Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO92/01419 PCT/US91/05182
METHOD OF DETECTING CANCER BY MEASURING
LIPID-PEROXIDATION USING NMR
BACKGROUND OF rrHE- INVENTION
Field of the Invention
The present invention relates to a diagnostic method and
apparatus for the detection of cancer in a living patient.
Prior Art
A~tempts utilizing the technique of nuclear magnetic
resonance (NMR) to aid in arriving at a clinical diagnosis of
cancer are well known in the prior art.
Damadian was the first to propose any medical use for
nuclear magnetic resonance (NMR) and that was for the detection
of malignancy in tissue. See R. Damadian, "Tumor Detection by
Nuclear Magnetic Resonance," Science 171:1151-1153 (1971).
U.S. Patent 3,789,832 issued to Damadian covers an apparatus
and method for application of nuclear magnetic resonance to
surgically removed specimens to measure Tl and T2 for proton
relaxation times, which values, compared against values for
healthy tissue, were taken as an indication of cancer. U.S.
Patent Nos. 4,411,270 and 4,354,499 issued to Damadian cover
apparatus and method for cancer detection with NMR lmaging and
scanning of whole-body specimens.
Cl I~CTITI ITF C3~JFFT
'
WO92/01419 PCT/US91/05182
~ ~ 8 2
A number of other investigators also reported that nuclear
magnetic resonance relaxation times (T1) for water pro~ons in
organs of tumor-bearing animals have higher values than the
corresponding T1 for water structure in organs of healthy
animals. See Frey et al, J._Natl. Cancer Inst. 49, 903 (1972);
Inch et al, J. Natl. Cancer Inst. 52, 353 (1974); Iijima et al,
Phvsiol. rhem. and Phvsics 5, 431 (1973); and Hazlewood et al,
J. Natl. Cancer Inst. 52, 1~49 (1974).
Today, despite uncertainty regarding mechanistic details,
it is well known that biophysical changes which occur in
malignant cells often result in alterations of the pro~on NMR
signal. See D.G. Taylor et al, "A Review of the Magnetic
Resonance Response of Biological Tissue and Its Applicability
to the Diagnosis of Cancer by NMR Radiology," Com~uted
Tomoara~hv, 5:122-133 (1981). Such changes form the physical
basis for detection of tumors by proton NMR imaging. See
R. Zimmerman et al, "Cerebral NMR: Diagnostic ~valuation of
Brain Tumors by Partial Saturation Technique with Resistive
NMR," Neuroradiolo~v 27:9-15 (1985) and K. Ohtomo, "Hepatic
Tumors: Differentiation by Transverse Relaxation Time (T2) of
Magnetic Resonance Imaging," Radioloav 155:421-423 (1985).
However, NMR imaging is not likely to be widely applied as a
screening test for malignancy because of accessibility and
economic factors.
.C~I IR~;TITIITE~ SHE~T
WO9Z/01419 PCT/US91/05182
Proton NMR studies on excised tumors, as well as on plasma
and serum, from experimental animals and patients have often
shown differences in the relaxation parameters T,, T2 and T2~,
T2~ being a combination of T2 from intrinsic relaxation and
relaxation induced by magnetic field inhomogeneities, as a
function of malignancy. Such findings have been reported by
the following:
L. McLachlan, "Cancer-induced Decreases in Human
Plasma Proton NMR Relaxation Rates," Phys. Med. Biol.
25:309-315 (1980);
F. Smith et ai, "Nuclear Magnetic Resonance Imaging
of the Pancreas," Radioloav 142:677-680 (1982),
P. Beall et al, "The Systemic Effect of Elevated
Tissue and Serum Relaxation Times for Water in Animals and
Humans with Cancers," NMR Basic Principles and Pro~ress,
P. Diehl et al, Eds., 19:39-57 (1981);
R. Floyd, "Time Course of Tissue Water Proton Spin-
lattice Relaxation in Mice Developing Ascites Tumor,".
Cancer Res. 34:89-91 (1974);
C. Hazlewood et al, "Relationship Between Hydration
and Proton Nuclear Magnetic Resonance Relaxation Times in
Tissues of Tumor Bearing and Nontumor Bearing Mice:
Implications for Cancer Detection," J. Natl. Cancer Inst.
52:1849-1853 (1974); and
R. Klimek et al, "A Discussion of Nuclear Magnetic
Resonance (NMR) Relaxation Time of Tumors in Terms of
Their Interpretation as Self-organizing Dissipative
Structures, and of Their Study of NMR Zeugmatographic
Imaging," Ginekol Pol. 52:493-502 (1981).
However, due to extensive overlap of groups and small
differences between the means of groups, these methodologies
are not clinically useful.
5UB5TITUTE ~ T
WO92/01419 PCT/US91/05182
While most of the prior art mentioned above describes
applications of NMR to analysis of tissue, it is also known to
subject bodily fluids to such analysis. This is described, fo~
example, by Beall et al., su~ra.
The foregoing prior art studies and methods rely on the
observation of the composite NMR signal arising from all
protons in the tissue or blood derived samples. This composi~e
signal is dominated by the protons of water, obscuring the NMR
signal from other proton-containing constituents of the sample.
Indeed, the prior art believed that the apparent correlation
between malignancy and observed changes in NMR parameters was
due to "changes in water structure," quoting Frey et al.,
su~ra.
In other applications of proton NMR spectroscopy, it was
known to suppress the signal from the solvent (such as water),
in a sample.
It was discovered that the components of the NMR spectrum
which have significant predictive value may be masked by other
materials in the sample. By eliminating the masking, as by
eliminating the water signal, the previously masked spectrum of
these components was revealed. In United States Patent Number
4,912,050 entitled "Process for the Screening of Cancer Using
Nuclear Magnetic Resonance," issued ~o Eric T. Fossel on Marcn
27, l990, those discoveries were incorpora~ed into a reliable
method of diagnosing the presence of cancer in a living
SO~STITUT~ 5HE~
WO92/01419 P~T/US~1/05182
patient. In accordance with that invention, a sample of a
patient's bodily fluid is subjected to nuclear magnetic
resonance spectroscopy to generate a nuclear magnetic resonance
spectrum. A resonance line generated by a non-water component
of the sample is selected, and the full width of this resonance
line, e.g., at half its height, is measured. The full width so
measured has proved to be a statistically reliable measure of
the presence or absence of cancer in the patient.
The above described test of water-suppressed proton NMR of
plasma discriminates between persons with untreated cancers and
others with better than 90% accuracy. No prior non-invasive
test for cancer had reached even close to that level of
accuracy. False positive results, however, have been obtained.
In accordance to a later invention, U.S. Patent No. 4,918,021
('021) entitled "Process for the Detection of Cancer" issued to
Eric T. Fossel on April 17, l990, it has been discerned that
the major source of false positive results are those persons
with high levels of plasma triglycerides. Thus, in accordance
with the '021 patent, a method and apparatus was developed to
improve upon the accuracy of a non-invasive method to determine
the presence of cancer in a living patient using C-13 NMR.
In the past in accordance with the teachings of the '021
patent, in the event that a positive reading is obtained in
accordance with the present invention, the level oE
triglycerides is determined. If the level of triglycerides is
high, then the patient's bodily fluid is further subiected to
~ TITUT~ 5HE~
,
WO92/01419 PCT/US9l/05182
,~ ~ 8 i 1~ 6
C-13 nuclear magnetic resonance spectroscopy. The resonance
spectrum of the plasma C-13 spectra discriminates between true
and false positive results to determine the presence or absence
of cancer in the patient with a higher degree of accuracy than
was previously possible. C-13 NMR looks at the ratio of fatty
acids with a single double bond versus fatty acids with two
double bonds. However, C-13 is costly and takes a relatively
long time to run.
The present invention is an improved method for screening
for the presence of cancer which would eliminate the need to
use C-13 to screen for false positives as disclosed in the '021
patent. The advantage to the present invention is the the
relatively short time to run the test and the relative decrease
in cost.
These and other objects and features of the present
invention will become apparent to those skilled in the art from
a reading of the description of the invention, taken together
with the drawing, which follow.
SUMMARY~OF THE INVENTION
Accordingly, the principal obiect of the present invention
is to provide a method of confirming a diagnosis by NMR water
suppressed proton method of the presence of cancer in a living
patient.
SUBSTITaJ~E 5~FFT
WO92/01419 PCT/US91/0518~
7 2~3`l~1
Another object of the present invention is to provide a
method to differentiate between true and false positive results
obtained in a water suppressed proton NMR test in diagnosing
the presence of cancer in a living patient.
Yet another object of the invention is to provide a method
for detecting the presence of cancer in a patient which can be
carried out on a sample of the patient's body fluid.
A further object of the present invention is to provide a
method of diagnosing the presence of cancer in a living patient
which is more accurate than previously known methods.
.
In accordance with the present invention, a sample of a
patient's bodily fluid is subjected to proton nuclear magnetic
resonance spectroscopy to generate a nuclear magnetic resonance
spectrum. A resonance line generated by a non-water component
of the sample is selected, and the area under the peak of the
resonance line or the intensity of the peak is measured. The
area or intensity so measured, as compared to a standard
control, provides a statistically reliable indication of the
presence or absence of cancer in the patient.
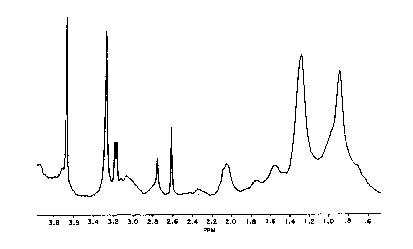
Normally the ratio of H-l resonances at 2.0 ppm (allylic)
to those at 2.8 ppm (bis-allylic) is between 2.0 and 2.5. This
arlses since in normal persons the ratio of polyunsaturated
fatty acids to monounsaturated fatty acids is more than 9:l.
SU~STITUIT~ S?~l~E~
,
WO 92tO14]9 PCI~US91/0~;182
~ ~jr 1~ ~
\/-V--\/' \/=V
2.0 2.8 2.0 2.0 2.0
ppm pp~ ppm ppm ppm
Double bonds ofDouble bond of
linoleic acidole1c acid
The most abundant polyunsaturated '~atty acid in plasma
lipoproteins is linoleic acid and the most abundant
monounsaturated fatty acid is olelc acid. In a cancer patients
where peroxida~ion of lipids occurs there will be a decrease o~
linoleic and othe_ polyunsaturated fa~ty acids relative to
monosaturated fatty acids because they are more reactive with
free radicals than monosaturated fatty acids. This results in
a decrease in the resonance at 2.8 ppm and an increase in the
ratio of the 2.0 ppm/2.8 ppm resonances. Ratios elevated above
2.5 indicate the presence of cancer. The xesonance at 2.0 and
2.8 ppm are illustrated in the spectrum in Figure 2.
In preferred embodiments, the bodily fluid is blood,
spinal fluid, or bone marrow plasma; although blood plasma or
serum is especially advantageous. ~alse positive results in
the initial pro~on NMR spectra due ~o hypertriglyceridemia can
be distinguished in the resulting spectra o~ peroxidized
lipoproteins by measuring the ratio of peroxidized lipoproteins
and comparing to a standard.
C~ C:TI'rl 1~ 5HIE~ET
WO92/01419 PCT/US~1/05182
9 ~ q~
BRIE~ DESCRIPTION OF THE DRAWING
FIG. 1 ls a typical 360 MHz NMR spectrum for the non-water
components (water-suppressed) of a plasma sample from a healthy
contrcl oblained in accordance with the present invention:
FIG. 2 is an expanded view of the reading of the sample of
FIG. ~ showing of the region of the spectra containing
resonances a_ 2.0 and 2.8 ppm ; and
FIG. 3 shows the results of a s~udy performed using the
method of the present invention.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
At the outset the invention is described in its broadest
overall aspects with a more detailed description following.
The present invention is a method to detect the presence of
cancer in a living patient. In accordance with the invention,
a sample of a patient's bodily fluid is subjected to proton
nuclear magnetic resonance spectroscopy to generate a nuclear
magnetic resonance spectrum. Since components of the NMR
spectrum which have significant predictive value may be masked
by other materials in the sample, the masking is eliminated to
produce the NMR spectrum. A resonance line generated by a
non-water component of the sample is selected, and the full
width of this resonance line, e.g., at half its height, is
measured to provide a reliable measure OL the presence or
U13S rl~UTF Sl IE:E~
WO92/01419 PCT/U~)1/05182
~, ~ 8 7 ~ ~ ~
absence of cancer in the patient. The above procedure is
described in '050 Fossel patent, the teachings o~ which are
incorporated herein by reference.
In the event that a positlve reading is obtained, this
reading may indicate the presence of cancer in the patient, or
it may be a false positive reading. It has been discovered
that a major source of false positive readings are persons with
high levels of plasma triglycerides.
Accordingly, in order to differentiate between true and
false positive readings, the sample tested previously is
subjected to a second proton NMR spectroscopy for those who
have elevated triglyceride levels. The false Positive results
due to hypertriglyceridemia and, conversely, the presence of
cancer in the patient, can be reliably determined from the
resulting ratios of peroxidized lipoprotelns as found in the
resulting spectra as compared to a standard.
In one important embodiment of this invention, proton NMR
spectroscopy is performed initially on the sample to be tested.
The water suppressed proton NMR spectrum obtained on human
blood plasma is dominated by the resonances of the plasma
lipoprotein lipids. As taught in accordance with '050 Fossel
patent, without water suppression, these non-water resonances
are virtually overwhelmed by the water. Signal averaging
allows observation of resonances OL some moieties associated
with non-water bodily fluid components, at high magnetic
SUE~iTITl~ SHEE:l
W092/01419 P~T/~S9l/05182
~871~1
11
fields, even in the presence of the water resonance. However,
the capability of modern NMR spectrometers to suppress nearly
completely the water proton resonance will facllitate this
reading. The water suppressed proton NMR spectrum of plasma ls
essentially that of plasma lipoproteins and a few low molecular
weight molecules.
The process of the present invention operates on any
lipid-containing bodily fluid, blood, or bone marrow plasma.
Plasma, whole blood, or serum may be used. While the test may
be performed on any such lipid-containing body fluid, work to
date has focused on blood plasma. In blood the lipids,
inclusive of choles~erol, triglycerides and phospholipids, are
present in the form of lipoproteins. The test for cancer will
typically be performed in vitro, preferably on serum or plasma.
The selected fluid of a suspect patient or other person to
be screened for cancer is exposed to a magnetic field and
radio-frequency energy to generate a nuclear magnetic resonance
signal which is then processed to obtain a value for the
selected parameter, e.g., Wl/2, for lipid methyl and/or
methylene protons. A relatively broad range of proton
frequencies may be employed, e.g., 60 MHz and higher; 360 MHz
or above is a preferrPd frequency. If cost is not a factor,
500 MHz may be thP preferred frequency.
5UB5~1~1LIT~ SH~ET
WO92/01419 PCT/USgl/OSt82
~ 38~
FIG. l shows a water suppressed proton spectrum of a
healthy control, and FIG. 2 shows an expanded spectrum of the
same sample showing the region from 2.0 to 2.8 ppm (parts per
million of resonance frequency). The resonances in the region
from 2.0 ~o 2.8 ppm arise from the fatty acid groups of the
lipoprotein lipids. Accordingly, in its preferred embodiments,
the present invention uses one cf a number of conventional
water suppression techniques, i.e., techniques for suppression
of the water proton NMR signal. Numerous techniques have been
devised ~o suppress the water proton NMR signal in other
contexts. These techniques have been set out in the '050
Fossel patent.
- In accordance wi~h the teachings in the '050 Fossel
patent, the linewidth at half-height of the resonances of
moieties, e.g., methyl and methylene groups, associated with
the lipids of plasma lipoproteins are treated as the variable
of interest. Full width at half-height Wl/2 (linewidth) of an
NMR resonance line is inversely proportional to the apparent
spin-spin relaxation ~ime (T2*), i.e. Wl/2 = l _
T2*
The detected value for the selected parameter is then
compared with the corresponding parameter for the healthy
controls. In a preferred embodiment, values for methyl and
methylene are averaged and an average value of 33 Hz or less a~
a proton frequency of 360 MHz (~.45T) or 400 MHz (9.40T) is
taken as an lndication of malignancy.
5UE~STITUTE 5H~ F~
WO92/01419 PCTIUS91/0~182
13 ~8 7~
If a positive reading is obtained from the water
suppressed proton NMR spectrum o~ a p~asma sample ~rom a
patient, a second level of testing to confirm the diagnosis is
performed. First, a conventional test, commonly called a
triglyceride analysis, is performed to determine the
triglyceride level of the patient. If the triglyceride level is
normal, the positive reading from the water-suppressed proton
NMR spectroscopy is a true positive and indicates the presence
of cancer in the patient. If the triglyceride level is above
normal, in order to differentiate between true and false
positive results, a second proton NMR spectra on the plasma
sample already obtained from the patient is conducted.
False positive results due to hypertriglyceridemia can be
reliably distinguished from true positive results by
substantial differences in ratio of oxidized lipoproteins in
the resulting spectra. Accordingly, the plasma sample already
obtained from the suspect patient to be screened is exposed to
a magnetic field and radio frequency energy to generate a
nuclear magnetic signal which is then processed to obtain a
second proton NMR value.
In accordance with the teachings of the '021 (or Carbon
13) Fossel patent, initially, the olefinic region, 120-140 ppm,
of the spectra is examined. Two peaks will appear, one at
approximately 128-129 ppm and another at approximately 130-131
ppm, said peaks appearing about 2 ppm apart. The ratio of the
resonance at the general region of 128 ppm to that at 130 ppm
SUE~STITUTE S~ T
.
W~92/01419 PCT/U~1/05182
~ i~ 8 7 1 1 1
14
is indicative. In readings of plasma from normal controls and
from persons with non-cancer disease, the ratio of the height
of those two resonances (128/130 ppm) is 0.9 or greater, i.e.
the resonance peak at 128 ppm is approximately equal to or
taller than that at 130 ppm. The heights of the peaks are
measured with a ruler or computer from the center of the
baseline noise to the top of the peak. In readings of plasma
from patients with untreated cancer, the ratio of the peak
heigh~s is less than 0.9, or the resonance peak at 130 ppm is
taller by at least 10% than that at 128 ppm. It should be
noted that in patients with hypertriglyceridemia, the ratio of
the height of the resonances (128/130 ppm) is the same as
normal control values.
The changes in the olefinic region of the spectra of
untreated cancer patients can be explained by increases in
peroxidized lipoDroteins.
:
Oleic acid is a monounsaturated fatty acid and is made by
the human body. Linoleic acid is a polyunsaturated fatty acid,
having two double bonds, and is not made by the human body, but
is obtained by consumption. Dietary fatty acids include
polyunsaturated acids, such as linoleic acid. A resonance peak
in the general region of 128-129 ppm evidences only linoleic
acid in the patient. A resonance peak at the general region of
130-131 ppm evidences both oleic and linoleic acid in the
patient.
Sl~JB5T~TUTE ~ffE~:~.T
WO92/0141~ PCT/US91/0518
It has been discovered that the height of those resonance
peaks. relative to each other, are affected by certain
conditions of the patient. For example, persons with high
triglyceride levels usually have a high ratio of linoleic acid
to oleic acid levels. Patients with untreated cancer are found
to have low levels of linoleic acid in their bodies,
presumably because cancer causes a oxidation of polyunsaturated
fatty acids, including linoleic acid. This is consistent with
the hypothesis that lipids are oxidized by hydroxyl free
radicals in cancer patients since polyunsaturated fatty acids
are most susceptible to oxidation.
Accordingly, if the subject patient has both high
triglycerides and untreated cancer, the resonance peak at 130
ppm will be higher, reflecting the decreased linoleic acid in
both peaks. If, however, the peak at 128 ppm is not shorter
than that at 130 ppm by more than 7%, no depression, or an
insignificant depression, of linoleic acid levels has occurred
and the positive result obtained from the proton NMR spectra is
confirmed as a false positive and no cancer is present.
In addition, the spectral region between 48 ppm and 80 ppm
is far more complex in untreated cancer plasma than in normal
control or hypertriglyceridemia plasma. By "more complex" is
meant that there are more resonance peaks in the region. A
resonance peak is counted if its height is 50% greater or more
than that of the background noise during a normal testing
5UB5TITUTE 51t~E~
',,
WO 92/01419 PCl/US91/0518~
~ a ~ r~
16
perlod. As those skilled in the art will know, the longer
data is collected, the noise will lessen and peaks will show
more clearly.
These parameters include the size of the sample tube, the
pulse width, the pulse repetitlon rate, and the exponential
multiplication of the free induction decay by different
factors, For example, it is obvious to those skilled in the
art that the bigger the sample tested, the faster spectra of
adequate quality will be obtained. Other changes to the
conditions given here will be evident to those skilled in the
art.
It is possible, as with C-13 NMR as practiced in the '021
Fossel patent, that spectroscopy according to the method of the
instant invention can be performed initially on a patient as a
method to diagnose the presence of cancer, without first
performing a proton NMR spectroscopy as described above. This
has not yet been tested, however.
Any conventional modern NMR spectrometer may be used in
the practice of the present invention. In the preferred
embodiments, an NMR spectrometer with a magnet at constant
field strength is used a~d the NMR signal is Fourier
transformed, with the full linewidth at half-height for proton
resonances of methyl and methylene s~oups, and then proton NMR
resonances of lipoproteins at 2.0 ard 2.8 ppm which are the NMR
parameters of interest.
5UBSTITUTE~: SH ~ T
WO92/01419 PC~/US91/05182
17 ~7~
As noted in the '050 Fossel patent, correct sample
preparation and execution is essential to carry out a
successful measurement on plasma. ~lood is collected in tubes
containing 70 l of a solution of 15~ Na2 EDTA. Blood was
maintained at 4C until centrifugation. Plasma was separated
and stored at 4C until NMR analysis. Plasma samples were
never frozen because freezing destroys lipoprotein lipid
structural integrity. Samples which showed any visible sign of
hemolysis were excluded.
In the preferred embodiment, spectra were obtained at
20-22C at magnetic field strengths of 360MHz or greater.
Other tests were conducted successfully at temperatures of 30C
and 37C. The samples were shimmed individually on the area of
the proton free induction deca~ until the full width at half
height of the water resonance was 4Hz or less. Of course,
careful shimming is an assumed component of good NMR laboratory
technique. Of course, the field strength used will determine
the length of time in which a sample is taken. In addition, to
the experimental conditions, accurate results re~uire careful
review of a patient's medical record to arrive at the patient
classification.
In accordance with the '050 Fcssel patent, the
spectrometer contains means for selecting at least one and
preferably a plurality such as two NMR resonance lines in the
NMR spectrum of the sample and, in the first step of the
5U~^~TITI.3T~ S~ T
..
WO92/01419 PC~/VS9~/05182
~ ~ ~ rl 1''~ 1
18
present invention, measuring the linewidth of the line or lines
so selected. Preferably, the linewidth is measured at half the
height of the line, but this is not necessary and linewidth can
be measured at any predetermined fraction of the height of the
line in ~uestion. Measurement at half of line height is
preferred because this is a standard measurement carried out in
the field of NMR spectroscopy. The spectrometer also con~ains
means for measuring selected peaks, useful for the examination
of the second proton NMR spectra. The spectrometer also is of
conventional construction and includes in addition to all its
other structure a means for storing a value or range of values.
In the preferred embodiment, an area or intensity which is
either measured directly or derived from a plurality of such
direct measurements is compared with a value or range of values
which represents the value or range of values to be expected
from normal patients, i.e. patients who are free of cancer. In
accordance with the invention, the spectrometer also includes
means for classifying the measured or derived areas of the 2.0
or 2.8 ppm resonances or intensities of the 2.0 or 2.8 ppm and
number of peaks as normal (i.e. cancer-free) or abnormal ~i.e.
cancerous) based upon the stored information. This may be done
by comparison, subtraction, or any other appropriate
mathematical operation.
In the preferred embodiment, the selecting and measuring
means is pre-adjusted to measure the areas or intensities of
the 2.0 ppm and 2.8 ppm resonances of the peroxidized
lipoproteins in the spectra. This may include suppressing the
~;UBSTlTVT S~:ET
WO92/01419 PCT/US91/051~2
~g~17i
19
signal of water from the NMR spectrum of the sample, or may
alternatively be done directly where the spectrometer is
sensitive enough t~ do so.
EXAMPLE
In this example, the method of the present invention was
applied to a group of 40 patients. The samples were tested in
accordance with the method of the instant i.nvention. The
results as show in Figure 3 show the clustering of those
samples indicating malignancy. The area of the resonance lines
generated according to the method of the instant invention were
measured. The results show that the ratio of the samples
tested (2.0 ppm to 2.8 ppm) is an indicator of the presence of
cancer.
The invention may be embodied in other specified forms
without departing from the spirit or essential characteristics
thereof. The present embodiments are therefore to be
considered in all respects as illustrative and not restrictive,
the scope of the invention being indicated by the appended
claims rather than by the foregoing description, and all
changes which come within the meaning and range or equivalency
of the claims are therefore intended to be embraced therein.
What is claimed is-
SUEi STITUTF SH~ T
: