Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
20917~ STLC 062
(2001840)
Integrated Process for Producing
Crystall;ne Fructose
This invention relates to edible sugars. More
particularly, it relates to fructose obtained by the
isomerization of dextrose. Of specific relevance is a
process for the concurrent production of anhydrous
crystalline fructose and a syrup consisting essentially
of fructose and dextrose.
Also of specific relevance are a process of -
crystallizing fructose by cooling a solution of fructose
such that differing levels of supersaturation are
produced during different periods of crystal growth and a
process for producing a purified and concentrated
fructose syrup.
LI~UID-PHASE FRUCTOSE PRODUCTS
Fructose is a monosaccharide highly valued as a
nutritive sweetener. The vast majority of fructose sold
in this country is derived from corn starch with the
principal form of the product being High Fructose Corn
Syrup (HFCS~. The syrups of commerce range from 42~ to
90% by weight fructose on a dry solids basis [(dsb)].
(As used hereinafter, including the claims, "dsb" shall
mean "by weight on a dry solids basis.") The remainder
is predominately dextrose. The HFCS commonly used as a
! '~
.:.
` -2- 2~91 7
,
~;
sucrose replacement in soft drinks typically comprises
55% fructose, 41% dextrose, and 4% higher saccharides
(all percentages dsb). The solids content of such a
syrup is usually about 77% by weight.
On an industrial scale, the production of HFCS
commences with the enæymatic liquefaction of a puri~ied
starch slurry. The principal source of raw material in
the United States is corn starch obtained by the wet
milling process. However, starches of comparable purity
!~ ~rom other sources can be employed.
In the first step of a typical process a starch
slurxy is gelatinized by cooking at high temperature.
The yelatinized starch is then liquefied and dextrinized
by thermostable alpha-amylase in a continuous two-stage
reaction. The product of this reaction is a soluble
dextrin hydrolysate with a dextrose equivalent (DE) of
6-15, suitable for the subsequent saccharification step.
Following liquefaction-dextrinization, the pH and
temperature oE the 10-15 DE hydrolysate is adjusted for
the saccharification step. During saccharification the
hydrolysate is further hydrolyzed to dextrose by the
enzymatic action of glucoamylaseO Although
saccharification can be carried out as a batch reaction,
a continuous saccharification is practiced in most modern
plants. In the continuous saccharification reaction,
glucoamylase is added to a 10-15 DE hydrolysate following
pH and temperature adjustmentO The carbohydrate
composition of typical high-dextrose ~accliarification
liquor is: 94-96% dextrose, 2-3% maltose; 0.3-0.5
maltotriose; and 1-2% higher saccharides (all percentages
dsb). The product will typically be 25 to 37% dry
substance. This high-dextrose hydrolysate is then
`'.
Z ~ ~3~ 20917~
refined to produce dextrose feedstock for the
isomerization reaction.
Preparation of very high-quality dextrose feedstock
5 for isomerization is necessary because of the very low
color and ash specifications of the finished HFCS. A
~ high~purity feedstock is also required for efficient
3 utilization of the immobilized isomerase enzyme column.
Immobilized isomerase enzyme columns are used
continuously over a period of several months. During
this period very large volumes of dextrose feedstock pass
through the columns. Extremely low levels of impurities
such as ash, metal ions, and/or protein in the feedstock
can accumulate and lead to decreased productivity of the
enzyme. For these reasons dextrose feedstock is refined
to a color of 0.1 (CRA x 100) and a conductivity of 5-10
micromhos.
Carbon-treated, filtered, and deionized,
high-dextrose liquor is evaporated to the proper solids
level for isomerization. In addition, the feedstock is
chemically treated by the addition of magnesium ions,
which not only activate the immobilized isomerase, but
25 also competitively inhibit the action of any residual ;~
calciums ions, which are potent inhibitors of isomerase.
~''
The isomerization reaction, which converts some of
the dextrose to fructose, is commonly carried out on a
stream comprising 94-96% (dsb) dextrose and 4-6~ (dsb)
higher saccharides, at 40-50% dry substance. The stream
has a pH of 7.5-8.2 at 25C and will be subjected to the
action of the isomerase enzyme for 1/2 to 4 hours at
55-65C.
~ 4_ 2~ ~ 7~
The conversion of glucose to fructose is a
reversible reaction with an equilibrium constant of about
1.0 at 60C. Thus, one would expect to obtain a fructose
, level of about 47-48% at equilibrium, starting from a
, 5 feedstock continuing 94-96% dextrose. However, the
¦ reaction rate of the isomerization near the equilibrium
point is so slow that it i~ prudent to terminate the
reaction at a conversion level of about 42~ fructose to
achieve practical reactor residence times.
In a given isocolumn ~immobilized isomerase column),
the rate of conversion of dextrose (glucose) to fructose
~ is proportional to the enzyme activity of the immobilized
1 isomerase. This activity decays over time in a nearly
exponential fashion~ When the column is new and the
activity is high, the flow of feedstock through the
column is relatively high, since a shorter residence time
is required to achieve the 42% fructose level. As the
usage life of the column increases, the flow through the i~
column must be reduced proportionately to provide a
longer residence time, compensating for the lowered
activity in order to achieve a constant conversion level.
In practice, parallel operation of multiple
isocolumns is used to minimize production fluctuations
with respect to capacity and conversion level. In this
arrangement each isocolumn can be operated essentially
independently of the others. The variation in total flow
of the isocolumns must be maintained within relatively
narrow limits because of the requirements of evaporation
and other finishing operations. In practice, flow cannot
be precisely controlled at all times so as to obtain a
42% fructose stream, but this can easily be achieved on
an average basis.
5- ~9:L7~
One of the most critical operating variables in such
a process is the internal isocolumn pH. The operating pH
is usually a compromise between the pH of maximum
activity (typically around pH 8~ and the pH of maximum
stability (typically pH 7.0-7.5). This is complicated by
the fact that the dextrose feedstock is not pH stable at
temperatures around 60C. Some decomposition occurs
producing acidic by-products which results in a pH drop
across the isocolumn during operation.
~1 10
Following isomerization, the typical manufacturing
process employs secondary refining ox polishing of the
42~ HFCS product. Some additional color is picked up
during the chemical treatment and isomerization when the
feedstock is held at a higher pH and temperature for a
period of time. The product also contains some
additional ash from the chemicals added for
isomerization. This color and ash are removed by
secondary carbon and ion exchange systems. The refined
42% HFCS is then typically evaporated to 71% solids for
shipment.
~. ':
The use of activated carbon to purify sugar syrups
is generally known. U.S. Patent No. 1,979,781 (van
Sherpenberg) discloses mixing a raw sugar syrup (i.e.,
one not mixed with glucose syrup or with invert sugar
syrup) at 60 Brix (60~ dry solids) with 1 to 2% by
weight activated carbon and heating to 134C for a short
period of time. U.S. PatPnt No. 2,763,580 (Zabor)
broadly discloses treatment of sugar liquors (e.g., cane,
~, beet or corn sugars) having solids contents of between 10
and 60%, especially 20 to 56%, by weight at 125 to 200F
with activated carbon. The patent discloses that partial
treatment can be carried out at one concentration or
condition, after which the treatment can be completed at
-` -6- 2~9~7~
a higher concentration (obtained by evaporation) or other
condition.
Various patents directed to the production of coxn
syrups containing fructose incidentally disclose-
carbon-treatment and subsequent concentration of aqueous
solutions having varying fructose concentrations (dsb)
and varying levels of dry solids. U. S. Patent Nos.
3~383,245 (Scalle-t et al.) and 3,690,948 (Katz et al.)
disclose carbon-treating fructose containing syrups
having about 20% (dsb) fructose at about 40~ dry solids
and subsequently concentrating the syrups (e.g., by
evaporation to 70-83% dry solids).
U. S. Patent No. 3,684,574 (Katz et al.) discloses
¦~ carbon-treatment of a syrup containing about 20% (dsb)
! fructose at a dry solids as low as 20% dry solids and
subse~uent eoncentration of the syrup. U.S. Patent No.
4,395,292 (Katz et al.) discloses feeding a
carbon-treated mixture of fructose and dextrose having
from 10 to 70% dry solids, preferably 40%, to a
fractionating column and concentrating the fructose
containing extracts. The '292 patent discloses that
extracts containing over 90% fructose can be obtained and
discloses an example (Example No. 7~ wherein a 40% dry
solids feed was fractionated to produce a fraction having
100% (dsb) fructose at 9% dry solids.
The HFCS product from the isomerization reaction
typically contains 42% fructose, 52% unconverted
dextrose, and about 6% cligosaccharides. For reasons
previously discussed, this product represents the
practical maxlmum level of fructose attainable by
isomerization. In order to obtain products with higher
levels of fructose, it is necessary to selectively
concentrate the fructose. Many common separation
2~3:~7~
~ -7-
¦ techniques are not applicable for this purpose, since
they do not readily discriminate between two isomers of
essentially the same molecular size. However, fructose
preferentially forms a complex with different cations,
5 such as calcium. This difference has been exploited to
develop commercial separation processes.
-..
There are basically two diEferent commercial
processes currently available for the large-scale
~ 10 purification of fructose. In both instances, resins in
3 the preferred cationic form are used in packed bed
3 systems. One process employs an inorganic resin leading
to a selective molecular absorption of fructose (see, R.
J. Jensen, "The Sarex Process for the Fractionation of
High Fructose Corn Syrup," Abstracts of the Institute of
Chemical Engineers, 85th National Meeting, Philadelphia,
Pa., 1978).
Chromatographic fractionation using organic resins
is the basis for the second commercial separation process
(see, K. Venkatasuhramanian, "Integration of Large Scale
Production and Purification of Biomolecules," Enzyme
En~ineerinq 6-37-43, 1982). When an aqueous solution of
, -
dextrose and fructose (e.g., 42% HFCS) is fed to a
fractionating column, fructose is retained by the resin
to a greater degree than dextrose. Deionized and
deoxygenated water is used as the eluent. Typically, the
separation is achieved in a column packed with a bed of
low crosslinked, fine-mesh, polystyrene sulfonate cation
exchange resin using calcium as the preferred salt form.
The. enriched product which contains approximately 90%
fructose is referred to as Very Enriched Fructose Corn
Syrup (VEFCS~. This VEFCS fraction can be blended with
the 42% HFCS feed material to obtain products having a
fructose content between 42% and 90%. The most typical
of these products is 55% Enriched Fructose Corn Syrup,
2 ~
,J
.~ :
which is sometimes referred to as EFCS or 55 EFCS. U. S.
Patent No. 4,395,292 (Katz et al.) discloses an example
(Example No. 1) of fractionating a mixture of fructose
and dextrose into various fractions and combining
y 5 fructose-enriched fractions to produce a syrup containing
55.8~ (dsb) fructose. This same example also discloses
single fractions having high concentrations (dsb) of
fructose (e.g., 75.1% (dsb)) and discloses combining
fractions containing lesser concentrations of fructose
~3 10 (e.g., 64.5% (dsb) with 58.2% (dsb) fructose).
The treatment of other raffinate streams in the
fractionation process is an important consideration. In
general, the dextrose-rich raffinats stream is rscycled
to ths dextrose fsed of the isocolumn system for further
conversion to 42% HFCS. A raffinate stream containing
dextrose and fructose and having a fructose lsvel higher
than that of the feed stream can be recycled through a
frac~ionator to maintain a high solids level and to
reduce water usage. A raffinats stream rich in
oligosaccharides can bs recycled to the saccharification
system.
Since water is used as the elution media, it has a
great impact on the overall evaporation load on the
system. Very low solids concentrations increase the risk
of microbial contamination within the system. Thus, the
most important design parameter dictating overall process
economics is the maximization of solids yield at
acceptable purity while minimizing the dilution effect of
the eluant rinse. The efficiency of feed and water usage
must be maximized for optimal yield. The yield is
important to reduce the cost of reisomerization.
~3
,l3 35 Procedures available for achieving these goals
include recycling techniques, higher equalization of the
..
` _9_ ~0917~
resin phase with proper redistribution in a packed
column, and the addition of multiple entry and exit ..
points in the column. These approaches can be used to
increase the purity and the yield.
In a batch fractionation system, a small apparent
increase in the purity of feed to the fractionating
column, that is, higher fructose levels, results in a
much larger gain in production through increased yield at
a given product purity. In practice, this translates
into maximization of the ratio of the sugar volume fed
per volume of resin per cycle, minimization of the ratio
of the water column required per volume of resin per
cycle, and careful fluid distribution to the columns.
SOLID-PHASE FRUCTOSE PRODUCTS
A number of processes are known for crystallizing
1 20 fructose. For example, crystalline fructose may be
¦ prepared by adding absolute alcohol to the syrup obtained
from the acid hydrolysis of inuline (Bates et al., Natl.
Bur. Std. Circ~ C440,399, 1942). The preparation of
fructose from dextrose is described in U.S. Patent
2,354,664 and U.S. Patent 2,729,587 describes its
preparation from sucrose by enzymatic conversion.
Fructose forms orthorhombic, bisphenoidal prisms
from alcohol which decompose at about 103-105C.
Hemihydrate and dihydrate crystalline forms are also
known, but it is preferable to avoid the formation of
these species inasmuch as they are substantially more
hygroscopic than the anhydrous form and have melting
points close to room temperature. These properties make
these crystalline forms of fructose very difficult to
handle.
.-
'.1 .
~ 209~7~
1 o--
Solvent Crystalline Fructose (SCF) is prepared by a
process wherein an organic solvent, such as denatured
ethyl alcohol, is mixed with a high-fructose stream (95%
dsb). This stream crystallizes as it is cooled to form
pure crystalline fructose. The product is centrifuged to
separate it from the mother liquor, desolventized, and
dried.
U.S. Patent 4,199,374 describes a process for
producing SCF. Fructose is crystallized from a solution
of VEFCS in ethanol. The solution is seeded with fine
crystals of fructose or glucose. The crystals are
harvested by filtration, centrifugation or other suitable
means. These crystals are then washed with alcohol and
dried under vacuum. The moisture content of the alcohol
and syrup must be carefully controlled in this process in
order to obtain free-flowing fine crystals of fructose.
It is also possible to simply produce a dried
fructose sweetener ~FS). In a DFS process, a high
fructose stream derivsd from fractionation is dried in a
rotary dryer, then sized in a classifier containing
screens and grinders. U.SO Patent 4,517,021 describes
the preparation of such a granular, semi-crystalline,
solid fructose which comprises less than about 2% water
by weight. The patent discloses that about 60 weight
percent of the product is crystalline fructose, and less
than 35 weight percent is amorphous fructose. A drum
dryer is used, with air having an initial temperature of
50-80C. A portion of the solid fructose product may be
recycled as the crystallization initiator.
One disadvantage of a DFS process is that the
product cannot be called pure fructose because it is a
total sugar product and does not meet the Food Chemicals
Codex criteria for "fructose." Moreover, since it is not
~ 2~917~
`. --11
i
J
completely crystalline, it is more hygroscopic and thus
harder to handle in humid conditions than crystalline
fructose O .:
' ' ~
An aqueous process can also be used to produce
crystalline fructose. An aqueous crystalline fructose
process typically starts with a high fructose feed stream
which is cooled to crystallize the fructose from
solution. A number of referencles describe such a
process.
In U.S. Patent 3,513,023 crystalline, anhydrous
fructose is obtained from an aqueous solution of fructose
(min. 95% ds). The pH of the solution must ba between
3.5 and 8Ø The fructose solution is concentrated under
vac~um until the water content is between 2 and 5%~ The
solution is cooled to 60-85C, seeded with crystalline
fructose, and stirred vigorously while the temperature is
maintained at 60~85C. The patentee states that a
crystalline mass results which, after slow cooling, can
be crumbled or ground and subsequently dried to produce a
non-sticking, free-flowing, finely-crystalline powder.
The process is said to avoid the formation of the glass
phase product which ordinarily results when fructose
solutions of this type are concentrated in a vacuum and
allowed to cool in the usual manner.
In U.S. Patent 3,883,365 fructose is crystallized
~rom an aqueous fructose/glucose solution of 90% ds and
containing 90-99% (dsb) fructose. The solution is
saturated (58-65C). The fructose is crystallized from
the solution by adding fructose crystals of homogeneous
size. The formation of new crystals is minimized by -`
keeping the distances of the seed crystals from each
other suitably short and maintaining the degree of
supersaturation between 1.1 and 1.2. The volume of the
. ~
2~917~ ~
12-
solution is increased, either continuously or stepwise,
as the crystallization proceeds. The optimum pH of the
fructose solution is said to be 5Ø The crystals so
obtained reportedly have an average crystal size between
200-600 microns. Centrifugation is used to separate the
I crystals from the solution.
I U.S. Patent 3,928,062 discloses that anhydrous
¦ fructose crystals are obtained by seeding a solution
¦ 10 containing 83-95.5% (dry basis) total sugar comprising
88-99% fructose. Crystallization may be accomplished by
simply cooling the solution under atmospheric pressure or
by evaporating water under reduced pressure. Formation
~ of the hemihydrate and dehydrate are avoided by carrying
f 15 out the crystallization within a certain range of
fructose concentrations and temperatures. This range
lies within the supersaturation area below the point at
which th~ he~ihydrate begins to crystallize out. It is
¦ said that the mother liquor may be used repeatedly for
20 the crystallization of further crops in the same manner
as the first crop without any additional treatment. The
addition of seed crystals may be achieved using a form of
massecuite which was previously prepared by suspending
the crystals in the fructose solution.
In U.S. Patent 4,199,373 crystalline fructose is
produced by seeding a fructose syrup (88-96~ dsb) with
2-15 weight percent fructose seed crystals and permitting
the seeded syrup to stand at about 50 to 90F at a
30 relative humidity below 70%. Crystallization is said to
require 2 to 72 hours. The crystalline product produced
by the process is in the form of large pellets.
U.S. Patent 4,164,429 describes a process and
35 apparatus for producing crystallization seeds. A series
of centrifuga:L separations are employed to select seed
2~7~
~, -13- ~
' ' :~
crystals from the seeded solution which fall within a
predetermined size range.
Crystallization Coolinq Curves
The cooling of a saturated or supersaturated
solution to crystallize material therefrom is, of course,
generally known.
It is also known that the natural cooling of a
saturated or supersaturated solution often results in
severe nucleation which contributes to a potentially
undesirably broad particle size distribution for the
crystalline product~ For example, the discussion of
crystallization in the Encyclopedia of Chemical
Technolo~y, Vol. 7, pp. 243-285, (Kirk-Othmer, Ed. John
Wiley & Sons, N.Y., 3rd ed., 1979), states that natural
cooling results in a supersaturation peak early in the
~, 20 cooling period thus inducing heavy nucleation. The
article teaches that by following a controlled cooling
curve, a constant level of supersaturation can be
maintained, thereby controlling nucleation within
acceptable limits. Figure 5 is a reproduction of the
~'; 25 natural and controlled cooling curves published in this
4- work.
!
The various aspects of this invention are briefly
discussed below.
~j 30
`.~. ::
'' : '
'& . ~
2~ 7~
-14-
I. INTEGRATED, MULTIPLE, FRUCTOSE SWEETENER
PRODUCTION
I In one asp~ct, this invention broadly relates to the
¦ 5 integrated production of a plurality of sweeteners which
I contain fructose.
A. Concurrent Production of a Liquid-Phase
Sweetener and Crystalline Fructose
In a particular aspect, this invention relates to a
process for producing crystalline fructose and a
liquid-phase sweetener comprising fructose and dextrose
which comprises:
crystallizing fructose in an aqueous solution of
fructose to produce a mixture comprising
crystalline fructose and mother liquor,
separating crystalline fructose from the mother
liquor; and,
~I mixing dextrose with the mother liquor to produce a
liquid-phase sweetener comprising dextrose and
fructose.
In the manufacture of crystalline sucrose from
aqueous solution, it is common practice to take repeated,
successive strikes of crystals to concentrate impurities
in the mother liquor, referred to as molasses. This
molasses is generally so impure that it has value only as
an animal feed supplement or fermentation media. U.S.
Patent No. 3,928,062 teaches that the mother liquor from
fructose crystallization can be used repeatedly for
crystallization of further crops of fructose crystals.
The comparatively low yield of fructose crystals from a
20~7a~
. .
single strike of crystals using common crystallization
techniques and the difficulties associated with
isomerizing and fractionating corn syrups to obtain a
crystallizer feed having a high concentration of fructose
makes the recycle of mother liquor by taking ~f
successive strikes of fructose crystals appear desirable.
However, the integration of the production of crystalline
fructose with a liquid-phase sweeten~r by adding dextrose
to the mother liquor allows one to obtain two premium
quality sweeteners. This in turn allows one to maximize
the yield of fructose useful as a sweetener and thereby
justify the difficulty of isomerization. This process
does, however, entail a sacrifice of gains made in
fractionation in that the whole point of fractionation is
to remove dextrose to prepare a crystallizer feed and
¦ thus the addition of dextrose to the mother liquor
sacrifices part of the enrichment achieved through
I fractionation.
In a particular embodiment of this aspect of the
invention, this invention relates to a process for
producing crystalline fructose and a stream comprising -~;
dextrose and fructose from a feed stream comprising
dextrose which comprises:
isomerizing a portion of the dextrose in the feed
stream to produce a first dextrose/fructose
stream comprising dextrose and fructose;
.
¦ 30 splitting the first dextrose/fructose stream into a
first feed stream and a second feed stream;
fractionating the first feed stream to produce a
high fructose stream;
2~71~
! crystallizing fructose in the high fructose stream
to produce a mixture comprising crystalline
I fructose and mother liquor;
! 5 separating crystalline fructose from the mother
I liquor; and,
I blending at least a portion o~ the mother liquor¦ with the second feed stream to produce a second
¦ 10 dextrose/fructose stream which has a higher
fructose-to-dextrose ratio than the first
dextrose/fructose stream. (As used
hereinafter, including the claims, the term
"dextrose, fructose stream, shall mean a stream
comprised of dextrose and fructose.")
In a related aspect, this invention relates to a
process for producing crystalline fructose and a
liquid-phase sweetener comprising fructose which ~-
20 comprises: ~
~ : .
crystallizing fructose in an aqueous solution of
fructose to produce a mixture comprising
crystalline fructose and mother liquor;
separating crystalline fructose from the mother
liquor; and,
~ .
inhibiting further crystallization in the mother ¦~
liquor to produce a liquid-phase sweetener
comprising fructose.
..
Th~ mother liquor remaining aEter crystallization is
a saturated solution of fructose. The prior art, e.g.,
U.S. Patent 3,928,062, teaches that the mother liquor can
be used repeatedly for the crystalli~ation of further
-17 2 ~ 7 ~ ~
crops of crystals. To produce further crops of crystals,
the saturated mother liquor must be heated and
concentrated to obtain a suitable supersaturated solution
of fructose and thus enable crystallization in the mother
liquor. It has been found that rather than enabling the
crystallization of further crops, one should inhibit
further crystallization so that the mother liquor can be
used to produce a liquid-phase sweetener. As noted
above, the mother liquor is a saturated solution of
fructose. To prevent precipitation of fructose crystals
therefrom during handling, transport, and/or storage,
steps must be taken to inhibit crystallization of
fructose in the mother liquor. This aspect of the
invention is related to the first aspect of this
invention discussed above, in that further crystalliza-
tion is avoided. However, this aspect does not
necessarily require the sacrifice of fractionation gains
because inhibiting further crystallization does not
necessarily require addition of dextrose, i.eO, simple
dilution of the mother liquor with water will serve to
inhibit crystallization without diluting the fructose
purity of the mother liquor on a dry solids basis.
B. Multi~le Hiqh_Fructose Sweetener Fractionation
In a related aspect, this invention relates to a
process for producing multiple fructose sweeteners, at
least one of said sweeteners comprising dextrose and
fructose, which process comprises:
fractionating a feed stream comprising dextrose and
fructose into a dextrose-enriched raffinate, a
lower-fructose extract, and a higher-fructose
extract, said higher-fructose extract being
greater than about 90~ (dsb) fructose; and,
~i ` 20~7~
18-
mixing the lower-fructose extract with a dextrose
composition having a greater concentration
(dsb) of dextrose than said lower-fructose
extract to produce a liquid-phase sweetener.
'~Fructose sweeteners" in this context includes any
¦ sweetener containing fructose without regard to whether
~ the fructose is in solution, dispersed, amorphous or
,j crystalline. For example, the higher-fructose extract
can be used to produce a syrup containing fructose,
crystalline fructose, or a semi-crystalline fructose
wherein at least a portion of the fructose is in an
amorphous solid phase.
The fractionation of an isomerized dextrose syrup,
i.e., one containing both fructose and dextrose, to
produce a fructose sweetener i5 commonly conduct~d by
taking a dextrose raffinate and a fructose extract, with
recycle of the remaining fractionation output. For
example, U.S. Patent No. 4,395,292 states that such an
operating condition is preferred. However, by taking two
extracts, one having a higher concentration (dsb) of
fructose (i.e., a higher-fructose extract) and one having
a lower concentration (dsb) of fructose, a fructose
extract having a higher concentration than a single
extract can be obtained without increasing the aggregate
degree of resolution of the isomerized feed and all of
the problems associated therewith (e.g., reduced
fractionation capacity, greater evaporation load from
increased Plution water, and/or deleterious pressur~ drop
due to higher elution water flow rates needed to increase
resolution).
~ ,:
The utility of the lower-fructose extract is of a
narrower scope than the utility of the higher-fructose
extract (i.e., it would be difficult to use the lower
-19- 2~7~i~
fructose extract to produce crystalline fructose), but
the fructose therein can be used to upgrade the fructose
content of corn syrups containing even less fructose,
e.g., by admixture with an isomerized corn syrup (e.g.,
42% fructose corn syrup) to procluce a higher fructose
corn syrup (e.g., a 55% fructose corn syrup).
i
In a particularly preferrecl embodiment of this
¦ aspect, the higher-fructose extract is used to prepare a
i 10 crystallizer feed for the crystallization of fructose.
Accordingly, in one aspect, this invention relates to a ~1
l process for producing crystalline fructose and a
3' liquid-phase sweetener comprising dextrose and fructose
which comprises:
fractionating a stream comprising dextrose and
fructose into a dextrose-enriched raffinate, a
lower-fructose extract, and a higher-fructose
extract;
crystallizing fructose from an aqueous solution
derived from the higher-fructose extract; and,
~ mixing the lower-fructose extract with a dextrose
i 25 composition having a dextrose concentration
(dsb) greater than the lower-fructose extract
to produce a liquid-phase sweetener comprising
~ dextrose and fructose.
;1 30 This embodiment is particularly advantageous because
the fructose concentration (dsb) commonly required to
feasibly crystallize fructose from an aqueous solution is
so high that fractionation of a dextrose/fructose feed
stream from an isomerization process to produce a single
extract may be impractical. In other words, the degree
of resolution needed to produce a single extract having a
.~
` -20~ 7 ~ 6
sufficiently high fructose purity to be useful as a
crystallizer feed will often so reduce the fractionation
capacity and/or increase other difficulties associated
with fractionation that such resolution is impractical.
A possible drawback of taking both higher-fructose
and lower-fructose extracts and separately using them to
produce a crystalline sweetener and a liquid-phase
sweetener, respectively, is that the amount of fructose
in the lo~er-fructose extract that is available for
upgrading the fructose content of an isomerized corn
syrup is less than that available in a single fructose
extract taken with the same aggregate degree of
resolution. Thus, the total amount of fructose (dsb)
available as a liquid-phase sweetener is reduced. This
drawback is ameliorated by the availability of the mother
liquor from the crystallization of part of the fructose
of higher-fructose extract. In other words, in an
especially preferred embodiment, mother liquor containing
fructos2, a lower-fructose extract and an isomerized corn
syrup are mixed to prepare a liquid-phase sweetener
(e.g., a 55% fructose corn syrup).
.
II. VARIABLE SUPERSATURATION COOLING CURVE
In another aspect, this invention relates to a
process for producing crystalline fructose from a
solution comprised of fructose comprising:
cooling said solution through an initial temperature
range at an initial rate of cooling;
then cooling said solution through an intermediate
temperature range at an intermediate rate that
is slower than the initial rate; and,
~ -21- 2~1706
,~
then cooling said solution through a final
temperature range at a final rate that is
faster than the intermediate rate.
Figure 5 shows typical cooling curves used in
crystallization processes. Curve A is a natural cooling
curve and curve B is a controlled curve designed to
achieve a constant level of supersaturation. Figure 4
shows a variable saturation cooling curve of this
invention. A comparison of the two figures shows the
stark diff~rences between the conventional curves and the
curve of this invention.
The use of a cooling rate in an intermediate cooling
period that is slower than the rates of cooling in the
initial and final rates allows one to minimize both
spontaneous nucleation in the solution and heat-induced
degradation of the fructose in the solution, especially
during the initial ~ooling period. The reduction in
nucleation results in a crystalline product having a more
nearly uniform particle size distribution and the
reduction in heat damage increases the yield of fructose
crystals and mother liquor and reduces the level of
degradation product impurities in the mother liquor, thus
improving its utility as a source of fructose for a
liquid-phase sweetener.
~ ~::
III. PURIFICATION OF HIGH-FRUCTOSE SYRUPS
BY CARBON-TREATMENT AT LOW SOLIDS
In another aspect, this invention relates to a
process for preparing a concentrated solution of fructos~
comprising:
~,.; ' ~ ; ., ~ ,`. .'.' " .
~ -22- 2~9~7~
obtaining a solution of fructose having a fructose
concentration of greater than about 75~ (dsb)
by weight and a dry solids concentration of
less than 40~;
~, contacting said solution with activated carbon to
¦ prepare a purified solution of fructose; and, ~:
evaporating said purified solution to a dry solids
concentration of greater than 40%.
.:
While the treatment of sugar syrups with activated
carbon to purify said syrups is generally known, it has --
been found that fructose syrups having a high
15 concentration (dsb) of fructose should have a relatively : :
low solids concentration when in the presence of .
activated carbon to reduce the formation of by-products
(e.g., difructose) which can reduce the availability of
fructose in the syrup~ inhibit crystallization of
fructose fxom the syrup, and/or affect the organoleptic
properties of the syrup nr a sweetener prepared
therefrom. Tables II and III show the effect of solids
concentration on difructose formation in a high :.
fructose(95+% dsb) syrup over time in contact with
25 activated carbon. ;
: In a related aspect, this invention also relates to
a process for producing crystalline fructose comprising:
.:
fractionating a stream comprised of dextrose and
fructose to produce a high-fructose stream
having greater than 90~ (dsb) fructose;
contacting said high-fructose stream with activated
carbon to produce a purified fructose stream;
l ' -23- 2~
then evaporating said purified fructose stream to
produce a solution of fructose; and
~l crystallizing fructose in said aqueous solution of
~ 5 fructose.
'1
~ The sequence of contacting and then evaporating the
3 high-fructose stream ensures that the contacting is
performed at comparatively low solids because
high-fructose extracts are typically at low solids upon
elution from a fractionation column.
i~ .
J~ In another related aspe~t, this invention relates to
a process for producing crystalline fructose comprising-
crystallizing fructose in a solution of fructose to
produce a mixture comprising crystalline
fructose and mother liquor comprised of
fructose;
separating crystalline fructose from the mother
liquor;
:
mixing at least a portion of the fructose of said
mother liquor with a liquid comprised of water
to form a lower solids solution of fructose
(e.g. less than about 70% dry solids);
contacting said lower solids solution of fructose
with activated carbon; and,
evaporating said lower solids solution of fructose
to form a higher solids solution of fructose.
In particularly preferred embodiments, the mother
liquor resulting from the crystallization of fructose
. '
.,
.'
i~ 2 ~ 7 ~ ~
',
' !
will be mixed with a liquid comprised of water ~e.g., tap
water, sweet water, saccharide syrups such as 42%
fructose corn syrups, and the like) to reduce the solids
content prior to treatment with activated carbon and then
evaporation to higher solids. The resulting higher
solids solution can be used in a variety of ways, eOg.,
as a crystallizer feed, a high .Fructose corn syrup
sweetener or production stream therefor, all of which
benefit from the advantages discussed above which result
from reducing the solids concentration of the mother
liquor before treatment with activated carbon and
subsequent evaporation.
~"i
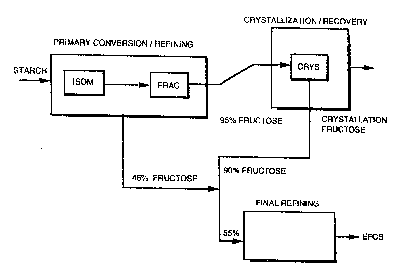
Figure 1 shows the various steps in a conven~ional
process for the production of 42% HFCS and 55% HFCS
~EFCS) from starch.
:
Figure 2 shows an integrated, starch-based process
for producing both crystalline fructose and EFCS.
Figure 3 shows in more detail the process
illustrated in Figure 2.
Figure 4 is a graph of massecuite temperature versus
time since seeding for a typical variable supersaturation
cooling program of the invention.
Figure 5 is a graph of temperature versus time in a
batch crystallizer for both the natural cooling curve
(Curve A) and a constant supersaturation cooling curve
(Curve B).
An important feature of the present invention is the
synergy which obtains when anhydrous crystallin~ fructose
(ACF) is produced in conjunction with EFCS. The yield of
fructose crystals from a fructose massecuite is typically
2~1~17~
on the order of 40-55%, e.g. 45%. Longer crystallization
times may increase the yield, but only at the expense of
process throughput. Thus, a significant advantage is had
by integrating fructose crystallization with a process
which not only provides the fructose feed for the ACF
crystallization process but also can accept without
penalty the non-crystallized fructose from the ACF
process.
In some crystalline fructose processes of the prior
art, the noncrystallized portion is recycled through the
crystallization process. The problem with this approach
is that undesirable by-products such as difructose,
5-(hydroxymethyl)-2~furfural (HMF) and higher saccharides
tend to build up in the recycle stream since
crystallization is essentially selective for fructose.
As a result, the recycle stream eventually becomes so
contaminat~d with by-products that it must be puryed from
the system with the concomitant loss of a substantial
1 20 quantity of fructose.
I The present invention solves the problem of
by-product built up by incorporating the solution phase
I material which remains after the crystallization of
fructose (the mother liquor) into a process which
produces high-fructose, liquid-phase sweetener(s). In
this fashion unwanted by-products are not concentrated in
that portion of the integrated process which produces
I ACF, but rather are continuously removed from that
1 30 system. This integration obviates the need for
fructose-containing purge streams thereby conserving
fructose in more economically valued products.
Referring to Fig. 1, it can be seen that the
production of 55~ HFCS (EFCS) requires a separation
(fractionation) step in the process stream. In general,
.
~l 2~7~
, i
-26-
ij :
fractionation is required to make syrups having a
fructose content higher than approximately 48%. For the
purpose of crystallizing fructose, a syrup containing
more than 95% fructose (dsb) is preferred. Although it
is possible to crystallize fructose from solutions
containing less fructose than this, lower yields will be
obtained and the process would not be as economically
desirable.
Fractionation techniques are known which will
~ provide a 95+% fructose stream from a feed comprising ~-
5~ about 42% (dsb) fructose (the typical output from
dextrose isomerization). Thus, it is possible to obtain
an ACF feed stream from an EFCS process with little or no
modification. Most preferably, the fractionation system
is of the simulated moving bed chromatographic type, as
is well-known in the art.
Referring now to Fig. 2, the details of the
integrated process will be described. As shown in the
block labeled "Primary Conversion/Refining," starch is
first converted to dextrose using the conventional
enzyme-based process described hereinabove.
Isomerization
The isomerization step employs an enzyme to convert
dextrose to fructose. The enzyme is fixed to a carrier
and is stationary in a column (isocolumn) until it is
replaced when it is exhausted. One advantage of the
present invention is that it permits the efficient
utilization of increased quantities of isomerase in the
isocolumns. Owing to seasonal fluctuations in the demand
for EFCS (55% fructose), a producer who invests in
additional isomerase to meet peak demand must pay for
j 2~1L7~
.' .:
~7-
~ that increased l~vel of isomerization capacity throughout
7 the year even when his EFCS production is at a relatively
low level. By selectively practicing the integrated
process disclosed herein, a producer can efficiently
5 utilize the increased level of isomerization by
channeling more of the high-fructose stream from the
fractionation train to EFCS procluction when demand for
that product is high and employing a greater portion of
that stream in ACF production when demand for EFCS is
10 lower. In this way an investment in increased
isomerization capability can be effectively utilized
throughout the year.
15 Fractionation
Fractionation occurs in a train, or group of vessels
containing resin which operate in sequence to separate
fructose from dextrose in the syrup feed stream. The
20 feed stream and elution water stream are fed into the
train and one or more high-fructose product streams, a
high-dextrose raffinate stream, and/or one or more
high-oligosaccharide raffinate streams are removed. As
shown in Figure 3, the high-dextrose stream is recycled
25 to isomerization for conversion to fructose while the
high-fructose stream(s) goes into the ACF portion of the
process or is blended to make EFCS.
Fractionation capacity is measured by the feed flow
30 rate, percent fructose in the product stream, and
recovery of fructose in the stream. For a given dsb,
fructose contentl the higher the fractionation capacity,
the lower the fructose conversion that is needed in
isomerization. Therefore, to lower the isomerase
35 ingredient cost, fractionation is preferably continuously
operated at its maximum capacity.
2 ~
To obtain practical crystallizer yields from the ACF
process the fractionation product must be greater than
about 90% (dsb) fructose and preferably greater than 95
(dsb) fructose. Since this is higher than the 90% (dsb)
fructose normally isolated in an EFCS process, special
operating conditions for conventional fractionation
systems have to be used that may result in decreased
fractionation capacity. These are: 1) slowing the syrup
feed rate without changing the elution water ratio to
enhance resolution and/or, 2) increasing the elution
water ratio to enhance resolution. These operating
conditions have the disadvantage of either decreasing
product throughput and/or adding water which must
subsequently be removed, entailing at least the
expenditure of additional energy. There is, however, a
preferred alternative.
As will be appreciated by those skilled in this art,
when an aqueous isiolution comprising dextrose and fructose
is passed through a suitable chromatographic column, at
least a partial resolution of the two species is
obtained. To achieve fractionation, the effluent from
the column must be diverted as appropriate in order to
isolate the desired fractions. The diverted portions are
commonly referred to as "cuts". A "narrow cut" contains
fewer volume elements of the effluent than does a "wide
cut". Thusi, in terms of purity, a separation may be
optimized for a particular species by taking an
appropriately narrow cut. The usual trade-off for taking
a narrow cut from the effluent is that the total recovery
of the selected species is adversely affected. .
It has been found that the 95+% (dsb) fructose
stream which is preferred as the feed for the crystalline
fructose portion of the disclosed process may be obtained
by taking an appropriately narrow cut from the product
~"~
) 9 1 7 ~ 6
I -29-
,~ .
!j
stream of the fractionation system of a conventional
process for the production EFCS. One such fractionation
system which is particularly preferred is described in
the commonly-owned United States patent application of
John F. Rasche, serial number 851,026, filed 5/8/86 which
is entitled "Simulated Moving Bed Chromatographic
Separation Apparatus." The teachings of this disclosure
are expressly incorporated herein.
A preferred way of operating the above-referenced
chromatographic separation apparatus when employed in the
fractionation system of the present invention is to
increase the eluant-to-feed ratio from about 1.7 to about
2Ø The syrup fe~d is preferably about 60% dry
substance by weight and is maintained at a temperature of
about 140Fo
The raffinate stream from the fractionation system
may be apportioned in a manner similar to that used to
divide the extract stream. In this way a stream
relatively rich in oligosaccharides may be isolated for
recycle to the saccharification system, sent to a
separate, dedicated saccharification system, or purged
from the system.
In the absence of a purge or recycle of
oligosaccharides to a saccharification system, the only
~; outlet for oligosaccharides from the system is the
extract stream inasmuch as the typical isomerization has
no effect on oligosaccharides. Thus, oligosaccharides in
the raffinate stream which are recycled to the
isomerization system simply pass through that system
unchanged and return in the feed to the fractionation
system.
20917~
-30-
Oligosaccharides are undesirable in the extract
stream since at least a portion of that stream is used as
feed to the fructose crystallization portion of the
process and the crystallization of fructose is preferably
accomplished from a solution containing a minimum of
other species. Likewise, oligosaccharides are
undesirable in the liquid-phase sweetener produced by the
process of the present invention, hence only a limited
quantity of such oligosaccharides can be removed from the
system via the liquid-phase product.
An additional advantage is had by recycling an
oligosaccharide-rich stream from the fractionation system
to the saccharification system. Such a stream will
typically be relatively low in dry substance content,
most commonly about 10% d.s.--i.e., it is about 90% water
by weight. The starch slurry resulting from the
liquefaction/dextrinization portion of the process must
typically be diluted prior to saccharification. The
water in the oligosaccharide stream can substitute for at
least a portion of the water used as a diluent for the
starch slurry thereby conserving water and decreasing the
evaporation capacity required for the system as a whole.
Blendinq
In a conventional EFCS process, the high-fructose
extract from fractionation is blended with the product of
isomerization (typically 42-48% (dsb3 fructose) to obtain
the desired fructose content in the final product (55%
(dsb) for EFCS). In the integrated process of the
present invention, mother liquor from the centrifugation - -
step of the crystallization process containing about
88-92% (dsb) fructose, preferably 90-92% (dsb) fructose,
at approximately 83% d.s. is additionally available for
2~9171D~
-31-
blending. This gives additional flexibility to the
process since various streams can be blended for i~put to
EFCS polishing steps where the blend is typically ion
exchanged, carbon treated, and then evaporated to 77~
d.s. as part of conventional EFCS production. The dashed
lines in Fig. 3 indicate some of the options for
blending. The ultimate choice of blend streams depends,
of course, on mass balance of the system as a whole.
Since nc chemicals are added to the high-fructose
stream in the ACF process other than the very small
quantities of hydrochloric acid or soda ash for pH
adjustment, significant quantities of new trace
components are not generated in the ACF process. Color
bodies, HMF, and difructose may be generated during the
carbon treatment and evaporation steps of the
crystallizer feed treatment. However, these compounds
can be removed by finish carbon and ion exchange
treatments in the EFCS process.
Inasmuch as most steps of the entire fructose
process can be operated at high dry substance levels,
microbial growth is inhibited and should not be of major
concern. As a result, the acetaldehyde level should not
increase significantly and can be reduced by the finish
ion exchange and final evaporation steps if necessary. ~-
~ :~
FRUCTOSE_FEED T0 CRYSTALLIZER
pH Ad~ustment
It has been found that the pH of the aqueous
fructose solution from which fructose crystals are to be
ohtained is preferably between about pH 3.7 and about pH
4.3, teachings to the contrary ( s~ee, e.g., U.S. Patent
20~17~fi
-32-
3,883,365) notwithstanding. Proper control of the pH of
the fructose feed to the crystallizer is necessary to
minimize the formation of difructose anhydrides. The
presence of difructose anhydrides in the crystallizer has
been found to result in lower crystallizer yields and
adversely affects the size distribution of the fructose
crystals that are formed. It is believed that the rate
of formation of anhydrides is at a minimum in the pH
range 3. 7 to 4.3. Higher anhyclride formation rates
obtain both above and below this range. It is further
believed that the formation of color formers is favored
at higher pH levels.
EXAMPLE
The effect of pH on the solubility of fructose and
the generation of impurities in a syrup containing
approximately 95% fructose on a dry solids basis were
investigated as described below. The syrups studied are
representative of those used as feed for the fructose
crystallization portion of the disclosed process.
Crystalline fructose was added to a sample of VEFCS
(90% fructose, dsb) to produce a syrup comprising
approximately 95~ (dsb) fructose. The syrup was
~, subsequently subjected to treatment with granular
activated carbon as d~scribed in the section o~ this ~ ~ ~
disclosure entitled "Carbon Treatment". Thus, this syrup ~-
was treated in the same way as feed to the crystallizer.
An aliquot of the above-described syrup was adjusted
to pH 3.94 and evaporated at 73C to high solids. Two
liters of this high-solids syrup were placed in a sealed,
stirred flask and immersed in a constant temperature bath
maintained at approximately 55C. This sample ("the pH 4
2~17~
-33-
sample") was stirred continuously in the constant
temperature bath while a second sample was prepared -~
(approximately 5 hours~.
A second aliquot of the 95~ fructose syrup was
adjusted to pH 5.48 and evaporated at 77C to high
solids. This evaporation was accomplished more slowly
than that of the pH 4 sample. Two liters of this sample
("the pH 5.5 sample") were placed in a sealed, stirred
flask and immersed in the constant temperature bath
containing the pH 4 sample.
After adjusting the temperature of the bath to
55.5C, 50 grams of fructose seed crystals were added to
both samples. Stirring was continued for 60 hours at
constant temperature. This is the approximate
crystallizer residence time of the syrup in the ACF
process disclosed herein.
The resulting massecuites were sampled, centrifuged,
and the mother liquor analyzed along with samples of the
feed syrup. Ths resulting analytical data are tabulated
below.
7 q~ ~
-34-
TABLE I
~ FEED EOUILIBRATED
J pH 4 pH 5.5 pH 4 pH 5.5
Fructose (% dsb)95.8195.86 95.33 95.24
Fructose (% dsb)96.0896.10 95.33 95.72
following hydrolysis
Mono-anhydrides (% dsb)0.27 0.24 nd* 0048
Solids (weight %)89.7989.13 88.90 88.86
HMF (ppm dsb) 5.71 4.03 25.96.58
i~ 10 Acetaldehyde (ppb total) 104 48 58 66
Furfural (ppm dsb) nd* nd* 0.29 0.44
Color (RBU units) 14.0 39.6 50.7163.1
Solubility -- -- 7.64 7.60
~g fructose/g water)
15 Supersaturation -- -- 1.01.0
*nd: none detected
Mono-anhydrides are calculated from the difference
in the fructose assay before and after hydrolysis of the
sample. Fructose solubility is calculated from the
fructose assay (before hydrolysis) and the solids content
of the sample. Some fructose crystallized out of both
sample solutions to establish equilibrium.
The color increase in the pH 5.5 sample was
significantl,v greater than that observed in the pH 4
~¦ sample. Higher color would result in lower yields for
the crystallization process inasmuch as more washing of
the centrifuge cake would be required. Mother liquor
~ refinement requirements would also likely be increased.
.
3 Both samples exhibited similar increases in mono-
i 35 anhydrides during preparation of feed (compare 0.27% dsb
¦ at pH 4 with 0.24% dsb at pH 5.5); however, the results
of liquid chromatographic studies (not shown above)
indicated that more difructose dianhydrides may have been
formed at pH 5.5.
2 ~
-35-
In summary, the pH 4 sample exhibited less color
formation, exhibited a decrease in total acetaldehyde
content, and had a solubility not significantly different
from the pH 5.5 sample. A pH 4 feed syrup for an ACF
~ 5 process therefore has advantages over a pH 5.5 feed with
.i, regard to product yield and mother liquor quality as a
result of its lower color content. Lower pH apparently
minimizes color and difructose :Eormation and has
negligible eEfect on solubility.
~, 10
As shown in Fig. 3, pH adjustment is conveniently
accomplished subsequent to fractionation and prior to
carbon treatment. The viscosity of the fructose solution
is relatively low at this point in the process and thus
is relatively easy to obtain thorough mixing of the
solution with the acid or base used for pH adjustment. A
number of acids and bases suitable for this purpose are
~l~ known in the art. Especially preferred are hydrochloric
acid ~HCl) to lower pH and anhydrous sodium carbonate
(Na2Co3, "soda ash") to raise pH.
' .
. . ~
Carbon Treatment
~ 25 The 95+~ (dsb) fructose feed stream for the
fl crystallization process is preferably carbon-treated
prior to concentration by evaporation. One purpose of
carbon treatment is to remove impurities that may inhibit
crystallization. Another purpose is to remove impurities
such as color bodies, HMF, furfural, and acetaldehyde
which adversely affect the quality of the mother liquor
and consequently impair its use as a component of a
liquid-phase sweetener. Carbon treatment is preferably
accomplished with granular carbon, at a dosage of about
1-3% dry substance, or powdered carbon, typically at a
lower dosage than granular carbon. The temperature of
*, "~:' . ~,'' ' .: : .:.. . . - : :
2~17~
-36-
the syrup is preferably about 160F and typically 15-30,
the syrup is preferably about 20 to about 25, percent by
weight dry substance.
Carbon treatment is most aclvantageously performed
immediately following fractionation and before
evaporation. Carbon treating at low solids concentration
has been found to keep fructose loss to difructose below
0.5%. If carbon treatment is accomplished after
10 evaporation, fructose losses greater than 2.5% can be ,c,;
expected. The syrup temperature should be approximately
160F (as compared to 140F) to prevent microbial growth
in the carbon adsorber and also to lower the syrup
viscosity to obtain better diffusion into the carbon
particles.
EXAMPLE
The amount of difructose formed in aqueous solutions
20 of at least 95% (dsb) fructose ~t varying dry solids was -
measured. In the first two trials, the aqueous solutions
I were mixed in a flask with 2.7% granular carbon (dry
I solids of granular carbGn by weight of the dry solids of
the aqueous solutions) and agitated at 160F for 24
hours. Samples were taken at 0, 6, 14 a~d 24 hours for
measurement of the difructose contained therein. The
results are shown below:
i ~9:l7~
-37-
TABLE II
Difru~tose L%dbs ~at:
Dry Solids: 25% ds 50% ds
35 Time lhrs)
0 0.25 0.47
6 0.32 0.85
14 0.38 1.62
~ 24 0.78 1.94
''~ 10
The above data shows that difructose formed much ~ :
faster (up to 4 times faster) in the solution at 50% ds
as compared with 25% ds.
si 15
The following four trials were designed to simulate
the operation of a commercial scale carbon-treating tower
in a plug flow manner, i~e, to allow measurement of
difructos~ formation in a dynamic flow system as compared
with the static system of an agitated flask.
Two 12-inch glass columns were run in series to
provide a residence time for the syrup feed of about 20
hours. The columns and a short coil of stainless tubing
used for feed preheat were immersed in regulated water
baths.
: :
To simulate a counter-current flow of carbon at
steady state, the columns were initially run to condition
and partially exhaust the carbon and the second column
w~s then slugged with virgin granular activated carbon,
placing about two inches of fresh carbon at the outlet of
this column.
.,
- 2 a~ 9 ~ r~ o ~
-38-
¦ Four different conditions were examined using this¦ apparatus: (with conditio~ed, new granular carbon for
I each condition)
70~ ds at 140F
70% ds at 160F
50% ds at 160F
25~ ds at 160F
Each of the condition6 listed was run with
continuous feed and no recycle for 35 hours and the
amounts of difructose in the column effluent at 0, 6, 14,
24 and 36 hours are shown below:
TABLE III
:~
Difructose (% dsb) at: __
Temp: 140F 160F
Dry Solids:70% ds 25%ds 50~ds 70~ds
Time (hrs)
0 0.32 0.32 0.35 0.32
6 0.83
14 0.26 0.92 0.95 --
24 0.39 0.61 1.35 1.83
36 0~24 0.64 1.72 2.24
While the formation of difructose at 140F at 70% ds
: appears to present no problem, the lower temperature
means an increased risk of microbiail growth and higher
syrup viscosity. Both of the higher solids tests at
160F (50% and 70% ds) showed difructose levels that
continued to substantially increase over time, although
the time of exposure to heat and carbon was the same for
all samples taken. Without wishing to be bound by any
particular theory, a possible explanation may be that the
formation of difructose is catalyzed and/or co-catalyzed
by material being removed from the aqueous solution by
2~17~
-39-
the carbon and thus the buildup of this material on the
carbon causes an increasing rate of conversion of
fructose to difructose over the time of use of the
carbon.
A carbon check filter may be used on the syrup ~ -
leaving tha carbon column to remove any carbon fines in
the stream. Efficient filtering is important because any
insoluble material that passes into the crystallizer will
be centrifuged into the crystalline fructose and directly
affect product quality.
Since the fructose that does not crystallize is
blended to make liquid-phase sweetener, the carbon
treatment enhances the quality of that material as well.
Since EFCS is normally carbon-treated near the end of the
process (i.e., after blending~, the mother liquor from
the centrifuge has been refined by two carbon treatments
by the time it reaches the final product. :~
Crystallizer Feed Evaporator
The driving force for the crystallization is
super-saturation obtained by cooling high-fructose syrup
to a point below its saturation temperature. The
saturation curve for fructose ~concentration vs.
~aturation temperature) is very steep. To achieve
theoretical crystallizer yields in the 40-55% range, e.g.
40-48~, a fructose feed syrup requires approximately
45-55F, e.g. 47F, of cooling.
During the evaporation step(s) water is removed from
the feed syrup to concentrate it to the point that
fructose will crystallize from the solution when it is
cooled. The evaporators are preferably designed and
2~7a~
-40-
. . , ,~, . .
operated to concentrate the solution with minimum heat
damage to the syrup. One preferred way of effecting
evaporation entails a two-step process. The feed syrup
is first concentrated in a 6-pass tube-type falling film
evaporator having multiple effects and mechanical
recompression. The 95+~ (dsb) fructose stream from the
carbon treatment step is supplied to the evaporator at
about 20 to about 25 percent by weight dry substance, at
a temperature of about 190F, and at a pH of about 3.7 to
about 4.3. The output of this step is a syrup having
about 55 to about 65 percent by weight dry substance.
In the second evaporation step, the output from the
first step is fed to a plate-type, rising film, single
effect evaporator operated at about 23 to about 24 in Hg
vacuum. The output of the second step is a syrup at
about 165 to about 175F having about 88 to about 90
¦ percent by weight dry substance. More preferably, the
¦ evaporator is operated at about 26 in Hg vacuum such that
~ ~0 the product temperature is about 140 to about 150F,
I thereby minimizing the loss of fructose.
The main criterion in crystallizer feed evaporator
design and operation is to concentrate the solution which
minimum heat damage to the syrup. The most troublesome
heat damage to crystallizer feed syrup is conversion of
fructose to difructose which reduces yield in the
crystallizer. The formation of difructose is favored by
high temperature, high concentration, and long residence
time in the evaporator. Since concentration is
essentially fixed, design and operating conditions should
be chosen to minimize temperature and residence time of
the syrup in the evaporator.
L 7 ~ 6
41-
Suitable evaporators such as the tube-type falling
film and the plate-type rising film are generally known
in the art.
Crystallization
Crystallization of fructose may be accomplished in
either bateh or continuous erystallizers. There are
advantages and disadvantages to both batch and continuous
erystallization. Batch crystall:ization has greater
flexibility in producing different erystal size
distributions, and can adjust for process upsets more
easily and quickly. However, batch erystallization has
lower crystallizer productivity (time required to load,
unload, and seed the crystallizer); it is more diffieult
to produee a eonsistent erystal size distribution from
batch ts batch; it requires larger storage tanks for feed
and for masseeuite in order to keep batch cycle times to
a minimum; and, it requires individual eoolin~ systems
for eaeh crystallizer. Continuous crystallization has
the opposite advantages and disadvantages.
Crystallization may be accomplished in either a
single pass or multiple passes. Single pass, howev~r, is
preferred. It is estimated that only 88% of the yield
per batch would be achieved and crystallization time
would be 87% longer for second pass crystallization.
Moreover, the mother liquor from a second pass
erystallization is more viscous due to greater levels of
higher saccharides and slurry density (pounds crystal per
pound masseeuite) is lower for second pass massecuite~
Both these factors tend to reduce centrifuge
productivity.
-42- 2~.917~
The utility of the mother liquor as blend stock for
a liquid-phase sweetener depends in large part on the
purity of the mother liquor. While the precise levels of
I by-products that can be tolerated in, or efficiently
¦ 5 removed from, the mother liquor will depend upon a
variety of factors, steps shoulcl be taken to minimize the
~ormation of by-products in the crystallization portion
of the process. Inasmuch as crystallization is
essentially selective for fructose, by-products tend to
become concentrated in the mother liquor with each
successive crystallization pass. Thus, the problem is
exacerbated in the case of multiple pass crystallizations
and the level of by-products in the mother liquor will
often impose an upper limit on the number of
crystallization passes which may actually be employed in
the integrated process.
It has been found that color, ash, HMF, furfural,
and acetaldehyde levels all tend to increase in the
mother liquor during multiple pass crystallization. Of
these, color increases most rapidly, and it is therefore
usually the determining factor in the number of
crystallization passes which may effectively be employed~
Appropriate measures to maintain the purity of the
mother liquor include careful control of evaporation,
carbon treatment, and crystallization conditions such as
pH, temperature, and residence times. Preferred
conditions are discussed in the sections of this
disclosure devoted to the various steps of the process.
.,
Syrup feed to the crystallizer is preferably cooled
to approximately 140F before entering the crystallizer.
To produce a 40-48% theoretical yield of crystalline
fructose it should contain a minimum of 95~ (dsb)
: :,
3 -~3- 2~7~6
`! .
fructose and have a solids content of 88.5-89.7% by
weight (nominal 8g% d . S . b~ .
The batch is seeded and thoroughly mixed with the
5 seed crystals. The seeding temperature (approximately
135F) is based on the estimatecl percent d.s. and percent
fructos~ of the crystallizer batch. Once the syrup is
~ thoroughly mixed with the seed crystals, a sample of the
¦ batch should be analyzed to determine the actual
10 saturation temperature. The cooling system of the
crystallizer should be adjusted to bring the batch into
the supersaturation range 1.00-1.05 (based on fructose
concentration). If the massecuite is already below this
range, but nucleation has not occurred, cooling should
15 continue.
Nucleation is a process hy which crystals are formed
from liquids! supersaturated solutions (gels), or
saturated vapor (clouds). A crystal originates on a
2Q minute trace of a foreign substance acting as a nucleus.
These are often provided by impurities. Crystals form
initially in tiny regions of the parent phase and then
propagate into it by accretion. In the process of the
subject invention, nucleation is undesirable inasmuch as
25 it gives rise to a produce of small crystal size.
Moreover, control of the crystal size distribution is
lost if appreciable nucleation occurs. For these
reasons, the use of seed crystals is preferred.
The progress of the crystallization can be
controlled indirectly by the rate of massecuite cooling,
the setpoint for the cooling water being adjusted
according to predetermined cooling curve such that the
supersaturation level is 1.0 to 1.35, e.g. 1.0 to 1.3.
'
~ _44_ 2~7~
More preferably, supersaturation is actually
measured in order to directly control the progress of the
crystallization. Supersaturation can be estimated from
percent d.s. of the mother liquor alone given the initial
p~rcent d.s. and percent fructose. Using the
supersaturation data, a decision can be made whether to
continue a batch on a predetermined cooling curve or to
modify the cooling rate so as to maintain the desired
degree of supersaturation.
One preferred way of effecting crystallization
comprises seeding a 95+% (dsb) fructose syrup having
about 88 to ~bout 90 percent by weight dry substance, a
pH of about 3.7 to about 4.3, and a temperature of about
lS 130 to about 138F with about 7 to about 10% by weight of
seed crystals having a mean particle size of about 150 to
about 250 micrometers. The seeded syrup is then
subjected to controlled cooling to cause the fructose in
solution to crystallize out.
The cooling can be accomplished as follows: from a
syrup temperature of about 138 to about 115F the syrup
is cooled at a rate of about 0.5F/hr; from about 115 to
about 86F the syrup is cooled at the rate of about 1.0
to about 1.5F/hr. It is recommended that the
supersaturation level be maintained below about 1.17 when
the syrup temperature is above about 115F and maintained
below about 1.25 if the syrup temperature is below about
115F. The maximum temperature difference between the
coolant and the massecuite is preferably about 10F. Too
high a temperature difference may cause nucleation to
occur.
Preferably, however, cooling is controlled at
differing rates in at least three periods. For example,
during the Initial Period, when the syrup is between
`~ -45~ 7Q~
about 13f~ and about 125F, the cooling is accomplished at
a rate between about 1.0 and about 1.5F/hr and the
supersaturation level is maintained below about 1.20.
During the Critical Period, when the syrup is between
about 125 and about 110F, the cooling rate is preferably
about 0.5 to about 1.0F/hr and the supersaturation level
is maintained below about 1.17. And, during the Rapid
Cooldown Period, when the syrup is between about 110 and
about 86F, the cooling rate is preferably about 1.5 to
about 2.5F/hr and the supersaturation level is
maintained below about 1.25.
~1 ,
3 It has been found that a preferred means of cooling
involves coupling a continuous monitor of the level of
supersaturation to an automatic control of the cooling
water temperature. In a particularly preferred means, a
data processor continuously receives information about
massecuite temperature, cooling water temperature and
supersaturation. The processor then uses this
information to control the cooling water temperature and
thus, the rate of cooling of the massecuite. The data
processor is programmed to first cool the massecuite from
its seeding temperature (Ts) to a predetermined critical
temperature (T~) at 2.5F/hr. (The critical temperature
is predetermined by calculating from the % fructose and %
ds of the crystallizer feed the temperature at which the
level of supersaturation will reach 1.17). The program
then prsvides for cooling of the massecuite from T' to
115F at 1F/hr and from 115F to final temperature
(typically 86F) at 1.5F/hr. However, the program has
overrides ko prevent excessive nucleation. First, the
program provides that, in any event, the temperature
difference between the massecuite and cooling water will
not at any time during cooling exceed a predetermined
temperature (typically about 14F). Second, the program
provides that, in any event, the level of supersaturation
- ~6- 2 ~ J~ ~
will not at any time during cooling exceed a
predetermined value (typically 1.28). The particular
temperatures and rates described above may be varied to
optimize the curve for a given set of crystallization
conditions without undue experimentation. The major
factors which affect the temperatures are the total dry
solids level (% ds) and the total surface area of the
seed. For example, increasing the dry solids level will
move the critical period to a range earlier in the
cooling curve and vice versa. Decreasing the total
surface area of the seed, e.g. by ~ecreasing the amount
of seed loaded, will broaden the critical period, and
vice versa.
Crystallization Kinetics
Supersaturation
In crystallization kinetics, the growth rate is a
function of a concentration driving force--the
concentration present in the mother liquor ~ersus the
concentration that would be present at that temperature
at equilibrium.
Supersaturation is a measure of the concentration
driving force. There are many ways of defining
supersaturation. For fructose crystallization, it has
been found that supersaturation defined on a water basis
is the most reliable for the purpose of monitoring the
progress of the batch. Thus, supersaturation is defined
as the ratio of the grams of fructose per gram of water
in the supersaturated syrup to that which obtains at
equilibrium:
~ ~~7~ 2~ 7~
(Fructose/Water)
Supersaturation = ACTUAL
(Fructose/water)EQuI~
Ideally, the batch cooling rate should be adjusted
to control the supersaturation level of the mother
liquor. For the crystallization of fructose it has been
found that the supersaturation range 1.0-1.30 produces an
acceptable yield of crystals in the desired size range.
Supersaturation levels below this range result in
extended batch cooling times while supersaturation levels
in excess of 1.35 result in severe nucleation.
Nucleation
There is a tradeoff in selecting a target value of
~upersaturation. Fructose does not appear to have a
detectable metastable zone, i.e., a range of
supersaturation wherein no nucleation occurs. The growth
of existing crystals i5 always competing with the birth
of new crystals (nucleation). As the supersaturation
level is raised, the crystal growth rate increases, but
so does the nucleation rate. The goal is to find a
2S supersaturation level that will produce the desired -~
crystal size in an economically advantagPous cycle time. - ~
-:
The nucleation referred to above is the "shower" or
"shock" type. As mentioned above, fructose
crystallization is always accompanied by nucleation.
Shock nucleation can occur at the start of the batch upon
occur at the start of the batch upon seeding. It is
contemplated that this is due to a low seeding
-~8- 2~ 7~
temperature. If nucleation occurs, the massecuite should
preferably be heated to remove the nuclei. Once the
nuclei have been dissolved, cooling can begin.
A preferred method of avoicling shock nucleation is
to maintain the supersaturation level below 1.30 aftPr
seeding. Massive nucleation will greatly increase the
viscosity of the massecuite, making centrifuging very
difficult by greatly increasing the purge time~ Fine
crystals separated from the massecuite are much more
difficult to dry and tend to agglomerate more easily.
Massive nucleation gives rise to a product with
undesirably small mean crystal si~e.
It has been observed that a 95-gallon batch of syrup
in a 100-gallon crystallizer will require about a 30- to
80-hour cooling cycle and usually about 35- to ~0-hour
cooling cycle for fructose crystallization. During that
period the syrup is preferably cooled at multiple,
preferable three, different rates. The requirement of
different cooling rates is a consequence of the -
nonlinearity of fructose crystallization. The various
rates correspond to the different periods o~ growth found
during cooling.
Initial cooling covers the temperature range down to
about 120F. The target cooling range is about l to
4F/hr; the typical rate is 2F per hour, which makes
this period require four to six hours, preferably about
eight hours. During this time growth occurs almost
entirely on the seed crystals and slurry density builds
slowly. Most of the heat load on the cooling water comes
from removal of sensible heat.
2~3~7~
-49-
Nucleation of the batch can occur in this region;
however, this will occur only if the seediny temperature
is too low or supersaturation exceeds 1.3.
In the "Critical Period" the growth rate increases
by a factor of 2 to 4. Slurry density increases rapidly
and new crystals are born and grow into the desired size
range. The competing processes of crystal growth and
nucleation both accelerate.
The boundaries of this region are not clearly
defined. Best estimates place it between 120 and 110F.
Caution is required in this region inasmuch as nucleation
processes can easily dominate and get out of control. By
maintaining a moderate level of supersaturation (1.05 -
1.20), it has been found that nucleation can be kept
within acceptable limits. A slower cooling rate is the
preferred way to control the degree of supersaturation.
In this region a cooling rate of about 0.5 to 3.0F/hr,
typically a 0.5 to 1.5F/hr cooling rate, is recommended.
At this rate the estimated time in the Critical Period is
about 10-40 hours, preferably about 18-22 hours.
In some situations, high supersaturation levels may
not result in nucleation. In that case, further cooling
could lead to the formation of fructose hemihdyrate.
This species occurs in the form of needle-shaped crystals
which form a slurry having a very high viscosity
(>800,000 cps). This slurry is impractical to centrifuge
30 and may even overload the crystallizer drive. The -~
hemihydrate can be detected during routine crystal
inspections which should be conducted throughout the
cooldown period.
Upon completing the Critical Period the slurry
density is high enough to support a faster cooling rate
2 ~
-50-
i
~i without nucleating. In this rapid cooldown region the
cooling water temperature can be dropped rapidly.
I Massecuite cooling rates of from about 1 to 7F/hr,
~ preferably about 1-4F/hr, are recommended. To cool from
i 5 110 to a final temperature of about 100-75F will
j require about 3-12, typically 8--12, hours. More rapid
;l cooling can be done without nucleation, but the growth
does not keep pace and one may be left with a higher
level of supersaturation at the end of the batch. Some
residual supersaturation can be relieved by placing the
, massecuite in a mingler or a mixer tank for a period of
time.
a
While cooling can be accomplished more rapidly in
the Rapid Cooldown Period than in the earlier phases of
the batch, there is a limit to how great a temperature
i difference can be tolerated between the cooling water and
the massecuite. This limit is not known with precision,
but cooling rates should not produce temperature
differences between the massecuite and the cooling
surfaces greater than about lSF. Temperature ~ -~
differences greater than this may cause nucleation and
fouling of cooling surfaces.
Seedinq
¦ The seeding temperature may be derived from the
saturation temperature of the full crystallizer mother
liquor. To obtain this information, a liquid
chromatogram of the feed syrup and the refractive index
can be taken. The percent fructose and the percent d.s.
of the feed syrup are then used to calculate a fructose
concentration. Seeding should be accomplished in the
supersaturation range of greater than 0.96, e.g. 1.0 to
1.10.
. ....
.~
2091~
-51-
Most preferably the seed is dried crystalline
fructose having a mean crystal size of about 100-400
microns. A 1 to 20% (dsb) loading is recommended. The
loading depends upon the particle size desired in the
~inal product. Seed should be added to the full
crystalli2er with every effort made to distribute the
seed uniformly in the crystallizer. As mentioned above,
U.S. Patent 4,164,429 describes a process and apparatus
for producing crystallization seeds.
Seeding is preferably accomplished by first mixing
the seed crystals with fructose feed syrup to obtain a
liquid slurry for addition to the crystallizer. This has
the effect of conditioning the surfaces of the seed
crystals. Preparing the seed crystals in syrup also
minimizes the formation of bubbles in the crystallizer
upon seeding. Bubbles are a possible site of nucleation.
, .
Consistent seeding is largely a matter of providing
the same surface area for growth of fructose crystals.
Since the surface-area-to-volume ratio of seed crystals
generally decreases with increasing particle size, if the -
size of the seed crystals is increased, a greater weight ~-
of seed crystals is required to obtain the desired
surface area.
Alternatively, a heel of about 5 to 30%, preferably
about 10 to 20%, may be left in the crystallizer to act
as seed. This procedure is much less labor intensive
j 30 than using dry seed, but produces a broader distribution
¦ of crystal sizes since fine particles remain in the heel
~ which would otherwise have been removed during the
¦ centrifuging and drying steps. With this method larger
crystals are obtained which may subsequently have to be
ground in order to meet final product crystal size
specifications.
7 1~ ~
The preferred procedure is to add hot syrup on top
of the heel. The hot syrup will raise the temperature of
the mass~cuite heel to the estimated saturation
temperature (approximately 133F) while ~he feed syrup is
5 cooled to seeding temperature. Some crystal mass is
probably lost during this process. Despite that ~act,
the final seed density should preferably be at lPast in
the rang~ of 2 to 10% (dsb). The critical poxtion of
this operation is the final temperature reached by the
feed syrup and the massecuite heel. This should result
in supersaturation levels of 1.00 to 1.10. In this range
the loss of seed will be minimized and the production of
nuclei will be small.
EXAMPLE
A fructose crystallization was conducted using a
feed syrup comprising 95.82% (dsb) fructose at 89.60% dry
substance in a pilot scale version of a conventional
crystallizer. The crystallizer employed had a center
shaft agitator. Cooling was achieved through internal
fins attached to the center shafk. The crystallizer was
nearly filled with 102 gallons of syrup. Cooling was
accomplished in about 40 hours from seeding; however,
considerable supersaturation (1.17) remained at the end
of the period. The batch was monitored by following the
change in supersaturation.
Seed was prepared by grinding crystalline product
through a 2A Fitzmill screen. The ground material was
screened through a 55-mesh screen and through a 100-mesh
screen. The seed had a mean size of 161 microns. Dry
seed was added directly to the syrup in the crystallizer.
''!7
2~17~
-53-
Table IV presents the cooling program actually used
during the crystallization. Supersaturation rose during
the first 18 hours of the run to a maximum of 1.26. It
then dropped to around 1.17 where it remained throughout
the remainder of the cool-down.
TABLE IV
Period Starting Ending Cooling
(hrs since seeding) Temp (F) Temp (F) Rate
(F/hr)
~ 2.0 - 10.8 133.5 122.5 1.~5
;~5 1~10 . 8 - 20.8 122.5 111.7 0.98
20.8 - 30O8 111.7 100.6 1.11
30.8 - 40.8 100.6 86.0 1.46
The product crystals had a mean size of 268 microns.
The crystal yield was 46% based on the fructose content
o~ the syrup.
'
Separation
A preferred method of separating fructose crystals
~rom the mother liquor is centrifugation in a basket
centrifuge. It has been found that about 4 gallons of
massecuite in a 14" x 6" centrifuge can be separated in
about 10-15 minutes. This period includes one to three,
typically two, washes with warm water (120-200F).
Higher washwater temperatures may result in a greater
dissolution oE fructose and loss of yield. Rerommended
washwater amounts are 1-5~ based on massecuite charge.
Deionized washwater can be used. It is preferred that
the pH of the washwater be in the range of about pH 3 to
5.
2~7~
54-
Preferred operating conditions for a basket
centrifuge used to remove crystalline fructose from the
mother liquor include: a g force of about 1400, a cake
thickness of about 2 to about 3 inches; cake moisture
5 between about 0.7 and about 1.5 percent by water; and a
product purity above about 99.5%, more preferably above
about 99.8%~ Cake moisture and purity are believed to be
important criteria for producing a nonagglomerated and
stable product. ~ ;
1 0
The product cake is preferably washed in the
centrifuge prior to removal. A preferred wash is water
at a temperature between about 150 and about 180F in a
~uantity of about 1 to about 1.5 percent by weight of the
massecuite charged to the centrifuge. Using this method,
loss of the product in the wash has typically been found
to be about 5 to about 10%. Washwater containing
dissolved fructose may be recycled to the carbon
treatment step for impurity removal and subsequent
reconcentration.
DrYinq
A variety of dryer types may be employed in the
process. Fluidized bed dryers, vibrating fluidized bed
dryers, tray and rotary dryers are all suitable.
Prefera~ly, wet cake from the centrifuge is metered into
a continuous mixer through a variable speed screw
conveyor. Dry recycle material is metered in through a
choked conveyor ~to prevent air bypassing) at a nominal
ratio up to 4:1 over the wet cake. Action in the mixer
must be sufficient to thoroughly blend the wet and dry
materials. The blended cake is then removed to the
dryer.
_55_ 2~7~
Preferably, the cake is dried concurrently to avoid
overheating the product. Room air should first be
cleaned by passage through an ultrafine borosilicate
filter rated for 35% removal of 0.5-micron particles.
The air is then heated to a temperature which, when mixed
with the exhaust air from the cooler, produces 160F air
at the dryer inlet.
The product leaves the dryer at about 130F and is
conveyed to the cooler. A controlled amount of the
produc~ is recycled without cooling to the dryer inlet
for treating wet centrifuge cake. The most critical
variable in dryer operation is moisture of the incoming
cake. If the moisture is too high, the dryer will
produce balls and agglomerated product. The moisture may
be controlled by the ratio of dry recycle to wet cake.
Although a 2:1 ratio of dry recycle to wet cake is
usually satisfactory for well-developed crystals,
nucleated crystals will not centrifuge well and may
require a 3:1 ratio to avoid agglomeration.
The centrifuge cake is preferably dried in a rotary
dryer to reduce the moisture of the fructose crystals to
below about 0.1 percent by weight. It has been found
that if the moisture content of the centrifuge cake
exceeds approximately 1.5 percent by weight, lumps will
form in the dryer. As noted above, dry product recycle
may be used to control the centrifuge cake moisture. It
is recommended that the product temperature not be
allowed to exceed about 140F. Preferred dryer opsrating
conditions are: an inlet air temperature of about 170 to
about 250F, more preferably about 170 to about 200F; an
outlet air temperature of about 130 to about 145F; a
product temperature of about 125 to about 135F; and, a
product moisture content of less than about 0.1%, more
preferably less than about 0.07%.
' -56~ 7~
¦ Conditioninq
, It has been found that if fructose crystals are
stored while still warm they will produce lumps during
storage. This same phenomenon exists in dextrose and
sucrose production. While the exact mechanism has not
been proven, it is contemplated that moisture migration
3 from the large crystals to the smaller ones causes
further crystallization at the boundariPs. This is the
~ result of either temperature variances or moisture
I variances, both of which occur because the crystal is not
~, at equilibrium. Tests have shown that drying the product
to very low moisture (around 0.05%) and cooling it to
room temperature will produce a free-flowing product. To
be in equilibrium with fructose crystals having 0.05~
~oisture, air at 70 must have a relative humidity below
50%.
A rotary cooler with countercurrent air works well
for this purpose. Refrigerated, dehumidified
(conditioned) air is used to cool the product crystals to
below about 75F, more preferably about 72F. It is
recommended that the inlet cooling air have a temperature
below about 70F and a relative humidity below about 40%.
Retention time in the cooler should be sufficient to
assure that the crystals are properly conditioned. The ~ ;
final product moisture content is preferably less than
about 0.07%.
The final product may be sized by screening and/or
grinding. Prolonged storage of product at high
temperatures will cause caking and color problems even if
it is stored in moisture barrier bags. Warehousing
should be done under controlled humidity conditions.
~ -57~ 7 ~ ~
Blending
The mother liquor separated from the crystalline
product in the centrifuge may be returned to the EFCS
portion of the process.
In addition to mixing dextrose with the mother
liquor which remains after separation of the crystalline
fructose, the mother liquor may simply be diluted with
water to produce a VEFCS.
Following separation of the crystalline fructose,
the mother liquor may be mixed with dextrose or
dextrose-containing solutions to ultimately produce a
liquid-phase sweetener comprising dextrose and fructose
such as 55% HFCS (EFCS). As shown in Fig. 3, a number of
dextrose-containing streams may be blended with the
mother liquor prior to input to the final finishing
operations. The choice of particular stream or streams
will be dictated by mass balance considerations, the goal
being the desired fructose level in the final liquid
phase sweetener product. Most commonly for the
integrated process this level will be 55% (dsb) fructose.
If sufficient fructose is available in the mother liquor,
it is even possible to use the dextrose product stream
from saccharification (typically 94-96% (dsb) dextrose)
to blend for input to EFCS finishing.
.:
Alternatively, the mother liquor which is typically
90-92% (dsb) fructose may simply be diluted with water to
produce a liquid-phase sweetener. Dilution is
recommended if it is desired to maintain the fructose
contained in the mother liquor in the liquid inasmuch as
additional fructose would likely crystallize from the
mother liquor if the solution is not diluted to below the
saturation point for all temperatures likely to be
~ ~ :
-58- 20917~6
encountered. In addition to water, other suitable
diluents include aqueous saccharide solutions such as
dextrose syrups, HFCS, EFCS, VEFCS, and production
streams for such syrups. Other means for inhibiting the
crystallization of ~ructose in the separated mother
liquor include measures for preventing or reducing the
evaporation of water from the solution and the
incorporation of crystallization-inhibiting additives.
¦ 10 Another use for the separated mother liquor or a
¦ portion thereof is production of a non-crystalline or a
semi-crystalline fructose sweetener. One way of
accomplishing this is to disperse the mother liquor on an
edible, particulate solid and then drying the dispersion
1 15 to produce a sweetener comprising fructose in an
¦ amorphous or semi-crystalline form. A preferred edible,
particulate solid for this purpose is crystalline
fructose.
United States Patent 4,517,021 describes a method
~or producing a semi-crystalline fructose composition.
The teachings of this patent are expressly incorporated
by reference into this disclosure. The separated mother
liquor of the present invention may be used as the
aqueous fructose syrup of that process and crystalline
fructose may be used as the crystallization initiator.
Thus, there is provided an integrated process for the
production of crystalline fructose, semi-crystalline
fructose, and one or more liquid-phase sweeteners
comprising fructose.
The foregoing description has been directed to
particular embodiments of the invention in accordance
with the requirements of the United States patent
statutes for the purpose of illustration and explanation.
It will be apparent to those skilled in this area,
~ _ 50 ~ 7~
~ .
however, that many modifications and changes in the
equipment, compositions and methods set forth will be
possible without departing from the scope and spirit of
the inventionO It is intended that the following claims
be interpreted to embrace all such modifications and
ohanges.