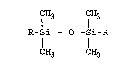Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2~33~2
METHYLPHENETHYL FUNCTIONAL SILICONES
The present invention relates to an optical
matching oil. ~ore specifically, the present invention
relates to an optical matching oil that consists of an
organosilicon compound whose molecule contains at least
one 2-methylphenethyl group.
Like optical adhesives and optical greases,
optical matching oils are employed as filling agents
between the lens and prisms present in optical devices
and as filling agents in optical fiber connectors. When
air bubbles remain in the optical matching oil filled
between ].enses, between prisms, or between the ends of
optical fibers, or when air bubbles are generated at the
contact surface between an optical matching oil and a
lens, prism, or optical fiber terminus, the transmitted
light or optical signal is scattered, causing a flare or
transmission loss. As a consequence, one type of optical
matching oil in use up to now has been based on a low-
viscosity phenylsilicone oil having a viscosity of 10 to
100 centistokes and a refractive index of 1.46 to 1.51.
However, phenylsilicone oils by themselves have
relatively high viscosities and must therefore be diluted
with low-molecular-weight silicone oils, such as,
dimethylsilicone oils. This has created the problem of
a timewise variation in the refractive index of the
optical matching oil due to volatilization of the low-
molecular-weight silicone oil. Another problem is
associated with the use of such optical matching oils in
ambients below room temperature. This type of optical
matching oil suffers from a deterioration in performance
in such ambients due to a sharp increase in the viscosity
~3~6~
of the phenylsilicone oil base or even
crystallization of part of the phenylsilicone oil. For
these reasons, there is a need for an optical matching
oil that is immune to timewise variations in its
refractive index~ that presents little increase in
viscosity below room temperature, and that resists
crystallization below room temperature.
The present invention -takes as its object the
introduction of an optical matching oil that is immune to
timewise variations in its refractive index, that
undergoes little increase in viscosity below room
temperature, and that also resists crystallization below
room temperature.
Figure 1 is a light transmittance chart for the
1-(2-methylphenethyl)-3-vinyltetramethyldisiloxane
prepared in Reference Example 1.
Figure 2 is a light transmittance chart for the
2-methylphenethylpentamethyldisiloxane prepared in
Reference Example 2.
Figure 3 is a light transmi-ttance chart for the
1,3-di(2-methylphenethyl)tetramethyldisiloxane prepared
in Reference Example 3.
Figure 4 contains an infrared absorpt.ion
spectrogram for the 1-(2-methylphenethylj-3-
vinyltetramethyldisiloxane synthesized in Reference
Example 1.
Figure 5 contains a IH nuclear magnetic
resonance spectrogram for the 1-(2-methylphenylethyl)-3-
vinyltetramethyldisiloxane synthesized in Reference
Example 1.
23~3~
Figure 6 contains an infrared absorption
spectrogram for the 2-methylphenylethylpentamethyl-
siloxane synthesized in Example 4.
Figure 7 contains a IH nuclear magnetic
resonance spectrogram for the 2-methylphenethylpenta-
methyldisiloxane synthesized in Example 4.
Figure 8 contains an infrared absorption
spectrogram for the 1,3-di(2-methylphenethyl)tetra-
methyldisloxane synthesized in Example 5.
Figure 9 contains a IH nuclear magnetic
resonance spectrogram for the 1,3-di(2-
methylphenethyl)tetramethyldisloxane synthesized in
Example 5.
The optical matching oil according to the
present invention comprises an optical matching oil based
on an organosilicon compound with the following general
formula
CH3 CH3
R-Si-O-Si-R
1H3 CH3
wherein R is selected from the group consisting o
methyll vinyl, and 2-methylphenethyl with the proviso
that at least one R group is the 2-methylphenethyl group.
The optical matching oil of the present
invention is based on the aforesaid organosilicon
compound that contains at least one 2-methylphene~hyl
group in each molecule, and this organosilicon compound
is exemplified by 1-(2-methylphenethyl)-3-vinyltetra-
.
.
2~
methyldisiloxane, 2-methylphenethylpentamethyldisiloxane,
and 1,3-di(2-methylphenethyl)tetramethyldisiloxane.
These organosilicon compounds have viscosities in the
range of 1 to 10 centistokes and refractive indexes in
the range of 1. 46 - 1. 51. II1 particular, because they
have relatively high boiling points, optical matching
oils based on these organosilicon compounds do not suffer
from timewise variations in refractive index. These
organosilicon compounds are also characterized by their
low viscosity increase at temperatures below room
temperature and by their resistance to crystallization at
temperatures below room temperature, for example, at -
10C. Another characteristic of the optical matching
oils of the present invention based on such organosilicon
compounds is their transparency in the visible region
(450 to 750 nm) and also in the near infrared region.
The synthesis of the organosilicon compounds
comprising the base or principal agent of the optical
matching oil of the present invention is exemplified by
the following methods:
(a) the addition reaction of 1,1,3,3-tetramethyl-
disiloxane with alpha-methylstyrene under platinum
catalysis,
(b) the re-e~uilibration reaction between the 1,3-
di(2-methylphenethyl)tetramethyldisiloxanepreparedasin
(a) with 113-divinyltetramethyldisiloxane or
hexamethyldisiloxane, and
(c) the cohydrolysis of 2-methylphenethyldimethyl-
halosilane with a halosilane selected from the group
,. , :~
2~33$~
consisting of trimethylhalosilane, vinyldimethylhalo-
silane, and 2-methylphenethyldimethylhalosilane.
Chloroplatinic acid, alcohol solutions of
chloroplatinic acid, platinum/olefin complexes,
platinum/vinylsiloxane complexes, and platinum-on-
inorganic powder, are examples of the platinum group
metal catalysts that can be deployed in the above-listed
platinum-catalyzed addition reaction (a) between 1,1,3,3-
tetramethyldisiloxane and alpha-methylstyrene.
With regard to the re-equilibration reaction
(b) between 1,3~di(2-methylphenethyl)tetramethyl-
disiloxane and 1,3-divinyltetramethyldisiloxane or
hexamethyldisiloxane, the hexamethyldisiloxane and 1,3-
divinyltetramethyldisiloxane used in this method are
available commercially while the 1,3-di(2-
methylphenethyl)tetramethyldisiloxane can be synthesized
by the method already described above. An acid or base
catalyst can be used as the re-e~uilibration catalyst.
Operable acid catalysts are specifically exemplified by
mineral acids such as hydrochloric acid, sulfuric acid,
nitric acid, and by solid catalysts such as activated
clay, and Filtrol. Operable base catalysts are
specifically exemplified by lithium hydroxide, sodium
hydroxide, potassium hydroxide, cesium hydroxide,
tetramethylammonium hydroxide, lithium silanolate,
potassium silanolate, phenyllithium, methyllithium, and
butyllithium. The ~uantities of addition of
hexamethyldisiloxaneorl,3-divinyltetramethyldisiloxane
and 1,3-di(2-methylphenethyl)tetramathyldisiloxane are
not critical however, preferred amounts are O.l to 10
- , :
2~3`3$2
moles hexamethyldisiloxane or 1,3-divinyltetramethyl-
disiloxane per 1 mole 1,3-di(2-methylphenethyl)tetra-
methyldisiloxane. The preparative conditions are also
not critical, but a preferred range for the reaction
temperature is 50 to 200C.
With regard to -the method comprising the
cohydrolysis of 2-methylphenethyldimethylhalosilane with
a halosilane selected from the group consisting of
trimethylhalosilane, ~rinyldimethylhalosilane, and 2-
methylphenethyldimethylhalosilane, the
trimethylhalosilane and vinyldimethylhalosilane are
available commercially while the 2-methylphenethyl-
dimethylhalosilane can be prepared by an addition
reaction between dimethylhalosilane and alpha-
methylstyrene under platinum catalysis. The reaction
temperature is not critical, and the reaction can be run,
or example, in the temperature range of from room
temperature to the boiling point of the particular
halosilane or the boiling point of any organic solvent
used. An organic solvent can be used in the reaction,
and operable organic solvents are exemplified by aromatic
solvents such as toluene, and xylena, and aliphatic
solvents such as hexane, heptane, and octane. In
addition, this halosilane cohydrolysis is preferably run
with the addition of a basic salt such as sodium
carbonate, potassium carbonate, calcium carbonate,
sodium bicarbonate, or an amine compound such as
trimethylamine, and triethylamine, in order to capture
the hydrogen chloride by-product.
2~8~ g3~
The optical matching oil of the present
invention is based on the aforesaid organosilicon
compound whose molecule contains at least one 2~
methylphenethyl group. The optical matching oil of the
present invention may consist of only this organosilicon
compound, or it may also contain other components, for
example, additives such as antioxidants, and refractive-
index regulators. Blendable antioxidants are exemplified
by 4,4'-thiobis(6-tert-butyl-m-cresol), 4,4'-
butylidenebis(6-tert-butyl-m-cresol), 2,2 -methylene-
bis(4-methyl-6-tert-butylphenol), 2,2'-methylenebis(4-
ethyl-6-tert-butylphenol), 2,5-di-tert-butylhydroquinone.
2,5-di-tert-amylhydroquinone, and 2,6-di-tert-butyl-p-
cresol. Operable refractive-index regulators are
exemplified by dimethylpolysiloxanes and
methylphenylpolysiloxanes that contain only small
quantities of volatile component and by organic
refractive-index regulators.
Because the optical matching oil of the present
invention has a high refractive index and low viscosity,
it can be employed as a filling agent between the lenses
in a video projector or as filler in optical fiber
connectors. It is suitable for use in optical fiber
transfer or diverter devices, optical fiber switches, and
movable optical fiber connectors.
The present invention will be further explained
below in the illustrative examples. In the examples, the
refractive index, viscosity, and light transmittance were
measured at 25C; the refractive index was measured using
' " ' "' ' ' ' '
2~833~2
the D-line of sodium (589 nm); and the light
transmittance was measured using water as the blank.
REFERENCE EXAMPLE 1
To prepare 1-(2-methylphenethyl)-3--
vinyltetramethyldisiloxane the following were charged to
a 1 L roundbottom flask equipped with a stirrer,
thermometer, and addition funnel and heated to 70C: 375
g (3.15 mol) alpha-methylstyrene and 0.6 mL 1%
isopropanolic chl.oroplatinic acid solution. 285 g (3.0
mol) dimethylchlorosilane was then dripped in from the
addition funnel over 1 hour. The reaction was
subsequently maintained at 80C for 1 hour and cooled.
212 g (1.0 mol) of the 2-methylphenethyldimethyl-
chlorosilane product and 217 g (1.8 mol)
dimethylvinylchlorosilane were dripped into a mixture of
300 g water and 100 g isopropyl alcohol. The resulting
hydrolyzate was washed with water 3 times, the water
(lower) layer was removed, and heating in vacuo produced
180 g (65% of the theoretical yield) 1-(2-
methylphenethyl)-3-vinyltetramethyldisiloxane. This 1-
(2-methylphanethyl)-3-vinyltetramethyldisiloxane had a
refractive index of 1.471, a viscosity of 2.2
centistokes, and a boiling point of 121C/5 mmHg.
REFERENCE EXAMPLE 2
2-methylphenethylpentamethyldisiloxane(71%of
tha -theoretical yield) was prepared as in Reference
Example 1 using trimethylchlorosilane in place of the
vinyldimethylchlorosilane used in Reference Example 1.
. , , ............................................ ~
,. ' " ' ' . ' ' :,' '' "',; .' ;', ' ~ .:' ': .'`' ' '
, . . -, . ~: :
:.. -
~3~
This 2-methylphenethylpentamethyldisiloxane had a
refractive index of 1.463, a viscosity of 2.1
centistokes, and a boiling point of 120C/10 mmHg.
REFERENCE EXAMPLE 3
1,3-di(2-methylphenethyl)tetramethyldisiloxane
(95% of the theoretical yield) was prepared as in
Reference Example 1 by omitting the
vinyldimethylchlorosilane and using only the 2-
methylphenethyldimethylchlorosilane. This 1,3-di~2-
methylphenethyl)tetramethyldisiloxane had a refractive
index of 1.505, a viscosity of 9.3 centistokes, and a
boiling point of 160C/0.5 mmHg.
EXAMPLE 4
The following were charged to a 1 L roundbottom
flask equipped with stirrer, thermometer, and addition
funnel and heated to 70C: 375 g (3.15 mol) alpha-
methylstyrene and 0.6 mL 1% isopropanolic chloroplatinic
acid solution. 285 g (3.0 mol) dimethylchlorosilane was
then dripped in from the addition funnel over 1 hour.
The reaction was subsequently maintained at 80C for 1
hour and cooled. 212 g (1.0 mol) of the 2-
methylphenethyldimethylchlorosilane product and 196 g
(1.8 mol) trimethylchlorosilane were dripped into a-
mixture of 300 g water and 100 g isopropyl alcohol. The
resulting reaction mixture was washed with water 3 times,
the water (lower) layer was removed, and the reac-tion
mixture (upper layer) was heated in vacuo to give
190 g (71% of the theoretical yield) 2-
.
~, .
methylphenethylpentamethyldisiloxane. This 2-
methylphenethylpentamethyldisiloxane had a refractive
index of 1.463, a viscosity of 2.1 centistokes, and a
boiling point of 120C/10 mmHg.
EXAMPL~E 5
The following were charged to a 1 L roundbottom
flask equipped with stirrer, thermometer, and addition
funnel and heated to 70C: 375 g (3.15 mol~ alpha-
methylstyrene and 0.6 mL 1% isopropanolic chloroplatinic
acid solution. 285 g (3.0 mol) dimethylchlorosilane was
then dripped in from the addition funnel over 1 hour.
The reaction was subsequently maintained at 80C for 1
hour and cooled. 424 g (2 mol) of the 2-
methylphenethyldimethylchlorosilane product was dripped
into a mixture of 300 g water and 100 g isopropyl
alcohol. The resulting reaction mixture was washed with
water 3 times, the water (lower) layer was removed, and
the reaction mixture (upper layer) was heated in vacuo to
give 350 g (95% of the theoretical yield) 1,3-
di(2-methylphenethyl)tetramethyldisiloxane. This 1,3-
di(2-methylphenethyl)tetramethyldisiloxane had a
refractive index of 1.505, a viscosity of 9.3
centistokes, and a boiling point of 160C/0.5 mmHg.
EXAMPLE 6
The following were charged to a 1 L roundbottom
flask e~lipped with a stirrer and thermometer: 200 g
1,3-di(2-methylphenethyl)tetramethyldisiloxane as
prepared in Example 3, 600 g hexamethyldisiloxane, and
,,s, ", :,, . ;~,., "~ ,",, ,~ "" "
~3~2
5 g thoroughly dehydrated activated clay. This was
followed by heating to 80 to 85C and stirring for 2
hours. The reaction mixture was then filtered and the
filtrate was heated in vacuo to afford 170 g 2-
methylphenethylpentamethyldisiloxane.
EXAMPLE 7
The following were charged to a 1 L roundbottom
flask e~uipped with a stirrer and thermometer: 200 g
1,3-di(2-methylphenethyl)tetramethyldisiloxane as
prepared in Example 3, 600 g 1,3-
divinyltetramethyldisiloxane, and 5 g thoroughly
dehydrated activated clay. This was followed by heating
to 80 to 85C and stirring for 2 hours. The reaction
mixture was then filtered and the filtrate was heated in
vacuo to afford 160 g 1-(2-methylphenethyl)-3-vinyltetra-
methyldisiloxane.
The light transmittance of the organosilicon
compounds prepared in Reference Examples 1, 2, and 3 was
measured using a light transmission meter with water as
the blank. The optical fiber connection loss was
measured on the organosilicon compounds prepared in
Reference Examples 1, 2~ and 3. Graded index optical
fibers (core diameter = 50 micrometers, clad diameter =
125 micrometers) were installed in a holding plate tool
having a V-shaped groove, the particular organosilicon
compound was filled between the ends of the optical
fibers, and measurement was then carried out. The
results for the connection loss are reported in Table 1.
. . . .. . . . . .
2 0 ~
TABLE 1
Reference Reference Refere~ce
Sample Number Example 1 Example 2 Example 3
conne~tion loss (dB) 2.0 1.8 1.5
The change in viscosity as a function of
temperature was measured on the 2-methylphenethyl~
pentamethyldisiloxane prepared in Reference Example 2 and
on a phenylsilicone oil (methylphenylsiloxane ol.igomer
with refractive index = 1.558). These results are
reported in Table 2. During this measurement, the
methylphenylsiloxane oligomer exhibited a sharp increase
in its viscosity at below room temperature and in fact
was partially crystallized at -10C.
TABLE 2
Temperature (C)
Viscosity (centistokes) -10 0 10 25 50
2-m~thylphenethyl-
pentamethyldisiloxane 7.1 5.5 4.0 2.9 1.7 ::
methylphenylsiloxane oligomer 800 232 108 41.4 12.5
Because the optical matching oil of the present
invention is based on an organosilicon compound that
contains at least one 2-methylphenethyl group in eacl
molecule, it is characterized by freedom from time-
dependent changes in its refractive index, by a small
increase in its viscosity below room temperature, and by
resistance to crystallization below room temperature.
Other variations and modifications may be made
in the compounds, compositions, and methods, described
3 $ ~
herein without departing from the essential features and
concepts of the present inventio:n. The forms of the
invention described herein are exemplary only and are not
intended as limitations on the scope of the invention as
defined in the appended claims.