Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~72 L7
BACKGROUND OF T~IE INVENTION
This invention relates to two pure strains of bacteria
capable of selective removal of organLcally bound sulfur from carbon-
aceous materials while maintaining the calorific value of the carbon-
aceous materials. The microorganisms of this invention are particu-
larly useful in removal of organic sulfur from fossil fuels such as
oils and coal3.
ConventLonal methodg for the removal of organic sulfur from
fuels rely on heterogeneous catalysis which is limited by the range of
compounds which can be successfully removed. Aromatic sulfur contain-
ing compounds make up a significant percentage of the organic sulfur
in fuels and these are generally the least susceptible to removal by
conventional methods. The most recalcitrant of the aromatic ~ulfur-
containing compounds are those in which the sulfur atoms are
sterically hindered.
Most microbial methods described to date for the removal of
~ulfur from petroleum products, coal, tar sands and shale oil are
limited to inorganic sulfur. The majority of the microbial methods
involving organic sulfur removal are non-~pecific in that they attack
the carbon skeletons of the organic constituents, thus resulting in a
high degree of unwanted side reactions and reduction of the calorific
value of the fuel. In addition, there have been no reports on the
selective removal of 8ulfur from those organic con~tituent~ in which
the sulfur atoms are sterically hindered.
~ he following is a brief description of the prior art.
Sulfur Ln carbonaceous fuels is undesirable due to its
ability to promote corrosion in storage and processing equipment and,
when the fuel is combu3ted, the release of sulfur oxide gases which
are attributed to detrimental affects on the environment. Sulfur is
found in two forms in the~e materials: 1) inorganic, with the
principle forms being elemental sulfur, sulfate, and pyrite, and 2)
organic sulfur, with the principle forms in crude oils being (Rall,
,
. . .
2~72~7
H. T., Thompson, C. J., Coleman, H. J., and Hopkins, R. L. (1972)Sulfur compound~ in crude oil. U. S. Department of the Interior,
Bureau of Mines, Bulletin 659) aliphatic and aromatic thiolq, dialkyl,
diaryl, alkyl-aryl and cyclic sulfide~, disulEides, and thiophenes
~uch as thiophene, benzothiophene, dibenzothiophene and various
derivative~ of these compound~ to which alkyl and/or aryl groups are
attached. Although the structure of coal i9 not known the sulfur
forms are believed to be similar to oils except that they are co-
valently bound in the complex coal matrix.
Coal can contain appreciable quantities of both forms of
sulfur with a total sulfur content, typically between 1 and 4%, but in
some cases being greater than 11% ~Chakrabarti, J. N. (1978) Analyti-
cal Methods for Coal and Coal Products, 1, 279-322. New York:
Academic). The sulfur content of crude oils varies widely ranging
from less than 0.1% to 5% and more in some of the heavy crudes (Inter-
national Petroleum Encyclopedia. ~1983) PennWell Publishing Co.,
Tulsa, OK). In crude oils sulfur i9 also found in both inorganlc and
organic forms, in addition, hydrogen ~ulfide may also be present. In
distillate fractiona of crude oil~ the sulfur content increases with
the boiling range of the fraction with the bulk of the sulfur found in
the middle distillates fractions and above.
A number of processe~ have been developed to remove inorganic
Rulfur forms from coal including oxidation to ~ulfur oxide gases and
reduction to hydrogen ~ulfide gas~ and phy~ical cleaning by froth
flotation and upward current classifiers based on the substantial
difference in density of coal and pyrite. In addition, a number of
microbial approaches have been developed which rely largely on the
oxidation of pyrite and reduced sulfur forms, including elemental
sulfur, by Thiobacillus species and Sulfolobus species, releasing
sulfur in the form of sulfuric acid and sulfates (reviewed in: Bo~, P.
and Kuenen, J. G. (1990) Microbial treatment of coal. In: Microbial
Mineral Recovery (Ehrlich, H. L. and 8rierley, C. L., eds) McGraw-
Hill, New York, pp. 343-377). Some of the microbial proce~s have been
patented, exemplified by Detz, C. M and Barvinchak, G. (1978) US
patent 4,206,288 in which Thiobacillus ferroxidans i3 employed in a
, , :, , :
' ' ' ' , ~ ' , '
. .
2~97~7
slurry reactor design to remove inorganic sulfur from coal, and
Attia, Y. ~. and Elzeky, M. A. (1988) US patent 4775.627 in which
Thiobacillus ferroxLdans is used in conjunction with physical separa-
tion to remove inorganic sulfur.
The removal of organic sulfur is more problematic. In coal
organic sulfur is covalently bound within the complex carbonaceous
structure. For pre-combustion sulfur removal this requires that the
coal matrix be exposed to the desulfurizing agent by depolymerization
and/or ~olubilized prior to treatment. Once pretreated in this way
the organic sulfur in the coal product is removed by similar tech-
niques a3 those used for oils. The requirement for pretreatment is a
major factor in determining costs for a coal desulfurization process.
Alternatively, organia sulfur in coal is removed by scrubbing of
sulfur dioxide after combustion. The use of scrubbers typiaally
limits the use of coal to large facilities were their use is economi-
cally fea~ible (Boq , P. and Kuenen, J. a. (lsso) Microbial treat-
ment of coal. In: Microbial Mineral Recovery (Ehrlich, H. L. and
Brierley, C. L., eds) McGraw-Hill, New York, pp. 343-377).
In standard refinery operations organic sulfur in oils is
removed by hydrodesulfurization using heterogenous inorganic cata-
ly~ts, high temperature and high hydrogen pressure. These method~ are
effective in removing thiols, most sulfides and disulfides but are
much le~s effective against thiophenic sulfur, particularly the
dibenzothiophenes containing sub3titution~ rendering the sulfur atom
sterically hindered, i.e., monobeta and dibeta substituted dibenzo-
thiophenes. ~ighter regulations on the maximum amount of sulfur
allowable in fuels will necessitate the removal of even the mo~t
recalcitrant sulfur compounds. This requires the development of new
technologies or the processing of fuels undsr much more severe condi-
tions, i.e., increased temperature and hydrogen pre~sure, thereby
greatly increasing the cost of fuel processing.
A large amount of research and many patents have been issued
on the use of microorganisms to remove organic sulfur form coal and
oils ~reviewed Ln: 1) Bos , P. and Kuenen, ~. G. tl990) Microbial
.
. . ,.: ,
:
,, : :
20~7217
treatment of coal. In: Microbial Mineral Recovery (Ehrlich, H. L. and
Brierley, C. L., eds) McGraw-Hill, New York, pp. 343-377; 2) Foght,
J. M., Fedorak, P. M., Gray, M. R. and Westlake, D. W. S. (1990)
Microbial desulfurization of petroleum. In:.Microbial Mineral Recovery
(Ehrlich, ~. L. and Brierley, C. L., ed~) McGraw-Hill, New York, pp.
379-4U7). The majority of this work ha~ focused on aerobic processes
uaing dibenzothiophene as the model compound to isolate organisms and
evaluate reaction mechani~ms. Re~earch on the use of anaerobic
bacteria to remove 3ulfur via a reductive process re~ulting in the
relea~e of ~ulfur as hydrogen sulfide i5 exemplified by Kim, B. H.,
Kim, T.-S. and Kim, H.-Y. ~1990) VS patent 4,954,229, in which
electrical energy i~ used to supply reducing power for the reduction
of organic ~ulfur by esulfovibrLo species.
The vast majority of microorganisms isolated which can
degrade dibenzothiophene aerobically do so by the initial oxidation
and cleavage of one of the aromatic ring~, and in 80 doing initiate
the complete degradation of the molecule to C02, H20 and S04=. This
pathway was initially described by Kodama et al. (Xodama, K.,
Nakatani, S. ~mehara, K., Shimizu, R., Minoda, Y. and Yamada, R.
~1970) Microbial conver~ion of petrosulfur compounds: Part III. ;;
I~olation and identification of products from dibenzothiophene. Agr.
Biol. Ch~m. 34, 1320-1324) and is fre~uently referred to as the Kodama
pathway.
Microbial deqradation of organosulfur containing carbonaceous
materials by the Kodama pathway, or related pathways involving C~C
bond cleavage, i~ undesirable due to the lack of ~pecificity for
~ulfur. These nonspecific pathways result in the degradation of
~tructurally related aromatic hydrocarbon compounds thereby greatly
reducing the efficiency of the proce~s and the calorific value of the
fuel. The lack of sulfur specificity inherent in Xodama like pathways
is illustratQd by the work of Monticello et al. (Monticello, D. J.,
Ba~.ker, D., Schell, M. and Finnerty, W. R. (1985) Appl. Environ.
Microbiol. ~9, 761-764) who, working with Pseudomona~ species, demon-
~trated that mutants unable to grow on dibenzothiophene were also
unable to oxidize naphthalene. It i~ therefore desirable to utilize a
- -, . , ;
. ~
:
: ' ~ : . i , . .
.
2 l 7
a microbial deculfurization process which removes organically bound
sulfur via a sulfur specific mechanism without removing carbon from
the molecule, thereby operating at an efficiency, and retaining the
calorific value of the fuel, in a manner not possible by carbon
degradative pathways.
Sulfur specific oxLdation of dibenzothiophene by ~TCC 39381
resulting in the release of sulfur as sulfate with out the degradation
of the carbon skeleton is described by Isbister and Doyle ~Isbister,
J.D. and Doyle, R.C. (Atlantic Res. Corp.) (1985) US patent 4562156).
However, the ATCC 39381 culture on deposit does not possess the C-S
cleavage trait and the depositors of the culture have stated that the
culture on deposit cannot be replaced as such culture3 having the C~S
cleavage trait to their knowledge do not exist (4th Department of
Energy Preparation, Uti}ization and Environmental Control Contractors
Sonference, U.S. Department of Energy, Pittsburgh Energy Technology
Center, Pittsburgh, PA 15236, U.S.A., 1988). More reaently, a patent
by Kilbane (Rilbane, J.J. (1991) US patent 5,002,888) describes the
use of a mutant strain of Bacillus sphaericus strain ATCC 53969 which
has the property of sulfur removal from organosulfur compound~ by
selective cleavage of C-S bonds. In the case of sulfur specific
metabolism of dibenzothiophene, the end products are 2-hydroxy bi-
phenyl and SO4=. This organism is unable to perform the desulfuriza-
tion of organosulfur compounds indPpendently, requiring the presence
of a "nutritional helper culture". Kilbane and Bielalga have reported
the i~olation of a mutant ~train of Rhodococcus rhodochrous strain
IGTS8 which performs the identical sulfur 3pecific metabolism of
organo3ulfur compounds but does not require a "helper cultur0"
~Kilbane, J.J. and Bielaga, B.A. (1990) Microbial removal of organic
sulfur from coal: a molecular genetics approach. In: Gas, Oil, Coal,
and Environmental biotechnology II ~Akin, C. and Smith, J. eds.)
Inatitute for gas technology, Chicago. pp. 317-330). van Afferden
et al. (van Afferden, M., Schacht,S., Klein, J. and Truper, B. G.
Degradation of dibenzothiophene by Brevibacterium sp. DO. Arch.
Mlcrobiol. 153, 324-328) reported the isolation of a Brevibacterium
specie3 which i3 able to use dibenzothiophene as a sole carbon, 3ulfur
and energy source and therefore completely degrades the molecule to
`.~
'
.... . .
., ~,.', 1
. ;; ' '
,.
'' ' .: :;, , ' ,
20~72~7
-- 6 --
C2 and H20, this process is initiated by oxidation of the sulfur to
dibenzothiophene sulfoxide and then to the sulfone, two intermediates
in the propo~ed pathway utilized by IGTS8. Omori et al. (omori, T.,
Monna, L., Saiki, Y. and Kodama, T. (1992) Desulfurization of
dibenzothiophene by Cory_ebacterium sp strain S~l. Appl. Environ.
Microbiol. 58, 911-915) reported the isolation of CorYnebacterium sp
strain SY1 which selectively removed sulfur from diben~othiophene and
a number of other organosulfur compounds. Corynebacterium sp strain
SYl metabolized dibenzothiophene to dibenzothiophene-5-oxide, dibenzo-
thiophene sulfone and hydroxybiphenyl, which was subsequently nitrated
to produce at least two different hydroxynitrobiphenyls.
In none of the above casea involving sulfur specific metabo-
lism were ~terically hindered organosulfur compounds demon~trated to
be sub~trates for sulfur removal. Further, it is evident that the
isolation of organisms capable of sulfur specific metabolism of
organosulfur compounds of the type found in carbonaceous materials
such as coal and oil is not obvious. This is demonstrated by the work
of Rilbane and Bielalga (Kilbane, J.J. and Bielaga, B.A. (1990)
Microbial removal of organic ~ulfur from coal: a molecular genetics
approach. In: Ga~, Oil, Coal, and Environmental biotechnology II
(Akin, C. and Smith, J. eds.) where three strains of Rhodococcus
rhodochrous different than the sulfur specific strain Rhodococcus
rhodochrous IGTS8 and five other Rhodococcus strains were shown to
lack sulfur specific metabolism of organosulfur compounds. This
evidence and work in our own laboratory show~ that sulfur speci~ic
metabolism of organosulfur compounds is strain specific and not a
general c~aract~ristic of any genera or ~pecies of microorganisms.
SUMMARY OF THE INVENTION
The present invention is two biologically pure cultures of
organic ~ulfur elective microorganisms, Arthrobacter species ATCC
55309 and 55310 respectively, which have the ability to selectively
reduce the organic sulfur content of ~ulfur containing organic
carbonaceous matsrial. The culture have been deposited with the
American Type Culture Collection, 12301 Park Lawn Drive, Rockville, MD
,. ,. ,,, ~
~; , , .
,:
~, .
20~7217
20852 and assigned ATCC Numbers 55309 and 55310. The two strains of
bacteria claimed herein po3se~ the same metabolic activity with
respect to sulfur and are of the same genus and species but differ in
colony morphology.
The ability to selectively remove sulfur from organosulfur
compounds pre~ent in organic carbonaceou~ material is based on the
~ulfur specific metabolism of these baateria which resulta in the
selective cleavage of carbon-sulfur bond~ and the release of sulfur
which is detected as sulfate or incorporated into biomass.
In a preferred embodiment the sulfur is ~terically hindered
in the organic compounds by the location of groups adjacent to the
carbon-sulfur bonds such as in the ca~e of mono and dibeta dibenzo-
thiophene~. The ability of these organisms to remove sulfur from
organosulfur compounds in which the sulfur is sterically hindered
allows for the removal of the all classes of organosulfur compounds
found in carbonaceous fuels such as coal3 and oils.
BRIEF D SCRIPTION OF T~E DRAWING
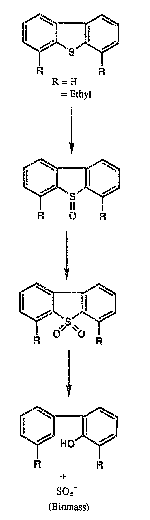
Figure 1 shows the biochemical pathway for the de~ulfuriiza-
tion of dibenzothiophene and the sterically hindered derivative
4,6-diethyl-dibenzothiophene by Arthrobacter specieq ATCC 55309 and
55310.
DESCRIPTION OF THE PREFERRED EMBODIMENT
The present invention i~ newly di~covered and i~olated
microorganisms which can effectively and specifically remove sulfur
from organic sulfur compounds of the type commonly found in petroleum
products, coal, tar sands and shale oil, including those compounds in
which the sulfur atom~ are sterically hindered. The unique ability of
these microorganisms to perform carbon-sulfur bond specific biochemis-
try on these organic sulfur compounds is the basi3 for the use of
these miaroorganisms in a process for the selective removal of organic
sulfur from fuels and related products.
.
.: ''.:
2~9 ~7
Microorganisms capable of selective sulfur removal from
organic sulfur compounds were i~olated from intertidal marine sedi-
ment3. This wa~ accomplished by enrichment culture of the sediments
employing a growth medium containing a sterically hindered organic
~ulfur molecule, 4,6-diethyl-dibenzothiophene (DEDBT) as the sole
source of culfur, and a readily as~imilable carbon/energy source
(~odium acetate). The formulation of thi~ growth medium wa~ such that
organisms capable of obtainin~ their required sulfur from the organic
sulfur compound could grow, while the growth of thoqe organi~ma which
could not would be discouraged. The addition of a readily as~imilable
carbon/energy source provided those organisms capable of sulfur
specific sulfur removal of growth advantage by eliminating the need to
obtain both carbon/energy and ~ulfur from the relatively poor
carbon/energy sourae provided by the organic sulfur compounds.
From such enrichment cultures, two nonsporing Gram-positive
irregular rod shaped bacteria were isolated, and identified as
Arthrobacter spec es, which are capable of removing the sulfur from
DEDBT, yielding primarily a product lacking sulfur and being mono-
hydroxylated in the position previously occupied by sulfur, namely
2-hydroxy-3,3-diethyl biphenyl. In addition, the isolated or~anisms
were 3hown to be able to perform the identical chemistry using di-
ben~othiophene as the ~ubstrate, demonstrating that sulfur removal is
not limited to sterically hindered organic suIfur compounds.
Separately, the two organisms were inoculated into 200 ml of
a mineral salt~/acetate medium containing DED8T a~ the sole source of
~ul~ur, in a one liter Erlenmeyer flask with a foam rubber stopper.
The culture~ were incubated on a shaker (200 rpm) at room temperature
for seven day~. The cultures were acidified to pH3 with HCl and
extracted three times with methylene chloride. The organic phases was
filtered through anhydrous sodium sulfate and the volume was reduced
by evaporation under a stream of N2 gas at room temperature to 0.1 ml.
The above methylene chloride culture extract was analyzed by
GC/FID, GC/MS and GC/SCD ~sulfur chemilumine~cense detection) (Figure
1), one major product was detected, and identified as 2-hydroxy
:
,
,
2~9~17
3,3-diethyl biphenyl, a sulfur-free derivative of the starting
material, with its carbon ~keleton intact and a hydroxyl group and a
hydrogen atom inserted in the location previously occupied by the
carbon-sulfur bonds. Analysis of the culture extract demonstrates the
formation of the sulfoxide and sulfone of the starting organosulfur
compound ~uggestlng tha~ these are intermediates in the pathway
resulting in ~ulfur removal. Sulfur i~ released as SO4= or a~similat-
ed into biomass. The ~ubstrate organosulfur compound (dibenzothio-
phene or the sterically hindered derivative 4,~-diethyl-dibenzothio-
phene), intermediate products and final products formed by the
d~ulfurization activity of the two claimed Arthrobacter species ~ATCC
55309 and ATCC 55310) are shown in Figure 1.
APPLICATION OF T~E MICROBIAL DESULFURIZATION PROCESS
To accomplish the desulfurization of a carbonaceous material
either of the claimed organisms, Arthrobacter species ATCC 55309 and
~5310 or prepared cell fractions containing the enzymes rssponsible
for deaulfurization or the isolated enzymes, are brought in contact
with the carbonaceous material (oil, coal, lignite, bitumen etc.) in
a manner sufficient to produce the desired degree of organic sulfur
removal. This can be accomplished in such configurations as described
below in which the system operates in a batch, semi-batch or con-
tinuous mode. In all examples the biological system which carries out
the de~ulfurization of organo~ulfur molecules, whether in the form of
whole cells of the organisms Arthrobacter species ATCC 55309 and/or
55310, or prepared cell fractions containing the enzymes responsible
for desulfurization or the isolated en7ymes, will be referred to below
as the bioaatal~st.
1) A slurry bio-reactor where the biocatalyst is free in an
aqueous solution comprising mineral nutrients and an assimilable
source of carbon and contacted with the carbonaceous material and
where the oxidlzed sulfur waste is removed from the aqueous phase
after separation from the carbonaceou~ material.
- , , - ' ' . ~ ~ , :
. . .
., , ,~
.
2-~972~7
-- 10 --
2) A slurry bio-reactor where the biocatalyst, essentially
free of non-adhering water i9 directly contacted with the carbonaceous
material and where the oxidized sulfur waste and biocatalyst is
removed by washing the carbonaceous material with minimal quantities
of water or by solvent extraction or both.
3) A fixed bed or slurry bio-reactor in which the bio-
catalyst is immobilized on a solid support in an aqueous solution
comprising mineral nutrients and an assimilable source of carbon and
contacted with the carbonaceous material and where the oxidized sulfur
waste is removed from the a~ueous phase after separation from the
carbonaceou~ material.
r
4) A fixed bed or slurry bio-reactor in which the bio-
catalyst i5 immobilized on a solid support and, essentially free of
non-adhering water, contacted with the carbonaceous material and where
the oxidized sulfur waste and biocatalyst is removed by washing the
carbonaceous material with minimal quantities of water or by solvent
extraction or both.
5) A membrane bio-reactor in which the biocatalyst, suspend-
ed in an aqueous solution comprising mineral nutrients and an assimil-
able source of carbon or nonaqueous solution, is separated from the
carbonaceous material by a membrane which prevent~ the mixing of
biocatalyst and carbonaceous material while allowing contact at the
membrane curface for desulfurization to occur, therefore, obviating
the need for qeparation of product and waste and/or biocatalyst.