found:
C,60.1?: H,4.67; N,9.57. C22HIa~3o~s~ HC1:0.75 H~0 requires
C,60.14; H~4.90; ~9.57Z.
SU8STITUTE SHEET
'~ W O 91/17162 PCT/EP91/00737
20780~
EXAMPLE 101
Methyl 4-chloro-2-~4-(2-methvlimidazo~4,5-clpyrid-l-yl)ben
thiol benzoate
4-(2-~ethylimidazo[4,5-c]pyrid-l-yl)benzyl alcohol (7.4 mmol,
1.76 g) was dissolved in 48% hydrobromic acid (25 ml) and heated
under reflux for 15 minutes then evaporated to dryness under
vacuum. The residue was dissolved in methanol (100 ml) and methyl
-chloro-thiosalic~late (10 mmol, 2.03 g) added. Sodiu~ hydrogen
carbonate (~ mmnl, 2.10 g~ ~-as adu~d por~ionwlse and the mixture
stirred overnight. The resulting suspension was poured into ethyl
acetate and washed with brine, dried over Na2SO4, filtered and
evaporated to dryness. The residue was chromatographed on silica
eluting with methanol, dichloromethane (97:3) to give the pure
protuct. l'his was redissolved in dichloromethane and treated with
ethanolic hydrogen chloride. The solution was evaporated to
dryness and the residue recrystallised from ethyl
acetate/methanol to give the hydrochloride salt (3.69 g, 95X),
m.p. 233-255C. Found: C,56.82; H,4.39; N,8.87. C22H18ClN302S.
HClØ25 H20 requires C,56.84; H,4.20; N,9.04%.
EXAMPLE 102
1-~4-(2-Chloro~hen~lthiometh~l)ohen,~1]-7-methyl-imidazo~4,j-c]-
p,~ridine
The above compound ~as made follo~ing the procedure o.
Example 101 using 2-chloro-~enzenethiol as starting ma~eria_.
~!.p. 278-280C. Found: C,58.75; H,4.31; N,10.38.
C20H16ClN3S.HCl. 0.25 H20 requires ~,59.04; H,4.31; N,10.33',.
SUBSTITUTE SHEET
, ' . ~ ' `
.. . .
. . . .
, . . . ; . .
.;, . - .. ,
., . ,, - ; . ,~,
..
2078~
W O 91/17162 PCT/~P91/00737,~`?
62
EXAMPLE 103
2-[4-(2-Methvlimidazo[4,5-c]pyrid-]-vl)benzvlthiolbenzoic acid
Methvl 2-~4-(2-methylimidazo~4,5-c!p~rid-1-yl)benzvlthiolbe~.zoate
(7.5 mmol, 1 g) was dissolved in ethanol (20 ml) and 2N sodiu~
hydroxide (1~ ml) was added. The mixture was stirred overnight
then poured onto water and washed with dicnlorometnane. ~he
aqueous phase was acidified with acetic acid and re-e~tracted with
dichloromethane (3 ~ 75 ml). I`he CG~3i ned e.~cracts ~ere dried
over NA ~n~, fi~e-eA a-nd e-vavu--d,~d ~o cr~rness. lhe resultin~
white solid was washed with ether. (0.~1 ~, 86%). ~I.p. 251-254~. -
Found C 65-95; ~,4.68; N,10.71. C21H17N302S 0 5 ~2 q
C,65.63; H,4.69; N,10.94Z.
EXAMPEE 104
4-Chloro-2-~4-(2-methylimitazol4,5-c]pvrid-1-yl)benzvlthio]benzoic
acid
The above E~ample was followed using the co~pound of Example
101 to give the title product in 78% yield. M.p. 264-267C.
Found: C,61.31; H,3.95; ~,10.11. C21P.16N3C102S requires C,61.54;
H,3.91; N,10.26%.
EX~IPLE 105
N-Methvl-2-~4-(2-meth~limidazo~4,5-c`~rid~ l)ben-vlthiol-
benzamide
2-~4-(2-Meth~limidazo~4,5-c]7~rià-:-~l)be~~~7:hio]benzoic
acid (1 mmol, 375 mg) was stirred e d chlûrome~hane (10 ml) anc
drop of dimethylformamide was added. C~:al~l chloride (2 m~.ol, 25-
mg) was added and the mixture stirred ^or 90 minutes under
SUBSTITUTE SHEET
,- W O 91/17162 2 D 7 8 Q O 7 PCT/EP91/00737
nitrogen. l'he solvent was removed under vacuum and the residue
dissolved in dichloromeehane (10 ml) and added dropwise to an lce
cold solution of meth~lamine (33,' sclution in ethanol 10 ~ . lhe
mixture was stirred at 0C for 30 minutes then poured into water
and extracted with dichloromethane (3 x 50 ml). Tne combined
extracts were d.ied over NaAS0 , filtered and evaporated to
dryness. Purification was effected b~ column ch omatog.ap, on
silica eluting with dichloromethane,iQ-rhanol (97:3). The product
cont~ining fr ^ti^r.s wer2 2V2Vu~ d ~0 cr~ness and cr~sta'lised
on trituration with ether. (0.28 g, 73%). M.p. 172-174C. Found:
C,67.26; H,5.25; N,13.92. C22H20N40S. 0.25 H20 requires C,67.26;
H,5.22; N,14.27%.
SU~STITUTE SHEET
. - ,
,
'
' . '` ,; "~ '
. ~ ..
W O 91/17162.~ 2 ~ 7 8 0 a 7 PCT/EP91/00737
64
EXAMPLES 106-107
lhe following compounds were prepared as described in the
previous Example using the appropriate acid and reactin~ with
dimethylamine.
; R18 CH3
~SC~
R17 ~ ~
Example R17 R18 m.p. Analysis %
No C (Theoretical in brackets~
106 H -CON(CH3)2 178- 1 68.17 5.53 13.60
180 1(67.90 5.54 13.78
¦ ,(0.25 mole H20)
. .~
107 ¦ Cl ~-CON(CH3)2 ll36- ' 58.38 5.08 10.82
139 (58.03 5.03 10.83)
` (HCl, 0.5 mole C4H80~)
.
SUBSTITUTE SHEET
,~ .... ~ . .
wo 9"".62 2 0 7 8 0 ~ 7 PCT/EP91/00737
: 65
EXAMPLE 108
Methvl 2-~v-(2-methylimidazo[4,5-c]pyrid-l-yl)benzxlthlolbenzoate
S-oxide
Methyl 2-[4-(2-methylimidazol4,5-c]pyrid-l-yl)benzylchio]-
benzoate (1 mmol, 389 mg) was dissolved in dichloromethane (15 ml)
and cooled in an ice bath, m-chloroperbenzoic acid (85~ 1 mmol,
203 mg) was added and the solution stirred for 2 hours then poured
into dilute sodium hydrogen carbonate solution and extracted with
~; rhloromerh.a"c ~3 A 50 ml) . The combined organic extracts were
dried over Na2S04, filtered and evaporated to dryness, the residue
being purified by column chromatography on silica eluting with
dichloromethane/methanol (97:3). The product-containing fractions
were evaporated to dryness and the residue re-crystallised from
dichloromethane. (0.33 g, 81X). M.p. 146-148C. Found: C,64.79;
H~4-74; N,10.24. C22H19N303S requires C,65.19; H,4.69; N,10.37X.
SUBSTITUTE SHEET
. `
.
WO 91~17162 2 G 7 8 0 0 7 PCr/EP91/00737 ~
- 66
EXAMPLES 109-112
Oxidation o~ the appropriate thio compound of Examples
102-107 with met2-chloroperbenznic acid as descrived in ~he
previous example gave the foll~ wing sulphoxides:
~1~ c~,-3
~ SCH.,
R-7
Example R17 R18 I m p Analysis %
llo ¦ ~ ¦ (Thcore~ical n brackets
109 U -CONHCH3 1 205- 64.46 5.11 13.46
. j 207 (64.39 5.04 13.66)
' (O.33 =~le U 0)
110 I H -CONtcH3)2 ! 183- 66.01 5.58 13.2_
~ 185 (66.03 5.26 13.40)`
. ._ .
111 H Cl ` 120- 62.67 4.18 10.86
i 1^' (6^.9' 4.19 11.01!
--- . I
-C0N(CH3~A I l'C- 60.9~ ~.66 1'.^3
I 1~, (50.99 ~.6~i 1^.38)
.
~ WO gl/l7l62 2 0 7 8 o ~ 7 PCT/EP9l/00737
67
EXAMPL~ I 13
N-Methyl-2-~4-(2-~ethvl.i~idazo-~4,5-c~p~rid-1-Ylbenzt~lthiolbenza-
mide-S,S-dioxide
N-Methyl-2-~4-(2-methvlimidazo~4,5-c~pyrid-1-yl)benzylthio]-
benzamide-S-oxide (0.25 mmol, 101 mg) was dissolved in
dichloromethane (10 ml~ an~ cooled in ar. ice bath. ~oncentrated
hydrochloric acid (; drops) waa adaed followed b~-
m-chloroperbenzoic acid (85%, 0.25 mmoi, 5i mg) and the mi.~:ture
s....eu d~ VC ror ^~ nours ~Hen at room ~em?erarure ~cr ? hcurs.
The mixture was basified with S~O scdium carbonate sclution and
extracted with dichloromethane (3 x 50 ml). The combined extracts
were dried over Na2S04, filtered and evaporated to dryness with
the residue being purified by column chromatography on silica
eluting with dichloromethane/~ethanol (96:4). Evaporation of the
product-containing fractions gave an oil which crYstallised on
trituration with ether, (14 mg, 13X). M.p. 241-243C. Found:
C,61.91; H,4.75; N.12.79. C2?H20N403S. 0.33 H?~ requires C,61.97;
H,4.85; N,13.14%.
EXAMPLE l14
N,N-Dimethyl-4-chloro- -~4-(2-metht~limidazo~4,5-c]p~rid~
benzvlthio]ben _mide-S,S-dioxide
1he above procedure was rollowed s-arting wit~. the
corresponding ~-chloro-derivative to git.e the title product. ~I.p.
193-194C; Found: C.57.81; ~i,4.4&; ~ '0. C?~U.~.u~ S. 0.; H~O
requires C,57.80; H,4.61; ~,11.~3..
SUBST~TUTE SHEET
.
:.'.
2078~07
W O 91/17162 PCT/EP91/00737 -
68
EXAMPLE l15
l-~t~-Dimethylaminocarbonyl)phenylsulphinyl~ 4-(~-methYl-
imidazo[4,5-c]pyrid-1-yl?phenvl]methanol
N,N-Dimethyl-2-[4-(2-methylimidazo[4;5-c]pyrid-1-yl)benz,l-
thio]benzamide-S-oxide (0.3 mmol, 125 mg~ was dissolved in dry
dichloromethane (110 ml) and anhydrous hydrogen chloride added.
m-Chloroperbenzoic acid (85X, 0.3 mmol, 61 mg~ was added and the
mixture s~irred at room temperature for 2 hours then poured into
," suuiu~ carbona~e solutlon and extracted with dichloromethare (3
x 50 ml). The combined organic extracts were dried over Na~S04,
filtered and evaporated to dryness. The residue was purified by
high pressure liquid chromatography and the product crystallised
from diethyl ether (17 mg, 13X). M.p. 174-176C. Found:
C,60.90; H,4.70; N,12.14. C23H22~4O3S. H20 requires C,61.06;
H,5.30; N,12.39~.
EXAMPLE 116
Dimethyl 2-(4,5-dichloro-2-nitrophenyl)-1-~4-(2-methylimidazo~4,5-
! c]p~rid-l-yl)phenyl]et lonate
a) Following the method of W. Lehrest, Tetrahedron 1973, 29,
! 635, titanium tetrachloride (4.40 ml, 40 mmol) was added at 5-lO
to dry tetrahytrofuran (80 ml) under nitrogen. Dimethvl malonate
(2.6~ g, 20 mmol) was added by syringe, followed b a solution c-
4-(2-methylimidazo[4,5-c]pyrid-1-yl)ben-aldehyde ~4.7~ g, 20 mmol)
SUBSTITUTE SHEET
`- ~. Wo 91/17t62 PCl/EP91/00737
2o78o~7
in dry tetrahydrofuran (80 ml). Finally dry pyridine (4.85 ml, 60
mmol) ~as added dropwise and the resulting sugpension was stlrred
at roo~ temperature for 24 hours. Methanol (30 ~1) was added, anc
the resulting white suspension was poured into a mixture of
dichloromethane (100 ml), ice, and saturated aqueous sodium
bicarbonate (500 ml). The titaniu~ salts were removed b~
filtration, and washed with dichloromethane. The filtrate layers
were separated, and the aqueous laver extracted with
dichLu~u~etnane t2 x lûû mij. ~l~ne combined organic extracts were
dried (MgSû4) and concentrated to give a yellow oil. On the
addition of diethyl ether (50 ml), the product crystallised, and
was filtered off and dried, to afford dimethyl 4'(2-methylimidazo-
~4,5-c]pyrid-1-yl)benzylidene malonate, as a white solid, 5.1 g
73X). A portion was recrystallised from hot ethyl acetate. M.p.
155-156C. Found: C,64.79; H,4.95; N,11.87. ClgH17N3O requires
C,64.95 N,/~.85 N,11.96X.
SUBSTITUTE SHEET
.. ~, . .
wo 91/17162 2 0 7 8 ~ ~ 7 PCT/EP91/00737,i-,
,
(b) A solueion of 4,5-dichloro-2-nitrotoluene (2.472 g, 12.~
mmol) in dry dimethylfor~amide (5 ml) was added over 2 mlnutes by
s~rin~e to a suspension of sodium hydride t600 mg, 60/~ dispersion
in oil, 15.0 mmol) and dimethyl 4'-(2-mèthylimidazo[4,5-
c]pyrid-l-yl)benzylidene malona~e (from part a) in dry
dimethylformamide (40 ml) whilst maint2ining the te~perature below
15C. The resulting bro~n solution ~as s;irrod at 20C for 3
hours, and glacial a.ecic ~cid (2 ml) was added. The mixture was
pOULCu - Lli~ ~n~i aceraee ~4~Ju ml) and the solution rendered basic
by the addition of saturaeed a~ueous sodium bicarbonate. The
organic layer was washed with water (3 x 100 ml), brine (100 ml),
dried (MgS04) and concentrated under reduced pressure. The crude
product was purified by chromatography, eluting with a gradient of
ethyl acetate/methanol, to glve the title compound as a white
solid, (3.119 g, S6~). m.p. 94-96C (methanol). Found: C,53.18;
26H22C12N406. 1-5 H20 requlres C,53.43; H,4 31;
N,9.59X.
EXAMPLE 117
Dimethyl 2-(2-amino-4,5-dichloro~henyl)-1-14-(2-methvlimidazo~4.5-
c]pyrid-l-yl~phen~l]ethvlmalonate
A solution of 25X aqueous eitanium trichloride (4 ml, 6.5
mmol) was added dropwise to a solution of dimethyl
2-t4,5-dichloro-2-nitro-phenyl)-1-[4-t~-meth~limidazo~,5-c]pyrid-
l-yl)phenyl]eeh~lmalona~e (557 mg, l.C cmo') in de~assed me~hanol
(15 ml) under nitrooen at room tempera;ur-. The solu~ion was
stirred for 1 hour, poured into dichloro~ethane (50 ml! and
rendered basic by the atdition of saturated aqueous sodium
SUBSTITUTE SHEET
2Q780~7
; ~ W O 91/17162 PCT/EP91/00737
bicarbonate. The precipi~ated salts were filtered off and washed
with dichloromethane (150 ml). The filtrate was separated, dried
(MgS0 ) and concentrated tlrder reduced pressure to tne ~ e tne
title compound as a whi~e solid, (45~ mg, 86%), m.p. 186-188C
(methanol~. H ~R (300 MHz, CD~13) : ~.52(3H,s~, ~.59(1H,d,J
12Hz), 3.15(1H,dd,J 1~ and 2H7), '7.55!3H,s), 3.70(1~.,dc, J 1~ and
2Hz), 3.91(3H,s), 3.95(7~.,d,J 1~ (2H,br s), 6.'5(1~,s),
6.78(1H,s), 7.03(1H,d, J 6'.~7), ,.29 (~H,s), 8.39(1H,d,J 6Hz),
.û/ (;n,s) -
EXAMPLE 11~
3-t2-ChloroPhenyl)-2-ethoxycarbonyl-1-~4-(2-methylimidazo~4,5-c]-
' pyrid-l-vl)phenyl]prop-2-ene-1-one
` A mixture of 2-chlorobeozaldehyde (2.8 g, 19.9 mmol)~ethyl
4'-(2-methylimidazo~4,5-c~pyrid-1-yl~benzoyl acetate (6.4 g, 19~9
mmol) and piperidine (100 microlitres) were stirred at room
temperature for 48 hours in acetonitrile (30 m7). The mixture was
evaporated eo dryness and the residue chromatographed on silica
eluting wi~h ethyl-acetate/meehanol (5:1). The fractions
containing product were combined and evaporated to give the title
compound (5.3 g, 60~). lH N~R (CDC13) 1.35(3H, t,J 8Hz),
2.53(3H, s), 4.27(2H, q,J 8H2), 7-9.1~1~H, m).
UBsTlTuTE SltEET
.: . .
.
` 2Q78~U ~
W O 91/17162 PCT/EP91/00737 -
EXAMPLES ll9-120
The following compounds were made by the method o~
E~ample 118 using the appropriate aro~atic aldehyde as s~artin~
~aterial.
C2CH2CH~ c~-3
R -CH=C-C ~ ~
~/>
Exa~ple R20 N.M.R data
No (CDC13)
._ _ . . .
119 < 01.38(3H,t,J=8); 2.6(3H,s);
0 ~4.32(2H,q,J=8); 6.02(2H,s);
6.7-9.1(llH,~)
120 ¦ I1.31(3H,t,J-8); 2.58(3H,s~;
.28(2H,q,J~8);
9.1(13H,~.).
__ . .
SUBSTITUTE SHEET
-` ` W O 91~17162 2 0 7 8 0 0 7 PCT/EPgl/00737
EXAMPLE 121
Ethyl 2-(2-chlorophenylmethyl)-3-hvdroxv-3-~4-(2-methvlimidazo-
~4,rl-c~vrid-l-yl?phenyl]propanoate
Sodium borohydride (0.012 g) was added in a single portion to
a solution of 3-(2-chlorophenyl~-2-ethox~carbonyl-1-~4-(2-meth~
imidazo~4,5-c]pyrid-1-yl)phenyl]prop-2-ene-1-one (0.13 g, 0.3
mmol) in ethanol (2 ml) at 20C. After 1 hour~ the mixture was
partitioned between ethyl acetate and brine. The organic layer
w~ dried ~-~g~u4j and evaporated ~o a gum. Purification bv
chromatography on silica afforded a colourless gum (0.12 g, 91%!.
Found: C,61.79; H,5.41; N,8.93. C25H24ClN3O3.2 H2O requires
C,61.79; H,5.81; N,8.65%.
EXA~PLE 122
2-Chlorobenzyl 2-(2-chlorophenylmethylidene)-3-~4-(2-methyl-
i
imidazo[4,5-c]pyrid-1-yl)phenyl]-3-oxopropanoate
a) 2-Chlorobenzyl 4'-(2-methylimidazo~4,5-c]pyrid-1-vl~-benzo~l-
acetate
A solution of ethyl 4'-(2-methylimidazo[4,5-c]pyrid-1-yl)-
benzoylacetate (1 g, 3 m~ol) and 2-chlorobenzyl alcohol (1.4 g, 10
mmol) in toluene (15 ml) was heated at reflux for 20 hours, then
cooled and evaporated. l`he residue was purified b~ chromatography
to artord a colourless roam (1.23g. C57;),
Found: C,64.15; H,4.38; ~,9.55. C~3HI~C1~30~. 0.5 H~0 requires:
C,64.~1; H,4.47; ~,9.80,o.
b) Piperidine (0.02 ml~ was added ~o a stirred solution Ot the
product from a) (0.7 g, 1.67 mmol) ar.d 2-chlorobenzaldehyde (0.28
g, 2 mmol) in acetonitrile (8 ml) a~ 2C. The mixture was allowed
SUBSTITUTE SHEET
2~78007
W O 9I/17162 YCT/EP9t/00737
to stand for 6~ hours. then evaporated and purified by
chromatography to give the title compound ag a colourless foam
(Q.39 g, 43"), ~.5. ~-51~. Found: C,65.5~; H,4.10; ~,7.40,
C30H21Cl~N303. 0.5 H~0 requires C,65.35; H,4.02; ~',7.62%.
E~AMPLE I23
3-(2-Chloroohenvl)-l-r.~ 2-~ethyli idazo[~',5-c]pyrid-1-Yl)-
.
phenvl]-l-o~c-2-pro~ene
Soliu suuiu~ nydrc~ide (O.u4 ~, 1 m~ol) was added to a
stirred solution of 2-chlorobenzaldehvde (û.194 g, 1.~ ~mol) and
4~-(2-methyli~idazo[4,5-c]pyrid-1-yl~acetophenone (0.35 g, 1.4
mmol) in methanol at 25C. Water (2-3 drops) was added to aid
dissolution. After 2 hours, the pH was adjusted to 8 by addition
of 2M hydrochloric acid followed by sodium bicarbonate solution,
then the mixture was extracted with ethyl acetate. The or~anic
layer was dried over MgS04 and evaporated to a gum. Flash
chromatography, eluting with lû% methanol in ethvl acetate,
afforded a solid which was recrystallised rrom methanol to give
the title compound (û.û8 g, 16%), m.p. 155-157C. Found:
C,70.67; H,4.22; ~,11.12. C22H16ClN30 requires: C,70.68; H,4.31;
~,11.24Z.
EXAMPL~ I2-
E and Z-l-Phen~ 4'-(~-meth~limidazo~4,~-clpvrid~ l)Phen~
ethene
~ =:
Sotium methoxide (û.ll g, 2 mmo;` was added to a stirrec
solution of 4'-(2-meth)~limidaæo~4,~-cj?yrid-1-vl~benzaldehyde
(0.24 g, 1 mmol) and benzyltriphenylphosphonium chloride (0.43 ~,
SUBSTITUTE SHEET
~ W O 91/17162 2 ~ 7 ~ O 0 7 PCT/EPg1/00737
1.1 mmol) in anhydrous dimethylformamide (5 ml). After 18 hours
at 23C the solvent was evaporated and the residue was particioned
between ethvl acetate and water. The or~anic extract was washed
three times with wa~er, dried (~SgS04) and evaporated to a gum.
Flash chromztography elutin~ with 5,~ methanol in ethyl acetate,
afforded an amorphous solid (0.13 g, 44%). (Ratio of E/Z isomers
was approximately 9:1)
lH N~R (CDC13): 2.57(3J.,s) 6.58 and 6.81 (each lH,d J=14Hz),
7.13(1H.d J=4.5H7~; ?.23(2U,d J-~H,c~, 7.34~n,brsi, 7.4Y~
J=8~z), 8.42(1H,d J=4.5Hz) and 9.07(1H,s).
EXAMPLE 1 25
1-~4-(2-Methylimidazo~4,5-c]pyrid-1-yl)phenyl]-2-pyrid-2-yl
ethanol
Sodium methoxide (0.33 g, 6 mmol) was added to a stirred
solution of 2-(trimethylsilylmethyl)pyritine (0.55 g, 3.3 mmol)
and 4'-(2-methylimidazo[4,5-clpyrid-1-yl-benzaldehyde (0.71 g, 3
mmol) in dry dimethylfor~amide (10 ml) under nitrogen. After 30
minutes, most of the solvent was removed and the residue
partitioned between eth~l acetate and water. The organic layer
was washed several times with water then dried (MgS04) and
evaporated to an oil. Purification by flash chromatograph~,
eluting with 20X methanol in ethyl acetate, followed b~
~rituration with ether, gave a white solid (0.1 ~ ). M.p.
160-16`'~C. Found: C,72.23; H,5.46; ~,16.6~ h'l~`.;,0. 0.1~5 H~0
requires C,7~.~1; H,5.53; ~,18.8~.
SUBSTlTlJTE SHEET
W 0 91/17162 2 ~ 7 8 0 0 7 PCT/EP91/00737 ~
; ' ;,
76
EXAMPLE I~6
E and Z-1-(2-~yanophenyl?-2-[4'-(2-methylimidazo~4,;-c~pyrid-1-
vl)-~henyl]ethane
Sodium methoxide (4.32 g, 80 mmol) was added to a stirred
suspension of 2-chlorobenzyltriphenylphosphoniu~ bromide (20.~ g,
44 m~ol) and 4'-(2-methylimidazo[4,5-c]pyrid-l-yl)benzaldehYde
(9.5 g, 40 mmol) in dimethylformamide (210 ml). The mixture was
heated to 100C for 3 hours then evaporated and the residue ~-2s
partitioned between ethvl 2e~tal e and water. Tll~ organic layer
was dried (MgS04) and evaporated co a gum (20 g) which was
:. .
chromatographed on silica eluting with a gradient of from 5% to
25% methanol in ethyl acetate, to afford two products:
a) E-isomer, colourless foam (0.5 g, 4%).
H NMR (CD3SOCD3): 2.46(3H,s), 6.91 and 7.05 (each lH,d J=15Hz),
7.15(1H,d J-5Hz) 7.33(2H,d J=7.5Hz), 7.49(4H,m), 7.66(1H,t
J=7.5Hz), 7.90 (lH,t J=7.5Hz), 8.28(1H,d J=5Hz) and 8.89tlH,s).
b) Z-isomer, recrystallised from toluene (7 g, 522). M.p.
213-215C.
H NMR (CD3SOCD3): 2.51(3H,s), 7.25(1~,d J=5Hz), 7.50(2H,m),
7.67(3H,~), 7.75(1H,t J=7.5Hz), 7.91 (3H,m), 8.07(1H,d J=7.5Hz),
8.30(1H,d J~5Hz) and 8.92 (lH,s).
EXAMP~E 127
1-~4-(7-Methvlimidazo~4,5-c]pYrid-l-Yl)~henYl]-l-oxo-
?,~,3,3,4,4,4-heptafluoro~utane
To a solution of heptafluoropropyliodide (60 mmol, 1 .76 ~!
in ether (200 ml) was added phenylmagnesium bromide (55 mmol 18.33
ml) (3M in ether) whilst keeping the internal temperature below
-60C. The m~xture was then stirred at -70C for 15 minutes
Sl)!?~STITUTE StlEET
.: . . .. ' . . . .. . : .
wo gl/17162 2 ~ 7 8 0 o 7 pcr/Ep9l/oo737
. ~ , ,
followed by the dropwise a'ddition of ethyl ~4-(2-methyllmldazo-
~4,5-c]pyrid-1-yl)benzoate (25 mmol, 7.05 g) in tetrahydrofuran
(40 ~1) again keeping the temperature below -60C. l'he ~ilxture
was allowed to warm to 0C very slowly over a 5 hour period then
quenched with ammonium chloride solution. After pouring the
mixture into water the product was extracted into ethyl acetate (~
x 300 ml) and the combined organic e~tracts dried over MgSO,,
filtered and evaporated to dryness. The residue was purified Dy
column chrnm3tQ~ arh~, ^r si' .a elu~ing with dlchloromethane/
methanol (95:5) then recrystallised from ethyl acetate/hexane.
(5.41 g, 53Z). M.p. 138-141C. Found: C,41.10; H,2.74; N,10.01.
HloF7N30 0.5 H20 requires C,49.29; H,2.68; N,10.14~.
EXAMPLE 128
1-[4-(2-MetbYlimidazo~4,5-c]pyrid-1-yl)phenyl]-2,2,3,3,4,4,4-
he~tafluoro-1-~4-fluorophenyl]butanol
1-14-(2-Methylimidazo[4,5-c]pyrid-1-yl)phenyl~ oxo-
2,2,3,3,4,4,4-heptafluorobutane (1 mmol, 405 mg) was dissolved in
tetrahydrofuran (10 ml) and cooled to -40C. 4-Fluorophenyl-
~agnesium bromide (5 mmol, 5 ~1, lM in teerahydrofuran~ was added
dropwise and the solution allowed to warm to room temperature over
1 hour and then stirred for a further ~ hours at room te~perature.
The reaction was quenched with ammonium chloride solution and
poured into water tlOO ml) then extracted with ethylacetate (3
50 ~1). l'he combined organic extracts were dried over MgSCI ,
filtered and evaporated to dryness. The residue was purified b~
chromatography on silica eluting with ethyl acetate/dietbylamine
SUBSTITUTE SHEET
. . . . . .. ... ~ ... . . . ... .. .
W O 91/~7162 2 0 7 8 0 O ~ PCT/EP9t/00737 ~
78
(98:2), the product containing fractions were evaporated to
dryness and the residue recrystallised fram ether (134 m~, ~;70),
M.p. 192-194C. Found C,54.77; H,3.31; `;,~.37 C~3,~1 r~-;
requires C,55.09; H,2.99; N,8.38~.
EXAMPLE l29
4-~4-(2-Methylimidazo~ -c]~vrid~ .l?~henvll-tetrado~a-~uc-o-
heptan-4-ol
The above w~s pre?o-od a..al~Oo-uslv Lo [he ?re~;lous ~et'.:Gd
using heptafluoropropyl magnesium bromide as the Grignard rea~ent
and was obtained in 5% yield. M.p. 221-223C. Found: C,41.74;
H~l-91; N~7-30- C20HllF14N30 requireS C,41.44; H,2.11; N,7.30%.
AMPLE 130
4-1- [4-(2-methylimidazo[4,5-c]pyrid-l-yl)phenyl]-l~ 2~2~3~3
heptafluoropentan-2-ol
The above was prepared analogousl to the previous method
using methylmagnesium bromide as the Grignard reagent to give the
produce in 56Z yield. M.p. 233-235C. Found: C,51.15; H,3.31;
N,9.82. C8H14F7~3Q requires C,51.31; h,3.33; N,9.98X-
EXA~LE l31
3-HePtafluoro~ropvl-3-hvdroxv-~-~4-t~-~sth~ ida-o~4.5-cl~rid-l-
yl)phenYl~propionamide
8is(trimethylsilvl)acetamide (5 mm31, i.~ ml) was cissolved
in tetrahydrofuran (15 ~1) and cooled :a -7S~ in a CQ~/ace~one
bath. Butyllithium (2.2M, in hexane, ; Fmol, 2.27 ml) was addec
dropwise over 5 minutes and the solution stirred at -78C for a
SUBSTITUTE StlEET
.
., ~
.: . .
j W O 91/17162 2 0 7 8 0 0 7 PCT/EP91/00737
7~
rurther 15 ~inutes. A solution of 1-~4-(2-methylimidazo[4~5-cl-
p~rid-l-yl)phenylJ-l-oxo-2,2,3,3,4,4,4-heptafluorobutane (1 m~ol
40O mg) fresh]~ dried by evaporation of a toluene solution w2.c
dissolved in tetrahydrofuran (15 ml) and added dropwise to the
anion solution over 20 ~inutes. lhe mixture was stirred at -7~CC
for 1'~ hours then the cooling bath removed and the mixture
~uenched with ammonium chloride solution. The reaction ~ix~urs
was poured in water (lnO ml) and extracted with ethyl acstate (3 A
50 ml). The ~mhined o ga.. c eAtrac;~ were dried over MgS~' ,
filtered and evaporated to dryness. Chromatography on silica
followed by recrystallisation from ethyl acetate/ether gave the
title product (145 mg, 31%), m.p. 233-239C dec. Found: C,48.93;
ClgH15F7~4o2 requires C,49.14; H,3.23; N,12.07%
EXAMPLE 132
l-Heptafluoropropy~ 4-(2-m~hy~dazor4~5-c]pyrid~ l)phen
2-~1?2,4-triazol-1-yllethanol
a) l-Heptafluoropropyl-1-~4-(2-methyli~idazo~4,5-c]pyrid-1-yl)-
ph ~ oxirane
1-14-(2-Methylimidazo~4,5-c]pyrid-1-)~l)phenyl]-1-oxo-
2,2,3,3,4,4,4-heptafluorobutane (6 ~mol. 2.43 g) was dissolved in
teerahydrofuran (30 ml) and cooled in an ice bath.
~imethylsulphoxonium methylide (0.~ `' in tetrahydroruran, lC! m~ol,
16.7 ml) was added dropwise and the solution stirred at ~C for ~G
minute~. lhe reaction mixture was then poured into brine ar.^
ex~rac~ed with ethvl acetate (3 x 5G ml). The combined organic
extracts were dried over MgS04, filtered and evaporated to
SUBSTITUTE SHEET
: ` :
W O 91/17162 2 0 7 8 0 0 7 PCT/EP91/00737 ~,
dryness. The residue was purifled by column chromatograph-~ on
silica eluting with dichloromethane/methanol (97:3) to glve the
oxirane a~ an unstable oil which was u~e~ immPdiatel.~.
b) l-Heptafluoropropyl-1-[4-t2-methylimidazo[4,5-c]pyrid-]-yl)
phenylo~irane (0.6 m~ol, ~0 mg) and sodium triazole (1 mmol, 93
mg) were stirred in dimeth~lformamide (5 ml) for 40 minu~es at
room temperature. The reac~ion mixturC was ?cu.~d in~o brine anG
extracted with ethyl aceta~e (3 ~ 50 ml). The comDined e~erac~s
were dri~d oVcr Mgc~ , f'l;e-Leu ar.r eva~orated to dr~ness. The
residue was purified by column chromatograph~ eluting with
dichloromethaneJmethanol (94:6). The product containing fractions
were evaporated to dryness and triturated with ether to give the
title product (80 mg, 27X), ~.p. 211-213C. Found: C,48.39;
C20al5F7N6' 0.1 (C2H5)2O, 0.5 H20 requires
C,48.53; H,3.37; N,16.65%.
EXAMPLE 133
2-C~ano-l-heptafluorooropvl-1-[4-(2-methvlimidazor4,5-c]pvrid-1-
yl)phenyl~ethanol
The above was prepared in a similar manner to the previous
example, but reacting the oxirane with sodium cyanide to give the
tltle product m.p. 290-29lC dec. Found: C,51.12; H,3.05;
N,12.48. ClgHI3F7N40 requires C,51.12; H,2.91; ~,12.5~%.
EYAMPLE 13~
N-~2-t2-Chlorophen~ -hvcro~eth~ ~-meth~ idazor~i~;-c]-
p,vrid~ benzamide
2-(2-Chlorophenyl)-2-hydroxyethylamine (^.5~ mmol, 440 mg) and
triethylamine ~3.15 m~mol, 318 mg) were dissolved in
SUBSTITUTE SHEET
, .. ' ! WO 91~17162 2 0 7 8 0 0 7 PCT/EP91/00737
.
~ 81
;dichloromethane (20 ml) and the solution cooled ln an lce bath A
suspension of 4-(2-methyl-imidazo[4,5-c~pyrid-1-yl)ben~oylchloride
(1.57 m~ol 426 ~g) in dichloromethane (20 ml) was added and the
mixture stirred at 0~ for 15 minutes then at room temperature for
45 minutes. The mixture was poured into lN hydrochlor~c acid an~
the organic phase separated. The aqueous phase was basified wi~:~
2N sodium hydroxide then evtracted with dichloromethane (3 x 100
ml). The co~bined organic extracts were dried over ~aAS04,
filt~red ar.d ev-~ora.ed ;o d-L-y!l~ss. rurirication was effec;ed b~
column chromatography on silica eluting with dichloromethane/
methanol/ ammonia 94:6:0.1 and the product-containing fractions
evaporated to dryness. The resulting oil was dissolved in a
little dichloromethane and the product precipitated with cold
ether, (0.24 g, 37X). M.p. 126-128C. Pound: C,63.52; H,4.61;
N,13.19. C22HlgClN402. 0.5 H20 requires C,63.54; H,4.81; N,13.47%.
EXAMPLE 135
3RS,4SR-7,8-Dichloro-3-methoxycarbonyl-4-14-(2-methvlimidazo[4,5-
c]pyrld-l-yl)phenyl-2~3~4~5-tetrahydro-lH-l-benzazepin-2-one
Sotium metal (846 mg, 36.8 mmol) was dlssolved in dry
methanol (250 ml) under nitro~en. Di~ethyl 2-(2-amino-4,5-
dichlorophenyl)-1-~4-(2-methylimidazo~4,5-c]pyrid-1-yl)phenyl]-
ethyl-~alonate (from Example 117, 16.15 g, 30.16 ~mol) was added
and the mixture was heated at reflux for 4.5 hours, cooled and
poured into 15 ml of 4N hydrochloric acid and ice. The pH was
ad~usted to 7 by the addition of saturated aqueous sodiu~
bicarbonate and the product was extracted into dichloromethane (4
;x 150 ml). The combined extracts were dried (MgS04) and
SUBSTITUTE SHEET
~ ~ . .. ..... ...... .... .. .. .
. ' ~" "'. '.
:
207~37
W O 91~17162 PCT/EP91/00737 ~~
concentrated under reduced pressure to give a white ~ol~d (14.6~
g, 96%). A portion, recrystallised fro~ methanol/dichloromethane
had m.p. 221-223C. lhe stereoche~istr; or ~he produc~ ~a~
assigned fro~ the H3-H4 coupling constant (9 Hz). Found: C,60.29;
25 20 2 43 requires C,60.62; H,4.07;
~,11.317~.
-
EXAM~LE 136
7,8-Dichlorn-6~ o~ r/.c-~llv-yïlu-~-v~ne~ 3
4,5-tetrahydro-lH-l-benzazepin-2-one
A mixture of 7,8-dichloro-3-methoxycarbonyl-4-~4-(2-methyl-
imidazo[4,5-c]pyrid-1-yl)phenyl]-2,3,4,5-tetrahydro-lH-l-
benzazepin-2-one (14.42 g, 29.1 mmol) and lithium iodide (19.36 g,
145.5 mmol) in try pyrldlne (200 ml) was heated at reflux under
nitrogen for 2 hours. The mixture was concentrated under reduced
pressure, and purified by flash chromatography (gradient elution
with ethyl acetate/methanol) to give the title compound (9.52 g,
75%), m.p. 314-316C (after recrystallisation from methanol/
dichloromet~ane). Found: C,62.14; H,4.24; N,12.23. C~3HI~Cl2N40.
0.5 H~0 requires C,61.89; H,4.29; N,12.557~.
EXAMPLE 137
7,8-Dichloro-l-methyl-4-~4-(2-meth~limidazo~4,j-cjD~rid~
phenvl]-2.3,4,5-tetrahvdro-lH-benzazepin-2-one
A mi~ture of 7,8-dichloro-'-t~-(2-met~ylimidazot4,;-cjpyrid-
l-yl)phenyl]-2,3,4,5-tetrahydro-lH-l-benzazepin-2-one (1.093 g,
2.5 mmol) and sodium hydride (150 m~. 60% dispersion in oii, 3.75
mmol), in dry dimethylformamite (10 ml) under nitrogen at room
SUBSTITUTE SHEET
.. ~ , . .
.
W O 91/17162 2 0 7 8 0 ~ ~ PCT/EP91/00737
83
temperature was sonicated for 5 minutes then stirre~ for a rurther
1 hour. ~ethyl iodide (171 microlitres, 2.75 mmol) was added tn
the light brown solution and the mixture was stirred fo~
hours , then poured into excess ice cold dilute hydrochloric acid.
The solution was reDdered basic by the addition of saturated
aqueous sodium bicarbonate and the product was extracted Wit;~
dichloromethane (4 x 125 m]!. The combined extracts were drie~
(MgS04) and concentrated under reduced pressure. The residue ~as
,ur~fiod b; f'ash chLumatu~L~hv eiu~ir.g with ethyi ace.e~e
methanol (9:1) to give a white solid (848 mg, 75%), m.p. 259-261
(after recrystallisation from ethyl acetate). Found: C,63.33;
24 20Cl2N4 0-25 CH3C2C2H5 requires C,63.43;
H,4.68; N,11.84%.
EXAMPLES 138-139
The following compounds were prepared from 7,8-dichloro-4-~4-
(2-methylimidazû~4,5-c]pyrid-l-yl)phenyl~-2,3,4,5-tetrahydro-ln-l-
benzazepin-2-one by the method of Example 137, using 2-picolyl
chloride and 4-chlorobenzyl chloride instead of methyl iodide.
R21
Cl~O
~ C''
SUBSTITUTE SHEET
W O 91/171 2 2 07 8 0 0 7
6 ~ PCT/EP91/00737
84
Example R21 ¦ m.p. I Analysls/. I
! No I O~ ¦(theoretical in brac'~ets~
C H
138 ~ l135-13765.024.39 13.1C
~ CH2- (6~.814.50 13.03)
.' ,
~39 j ~ 208~ 63.974.05 9.86
j 1 2 ~ (64.124.13 9.97)
~ Calculated for hemihydrate
.
EXA _ LE 140
6-~4'-(2-Meth,vlimidazol4,5-c~p~lrid-1-yl)Dhenyl]-5-cyano-2H-1~7-
dih~dro~3,4]benzazePin-2-one
A solution of hexane-washed sodium hydride in dimethyl-
sulphoxide (4 mg in 0.5 ml) was added to a stirred solution of
2-(2-cyano~ethylbenzylamido)-4'-(2-methylimidazo~4,5-c]pyrid-1-
yl)acetophenone (0.05 g, 0.12 mmol) in dimethylsulphoxide (1 ml).
The solution was stirred at ambient temperature for 16 hours, then
partitioned between ethyl acetate and ~rine. ~he organic lave
was washed with brine, dried ~M~S0 ) and the solvent eVaporateG .c
give a yellow solid (0.028 g). Flash chromatography, eluting witri
5 then 15X methanol in ethyl acetate, afforded the title product
as a yellow solid (0.011 g, 23~), m.p. above 320C. lH NMR
SUBSTITUTE SHEET
`
:. ,~ .. . `
:. :
- ,
., . . ,
.. ` ,
wo 91/17162 2 0 7 ~ o ~ 7 PCT/EP9l/00737
~'
(CDC13): 2.62(3H,s), 4.58(2H,s), 7.17(1H,d Jn5.7Hz) 7.45(2H,d
J=8.5Hz), 7.60(2H,m), 7.76(1H, t J=7.5Hz), 8.13(2H,d J=8.5Hz),
: 8.17(1H,s), 8.43(2H,m) and 9.08(1H,s).
,~
; EXAMPLE 141
Ethyl 4'-(2-methylimidazo~4.5-c]~ -l-yl)-2-(2~-nitro~hen
acetYl) -2-benz~e
Hexane-washed sodium hydride (0.053 g, 2.2 mmol) was added to
a sti..e' sulutiuu ~i e~hyi 4T-~2-methylimidazol4~5-c~pyrid-l-yl)
benzoylacetate (0.65 g, 2 mmol) in dry tetrahydrofuran (8 ml).
After 0.5 hours, solid 2-nitrophenacyl bromide was added in
; portions and the resultant brown solution was stirred for 1 hour.
The mixture was partitioned between ethyl acetate and water,
buffering the aqueous phase to pH 7 with lM hydrochloric acid.
The organic layer was dried (MgSO4) and evaporated to dryness.
Flash chromatography eluting with 5% methanol in ethyl acetate
afforded the title product as a foam (0.61 g, 63~). lH NMR
(CDC13): 1.29 (3H,t J=7.5Hz), 2.67(3H,s), 3.68(1H,dd J-16,5Hz~,
3.79(1H,dd J=16,8Hz), 4.28(2H,q, J=7.5Hz), 5.23(1H,dd J=8,5Hz),
7.19(1H,d J25.3Hz), 7.60~2H,d J=8.5Hz), 7.69(2H,m), 7.83~1H, t
J~5.5Hz), 8.18(1H, d J-9Hz), 8.40(2H,d J-8.5Hz), 8.50(1H,d
J~5.4Hz), 9.12(1H,s).
EXAMPLE 142
.
(2-Methvlimidazol4.5-c]pvrid-l-yl~henyl~ -(2~-nitr
phenvl)butane-1,4-dione
A solution of ethyl 4'-(2-meehylimidazo[4,5-c]pyrid-1-yl)-2-
(2'-nitrophenylacetyl)-2-benzoylacetate (0.6 g, 1.23 mmol) in 2M
SU8STITUTE SHEET
.
: ' ' '
20780~7
W O 91/17162 PCT/EP91/0073
86
hydrochloric acid (12 ml) was heated at 100C for 5 hours then
cooled and basified with solid sodium hydrogen carbonate and
partitioned between ethyl acetate and ~ater. The aq~eous la~e~
was re-extracted with ethyl acetate and the combined organic
layers were dried (MgS04) and the solvent evaporated. Flash
chromatography, eluting with 3~0 methanol in ethvl acetate ~ffor~ed
the title compound as a yellow solid (0.186 g, 37~ H ~
(CDC13): 2.62(3H,s), 3.37 and 3.62 (each 2H, t J=5.9Hz), 7.15(1H.,c
~=5~T~, 7..J~d,u ô.~az~, 7.7û(2-~ ), 7.~0(1~3~),
8.18(1H,d,J=8.2~z), 8.31(2H,d J=8.4Hz), 8.44tlH,d J=5.5'zi and
9.10(1H,s).
EXAMPLE 143
1-(2'-Aminophenyl)-4-~4'-(2-methylimidazo~4,5-c]pyrid-1-yl)-
phenyl]butane-1,4-dione
A solution of 1-~4'-(2-methylimidazo~4,5-c]pyrid-1-yl)-
phenyl]-4-(2'-nitrophenyl)butane-1,4-dione (û.18 g, û.43 mmol) ie
ethanol (8 ml) was hydrogenated over 5X palladium on carbon (0.3
g) at 30 p.s.i. (2.1 bar) and 22C for 2 hours. ~he catalyst was
flltered off and the filtrate was evaporated to vield a
pale-yellow foam (0.166 g, lOû~). H NMR (CDC13): 2.63t3P.,s),
3.45 and 3.54 (each 2H,t J~7Hz), 6.25 br (2H,s), 6.70(2H,m),
7.27t2H,m), 7.52(2H,d J=8.5Hz), 7.90(2~';,d J=9.5H~), 8.32( n,d
J-8.5Hz) 8.46(1H,d J-4.5Hz) and 9.û8(1n,s).
SUBSTITUTE SHEET
: ..
. ,, . '
W O 91/17162 2 0 7 8 ~ ~ 7 PCT/EP91/00737
~7
F,XAMPLE 146
7-~4'-(2-Methylimidazor4,5-c]pyrid-1 ~l)phenyl]-lH-4,5-d_hvdro-
~2,3~-benzazepin-4-one
A solution of 1-(2'-aminophenyl)-4-~4'-(2-methylimidazo~4,5-
c]pyrid-l-yl)phenyl]butane-1,4-dione (0.166 g, 0.43 mmol) in
toluene (16 ~1) and acetic acid (2 ml) was heated at reflux for 5
hours, then evaporated to dryness. Flash chromatography, eluting
with 5% methanol in ethyl acetate afforded a foam whic~ was
crystaliised ~rom ethyl acetate to give the title 4,5-dihYdro~2.
3~benzazepin-4-one (0.036 g, 23%), M.p. 223-227C. lH ~MR
(CDC13): 2.60(3H,s), 3.35(2H,d J=7.5~z), 5.36~1H,t J=7.5Hz),
6.59br(1H,s), 7.10(3H,m), 7.43(2H,d J=9Hz), 7.53(2H,d J=9H~),
7.72t2H,d J~8.5Hz), 8.11(1H,d J=8Hz), 8.42(1H,d, J-4.5Hz),
9.10(1H,s).
:;
EXAMPLE 145
3-(7-Carboxyphenyl)-2-r6-(2-methylimidazo~4,5-c]pyrid-l-,vl~-
phenyl]propenenitrile
(a) A mixture of 4-aminobenzyl cyanide (29.2 g, 0.22 ~mol) and
4-chloro-3-nitropyridine (42.0 g, 0.27 mmol) in ethanol t550 ml)
was stirred at room temperature overnight. The solid which had
precipitated was dissolved in water (1 litre) and neutralised with
saturated aqueous sodium bicarbonate. The product was extracteG
into dichloromethane (1 x 100 ml and 2 ~ 500 ml), and the combined
extracts were dried (MgS06) and concentrated under reduced
pressure to give a 4-(4-cyanomethylphenvl)amino-3-nitropvridine
(55.5 g, 99Z) as a bright yellow solid.
(b) A solution of the nitropyridine (10.0 g, 39.4 mmol) prepared
SUBSTITUTE SHEET
;.
.. . ~ .
W O 91/17162 2 0 7 8 0 a ~ PCl/EP91/00737
in (a~ above in ethanol:dichloro~ethane (2:1, 300 ml) was
hvdrogenated over 10% palladium on carbon (1.0 ~) at 20 p.s.l.
(1.4 bar) and at room temperature for 2 hours. lne ca~al~
filtered off and the solvent removed under reduced pressur~ tO
give 3-amino-4-(4-cyano~ethylphenvl)aminopyridine (8.5 g, 6"~,
which was used directlv for the next reactio~,.
(c) A mixture of the diaminopyridine (8.5 ~, 37.5 mmo') (preDar2c
in (b) above), acetic acid (25 ml) and ace~ic annydride (2~ ml)
was heated at reflux for 16 hours. Arter bein~ cooled~ ~he e.cess
reagents were removed under reduced pressure and the residue W2S
dissolved in water (150 ml). This solution was rendered basic bv
the additioD of concentrated aqueous ammonia solution and the
product was extracted into dichloromethane (3 x 100 ml). The
combined extracts were dried (MgS04) and concentrated under
reduced pressure. The residue was purified by flash
cbromatography, eluting wlth ethyl acetate/methanol (9:1) to give
l-(cyanomethyl)-4-(2-methylimidazo[4,5-c]pyrid-1-yl~benzene (7.43
g, 79Z), as a brown solit.
(d) l-(Cyanomethyl)-4-(2-methylimidazo[~,5-c]pyrid-1-yl)benzene
(272 mg, 1.1 mmol~ in dry methanol (1 ml) was treated with a
solution of sodium methoxide (250 microlltres, 5.4M in methanol,
1.1 mmol) at room temperature under nitrogen. After the mixture
had been stlrred for 20 minutes, 2-carboxybenzaldehvde (ljO m~,
1.0 mmol) was added, and the mixture was heated at reflux for 2
hours. The mixture was cooled, neutra ~sed wit;n acetic acid, anc
concentrated under reduced pressure to ~ive the title compound
(417 mg) which was used directly for E~:ample 146 without rurther
purlfication. I~ NMR (CD30D): 2.65t3H,s), 7.38(1H,d,J 5Hz),
gU~STlTUTE SHEET
,.. ~, . . , . :,
.,;
-, , : -: . . :, `
wo gl/17162 2 0 7 8 0 ~ 7 PCT/EP91/00737
~ s-
.~ ; :. `.; .;
89
7.54(2H,m), 7.69(2H,d,J 8Hz), 7.89(1H,m), 8.0~(1H,m), 8.07(2H,d, J
8~z), 8.39(1H,d,J 5Hz), 8.65(lH~s?~ 8.94(1H,s~.
EXAMPLE 146
4-~4-(2-Methvlimidazo!4,5-c]pyrid-1-yl2 ~ envll-2,3-dihvdro-lH-2-
benzaze~in-1~3-dione
A mixture of 3-(2-carboxyphenyl)-2-[4-(2-methylimidazor4,5-
cjpyrid-l-vl)phenyl]propenenitrile (410 mg, 1.08 mmol) and
polypnosphoric acid (5 ml~ was heated at 100C for 2.5 hours,
cooled and treated with ice water (20 ml). The resulting solution
was neutralised with dilute aqueous ammonia and the product was
extracted into dichloromethane. The extracts were dried (MgSO4)
and concentrated under reduced pressure, and the residue W25
purified by flash chromatography, eluting with ethyl acetate/
methanol (4:1) followed by recrystallisation from hot ethanol to
give the title 2,3-dihydro-lH-2-benzazepin-1,3-dione (49 mg, 12~)
m.p. 273-276C. Found: C,71.64; ~,4.34; N,14.37. C23H16~4O2.
0.25 H20 requires C,71.76; H,4.32; N,14.56%.
EXAMPLE 14/
2-Methvl-l-r4-(3-py _ dYloxvmethv1)phenvl]-imidazo~4,5-c]PYridine
fumarate
4-( -Methylimidazol4,5-c]pyrid-1-yl)benz~l alcohol (0.48 g, ~
mmol) was treated with 48% hydrobromic acid (8 ml~ as described in
Example 101. The crude benzylic bromide was dissolved in
dimethylsulphoxide (2 ml) and added to a mixture of 3-hydrox.v
pvridine (0.38 g, 4 mmol~ and flake po~assium hydroxide (0.68 g,
12 mmol) in dimethylsulphoxide (8 ml) at room temperature. After
SUBSTITUTE SHEET
" ' ' ~ ~ '
.... ~
. . . ~
wo gl/17162 2 0 7 8 0 0 7 PCT/EP91/00737,-,
... ... ..
9~
being stirred for 16 hour.s, the mixture was poured onto ice,
neutralised with diluee hvdrochloric acid, and extrac~ed with
dichloromethane (3 x 50 ml). The comb ned extracts wer~ ~ ed
(MgSO4) and concentrated under reduced pressure. The residue was
purified by flash chromatographv (gradient elution witb
dichloromethane/methanol). Fractions containing the produc: were
combined, concentrated under reduced pressure, and treeted ~ith
fumaric acid (60 mg) in methanol. The methanol was re~oved unae-
red--cd prassu.e, tll~ residue was dissolved in water ar.c' f-ee7e-
dried, to give a pale ye11Ow powder, ~145 mg, 15%), m.p. 84C.
Found C,61.59; H,4-68; N,11.37. ClgH16N4O. 1.5 C4H404 re~uires
C,61.22; H,4.52; N,11.42~.
EXAMPLES 148-152
Examples 148-151 were prepared by the method of Example 1
using 4-(2-methylimidazor4,5-c]pyrid-1-yl)benzyl alcohol and the
appropriate phenol. Example 152 was prepared similarl~ using the
procedure of Example 147 but isolating the product as the rree
base.
~10 CH3
R8 ~ 0 _ CH~
SUBSTITUTE StlEET
~ , .
. . ~' . ~ ` ` ' ,` ' ,
,. ' ' ' '
W 0 91/17162 2 0 78 ~ o 7 PCT/~P91/00737
91
_ _
Example i R8 R10 m.p. Analvsls ~'
_ _ C( c he ore t lc.! l L ~ b r.c Ke ~ s )
148 ~ Br 151-60.59 4.16 10.42
L 152(60.93 4.09 10.66)
1 6 G r 1 -- C~`' ~ 5 ) ,~ ; 7-- D ~ . o h 1 1 . 4 4
60(65.56 5.72 12.23)
(hemihydrate)
., _ _ _ _._
150 Cl -CO-N ~ 0 119- 63.34 5.31 11.26
120(63.35 5.11 11.37
(hydrate~
~151 Cl-CO-~ ~ 0 17564.-24 5.10 11.92
~, (64.86 5.01 12.10)
._ _
152 Cl-CO-N ~ 159-67.36 5.39 12.08
162(67.75 5.47 12.15)
l_ .
`E.YAMPLE lS3
1-(2-Hvdroxv-~ ethvlDhenvl~-3-~4-t~-methvlimidazo[4~5-c]D~rid-l-
v])Dhen,vl]-2-proDen-l-one
A mixture of 4-(2-methylimidazo~4 "-c]pyrid-1-yl)benzaldehvde
SUBSl lTUTE SHEET
, . ~ ~ ' -
.
W O 91/17162 ~ ~ 2 ~ q ~ ~ O ~ 7 PCT/EP91/00737~
,. ~
.
(Preparation 13) (3.555 g, 15 mmol), 2-hydroxy-5-methyl-
acetophenone (2.225 g, 15 mmol) and lithium hydroxide hydrate
(3.720 g, 75 mmol) in ethanol/water (96:4) (100 ml) was stirred
under nitrogen at room temperature for 18 hours. The red
suspension was concentrated under reduced pressure, and the
residue was dissolved in excess dilute hydrochloric acid. The
solution was poured into a mixture of excess saturated aqueous
sodium bicarbonate and dichloromethane (100 ml). The orvanic
iajel w~ ~epara~ed and the aqueous laver was extracted ~-ith
dichloromethane (2 x 200 ml). The combined organic solutions were
dried (MgS04) and concentrated under reduced pressure. The
residue was recrystallised from ethyl acetate/dichloromethane to
give orange prisms (2.42S g, 44%), m.p. 229-230C. Found:
C,73.99; H,5.28; N,11.30. C23HlgN3O2. 0.25 H2O requires C,73.88;
H,5.26; N,11.24%.
EXAMPLE 154
1-(5-Fluoro-2-h~droxyphenyl)-3-~4-t2-methylimidazo~4,5-c]pvrid-1-
yl)phenyl]-2-propen-1-one
The tltle compound was prepared by the method of Example 15i,
using the appropriate 2-hydroxyacetophenone and 4-(2-methyl-
imidazo~4,5-c]pyrid-1-yl)benzaldehyde. The product was obtained
as a bright orange solid. lH NMR (300 ~.H , CDC13): 2.60(3H,s~,
; 7.02(1H,m), 7.13tlH,d,J 4Hz), 7.30(1H,m~, 7.48(2H,d,J 8Hz!~
7.61(1H,m), 7.64(1H,d,J 18Hz), 7.92(2H,d,J 6Hz~, 8.00tlH,d,J
18Hz), 8.41(1H,d,J 4Hz), 9.08(1H,s~, 12.'2(1H,s).
SUBSTITUTE SHEET
.
- W O 91/17162 2 0 7 8 0 ~ 7 PCT/EP91/00737
93
EXAMPLE 155
1-(4,5-Dimechyl-2-hydrox~phenx1?-3-~4-!2-methylimidazo~4,5-C]-
p~rid~ Dherv]~-2-propen-1-one
The title compound was prepared by the method of Example
153,and was obtained as a bright yellow solid. lH NMR (300 MHz,
CDC13) 2.28(3H,s), 2.31(3H,s), 2.60(3H,s), 6.86(1H,s), 7.13(1H,d,J
5Hz), 7.'5(2Y.,d,J 8Hz), 7.64(1H,S!, 7.73tlH,d,J 14Hz), 7.91(2H,d,J
8Hz), 7.9,(l~.,d,J 14Hz), 8.41(1H,d,J 5Hz), 9.07(1H,s),
~.JO~in~s l .
EXAMPLE 156
2RS-6-Methyl-2-~4-(2-~o~4,5-c~pyrid-l--yl-)phenyl]-2~3
dihytro-4H-benzolb]pyran-4-one
~' A mixture of 1-(2-hydroxy-5-methylphenyl)-3-~4-t2-methyl-
, imidazo~4,5-c]pyrid-1-yl)phenyl]-2-propen-1-one (400 mg, 1.08
mmol) and anhydrous potassium fluoride t50% on Celite, 200 mg) in
dry methanol (20 ml~ was heated under reflux for 48 hours, then
cooled and filtered. The filtrate was concentrated under reduced
pressure, and purified by flash chromatography on silica gel,
eluting with dichlorometbane/isopropanol t20-10:1). Fractions
containing the product were combined. concentrated and
recrvstallised twice from ethanol ~o give a creamy-coloured solid
(42 m~ ), m.p. 202-203C. Found: C,73.72; H,5.10: ~,11.08.
C 3H19r~3C~. 0.33 H~0 requires C,73.59; Y.,5.29; ~;,11.19,~,.
SUBSTITUTE Sl IEET
W O 9t/17162 2 0 7 8 0 0 7 PCr/EP91/00737 A~
94
EXAMPLE 157
2RS,4RS-4-Hvdroxy-6-methyl-2-~4-(2-me~hylimidazor4~5-c~pvrid
l?phenyl~-2,3-dihydro-4H-benzo~b,lpYran
The product from Example 153 (2.43 g, 6.54 mmol) was
dissolved in a mixture or methanol (60 ml), water (40 ml) and
aqueous sodium hydroxide (lM, 3.2/ ml, 3.27 mmol~ at roo~
temperature with stirring, and then sodium borohydride (7jb mg,
6.54 mmol) was added. The mixture was stirred for 18 hours at
room. t~mr''.~ .r~ CrlJ ;he-ll y.lL;itioned be~ween dichloromethane (i
x 50 ml) and O.lM aqueous sodium hydroxide (200 ml). The organic
` solutions were dried (MgS04) and concentrated under reduced
pressure, and the residue was purified by flash chromatography,
eluting with ethyl acetate:methanol:diethylamine (90:5:5~ to give
a white solid tl.57 g, 65X), m.p. 238-241C (after trituration
with methanoliethyl acetate). Fount: C,73.73; H,5.62; N,11.38.
C23H21N3O2. 0.2 H20 requires C,73.66; H,5.75; N,11.20~.
The stereochemistry of the product was assigned from the
coupling constants in the lH NMR spectrum (300 MHz, CDC13) as
follows: H-2 (dd, J 1 ant 11.6Hz) and H-4 (dd, J 6 and 10.5Hz),
hence both hydrogens are axial.
EXAMPLE 158
2RS,4RS-6-Fluoro-4-hvdrox -2-~4-(7-methvlimidazol4,5-c]~rid-l-
yl)~henyl]-2.3-dih,~dro-4H-benzo~blpvran
The product o~ example 154 was cyclised following ~he me~hod
:
SU~STITUTE SHEET
- ` . . :
... . . .. .
" , . .. , . .~
.; ~ - ' ` ~ ,~
W O 91/17162 2 0 7 ~ O ~ 7 PCT/EP91/D0737
;~;t ~
of Example 157 to give the title compound, m.p. 219-220C (from
methanol~. Found: C,70.50; H,4.73; N,11.21- C22Hl8FN307 requires
C,70.38; H.4.83; N,ll.l9~',.
EXAMPLE 159
2RS, 4RS-6,7-Dimethyl-L-hvdroxv-2-~4-(2-methYlimidazo~4,5-c~P~rid-
l-~l)phenyl~-2,3-dihYdro-4H-benzu[b] yran
The procuc~ of E~ample 155 was cyclised following the method
OT E~mrl_ 15~ U ~ive sne t~tle comDound. m.p. 243-246C (~ror.
: me~hanol/dichloromethane). Found: C,74.94; H,6.05; N,10.90.
C24H23N303 requires C,74.78; H,6.01; N,10.90%.
.i
EXAMPLE 160
2RS 4RS-4-Methoxy-6-methvl-2-~4-(2-methylimidazo[4,5-c]P~rid-l-
yl~ph ~ ,3-dihydro-4H-benzolb]pyran
The compound from Example 157 (371 mg, 1.0 mmol) was
dissolved in dry dimethylformamide t8 ml) and sodium hydride (48
mg, 60~ dispersion, 1.2 mmol) was added at room temperature.
After 45 minutes methyl iodide t68 ~1, 1.1 mmol) was added and the
mixture was stirred for a further 1 hour. The mixture was
concentrated unter reduced pressure, dissolved in ethyl acetate
(20 ml), washed with water tlO ml), dried (MgS04) and concentrated
under reduced pressure. The residue was purified by flash
chromato~raphy, eluting with dichloromethane:methanol (16:1),
followed by recrystallisation from diethy: ether to give a white
solid (200 mg, 52~), m.p. 151-153C. Found: C,74.74; H,6.03;
~10-91- C~4H23N302 requires C,74.78; H,6.01; N,10.90~.
SUBSTlTUtE SHEET
W O 91/17162 2 0 7 8 0 q 7 . PCT/EPg1/00737 --1
96
EXAMPLE 161
2RS,4RS-4-(4-Fluorophenyl)-2-~4-(2-methylimidazo~4,5-c~pvrid-1-
l)phenvll-',3-dioxane
The method of Example 37 was followed using 4-fluoro-(1,3-
dihydroxypropyl)benzene to give the title compound, m.p. 70C.
Found C,69.48; H,5.29; N,9.99. C23H20FN302. 0.5 H 0 requires
C,69.33; H,5.31; N,10.55~.
~XAMPLE 162
.
2-Methyl~ 4-(rhenYlmethyl)phenyl]-imidazo~4,5-c]~vridine
a~ A mixture of 4-chloroimidazor4,5-c]pyridine (670 mg, 4.0
mmol), p-fluorobenzophenone (880 mg, 4.4 mmol) ant anhydrous
potassium carbonate (607 mg, 4.4 mmol) in dry dimethylformamide (8
ml) was stirred at reflux for 3 hours. The mixture was then
concentrated under reduced pressure, dissolved in dichloromethane
(75 ml) and washed with water. The organic solution was dried
(MgS04~ and concentrated under reduced pressure, and the residue
was purified by flash chromatography eluting with ethyl acetate/
dichloromethane (3:2~ to give 1-(4-benzoyl)phenyl-2-methyl-
imidazo~4,5-c]pyridine, (1.14 g, 82X~, m.p. 205-207C (from ethyl
acetate~.
b) The compound from (a) above (612 mg, 1.94 mmol) was
hydrogenated over 30Z palladium on carbon (500 mg) in a mi~ure o-
ethanol t60 ml~ and dichloromethane (15 ml~ in the presence of
magnesium o~ide (61? mg~ at 60 p.s.i. (4.1 bar) and 40C fo-
hours. The mixture was cooled, filtered, and concentrated under
reduced pressure. The residue was purified by flash
chromatography on silica eluting with dichloromethane/methanol
SUBSTITUTE SI~IEET
., . . - . ` : : :,
. .
` ;.
~ wo gl/17162 2 0 7 8 0 ~ 7 PCT/EP91/00737
97
(16:1) to give 2-meth~ 4-(hydroxy(phenyl~methyl~pheny]l-
imidazo!4,5-c]pyridine (430 mg, 77%), m.p. 232-234C (from
me~hanol".
(c~ The product from s~ep (b) above, (240 mg, 0.76 mmol) was
added co a mixture or trifluoroacetic acid (8 ml) and triethyl-
silane (14s ~1, 0.91 m~ol), and the mixture was stirred at 50C
for 1 hour. The m-:iture was concentrated under reduced pressure,
and the residue W2S dissolved in dichloromethane (20 ml) and
Pn,~Pre~ ~7S' C ~-,.' ~hc a'~Ui;LV~I ~f salura~ea aqueous sodium
bicarbonate. The aqueous layer was separated, extracted with
dichloromethane (2 x 15 ml), and the combined organic layers were
dried (MgSO4) and concentrated under reduced pressure. The
residue was purified by flash chromatography on silica, eluting
with ethyl acetate/methanol (6:1) to give the title compound t205
mg, 90%), which was further purified by reverse-phase h.p.l.c.
(C18 silanized silica, eluting with methanol/water, 85:15) to give
a white solid, m.p. 131-132C (from aqueous methanol). Found:
C~80-79; H~5-75; N~13.70- C20~17N3 requires C,80.24; H,5.72;
N,14.04%.
SUBSTITUTE S~IEET
.
.
.
.
W O 91/17162 PCT/EP91/00737 ~
2 0 7 ~ . ~
98
PREPARATION I
Ethvl ~4-(2-methylimidazor4,5-c]pvrid-1-yl)phenvl~acetate
a) Ethvl 4-aminophenvlacetate (17.7 g, 0.1 mole) and sodium
bicarbonate (8.4 g, 0.1 mole) were stirred in ethanol (200 ml).
4-Chloro-3-nitropyridir.e (15.9 g, 0.1 mole) was added as A
solution in ethanol (50 ml) and the whole stirred at roo~
temperature ror 3 hours. The mixture was then evaporated to low
bulk and poured into ethyl acetate (500 ml) and washed with water
(~00 ~ij. Tne organic phase was then extracted with 0.5~
hydrochloric acid and the combined aqueous extracts basiried with
2N sodium hydroxide and extracted with dichloromethane. The
combined organic extracts were dried over Na2S04, filtered and
evaporated to dryness. rhe residue was recrystallised from
aqueous ethanol to give ethyl 4-(3-nitro-pyrid-4-ylamino)phenyl
acetate (7.32 g), m.p. 124-126C. A further 8.56 g was recovered
from the mother liquors.
b) The above product (15.7 g) was hydrogenated at 60 p.s.i. (4.1
bar) over 5% palladium on charcoal for 3 hours at room
temperature. Filt tion and evaporation of the solvent gave ethyl
4-t3-amino-pYrid-4-Ylamino)PhenYl acetate (14.1 g).
c) Ethyl 4-(3-amino-pyrid-4-yla~ino)phenyl acetate (14.1 g, 52
mmol), glacial acetic acid (100 ml) and acetic anhytride tlOO ml)
were mixed and heated at reflux under nitrogen for 1~ haurs. The
coolet solution was evaporated to dryness and basified with lOX
aqueous sodium carbonate solution then e~:;racted with
dichloromethane (3 .~: 100 ml!. The combir.ed organic extracts were
evaporated to dryness and purified by chromatography on silica
elutlng with dichloromethane/eehanol to give ethyl ~4-t~-methyl-
SUBSTITUTE S1~EET
,
.
.
;- W O 91/17162 ~ ~ 7 8 G ~ 7 PCT/EP91/00737
99
imidazo~4,5-c]pyrid-l-yl!phenyl~acecate (13.6 g~.
PREPAF;ATION 7
Ethy1 2-[4-(2-methvlimidazo[4,5-c]pvrid-l-yl)phenyl~proDanoate
Ethyl ~4-(2-methyl-imidazo~4,5-c]pyrid-l-yl)phenyl~acetate
(7.4 g, 25 mmol) was dissolved in tecrahydrofuran (lO0 ml) and the
solution cooied tO -/0C. Lithium diisopropylamide (1.5M, 18.4
ml, 27.5 mmol! was added under nitrogon ard the resultir.g
susye~1~ion sz-rrer ror 15 m nu.es. Methyl iodide (3.91 c, 27.5
mmol) was added and the mixture allowed to come to room
temperature over 2~2 hours. The reaction was then quenched with
hydrochloric acid (25 ml, lN) and evaporated to low bulk, the
solution was basified with sodium carbonate solution and extracted
wlth dichloromethane (3 x lOO ml). The organic extracts were
dried, filtered and evaporatet to tryness. The resitue was
purifiet by column chro~atography on silic2 to yielt ethyl
2-t4-(2-methylimidazo~4,5-c]pyrid-l-yl~-phenyl]propanoate (4.5 g,
58X), m.p. 78-80C.
PREPARATION 3
2-Me~ y ~ -methylimitazor4,5-c~pyrid-l-yl)phenyl]propan
Ethyl 2-[4-(2-methylimidazot4,5-c]pyrid-l-yl)-
phenyl]propanoate (~.94 g, 9.5 mmol) was dissolved in
tetrahydrofuran ~50 ml) and cooled in an ice bath under nitrogen.
Lithium aluminium hydride (0.19 g, 5 ~ol) was added portionwise
over 2 minutes~ The mixture was stirred at 0C for l0 minutes
then at room temperature for 3 hours. Fu.ther lithium aluminiu~
hydride (0.l9 g) was added and after l; minutes water was added
cautiously. The mixture was acidified with N hydrochloric acid (15
SUBSTITUTE Sl~EET
,
.
.
W O 91/17162 PCT/EP9t/00737~
2 0 7 8 ~3~
100
ml~ then basified with sodium carbonate solution and extracted
with dichloromethane (2 x 100 m]). The combined organic extracts
were dried over ~a~S04, ~iltered and evaporated to dryness. The
residue was purified by column chromatography on silica eluting
with 10% methanol in dichloromethane to give the title product
(2.3 g). Found: C,71.78; H,6.45; ~,15.56. C16H17N3O requires
C,71.91; H,6.37; ~,io.73%.
PREPARATION 4
'-~bl-n--r-l- ,3-Dro~aneaioi
A solution of ethyl 3-phenyl-3-hvdroxy-propanoate (4.7 g, 26
mmol) in anhydrous ether (20 ml) was added dropwise to a cold
(0C), stirred suspension of lithium aluminiu~ hydride in diethyl
ether (50 ml). A vigourous reaction ensued during the addition.
The resultant mixture was stirred at 18C for 15 hours, then
cautiously treated with water (1.1 ml), 15% aqueous sodium
hydroxide (1.1 ml) and water (3.1 ml). The mixture was filtered
through a filter pad and evaporated to a pale-yellow oil. Silica-
gel chromatography afforded the title diol as a colourless,
viscous oil (3.27g, 91%).
PREPARATIO~' 5
2-Phenvl-1,3-propanediol
A solution of tropic acid (3 ~, 18 mmol~ in tetrahydroruran
(20 ml) was added dropwise to a cold (0C), stirred suspension of
lithium aluminium hydride in tetra~yd-oruran (40 ml~. Af~er ~
hours, the mixture was cautiously hydrolysed by addition or water
(0.8 ml), 15Yo aqueous sodium hydroxide (0.8 ml) and water (2.5
ml). The mixture was filtered through a filter pad and evaporated
SUBSTITUTE SHEET
:`. . `.
` ~ . . . : .
.
- .- wo 9l/17162 2 0 7 8 ~ ~ 7 PCT/EP91/00737
101
to a pale-yellow oil. Ch~omatographv on silica elutin& wlth
diethyl ether afforded the title diol as a colourless oil which
crs~stallised on standing. M.p. 41-~5~C.
, .
PREPARATION 6
1,2-Bis(trimethylsil~lo ~ hen~-lethane
Butyllithium (1.6H in hexane; 19 ml, 30 mmol) was added
drop~ise to a cold (-20C), stirred solution of phen~lethanediol
~ L/.~ iJ in ~e~ranydro~uran (80 ml). A}ter iO minutes~
neat chlorotrimethyl-silane (4.5 ml, 3j mmol) was added. After 3C
minutes, all volatiles were removed under vacuum and the residue
was partitioned between hexane and saturated sodium bicarbonate
solution. The hexane layer was dried over magnesium sulphate and
evaporated to give the title product as a pale-yellow oil (4g,
97%).
.,~
PREPARATIO~ 7
., .
2-~4-(2-Meth~limidazol4,5 ~ ~y ~d-l-~l)phenyl]-~-methyl-oxirane
4-(2-Methylimidazo~4,5-c]pyrid-1-yl)acetophenone (430 mg 1~7
mmol) was dissolved in tetrahydrofuran and cooled in an ice bath
under nitrogen. Dimethylsulphoxonium methylide (0.6M in
tetrahydrofuran, 5 ml, 3 m~ol) was added and the solution stirred
at room temperature for 4 davs. The solvent ~as remo~ed under
vacuum and the residue purified by column chromatographY on silica
eluting with dichloromethane/methanol (9~:~). The title comround
crystallised on removal of the solver.~ under vacuum (416 mp, 92,~'.
lH NMR CDC13: 9.08(1H,s), 8.40(1H,d J~6Hz?~ 7.57(2H,d,J=
9Hz), 7.38(2H,d,J~9Hz), 7.10~1H,s), 4.02(1H,t~, 3.28(1H,m),
SUBSTITUTE S1 IEET
.
.
. . '~, ~: '`': '
W O 91/17162 2 0 7 8 0 0 ~ PCT/EP91/00737 ~-
102
2.90(1H,~), 2.63(3H,s~.
PREPAR~TTO~' 8
2-~4-(2-Methylimidazo~4,5-c]pvrid-l-vl)~7henvl~oxirane
4-(2-Methvlimidazo[4,5-c]pyrid-1-y])benzaldehyde !1.19 g, 5mmol~
waS dissolved in tetrahydroruran (20 ml) and the solution cooled
in an ice bash. Dimethyl-sulphoxonium methylide (0.6M in
tetrahydrofuran; 15ml, 9 rmol) was added and the resulting white
suspenslon stirred at O~C for 1 hour then at room temperature for
1 hour. The mi~ture as evaporated to 5 ml, poured into brine and
extracted with dichloromethane (3 x 50 ml). The combined extracts
were dried (MgS04), filtered and evaporated to dryness. The
residue was further purified by column chromatography on silica
eluting with dichloromethane/methanol (97:3) to give the title
oxirane as a clear, unstable oil which turned red on standing (362
mg, 29g).
PREPARATION 9
4-(2-Methylimidazo[4,5-c]pvrid-l-yl)acetophenone
(a) -(4-Acetylph~v~ n~-]-nitropyridine hvdrochloride
A solution of 4-chloro-3-nitropyridine hydrocbloride (9,75 g,
50 mmol) in ethanol (40 ml) was added to a slurry of p-aminoaceto-
phenone (6~76 g, 50 mmol) in ethanol (2j ml), and the mixture was
stirred at room temperature overnight. The mixture was chilled in
ice, and the yellow solid fil~ered o~f and dried (10.] g, 69%).
m.p. 197-200~C.
b) 4- ~ henyl)amino-3-aminorvridine
4-(4-Acetylphenyl)amino-3-nitropyridine hydrochloride (2.0 g,
SUBSTITUTE S'rlEET
. . - ,
,' , ~
. W O 91/17162 2 ~ 7 ~ O '~ 7 PCT/EP91/00737
103
78.8 mmol) was partitioned between aqueous sodlum hydro~ide and
dichloromethane (3 x 20 ml). The combined organic phases were
washed with water (20 ml) and concentrated under reduced pressurr
to give a solid. Ethanol (20 ml~ was added, and the solution was
hydrogenated over 5% palladium on carbon (0.2 g) at 50 p.s.i. (3.4
bar) for 3.5 hours. The catalyst was filtered of-, and the
solvent removed under reduced pressure tO give a brown solid.
(l.8 g, ca 100%) which was used directly for the nexr stage
wirhou~ purification.
(c) 4-2(-Methylimidazo[4,5-c]pvrid-l-yl)aceto~henone
A solution of 4-(4-acetylphenyl)amino-3-aminopyridine (68.0
g, 0.3 mmol) in acetic acid (204 ml) and acetic anhydride (204 ml)
:~
was heated ~t 95C for 1.5 hours then cooled and concentrated
under reducet pressure. The residue was dissolved in water (500
ml) and rendered basic by the addition of saturated aqueous
ammonia. The product was filtered off, washed with water (2 x lOO
ml) and dried under vacuum to give the title compound, (61.0 g,
81%) as a brown solid, m.p. 155-156C tfrom water).
PREPARATION lO
Ethyl 4-(2-methylimidazo[4,5-c]pyrid-l-yl)benzoYlacetate
A solution of 4-(2-methylimidazo~4,5-c]pyrid-l-yl-
acetophenone~l7.5 ~, 69.7 mmol) in drv tetrahydroruran (175 ml~
was added to a slurry of sodium hydride (3.68 g, 153 mmol~ in a
mix~ture of drv tetrahydrofuran (35 r'~ and dieth.l carbonate (^~.,
g, 209 mmol) at reflux wich stirring over 45 minutes~ After a
further l hour, the mixture was cooled, hexane (200 ml) was added,
` ant the resulting precipitate was fileered off and washed with
SUE~STITUTE Sl-IEET
,, ` , .
,
W O 91/17162 PCT/EP91/00737 ~
20780~':
,
104
hexane (2 x 100 ml). The solid was suspended in ethyl acets~e
(200 ml) and acetic acid (10.2 g) was added. After stirring for
15 minutes, water !200 ml) was added, and the orranic laver was
separated. The aqueous phase was eY.tracted with ethyl acetate
(100 ml) and the combined organic solutions were washed with w~ter
(200 ml), dried (MgS04~ and concentrated to give the title product
as a gum (17.3 g, 77"). Further purification by flash
chromatography (eluting wi.h ethyl acetate/methanol (7:1) gave the
;iLi~ co~pound as a white solid. m.p. 16;-166 C.
PREPARATION 11
3-Hydroxy-3-[4-(2-methylimidazo[4,5-c]pyrid-1-yl)phenyl)propanol
Sodium borohydride (0.38 g, 10 mmol) was added to a stirred
suspension of ethyl 4-(2-methylimitazot4,5-c~pyrid-1-yl]-
benzoyl acetate (2.4 g, 7.4 mmol) in isopropanol (20 ml) and the
mixture stirred at ambient temperature for one wee~. The solution
was concentrated and partitioned between water and
dichloromethane. The organic layer was dried over magnesium
sulphate and evaporated to an oil. Chromatography on silica
eluting with methanol in ethyl aceeate afforded a colourless foam
which crystallised from dichloromethane (0.25 g, 12,',). M.p.
148-50~C. Found: C,66.80; H,6.04; ~,14.57. C16H17~3O~Ø25 H~0
requires C,66.76; H,6.13; ~,14.60~;.
PREPAR~T-~O~ 12
4-(2-Methvlimidazo~4.5-cjpvrid-1--})benzonicrile
a~ 4-Cyanoaniline (6.894 g, 58.4 mmol) was added to a solution
of 4-chloro-3-nitropyridine (9.26 g, ;8.4 mmol) in ethanol (200
S~IBSTITUTE StlEET
. ` .. ~ ; ........
:
. .
'
- . .~ . ~
-~ W O 91/17162 2 ~ 7 8 0 0 7 PCT/EP91/00737
105
ml) and the mixture was stirred at room te~perature for 18 hours.
The resulting yellow suspension was poured into 500 ml of ice-cold
dilute ammonia and filtered. The solid was treated with 150 ' o'
boiling ethanol, cooled in ice, and filtered to give ~-(4-cyano-
phenyl)-4-amino-3-nitropyridine, 12.15 g, as a bright yellow
powder, m.p. 210-211C.
b) According to a modification of the method of Phar~. Hel~.
Acta, 1975, 50, 188., tin dichloride dihydrate (56.4 g, ~50 mnol)
was dUU~U to a suspension o~ ~-(4-cyanophenyl)-4-ami~o-3-
nitropyridine (12.0 g, 50 mmol) in 2N aqueous hydrochloric acid
(35 ml), water (150 ml) and ethanol (75 ml) and the resulting
mixture was heated to reflux for 10 minutes under nitrogen. The
mixture was cooled in ice, poured into ice-cold 2N aqueous sodium
hydroxide (400 ml) and filtered. The creamy coloured solid was
washed with 2N aqueous sodium hydroxide and ~ater, and then dried
in a vacuum tesiccator. The product, 3-amino-4-(4'-cvanophenyl)-
aminopyridine, 9.31 g, gradually turned reddish brown on exposure
to light and air.
c) A mixture of 3-amino-4-(4'-cyanophenyl)aminopyridine (9.31 g,
44.3 mmol)~ triethyl-orthoacetate (40 ml) and acetic anhydride (30
ml) was heated at reflux for 2 hours ~nder nitrogen, cooled, then
concentrated under reduced pressure. The brown residue was
dissolved in lM hydrochloric acid and washed with ethyl acetate
~200 ~1). The aqueous layer was rendered basic with saturated
aqueous ammonia and extracted with dichloromethane (3 x 200 ml).
The combined extracts were washed with water, dried (MgS04) and
concentrated to give the title product as a brown solid (6.j g).
H NMR (CDC13): 2.61t3H,s), 7.13(1H,d, J 6Hz), 7.58(2H,d,J 9Hz~,
S~JBSTITUTE S'.IEET
`
.
W O 91/17162 2 0 7 8 O ~ 7 PCT/EP91/00737 --.
106
7.98(2H,d,J 9Hz~, 8.45(1H,d,J 6hz~, 9.11(1H,s).
PREPARATIO~l l3
4-(2-Methvlimid2zor4,5-c~l~vrid-l-vl?benzaldehvd~
Nickel-aluminium alloy (1 g) was added to a stirred solution
of 4-(2-methylimidazo~4,5-c]pyrid-1-yl)benzonitrile (1 g, 4.3
m~ol) in 90% formic acid (13 ml) and weter (3 ml). The mixture
was heated to 120C when an e~:othP~i~ r~2ction .n~t_ated, ;hen
heated under reflux for an additional 1 hour. The solution was
~led and ril~ered usir.~ a filter aid, was:~ing the rilter cake
with methanol. The filtrate was concentrated and partitioned
between ethyl acetate (100 ml) and saturated aqueous sodium
bicarbonate (100 ml). The organic layer was separated, dried over
magnesium sulphate and evaporated to dryness. Trituration with
ethyl acetate, followed by recrystallisation from isopropanol
afforded the aldehyde as a colourless solid tO.2 g, 20X), m.p.
158-160C. Found: C,70.31; H,4.63; N,17.38. C14HllN3O requires
C,70.87; H,4.67; N,17.71~.
PREP~RATION 14
Methyll4-(2-methylimidazo~4,5-c]pyrid-1-yl)~henyl]pro~anoate
The procedure described under Preparation 9 was followed but
.i using p-aminophenylpropanoate in place of p-~minoacetophenone to
give the title compound (65X) m.p. 88-91C. Found: C,67.16;
i H,5.84; N,13.55. C17H17N302. 0.5 H~0 requires C,67.11; H,5.92;
N,13.82~.
,
SUBSTlTUTEi Sl IEET
,
.
:, ' ' : :.
-~ W O 91/17162 2 0 7 8 0 ~ 7 PCT/EP91/00737
107
PREPARATION 15
4-(2-~lethylimidazo[4,5-c]pyrid-1-Yl~benzyl alcohol
The procedure described under Preparation 9 was follo~e~ bu~
using 4-aminobenzyl alcohol instead of 4-aminoacetophenone to give
the title product (83%) m.p. 154-156C. Found: C,70.10; H"5._2;
~17-89- C14H13N30 requires C,70.29; H,5.44; N,17.577O.
PREPARATION 16
. .
2-'~varoxyetnvl~henyij~ -methvlimidazol4~5-c]~yridine
;; The procedure described under Preparation 9 was foliowed but
; using 4-aminophenethyl alcohol instead of 4-aminoacetophenone to
give the title product (67%) m.p. 196-198C. Found: C,70.99;
H,6-16; N,16.50- C15H15N3O requires C,71.15; N,5.93; N,16.60%.
PREPARATION 1?
4-(2-Methylimidazo~4,5-c]pyrid-1-yl)benzoic acid
A mixture of 4-(2-methylimidazo~4,5-c]pyrid-1-yl?benzonitrile
(12.0 g, 51.3 mmol) and sodium hydroxide (22.0 g, 0.55 mmol) in a
mixture of ethanol (55 ml) and water (55 ml) was heated under
nitrogen at refluY. for 1~ hours, cooled ant concentratet under
reduced pressure. The brown resitue was tissolved in iced water
and glacial acetic acid (33 ml) was added, at which pcint a bufr-
coloured solid precipitated. The solid was washed with ~ater and
dried under vacuum at 70C to gi~e the title compound, (9.139 &,
70%). H ~R (DMSO-d6), 2.50(3H,s!, 7.25(1H,d,J=5Hz~,
7.72(2H,d,J=8Hz), 8.16(2H.d,J=8Hz), 8.30~1H,d,J=5Hz), 3.9^(1'n.s~.
SUBSTlTU~E St~EET
:
, . . .
WO 91/17162 ` 2 0 7 ~3 o ~ '7 PCI/EP91/00737 ,~
].08
PUEPARATIo~ 1 8
4-(2-Methylimidazor4,5-cl ~ rid-1-vl)benzar!~.ide
A mixture of 4-(2-meth!~limida7O[4,5-cjp~.~ri~ cer.2onrril^-
(4.69 g, 20 mmol) and concentrated sulphuric acid (50 ml~ was
heated at 110C with stirring for one hour, then slowlv poured
onto crushed ice (200 g~. The pH of the mi~ture was adjusted to c
.. by addition of aqueous sodium hydroxide and the soiution e~tractec
with ethyl ace~ate. The organic pnase was dried (MgSOL~ and
evaporated to a yellow solid (5 g) which was re^rystalll.sed r^ro...
isopropanol to give the title product (2.19 g, 44"), m.p.
194-195C- Found C~65-46; H~6-35; N,18.40. C14N12N4O, (CH3)2CHo,L
requires C.65.36; H,6.45; N,17.94%.
SURSTITUTE SltEE~
.. ,, . :
~; " . . ..
. .
.. . ` . ..
. . ~ . . .
,,. ; ` ~, ' `` ` : ~