Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
20817~
PF/5- 1 ~834/A/GBF
Microbicides
The present invention relates to a macrocyclic compound of formula I, to a process for its
preparation and to the use thereof for controlling plant diseases, as well as toplant-protective microbicidal compositions which contain this compound as activeingredient.
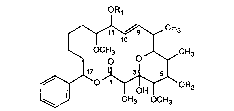
OR1
~,CH3
OCH3 J~CH3 (I)
~\
CH3 OCH3
In formula I the side chain Rl contains not more than 15 carbon atoms and is defined as
follows:
- a) an unsubstituted or a substituted hydrocarbon radical selected from the group
consisting of C~-C6alkyl, C3-C6alkenyl, C3-C6alkynyl, C3-C6cycloalkyl,
C3-C6cycloalkyl-(Cl-C6)alkyl, phenyl, xylyl and tolyl, possible substituents being
selected from the group consisting of halogen, CP3, carboxy, phenyl, mono- and
disubstituted phenyl, hydroxy, Cl-C4alkoxy, Cl-C4aLkylthio, alkoxyalkoxy, amino,monoalkylamino and dialkylamino; and also a saturated unsubstituted or
substituted heterocyclic 5- or 6-membered ring which is linked to the oxygen atom
in 1 l-position through a carbon atom which is a-oriented to the hetero atom 0, S
or N and so forms a cyclic acetal, thioacetal or aminal; with the proviso that Rl is
not methyl;
- b) hydrogen, provided the substituent R2 has a meaning other than hydrogen;
-` 2081700
- c) an acyl radical -COR3, wherein R3 is hydrogen or an unsubstituted hydrocarbon
radical or a hydrocarbon radical which is substituted by halogen, phenyl, phenoxy,
Cl-C4alkoxy, Cl-C4aLkylthio, amino, carboxy or otherwise in the manner of an
amino acid, which radical is selected from the group consisting of Cl-C6alkyl,
C2-C6aL~cenyl, C2-C6aLkynyl and C3-C6cycloalkyl; or wherein R3 is an
unsubstituted or substituted phenyl radical or a benzyl radical which is substituted
in the aromatic nucleus by one to three substituents selected from the group
consisting of methyl, hydroxy, methoxy, nitro, halogen and/or tnfluoromethyl;
and R2 is:
- hydrogen;
- a silyl group -SiR'R"R"', wherein R', R" and R"' are identical or different Cl-C6alkyl or
phenyl substituents;
- an acyl radical -COR4 in which R4 is hydrogen, phenyl or a Cl-C6alkyl group which is
unsubstituted or substituted by one or more than one halogen atom or by one or two
Cl-C4alkoxy or C3-C6alkoxyaLkoxy groups.
The general terms as employed above are de~med as follows:
Halogen will be understood as meaning fluoro, chloro, bromo or iodo.
C3-C6Cycloalkyl will be taken to mean cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl.
Depending on the chain length, alkyl will be understood as comprising methyl, ethyl,
propyl, butyl, amyl, hexyl, and the isomers thereof, typically isopropyl, isobutyl,
sec-butyl, tert-butyl, neopentyl and the lil~e.
Halogen-substituted alkyl is a mono- to perhalogenated alkyl substituent such as CHCl2,
CH2CI, CC13, CF3, CH2F, CH2Br, CH2CH2Cl, CH~7-CH3, CHBr2 and the like.
Alkenyl denotes an aliphatic hydrocarbon radical having a double bond, typically vinyl,
propen-l-yl, allyl, buten-l-yl, buten-2-yl, hexen-2-yl and the like.
Alkynyl denotes an aliphatic hydrocarbon radical having a triple bond, typically ethynyl,
,' : ' ' '
.
` - ~
208~73~
propyn-l-yl, propargyl, butyn-l-yl and the like.
Possible substituents of mono- or disubstituted phenyl are methyl, hydroxy, methoxy, nitro
and/or CF3.
Exemplary of aL~cyl radicals which are substituted by one or more aLlcoxy or aLkoxyallcoxy
groups are-CH2CH20CH3, -CH2CH(CH3)0CH3, -CH2CH2CH20CH3,
-CH2CH20CH20CH3, -C(CH3)2-CH20CH3, -CH2CH2~:)CH2CH20CH3 and other
branched or unbranched radicals.
Acetals, thioacetals or aminals are present in the 1 l-position of the macrocycle if as stated,
a Clbridge is next to the oxygen atom in this position and then either an oxygen, a sulfur
or a nitrogen atom. Illustrative examples of open-chain derivatives are -CH20CH3,
~CH20c2Hs~ -CH20C3H7(i). -CH(CH3)0C3H7(i), -CH20CH2CH20CH
-CH(CH3)0CH3, -CH20C4Hg(n), -CH(CH3)0CH2CH2-OCH3,
-CH(OCH3)CH2CH20CH3, -CH2-S-CH3, -CH2-S-CH2CH20CH3, -CH2-N(C2Hs)2,
-CH2-N(CH3)(C2Hs). Suitable cyclic derivatives are a-tetrahydropyran, a-tetrahydrofuran,
a-tetrahydrothiopyran, a-te~rahydrothiophene and a-piperidine which, in the manner of a
furanose or pyranose, may be wholly or partially substituted by -OH, -CH20H,
-CHOH-CH20H, NH2, -CH2NH2 or by appropriate acetyl derivatives. This recitation
implies no restriction.
The substituent -COR3 can be the radical of a (d)- or (I)-amino acid which is esteri~led
with the 1 l-hydroxy group. Exemplary of amino acids are glycine, a- and ,B-alanine,
valine, (iso)leucine, phenylalanine, (hydroxy)proline, tyrosine, serine, threonine, cysteine,
methionirie, tryptophan, aspartic acid, glutarnic acid (or pyrrolid-4-one-2-carboxylic acid),
arginine, Iysine, histidine, (x- and ~-aminobutyric acid and others. This recitation implies
no limitation.
In the narrower sense, the inventive compound of formula I is derived from macrocyclic
Soraphen C, for which the following spatial structure is assumed, and its hemiacetal
structure, depending on the pH and the solvent, is partly in the cyclic form and partly in
the seco-form:
. : ~
. .
20817~0
0~1 , .
- OH
~" oJ~ 'OH ~"" o~3~ 'OH
b ~ CH3 OCH3 ~ CH3 OCH3
Soraphen C
cyclic hemiacetal seco-form
Soraphen C is disclosed in EP-A-0 358 606 as a macrolide which is obtainable by
microbiological processes and is formed, in addition to other Soraphens of the same
molecular structure, by culturing Sorangium cellulosum strains.
The derivatives of formula I described herein are obtained by chemical means from
Soraphen C. It is therefore assumed that the spatial structure of Soraphen C will also apply
to the novel dcrivatives. The present invendon relates to all isomeric structures of
formula I and encompasses also the possible seco-form of the 3,7-hemiacetal. Likewise,
formula I encompasses all possible diastereoisomers which are addidonally formed by the
derivatisation with Rl and/or R2. For the sake of simplicity, the planar representation of
the novel Soraphen C derivatives of formula I will be retained throughout the remainder of
this description.
In inventive compounds of formula I and in corresponding intermediates, the reactivity of
a hydroxyl group in 5-position differs from that of a hydroxyl group in 1 l-position of the
molecule. The accessibility of e.g. compounds which are subsdtuted differently or
partially in these positions is therefore ensured.
Compared with the acdvity of the basic compound Soraphen C, the novel compoùnds of
formula I have a surprisingly enhanced microbicidal activity.
Preferred compounds of formula I are those wherein Rl has the given meaning and R2 is
hydrogen or an acyl radical -COR4, in which R4 is hydrogen or a Cl-C4alkyl group which
is unsubstitu~ed or may be substituted by Cl-C4alkoxy.
- ` 20817~
These compounds shall be designated here and subsequently as subgroup Ia.
Among this group of compounds, those compounds are especially preferred in which Rl,
together with the oxygen atom in 1 l-position, is an open acetal of formula
-O-~-Y-R7
R6
wherein Y is oxygen or sulfur, Rs and R5 are each independently of the other hydrogen or
Cl-C4alkyl, and R7 is Cl-C4aLkyl radical which is unsubstituted or, where R7 --C2-C4alkyl,
may be substituted in the ,B-position to the terminal position (~) by 1-3 substituents
selected from OH, alkoxyalkoxy of at most 6 carbon atoms and Cl-C4aL~coxy;
or wherein Rl is a cyclic acetal of forrnula
~ T~ oder ~7G, T2
which, in the manner of a furanose or pyranose, is wholly or partially substituted by -OH
or -O-COCH3, and wherein Tl is hydrogen, OH, -CH20H, -CHOH-CH20H or the acetyl
derivatives thereof, and T2 is hydrogen, OH, CHzOH or the acetyl derivatives thereof;
or wherein Rl is a thioacetal of formula
~7~ or ~)
(subgroup Ib), preferably those in which R2 is hydrogen (subgroup Ic).
Preferred compounds within subgroup Ib are those compounds wherein Rl, together with
the oxygen atom in 1 l-position, is an open acetal of formula
I
-O-CH-OR7
208~7~Q
in which Rs is hydrogen or methyl and R7 is a Cl-C4aL~cyl radical which is unsubstituted
or, where R7 = C2-C4aL~cyl, may be substituted in the ~- to terminal position by up to three
Cl-C3alkoxy groups (subgroup Id), more particularly those in which R2 is hydrogen,
formyl, acetyl or methoxyacetyl (subgroup Ie).
An important group of compounds from among the compounds of ~ormula Ia also
comprises those wherein Rl is a C3-CscycloaL~cyl radical or a hydrocarbon radical selected
from the group consisting of C2-C3aL~yl, C3-C4aLkenyl and C3-C4aL~cynyl, which radical is
unsubstituted or substituted in the ,B- to terminal position and the substituents are selected
-from the group consisting of OH, Cl-C4alkoxy and alkoxyaLlcoxy containing at most
6 carbon atoms (subgroup If).
Preferred compounds within subgroup If are those in which R2 is hydrogen, foTmyl, acetyl
or methoxyace~yl (subgroup Ig).
An important subgroup of the compounds of formula I also comprises those compounds
wherein Rl is the carboxyl group -COR3 of an amino acid (subgroup Ih), more particularly
those in which R2 is hyArogen, formyl, acetyl or methoxyacetyl (subgroup Ii).
An important group of derivadves furthermore comprises those compounds of forrnula I
wherein Rl is hydrogen and R2 is a silyl group -SiR'R"R"', wherein R', R" and R"' are
identical or different substituents selected from Cl-C6alkyl and phenyl. On account of its
character, this group Ii merits special mention as a group of intermediates for
derivatisations in 11-posidon.
From among the particularly important individual compounds, the following compounds
may be cited:
-5-tert-butyldimethylsilyl-Soraphen C (No. 2)
- l l -methoxymethoxy-Soraphen C (No. 68)
-1 l-ethoxymethoxy-Soraphen C (No. 82)
-1 l-methylthiomethoxy-Soraphen C (No. 73)
-1 l-methoxyethoxymethoxy-Soraphen C (No. 94)
-11-a-tetrahydropyranyloxy-Soraphen C (No. 108/109)
-1 l-isopropoxy-Soraphen C (No. 13)
-1 l-allyloxy-Soraphen C (No. 23)
20%17Q~
- 7 -
-11-methoxy-a-ethoxy-SoraphenC (No. 104)
-11-isopropoxymethoxy-Soraphen C (No. 87)
-11-isopropoxy-oc-ethoxy-Soraphen C (No. 224)
Compounds of formula I can be derivatised in 5- or 11-position, starting from Soraphen C,
by methods which also fall within the scope of the invention.
The process for the preparation of compounds of formula I comprises subjecting
"Soraphen C", in the appropriate choice and order of the reaction steps, to etherification,
silylation, (thio)acetylation, aminalation, acylation, silyl ether cleavage and/or ester
cleavage.
Conventional acylation of an OH group with the appropriate (thio)carboxylic acid or with
an appropriate acyl halide, acyl anhydride or ester, or by reaction of the ~-OH group with
the suitably substituted silane derivative of formula
/R'
X-Si--R"
\R~
results in all those derivatives in which Rl and/or R2 are the group -COR3 or -COR4
respectively, wherein R3 and R4 each have one of the meanings given for formula I, or R2
is the -SiR'R"R"' group, and the term "acyl halide" denotes acyl chloride or acyl bromide,
and X is a silyl leaving group. Silyl leaving groups X typically include bromide, chloride
and trifluoromethanesulfonate. This recitation does not constitute a limitation. Further
typical silyl leaving groups are known to those skilled in the art. Suitable silyl groups
-SiR'R"R"' include trimethylsilyl, diphenyl-tert-butylsilyl, bis(isopropyl)methylsilyl,
triphenylsilyl, thexyldimethylsilyl and the like and, preferably, tert-butyl-dimethylsilyl.
O-Acylations and O-silylations are carried out in anhydrous medium, preferably in inert
solvents and, most preferably, in aprotic solvents. The reaction is conveniently run in the
temperature range from 0 to 80C, preferably from 10 to 50C. It is preferred to add an
organic base. Suitable bases are typically amines such as triethylamine,
triethylenediamine, triazole and, preferably, pyridine, imidazole or
, .
2~8~7~0
1,8-diazabicyclo[5.4.0]undec-7-ene (DBU).
Exempla~y of suitable solvents are: ethe}s and ethereal compounds including diaLkyl
ethers (diethyl ether, diisopropyl ether, tert-butylmethyl ether, dimethoxyethane, dioxane,
tetrahydrofuran, anisole, and the like); halogenated hydrocarbons such as chlorobenzene,
methylene chloride, ethylene chloride, chloroform, carbon tetrachloride,
tetrachloroethylene, and the like; or sulfoxides such as dimethyl sulfoxide, and aromatic or
aliphatic hydrocarbons such as benzene, toluene, xylene, petroleum ether, ligroin,
cyclohexane and the like may also be present. In some cases it can be advantageous to
carry out the reactions in an inert gas atmosphere (e.g. argon, helium, nitrogen, etc.) and/or
in absolute solvents.
If a free carboxylic acid is used as reactant for the acylation, then this reacdon will
preferably be calTied out in the presence of condensing agents, typically in the presence of
dicyclohexylcarbodiimide and pyridine or of dialkyl azodicarboxylates and
triphenylphosphine.
If acyl halides or acyl anhydrides are used for the acylation, then it is expedient to add a
neutralising agent. Suitable neutralising agents are tertiary amines such as trialkylamines,
pyridine or pyridine bases (e.g. 4-dimethylaminopyridine), which may also act as solvents.
Compounds of formula I which have a sugar residue attached in 11-position to the oxygen
atom are prepared by one of the linkage reactions employed in sugar chemistry, for
example by the Koenigs-Knorr synthesis, the Ag-triflate process, the orthoester process,
the phenylthio synthesis, the trichloroacetimidate process or the 2-pyridylthio synthesis.
A) In the Koenigs-Knorr synthesis or the silver triflate process, the 5-hydroxy group of the
compound of forrnula I (R2=H) can be obtained in the presence of a silver salt or mercury
salt as condensing agent with the sugar residue to be introduced, wherein all OH groups
are protected by e.g. acetylation, with the exception of chlorine- or bromine-substituted
1-OH groups, in the temperature range from -30C to +60C, preferably from -5C to
+30C, excluding light.
The protected 1-chloro- or l-bromo-sugar is added to the Soraphen in not less than
equimolar amount, but preferably in a 1.5- to 3-fold excess.
20817~0
Fresh1y disdlled Ag20 may be used as silver salt; however, it is preferred to use Ag2CO3
or CF3-COOAg. It is particularly prefer~ed to use silver trifluoromethanesulfonate
(=Ag-trillate = CF3-SO3Ag), in the presence of which glycosidation proceeds rapidly even
at minus temperatures. To activate the S-OH group and to neutralise the CF3-SO3H or
CF3-COOH which may be formed, it is useful to add a tertiary amine (triethylamine,
diisopropylethylamine, diazabicycloundecane etc.) to the reaction solution.
Instead of the silver salt, it is also possible to use mercury(I) cyanide or a combination of
HgO with either mercury(I) chloride or mercury(I) bromide (Helferich synthesis).
In a furth~er variant, the reaclivity in the l'-position of the sugar in which the glycosidic
linkage is to be formed and the further OH groups of which must be protected can be
enhanced by inidal conversion into the 1'-phenylthio derivadve and subsequent reacdon
with DAST (= diethylamino sulfur trifluoridc) in absolute dry dichlo~omethane (molecular
sieve) at +5C to -30C to give the l'-fluoro derivadve. More reactive than the l'-chloro or
l'-bromo derivative used in the Koenigs-Knorr synthesis, the l'-fluoro derivadve so
obtained of the sugar reactant can be linked to the S-hydroxy group of the Soraphen in the
presence of SnC12 and AgCI04 in a dry aprotic solvent such as diethyl ether in an inert gas
such as argon at ~5C to -30C (1. Am. Soc. 1984, 106, 4189-4192).
B) Further, the protected monosaccharide to be activated in l'-position can be converted
with 2,2'-dithiopyridine in d~ dichloromethane at -10C to +10C and in an inert gas
atmosphcre (e.g. argon) into the 1'-S-(2-pyridyl)-monosaccharide, which reacts readily
with the free l l-OH group of the Soraphen in the presence of Pb(CI04)2j AgS03CF3 or
AgCI04 as condensing agent, at room temperature in tetrahydrofuran (~) as solvent, to
form the glycosidic linkage (J. Org. Chem. 1983, 48, 3489-3493).
.
The protected monosaccharide which is acdvated in 1'-posidon as trichloroacetimidate can
also be reacted with Soraphen C in dry aprodc solvcnts with Lewis acids such as
(CH3)3SiOS02CF3 at -50C to +50C.
C) Glycosidic linkages can also be formed in the presence of Lewis acids such as AICI3,
AlBr3, SnC4, ZnCl2, BF3 (and especially the etherate), for which purpose in particular
acetylated sugars are very suitable (Chimia 21, 1967, pp. S37-538).
D) lt is also possible to form glycosidic linkages by the so-called orthoester method by
'
. ~ :
:
' ., ~ ~ '
20817~13
- 10-
reacting Sorophen with the protected sugar in which the glycosidic linkage is to be formed
in the presence of the orthoester of a lower alcohol whose one alcoholic component is the
sugar reactant.
The process for the preparation of Soraphen-l l-glycosides of formula I comprises in the
narrower sense reacting Soraphen C
a) in the presence of a silver salt or mercury salt as condensing agent with the sugar
residue to be introduced, all of whose OH groups are protected, with the exception of
the chlorine- or bromine-substituted anomeric l-OH groups in l-position, excluding
light and in the temperature range from -30C to +60C, preferably ~orm -5C to
+30C; or
b) in the presence of SnCI2 and AgCl04 as condensing agents with the sugar residue to
be introduced all of whose OH groups are protected, with the exception of the
fluorine-substituted anomeric l-OH groups in l-position, excluding light and in the
temperature range from -5C to ~30C;
followed by mild saponification of the hydroxyl protective groups at the sugar resldue.
The protectivc groups can normally be split off by mild saponification with e.g.NH3/methanol. Suitable solvents for this partial step are preferably anhydrous aprotic
representatives, conveniently dichloromethane, acetonitrile, benzene, toluene, nitro-
methane, dioxane, THF, ethylene glycol dimethyl ether. Diethyl ether is particularly
preferred.
The etherification of the 1 l-OH group is carried out in the free form or in thealkali-alcoholate form by organic halides or sulfates. If the OH group is in the free form,
the reaction will preferably be carried out in the presence of an acid acceptor such as an
alkali metal (hydrogen)carbonate or a tertiary amine such as trialkylamine, 2,6-di-tert-but-
ylpyridine or pyridine. Preferred halides are suitably bromides and iodides such as methyl
iodide, propargyl bromide, allyl bromide, cyclopropyl bromide, cyclopentyl bromide,
conveniently in the presence of catalytic amounts of NaI. Lower alkyl groups canconveniently be introduced as alkyl sulfates, typically dimethyl sulfat, diethyl sulfate and
others.
208171~
11 -
Suitable solvents are typically acetone, dimethyl formamide, pyridine, 2,6-di- ~
tert-butylpyridine or ethereal compounds such as tetrahydrofuran, diethyl ether,1,2-dimethoxyethane. The reaction temperatures are in the range from 0C to 10()C.
It may also be useful to add a halogen acceptor, suitably a silver salt such as AgNO3,
AgOSO2CF3 and the like.
If interfering functional groups such as OH, NH2, -COOH, are present in the molecule or
in the reactant, these can be masked at the outset, as already mentioned above, by
acetylation or the introduction of other protective groups and removed again from the final
product [T.W. Green "Protective Groups in Organic Synthesis", J. Wiley & Sons, 1981
(New York)].
Open or cyclic acetals (e.g. methoxymethyl ether or tetrahydropyranyl ether) can be
prepared on the one hand from the corresponding l-halo-l-alkoxymethanes (e.g.
chloromethyl methyl ether, bromomethyl benzyl ether, methoxyethoxy methyl chloride
etc.) in aprotic solvents such as ethers (e.g. diethyl ether, dimethoxyethane, dioxane,
tetrahydrofuran and the like), halogenated hydrocarbons (methylene chloride, chloroform,
chlorbenzene and the like); aliphatic and aromatic hydrocarbons (e.g. toluene, xylene,
hexane, petroleum ether and the like), nitriles (e.g. acetonitrile, propionitrile and the like),
esters (e.g. ethyl acetate, propyl acetate, butyl acetate and the like), ketones (e.g. acetone,
methyl ethyl ketone and the like), dimethyl sulfoxide, dimethyl formamide and others.
Addition of a base such as sodium hydride, triethylarnine, diisopropylamine, diisopropyl
ethylamine, pyridine, p-dimethylaminopyridine, potassium tert-butanolate and the like, is
useful. The reaction is carried out in the temperature range from -50 to 100C, preferably
from -20 to 25C. On occasion it is advisable to add a halogen acceptor such as a silver
salt (silver nitrate, silver triflate and others).
On the other hand, the acetals can be converted from the corresponding enol ethers such as
dihydropyran, methyl vinyl ether etc., in aprodc solvents (e.g. one of those cited above),
under acid catalysis, into the acetals. Suitable acids are typically anhydrous hydrochloric
acid, sulfuric acid, p-toluenesulfonic acid, pyridinium p-toluenesulfonate, phosphoroxy
chloride and the like, or else also polymers modified with acid groups such as
Amberlite H-15. The reaction can be carried out in the temperature range from -50C to
+100C, preferably from -10C to +30C.
208~7~0
- 12-
An alternative route of synthesis is transacetalation, wherein acetals such as di-
methoxyethane or diethoxymethane and the like are reacted with the correspondingalcohols under acid catalysis. The reaction can be calTied out in the acetal itself as solvent
or with the addition of aprotic solvents cited above. Suitable acid catalysts are phosphorus
pentoxide, hydrochloric acid, p-toluenesulfonic acid or conveniendy the catalysts
mentioned above. It can be useful to remove the alcohol formed from the reaction mixture
(by distillation, molecular sieve or the like). The reaction temperature is from -20 to
+100C.
For the other derivatisations of the Soraphen structure referred to above it is advisable to
use in general the following solvents or mixtures of solvents:
aliphatic and aromatic hydrocarbons such as benzene, toluene, xylenes, petroleum ether,
hexane; halogenated hydrocarbons such as chlorobenzene, methylene chloride, ethylene
chloride, chloroform, carbon tetrachloride, tetrachloroethylene; ethers and ethereal
compounds such as dialkyl ethers (diethyl ether, diisopropyl ether, tert-butylmethyl ether
and the like), anisole, dioxane, tetrahydrofuran; nitriles such as acetonitrile, propionitrile;
esters such as ethyl acetate, propyl acetate or butyl acetate; ketones such as acetone,
diethyl ketone, methyl ethyl ketone; dimethyl sulfoxide (DMSO); dimethyl formamide
(DMF) and others.
The recitation of all the methods set forth above does not constitute any limitation. If
desired, the final products can be purified in conventional manner by washing, digestion,
extraction, recrystallisation, chromatography and the like.
The syntheses described above including all partial steps likewise fall within the scope of
this invention.
It has been found that the compounds of formula I have, for practical purposes, a very
advantageous biocidal spectrum against phytopathogenic microorganisms, especially
against fungi. They have very useful curative, systemic and, in particular, preventive
properties, and are used for protecting numerous cultivated plants. The compounds of
formula I can be used to inhibit or destroy the pests which occur on plants or parts of
plants (fruit, blossoms, leaves, stems, tubers, roots) in different crops of useful plants,
while at the same time the parts of plants which grow later are also protected from attack
by phytopathogenic microorganisms.
20~17~0
- 13 -
The compounds of formula I are effective microbicides against the phytopathogenic fungi
belonging to the following classes: Fungi imperfecti (e.g. in particular Botrytis and also
Pyricularia, Helminthosporium, Fusarium, Septoria, Cercospora, and Alternaria);
Basidiomycetes (e.g. Rhizocotonia, Hernileia, Puccinia). They are also effective against
the class of the Ascomycetes (e.g. Venturia and Erysiphe, and also Podosphaera,
Monilinia and Uncinula), and of the Oomycetes (e.g. Phytophthora, Plasmopara). The
compounds of formula I can also be used as dressing agents for protecdng seeds (fruit,
tubers, grains) and plant cuttings against fungus infections as well as against
phytopathogenic fungi which occur in the soil.
The invention also relates to compositions which contain the compounds of formula I as
active components, in particular plant-protective compositions and to the use thereof in the
field of agriculture or related fields. The invention further relates to a method of treating
plants, which comprises applying thereto the compounds of formula I or the novelcompositions which contain them.
Target crops to be protected within the scope of the present invention typically comprise
the following species of plants:
cereals (wheat, barlcy, rye, oats, rice, maize, sorghum and related species), beet (sugar
beet and fodder beet), pomes, drupes and soft fruit (apples, pears, plums, peaches,
almonds, cherries, strawberries, raspberries and blackberries), leguminous plants (beans,
lentils, peas, soybeans), oil plants (rape, mustard, poppy, olives, sunflowers, coconut,
castor oil plants, cocoa beans, groundnuts), cucumber plants (cucumber, marrows,melons), fibre plants (cotton, flax, hemp, jute), citrus fruit (oranges, lemons, grapefruit,
mandarins), vegetables (spinach, lettuce, asparagus, cabbages, carrots, onions, tomatoes,
potatoes, paprika), lauraceae (avocados, cinnamon, camphor), or plants such as tobacco,
nuts, coffee, pineapples, sugar cane, tea, pepper, vines, hops, bananas and natural rubber
plants, as well as ornamentals (composites). This recitation constitutes no limitation.
The compounds of formula I are normally applied in the form of compositions and can be
applied to the crop area or plant to be treated, simultaneously or in succession, with further
compounds. These further compounds can be both fertilisers or micronutrient donors or
other preparations that influence plant growth. They can also be selective herbicides,
insecticides, fungicides, bactericides, nematicides, mollusicides or mixtures of several of
these preparations, if desired together with further carriers, surfactants or application
.
- ` 2~81~
promoting adjuvants customarily employed in the art of formulation. Application
promoting adjuvants are vegetable oils such as cottonseed oil, castor oil, flax oil,
rape-seed oil, olive oil, sunflo~.ver oil, maize gerrn oil, sesame oil and the like.
Suitable carriers and adjuvants can be solid or liquid and correspond to the substances
ordinarily employed in formulation technology, e.g. natural or regenerated mineral
substances, solvents, dispersants, wetting agents, tackifiers, thickeners, binders or
fertilisers.
A preferred method of applying a compound of forrnula I, or an agro-chemical
composition which contains at least one of said compounds, is foliar application. The
number of applications and the rate of application depend on the risk of infestation by the
corresponding pathogen. However, the compound of formula I can also penetrate the plant
through the roots via the soil (systemic action) by drenching the locus of the plant with a
liquid formulation, or by applying the compounds in solid form to the soil, e.g. in granular
form (soil application). The compounds of formula I may also be applied to seeds(coating) by impregnating the seeds either with a liquid formulation containing a
compound of formula 1, or coating them with a solid formulation.
The compounds of formula I are used in unmodified form or, preferably, together with the
adjuvants conventionally employed in the art of formulation, and are therefore formulated
in known manner to emulsi~lable concentrates, coatable pastes, directly sprayable or
dilutable solutions, dilute emulsions, wettable powders, soluble powders, dusts,granulates, and also encapsulations in e.g. polymer substances. As with the nature of the
compositions, the methods of application, such as spraying, atomising, dusting, scattering,
coating or pouring, are chosen in accordance with the intended objectives and the
prevailing circumstances. Advantageous rates of application are normally from 5 g to
500 g of active ingredient (a.i.) per hectare, preferably from 50 g to 200 g a.i./ha. For
treating plant propagation material, the rates of application are 1 g to 100 g active
ingredient/100 kg of plant material, typically seeds.
The formulations, i.e. the compositions containing the compound (active ingredient) of
formula I and, where appropriate, a solid or liquid adjuvant, are prepared in known
manner.
Suitable solvents are: aromatic and aliphatic hydrocarbons, e.g. xylene mixtures,
-- ` 20817~
- 15 -
cyclohexane or paraffins; also alcohols and glycols and their ethers and esters, such as
ethanol, ethylene glycol, ethylene glycol monomethyl or monoethyl ether and acetates;
ketones such as cyclohexanone, strongly polar solvents such as N-methyl-2-pyrrolidone,
dimethyl sulfoxide or dimethyl formamide, as well as vegetable oils or epoxidised
vegetable oils such as epoxidised coconut oil or soybean oil; or water.
The solid carriers used e.g. for dusts and dispersible powders, are normally natural mineral
fillers such as calcite, talcum, kaolin, montmorillonite or attapulgite. In order to improve
the physical properties it is also possible to add highly dispersed silicic acid or highly
dispersed absorbent polymers. Suitable granulated adsorptive carriers are porous types, for
example pumice, broken brick, sepiolite or bentonite; and suitable nonsorbent ca~iers are
materials such as calcite or sand. In addition, a great number of pregranulated materials of
inorganic or organic nature can be used, e.g. especially dolomite or pulverised plant
residues.
Depending on the nature of the compound of formula I to be formulated, suitable
surface-active compounds are non-ionic, cationic and/or anionic surfactants having good
emulsifying, disper8ing and wetting properties. The term "surfactants" will also be
understood as comprising mixtures of surfactants.
Suitable anionic surfactants can be both water-soluble soaps and water-soluble synthetic
surface-active compounds.
More frequently, however, so-called synthetic surfactants are used, especially fatty
sulfonates, fatty sulfates, sulfonated benzimidazole derivatives or aLIcylsulfonates.
Nonionic surfactants are polyglycol ether derivatives of aliphatic or cycloaliphatic
alcohols, or saturated or unsaturated fatty acids and alkylphenols, said derivatives
containing 3 to 30 glycol ether groups and 8 to 20 carbon atoms in the (aliphatic)
hydrocarbon moiety and 6 to 18 carbon atoms in the alkyl moiety of the alkylphenols.
Further examples of nonionic surfactants are nonylphenolpolyethoxyethanols, castor oil
polyglycol ethers, polypropylene/polyethylene oxide adducts, tributylphenoxypoly-
ethyleneethanol, polyethylene glycol and octylphenoxypolyethoxyethanol. Fatty acid
esters of polyoxyethylene sorbitan, e.g. polyoxyethylene sorbitan trioleate, are also
suitable nonionic surfactants.
.. . .
20817~
- 16-
Further surfactants customa~ily employed in formulation technology are known to those
skilled in the art or may be found in the relevant literature.
The agrochemical formulations usuall~ contain 0.1 to 95 % by weight of a compound of
formula I, 99.9 to 5 % by weight of a solid or liquid adjuvant, and 0 to 25 % by weight of
a surfactant.
Whereas commercial products will preferably be formulated as concentrates, the end user
will normally employ dilute formulations.
The compositions may also contain further auxiliaries such as stabilisers, antifoams,
viscosity regulators, binders, tackifiers as well as fertilisers or other chemical agents for
obtaining special effects.
The inventdon is illustrated in more detail by the following non-limitative Examples.
Preparation of the compounds of formula I
Example Pl: PreParation of S-tert-butvldimethvlsilvl-Soraphen C (comPound 2)
15.0 g of Soraphen C are dissolved in 50 ml of dimethyl formamide and to the solution are
added at 0C 17.8 g of tert-butyldimethylsilylchloride and 8.05 g of imidazole. After
stirring for 16 h at room temperature, the reaction mixture is poured into water and
extracted with 3 x 50 ml of ethyl acetate. The organic phase is washed with
lN hydrochloric acid and a saturated solution of sodium chloride, dried over sodium
sulfate and concentrated. Chromatography on silica gel with ethyl acetate/hexane (9:1)
gives 14.10 g (65 %) of 5,1 l-di-tertbutyldimethylsilyl-Soraphen C. This compound is
stirred for 5 hours at -20C to -10C in a solution of hydrogen fluoride/water/acetonitrile
(2:3:95). The reaction mixture is poured into a saturated solution of NaHCO3 andextracted with 4 x 20 ml of ethyl acetate. The organic phase is washed with a saturated
soludon of NaHCO3 and a saturated solution of sodium chloride (= brine), dried over
sodium sulfate and concentrated. Chromatography on silica gel with ethyl acetate/hexane
(1:3 to 1:1) gives 2.92 g (21 %) of educt and 8.74 (73 %) of 5-tert-butyldi-
methylsilyl-Soraphen C.
` ` 208~7~0
- 17-
Example P2: Preparation of 5-tert-butvldimethYlsilvl-l l-ethoxY-Soraphen C
(compound 11~
52.4 mg of compound 2 are dissolved in 2 ml of methylene chloride and to the solution are
added 180.9 mg of 1,8-bis(dimethylarnino)naphthalene. After addition of 54.2 ml of ethyl
trifluormethanesulfonate, the reaction mixture is sti~red for 18 hours at room temperature.
Then 2 ml of triethylamine are added and stirring is continued for a further 10 minutes.
The reaction mixture is taken up in ether and the ether solution is washed with
lN hydrochloric acid, a saturated solution of NaHCO3 and a saturated solution of sodium
chloride, dried over sodium sulfate and concentrated. Chromatography on silica gel with
ethyl acetate/hexane (1:3) as eluant gives 31.4 mg of the tide compound.
Example P3: Preparation of 1 l-ethoxY-SoraPhen C tcompound 10)
15 mg of compound 11 are stirred for 2 days in a solution of hydrogen
fluoride/water/acetonitrile (2:3:95) and the mixture is then taken up in ethyl acetate. The
ethyl acetate solution is washed with a saturated solution of sodium hydrogencarbonate
and a saturated solution of sodium chloride, dried over sodium sulfate and filtered. The
filtrate is concentrated and the residue is chromatographed on silica gel with ethyl
acetate/hexane (S:l) as eluant, giving 7.6 mg of 1 l-ethoxy-Soraphen C.
ExamDle P4: PreParadon of 5-tert-butvldimethYlsilYl-l l-allYloxv-SoraPhen C
(compound 24~
52 mg of compound 2 are dissolved in 1 ml of tetrahydrofuran and to the solution are
added 10 mg of sodium hydride and 0.2 ml of allyl iodide. The reasction mixture is stirred
for 2 hours at room temperature and then taken up in 5 ml of ethyl acetate, washed with
lN hydrochloric acid and a saturated solution of sodium chloride, dried over sodium
sulfate and filtered. The fi1trate is concentrated and the residue is chromatographed on
silica gel with ethyl acetate/hexane (1:5 to 1:1), giving 32.2 mg of the title compound.
Example P5: E'reParation of 1 l-allvloxv-SoraPhen C (compound 23)
32.2 mg of compound 24 are stirred for 2 days at~ room temperature in 2 ml of a solution of
7.8 g of 70 % hydrofluoric acid (dissolved in pyridine) in 18.8 ml of pyIidine and 60 ml of
tetrahydrofuran. The reaction mixture is taken up in ethyl acetate and the solution is
washed with lN hydrochloric acid and a saturated solution of sodium chloride, dried over
sodium sulfate and filtered. The filtrate is concentrated and the residue is
chromatographed on silica gel with ethyl acetate/hexane (2:3 to 2:1) as eluant, giving
20817~
- 18-
11.6 mg of the title compound.
Example P6: Preparation of 5-tert-butvldimethvlsilvl-1 1-benzvl-Soraphen C
(compound 44)
534 1ll of 2,6-di-tert-butylpyridine and 207 mg of silver trifluoromethanesulfonate are
dried under a high vacuum and 50 mg of compound 2 and 96 ~LI of benzyl bromide are
added in succession under argon. After 2 minutes, ethyl acetate is added and the sus-
pension is filtered. The filtrate is washed with lN hydrochloric acid, then successively
with a saturated solution of sodium hydrogencarbonate and with brine, dried over sodium
sulfate and filtered. The filtrate is concentrated and the residue is chromatographed on
silica gel with ethyl acetate/~exane (1:10) as eluant, giving 30.9 mg of the title compound.
Example P7: Preparation of 5-tert-butYldimethvlsilYl-1 1-methoxymethoxY-Soraphen _
(compound 69)
60.8 mg of compound 2 are dissolved in 2 ml of methylene chloride and to the soludon are
added 0.3 m1 of diisopropyl e~hylamine and 0.2 ml of bromomethyl methyl ether. The
mixture is stirred for 16 hours at room temperature and then taken up in ethyl acetate. The
ethyl acetate solution is washed with lN hydrochloric acid, then successively with a
saturated solution of sodium hydrogencarbonate and with brine, dried over sodium sulfate
and filtered. The filtrate is concentrated and the residue is chromatographed on silica gel
with ethyl acetate~exane (1:3 to 1:1) as eluant, giving 39 mg of the title compound.
xamDle P8: PreParation of 11-methoxvmethoxY-Soraphen C (com~Pound 68~
30,1 mg of compound 69 are stilred for 4 days at room temperature in 2 ml of a solution
consisting of 7.8 g 70 % hydrofluoric acid (dissolved in pyridine ) in a mixture of 18.8 ml
of pyridine and 60 ml of tetrahydrofuran. The reaction mixture is taken up in ethyl acetate
and the solution is washed with lN hydrochloric acid and a saturated solution of sodium
hydrogencarbonate, dried over sodium sulfate and filtered. The filtrate is concentrated and
the residue is chromatographed on silica gel with ethyl acetate/hexane (1:2 to 1:1) as
eluant, giving 19.9 mg of the title compound.
Example P9: Preparation of 5-tert-butvldimethYlsilvl-1 l-methYlthiomethoxv-Soraphen C
(compound 74)
162 mg of compound 2 are concentrated for 36 hours at room temperature in a solution of
1 ml of dimethyl sulfoxide and 1 ml of acetic anhydride. The mixture is concentrated to
dryness under a high vacuum and the residue is taken up in ethyl acetate. The ethyl acetate
20817~0
- 19-
solution is washed with a saturated solutdon of sodium hydrogencarbonate and brine, dried
over sodium sulfate and filtered. The filtrate is concentrated and the residue is
chromatographed on silica gel with ethyl acetate/hexane (1:4 to 1:2) as eluant, giving
61.1 mg of the title compound.
Example P10: PreParadon of 5-tert-butvldimethYlsilvl- 11-(0-
tetraacetvl lucosidvl-Soraphen C (compound 134~
51.1 mg of compound 2 are dissolved with 370 mg of l-thiopyridylacetyl glucose in 9 ml
of toluene. 5 g of molecular sieve (4 ~) and 140 mg of silver trifluoromethanesulfonate
are added. The reactdon mixture is sdrred for 16 hours at room temperature, then charged
to silica gel and chromatographed with ethyl acetat/hexane (1:1), giving æ mg ofcompound 134.
Example Pl 1: Preparadon of Soraphen C- 11-maleate (comPound 181)
To 100 mg (0.197 mmol) of Soraphen C in 3 ml of dichloromethane are added 149 mg(1.519 mmol) of maleic anhydride and 171.5 mg (1.52 mmol) of 2,4,6-collidine, and the
mixture is thereaher sdrred at 40C for 3 hours. For working up the reactdon mixture is
hydrolysed with lN hydroch10ric acid and extracted with ethyl acetate. The combined
organic phases are then washed with lN hydrochloric acid and a saturated solution of
sodium chloride and dried over sodium sulfate. The crude product is purified by PSC or by
column chromatography. The resultant Soraphen C-5,11-dimalea~e is dissolved in 1 rrll of
methanol and thereafter treated with 1 ml of an anhydrous soludon of methanol inammonia (2 mmol NH3tml). The progress of the reaction is monitored by thin-layerchromatography and discontinued after c. 4-8 hours before substandal amounts of
Soraphen C have formed. The reaction mixture is acidified with lN hydrochloric acid and
extracted with ethyl acetate. The combined organic phases are once more acidified with
lN hydrochloric acid and extracted with a saturated soludon of sodium chloride. The
crude rnixture is purified by preparadve layer chromatography (eluant: dichloromethane-
/methanol 90:10, 1 % acetic acid, vtv), and then over Sephadex LH-20 (height of
column: 30 cm, column diameter: 3 cm; eluant: methanol), to give 32 mg of
compound 181.
Example P12: Preparadon of 11-0-tetrahvdropvranvl-Soraphen C (compound 108/109)
935 mg (1.846 mmol) of Soraphen C are dissolved in 10 ml of ethyl acetate and to the
solution are added 337 ,ul (3.691 mmol) of 3,4-dihydro-2H-pyran and 3 mg (0.016 mmol)
of p-toluenesulfonic acid (PTS) as catalyst. The reacdon mixture is stiIred for 30 minutes
208~7~
- 20 -
at room temperature. The reaction solution is then diluted with water, and the phases are
separated. The aqueous phase is extracted with ethyl acetate. The organic phase is washed
with a saturated solution of sodium hydrogencarbonate and with a saturated solution of
sodium chloride. The combined organic phases are dlied over sodium sulfate. The crude
product is purified by preparative column chromatography (silica gel 60, eluant:dichloromethane/methanol, l99:1, v/v). Two mixtures of
5, l l-di-O-tetrahydropyranyl-Soraphen C isomers as well as the two isomers of
l l-O-tetrahydropyranyl-Soraphen C (108/109) can be isolated.
Example P13: Preparadon of 5-formvl-SoraPhen C (compound 1) and
l l-formvl-Soraphen C (comPound 147)
40 mg (75 ~mol) of Soraphen C, 10 mg (80 ~,Imol) of 4-dimethylaminopyIidine, 3.8111
(4.6 mg, 100 ~mol) of formic acid and 41111 (30 mg, 300 ~mol) of triethylamine are
dissolved in 4 ml of dry dichloromethane. The solution is cooled to - 15C, then 7.6 ~
(8,2 mg, 801,1mol) of acetic anhydride are added through a septum and the mixture is
stirred for 30 minutes at this temperature. The coo1ing bath is then removed and stilTing is
continued for another 30 minutes. A few drops of methanol are added to the reaction
mixture, which is concentrated under vacuum. The residue is acidified with
lN hydrochloric acid and extracted twice with ethyl acetate. The combined organic phases
are washed with a 5 % solution of NaHCO3 and with a concentrated solution of NaCl and
dried over sodium sulfate, giving 43 mg of crude product which is purified by PSC. In
addidon to 11 mg of compound 1, 4.5 mg (8 ,umol, 11 %) of compound 147 are also
isolated.
Example P14: PreParation of 5.11-diformYl'SoraPhen C (compound 148)
42 mg (83 ~Imol) of Soraphen C, Z3 mg (190 ~mol) of 4-dimethylaminopyridine,17 111
(21 mg, 450 ~mol) of formic acid and 134 ~,11 (97 mg, 960 ~,lmol) of triethylamine are
dissolved in 3 ml of dry dichloromethane. The solution is cooled to -15C, then 36111
(39 mg, 380 ~mol) of acetic anhydride are added through a septum and the mixture is
stirred for 30 minutes at this temperature. The cooling bath is then removed and stirring is
continued for another 30 minutes. A few drops of methanol are added to the reaction
mixtwre, which is concentrated under vacuum. The residue is acidified with
lN hydrochloric acid and extracted twice with ethyl acetate. The combined organic phases
are washed with a 5 % solution of NaHCO3 and with a concentrated solution of NaCl and
dried over sodium sulfate, giving 47 mg of compound 148, which spectroscopic analysis
shows to be homogeneous.
;
2 ~$ n~ 3 0
- 21 -
Example P15: Preparation of 11-formvl-Soraphen C (compound 147)
27.0 mg (48 llmol) of compound 148 (Example 14) are dissolved in 1 ml of methanol and
to the solution are added 10 ~,11 of a 25 ~O solution of ammonia. The reaction is monitored
by thin-layer chromatography. After 10 minutes the reaction solution is concentrated
under vacuum, the residue is acidified with lN hydrochloric acid and extracted twice with
ethyl acetate. The combined organic phases are washed with a 5 % solution of NaHCO3
and with a concentrated solution of NaCI and dried over sodium sulfate, giving 23 mg of
compound 147, which is purified by PSC.
Example P16: Preparation of 5.11-diacetvl-Soraphen C (compound 152~ and5-acetYI-Soraphen C (compound 3)
50 mg (98.5 ~lmol) of Soraphen C are dissolved in 0.5 ml of dry pyridine and to the
solution are added 0.3 ml (325 mg, 3.2 mmol) of acetic anhydride and the mixture is
stirred for 1 hour at room temperature. The reaction mixture is acidified with
lN hydrochloric acid and extracted twice with ethyl acetate. The combined organic phases
are washed with a 5 % solution of NaHCO3 and with a concentrated solution of NaC1 and
dried over sodium sulfate, giving 68 mg of crude product which is purified by PLC
(column size: 239 cm2, column material: Lichroprep Si60, 15-25 ~lm, Merck Art. 9336;
eluant: dichloromethane/mcthanol 99:1, v/v; flow: 6 ml/min; pressure 12 bar; Rt 152 =
6.5 min; R, 3 = 13 min).
Yield: 34 % (33 mg, 56 ,umol) of compound 152
9 % (5 mg, 9 ~mol) of compound 3.
Where present, a silyl group in 5-position (e.g. compounds 24, 44, 69 etc.) can be split off
according to the general procedure of Examples 3, 5 or 8, to give the corresponding
Soraphen C derivatives.
ExamPle P17: PreParation of 5-tert-butYldimethYlsilYI-l l-methioninYI-Soraphen C(comPound 21
50.9 mg of compound 2 together with 24.5 mg of N-BOC-methionine, 22.7 mg of dicyclo-
hexylcarbodiimide and a trace of 4-dimethylaminopyridine are dissolved in 2 ml of
methylene chloride and the solution is stiIred for 3 hours at room temperature. The
mixture is taken up in ethyl acetate and the solution is washed in succession with
lN hydrochloric acid, a saturated solution of sodium sodium hydrogencarbonate and brine,
dried over sodium sulfate and concentrated. Chromatography of the residue on silica gel
2~ 7~ `
wilh ethyl acetate/hexane (1:3) as eluant gives the compound protected at the arnine.
44 mg of this compound are dissolved in 7 ml methylene chloride and the solution is
acidified with 1 ml of formic acid. After stirring for 6 hours at room temperature, the
mixture is taken up in in ethyl acetate and the solution is washed with a saturated solution
of sodium hydrogencarbonate and brine, dried over sodium sulfate and concentrated.
Chromatography of the residue on silica gel with methylene chloride/acetone (8:2) gives
41.3 mg of compound 211.
The following Soraphen C derivatives of forrnula I can be prepared in accordance with the
foregoing Examples or by one of the methods described hereinabove.
OR1
~CH3
OCH3 J~CH3 (I)
~ ~L~OR2
CH3 OCH3
Herein
Si-tBu-d = tert-butyldimethylsilyl
~OH
~0
glucosyl = ~ (either a- or ,B-forrn)
HO
OH
~ OAc
~0
acetyl glucosyl = ~ (either a- or ,B-form)
AcO
OAc
20817~
- 23 -
Table
No. Rl R2
H CHO
2 H Si-tBu-d
3 H COCH3
4 H COCH2OCH3
H Si(CH3)3
6 H Si(CH3)2-thexyl
7 H Si(C6Hs)2(t-butyl)
8 H cO-nCsH
9 H COCCl3
C2Hs H
11 C2Hs Si-tBu-d
12 C2Hs COCH3
13 i-C3H7 H
14 i~C3H7 Si-tBu-d
n-c3H7 H
16 n-c3H7 CHO
17 n-Bu H
18 n-Bu Si-tBu-d
19 n-Bu COCH2OCH3
t-C4Hg H
21 t-C4Hg Si-tBu-d
22 t-C4Hg CO-nCs
23 allyl H
24 allyl Si-tBu-d
allyl Si(CH3)2thexyl
26 allyl COCH2OCH3
27 -CH2CH=CHCH2CH2CH3 H
28 -CH2CH=CHCH2CH2CH3 Si(CH3)2t-Bu
29 -CH2CH2CH2CH2CH=cH2 H
-CH2C_CH H
.
.
20817~0
- 24 -
Nr. Rl R2 -
31 -CH2C-CH Si-tBu-d
32 -CH2C~CCH2CH3 H
33 -CH2CH2CH2C_CCH3 H
34 -C6H5 H
-C6Hs Si-~?.u-d
36 cyclopropyl H
37 cyclopropyl Si-d3u-d
38 cyclohexyl H
39 cyclopentyl H
cyclopentyl Si(CH3)3
41 n-C6Hl3 H
42 n-C6Hl3 Si-tBu-d
43 -CH2c6Hs H
44 -CH2c6Hs Si-tBu-d
-CH2c6Hs COCF3
46 -CH2COOH H
47 -cH2cH2NH2 H
48 -CH2C6H4t2-~O2) H
49 -CH2-C6H4(4-OCH3) H
-CH2-C6H4(3-CF3) H
51 -C6H4(3-OH) H
52 -CH2-C6H3(2,6-dimethyl) H
53 -CH2-C6H4(3-Br) H
54 -CH2-C6H4(4F) H
CH2CF3 H
56 CH2CF3 Si-tBu-d
57 CH2CH2Br H
58 CF3 H
59 CF3 Si-tBu-d
CF2CF3 H
61 CF2CHF2 H
62 CHF2 H
63 CHF2 Si-t-Bu-d
..
,
'
-
2~817~0
- 25 -
Nr. Rl R2 ~
.
64 CHF2 CHO
CHF2 COCH2OCH3
66 CH2CH(OH)phenyl H
67 (cF2)scF3 H
68 CH2OCH3 H
69 CH2OCH3 Si-tBu-d
CH2OCH3 CHO
71 CH2OCH3 COCH3
72 CH2OCH3 Si(CH3)2-thexyl
73 CH2SCH3 H
74 CH2SCH3 Si-tBu-d
CH2SCH3 CHO
76 CH2SCH3 COCH2OCH3
77 CH2N(CH3)2 H
78 CH2N(CH3)2 Si-tBu-d
79 CH2N(CH3)(C6H~3)(n) H
CH2NHCH3 H
81 CH2NHCH3 Si-tBu-d
82 CH2OC2Hs H
83 CH2Oc2Hs Si-tBu-d
84 CH2OnC6Hl3 H
CH2O-t-but. H
86 CH2O-t-but. Si-tBu-d
87 CH2O-i-prop. H
88 CH2O-i-prop. Si-tBu-d
89 CH2OCH2phenyl H
CH2OCH2Phenyl Si-tBu-d
91 CH2OCOCH3 H
92 CH20COCH3 Si-tBu-d
93 CH2OCOCH3 COCH3
94 CH2OCH2CH2OCH3 H
CH20CH2CH20CH3 Si-tBu-d
96 CH2OCH2CH2OCH3 CHO
20817~
- 26 -
Nr. Rl R2 ~
97 CH20CH2CH20CH3 COCH20CH3
98 CH2OCH3 COCH2OCH3
99 CH20CH2CH20CH3 COCH3
100 CH2CH2OCH2CH2OCH3 H
101 CH2CH2OCH2CH2OCH3 Si-tBu-d
102 CH2CH2O(CH2CH2O)2CH3 H
103 CH2CH2O(CH2CH2O)2CH3 Si-tBu-d
104 -CH(CH3)0CH3 H
105 -CH(CH3)0CH3 Si-tBu-d
106 2-tetrahydrofuryl H
107 2-tetrahydrofuryl Si-tBu-d
108 2-tetrahydropyranyl isomer A H
109 2-tetrahydropyranyl isomer B H
110 2-tetrahydropyranyl Si-tBu-d
111 2-tetrahydropyranyl COCH3
112 2-tetrahydrothienyl H
113 2-tetrahydrothiopyranyl H
114 -CH(nC4Hg)OCH3 H
115 -CH(CH3)OnC6HI3 H
116 -c(cH3)2ocH3 H
117 -c(cH3)2ocH3 Si-tBu-d
118 -CH2OCH(cH2OcH3)2 H
119 -CH2O-C6Hs H
120 -CH2O-C6Hs Si-tBu-d
121 -CH2O-C6H4(4-OCH3) H
122 -CH2O-C6H4(2-NO2) H
123 CH2O-C6H4(3-F) H
124 CH2-O-C6H3(2,6-dimethyl) H
125 CH2OCH2CH2OH H
126 CH2OCH2CH2OH Si-tBu-d
127 glucosyl ,3-Fonn H
128 glucosyl a-Folm H
129 glucosyl a-Form Si-tBu-d
.
208~7~0
- 27 -
Nr. Rl R2
130 glucosyl ,B-Form Si-tl3u-d
131 acetyl glucosyl a-form H
132 acetyl glucosyl ,B-form H
133 acetyl glucosyl a-form Si-tBu-d
134 acetyl glucosyl ,B-forrn Si-tBu-d
~OH
HO; O
135 ~OH >--~-form H
OH
OAc
AcOLo
136 I~OAC >--3-form H
OAc
OAc
137 l ) ,B-form ~ Si-tBu-d
138 J ~ ~ l H
Ac
OH
Lo
139 ~ ,B-form H
HO
140 acetyl glucosyl ,B-form -COCH3
~ OAc
)--O
141 ~ ,B-form -COCH3
AcO
-` 20817G~
- 28 -
Nr.Rl R2
lOAco
AcO--/
OAc
142 ~ forrn -COCH3
OAc
~OAco
AcO V
\ OAc
143 \ /,B-form Si-tBu-d
OAc
Ac
AcO--/O\
144 ~ ,B-form H
OAc
OH
\
145 \~ ,B-form . Si-tBu-d
OH
V
146 \~ H
OH
147 -CHO H
148 -CHO ClHO
149 -CHO Si-tBu-d
150 -CHO COCH3
151 -COCH3 H
152 -COCH3 COCH3
153 -COCH3 CHO
154 -COCH3 Si-tBu-d
155 -COC2Hs H
156 -COt-but. H
20817~0
- 29 -
Nr. Rl R2
157 -COnC6HI3 H
158 -COCH2OCH3 H
159 -COCH2OCH3 COCH2OCH3
160 -COCH2OCH3 Si-tBu-d
161 -COCH2OCH3 Si(Ph)2t-but.
162 -COCH2CH2COOH H
163 COCH2CH2COOH Si(CH3)2-thexyl
164 -coc6Hs H
165 -COC6Hs Si-tBu-d
166 -COC6Hs -coc6H5
167 -COC6H4~4-OCH3) H
168 -CO-C6H4(2-NO2) H
169 -CO-C6H4(3-CF3) H
170 -CO-C6H4(3-OH) H
171 -COCF3 H
172 -COCF3 COCF3
173 -COCF3 Si-~Bu-d
174 -COCCI3 H
175 -COCCI3 COCCI3
176 -COCC13 Si-~Bu-d
177 -COi-prop. H
178 -COCH2SCH3 H
179 -COCH2O ~ H
180 `-COCH2O~ Si-tBu-d
181 -CO COOH H
182 -CO COOH Si-tBu-d
20817~0
- 30 -
Nr. Rl R2 ~
CO
183 \=~ H
COOH
184 -CO COOH COCH3
185 -CO~
186 CO-(CH2)4-CH=CH2 H
187 CO-C--CH H
188 CO-C-CH Si-tBu-d
189 CO-ICH2)4-C=CH H
190 -CO-cyclopropyl H
191 -CO-cyclopropyl Si-~Bu-d
192 -CO-cyclohexyl H
193 -CO-cyclohexyl Si-tBu-d
194 -CO-cyclohexyl COCH3
195 -CO-benzyl H
196 -CO-benzyl Si-tl3u-d
197 -CO-CH2-C6H4(4-OCH3) H
198 -CO-CH2-C6H4(2-NO2) H :
199 -CO-CH2-C6H4(4-OCH3) Si-tBu-d
200 -co-cH2-c6H4(4-cF3)
201 -CO-CH2-C6H3(2,4-dimethyl) H
202 -COCH2CH2N(CH3)2 H
203 -COCHF2 - H
204 -COCF2Cl H
205 -COCF2Cl Si-tl3u-d
206 COC3F7 H
207 COC3F7 Si-tBu-d
o
208 J~NH2 H
CH3
~' -' -' '
~ . . -, .
... '
.
- ,:
20817~
- 31 -
Nr. Rl R2
209 ~I~NH2 Si-tBu-d
O CH3
J~, NH2
210 ~ H
SMe
J~, NH2
211 l Si-tBu-d
SMe
J~, NH2
212 ~ H
O ~
J~NH2
213 ~ Si-tBu-d
o
214 J~NH2 H
OH
208i7~
- 32 -
Nr. Rl R2
215 J~NH2 Si-tBu-d
OH
O
216 J~,NH2 ~ H
217 ~ J Si-tBu-d
NH2
O
218
~ NH2 ¦-
219 ~ J Si-tBu-d
COOH
O
220 J~, NH2 l H
221 1~< J Si-tBu-d
222 CH2OC4Hg(n) Si-tBu-d
223 CH20C4Hg(n) H
224 CH(CH3)0C3H7(i) Si-tBu-d
225 CH(CH3)0C3H7(i) H
226 CH(CH3)0C3H7(i) -CHO
227 C(CH3)2OC3H7ti) Si-tBu-d
228 C(CH3)2OC3H7(i) H
229 CH(CH3)OC3H7(n) Si-tBu-d
230 CH(CH3)OC3H7(n) H
231 CH(CH3)0C4Hg(sec) Si-tBu-d
232 CH(CH3)0C4Hg(sec) H
233 CH(CH3)0C4Hg(sec) -COCH3
--` 208~7~
- 33 -
Nr. Rl R2
234 CH(CH3)0C4Hg(tert.) Si-tBu-d
235 CH(CH3)0C4Hg(tert.) H
236 CH(CH3)0CH(CH3)CH2-OCH3 Si-tBu-d
237 CH(CH3)0CH(CH3)CH2-OCH3 H
238 CH(CH3)0CH(CH3)CH2-OCH3 -COCH20CH3
239 CH2SC3H7(i) Si-tBu-d
240 CH2SC3H7(i) H
241 CH2SC3H7(i) -CH0
20817~
- 34-
Table 2 (Physico-chemical data of some Soraphen C derivatives)
? = assignment uncertain
Individual
exchanged
MS lH-NMR proton from
No. M+ Solv.* 2-H 4-H 5-H l l-H R2 Rf (solv)~*
533 (M+-H) A 3.14 3.14 5.21 4.16 8.13 0.34 (1)
2 621 (M+-H) B 2,96 3.10 4.38 4.08 0.94 0.39 (2)
3 547 (M~-H) A 3.12 3.10 5.04 4.10 2.12 0.28 (1)
534 A 3.15 3.19 4.02 3.81 1.21(CH3) 0.60 (3)
11 648 A 3.03 2.99 4.16 3.85 1.22 0.90 (2)
13 548 A 3.15 3.19 4.01 3.85 1.15 0.36 ~2)
14 662 A 3.05 2.99 4.15 3.97 1.16 0.45 (4)
17 562 A 3.12 3.18 4.02 ? 0.93 0.36 (2)
18 676 A 3.04 2.98 4.16 3.85 0.93 0~85 (2)
561 (M+-H) A 3.14 3.18 4.04 3.82 1.22 0.43 (2)
21 676 A 3.07 2.99 4.13 3.65 1.23 0.40 (4)
23 546 A 3.15 3.19 ? ? 5.96 0.41 (2)
24 660 A 3.04 2.98 4.14 ? 5.98 0.44 (4)
43 596 A 3.16 3.19 4.02 3.84 4.65 0.47 (2)
44 710 A 3.10 2.98 4.16 4.01 4.63 0.54 (4)
68 549 (M~-H) A 3.15 3.19 4.03 4.14 4.66 0.36 (2)
AB-system
69 - A 3.03 2.98 4.14 4.19 4.66 0.57 (2)
AB-system
73 - A 3.15 3.18 4.03 4.26 2.21 0.31 (2)
74 680 A 3.04 2.98 4.14 4.28 2.20 0.22 (4)
82 564 A 3.14 3.18 4.01 4.15 4.71 0.24 (2)
83 - A 3.03 2.97 4.14 4.20 4.72 0.65 (2)
89 626 A 3.15 3.18 4.02 4.22 4.66 0.31 (2)
AB-system
- A 3.05 2.99 4.14 4.19 4.67 ().44 (4)
AB-system
2~817~
- 35 -
Einzelnes
ausgew.
MS lH-N~DR Proton aus
Nr. M+ Lsgm.* 2-H 4-H 5-H 11-H R~ Rf ~Lsgm)~
91 577 (M--H) A 3.15 3.18 4.03 4.18 2.160.25 ~2)
92 692 A 3.03 2.97 4.14 4.22 2.11 0.23 ~4)
94 593 (M--H) A 3.13 3.18 4.01 4.18 4.75 0.13 (2)
AB-system
708 A 3.04 2.98 4.15 4.24 4.?8 0.46 (2)
AB-system
101 - A 3.04 2.99 4.16 ? 3.83 0.26 (4)
103 - A 3.04 2.99 4.16 3.94 3.82 0.28 ~4)
105 - A 3.04 2.98 4.12 4.20 4.62/4.68 0.65 (2)
108 590 A 3.12 3.17 4.00 4.24 4.54 0.37 (5)
109 590 A 3.12 3.16 3.99 4.18 4.78 0.29 (5)
110 - A 3.08 2.99 4.15 4.16 4.67/4.79 0.13 (4)
4.32
134 951 (M--H) A 3.02 2.99 4.15 ? 2.01/2.03 0.48 (2)
2.06/2.15
147 534 A 3.12 3.17 4.01 5.49 8.11 0.51 (1)
148 562 A 3.13 3.13 5.20 5.49 8.12 0.66 (1)
151 548 A 3.13 3.16 4.00 5.36 2.09 0.41 (1)
152 590 A 3.12 3.09 5.03 5.37 2.11 0.63 (1)
158 578 A 3.13 3.16 4.00 5.45 4.06 0.44 (1)
159 650 A 3.13 3.13 5.11 5.47 4.08 0.50 (1)
160 691 (M--H) B 2.98 3.12 4.38 5.31 4.03 0.75 (2)
162 605 (M--H) A 3.12 3.16 4.02 5.39 2.69 0.24 (6)
164 610 B 3.01 3.10 4.32 5.14 8.08 0.56 (2)
165 724 B 3.00 3.14 4.42 5.33 8.22 0.86 (2)
181 627(M--Na) A 3.02 3.17 4.23 5.54 5.83 0.43 (7)
182 - C 2.97 3.11 4.37 5.50 6.36 0.52 (8)
210 638(M++H) A 3.15 3.18 4.01 5.41 2.11 0.12 (9)
211 - - - - - - - 0-35 (9)
212 654(M++H) A 3.15 3.18 4.03 5.41 7.35 0.21 (9)
20~17~
- 36-
Individual
exchanged
MS lH-NMR proton from
No. M+ Solv.* 2-H 4-H 5-H ll-H R2 Rf (solv)*~
213 - A 2.98 ? 4.13 5.48 7.25 0.34 (9)
220 620(M+~H) A 3.14 3.18 4.02 5.39 0.95 0.16 (9)
221 - - - - - - - 0.29 (9)
104 - A 3.14 3.18 4.02 4.06 4.72 0.28 (2)
(Isomer A)
104 - A 3.14 3.18 4.02 4.16 4.65 0.32 (2)
(Isomer B)
88 - A 3.05 2.98 4.15 4.24 3.90 0.61 (2)
87 577(M-H) A 3.14 3.18 4.03 4.20 3.94 0.23 (2)
222 - A 3.02 2.96 4.11 4.20 4.71 0.60 (2)
223 - A 3.13 3.17 4.01 4.18 4.68 0.29 (2)
* Solvents for lH-NMR are:
A = CDCl3
B = CD3COCD3
C = CD3COCD3-CD3COOD
*~ Solvents for Rf determination are:
1 = dichloromethane/acetone 90:10
2 = ethyl acetate/hexane 50:50
3 = ethyl acetate/hexane 75:25
4 = ethyl acetate/hexane 25:75
S = dichloromethane/acetone 92:8
6 = methanoVH20/NH3 599:400:1
7 = methanol/H2O/acetic acid 69:30: 1
8 - chloroform/methanol 85:15
9 = dichloromethane/acetone 80:20
2~817~0
- 37 -
Formulation Examples for compounds of formula I
(% = percenta e bv wei~ht)
F1. Solutions a) b) c) d)
compound of the Tables 80 % 10 % 5 % 95 %
ethylene glycol monomethyl ether 20 % ^ - -
polyethylene glycol 400 - 70 % - -
N-methyl-2-pyrrolidone - 20 %
epoxidised coconut oil - - 1 % 5 %
petroleum distillate
boiling range: 160-190C) - - 94 %
These solutions are suit~ble for application in the form of microdrops.
F2. Granulates a) b)
compound of the Tables 5 % 10 %
kaolin 94 %
highly disperse silica 1 %
attapulgite - 90 %
The compound is dissolved in methylene chloride, the solution is sprayed on to the carrier
and the solvent is then evaporated under vacuum.
F3. Dusts a) b)
compound of the Tables 2 % 5 %
highlydispersesilica 1% 5 %
talcum 97 %
kaolin - 90 %
Ready-for-use dusts are obtained by intimately the carriers with the compound.
F4.Wettablepowders a) b) c)
compoundof theTables 25 % 50 % 75 %
sodium ligninsulfonate 5 % 5 %
sodium lauryl sulfate 3 % 5 %
2asl7~
sodium diisobutylnaphehalene
sulfonate - 6 % 10 %
octylphenol polyethylene glycol
ether 2 %
(7-8 mol ethylene oxide)
highly disperse silica S % 10 % 10 %
kaolin 62% 27 %
The compound is thoroughly mixed with the adjuvants and the mixture is well ground in a
suitable mill to give wettable powders which can be diluted with water to suspensions of
any desired concentration.
F5. Emulsifiable concentrate
compound of the Tables 10 %
octylphenol polyethylene glycol
ether 3 %
(4-S mol ethylene oxide)
calcium dodecylbenzenesulfonate 3 %
castor oil polyglycol ether4 %
(35 mol ethylene oxide)
cyclohexanone 30 %
xylene mixture 50 %
This concentrate can be diluted with water in all proportions.
F6. Coated ranulate
Compound of the Tables 3 %
polyethylene glycol 200) 3 %
kaolin 94 %
The finely ground compound is applied uniformly in a mixer to the kaolin which is
moistened with polyethylene glycol to give non-dusting coa~ed granulates.
20~1700
- 39 -
Biolo~ical Examples:
Example B 1: Action a~ainst Puccinia grarninis on wheat
a) Residual protective action
Wheat plants are treated 6 days after sowing with a spray rnixture (60 ppm a.i.) prepared
from a wettable powder foImulation of the test compound. After 24 hours the treated
plants are infected with a uredospore suspension of the fungus. The infected plants are
incubated for 48 hours at 95-100 % relative humidity and c. 20C and then stood in a
greenhouse at c. 22C. Evaluation of rust pustule development is made 12 days after
infection.
b) SYstemic action
Wheat plants are treated 5 days after sowing with a spray mixture (6 ppm a.i., based on the
volume of the soil) prepared from a wettable powder formulation of the test compound.
After 48 hours the treated plants are infected with a uredospore suspension of the fungus.
The plants are then incubated for 48 hours at 95-100 % relative humidity and c. 20C and
then stood in a greenhouse at c. 22C. Evaluation of rust pustule development is made
12 days after infection.
Puccinia infestation was 100 % on untreated, infected control plants. Compounds of the
Tables exhibited good action against Puccinia fungi (5-20 % infestation). Compounds 1,3,
10, 13, 17, 20, 23, 43, 68,73, 82,87, 89, 91, 94, 104, 108, 109, 147, 148, 151, 158, 159,
181, 212, 213, 214, 223 and others inhibited infestation to 0-5 %. In the systemic test the
basic compound, Soraphen C, inhibited infestation to 10-20 %.
Example B2: Action a ainst Cercospora arachidicola on roundnut Plants
a) Residual Protective action
Groundnut plants 10-15 cm in height are sprayed with a spray mixture (20 ppm a.i.)
prepared from a wettable powder formulation of the test compound and infected 48 hours
later with a conidia suspension of the fungus. The infected plants are incubated for
72 hours at c. 21C and high humidity and then stood in a greenhouse until the typical leaf
specks occur. Evaluation of the fungicidal action is made 12 days after infection and is
based on the number and size of the specks.
Compared with untreated, infected control plants (number and size of the
20817~0
- 40 -
specks = 100 %), Cercospora attack on groundnut plants treated with compounds of the
Tables was strongly reduced. Thus in the above tests compounds 1, 2, 3, 10, 11, 13, 14, 17,
18, 20, 21, 23, 24,43, 68,73,74, 82, 83, 87, 88, 89, 90, 91, 92, 94, 95, 101, 103, 104, 105,
108, 109, 110, 134, 147, 148, 151, 152, 158, 159, 160, 162, 164, 165, 181, 182, 210-213,
215, 220-223, 227, 228 inhibited speck infestation almost completely (0-10 %). In a spray
concentration as low as 2 ppm a.i. compounds Nos. 68 and 94 inhibited the infestation
completely (0-5 %).
Example B3: Action a~ainst EerYsiphe raminis on barlev
a~ Residual protective acdon
Barley plants about 8 cm in height are sprayed with a spray mixture (6 ppm a.i.) prepared
from a wettable powder forrnulation of the test compound. The treated plants are dusted
with conidia of thc fungus after 3 to 4 hours. The infected barley plants are stood in a
greenhouse at c. 22C and fungus infestation is evaluated after 10 days.
b) Svstemic acdon
A spray mixture (2 ppm a.i., based on the volume of the soil) prepared from a wettable
powder folmulation of the test compound is poured on to barley plants about 8 cm in
height Care is taken that the spray mixture does not come in contact with the growing
parts of the plants. The treated plants are infected 48 hours later with a conidia suspension
of the fungus. The infected plants are then stood in a ~eenhouse at c. 22C and evaluation
of infestation is made after 10 days.
Compounds of formula I exhibited good action against Erysiphe fungi. Erysiphe
infestation on untreated, infected control plants was 100 %. Among other compounds of
theTables, compounds 1, 2, 3, 10, 11, 13, 14, 17, 20, 23, 43, 68, 73, 82, 87, 89, 91, 94,
108, 109, 147, 151, 158, 181, 212, æ3-æs, 234, 236 inhibited fungus infestation on
barley to 0 to 5 %. At the same concentrations Soraphen C inhibited fungus infestation to
10-20%.
Example B4: Residual protective action avainst Venturia inaequalis on apple shoots
Apple cuttings with 10-20 cm long fresh shoots are sprayed with a spray mixture (20 ppm
a.i.) prepared from a wettable powder formulation of the test compound. The plants are
infected 24 hours later with a conidia suspension of the fungus. The plants are then
incubated for 5 days at 90-100 % relative humidity and stood in a greenhouse for a further
10 days at 20-24C. Scab infestation is evaluated 15 days after infection. Compounds of
208170~
the Tables inhibited infestation markedly. Thus the compounds listed in Example B2
inhibited fungus infestation almost completely (0-5 %). Venturia infestation on untreated,
infected shoots was 100 %. Soraphen C inhibited fungus infestation to 10-20 %.
Example B5: Action a~ainst BotrYtis cinereas on beans
Residual protective action
Bean plants c. 10 cm in height are sprayed with a spray mixture (60 ppm a.i.) prepared
from a wettable powder formulation of the test compound. After 48 hours the treated
plants are infected with a conidia suspension of the fungus. The infe ted plants are
incubated for 3 days at 95-100 % relative hurnidity and 21C and then fungus infestation
is evaluated.
Botrytis infestation on untreated, infected plants was 100 %. Infestation after t~eatment
with one of the compounds of formula I was ~20 %. Infestadon was 0-5 % after treatment
with the compounds listed in Example B3. Soraphen C inhibited the infestation to 0-5 %.
Example B6: Action against Rhizoctania solani (soil fungus on rice plants)
a) ~rotective local soil aDplication
A spray mixture (6 ppm a.i.) prepared *om a formulation of the test compound is poured
on to 12-day-old rice plants without wetting the growing parts of the plants. The treated
plants are infected by applying a suspension of mycelium and sclerotia of R. solani to the
surface of the 80iL After incubation for 6 days at 27C (by day) and 23C (by night) and
100 % relative humidity (humidity box) in a controlled environment chamber, fungus
infestation on the leaf sheath, leaves and stems is evaluated. .
b) ~rotective local foliar aPPlication
l~day-old rice plants are treated with a spray mixture (6 ppm a.i.) prepared from a
formulation of the test compound. One day later the treated plants are infected with a
suspension of mycelium and sclerotia of R. solani. After incubation for 6 days at 27C (by
day) and 23C (by night) and 10~ % relative humidity (humidity box) in a controlled
environment chamber, fungus infestation on the leaf sheath, leaves and stems is evaluated.
Compounds of the Tables exhibited very good activity by inhibiting Rhizoctania
infestation to less than 10 %. Compounds Nos. 68 and 94 inhibited the fungus infestation
to 0-5 %, Soraphen C inhibited to 10-20 %. Infestation was 100 % on untreated, infected
control plants.
,. - -
-
' " ' '. ~
-;
'
: .
2~ 7~0
- 42 -
Example B7: Action a~ainst Plasmopara viticola (Bert. et Curt.) (Berl. et DeToni) on
vines
Vine cuttings of the Chasselas variety are reared in a greenhouse. Three plants in the
10-leaf stage are sprayed with a rnixture (60 ppm a.i.) prepared from a wettable powder
formulation of the test compound. After the spray coating has dried, the plants are infected
uniformly on the underside of the leaves with a spore suspension of the fungus. The plants
are then kept for in a humidity chambcr for 8 days, after which time marked symptoms of
infestation are observed on the control plants. The number and size of the infected areas
on the untreated plants act as an indicator of the efficacy of the tested compounds.
Compounds 87, 104, 108, 109, 212, 223, 225, 226, 232, 233, 235 inhibited infestation to
less than 10 %. Compounds Nos. 68 and 94 inhibited infestation to 0-5 % and Soraphen C
to 10-20 %.
Example B8: Action a~ainst PYricularia oNzae on rice plants
a) After a cultivation period of 2 weeks, rice plants are sprayed with a spray mixture
(60 ppm a.i.) prepared from a wettable powder formulation of the test compound. After
48 hours the treated plants are infected with a conidia suspension of the fungus.
Evaluation of fungus attack was made after incubation for 5 days at 95-100 % relative
humidity and 24C.
b) A spray mixture (20 ppm a.i., based on the volume of the soil), is poured on to
2-week-old rice plants growing in conventional flower pots. The pots are then filled with
water until the lowermost parts of the rice stalks are standing in water. After 48 hours the
treated rice plants are infected with a conidia suspension of the fungus. Fungus infestation
is evaluated after incubating the infected plants for S days at 95-100 % relative humidity
and c. 24C.
Compared with untreated plants (100 % infestation), fungus infestation on rice plants
treated with a spray mLlcture containing a compound of Tables 1 and 2 is only minor. Thus
e.g. compounds 68, 87, 104, 108, 109, 212, 223, like Soraphen C, reduced infestation to
10-20 %.
,