Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2~89~6~
3-Aryl or 3-~hetaryl~-carbolines, their production
and use in pharmaceutical agents
The invention relates to new 3-hetaryl or 3-aryl-~-
carbolines, their production and use in pharmaceutical agents.
From numerous publications, such as, for example, from EP-A
110814, it is known that ~-carbolines affect the central nervous
system and are suitable as psychopharmaceutical agents. It was
shown in a surprising way that the ~-carbolines substituted
according to the invention in 3-position are bioavailable over a
prolonged period and at the same time have a good affinity for
the benzodiazepine receptors.
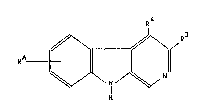
The compounds according to the invention have formula I
R
~ ¦~
in which
RA means halogen, -CHR1-R2, phenyl optionally substi~uted
with halogen, C14-alkoxy or amino, hetaryl or oR5 and can be
single to double and
R1 represents hydrogen or C14-alkyl,
R2 represents hydroyen, C14-alkyl, -0-Cl~-alkyl or an
optionally substituted phenyl, benzyl or phenoxy radical and
2 2~89~69
Rs represents hydrogen, C16-alkyl, C37-~ycloalkyl or an
optionally substituted phenyl, benzyl, hetaryl or benzocondensed
hetaryl radical,
R~ represents hydrogen, C14-alkyl or Cl4-alkoxy-C12-alkyl and
R3 represents a C612-aryl or hetaryl radical optionally
substituted singly or multiply with C14-alkyl, C37-cycloalkyl,
halogen, C14-alkoxy-C1~-alkyl, phenyl or amino as well as their
isomers and acid addition salts.
Substituent RA can be in the A ring in 5-8 position,
preferably in 5, 6 or 7 position.
An alkyl contains respectively straight-chain as well as
branched-chain radicals such as, for example, methyl, ethyl,
propyl, isopropyl, butyl, isobutyl, sec. butyl, tert. butyl,
pentyl, isopentyl and hexyl.
By halogen is meant fluorine, chlorine, bromine and iodine
respectively.
Cycloalkyl respectively can stand for cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl and 2-methyl-
cyclopropyl, and 3-5 carbon atoms are preferred.
If Rs or RA means a hetaryl radical, then the latter is 5-
or 6-membered and contains 1-~ heteroatoms such as nitrogen,
oxygen and/or sulfur. For example, the following 5- and 6-ring
heteroaromatic compounds can be mentioned: pyridine, pyrimidine,
pyrazine, pyridazine, furan, thiophene, pyrrole, thiazole,
imidazole, triazine.
Pyridine, thiophene and furan are to be considered as the
preferred hetaryl radical RA.
2~9~
If R5 is a benzocondensed hetaryl radical, then it
preferably contains 1-2 nitrogen atoms such as quinoline,
isoquinoline, quinoxaline or benzimidazole.
The substituent of phenyl, benzyl, hetaryl and
benzocondensed hetaryl radical Rs can be single to triple in any
position. ~uitable substituents are halogens, nitro, cyano, C14-
alkyl, C14-alkoxy, amino, C14-alkoxycarbonyl, C14-alkylthio and
trifluoromethyl, and for the phenyl and benzyl radical, the
single to double substitution with halogens is preferred.
As preferred hetaryl radicals and benzocondensed hetaryl
radicals R5 nitrogen-containing heterocycles are to be
considered, that optionally are single to double substituted with
halogen, C1~4-alkyl, C14-alkoxy or trifluoromethyl, especially
with halogen.
As substituents of phenyl, benzyl and phenoxy radical R2 the
substituents of the aromatic compounds mentioned for Rs are
suitable, especially halogen such as chlorine and bromine.
Aryl and hetaryl radical R3 can be present as monocyclic or
bicyclic compounds and contain 5-12 ring atoms, preferably 5-9
ring at s, such as, for example, phenyl, biphenylyl, naphthyl,
indenyl as aryl radical and thienyl, furyl, pyranyl, pyrrolyl,
pyrazolyl, pyridyl, pyrimidinyl, pyridazinyl, oxazolyl, iso-
oxazolyl, thiazolyl, isothiazolyl, 1,3,4-oxadiazol-2-yl,
quinolyl, isoquinolyl, benzo[1]thi-enyl, benzofuryl as hetaryl
radical with 1-3 heteroatoms such as sulfur, oxygen and/or
nitrogen.
2 ~ 6 ~
The substituent of aryl and hetaryl radical R3 can be single
or triple~ especially single.
As preferred embodiments there can be mentioned; RA in the
meaning of oR5, and R5 means Cl6~alkyl or an optionally single or
double substituted phenyl or benzyl radical or a five or six
membered optionally benzocondensed heterocyclic compound with 1-3
nitrogen atoms, that is optionally single to double substituted,
and R3 meaning phenyl optionally substituted with halogen or C14-
alkoxy or an optionally substituted five or six membered
heterocyclic compound with 1-3 heteroatoms, such as 1,3,4-
oxadiazol-2-yl, thienyl, pyrrolyl, pyridyl, thiazolyl, oxazolyl
or a benzocondensed heterocyclic compound such as benzothienyl.
As substituents of the thiazolyl radical, C14-alkyl and phenyl
are to be considered as preferred and as substituent of the
1,3,4-oxadiazolyl radical, C14-alkyl, C14-alkoxy-C12-alkyl, C37-
cyclopropyl and amino are preferred.
If a chiral center is present the compounds of formula 1 in
the form of stereoisomers and their mixtures can be present.
The physiologically compatible acid addition salts are
derived from the known inorganic and organic acids such as, for
example, hydrochloric acid, hydrobromic acid, sulfuric acid,
phosphoric acid, formic acid, acetic acid, benzoic acid, maleic
acid, fumaric acid, succinic acid, tartaric acid, citric acid,
oxalic acid, glyoxylic acid as well as from alkanesulfonic acids
and arylsulfonic acids, such as, for example, methanesulfonic
acid, ethanesulfonic acid, benzenesulfonic acid, p-
toluenesulfonic acid, i.a.
20~6~
The compounds of formula I as well as their acid addition
salts are usable as pharmaceutical agents because of their
affinity to benzodiazepine receptors and have an antagonistic,
inverse agonistic and agonistic effect on the properties known of
the benzodiazepines~ At the same time the compounds according to
the invention show an extended duration of action and are
distinguished by anxiolytic effectiveness. The affinity to the
benzodiazepine receptors is determined by tests of th~
displacement capacity of radioactively labeled flunitrazepam by
the benzodiazepine receptors. For examination of the anxiolytic
effect the compounds are tested in a 4-plate test according to
the methods of Boissier et al, Eur. J. Pharmacol. 4, 145-150
(1968). Thus the minimal effective dosage (MED) is given, that
increases the locomotor activity of the afflicted mice after i.p.
treatment. A reduction of the activity in the 4-plate test
without being afflicted indicates a sedative effect.
The compounds of formula I are suitable especially for the
treatment of anxiety accompanied by depression and disturbed
sleep.
For use of the compounds according to the inv~ntion as
pharmaceutical agents they are put in the form of a
pha~naceutical preparation, that, besides the active ingredient
for enteral or parenteral administration, contains suitable
pharmaceutical, organic or inorganic inert vehicles such as, for
example, water, gelatin, gum arabic, lactose, starch, magnesium
stearate, talc, vegetable oils, polyalkyleneglycols, etc.
6 2089~
The pharmaceutical preparations can be available in solid
form, for example, as tablets, coated tablets, suppositories,
capsules or in liquid formJ for example, as solutions,
suspensions or emulsions. Moreover, optionally, they contain
auxiliary agents such as preservatives, stabilizers, wetting
agents or emulsifiers, salts for changing the osmotic pressure or
buffers.
Especially suitable for parenteral use ars injection
solutions or suspensions, especially aqueous solutions of the
active compounds in polyhydroxy-ethoxylated caster oil.
As vehicle systems surface-active auxiliary agents such as
salts of bile acids or animal or vegetable phospholipids, but
also their mixtures as well as liposomes or their components can
be used.
. .
Especially suita~le for oral use are tablets, coated tablets
or capsules with talc and/or hydrocarbon vehicles or binders,
such as, for example, lactose, corn or potato starch. The use
can even take place in liquid form, such as, for example, as
juice to which a sweetener is optionally added.
The compounds according to the invention are generally
introduced in a dose unit of 0.05 to 100 mg of active substance
in a physiologically compatible vehicle.
The compounds according to the invention generally are used
in a dose of 0.1 to 300 mg/day, preferably 0.1 to 30 mg/day,
especially preferred 1-20 mq/day, for example as anxiolytic
agents analogous to diazepam.
208~rj6~
The production of the compounds according to the invention
takes place according to methods known in the art. For Pxample,
compounds of formula I are achieved in that
a) a compound of formula II
R
// H a l
H
in which
RA and R4 have the above meaning and Hal is halogen, is
arylated in the presence of a nickel or palladium catalyst with
an organometallic compound of formula III
R -Me-Xn I I I .
in which
R3 has the above meaning,
Me means a metal atom,
X means halogen, hydroxy or C14-al:kyl and
n means 1 to 3, or
b) a compound of formula IV
R~
C O - N ~I - N N 2
. 8 208~69
in which RA and R4 have the above meaning, is cyclized with
orthocarboxylic acid esters to compounds of formula I with ~3
meaning a l,3,~-oxadiazol-2-yl radical, that is optionally
substituted with C14-alkyl, C14-alkoxy-C12~alkyl, phenyl or C3 7-
cycloalkyl or is cyclized with bromocyanogen to a compound of
formula I with R3 meaning a 5-amino-l,3,4-oxadiazol 2-yl radical
or
c) a compound of formula V
R~
CO-CH _Z
R9
in which
RA and R4 have the above meaning, Z is halogen and R9
represents hydrogen or a protective group, is cyclized with
thiocarboxylic acid amides to compounds of formula I with R3
meaning a thiazol-4-yl radical optionally substituted in 2-
position and then the protective group is optionally cleaved off
or
d) a compound of formula VI
R~
~k ~ N
in which
2 0 ~
RA and R4 havs the above meaning is cyclized with
tosylmethylisocyanide in the presence of a base to compounds of
formula I with R3 meaning an oxazol-5-yl radical and optionally
then thP benzyl group R5 is cleaved off or RA meaning hydroxy is
etherified or the isomers separated vr the acid addition salts
formed.
The arylation according to process variant a) takes place in
a solution or in a suspension in inert solvents at temperatures
of 0C up to the boiling temperature of the reaction mixture.
A~ solvents, for example, cyclic and acyclic ethers such as
diethyl ether, tetrahydrofuran and dioxane, hydrocarbons such as
toluene and benzene as well as aprotic, polar solvents such as
dimethylformamide, dimethylacetamide, N-methylpyrrolidone, i.a.,
are suitable. In the case of boron an addition of protic
solvents such as, e.g., alcohol, is not harmful.
As halogen hal, especially bromine and iodine are to be
considered.
The organometallic compound of general formula III contains
as metal atom lithium, magnesium, zinc, tin or boron, and
substituent X, depending on the valence of the metal atom, can be
single to triple, and X as halogen is especially chlorine or
bromine.
Suitable nickel and palladium catalysts are, for example,
1,3-diphenylphosphinopropane-nickel-II-chloride, bis-tri-o-
tolylphosphine-palladium-II-chloride, bis-triphenylphosphine-
palladium-II-chloride, tetrakis-triphenylphosphine-palladium-(O)
and l,l'-bis-diphenylphosphinoferrocenepalladium-II-chloride.
lo ~`8~
The representation of 1,3,4-oxadiazoles according to process
variant b) takes place by heating of B-carboline-3-carboxylic
acid hydrazdes with orthocarboxylic acid esters in solution or in
suspension and then cyclization in the presence of a base such
as, for example, alkali alcoholates such as sodium or potassium
ethylate, -tert. butylate.
As solvents the corresponding alcohols are suitable.
The reaction takes place at temperatures up to the boiling
temperature of the reaction mixture and is completed after
approximately 2-10 hours.
5-Amino-1,3,4-oxadiazoles are produced according to process
variant b) by cyclization of the compounds of formula IV with
bromocyanogen in protic solvents such as, for example, alcohols
at elevated temperature, preferably 20-50C, and then treated
with a base such as, for example, ammonia.
For the introduction of the thia~ole group according to
process variant c) compounds of formula V, especially 3-
bromoacetyl or 3-chloroacetyl-~-carbolines with thiocarboxylic
acid amides such as thioformamide, C1~-alkyl-CSNH2, thiobenzoic
acid amide in protic solvents such as alcoholates, are refluxed.
Optionally present protective groups in 9-position of the ~-
carboline such as, for example, alkanoyl, arylsulfonyl,
alkylsulfonyl or trialkylsilyl protective groups can be cleaved
off with the usual methods such as treatment with a base, for
example alkali alcoholate or hydroxide or acids such as dilute
mineral acid or with fluorides such as cesium fluoride at room
temperature or elevated temperature.
11 2~ 9
For the production of oxazoles according to process variant
d) 3-carbaldehyde-~-carbolines of formula VI are reacted with
tosylmethyl isocyanide in suspension or in solution. The
reaction takes place in the presence of a base such as
alkalicarbonates or alkali alcoholate~ in protic solvents such as
alcohols at temperatures up to the boiling temperature of the
reaction mixture and is completed after about 1-3 hours.
If cleaving off of radical R5 is desired, then it takes
place according to the pr~cesses described in EP-A-130 140 or by
hydrogenolytic cleavage.
The optionally following etherification of the free hydroxy
group takes place according to the process described in EP-A-237
467, by reactive compound RA-Y in which Y means, for example,
halogen, tosylate, mesylate or triflate, being reacted in the
presence of a base such as alkali alcoholate or hydroxide in
polar solvents such as dimethylsulfoxide, dimethylformamide,
acetonitrile or alcohols at room tempexature or elevated
temperat~lre, optionally in the presence of phase transfer
catalysts~
The isomer mixtures can be separated according to the usual
methods, such as, for example, crystallization, chromatography or
salt formation, in the diastereomers or enantiomers.
For the formation of the physiolo~ically compatible acid
addition salts a compound of formula I, for example, is dissolved
in a little alcohol and mixed with a concentrated solution of the
desired acid.
12 2 ~ 8 ~
If the production of the initial compounds is not described,
they are known or are producible analogously to known compo~mds
or processes described here.
3-Halogen-~-carboline derivatives of formula II are obtained
according to the methods described in EP-A-110 814 or according
to a Sandmeyer variant in bromoform as solvent with
isoamylnitrite in the presence of polyethylene glycolO
The representation of the compounds of formula IV takes
place by heating of ~-carboline-3-carboxylic acid alkyl ester
with hydrazine hydrate.
By oxidation of 3-hydroxymethyl-~-carbolines according to
the process described in EP-A-54 507 or according to the
syntheses described in EP-A-305 322, 3-carbaldehyde-~-carbolines
of formula VI are obtained.
The following examples are to explain the process according
to the invention.
- 13
~89~
Production of the initial materials:
A.~ 6-Ben2yloxy-4-methoxymQthyl-~-c~rboline-3-car~oxylic
a~i~
is produced according to the process given in EP-A-161 574
from 6-benzyloxy-4-methoxymethyl-~-carboline-3-carboxylic acid
isopropyl ester.
Melting point 243C.
B.~ 3-Trimethylsilylethyloxycarbonylami~o-6-benzyloxy-4-
metho~ymethyl-~-carboline
3.62 g of 6-benzyloxy-4-methoxymethyl-~-carboline-3-
carboxylic acid is dissolved clear in 50 ml of dimethylformamide,
mixed with 4.3 g of phosphoric acid diphenylesterazide and 1.4 ml
of triethylamine and stirred for 2 hours at 80C bath temperature
under argon. After cooling it is mixecl with 2.9 ml of 2-
trimethylsilylethanol and heated for 4 hours to 80C bath
temperature. After standing overnight it is concentrated by
evaporation in a vacuum. The residue is taken up in ethyl
acetate and washed once each with saturated sodium bicarbonate
solution and common salt solution. The organic phase is dried,
filteredr and concentrated by evaporation. The residue is
introduced without further purification in the next step.
C.) 3-Amino-6-benzyloxy-4-methoxymethyl-~-carboline
5 g of 3-trimethylsilylethoxycarbonylamino-6-benzyloxy-4-
methoxymethyl-~-carboline in 40 ml of tetrahydrofuran is heated
with 22 ml of tetrabutylammonium fluoride for 3 hours to 50C.
2 ~
After distilling off the solvent it i5 taken up in ethyl acetate
and washed once each with a saturated sodium-carbonate and
saturated common salt solution. The organic phase is dried,
filtered and concentrated by evaporation. The residue is
chromatographed on silica gel with methylene chloride : ethanol =
10 : ~ as eluant. After concentration by evaporation of the
corresponding fractions and absorptive precipitation with
cyclohexane/ethyl acetate, 1.32 g of 3~amino~6-benzyloxy-4-
methoxymethyl-~-carboline is obtained.
D.) 3-Bromo-6-benzyloxy-4-methoxymethyl-~-carboline
1 g of 3-amino-6-benzyloxy-4-methoxymethyl-~-carboline in 32
ml of bromoform is mixed with 0.6 ml of HBr/glacial acetic acid
(33%) and 5 g of PGE2~0. 0.46 ml of isoamylnitrite is added at
9C and it is maintained for 30 minukes at this temperature.
Then 0.56 g of copper(I) bromide is adcled to this batch. After
heating of the batch to room temperature it is diluted with
methylene chloride within one hour and extracted once each with
dilute ammonia solution and with water. The organic phase is
dried, liltered and concentrated by evaporation. The residue is
chromatographed on silica gel with methylene chloride : ethanvl =
15 : 1. The corresponding combined fractions are concentrated by
evaporation and absorptively precipitated with ethyl
acetate/hexane. 0.54 g of 3-bromo-6-benzyloxy-4-methoxymethyl-~-
carboline is obtained.
2 ~
E.) 6-Benzylo~y-4-methoXymethyl~3-acetyl-~tosyl-~-carboline
8.4 g of 6-benzyloxy-4-methoxymethyl-9-tosyl-~-carboline-3-
carboxylic acid isopropyl ester is dissolved in 500 ml absolute
tetrahydrofuran and cooled to -60C. 15 ml of a 1.5 molar
ethereal methyllithium solution i5 added drop by drop to this
solution under argon atmosphere and then stirred for anothsr 4
hours at -60C. After heating to room temperature the reaction
solution is mixed with saturated ammonium chloride solution,
extracted with methylene chloride, dried and concentrated by
evaporation. The resulting crude product is chromatographed on
silica gel with cyclohexane : ethyl acetate = 8 : 2. 4.1 g o 6-
benzyloxy-4-methoxymethyl-3-acetyl-9-tosyl-~-carboline with a
melting point of 173-175C is obtained.
F.) Bromination
2.29 g of 6-benzyloxy-4-methoxymethyl-3-acetyl-9-tosyl-~-
carboline is stirred with the double molar amount of
phenyltrimethylammonium tribromide in 100 ml of absolute
tetrahydrofuran for 4 days at room temperature. The reaction
mixture is evaporated to dryness, taken up in methylene chloride,
washed with saturated NaCl solution, dried on Sikkon and
concentrated by evaporation. The crude product is
chromatographed on silica gel (cyclohexane/ethyl acetate = 8 +
2). 1.9 g of 6-benzyloxy-3-bromoacetyl-4-methoxymethyl-9-tosyl-
~-carboline is obtained. Melting point 165C (decomposition).
16
2 ~ 6 ~
~ .) 5 Benzyloxy-4-methogymethyl~ arboline-3-carboxylic
acid-hydrazide
1.56 g of 5-benzyloxy-4-methox~nethyl-~-carboli~e-3-
carboxylic acid-ethyl ester is refluxed in 20 ml of hydrazine
hydrate (80~) for 4 hours. After cooling, the precipitated
crystals are suctioned off and dried. 1.2 g of 5-benzyloxy-4-
methox~nethyl-~-carboline-3-carboxylic acid-hydrazide of a
melting point of 212-213C is obtained.
In a analogous way, there are produced:
6-benzyloxy-4-methoxymethyl-~-carboline-3-carboxylic acid-
hydrazide, melting point 228-231C
5-(4-fluorobenzyloxy~-4-methoxymethyl-~-carboline-3-
carboxylic acid-hydrazide, melting point 218-225C
5-(4-chlorophenoxy)-4-methoxymethyl-~-carboline-3-carboxylic
acid-hydrazide, melting point 194-198C
5-isopropoxy-4-methyl-~-carboline-3-carboxylic acid
hydrazide, melting point 240-243C
6/7-dimethoxy-4-ethyl-~-carboline-3-carboxylic acid
hydrazide, melting point Z53-255C
6-benzyloxy-4-ethyl-~~carboline-3-carboxylic acid hydrazide,
melting point 265-268C
Exampla 1
6-Benzyloxy-3-phenyl-4-methoxymethyl-~-c~rboline
476 mg of 3-bromo-6 benzyloxy 4 methoxymethyl-~-carboline is
introduced in 10 ml of toluene and 5 ml of ethanol, mixed with 41
mg of tetrakis(triphenylphosphine)-palladium(0) and stirred for 5
2 ~
minutes. Then 177 mg of phenylboronic acid and 1.5 ml of a 2 m-
soda solution are added and then heated for 3 hours to 95C.
Aîter concentration by evaporation it is taken up in a lot of
ethyl acetate and washed once each with water and saturated
common salt solution. After drying, filtration and concentration
by evaporation, it is recrystallized. 372 mg of 6-benzyloxy-3-
phenyl-4-methoxymethyl~ carboline of a melting point of 205-
206C (ethanol, methylene chloride, hex~ne) is obtained.
In a analogous way, there are produced:
5-benzyloxy-4-methoxymethyl-3-(2-thienyl)-,B-carboline,
melting point 189-191C
5-benzyloxy-4-methoxymethyl-3-(2-pyrrolyl)-,B-carboline,
melting point 203-205C
6-benzyloxy-4-methoxymethyl-3-(2-thienyl)-,B-carboline,
melting point 179C
6-benzyloxy-4-methoxymethyl-3-(3-thienyl) ,B-carboline,
melting point 211-213C
6-benzyloxy-4-methoxymethyl-3-(3-pyridyl)-~-carboline,
melting point 195-196C
6-benzyloxy-4-methoxymethyl-3-(2-benzothienyl)-,B-carboline,
melting point 224-226C
6-benzyloxy-4-methoxymethyl-3-(2-methoxyphenyl)~ carboline,
melting point 202-20C
18
~ ~ `8 ~
E~ample 2
6-Benzyloxy-4-metho~ymethyl 3-(2-methyl-4-thiazolyl)-~-
carboline
a.)
0.530 g of 6-benzyloxy-3-bromoacetyl-4-methoxymethyl-9-
tosyl-~-carboline is refluxed for 3 hours in 50 ml of ethanol
with 0.07 g of thioacetamide. After concentration by evaporation
the residue is chromatographed on silica gel with cyclohexane and
ethyl acetate = 1 ~ 1 as eluant. 0.260 g of 6-benzyloxy-4-
methoxymethyl-3-(2-methyl-4-thiazolyl)-9-tosyl-~-carboline of a
melting point 181-182C is obtained.
In a analogous way, there is produced:
6-benzyloxy-4-methoxymethyl-3-(2-phenyl-4-thiazolyl)-9-
tosyl-~-carboline, melting point 173-175C
b.)
0.260 g of 6-benzyloxy-4-methoxy-3-(2-methyl-4-thiazolyl)-9-
tosyl ~-carboline is refluxed for 3 hours in 30 ml of a sodium
alcoholate solution (0.015 g of sodium in 30 ml of methanol).
After concentration by evaporation the organic phasej it is
chromatographed on silica gel with hexane + acetone = 1300 + 700.
0.180 g of 6-benzyloxy-4-methoxymethyl-3-(2-methyl-4-thiazolyl)-
~-carboline of a melting point of 260C (decomposition) is
obtained.
In a analogous way, there is produced:
6-benzyloxy-4-methoxymethyl-3-(2-phenyl-4-thiazolyl)-~-
carboline, melting point 134-135C
19
2 ~
Example 3
6-Benzylo~y-~-methoxymethyl-3-~5-oxazolyl)-carboline
1 04 g of S-benzyloxy-4-methoxymethyl-~-carboli~e-3-
carbaldehyde and 0.68 g of tosylmethylisocyanide are added to a
suspension of 1 g of powdered potassium carbonate in 30 ml of
methanol. The batch is stirred first for 2 hours at room
temperature and then refluxed for 1 hour. After concentration by
evaporation it is taken up in 100 ml of ethyl acetate and the
organic phase is washed twice each with 50 ml of im-sodium
hydroxide solution, dried, filtered and concentrated by
evaporation. The residue is absorptively precipitated with ether
`and 550 mg of 6-benzyloxy-4-methoxymethyl-3-(5-oxazolyl)-~-
carboline of a melting point of 208-210C is obtained.
E~ample 4
5-Benzyloxy-4~methoxymethyl-3-(5-ethyl-1,3,4-oxadiazol-2-
yl)-~-carboline
1.6 Orthopropionic acid triethyl ester is added to a
suspension of 0.3 g of 5-benzyloxy-4-methoxymethyl-~-carboline-3-
carboxylic acid hydrazide in 20 ml of methanol and then refluxed
for 4 hours. After the concentration by evaporation the residue
is mixed in 20 ml of n-butanol with 0.14 g of potassium-tert.-
butylate and refluxed for another 5 hours. After the distilling
off of the solvent it is taken up in methylene chloride and
washed once each with saturated sodium bicarbonate and common
salt solution. The organic phase is dried, filtered and
concentrated by evaporation. The residue is chromatographed on
2 ~
silica gel with methylene chloride : ethanol = 1 : 1. 0.236 g of
5-benzyloxy-4-methoxymethyl-3-(5-ethyl 1,3,4-oxadiazol-2-yl)-~-
carboline of a melting point of 230-233C is obtained.
In a analogous way, there are produced:
6-benzyloxy-4-methoxymethyl-3-(5-methyl-1,3,4-oxadiazol-2-
yl)-~-carboline, melting point 234-235C
6,7-dimethoxy-4-ethyl-3-(5-methyl-1,3,4-oxadiazol-2-yl)-~-
carboline, melting point 265-268C
6-benzyloxy-4-ethyl-3-(5-methyl-1,3,4-oxadiazol-2-yl)-~-
carboline, melting point 259-260C
5-(4-chlorophenoxy)-4-methoxymethyl~3-(5-methyl-1,3,4-
oxadiazol-2-yl)-~-carboline, melting point 257-259C
5-(4-chlorophenoxy)-4-methoxymethyl-3-(5n-butyl-1,3,4-
oxadiazol 2-yl)-~-carboline, melting point 215-217C
6-benzyloxy-4-methoxymethyl-3-(5-methoxymethyl=1,3,4-
oxadiazol-2-yl)-~-carboline, melting point 180-182C
5-(4-chl~rophenoxy)-4-methoxymethyl-3-(5-methoxymethyl-
1,3,4-oxadiazol-2-yl)-~-carboline, melting point 216 218C
6-benzyloxy-4-methoxymethyl-3-(5-cyclopropyl-1,3,4-
oxadiazol~2-yl)-~-carbolinel melting point 235-237C
5-isopropyloxy-4 methyl-3-(5-cyclopropyl-1,3,4-oxadiazol-2-
yl)-~-carboline, melting point 228-232C
5-isopropyloxy-4-methyl-3-(5-methoxymethyl-1,3,4-oxadiazol-
2-yl~-~-carboline, melting point 220-222C
5-benzyloxy-4-methoxymethyl-3-(5-methoxymethyl-1,3,4-
oxadiazol-2-yl~-~-carboline, melting point 195-198C
21
5-benzyloxy 4-methoxymethyl-3-(5-methyl-1,3,4-oxadiazol-2-
yl)-,~-carboline, melting point 215-216C'
5-benzyloxy-4-methoxymethyl-3-(5-H-1,3,4-oxadiaY.ol-2 yl)-,B-
carboline, meltin~ point 200-205C
6-benzyloxy-4-methoxymethyl-3-(5H-1,3,4-oxadiazol-2-yl)-,B-
carboline, melting point 233-235C
5-(4-fluorobenzyloxy)-4-methoxymethyl-3-(5-ethyl-1,3,4-
oxadiazol-2-yl)-,B-carboline, melting point 243-244C
Example 5
6-Ben~yloxy-4-methoxymethyl-3-~5-amino-1,3,4-oxildiazol-2-
yl~ -carboline
O.376 g of 6-benzyloxy-4-methoxymethyl-,B-carboline-3-
carboxylic acid hydrazide is mixed in 10 ml of methanol with
0.106 g of bromocyanogen and heated for 1 hour to 40-45C. After
cooling the reaction solution is made alkaline with ammonia
solution and the precipitate is suctioned off, washed with water
and dried. After absorptive precipitation with cyclohexane/ethyl
acetate, 0.372 g of 6-benzyloxy-4-methoxymethyl-3-(5-amino-1,3,4-
oxadiazol-2-yl)-,8-carboline of a melting point of 290-293C is
obtainecl.