Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02098848 2000-11-02
-1-
A TRUNCATED INTERLEUKIN.I RECEPTOR GENE FOR THE TRE.1TMENT OF AR-
TH RITIS
BACKGROUND OF THE INVENTION
Field of the Invention
The present invention relates to a method of using
a gene encoding a truncated interleukin-1 receptor to resist
the deleterious pathological changes associated. with
arthritis. More specifically, this invention provides a
method Wherein a~gene coding for an extracellular
interleukin-1 binding domain of an interleukin-1 receptor is
introduced into synovial cells of a mammalian host _in vivo
l0 for neutralizing the destructive activity of interleukin-1
upon cartilage and other soft tissues. As an alternative,
the patients own cells are transduced _in vitro and
introduced back into the affected joint, using surgical
transplantation procedures.
Brief Description of the Prior Art
Arthritis involves inflammation of a joint that is
usually accompanied by pain and frequently changes in struc-
ture. Arthritis may result from or be associated with a
number of conditions including infection, immunological
disturbances, trauma and degenerative joint diseases such
as, for example, osteoarthritis. The biochemistry of
cartilage degradation in joints and~cellular changes have
received considerable investigation.
In a healthy joint, cells in cartilage
(chondrocytes) and the surrounding synovium (synoviocytes)
are in a resting state. In this resting state, these cells
secrete basal levels of prostaglandin EZ and various neutral
proteinases, such as, for example, collagenase, gelatinase
and stromelysin, with the ability to degrade cartilage.
During the development of an arthritic condition, these
CA 02098848 2000-11-02
-2-
cells become activated. In the activated state,
synoviocytes and chondrocytes synthesize and secrete large
amounts of prostaglandin EZ and neutral proteinases.
In efforts to identify pathophysiologically
relevant cell activators, it has been known that the
cytokine interleukin-1 activates chondrocytes and
synovio~ytes and induces cartilage breakdown _in vitro and _in
vivo. Additionally, interleukin-l is a growth factor for
synoviocytes and promotes their synthesis of matrix, two
l0 properties suggesting the involvement of interleukin-1 in
the synovial hypertrophy that accompanies arthritis. In
contrast, interleukin-1 inhibits cartilaginous matrix
synthesis by chondrocytes, thereby suppressing repair of
cartilage. Interleukin-1 also induces bone resorption and
thus may account for the loss of bone density seen in
rheumatoid arthritis. Interleukin-1 is inflammatory, serves
as a growth factor for lymphocytes, is a chemotactic factor
and a possible activator of polymorphonuclear leukocytes
(PMNs). When present in a sufficient concentration;
2o interleukin-1 may cause Fever, muscle wasting and
sleepiness.
The major source of interleukin-1 in the joint is
the synovium. Inte-rleukin-1 is secreted by the resident
synoviocytes, which are joined under inflammatory conditions
by macrophages and other white blood cells.
Nuch attention has been devoted to the development
of a class of agents identified as the "Non-Steroidal Anti-
Inflammatory Drugs" (hereinafter "NSAIDs"). The NSAIDs
inhibit cartilage synthesis and repair and control
inflammation. The mechanism of action of the NSAIDs appears
to be associated principally with the inhibitiow of prosta-
glandin synthesis in body tissues. Most of this development
has involved the synthesis of better inhibitors of cyclo-
CA 02098848 2000-11-02
-3-
oxygenase, a key enzyme that catalyzes the formation of
prostaglandin precursors (endoperoxides) from arachidonic
acid. The anti-inflammatory effect of the NSAIDs is thought
to be due in part to inhibition of prostaglandin synthesis
and release during inflammation. Prostaglandins are also
believed to play a role in modulating the rate and extent of
leukocyte infiltration during inflammation. The NSAIDs
include, such as, for example, acetylsalicylic acid
(aspirin), Fenoprofen calcium (Nalfon~ Pulvules~, aista
i0 Products Company)., ibuprofen (Motrin~, The Upjohn Company).
and indomethacin (Indocin~, Merck, Sharp & Dohme).
In contrast, the studies upon which the present
invention is based show that production of the various
neutral proteinases with the ability to degrade cartilage
occurs even if prostaglandin synthesis is completely
blocked.
It has been shown that genetic material can be
introduced into mammalian cells by chemical or biologic
means. Moreover, the introduced genetic material can be
2o expressed so that high levels of a specific protein can be
synthesized by the host cell. Cells retaining the
introduced genetic material may include an antibiotic
resistance gene thus providing a selectable marker for
preferential growth of the transduced cell in the presence
of the corresponding antibiotic. Chemical compounds for
inhibiting the production of interleukin-l are also known.
U.S. Patent No. 4,778,806 discloses a method of
inhibiting the production of interleukin-1 by monocytes
and/or macrophages in a human by administering through the
parenteral route a 2-2'-(1,3-propan-2-onediyl-bis (thio)~
bis-1 H-i~idazole or a pharmaceutically acceptable salt
thereof. This patent discloses a chemical compound for
inhibiting the production of interleukin-1. Hy contrast, in
CA 02098848 2000-11-02
-4-
the present invention, gene therapy is employed that is
capable of binding to and neutralizing interleukin-1.
U.S. Patent No. 4,780,470 discloses a method of
inhibiting the production of interleukin-1 by monocytes in a
human by administering a 4,5-diaryl-2 (substituted)
imidazole. This patent also discloses a chemical compound
for inhibiting the production of interleukin-1.
0.S. Patent No. 4,?94;114 discloses a method of
inhibiting the 5-lipoxygenase pathway in a human by
administering a diaryh-substituted imidazole fused to a
thiazole. pyrrolidine or piperidine ring or a
pharmaceutically acceptable salt thereof. This patent also
discloses a chemical compound for inhibiting the production
of interleukin-1.
U.S. Patent No. 4,870,101 discloses a method for
inhibiting the release of fnterleukin-1 and for alleviating
interleukin-1 mediated conditions by administering an
effective amount of a pharmaceutically acceptable anti-
oxidant compound such as disulfiram, tetrakis [3-(2,6-di-
2o tert-butyl-4-hydroxyphenyl) propionyloxy methyl] methane or
2,4-di-isobutyl-6-(N,N-dimethylamino methyl)-phenol. This
patent discloses a chemical compound for inhibiting the
release of interleukin-1.
U.S....Patent No. 4,816,436 discloses a process for
the~use of interleukin-1 as an anti-arthritic agent. This
patent states that interleukin-1, in association with a
pharmaceutical carrier, may be administered by intra-
articular injection for the treatment of arthritis or
inflammation. In contrast, the present invention discloses
a method of using and preparing a gene that is capable of
binding to and neutralizing interleukin-1 as a method of
resisting arthritis.
CA 02098848 2000-11-02
-S-
U.S. Patent No. 4,935,343 discloses an immunoassay
method for the detection of interleukin-lp that employs a
monoclonal antibody that binds to interleukin -la but does
not bind to interleukin -la . This patent discloses that
the monoclonal antibody binds to interleukin-la and blocks
the binding of interleukin -la to interleukin-lareceptors,
and thus blocking the biological activity of interleukin
-1R . The monoclonal antibody disclosed in this patent may
be obtained by production of an immunogen through genetic
engineering using recombinant DNA technology. The immunogen
is injected into a mouse and thereafter spleen cells of the
mouse are immortalized by fusing the spleen cells with
myeloma cells. The resulting cells include the hybrid
continuous cell lines (hybridomas) that may be later
screened for monoclonal antibodies. This patent states that
the monoclonal antibodies of the invention may be used
therapeutically, such as for example, in the immunization of
a patient. or the monoclonal antibodies may be bound to a
toxin to form an immunotoxin or to a radioactive material or
10 drug to~form a radio ph8rmaceutical or pharmaceutical.
U.S. Patent No: 4,766,069 discloses a recombinant
DNA cloning vehicle having a DNA sequence comprising the
human interleukin-1 gene DNA sequence. This patent provide=_
a process for preparing human interleukin-1R , and
recovering the human interleukin -1~ , This patent
discloses use of interleukin-1 as an immunological reagent
in humans because of its ability to stimulate T-cells and 3-
cells and increase immunoglobulin synthesis.
U.S. No. 4,396,601 discloses a method for
providing mammalian hosts with additional genetic
capability. This patent provides that host cells capable ~:
regeneration are removed from the host and treated with
genetic material including at least one marker which allows
CA 02098848 2000-11-02
-6-
for selective advantage for the host cells in which the
genetic material is capable of expression and replication.
This patent states that the modified host cells are then
returned to the host under regenerative conditions. In the
present invention, genetic material may be directly
introduced (a) into host cells in vivo or (b) into
synoviocytes in vitro for subsequent transplantation back
into the patient's joints.
In spite of these prior art disclosures, there
l0 remains a very real and substantial need for a process
wherein a gene encoding a truncated interleukin-1 receptor
is used to resist the deleterious pathological changes
associated with arthritis. More specifically there is a
need for such a process where a gene coding for the
extracellular interleukin-1 binding domain of the
interleukin-1 receptor, capable of binding to and
cieutralizing interleukin-1 is expressed in host synovial
cells in vivo.
SUMMARY OF TFiE INVENTION
The present invention has met the hereinbefore
described need. A method of using the gene encoding an
extracellular interleukin-1 binding docaain of the
interleukin-1 receptor is provided for in the present
invention. This gene is capable of binding to and
neutralizing interleukin-1 in vivo to substantially resist
the degradation of cartilage in a mammalian host. Unlike
previous pharmacological efforts, the method of this
invention employs gene therapy in vivo to address the
chronic debilitating effects of arthritis.
CA 02098848 2000-11-02
A preferred method of using the gene coding for
the truncated interleukin-1 receptor of this invention
involves employing recombinant techniques to generate a cell
line which produces infectious retroviral particles
5 containing the gene coding for the truncated interleukin-1
receptor. The producer cell line is generated by inserting
the gene coding into a retroviral vector under the
regulation of a suitable eukaryotic promoter, transfecting
the retroviral vector containing the gene coding into the
l0 retroviral packaging cell line for the production of a viral
particle that is capable of expressing the gene coding, and
infecting the synovial cells of a mammalian host using the
viral particle.
More specifically, the method of using the
15 hereinbefore described gene involves introducing the viral
particles obtained from the retroviral packaging cell line
directly by intra-articular injection into a joint space of
a mammalian host that is lined with synovial cells. The
method of using the gene of this invention may be employed
20 both prophylactically and in the treatment of arthritis.
In another embodiment of this invention, a method
of using the hereinbefore described gene involves infecting
synovial cells in culture with the viral particles and
subseguently transplanting the infected synovial cells back
25 into the joint. This method of using.the gene of this
invention may also be employed prophylactically and in the
treatment of arthritis.
In another embodiment of this invention, a method
of using the gene coding for an extracellular interleukin-'
30 binding domain of the interleukin-1 receptor that is capable
of binding to and neutralizing interleukin 1 includes
employing recombinant techniques to produce a retrovirus
vector carrying two genes. The first gene encodes the
CA 02098848 2000-11-02
- g -
extracellular interleukin-1 binding domain of the interleukin
receptor, and the second gene encodes for selectable
antibiotic resistance. This method of use involves
transfecting the retrovirus vector into a retrovirus
packaging cell line to obtain a cell line producing
infectious retroviral particles carrying the gene.
Another embodiment of this invention provides a method
of preparing a gene encoding an extracellular interleukin-1
binding domain of the interleukin-1 receptor including
synthesizing the gene by a polymerase chain reaction,
introducing the amplified interleukin-1 receptor coding
sequence into a retroviral vector, transfecting the
retroviral vector into a retrovirus packaging cell line and
collecting viral particles from the retrovirus packaging cell
line.
In another embodiment of this invention, a
pharmaceutical composition for parenteral administration to a
patient in a therapeutically effective amount is provided for
that contains a gene encoding an extracellular interleukin-1
binding domain of the interleukin-1 receptor and a suitable
pharmaceutical carrier.
Another embodiment of this invention provides for a
pharmaceutical composition for parenteral administration to a
patient in a prophylactically effective amount that includes
a gene encoding an extracellular interleukin-1 binding domain
of the interleukin-1 receptor and a suitable pharmaceutical
carrier.
In one aspect, the invention provides for the use of a
gene encoding soluble interleukin-1 receptor that is capable
of binding to and neutralizing interleukin-1 for the
prevention and/or treatment of cartilage degradation,
CA 02098848 2000-11-02
- 8a -
deleterious pathological changes associated with arthritis,
arthritis, and inflammation.
In another aspect, the invention provides a genetically
modified synovial cell that expresses a gene encoding a
soluble interleukin-1 receptor that is capable of binding to
and neutralizing interleukin-1.
In a further aspect, the invention provides a
pharmaceutical composition comprising a synovial cell as
described herein and a pharmaceutical carrier.
It is an object of the present invention to provide a
method of using in vivo a gene coding for the extracellular
interleukin-1 binding domain of the interleukin-1 receptor
that is capable of binding to and neutralizing substantially
all isoforms of interleukin-1, including interleukin-la and
interleukin-1(3 .
CA 02098848 2000-11-02
.,.
_g_
It is an object of the present invention to
provide a method of using a gene is vivo in a mammalian hose
that is capable of binding to and neutralizing substantially
all isoforms of interleukin-1 and thus, substantially resist
the degradation of cartilage and protect surrounding soft
tissues of the joint space.
It is an object of the present invention to
provide a method of using in vivo a gene coding for the
extracellular interleukin-1 binding domain of the
to interleukin-1 receptor that is capable of binding to and
neutralizing substantially all isoforms of interleukin-1 for
the prevention of arthritis in patients that demonstrate a
high susceptibility for developing the disease.
It is an object of the present invention to
provide a method of using in vivo a gene coding for an
extracellular interleukin-1 binding domain of an
interleukin-1 receptor that is capable of binding to and
neutralizing substantially all isoforms of interleukin-1 for
the treatment of patients with arthritis.
It is an object of the present invention to
provide a method of using in vivo a gene or genes that
address the chronic debilitating pathophysiology of
arthritis.
It is a further object of the present invention to
provide a compound for parenteral administration to a
patient which comprises a gene encoding an extracellular
interleukin-1 binding domain of the interleukin-1 receptor
and a suitable pharmaceutical carrier.
These and other objects of the invention will be
3o more fully understood from the following description of the
invention. the referenced drawings attached hereto and the
claims appended hereto.
CA 02098848 2000-11-02
.. . ,
-10-
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows the interleukin-1 binding domain
amino acid arrangement.
Figure 2 shows the amino acid and nucleotide
sequence of the human and mouse interleukin-1 receptors.
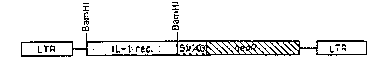
Figure 3 shows gene encoding a truncated
interlet~,kin-1 receptor inserted into a retroviral vector.
DESCRIPTION OF THE'PREFERRED EM80DIMENTS
As used.herein. the term "patient" includes
l0 members-of the animal kingdom.including but not limited to
human beings.
The gene and method of using the gene of this
invention provide for the neutralization of interleukin-1:
Interleukin-1 is a key mediator of cartilage destruction in
arthritis. Interleukin-1 also causes inflammation and is a
very powerful inducer of bone resorption. Many of these
effects result from the ability of interleukin-1 to increase
enormously the cellular synthesis of prostaglandin E2, the
neutral proteinases-- collagenase, gelatinase, and
2o stromelysin, and plasminogen activator. The catabolic
effects of interleukin-1 upon cartilage are exacerbated by
its ability to suppress the synthesis of the cartilaginous
matrix by chondrocytes. Interleukin-1 is present at high
concentrations in synovial fluids aspirated,from arthritic
joints.and.it has been demonstrated that intra-articular
injectioa.of recombinant interleukin-1 in animals causes
cartilage breakdown and inflammation.
Interleukin-1 exists as several species, each an
unglycosylated polypeptide of 17,000 Oaltons. Two species
have previously been cloned, interleukin -la and
interleukin~-lp . The a form has a pI of approximately 5,
and the ~ form has a-pI around 7. Despite the existence ci
these isoforms. interleukin -la and interleukin -lei have
CA 02098848 2000-11-02
Y n 1
substantially identical biological properties and share a
common cell surface receptor. The interleukin-1 receptor is
a 80kDa (kilodalton) glycoprotein and contains an
extracellular. interleukin-1 binding portion of 319 amino
acids which are arranged in three immu~oglobulin-like
domains held together by disulfide bridges as shown in
Figure 1. A 21 amino acid traps-membrane domain joins the
extracellular portion to the 217 amino acid cytoplasmic
domain. Figure 2 shows the amino acid and nucleotide
sequence of the human and mouse interleukin-1 receptors. In
Figure 2, the 21 amino acid traps-membrane region of the
interleukin-1 receptor is marked by the solid line. The
position of the 5' and 3' oligonucleotides for PCR are also
marked by a short solid line. The lysine amino acid just 5'
to the traps-membrane domain to be mutated to a stop codon
is marked by a solid circle in Figure 2.
Synovium is by far the major, and perhaps the
only, intra-articular source of interleukin-1 in the
arthritic joint. Snyovia recovered from arthritic joints
secrete high levels of interleukin-1. Hoth the resident
synoviocytes and infiltrating blood mononuclear cells within
the synovial lining produce interleukin-1.
The present invention provides a method of using
in vivo a gene coding for a truncated form of the
interleukin-1 receptor which retains its ability to bind
interleukin-1 with high afginity but which is released
a:tracellularly and therefore inactive in signal
transduction. The binding of this truncated and modified
receptor to interleukin-1 inhibits the intra-articular
activity of interleukin-1.
This method of using a gene encoding the
extracellular interleukin-1 binding domain of an
interleukin-1 receptor that is capable of binding to and
CA 02098848 2000-11-02
~~
-12-
neutralizing interleukin-1 includes employing a tetroviral
vector carrying a truncated interleukin-1 receptor gene
which encodes a truncated arid soluble active form of the
receptor. The expression of the novel interleukin-1
receptor gene is controlled by regulatory sequences
contained within the vector that are active in eukaryotic
cells.~~~This recombinant viral vector is transfected into
cell lines stably expressing the viral proteins _in traps
required for production of infectious virus particles
carrying the recombinant vector. These viral particles are
used to deliver the recombinant interleukin-1 receptor to
the recipient synovial cells by direct virus infection _in
vivo.
The soluble human interleukin-1 receptor to be
inserted into the retroviral vector may be generated by a
polymerase chain reaction (PCR). An oligonucleotide
complementary to the 5' leader sequence of the human
interleukin-1 receptor (GCGGATCCCCTCCTAGAAGCT) and an
oligonucleotide complementary to a region just upstream from
2o the traps-membrane domain of the interleukin-1 receptor
(GCGGATCCCATGTGCTACTGG) are used as primers for PCR. The
primer for the region of the interleukin-1 receptor adjacent
to the traps-membrane domain contains a single base change
so that the lys codon at amino.acid 319 (AAG) is changed to
a stop codon (TAG). By inserting a translation stop codon
just upstream from the transmembrane domain, a truncated
form of interleukin-1 receptor. that is secreted by the cell
is generated.. A HamBI recognition sequence (GGATCC) is
added to the 5' end of the PCR primers, and following
amplification, the resulting interleukin-1 receptor fragmen=
is cloned into a BamBI site. A cDNA library from human T-
cells is used as a source for the interleukin-1 receptor
cDNA. To amplify the appropriate region of the interleuki::-.
CA 02098848 2000-11-02
-13-
receptor from the cDNA library, the complementary primers
are added to the DNA and 50 cycles of annealing, primer
extension and denaturation are performed using a
thermocycler and standard PCR reaction conditions well known
by those persons skilled in the art. Following
amplification of the interleukin-1 soluble receptor using
the PCR~process, the resulting fragment is digested with
HamBI and inserted into the pLJ retroviral vector. The pLJ
retroviral vector.is available from A. J. Korman and R. C.
1o Mulligan. See also Proc. Natl. Acad. Sci:, Vol. 84, pp.
2150-2154 (April 1987) co-authored by Alan J. Korman, J.
Daniel Frantz, Jack L. Strominger and Richard C. Mulligan.
Restriction analysis was performed to determine thecorrect
orientation of the insert.
The retrovirus vector carrying the truncated
interleukin-1 receptor is transferred into the CRIP (Proc.
Natl. Acad. Sci., Vol. 85, pp. 6460-6464 (1988), O. Danos
and R. C. Mulligan) packaging cell line using a standard
CaP04 transfection procedure and cells wherein the viral
vector is stably integrated and is selected on the basis of
resistance to the antibiotic 6418. The viral vector
containing the neomycin resistant (neo-r) gene is capable of
imparting resistance of the cell line to 6418. The CRIP
cell line expresses the three viral proteins required for
packaging the vector viral RNAs into infectious particles.
Moreover, the viral particles produced by the CRIP cell line
are able to efficiently infect a.wide variety of mammalian
cell types including human cells. All retroviral particles
produced by this cell line are defective for replication but
retain the ability to stably integrate into synovial cells
thereby becoming an heritable trait of these cells. Virus
stocks produced by this method are substantially free of
CA 02098848 2000-11-02
-14-
contaminating helper-virus particles and are also non-
pathogenic.
More specifically, the truncated interleukin-1
gene can be inserted into a retroviral vector under the
regulation of a suitable eukaryotic promoter such as the
retroviral promoter already contained within the gene
transfer vector, such as for example, the pLJ.vector shown
in Figure 3. It will be understood by those persons skilled
in the art that. other vectors containing different
l0 eukaryotic promoters may also be utilized to obtain a
generally maximal level of interleukin-1 receptor
expression. The vectors containing the truncated, and
modified interleukin-1 receptor will be introduced into a
retroviral packaging cell line (CRIP) by transfection and
stable transformants isolated by selection for the
expression of the neomycin resistance gene also carried by
the pLJ vector. The CRIP cell line expresses all the
proteins required for packaging of the exogenous retroviral
RNA. Viral particles. produced by the 0418-selected CRIP
2o cell lines will carry a recombinant retrovirus able to
infect mammalian cells and stably express the interleukin-1
truncated receptor. The viral particles are used to infect
synovial cells directly _in vivo by injecting the virus into
the joint space.
. Another embodiment of this invention provides a
method for using the hereinbefore described viral particles
to infect in culture synovial cells obtained from the lining
of the joint of a mammalian host. The advantage of the
infection of synovial cells in culture is that infected
cells harboring the interleukin-1 receptor retroviral
construct can be selected using Gala for expression of the
neomycin resistance gene. The infected synovial cells
expressing the interleukin-1 receptor can then be
CA 02098848 2000-11-02
-15-
transplanted back into the joint by fntra-articular
injection. The transplanted cells will express high levels
of soluble interleukin-1 receptor in the joint space thereby
binding to and neutralizing substantially all isoforms of
interleukin-l, including interleukin -la and interleukin
-1 (3 .
The method used for transplantation of the
synovial cells within the joint is a routine and relatively
minor procedure used in the treatment of chronic
l0 inflammatory joint disease. Although synovium can be
recovered from the joint during open surgery, it is now
common to perform synovectomies, especially of the knee,
through the arthroscope. The arthroscope is a small, hollow
rod inserted into the knee via a small puncture wound. In
addition to permitting the intra-articular insertion of a
fibre-option system, the arthroscope allows access to
surgical instruments, such that snyovial tissue can be
removed arthroscopically. Such procedures can be carried
out under "spinal" anesthetic and the patient allowed home
the same day. In this manner sufficient synovium can be
obtained from patients who will receive this gene therapy.
The synovial cells (synoviocytes) contained within
the excised tissue may be aseptically recovered by enzymic
digestion of the connective tissue matrix. Generally, the
syno~rium is cut into pieces of approximately 1 millimeter
diameter and digested sequentially with trypsin (0.2t w/v in
Grey's Balanced Salt Solution) for 30 minutes at 37°
centigrade, and collagenase (0.2% w/v in Grey's Balanced
Salt Solution) for 2 hours at 37° centigrade. Cells
recovered from this digestion are seeded into plastic
culture dishes at a concentration of 104 - 105 cells per
square centimeter with Hank's F12 medium supplemented with
10% foetal bovine serum and antibiotics. After 3-7 days.
CA 02098848 2000-11-02
-16-
the culture medium is withdrawn. Non-adherent cells such as
lymphocytes are removed by washing with Grey's Balanced Salt
Solution and fresh medium added. The adherent cells can now
be used as they are. allowed to grow to confluency or taken
through one or more subcultures. Subcultivating expands the
cell number and removes non-dividing cells such as
macrophages.
Following genetic manipulation of the cells thus
recovered, they can be removed from the culture.dish by
to trypsinising, scraping or other means. and made into a
standard suspension. Grey's Balanced Salt Solution or other
isogenic salt solutions of suitable composition, or saline
solution are suitable carriers. A suspension °of cells can
then be injected into-the recipient mammalian joint. Intra-
articular injections of this type are routine and easily
carried out in the doctor's office. ~No surgery is
necessary. Very large numbers of cells canwbe introduced in
this way and repeat injections carried out as needed.
Another embodiment of this invention is the gene
2o produced by the hereinbefore described method of
preparation. ~ This gene carried by the retrovirus may be
incorporated in a suitable pharmaceutical carrier, such as
for example, buffered physiologic saline, for parenteraT
administration. This gene may be administered to a patient
in a therapeutically effective dose. More_specifically,
this gene may be incorporated in a suitable'pharmaceutical
carrier at a therapeutically effective dose and administered
by intra-articular injection.
In another embodiment of this invention, this gene
may be administered to patients as a prophylactic measure to
prevent the development of arthritis in those patients
determined to be highly susceptible of developing this
disease. More specifically, this gene carried by the
CA 02098848 2000-11-02
,~
-17-
retrovirus may be incorporated in a suitable pharmaceutical
carrier at a prophylactically effective dose and
administered by parenteral injection, including intra-
articular injection.
It will be appreciated by those persons skilled in
the art that this invention provides a method of using and a
method of preparing a gene encoding an extra cellular
interleukin-1 binding domain of an interleukin-1 receptor
that is capable of binding to and neutralizing substantially
l0 all isoforms of interleukin-1, and thus substantially
protect cartilage of a mammalian host from pathological
degradation. In addition, it will be understood by those
persons skilled in the art that the method of using the gene
of this invention will reduce inflammation, protect soft
15 tissues of the joint and suppress the loss of bone that
occurs in patients suffering with arthritis.
It will be appreciated by those persons skilled in
the art that the viral vectors employed in the hereinbefore
described invention may be employed to transfect synovial
2o cells in vivo or in culture, such as by direct intra-
articular injection or transplantation of autologous
synovial cells from the patient transduced with the
retroviral vector carrying the truncated interleukin-1
receptor gene.
25 ~. Whereas particular embodiments of this invention
have been described above for purposes of illustration, it
will be evident to those persons skilled in the art that
numerous variations of the details of the present invention
may be made without, departing from the invention as defined
3o in the appended claims.