Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
HOECHST AXGIENGES~LLSCHAFT HOE 92/F 049 Dr. Th/wo
Description
The use of xanthine derivatives for the treatment of
muscle damage after interruption of the blood circulation
A number of oxoalkyl- and hydroxyalkylxanthine~ promote
S blood flow and can also be employad for the treatment of
disorder~ of muscular energy metabolism as are present,
in particular, in cases of mitochondrial myopathy
(EP 0 268 585).
Tourniquets axe routinely used in surgery on the extrem-
ities in order to obtain an operation area free ~f blood.
Although there are reports that interruptions of blood
flow are poRsible for up to three hours, surgeons gener-
ally restrict the interruption of blood flow to 90 -
120 minutes (Tountas and Bergman, J. Hand. Surg., 1977
2 : 31 ~ 37) because more prolonged i~chemia with sub~e-
quent restoration of blood flow leads to a number of
types of muscle damage, for example swelling of the
muscle fibers, degeneration of the muscle cells, slight
sensory and motor damage, destabilization of the membrane
of muscle cells, in which case there is disturbance of
the intracellular medium, or difficulties in rehabilita-
tion. It is known that damage may occur a~ter restoration
of blood flow in ischemic tissue in the heart, kidney,
intestine and skeletal muscles ( McCord, ~ew England,
J. Med., 1985, 312 (3), 159 - 163). To date, no pharma-
ceuticals which allow the time of interruption of blood
circulation to be prolonged or the disturbances of
function caused by the interruption of the blood circula-
tion to be significantly reduced are known.
It has now been found that certain xanthine derivatives
are suitable for prolonging the tLme of interruption of
blood flow, for improving the syndromes which occur after
interruption of blood flow and subsequent restoration of
blood flow, and for reducing the changes in the intra-
compartmental pressure.
20899~9
-- 2 --
Interruption of the blood circulation in tissues, organsor extremities occurs, for example, in operations after
injuries to the large arteries or vein~, after emergency
opexations in case~ of severance of limbs, removal of
thrombi or other occlusions in arteries or veins or in
tran~plantations, for example of the kidney or heart.
Depending on the duration of the operation, there may
then be more or less pronounced disturbances of function
of the muscle tissue, which may range from slight dis-
turbances to motor deficits, for example paralysis.Partial or brief interruptions of the blood circulation
lead as a rule only to reversible damage at the start of
the interruption. Intact muscle tissue with a low extra-
cellular potassium content and the possibility of com-
plete regeneration remains. Surprisingly, experimentalanimals with vascular occlusion in the rear extremities
show a rapid recuperation o:E the muscle fibers on treat-
ment with the xanthines of the formula I.
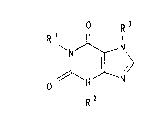
The invention therefore relates to the use of at least
one xanthine derivative of the formula I
R3
N ~ N
il \ (I)
O //~ N--~
R 2
in which at least one of the radicals R1 and R3 is a
tertiary hydroxyalkyl group of the formula Ia
R~
~ ( C H 2 ) n~ C ~ C H3 ~Ia)
O H
2 0 ~ 9
-- 3 --
in which R4 is an alkyl group with 1, 2 or 3 carbon atoms
and n is an integer of 2 - 5 and - if only one of the
radicals R1 or R3 is such a tertiary hydroxyalkyl group of
the formula Ia - the other radical is a hydrogen atom or
an aliphatic hydrocarbon radical R5 which has 1 to 6 car-
bon atoms and who~e carbon chain can be interrupted by 1
or 2 oxygen atoms or substituted by an oxo group or by 1
or 2 hydroxyl groups, these oxo and hydroxyl groups being
separated by at least 2 carbon atoms from the ring
nitrogen, and R2 is an alkyl group with 1 - 4 carbon
atoms, for the preparation of pharmaceuticals for the
prophylaxis and treatment of muscle damage which may
occur af~er interruption of ~he blood circulation.
Xanthine derivatives of the formula I which are preferab-
ly used are those in which R2 is methyl or ethyl and/oronly one of the two radicals R1 or R3 is the tertiary
hydroxyalkyl group of the formula Ia.
Xanthine derivatives of the formula I which are further-
more preferably used are those in which R1 or R3 is
t(~-1)-hydroxy-~-1)-methyl]-pentyl, -he~yl or -heptyl.
In addition, xanthine derivatives of the formula I which
are preferably u~ed are those in which R1 is t(~
hydroxy-(~-l)-methyl]-pentyl, -hexyl or ~heptyl~ R2 is
methyl or ethyl and R3 i6 hydrogen, alkyl or alkoxyalkyl
with, in each case, 1 - 4 carbon atoms or hydroxyalkyl
with 2 - 5 carbon atoms.
7-Ethoxymethyl-1-(5-hydroxy-5-methylhexyl~3-methylxan-
thine is particularly preferably used.
By muscle cells, muscle fibers or muscle tissues are
meant all cells, tissues or organs which are capable of
contraction, especially smooth or striated muscle
tissues, as well as muscle fibers of the heart.
:: 2~99~9
- 4 --
The xanthine derivatives of the formula I are prepared,
for axample, by the following process:
3-Alkylxanthines of the formula II
0 B
A -N ~ ~ (II)
o ~ N N
R 2
in which R2 is an alkyl group with 1 to 4 carbon atoms,
A is a hydrogen atom, R5 or the radical of the
formula Ia and
B is a hydrogen atom, R5, benzyl or diphenylmethyl
radical,
but where at least one of these radicals A and B i~ a
hydrogen atom, are alkylate~ in the presence of at least
one basic condensing agent or in the form of theix salts
in position 1 and/or 7, in one step or ~tepwise, with
appropriate alkylating agents of the formula III
X Q (III)
in which
X is a halogen atom or a sulfonic ester or pho~phoric
ester group and
Q is a tertiary hydroxyalkyl group of the formula Ia,
R5, benzyl or diphenylmethyl xadical,
with ~ubsequent reductive elLmination of the radical B
when this is a benzyl or diphe~ylmethyl group, ort where
appropriate, hydrolytic elLmination of an alkoxymethyl or
alkoxyalkoxymethyl radical from the position of the
radical B and reduction of the keto group to the alcohol
functionality when A or B i~ n oxoalkyl radical, at a
2~ 5 ~
-- 5 --
reaction temperature between 0C and the boiling point of
the particular reaction medium used.
The abovementioned reactionq take place under standard
conditions in a known manner tEP 0 268 585,
US 4 833 146).
The starting materials for the reactions are known or can
be easily prepared by methods known from the literature
(EP 0 268 585).
The invention also relates to phanmaceuticals which
contain at least an effective amount of a xanthine
derivative of the formula I, in addition to pharmaceutic-
ally suitable and physiologically tolerated excipients,
diluents and/or other active and ancillary substances.
The pharmaceuticals according to the invention are
administered parenterally, orally, rectally or, where
appropriate, also topically. The administration takes
place before, during or after an interruption in the
blood circulation.
The invention also relates to processes for the prepara-
tion o~ a pharmaceutical wherein at least one ~anthinederivative of the formula I is converted with a physio-
logically acceptable vehicle a~d other suitable active,
additional or ancillary substances into a form ~uitable
for administration.
~xamples of suitable solid or liquid pharmaceutical
formulations are granule~, powders, coated tablets,
tablets, (micro)capsules~ suppositories, syrups, 801u-
tions, suspensions, emulsion~, drops or injectable
solutions as well as productæ with protracted release of
active substance, in the preparation of which convention-
al aids such as excipients, disintegrants, binders,
coating agents, swelling agents, glidants or lubricants,
.
:
.
2 ~ 8 ~
flavorings, sweeteners or solubilizers are used. Examplea
of ancillary substances which are frequently used and
which may be mentioned are magnesium carbonate, titanium
dioxide, lactose, mannitol and other sugars, talc,
lactalbumin, gelatin, ~tarch, cellulose and its deriva-
tives, animal and vegetable oils, polyethylene glycols
and solvents such as, for example, sterile water and
monohydric or po].yhydric alcohols, for example glycerol.
Because of the pharmacological propertie~ of the xanthine
derivatives, these compounds can be used in all opera-
tions in hospital or outpatient procedures which are
associated with use of a tourniquet and thus interruption
of the blood circulation in the muscle tissue, for
example for vascular injuries in which there is prolonged
bloodlessnes~ in the extremities, uch as clamping of the
aorta, embolus removal by surgical means or delayed
treatment of severed large blood vessels. They can
furthermore be employed to reduce the damage which may
occur af~er restoration of blood flow to muscle fibers of
the heart, or ensure the survival of muscle or skin
grafts permeated by blood vessels.
The pharmaceutical product~. are preferably prepared and
administered in dosage units, where each unit contains as
active constituent a defined dose of at least one xan-
thine derivative of the formula I. In the case of solid
dosage units such as tablets, capsules, coated tablets or
suppositories, this dose is up to about 1000 mg, but
preferably about 100 - 600 mg, and in the case of injec-
tion solutions in ampule form is up to 300 mg, preferably
20 200 mg.
Indicated ~or the treatment of a patient (70 kg) whose
blood circulation has been interrupted is an intravenous
infusion treatment of 100 - 2000 mg per day in early
phaseR and an oral administration of 400 mg 3 times a day
in the later rehabilitation phase, especially of
2û899~9
-- 7 --
7-ethoxymethyl-1-(5-hydroxy-5-methylhexyl)-3-methyl-
xanthine.
However, higher or lower doses are also appropriate in
some circ~6tances. ~he administration of the dose can
take place either by a single administration in the form
of a single dosage unit or of several smaller dosage
units, or by multiple admini~tration of divided doses at
particular intervals.
Finally, the xanthine derivatives of the formula I and/or
their appropriate salts can also be formulated together
with other suitable active substances, for example active
substances which trap free oxygen radicals, for example,
1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one, superoxide
dismutase, dimethyl sulfoxide or mannitol, heparin,
ascorbic acid or deferoxamine, to prepare the abovemen-
tioned pharmaceutical formulations.
Example 1:
Preparation of 7-ethoxymethyl-1-(5-hydroxy-5-methyl-
hexyl)-3-methylxanthine (Compound 1)
a) 7-Ethoxymeth~1-3-methylxanthine
O CH2-O-~H2-CH3
H N ~\1~ ~
o i~\ N N
CH3
83 g (0.5 mol) of 3-methylxanthine are dissolved in a hot
solution of 20 g (Q.5 mol) of sodium hydroxide in 400 ml
of water. After filtration, the filtrate is concentrated
under reduced pressure, methanol is distilled over
, . . ~ ., ~
,
` 208~9~9
-- 8 --
~everal times/ and then the sodium salt is dried under
hi~h vacuum. ~he dried salt is suspended in 1.3 l of
dimethylformamide and, while stirring, 47.3 g (0.5 mol)
of etho~ymethyl chloride are added to the suspension,
which is then stirred at 110C for 18 hours. It i~
subsequently filtered hot, the ~iltrate is evaporated in
vacuo, the residue is dissolved in 500 ml of 2 N sodium
hydroxide solution, and extraction with chloroform is
carried out to remove the 1,7 dialkylated 3-methyl-
xanthine which is formed as by-product. The aqueous
alkaline solution is hd~usted to pH 9 with 2 N hydro-
chloric acid while stirri~g, the crystals which form are
separated off, and the crystals are washed initially with
water until free of chloride and then with methanol and
dried in vacuo.
Yield- 77.6 g (69.2 % of theory)
Melting point: 263 - 264C
CgHl2N403 (MW = 224.2)
b) 7-Ethoxymethyl-1 (5-hydroxy-5-methylhexyl~-3-
methylxanthine
CH3 CH2-O-CH2-cH3
H,C-C- ( CH2 )~-N/~
C H o N N
C H 3
11.2 g ~O.OS mol) of 7-ethoxymethyl-3-methylxanthine in
300 ml of dimethylformamide are mi~ed with 7.5 g
(0.054 mol) of potassium carbonate and 8.2 g (0.054 mol)
of l-chloro-5-hydroxy-5~methylhexane and heated at 110C
while stirring for 5 hours. The mixture is filtered hot
with suction, the filtrate is concentrated in vacuo, the
residue is taken up in chloroform, the solution is washed
first with 1 N sodium hydro~ide solution and then with
.~ ~
20899S9
g
water until neutral and is dried over sodi~m sulfate, the
solvent is removed by distillation under reduced pres-
sure, and the residue i8 recrystallized from diisopropyl
ether with the addition of ethyl acetate and petroleum
ether.
Yield: 14.1 g (83.3 ~ of theory)
Melting point: 102 - 103C
Cl5H26N404 (MW = 33a.4)
Analysis: Calculated: C 56.79 % H 7.74 % N 16.56 %
Found: C 56.76 % H 7.8~ ~ N 16.59
Example 2:
Preparation of a pharmaceutical
An iniectable pharmaceutical is prepared as follows:
10 g of the compound from Example l are di~solved in
water by stirring and 810wly heating, and the volume is
finally made up to 1000 ml with water. The resulting
solution is filtered through a 0.2 ~m membrane filter and
dispensed into ampules containing 5 or 10 ml which, after
sealing, are sterilized at 120C for 15 minutes.
Tablets which contain 250 mg of the compound from
Example 1 are prepared in a conventional way by mixing
200 g of the compound from Example I, 150 g of lactose,
30 g of starch, 10 g of crospovidone, lO g of talc, 2 g
of colloidal silicon dioxide and 1.5 g of magnesium
stearate. The tablets receive a coating of an aqueous
suspension composed of 40 g of hydroxypropylmethylcellu-
lose, 2 g of polyethylene glycol with an avexage molecul-
ar weight of ~000, 3.5 g of ~itanium dioxide, 3 g of talc
and 451.5 g of water. About 5 to 10 mg of solids are
applied as coating to each tablet.
Example 3:
Two groups of eight adult male South African long-tailed
monkeys (Cercopithecus pygerythrus) are investigated. The
.
-' ' ' ~
20~99~9
-- 10 --
anesthesia of the animals is induced with 15 mg/kg
ketamine (Warner Lambert) intramuscularly and is main-
tained with thiopentone sodium (25 mg~kg, May of Baker)
intravenously. One rear extremity is emptied of blood by
S a pneumatic tourniquet and an Esmarch bandage. The
interruption of the blood circulation is maintained for
three hours, and then the interruption is terminated and
the animals are able to awake from the anesthesia. The
removal of samples of muscle tissue takes place under
anesthesia (15 mg!kg ketamine).
20 mg/kg compound 1 is adminiæt~red intravenously to the
animals over 30 minutes before the interruption of the
blood circulation.
Muscle preparation:
The removed muscle tissue is immediately frozen in
isopentane at -183C. The muscle tissue is prepared as
described by Dubowitz (Dubowitz et al. Muscle Biopsy A
Practical Approach, London, Bailliere Tindall, 1985,
pp. 82 - 128). Microscopic sections are stained with
muscle fi~er ATPase at pH 9.4 for 30 minutes, embedded
and examined under a light microscope. The diameters of
at least 200 muscle fibers are evaluated with a computer-
ized video image analyzer.
The average diameter of each type of muscle fiber is
indicated in Table 1.
2~89~9
11 -
Table 1
Before inter- Interruption After After
ruption of of the 3 hrs 6 hrs
the blood blood
circulation circulation
Muscle
Fiber
Type 1
Control 34.45 34.07 39.2 38.85
Compound 1 36.50 36.28 39.89~ 43.42
Muscle
Fiber
Type 2a
Control 39.99 37.05 41.91 44.08
Compound 1 45.09 44.75 48.55~ 53.37
Before interruption Interruptlon of After 12 After 18 After 24
of the blood the blood hours hours hours
circulation circulatlon
Muscle
Fiber
Type 1
Control 34.45 33.63 37.03' 37.99' 40.26'
Compound 1 36.50 33.21 35.79 34.68 33.00
.
: Muscle
Fiber
Type 2a
Control 39.99 45.86 49.98' 51.55- 54.62'
Compound 1 45.09 38.69 42.28 41.31 40.24
. ~ ~ _ .. ._.__ ___ _.___ --T . . . , _
.' .
.
-` ~0899S~
- 12 -
The values identified by ~ differ significantly from the
values before the interruption of the blood circulation;
p < 0.05.
The values in Table 1 show that the muscle tissues of the
animals treated with compound 1 reveal a significantly
reduced swelling of the muscle fibers of Type 1 and 2a
after 18 - 24 hours restoration of blood flow.
-