Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~9~~~~
_1_
Use of ~yridylmeth l~phinyl--1H-benzimidazole derivates in the
treatment of illnesses caused by helicobacter laacteria
Scope of application of the invention
The invention relates to new or-al drug forms. The new drug forms are employed
for the treatment of diseases of the stomach and/or intestine caused by
Helicobacter bacteria.
Prior art
Pyridylmethylsulphinyl-1H-benzimidazoles which have gastric acid secretion-
inhibiting properties-are described in a large number of patent applications.
The following patent applications and patents may be mentioned in particular
here in connection with the p-resent invention: EP-A-134 400 (--- USP
4,555,518),
EP-A-127 763 (= USP 4,560,693), EP-B-166 287 (= USP 4,758,579), EP-A-201 575
(_
USP 4,686,230), W089/05299 and W089/11479. - European Patent Application EP-A-
382 489 describes and clay-ms the suitability of certain
pyridylmethylsulphinyl-
1H-benzimidazoles, which are substituted in the benzimidazole part, if
desired,
by methoxy or trifluoromethyl, for the treatment of infectious diseases caused
by bacteria of the Campylobacter (= Helicobacter) strain. International Patent
Application Wo90/09175 discloses the use of omeprazole in the treatment of
infectious diseases, in particular those caused by Campylobacter pylori. -
Because the pyridylmethylsulphinyl-1H-benzimidazoles have a low stability and
are easily decomposed by acid, various patent applications (e.g. EP-A-244 380
or
EP-A-247 983) refer to the need to administer these active compounds in a form
which is resistant to gaastric juice in the case of oral administration. An
"enteric coated" formulation is also used in the abovementioned EP-A-382 489
as
the oral presentation form for combating Campylobacter.
Description of the invention
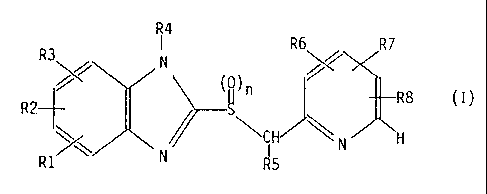
The invention relates to the use of compounds of the formula 2
~09~694
- 2 -
R4
R3 ~ R6 /, R7
/ N ~/
R8 (I)
RZ-~-
~\ ~ \~IH ~ N f1
R1 N R5
wherein
R1 denotes hydrogen, 1-4C-alkyl or 1-4C-alkoxy,
R2 denotes hydrogen, 1-4C-alkyl, 1-4C-alkoxy, 1-4C-alkoxy which is substituted
completely or predominantly by fluorine, chlorodifluoromethaxy, 2-chloro-
1,1,2-trifluoroethoxy or, together with R3, 1-2C-alkylenedioxy which is
substituted completely ox partly by fluorine, or
chlorotrifluoroethylenedioxy,
R3 denotes 1-~C-alkoxy which is substituted completely or predominantly by
fluorine, chlorodifluoromethoxy, 2-chloro-1,1,2-trifluoroethoxy or,
together with R2, 1-2C-alkylenedioxy which is substituted completely or
partly by fluorine, or chlorotrifluoroethylenedioxy,
R4 denotes hydrogen or a group which can easily be split off under
physiological conditions,
RS denotes hydrogen or 1-4C-alkyl,
R6 denotes hydrogen or 1-4C-alkyl,
R7 denotes hydrogen, 1-4C-alkyl or 1-4C-alkoxy,
R8 denotes 1-4C-alkoxy, 1-4C-alkoxy which is substituted completely or
predominantly by fluorine. or benzyloxy and
n represents the number 0 or 1,
and their pharmacologically tolerated salts, for the preparation of
medicaments
to be administered orally for combating Helicobacter bacteria.
Z-4C-Alkyl represents straight-chain or branched alkyl radicals; examples
which
may be mentioned are the butyl, i-butyl, sec.-butyl, t-butyl, propyl,
isopropyl,
ethyl and in particular the methyl radical.
1-4C-Alkoxy represents straight-chain or branched alkoxy radicals; examples
which may be mentioned are the butoxy, i-butoxy, sec.-butoxy, t-butoxy,
propoxy,
isopropoxy, ethoxy and in particular the methoxy radical.
Examples which may be mentioned of 1-4C-alkoxy which is substituted completely
or predominantly by fluorine are the 1,2,2-trifluoroethoxy, the 2,2,3,3,3-
pentafluoropropoxy, the perfluoroethoxy and in particular the 1,1,2,2-
tetrafluoroethoxy, the trifluoromethoxy, the 2,2,2-trifluoroethoxy and the
difluoromethoxy radical.
Examples which may be mentioned of 1-2C-alkylenedioxy which is substituted
completely or partly by fluorine are the 1,1-difluoroethylenedioxy (-O-CFZ-CHZ-
O-), the 1,1,2,2-tetrafluoroethylenedioxy (-O-CFZ-CFZ-O-) and in particular
the
difluoromethylenedioxy (-O-CFZ-O-) and the 1,1,2-trifluoroethylenedioxy
radical
(-O-CF2-CHF-O-).
If R2 and R3 together denote 1-2C-alkylenedioxy which is substituted
completely
or partly by fluorine, or chlorotrifluoroethylenedioxy, the substituents R2
and
R3 are bonded in adjacent positions to the benzo part of the benzimidazole
ring.
A group R4 which can easily be split off under physiological conditions is a
substituent which is separated off from the nitrogen atom by hydrolysis - if
appropriate under enzymatic catalysis - to form an N-H bond, the group itself -
bonding a hydroxyl group - being converted into a physiologically acceptable
and
in particular pharmacologically tolerated compound. Groups R4 which can be
split off and which may be mentioned are, in particular, all types of
substituted carbonyl groups, such as the alkylcarbonyl, arylcarbonyl,
aralkylcarbonyl, alkoxycarbonyl, aryloxycarbonyl or aralkoxycarbonyl group, or
the optionally substituted carbamoyl group. Examples which may be mentioned
are
the methoxycarbonyl, t-butoxycarbonyl, benzoyl, phenylcarbamoyl and the
dimethylcarbamoyl group.
Preferred possible salts for compounds of 'the formula I in which n denotes
the
number 0 (sulphides) are all the pharmacologically tolerated acid addition
salts. Salts which may be mentioned in particular are the pharmacologically
tolerated salts of the inorganic and organic acids usually used in galenics.
Examples of such suitable salts are water-soluble and water-insoluble acid
addition salts, such as the hydrochloride, hydrobromide, hydriodide,
phosphate,
nitrate, sulphate, acetate, citrate, gluconate, benzoate, hibenzate,
fendizoate,
butyrate, sulphosalicylate, maleate, laurate, malate, fumarate, succinate,
- 4 -
oxalate, tartrate, amsonate, embonate, metembonate, stearate, tosylate, 2--
hydroxy-3-naphthoate, 3-hydroxy-2-naphthoate or mesylate.
Preferred possible salts for compounds of the formula I in which n denotes the
number 1 (sulphoxides) are pharmacologically tolerated basic salts, in
particular pharmacologically tolerated salts with the inorganic and organic
bases usually used in galenics. Examples of basic salts which may be mentioned
are the lithium, sodium, potassium, calcium, aluminium, magnesium, titanitun,
ammonium or guanidinium salts.
Of the Helicobacter strains against which the compounds of the formula I have
proved to be active, the Helicobacter pylori strain may be mentioned in
particular.
Examples which may be mentioned of medicaments to be administered orally are
tablets, coated tablets, hard and soft capsules, for example of gelatine,
dispersible powders, granules, aqueous and oily suspensions, emulsions,
solutions or syrups, it being advantageous far the tablets, coated tablets,
capsules or granules to be such that they readily dissolve in gastric juice
and
release the active compound in the stomach.
For combined treatment of gastric diseases which are based both on an
increased
secretion of gastric acid and on damage to the stomach by Helicobacter pylori,
there may also be mentioned those drug formulations to be administered orally
which contain active compounds of the formula I both in a form which ie
resistant to gastric juice and in a form which is not resistant to gastric
juice
simultaneously in an individual dose. Examples which may be mentioned are
tablets which contain the active compound both in a core which is resistant to
gastric juice and in a shell which is not resistant to gastric juice, or
capsules filled with pellets or (mini)tablets which are resistant to gastric
juice and those which are not resistant to gastric juice.
In human medicine, the active compounds are in general administered in a daily
dose of about o.05 to about 5, preferably 0.1 to 2.5 mg/kg of body weight, if
appropriate in the form of several, preferably 2 to 6, individual doses, to
achieve the desired result.
2~1~~6~~
- 5 -
If the compounds of the formula I and/or their salts are to be employed for
the
treatment of diseases of the stomach based on the presence of Helicobacter
pylori, the medicaments to be administered can also contain one or more
pharmacologically active constituents of other groups of medicaments.
Combination of compounds of the formula I and/or their salts with
antimicrobial
substances which have an action against Helicobacter pylori, such as, for
example, penicillin G, gentamycin, erythromycin, nitrofurazone,
nitrofurantoin,
furazolidone, metronidazole and in particular amoxycillin, with the aim of
intensifying the main action in the super-additive sense, is to be emphasized
in
particular in this connection. Combination of the active compound 5-
difluoromethoxy-2-[(3,4-dimethoxy-2-pyridyl)methylsulphinyl)-1H-benzimidazole
[=
pantoprazole (INN)) and its salts with substances having an antimicrobial
action, in particular with amoxycillin, is particularly preferred in this
connection and the invention therefore furthermore relates to this
combination.
It has been found, surprisingly, that the compounds of the formula I are
considerably more active against Helicobacter bacteria in an acid medium than
in
a neutral medium, and that they accordingly - in Contrast to the doctrine to
be
found in the prior art - should appropriately not be administered in a form
which is resistant to gastric juice.
The invention thus preferably relates to the use of compounds of the formula I
and their pharmacologically tolerated salts for the preparation of medicaments
which are not in a formulation which is resistant to gastric juice and are to
be
administered orally for combating Helicobacter bacteria.
One embodiment of the invention which is worth mentioning (embodiment a)
comprises the use, according to the invention, of compounds of the formula Ia
R4
I
R3
(0
(Ia)
RZ y\
R1
- 6 -
wherein
R1 denotes hydrogen or methyl,
R2 denotes hydrogen, methyl, ethyl, methoxy, ethoxy, 1,1,2,2-
tetrafluoroethoxy, trifluoromethoxy, 2,2,2-trifluoroethoxy,
difluoromethoxy, 2-chloro-1,1,2-trifluoroethoxy or, together with R3,
difluoromethylenedioxy, 1,1,2-trifluoroethylenedioxy or 1-chloro-1,2,2-
trifluoroethylenedioxy,
R3 denotes 1,1,2,2-tetrafluoroethoxy, trifluoromethoxy, 2,2,2-trifluoroethoxy,
difluoromethoxy, 2-chloro-1,1,2-trifluoroethoxy or, together with R2,
difluoromethylenedioxy, 1,1,2-trifluoroethylenedioxy or 1-chloro-1,2,2-
trifluoroethylenedioxy,
R4 denotes hydrogen,
R5 denotes hydrogen, methyl or ethyl,
R6 denotes hydrogen or 1-4C-alkyl,
R7 denotes hydrogen or 1-4C-alkyl,
R8 denotes 1-4C-alkoxy and
n represents the number 0 or 1,
and their pharmacologically tolerated salts.
The use, according to the invention, of the following compounds of embodiment
a
and their pharmacologically tolerated salts is particularly worth mentioning:
2-[(4-methoxy-2-pyridyl)methylsulphinyl]-5-trifluoromethoxy-1H-benzimidazole,
2-[(4-methoxy-3-methyl-2-pyridyl)methylsulphinyl]-5-trifluoromethoxy-1H-
benzimidazole,
2-[(4-methoxy-5-methyl-2-pyridyl)methylsulphinyl]-5-trifluoromethoxy-1H-
benzimidazole,
2-((4-methoxy-2-pyridyl)methylsulphinyl]-5-(1,1,2,2-tetrafluoroethaxy)-1H-
benzimidazole,
2-[(4-methoxy-3-methyl-2-pyridyl)methylsulphinyl]-5-(1,1,2,2-
tetrafluoroethoxy)-
18-benzimidazole,
2-[(4-methoxy-3,5-dimethyl-2-pyridyl)methylsulphinyl]-5-trifluoromethoxy-1H-
benzimidazole
2-[(4-methoxy-3-methyl-2-pyridyl)methylsulphinyl]-5-(2,2,2-trifluoroethoxy)-1H-
benzimidazole,
5-difluoromethoxy-2-[(4-methoxy-3-methyl-2-pyridyl)-methylsulphinyl]-~1.H-
benzimidazole,
~~~~~4
7
5,6-bis(difluoromethoxy)-2-[(4-methoxy-2-pyridyl)methylsulphinyl]-1H-
benzimidazole,
5,6-bis(difluoromethoxy)-2-[(4-methoxy-3-methyl-2-pyridyl)methylsulphinyl]-1H-
benzimidazole,
5-difluoromethoxy-6-methoxy-2-((4-methoxy-3-methyl-2-pyridyl)methylsulphinyl-
1H-
benzimidazole,
5-chlorodifluoromethoxy-2-[(4-methoxy-2-pyridyl)methylsulphinyl]-1H-
benzimidazole,
2,2-difluoro-6-[(4-methoxy-2-pyridyl)methylsulphinyl]-5H-(1,3]-dioxolo(4,5-f]
benzimidazole,
2,2-difluoro-6-((4-methoxy-3-methyl-2-pyridyl)methylsulphinyl]-5H-(1,3]-
dioxolo[4,5-f]benzimidazole,
6,6,7-trifluoro-6,7-dihydro-2-[(4-methoxy-2-pyridyl)methylsulphinyl]-1H-[1,4]-
dioxino[2,3-f]benzimidazole,
6,6,7-trifluoro-6,7-dihydro-2-[(4-methoxy-3-methyl-2-pyridyl)methylsulphinyl]-
1H-[1,4]-dioxino[2,3-f]benzimidazole,
2,2-difluoro-6-[(4-methoxy-5-methyl-2-pyridyl)methylsulphinyl]-5H-(1,3]-
dioxolo[4,5-f]benzimidazole,
6,6,7-trifluoro-6,7-dihydro-2-[(4-methoxy-3,5-dimethyl-2-pyridyl)
methylsulphinyl-1H-(1,4]-dioxino[2,3-f]benzimidazole,
6-chloro-6,7,7-trifluoro-6,7-dihydro-2-((4-methoxy-3-methyl-2-pyridyl)-
methylsulphinyl)-1H-[1,4]-dioxino[2,3-f]benzimidazole,
5-difluoromethoxy-2-[(4-mathoxy-3-methyl-2-pyridyl)methylsulphinyl]-4,6-
dimethyl-1H-benzimidazole,
5-difluoromethoxy-6-methoxy-2-{[1-(4-methoxy-2-pyridyl)ethyl]sulphinyl}-1H-
benzimidazole,
5-(1,1,2,2-tetrafluoroethoxy)-2-{1-(4-methoxy-2-pyridyl)ethyl]sulphinyl}-1H-
benzimidazole,
2,2-difluoro-6-([1-(4-methoxy-2-pyridyl)ethyl]sulphinyl}-5H-[1,3]-dioxolo
[4,5-f]benzimidazole,
5-(2-chloro-1,1,2-trifluoroethoxy)-2-((4-methoxy-2-pyridyl)methylsulphinyl]-1H-
benzimidazole,
5-(2-chloro-1,1,2-trifluoroethoxy)-2-{[1-(4-methoxy-2-pyridyl)ethyl]sulphinyl}-
1H-benzimidazole,
5-(2-chloro-1,1,2-trifluoroethoxy)-2-[(4-methoxy-3-methyl-2-
pyridyl)methylsulphinyl]-1H-benzimidazole,
2[(4-methoxy-2-pyridyl)methylthio]-5-trifluoromethoxy-1H-benzimidazole,
- a -
2-[(4-methoxy-3-methyl-2-pyridyl)methylthio]-5-trifluoromethoxy-1H-
benzimidazole,
2-[(4-methoxy-5-methyl-2-pyridyl)methylthio]-5-trifluoromethoxy-1H-
benzimidazole,
2-[(4-methoxy-2-pyridyl)methyithio]-5-(x,1,2,2-tetrafluoroethoxy)-1H-
benzimidazole,
2-[(4-methoxy-3-methyl-2-pyridyl)methylthio]-S-(1,1,2,2-tetrafluoroethoxy)-1H-
benzimidazole,
2-[(4-methoxy-3,5-dimethyl-2-pyridyl)methylthio]-5-trifluoromethoxy-lli-
benzimidazole,
2-[(4-methoxy-3-methyl-2-pyridyl)methylthio]-5-(2,2,2-trifluoroethoxy)-1H-
benzimidazole,
5-difluoromethoxy-2-[(4-methoxy-3-methyl-2-pyridyl)-methylthio]-1H-
benzimidazole,
5,6-bis(difluoromethoxy)-2-((4-methoxy-2-pyridyl)methylthio]-1H-benzimidazole,
5,6-bis(difluoromethoxy)-2-[(4-methoxy--3-methyl-2-pyridyl)methylthio]-1H-
benzimidazole,
5-difluoromethoxy-6-methoxy-2-[(4-methoxy-3-methyl-2-pyridyl)methylthio-1H-
benzimidazole,
5-chlorodifluoromethoxy-2-[(4-methoxy-2-pyridyl)methylthio]-1H-benzimidazole,
2,2-difluoro-6-[(4-methoxy-2-pyridyl)methylthio]-5H-[1,3]-dioxolo[4,5-f]
benzimidazole,
2,2-difluoro-6-[(4-methoxy-3-methyl-2-pyridyl)methylthio]-5H-[1,3]-dioxolo
[4,5-f]benzimidazole,
6,6,7-trifluaro-6,7-dihydro-2-[(4-methoxy-2-pyridyl)methylthio]-1H-[1,4]-
dioxino[2,3-f]benzimidazole,
6,6,7-trifluoro-6,7-dihydro-2-[(4-methoxy-3-methyl-2-pyridyl)methylthio]-1H-
[1,4]-dioxino[2,3-f]benzimidazole,
2,2-difluoro-6-[(4-methoxy-5-methyl-2-pyridyl)methylthio]-5H-[1,3]-dioxolo
[4,5-f]benzimidazole,
6,6,7-trifluoro-6,7-dihydro-2-[(4-methoxy-3,5-dimethyl-2-pyridyl)methylthio-1H-
[1,4]-dioxino[2,3-f]benzimidazole,
6-chloro-6,7,7-trifluoro-6,7-dihydro-2-[(4-methoxy-3-methyl-2-pyridyl)
methylthio]-1H-(1,4]-dioxino[2,3-f]benzimidazole,
5-difluoromethoxy-2-[(4-methoxy-3-methyl-2-pyridyl)methylthio]--4,6-dimethyl-
1H-
benzimidazole,
- 9 -
5-difluoromethoxy-6-methoxy-2-{(1-(4-methoxy-2-pyridyl)ethyl]sulphinyl}-1H-
benzimidazole,
5-(1,1,2,2-tetrafluoroethoxy)-2-{[1-(4-methoxy-2-pyridyl)ethyl]sulphinyl}-1H-
i
benzimidazole,
2,2-difluoro-6-{(1-(4-methoxy-2-pyridyl)ethyl]sulphinyl}-5H-(1,3]-dioxolo
[4,5-f]benzimidazole,
I
5-(2-chloro-1,1,2-trifluoroethoxy)-2-((4-methoxy-2-pyridyl)methylthio]-lH-
benzimidazole,
I
5-(2-chloro-1,1,1-trifluoroethoxy)-2-{(1-(4-methoxy-2-pyridyl)ethylj-
sulphinyl}-
,i
1H-benzimidazole, I
5-(2-chloro-1,1,2-trifluoroethoxy)-2-((4-methoxy-3-methyl-2-
pyridyl)methylthio]-
1H-benzimidazole. '.-
Another embodiment of the invention which is worth mentioning (embodiment b)
comprises the use, according to the invention, of compounds of the formula Ib,
R
I
R3 ~ N
(Ib)
R2R1~ N
wherein
R1 denotes hydrogen or methyl,
R2 denotes hydrogen, methyl, ethyl, methoxy, ethoxy, 1,1,2,2--
tetrafluoroethoxy, trifluoromothoxy, 2,2,2-trifluoroethoxy,
difluoromethoxy, 2-chloro-1,1,2-trifluoroethoxy or, together with R3,
difluoromethylenedioxy, 1,1,2-trifluoroethylenedioxy or 1-chloro-1,2,2-
trifluoroethylenadioxy,
R3 denotes 1,1,2,2-tetrafluoroethoxy, trifluoromethoxy, 2,2,2-trifluoroethoxy,
difluoromethoxy, 2-chloro-1,1,2-trifluoroethoxy or, together ~:ith R2,
difluoromethylenedioxy, 1,1,2-trifluoroethylenedioxy or 1-chloro-1,2,2-
trifluoroethylenedioxy,
R4 denotes hydrogen,
R5 denotes denotes hydrogen, methyl or ethyl,
R6 donotes hydrogen or 1-4C-alkyl,
-
R7 denotes 1-4C-alkoxy,
R8 denotes 1-4C-alkoxy and
n represents the number 0 or 1,
and their pharmacologically tolerated salts.
The use, according to the invention, of the following compounds of embodiment
b
and their pharmacologically tolerated salts is particularly worth mentioning:
5-difluoromethoxy-2-[(3,4-dimethoxy-2-pyridyl)methylsulphinyl]-4,6-dimethyl-1H-
benzimidazole,
5-difluoromethoxy-2-[(3,4-dimethoxy-2-pyridyl)methylsulphinyl]-1H-
benzimidazole,
5-difluoromethoxy-2-((4,5-dimethoxy-3-methyl-2-pyridyl)methylsulphinyl]-1H-
benzimidazole,
2-[(4,5-dimethoxy-3-methyl-2-pyridyl)methylsulphinyl]-5-trifluoromethoxy-1H-
ben~zianidazole,
2-[(3,4-dimethoxy-2-pyridyl)methylsulphinyl]-5-(1,1,2,2-tetrafluoroethoxy)-1H-
benzimidazole,
2-[(3,4-dimethoxy-2-pyridyl)methylsulphinyl]-5-(2,2,2-trifluoroethoxy)-1H-
benzimidazole,
5-difluoromethoxy-6-methoxy-2-[(3,4-dimethoxy-2-pyridyl)methylsulphinyl]-iH-
benzimidazole,
2,2-difluoro-6-[(3,4-dimethoxy-2-pyridyl)methylsulphinyl]-5H-[1,3]-dioxolo[4,5-
f]benzimidazole,
6,6,7-trifluoro-6,7-dihydro-2-[(3,4-dimethoxy-2-pyridyl)methylsulphinyl]-1H-
[1,4]-dioxino[2,3-f]benzimidazole,
2-[(4,5-dimethoxy-2-pyridyl)methylsulphinyl]-5-trifluoromethoxy-1H-
benzimidazole,
2-[(4,5-dimethoxy-2-pyridyl)methylsulphinyl]-5-(1,1,2,2-tetrafluoroethoxy)-1H-
benzimidazole,
2,2-difluoro-6-[(4,5-dimethoxy-2-pyridyl)methylsulphinyl]-5H-(1,3]-dioxolo[4,5-
f]benzimidazole,
5-difluoromethoxy-2-[(3,4-dimethoxy-2-pyridyl)methylthio]-4,6-dimethyl-1H-
benzimidazole,
5-difluoromethoxy-2-[(3,4-dimethoxy-2-pyridyl)methylthio]-~H-benzimidazole,
5-difluoromethoxy-2-[(4,5-dimethoxy-3-methyl-2-pyridyl)methylthio]-1H-
benzimidazole,
- il -
2-((4,5-dimethoxy-3-methyl-2-pyridyl)methylthio]-5-trifluoromethoxy-1H-
benzimidazole,
2-[(3,4-dimethoxy-2-pyridyl)methyl.thio]-5-(1,1,2,2-tetrafluoroethoxy)-iH-
benzimidazole,
2-[(3,4-dimethoxy-2-pyridyl)methylthio]-5-(1,1,2,2-tetrafluoroethoxy)-1H-
benzimidazole,
2-[(3,4-dimethoxy-2-pyridyl)methylthio]-5-(2,2,2-trifluoroethoxy)-1H-
benzimidazole,
5-difluoromethoxy-6-methoxy-2-[(3,4-dimethoxp-2-pyridyl)methylthio]-1H-
benzimidazole,
2,2-difluoro-6-[(3,4-dimethoxy-2-pyridyl)methylthio]-5H-[1,3]-dioxolo[4,5-
f]benzimidazole,
6,6,7-trifluoro-6,7-dihydro-2-[(3,4-dimethoxy-2-pyridyl)methylthio]-1H-[1,4]-
dioxino[2,3-f]benzimidazole,
2-[(4,5-dimethoxy-2-pyridyl)methylthio]-5-trifluoromethoxy-iH-benzimidazole,
2-[(4,5-dimethoxy-2-pyridyl)methylthio]-5-(1,1,2,2-tetrafluoroethoxy)-1H-
benzimidazole,
2,2-difluoro-6-[(4,5-dimethoxy-2-pyridyl)methylthio]-5H-[1,3]-dioxolo[4,5-
f]benzimidazole.
Another embodiment of the invention which is worth mentioning (embodiment c)
comprises the use, according to the invention, of compounds of the formula rc
R4
I R6 R7
N
R3 ~ (o) n
~RB (Ic)
R2~ ~\ ~ \~H ~ N H
R 1 N E~5
wherein
R1 denotes hydrogen,
R2 denotes hydrogen, methyl, ethyl, methoxy, ethoxy, 1,1,2,2-
tetrafluoroethoxy, trifluoromethoxy, 2,2,2-trifluoroethoxy,
difluoromethoxy, 2-chloro-1,1,2-trifluoroethoxy or, together with R3,
difluoromethylenedioxy, 1,1,2-tri:cluoroethylenedioxy or i-chloro-1,2,2-
trifluoroethylenedioxy,
- 12 -
R3 denotes 1,1,2,2-tetra.fluoroethoxy, trifluoror~ethoxy, 2,2,2-
trifluoroethoxy,
difluoromethoxy, 2-chloro-1,1,2-trifluoroethoxy or, together with R2,
difluoromethylenedioxy, 1,1,2-trifluoroethylenedioxy or 1-chloro-1,2,2-
trifluoroethylenedioxy,
R4 denotes hydrogen,
R5 denotes hydrogen, methyl or ethyl,
R6 denotes hydrogen or 1-4C-alkyl,
R7 denotes i-4C-alkoxy,
R8 denotes benzyloxy and
n represents the number 0 or 1,
and their pharmacologically talerated salts.
The use, according to the invention, of the following compounds of embodiment
c
and their pharmacologically tolerated salts is particularly worth mentioning:
2,2-difluoro-6-[(5-benzyloxy-4-methoxy-2-pyridyl)methylsulphinyl]-5H-[1,3]-
dioxolo[4,5-f]benzimidazole,
2-((4-benzyloxy-3-methoxy-2-pyridyl)methylsulphinyl]-5-difluoromethoxy-1H-
benzimidazole,
2-[(3-benzyloxy-4-methoxy-2-pyridyl)methylsulphinyl]-5-difluoromethoxy-1H-
benzimidazole,
2-[(5-benzyloxy-4-methoxy-3-methyl-2-pyridyl)methylsulphinyl]-5-
difluoromethoxy-
1H-benzimidazole,
2-((5-benzyloxy-4-methoxy-2-pyridyl)methylsulphinyl]-5-trifluoromethoxy-1H-
benzimidazole,
2,2-difluoro-6-((5-benzyloxy-4-methoxy-2-pyridyl)methylthio]-5H-[1,3]-
dioxolo[4,5-f]benzimidazole,
2-[(4-benzyloxy-3-methoxy-2-pyridyl)methylthio]-5-difluoromethoxy-1H-
benzimidazole,
2-[(3-benzyloxy-4-methoxy-2-pyridyl)methylthio]-5-difluoromethoxy-1H-
benzimidazole,
2-[(5-benzyloxy-4-methoxy-3-methyl-2-pyridyl)methylthio]-5-difluoromethoxy-1H-
benzimidazole,
2-[(5-benzyloxy-4-methoxy-2-pyridyl)methylthio]-5-trifluoromethoxy-1H-
benzimidazole.
- 13 -
Another embodiment of the invention which is worth mentioning (embodiment d)
is
the use, according to the invention, of compounds of the formula Id
R4
I
R3
(0
(Id)
R2 ~\
R1
wherein
R1 denotes hydrogen,
R2 denotes hydrogen, methyl, ethyl, methoxy, ethoxy, 1,1,2,2-
tetrafluoroethoxy, trifluoromethoxy, 2,2,2-trifluoroethoxy,
difluoromethoxy, 2-chloro-1,1,2-trifluoroethoxy os, together with R3,
difluoromethylenedioxy, 1,1,2-trifluoroethylenedioxy or 1-chloro-1,2,2-
trifluoroethylenedioxy,
R3 denotes 1,1,2,2-tetrafluoroethoxy, trifluoromethoxy, 2,2,2-trifluoroethoxy,
difluoromethoxy, 2-chloro-1,1,2-trifluoroethoxy or, together with R2,
difluoromethylenedioxy, 1,1,2-trifluoroethylenedioxy or 1-chlora-1,2,2-
trifluoroethylenedioxy,
R4 denotes hydrogen, i
R5 denotes hydrogen, methyl or ethyl,
i
R6 denotes hydrogen or i-4c-alkyl, ,
R7 denotes hydrogen, 1-4C-alkyl or i~-4c-alkoxy,
t
RS denotes 1,1,2,2-tetrafluoroethoxy, trifluoromethoxy, 2,2,2-trifluoroethoxy
or difluoromethoxy and
n represents the number 0 or 1,
and their pharmacologically tolerated salts.
The use, according to the invention, of the following compounds of embodiment
d
and their pharmacologically tolerated salts is particularly worth mentioning:
5-difluoromethoxy-2-{[3-methoxy-4-(2,2,2-trifluoroethoxy)-2-pyridyl]
methylsulphinyl}-lI3-benzimidazole,
5-difluoromethoxy-2-{[3-methyl-4-(2,2,2-trifluoroethoxy)-pyridyl]
methylsulphinyl}-1H-benzimidazole,
~~~r'~~~~~
- 14 -
2-{[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methylsulphinyl}-5-(2,2,2-
trifluoroethoxy)-1H-benzimidazole,
2,2-difluoro-6-{[3-methyl-4-(2,2,2--trifluoroethoxy)-2-
pyridyl]methylsulphinyl}-
5H-[1,3]-dioxolo[4,5-f]benzimidazole,
5-difluoromethoxy-2-{[3-methoxy-4-(2,2,2-trifluoroethoxy)-2-
pyridyl]methylthio}-
1H-benzimidazole,
5-difluoromethoxy-2-{[3-methyl-4-(2,2,2-trifluoroethoxy)-pyridyl]methylthio}-
1H-
benzimidazole,
2-{[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methylthio}-5-(2,2,2-
trifluoroethoxy)-1Fi-benzimidazole,
2,2-difluoro-6-{[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methylthio}-5H-
[1,3]-dioxolo[4,5-f]benzimidazole.
The compounds of the formula I and their pharmacologically tolerated salts are
known from the following patent applications and patents: EP-A-134 400 (= USP
4,555,518), EP-A-127 763 (= USP 4,560,693), EP-B-166 287 (= USP 4,758,579), EP-
A-201 575 (= USP 4,686,230), Wo89/05299 and WO89/11479.
The medicaments to be administered orally are prepared using the active
compounds of the formula I in a manner which is known per se to the expert.