Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2~9~
-- 2 --
BACRGROU~ID OF THE INVBNTION
Fatty acid-based materials (fatty materials) such as
glyceride oils, wax esters, milk fat, and other fatty
acid compounds have a long history of use since many of
these materials are naturally derived from plants (e.g.
vegetable oils) or animals (e.g. tallow, milk fat, etc.).
While these fatty materials often have been directly
used in their crude state, modern commercial products
based on these materials are typically subjected to a
refining process. Refining processes may be used to
remove various impurities which are undesirable for
reasons of health, performance, aesthetics, etc.
The ~atty material may contain impurities such as
color bodies, chlorophyll, phospholipids (phosphatides),
trace metals (e.g. Ca, Mg, Fe), free fatty acids (FFA),
gums, soaps and/or other impurities. This variety of
divçrse impurities has led to the development of numerous
refining processes involving particular combinations of
chemical and/or physical treatment steps. A detailed
review of refining processes may be found in the
"Handbook for Soy Oil Processing and Utilization," ed. by
David R. Erikson et al., ASA/AOCS Monograph, 1980.
Recently, new refining processes have been developed
which share the common feature that an amorphous
adsorbent is contacted with a soap-containing fatty
material whereby soap and possibly other impurities in
the fatty material are adsorbed by the amorphous
adsorbent. Such refining processes are disclosed in
European Patent Application 0247411 (EP'411 - "MCR") and
U.S. Patent Application Serial No. 07/677455 (SN'455 -
"MPR"), filed April 3, 1991. The disclosures of these
applications are incorporated herein by reference.
20921~5
In the EP'411 process, large amounts of soap are
intentionally created in the fatty material by the
addition of a chemical base (e.g. NaOH) to the fatty
material at a point in the '411 process to eliminate free
fatty acid and to facilitate removal of other impurities.
The bulk of the soap is then separated from the fatty
material by a primary centrifuge. The fatty material
output from the primary centrifuge is then contacted with
an amorphous adsorbent (e.g. a silica gel) to remove
residual soaps and impurities from the fatty material.
Immediately after the adsorption or at some point
downstream in the process, the soap-containing adsorbent
is separated from the fatty material. The fatty material
may be subjected to additional refining steps (e.g.
bleaching, deodorizing, etc.) before and/or after the
separation of the amorphous adsorbent.
The SN'455 process differs from the EP'411 process
by creating only a small amount of soap by addition of
chemical base. The smaller amount of soap can be removed
from the fatty material by simply contacting with
amorphous adsorbent, i.e. without need for the primary
centrifuge step. As with the EP'411 process, the fatty
material may be subjected to additional refining steps
before and/or after separation of the amorphous
adsorbent.
A problem exists with these amorphous adsorbent-
based processes if one tries to subject the fatty
material to vacuum bleaching before removal of the soap-
containing adsorbent. Namely, adsorbed soap appears to
~0 leach out of the amorphous adsorbent in the vacuum
bleacher. This problem occurs even if the amount of soap
initially created is decreased relative to the amount of
amorphous adsorbent used. Soap leaching is undesirable
2~214~
4 --
since it causes sliming of packed beds and filters used
in the refining process. Since commercial refining
processes are typically continuous processes with large
throughput, even concentrations of a few ppm of soap are
generally unacceptable since the effect of the soap is
magnified by the large throughput. Also, the presence of
soap in the fatty material after vacuum bleaching can
have adverse effects in subsequent processing steps.
To date, the only solution to this problem has been
to separate out the soap-containing adsorbent prior to
vacuum bleaching. However, the insertion of a filtration
step before the vacuum bleacher may be commercially
undesirable or difficult to install in an existing
refining set up. Thus, there is a need for a way to
overcome the soap leaching problem without resorting to
filtration before vacuum bleaching.
8UMMARY OF THE INVENTION
The invention improves on the amorphous adsorbent-
based processes by overcoming the soap leaching problem
without using filtration before vacuum bleaching. For
fatty materials containing only minor amounts of
phospholipid and trace metal impurities, simply vacuum
drying at least a portion of the soap-containing fatty
material prior to contacting with the amorphous adsorbent
eliminates the soap leaching problem. For fatty
materials containing higher amounts of phospholipid and
trace metals, simply using an acid pretreatment and the
vacuum drying step eliminates the soap leaching problem.
2092145
In a process for treating a fatty material
containing water and soap, the process comprising:
a) contacting the material with a silica-based
amorphous adsorbent whereby soap is adsorbed by
the adsorbent, and
b) subsequently removing the soap-containing
adsorbent from the material,
the invention comprises the improvement of drying at
least a portion of the fatty material prior to the
contacting with adsorbent.
In a more specific process for treating a fatty
material, the process comprising
a) combining the material with a base to form
soap,
b) contacting the soap-containing material with a
silica-based amorphous adsorbent whereby soap
is adsorbed by the adsorbent, and
c) removing the soap-containing adsorbent from the
material,
the invention comprises the improvement of:
i) acid pretreating the fatty material prior
to or during step a), and
ii) drying at least a portion of the material
between steps a) and b).
In another preferred embodiment, the improvement may
also comprise:
iii) bleaching the material between steps b)
and c).
Preferably, the drying step of the invention
comprises vacuum drying at least a portion of the fatty
material. More preferably, all of the fatty material is
dried in the drying step. Preferably, the drying is
carried out so the fatty material to be contacted with
2~92145
the amorphous adsorbent has a water content of less than
about 0.6 wt.%, more preferably about 0.1-0.2 wt.%.
The acid pretreatment step preferably comprises
mixing into the fatty material, a minor amount of an
acid. Phosphoric acid is preferred. The bleaching step
preferably is a vacuum bleaching.
These and other aspects of the invention will be
discussed in more detail below.
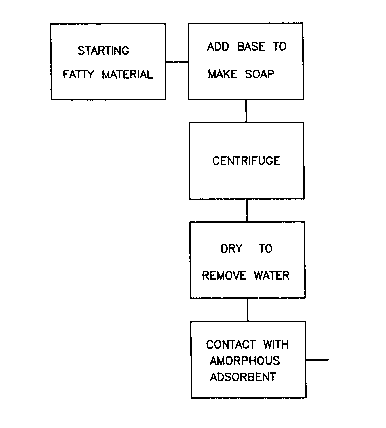
BRIEF DESCRIPTION OF THE D~A~INGS
Figure 1 is a flow diagram for the improvement of
the invention as applied to a modified caustic refining
process.
Figure ~ is a flow diagram for the improvement of
the invention as applied to an MPR type process.
Figure 3 is a flow diagram for the improvement of
the invention as applied to a slip stream configuration
of the process shown in figure 1.
Figure 4 is a flow diagram of the process
illustrated in fiqure 3 with the addition of acid
pretreatment and vacuum bleaching steps.
DET~ILED DE8CRIP~ION OF THE INVENTION
Broadly, the invention relates to the treatment of
any fatty material containing soap and water where the
material is to be contacted with an amorphous adsorbent
for purposes of removing soap and possibly other
contaminants from the fatty material. The improvement of
the invention encompasses drying the fatty material prior
to the contacting step.
2~2~
This drying step results in improved adsorption
efficiency and/or reduction in the required amount of
adsorbent. Additionally, for fatty materials containing
only minor amounts of phospholipid and trace metal (e.g.
corn oil) the drying step results in improved retention
of the adsorbed soap in the adsorbent whereby the fatty
material containing the adsorbent can be sent to a
downstream vacuum bleacher without prior removal of the
adsorbent and without leaching of the soap.
The initial fatty material containing soap and water
may be generated by any desired series of process steps,
even crude fatty materials or used fatty materials may be
used, assuming they contain soap and water. Preferably,
the initial fatty material to be treated by the process
of the invention is prepared by the steps of a caustic
refining process up through the primary centrifuge or by
the caustic addition step of the MPR process described in
SN'455.
For purposes of illustration only, the invention
will be described with respect to the process flow
diagrams shown in figures 1-4. The invention is not
limited to the particular embodiments shown in the
figures.
Figure 1 shows an example of the improvement of the
invention in the general context of a modified caustic
refining process. In figure 1, a fatty material is
treated with a chemical base to form soap (typically
about 7000 - 10000 ppm). The resulting mixture is fed to
a primary centrifuge where the bulk of the soap and water
is removed. The centrifuged fatty material still
contains a significant amount of soap and water due to
the limitations of centrifuge separation. According to
the improvement of the invention, the centrifuged
20921~
material is then dried prior to contact with the
amorphous adsorbent.
While figure 1 shows the entire flow of fatty
material going to the dryer, another option is shown in
figure 3 where a slip stream of the centrifuge output is
combined with the amorphous adsorbent while the major
portion of the centrifuge output is dried before contact
with the amorphous adsorbent.
Once the fatty material has been contacted with the
amorphous adsorbent, the fatty material may be subjected
to any desired processing steps known in the art.
Typically, it is often desired to feed the fatty material
to a vacuum bleacher followed by filtration. This can
easily be done with the amorphous adsorbent-contacted
material of the invention without leaching of the soap
from the adsorbent~ If desired, the amorphous adsorbent
contacting step and the bleaching step may be combined by
use of sequential packed beds or other expedients known
in the art.
Figures 2 and 4 show examples of the invention as
applied to an MPR-type process as described in SN'455.
Figure 2 shows the first required step of which is the
creation of soap (typically about 20-3000 ppm) by
treating the fatty material with a chemical base. The
soap-containing material is then dried prior to contact
with the amorphous adsorbent. As with the process shown
in figure 3, a slip stream could be used for addition of
the amorphous adsorbent while the majority of the soap-
containing fatty material is dried prior to contact with
the amorphous adsorbent.
As with the modified caustic refining process of
figure 1, once the fatty material has been contacted with
the amorphous adsorbent, the fatty material may be
20921~
subjected to any desired processing steps known in the
art. Advantageously, the fatty material can be fed to a
vacuum bleacher prior to removal of the amorphous
adsorbent without leaching of the soap from the
adsorbent. If desired, the amorphous adsorbent
contacting step and the bleaching step may be combined by
use of sequential packed beds or other expedients known
in the art.
For fatty materials containing substantial amounts
of phospholipids and trace metals, vacuum drying alone
may not result in complete prevention of soap leaching in
the vacuum bleacher. With such fatty materials, the use
of an acid pretreatment, prior to or in conjunction with
the soap forming step, in addition to drying after soap
formation surprisingly solves the soap leaching problem.
It should be understood that this embodiment would also
work for low phospholipid starting materials such as corn
oil.
Figure 4 shows another MPR process variation where
the fatty material is specifically subjected to an acid
pretreatment prior to or in conjunction with the soap
formation step. This embodiment which uses both acid
pretreatment and the drying step of the invention is
especially preferred as providing the best performance in
terms of removal of soap and phospholipids and resistance
to soap leaching in the vacuum bleacher.
The drying step of the invention is preferably
performed to achieve a water content in the fatty
material of about 0.6 wt.% or less, more preferably
about 0.1-0.2 wt.~. While drying to less than 0.1 wt.%
moisture can be used under the invention, excess drying
is preferably avoided otherwise inversion of the soap may
occur making removal of the soap extremely difficult.
209214~
-- 10 --
The drying may be performed using any known
technique, however vacuum drying is generally preferred.
Preferably, the dryin~ is performed at about 70-110C. The
temperature, degree of vacuum, and retention time in the
dryer may be adjusted easily to achieve the desired
amount of drying (i.e. the desired water content).
The amorphous adsorbent may be any known silica-
based amorphous adsorbent. Preferably, the amorphous
adsorbent is a silica-based amorphous adsorbent
containing up to 10 wt.% of other oxides. The silica-
based amorphous adsorbent is preferably selected from the
group consisting of silica gel, precipitated silica,
dialytic silica, fumed silica, silica-alumina and
mixtures thereof. The silica-based adsorbent may contain
water (e.g. a hydrogel) or may be completely dried (e.g.
a xerogel). The silica-based adsorbent may also
optionally be pretreated with an acid or base. The most
preferred amorphous adsorbents are acid-treated silica
gels. The amorphous adsorbent may be used in admixture
with other materials, such as clays, earths, etc., as
long as those other materials do not substantially
prevent the amorphous adsorbent from performing its
adsorbing function in the contacting step.
While amorphous adsorbent can be added to the fatty
material of the invention before the drying step (e.g.
with the addition of chemical base), the invention would
re~uire a separate amorphous adsorbent contacting step
(i.e. with additional amorphous adsorbent) which is
preceded by a fatty material drying step.
2092145
-- 11 --
The acid pretreatment step described above may be
conducted in any known manner with any suitable acid. In
general, the amount of acid needed may depend on the
amount of phospholipid present in the oil initially;
preferably, about 50-1000 ppm acid is used based on the
fatty material. Examples of suitable acids are
phosphoric acid and citric acid. Phosphoric acid or other
strong acids are most preferred.
The soap creation step of the MPR process may be
carried out by any of the methods described in SN'455.
Surprisingly, it has been found that the amount of soap
created needed is only about 20-300 ppm, more preferably
about 100 ppm. Also, the use of acid pretreatment does
not necessarily require the addition of a higher amount
of base in the soap creation step as long as the soap
level generated is in the preferred range.
The bleaching step referred to above may be any
conventional bleaching step, however vacuum bleaching is
generally preferred as having the least adverse effect on
the fatty material. In the vacuum bleaching, any
conventional bleaching earth or clay may be used.
Preferably, the amorphous adsorbent-containing fatty
material is fed to the vacuum bleacher without any
intermediate filtration steps.
If bleaching is unnecessary for a particular
situation, the amorphous adsorbent may be separated from
the fatty material after the contacting step by any
conventional means. The fatty material may then be
further treated by any desired processing steps such as
deodorizing, hydrogenation, etc.
The fatty material treated according to the
invention may be any fatty acid-based material such as
glyceride oils (e.g. corn oil, soybean oil, etc.), wax
20g214~
- 12 -
esters, milk fat, other fatty acid compounds and mixtures
thereof.
The invention is further illustrated by the
following examples. The soap levels were determined by
AOCS Recommended Practice Cc 17-79. The invention is not
limited to the details recited in the examples.
EXANP~E8
Example 1
1000 g water-degummed soybean oil (SBO) (analysis
given table I below) was heated to 50C in a water bath.
5.0 g 18 Be (13 wt.%) NaOH solution was added to the oil
and mixed with constant agitation for 30 min. at 50C and
atmospheric pressure. This resulted in an oil having a
soap content of 445 ppm and a moisture content of 0.567
wt.%.
The soap-containing oil was then heated to 70~C in a
water bath and a vacuum (30 inches - water) was applied
for 10 minutes with constant agitation to dry the oil.
The dried oil had a moisture content of 0.169 wt.%.
The 300 g of the dried oil was then combined with
1.8 g (0.6 wt.%) TriSyl~600 amorphous silica hydrogel
(64.44 wt.% total volatiles), sold by W.R. Grace & Co.-
Conn., Davison Chemical Division, under agitation for 30
minutes at 70C and atmospheric pressure. The mixture was
then heated in a 100C water bath and vacuum (30 in.
water) was applied with constant agitation for 20 minutes
to vacuum bleach the oil. The bleached oil was cooled to
70C and filtered to remove the amorphous silica. The
impurities content of the resulting oil was measured and
is shown in Table 1 below.
2~921~5
- 13 -
Comparison Example
As a control example for comparison, an identical
water-degummed soybean oil was treated in the same manner
as above except that the vacuum drying step before
contact with the amorphous adsorbent was omitted. The
values for the control example are also reported in Table
1.
The treated oil of Example 1 shows decreased soap
content as well as substantially decreased P, Ca, and Mg
content compared to the control.
Example 2
1000 g water degummed SBO of Example 1 was heated to
50C in a water bath. 0.144 g 85% H3PO4 was added to the
oil at atmospheric pressure with constant agitation and
mixed for 10 min at 50C. Next, 5.0 g 18 Be (13 wt.%)
NaOH solution was added to the acid-treated oil and mixed
with constant agitation for 30 min at 50C at atmospheric
pressure. The soap content of the oil was 107 ppm. The
moisture content of the oil was 0.534 wt.%.
In the adsorption step, 300 g soapy oil was treated
with 1.8 g (0.6 wt.%) TriSyl~ 600 silica under agitation
for 30 min at atmospheric pressure and 70C. The mixture
was then transferred to a 100C water bath where vacuum
(30 in. water) was applied with constant agitation for 20
min at 100C to bleach the oil. The mixture cooled to
70C and filtered.
The results in Table l show decreased levels of all
impurities yet a residual amount of soap remains.
Example 3
1000 g water degummed SBO of Example 1 was heated to
50C in a water bath. 0.144 g 85% H3PO4 was added to the
209~1~5
-- 14 --
oil at atmospheric pressure with constant agitation for
10 min at 50C. 5.0 g 18 Be (13 wt.%) NaOH solution was
added to the acid-treated oil at atmospheric pressure
with constant agitation for 30 min at 50C. The soap
content of the oil was 125 ppm. The moisture content of
the oil was 0.537 wt.96.
700 g soapy oil was heated to 70C in a water bath.
Vacuum (30 in. water) was applied and with constant
agitation mixed for 10 min at 70C to remove the moisture.
The moisture content of the dried oil was 0.189 wt.%.
In the adsorption step, 300 g dried soapy oil was
treated with 1.8 g (0.6 wt.%) TriSyl~ 600 silica under
agitation for 30 min at atmospheric pressure and 70C.
The mixture was then transferred to a 100C water bath
where vacuum (30 in. water) was applied with constant
agitation for 20 min at 100C to bleach the oil. The
mixture cooled to 70C and filtered.
The results of this example in Table 1 show a
substantial reduction in all impurities. No residual
soap remains.
~ Example 4
1000 g water degummed SBO of Example 1 was heated to
50C in a water bath. 0.18~ g 85% H3PO4 was added to the
oil at atmospheric pressure with constant agitation for
10 min at S0C. 5.0 g 18 ~e (13 wt.%) NaOH solution was
added to the acid-treated oil at atmospheric pressure
with constant agitation for 30 min at S0C. The soap
content of the oil was 8S ppm. The moisture content of
the oil was 0.S40 wt.%.
In the adsorption step, 300 g soapy oil was treated
with 1.8 g (O.6 wt.96) TriSyl~ 600 silica under agitation
for 30 min at atmospheric pressure and 70C. The mixture
2~921~5
was then transferred to a 100C water bath where vacuum
(30 in. water) was applied with constant agitation for 20
min at 100C to bleach the oil. The mixture cooled to
70C and filtered.
The results of this example in Table 1 show a
reduction in all impurities, yet residual soap remains.
Example 5
1000 g water degummed SBO of Example 1 was heated to
50C in a water bath. 0.187 g 85% H3PO4 was added to the
oil at atmospheric pressure with constant agitation for
10 min at 50C. 5.0 g 18 Be (13 wt.%) NaOH solution was
added to the acid-treated oil at atmospheric pressure
with constant agitation for 30 min at 50C. The soap
content of the oil was 85 ppm. The moisture content of
the oil was 0.399 wt.%.
- 700 g soapy oil was heated to 70C in a water bath.
Vacuum (30 in. water) was applied with constant agitation
for 10 min at 70C to remove the moisture. The moisture
content of the dried oil was 0.203 wt.%.
In the adsorption step, 300 g dried soapy oil was
treated with 1.8 g (0.6 wt.%) TriSyl~ 600 silica under
agitation for 30 min at atmospheric pressure and 70C.
The mixture was then transferred to a 100C water bath
where vacuum (30 in. water) was applied with constant
agitation for 20 min at 100C to bleach the oil. The
mixture cooled to 70C and filtered.
The results in Table 1 show substantial removal of
all impurities. No residual soap remains.
Example 6
1000 g water degummed SBO of ~xample 1 was heated to
50C in a water bath. 0.100 g 85% H3PO4 was added to the
2092145
- 16 -
oil at atmospheric pressure with constant agitation for
10 min at 50C. 5.0 g 18 Be (13 wt.%) NaOH solution was
added to the acid-treated oil at atmospheric pressure
with constant agitation for 30 min at 50C. The soap
content of the oil was 255 ppm. The moisture content of
the oil was 0.515 wt.%.
700 g soapy water degummed oil were heated to 90C in
a water bath. vacuum (30 in. water) was applied with
constant agitation for 3 min at 90C to remove the
moisture. The moisture content of the dried oil was
0.208~.
In the adsorption step, 300 g dried soapy oil were
treated with 1.8 g (0.6 wt.%) TriSyl~ 600 silica under
agitation for 30 min at atmospheric pressure and 70C.
The mixture was then transferred to a 100C water bath
where vacuum (30 in. water) was applied with constant
agitation for 20 min at 100C to bleach the oil. The
mixture cooled to 70C and filtered.
The results in Table 1 show a substantial reduction
in the levels of all impurities. No residual soap
remains.
20~2145
=_ = = = =
~o ~ o ~ o o ~ I
~ _ _ _ _ _ __
h o o~ _I ~ ~o t~ ~ o l
~ o o o o o o o o I
~7 o o o o o o o o
__ ~ o u~ _ o ~r I
~3 o O o o o o o o
_ _ _ _ _ _
o o o o o o 1` o I
o O O O O o ~ O I
~ o o O o O o o O I
r/ ~O O O O _ ~D l~') _
O O O O O ~D
. ~ O O O O O O ~ ~i
I~ _ _ _ _ ~ U~ _
,~ o o o O O o I
I
_ _ _ _ _ _
~ ~ ~ a~ o a~ ~1 ~ I
t~ P~ ~3 U~ ~r u~ ~r ~ ~ ~r t~ I
,~ ~ _l O O O O O ~ U~ I
_ _ _ _ ___
~ ~ ~ u~ I~ ~n u~ ~n ~ ~ u~ I
~r o ~ co co ~ ~ ~ I
EZ ~-I ~ ~ ~ lo ~D h O
_= = = = _ _
209214~
- 18 -
Example 7
1000 g degummed corn oil, analysis listed in Table
2, was heated to 50C in a water bath. lO.O g 18 Be (13
wt.%) NaOH solution was added to the oil at atmospheric
pressure with constant agitation for 30 min at 50C. The
soap content of the oil was 1126 ppm. The moisture
content of the oil was 1.118 wt.%.
In the drying step, 350 g soapy oil was heated to
70C in a water bath. Vacuum (30 in. water) was applied
with constant agitation for 25 min at 70C to remove the
moisture. The moisture content of the dried oil was
0.157 wt.%.
In the adsorption step, 300 g dried soapy oil was
treated with 1.8 g (0.6 wt.%) TriSyl~ 600 silica under
agitation for 30 min at atmospheric pressure and 70C.
The mixture was then transferred to a 100C water bath
where vacuum (30 in. water) applied with constant
agitation for 20 min at 100C to bleach the oil. The
mixture was cooled to 70C and filtered.
The results in Table 2 show a substantial reduction
in all impurities with no residual soaps.
,Comparison Example
For comparison, an identical corn oil was treated by
the same process as Example 7 above except the vacuum
drying step was omitted. The results in Table 2 show
substantial residual soap.
209214S
- 19 --
. _ : __
Soap Moisture FFA ~ P Ca Ng ~ Fe
¦ . ppm wt.% wt.% ppD ppm PP~ ppm
Degummed N/A 0.138 0.97 4.01 0.03 0.23 0.15
Corn~Oil ~ ~ ~
5 : ¦Comparison 548 0.032 0.09~ ~<0.26 0.02 ~ 0.02~ <0.03
~:Example ~ :
: :
e O ~.0~0 0.08 <0 26 <o.01 0.01 <0.03
,"~: - ~ !, '
:'~"'
.~ ,