Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
20919~2
J
: ~erc~ Patent Ge~ellschaft
~it beschr~nkter Haftung
6100 D a r m 8 t a d t
_ armaceut~al compo~it~on
The invention relate~ to a new pharmaceutical
composition containing a potassium channel activator
("PCA") and an angioten6in II receptor antagonist ~"AR").
A particularly preferred embodiment i8
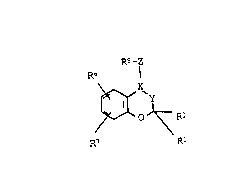
characteri~ed in that it contains a PCA o~ the formula I:
Rs -z
R6
\~ O J~ R-
F.7 R
10 in which
: X-Y i8 CH-CHR8-, -CR4-CR3A- or -CH-CA(OA)-, and, if
the radical R5 is neither completely nor parti-
ally hydrogenated, also -CR4 CHR3 or
-C~-CH(OA)-,
15 R1 is A,
R2 and Ra are each H:or A,
Rl and R2 together are al o alkylene wi~h 3-6 G at~ms,
~: R3 i~ 0~ or OAc,
R4 is H,
R3 and Rb t~ge her are also a bond,
R5 i~ a pyridyl, pyxidazinyl, pyrimidinyl,
pyra2inyl, oxo-dihy~ro-pyridyl, oxo-dihydro~
:~ pyridazinyl, oxo-dihydro-pyrimidinyl, oxo
dihydro-pyrazinyl, 3~- or 5H 2-pyrrolinon-1-yl,
2H~ oquinolinon-2-yl, 2H-l-phthalazinon-2
yl, 3~-4-quinazolinon-3-yl, or lH-2-thio-
pyridon-l-yl radical, each of which iB
unsubstituted or substituted once or twice by
A, F, Cl, Br, I, OH, OA, OAc, NO2, NH2, AcNH,
HOOC and/or AOOC, it al~o being possible for
the~e radicals to be completely or par~ially
~ .
.
2~19~2
-- 2 --
hydrogen~ted,
and R7 are each H, A, HO, AO, CHO, ACO, ACS, HOOC,
~OOC, AO-CS, ACOO, A-CS-O, hydroxy-alk,
mercapto-alk, NO2, NH2, NHA, NA2, CN, F, Cl, Br,
I, CF3, ASO, ASO2, AO-SO, AO-SO2, AcNH,
AO-CO-NH, H~NSO, HANSO, A~NSO, H2NSO2, HANSO2,
A2NSO2, H2NCO, ~ANCO, A2NCO, H2NCS, HANCS, A~NCS,
ASONH, A502NH, AO50NH, AOSO2NH, ACO-alk, nitro-
alk, cyano-alk, A-C(=NOH) or A-C(=NH2),
10 Z i5 a bond, O, S or NH,
R7 is al~o pyridyl, pyridazinyl, pyrimidinyl or
pyrazinyl,
A i~ alkyl with 1-6 C atoms (whare several
group3 .~ can, independently of one ano~her, be
different alkyl groups)~
-alk- i8 alkylene with 1~6 C atoms and
Ac is alkanoyl with 1-8 C a~oms or aroyl with 7-11
C atoms,
and/or one of the phy~iologically accep~able salts
thereof.
:Some of the compounds of the formula I are Xnown
(compare~ for example, DE-A-3,726,261).
: Preferred compound~ of the formula I are those in
: which the~radical R1 and:R2 are Qach CH3, the radical R7
i~ H and/or the radical R6~is CN, with the radical RB
~:~ :: preferably bei~g i~ the 6 position. ~he group X-Y i~
; ~: preferably~ -CR~-CR3A-~[especially; -C(OH)A or -C=~A-j
or;~ else, if the~radical ~5 i 8: neither completely nor
~ partially hydrogenated, -CR'-CHR3- (e~peclally~-CH-CHO~-
: ~ 3~ o~ -C-C~-)O
The group R~-Z i~ preferably l~-2-pyridon-1-yl,
~:~ 2-hydro~y-4-pyridyl-oxy, :~6-hyd~oxy-3-pyridazinyl-oxy,
~ 1,6-dihydro-1-methyl- or 1,:6-dihydro~1-ethyl-6-oxo-3-
:; : ~ pyridazinyl-oxy.
:~ 35 ~pecific preferred compound~ of the f~rmula I ars
~2-dimethyl-4-llH-2-pyridon 1-yl~-6-cyano-3-chroManol
(Ia), e~p~cially it~ enan~iomer, 2,2-dimethyl-4 tlH-
2-pyridon-l-yl)-6-cyano-2H-chromene (~Bimakalim"; Ib),
: :
,
:
_ 3 _ 209~942
2,2-dimethyl-4-(6-hydroxy-3-pyridazinyl-oxy3-6-cyano-
chromanol~ especially its (-) enantiomer, 2,2,3-
trimethyl-4-(6-hydroxy-3-pyrldazinyl~oxy)-6-cyano-3-
chromanol, e~pecially it~ (-) enantiomer, and 2,2-
dimethyl-4-(1,6-dihydro-1-methyl-6-oxo-3-pyridazinyloxy)-
6-cyano-3-chromanol (Ic), especially its (-) enantiomer.
AR, especially angiotensin II subtype l receptor
antagonists ("AT1 antagoni~ts"), are substance4 which
displace the hormone angiotensin II from its receptor
(ATl rsceptor). AR, especially ATl antagonists, lower the
blood pre~sure when the hypertension i~ associated with
an increased activity of the renin-angiotensin ~y~tem.
Preferred AR, and proces3e~ for the preparation
thereof, are indicated, for example, in the following
patent applications. DE-40 06 693, EP-28 833, EP-230 922,
EP-245 537, EP-253 310, EP-323 841, EP 324 377,
EP-392 317, EP-399 731, ~P-400 835, EP-400 974,
EP-401 030, EP-403 158, EP-403 159, ~P-407 102,
EP-407 342, EP-409 332, EP-411 507, EP-411 766,
EP-412 594, EP-415 886~ EP-419 048, EP-420 237,
EP-424 317, EP-425 211, EP-425 921, EP-426 021,
EP-427 463, EP-429 257, EP~430 300, EP-430 709,
EP-432 737, EP-434 038, EP-434 249, EP-435 827,
EP-437 103, EP-438 869, EP-442 473, EP-443 568,
EP-443 983, EP-445 811, EP-446 062, EP-449 699,
EP-450 566, EP-453 210, EP-454 831, EP-456 442,
EP-456 510, EP-459 136, :EP~461 039, EP-461 040,
US-4 88~ 804, US-4 916 129, US-5 ~41 552, US-~:045 540,
US-5 049 56~, US-5 053 3~9, W0-83 03 250, ~0-87 05 0~9,
WO-91 00 277, WO-91 00 281, WO-gl 07 404, WO-91 09 8~7,
WO-91 10 140, WO-91 11 909, WO-91 11 999, ~O-~l 12 001,
WO-91 12 002, WO-91 12 271, WO-91 13 088, WO-91 1~ 367,
WO-91 14 679, WO-91 15 206, WO-91 15 ~09, WO-91 15 47g,
WO-91 16 313, WO-91 17 148.
Further preferred AR corre~pond ~o the formula II
209~9~2
-- 4 --
Rll
R -CH2 - ~-X-~ I I
Rl
in which
R is
Rl4 Q
(a) N ~
R9 ~N~NRl' (b)N~Rl2
Q R19
Rl4 Q
R9 ~ R3 ~
H R~4
R16
R~7~ Rls
o r ( e )
R18
: R~ is A, alkenyl or alkynyl each with up to
: ` 6 C atoms,
Rl is H, COO~, COOA, CN, ~2~ NH2t ~HcoR13J NHSo2R13
or lH-S-tetrazolyl,
; R11 is H,~ Hal, A,: OA~or NO2,
~: R~ is:H, R13, Gyano-alk-~; ~ooc alk-, carboxy-alk-,
~ lH-5-tetra~olyl-alk , Ar-alk-, Ri3-CO-alX-, Ar-
- 10: : CO-alk-, He~-CO-alk-, ~et-alk-, alkenyl or
alkynyl, each with up to 6 C atoms
Rll
or -CH2-~W~
Rl:
Rl3 : i5~ alkyl with 1-4 C atoms, iTl which it is al50
po~sible for one or more H atom( 8 3 to be
`
.
. ~
2~91942
replaced by F,
R14 is H or Hal,
R15 is H, COOH, COOA, CHO, CN, NO2, CH2R19, CH2OR2,
NR21R22 CH NR21R22, CONR21~22 or lH-s-tetra
5 R16 is H or A,
17
R ,
and R21 are each H, A, alkenyl with 2-6 C atoms,
alkynyl with 2-6 C atoms, Ar or Ar-alk-,
Rl9 i8 H, Hal, A, Ar, CN, COO~, COOA, CH2COOH,
CH2COO~ or lH-5-tetrazolyl,
R20 is H, A, Ar, Ar~alk-, COA, COAx, COOA, COO~r,
CoNR23R24, COQ-alk-Ar or A-O-alk-,
~21 iS H, A, mono~ or poly-F-sub~tituted A, Ar,
Ar-alk~, CO-~, CO-Ar, CO-alk-Ar, COOA, COOAr,
COO-alk-~r or CoNRZ3R24,
NR21R22 i8 also pyrrolidino, piperidino, morpholino,
3uccinLmido or phthalimido,
R23 and R24are each H, A, cycloalkyl with 3-8 C a~oms,
alkenyl with 2-6 C atoms, alkynyl with
2-6 C atoms or Ar,
W is absent, -CO-, -O-, -NH-CO-, -CO-NH-, -C~2--,
-O-CH2-, -O-CH(COOH)-, -NH-CH(COOH)-, -NA-
CH ( COOH ) -, -CH=C ( COOH ) -, -CEI=C ( CN ) - or
-CH=C(lH-5-tetrazolyl)-,
25 Q i~ O or S,
: A~ i~ a phenyl group which i5 un~ub~tituted or
mono- or disubstituted~y ;Hal, R13, OH, oR13,
COOH, CooR13, C~, ~O2~,:NH2, N~A, N(A)~2, ~HCOR13t
; NHCOOA, NHSo2R13 and/ox l~-5-tetrazolyl,
30 Het i~ a 5- or 6-membered heteroaromatic: radical
with l to 3 ~, O and/or S atom~, which can ~l~o
be condensed with a benzene or pyridine ring~
Hal iB F, Cl ~ Br o~ I, ~nd
and -alk- hav~ the meanings indicated for fo~mula I,
and the ~alt~ thereof.
Additional preferred AR correspond to the
formul~ III
209~42
~ 6 -
!
R'
R~s-C~ ~ III
in which N--~A~
: : R25 is the radical R~3 ~ J ~ C
: N D
R26 is ~,: Hal, COOH, CONH2, CHO, CN, NH2 or
~: ` lH-5-tatrazolyl,
R27 is H, COOH, COOR26, CN, NO2, NH2, NHCOCF3,
NHSO2CF3 or lH 5-tetrazolyl,
`~ RZ~ i8 H, A, alkenyl or alkynyl, each with up to
.
:~ : 6 C atoms,
-A-B-C-D- is one o~ the groups -CH=CH-CH=N-,
-CH=CH-N=CH-, -CH=N-CH=CH-, -N=CH-CH=CH-,
-CH-CH-CO-NR29-, -CH=CH-NR2~-CO-, -CO-N*9-CH=CH-
or -NR29-C~-CH=CH-, in which the H atom~ of the
-CH~ qroups can be replaced by A, Hal, COOR2~,
CN and/or lH-5-tetrazolyl,~
~29 i8 ~ ~j A,~ ~cyano-alk-, R28OOC_alk_,
lH-~-tetrazolyI-alk-:or ~r-alk-, and
A, -alk-,:Ar and Hal have ~the~:~meanings indicat~d for
formula~I or II,~and the s~lt~ thereo~
Som~ ~pecifically preferred AR~are.~
2-butyl-4-chloro-5-hydroxyT~hyl-1-[2'-ilH-5 tetrazolyl)-
biphenylyl-4 ethyl]-imidazole ~ and ~ lt : K salt
nDUP:~753"); ~6~-butyl-1,2~-dlhydro-2-oxo-1-C2'-~lH-5-
tetrozolyl)~-biphenylyl-4-methyl]-p~ridine (m.p.~138~,
and the following::2-butyl-4/5-~ihydro-4-oxo-3-~2'-(lH-5-
: 25 tetrazolyl)-biphenyly~ 4-methyl3 -3~-imida~o~4,5-c)
~ ~ pyridine6. :~
: : 5-o-fluorobQn~syl~ ;(m.p.:~118~
: : 5-~2-thienylmethyl)-:(m.p. 14S~:
5-(3,3-dimethyl-2-oxo-butyl)- (m.p.: 203)
: 33 5-(o-methoxyphenacy})- (m.p.~137~
~ ~ 5-(o-methoxycarbonylbenzy1)- (~m.p. 124-).
:
::
~ '
: . ' ,
2~919~2
_ 7 -
It i8 remarkabla that concurrent administration
of AR di~tinctly enhances the action of the ~aid PCA in
lowering blood pressure, lowers the heart rate and
reduce~ the activity of the renin-angiotensin system.
This effect can be found, for example, in standard tests
on anaesthetised or conscious rats, dogs, cats, monXeys
or minipig~, for example by methods as described in
EP~A2-0,271,271.
The new pharmaceutical compo~ition can be pre-
pared by converting (at least) one PCA and (at least) one
AR together with at least one solid, liquid or 8emi-
liquid vehicle or auxiliary into a sui~able do3age form.
The composition~ obtained in thi~ way can be u~ed a~
pharmaceutical~ in human or veterinary medicine, e~-
pecially for lowering blood pressure. Suitable vehicles
~ are organic or inorganic substances which are suitable
: for enteral (for example oral or rec~al), parenteral or
topical administration and ~o not react with the new
compounds, for example water, vegetable oils, benzyl
alcohol~, polyethylene glycols, glycerol triacetate and
other fatty acid glyceride~, gelatin, ~oya lecithin,
carbohydrates such as lacto~e or starch, magnesium
stearate, talc or cellulose. U~ed for oral administration
are, in particular, tablet~, coated tablet~, capsules,
25 SylUp8~ ~olutions or drop8, for rectal administration are
suppositorie~, for parenteral ad~inistration are solu-
tions, preferably oily or aqueous solutions, as well a~
: suspen ion~, emul3ion~ or:~ implants, and for topical
adminiYtrat~on are o-n~ment~, cr~am~ or pla~ters. The
active ~ubstances can also be freeze-dried and the
reffulting lyophili~ate~ used,:for example~ for:preparing
in~ection products. ~he compositions can be s$erilised
: and/or contain auxiliarie~ 3uch as preservatives~ stabi-
liser~ and/or wetting agents, emul~ifier~, salts to
influence ~he o~motic pre~sure, bufer su~tance~,
: colorants and/or flavouring~. They can also contain other
active sub~tances, for example other ~ubstance~ actiny to
lower blood pressure, or diuretics.
";
2~919~2
-- 8 --
The compo~itions according to the invention are,
a~ a rule, adminis~ered for the therapy and/or prophy-
laxis of di~order~ of the cardiovascular 8y8t~m, e~-
pecially decompensated heart failure, angina pectoris,
peripheral or cerebrsl vessel di~orders and pathological
states associated wlth high blood pressure, in analogy to
known substances acting to lower the blood pressure,
especially the AR themselves. The dosages o the PCA are
prefarably between about 0.01 mg and 50 mg, especially
0.02 and 5 mg, very particularly preferably between 0.1
and 1 mg, per do~age unlt. The AR are preferably used in
do~ages between 0.5 and 500 mg, especially between 1 and
200 mg, per dosage unit. Preferred do~ages of the
indicated individual AR are between 2 and 200, especially
between S and 100 mg per dosage unit. The daily dosage of
the PCA is preferably between about 0.0001 and 1,
preferably between 0.0002 and 0.1 mg/kg of body weight,
and those of the AR are preferably between about 0.01 and
10 mg/kg of body weight. The specific do~e for each
particular patien~ depend~, however, on a wide variety of
factors, for ex~mple on the activity of the specific
compound employed, the age, body weight, general state of
health, ~ex, the diet, the time and route of
administration, the rate of elimination, drug combination
and severity of the particular disea~e for which the
therapy is applied. Oral administration is preferred.
The components of the~ new pharmacsutical com-
position are preferably adminis~ered in combination.
However, ~they can al~o be admini~tered ~ingly, con-
currently or uccessively.Example A: ~ablets
A mixture of 20 g of PCA active substance ~e.g.
Ia), 0.4 kg of AR active ~ub~tance (e.g. D~P 753), 4 kg
of lactose, 1.2 Xg of potato starch, 0.2 kg of talc and
0.1 kq of magnesi~m ~tearate i~ compressed in a customary
manner to give tablets such tha~ each tablet contains
1 mg of PCA active sub~tance and 20 mg of AR active
substance.
2~19~2
g
~ample B: Coated tablet~
Tablets are compressed in analoyy to Example A
and then coated in a conventional manner with a coating
composed of sucrose, potato starch, talc, tragacanth and
S colorant.
~ample C: Cap~ules
A screened mixture of 30 g of PCA active
~ubstance (e.g. Ib), 1 kg of AR active substance and 6 kg
of lactose i~ used to fill hard gelatin capsuleR in a
customary manner ~uch that each capsule contains 0.3 mg
of PCA active ~ubstance and 10 mg of AR active su~stance.
~xample D: Ampoule~
A solution of 2 g of PCA active substance (e.g.
Ic) and 0.1 kg of AR active sub~tance in 30 1 of double-
distilled water i8 filtered qterile, dispensed intoampoules, freeze-dried under ~terile condltions and
saalad sterile. Each ampoule contain~ 0.1 mg of PCA
active ~o~tance and S mg of ~R actlve s~o~tance.
,' ' ~