Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
W092/08973 PCT/US91/0~573
l ~100363
IMPROVED AGGLUTINATION ,REACTION DEVICE
UTILIZING POROUS ABSORBENT MATERIAL
BACKGRO~ND OF THE INVENTION
The present invention is directed to a device for
performing an agglutination reaction of immunochemical
particles. The agglutination reaction device is designed to
provide a convenient means for performing and reading the
results of an agglutination reaction.
Agglutination reactions and their procedures are
generally well known in the art. A typical agglutination
reaction consists of the clumping together (or aggregation)
in suspension of antigen- or antibody-bearing cells,
microorganisms, or particles in the presence of specific
analytes. This clumping or agglutination of particles is
then monitored to determine the absence or presence of an
analyte sought to be detected.
One method for reacting immunochemical particle
reagents is to place liquid reagents on a glass slide and
generally rock or swirl the slide back and forth to cause
the reagents to mix and form agglutinations. Methods have
also been developed to avoid the necessary swirling of the
particle reagents in order to visualize the agglutinations.
For example, U.S. Patent 4,596,695 discloses an
agglutination reaction chamber for reacting immunochémical
particle reagents. The chamber includes a first
transparent panel having a first surface and a second panel
having a second surface spaced apart from the first surface
to define a chamber inbetween. The chamber intrinsically
causes immunochemical particle reagents to flow by
capillary action without an external motion imparted to the
chamber during which fIow the immunochemical particle
reagents can react.
An object of the present invention is to provide
SUBS T ITUTE SHEI~
W092/0~973 PCT/US91/0~573
2~1~0~3 2
a device that can be easily adapted for use in the
automated diagnosis of a plurality of samples. Another
object of the present invention is to provide a device
~apable of performing multiple, highly sensitive,
diagnostic tests simultaneously on a single sample in a
single device. In particular, the present invention is
directed toward a device that can be used in an automated
fashion where the reaction can be rapidly performed and
monitored with a minimum of sample material. In another
aspect, the present invention is directed toward a device
having multiple channels radiating from a central well
where multiple reactions on a single sample can be rapidly
performed and monitored with a minimum of sample material
with the results of such reactions being easily, visibly
observable. In particular, the present invention is
directed to an improved device for performing agglutination
reactions which device has means for controlling the
overall rate of liquid flow through the device comprising
a porous absorbent material in liquid communication with a
capillary chamber in the device.
SUMMARY OF THE INVENTION
The present invention is directed to a device
having an agglutination reaction chamber for performing
agglutination immunoassay reactions. In one aspect, the
device comprises a first wettable (hydrophilic) layer and
a parallel second layer wherein the first layer has
channels such that when the first and second layers are
brought into contact with each other an aggl1~tination
reaction chamber is formed for conducting fluid by
capillary action. The agglutination reaction chamber can
additionally include a sample recei~ing well contiguous
with the ingress of the chamber formed by the first and
5UBSTITUTE SHEET
W~92/08973 PCT/US91/~8573
(.. .. .
3 210~ 3
second layers. The chambers have means for controlling the
flow of liquid (fluid) when a predetermined amount of
reagent for performing an assay is dispersed in them, for
example in a well. The means for controlling the overall
rate of liquid flow thro~gh the chamber comprises utilizin~
a porous absorbent material such as an absorbent paper in
liquid communication with the chamber, typically at the
distal end of the chamber.
In the agglutination reaction chamber of the
present invention the reagent can be present in dried spots
or strips. It is also possible to suspend the reagent in
a water-soluble polymer.
A copending United States Patent Application,
Serial Number 07/138,253, filed on December 23, 1987,
entitled "Agglutination Reaction Device" (the disclosure of
which is hereby specifically incorporated herein by
reference), teaches an agglutination reaction chamber which
is constructed to be very small in size to accommodate
automated and efficient use of sample and reagents.
Typically, the length of such a chamber is from about 10 to
about 75 millimeters (mm), the channels are from about 0.01
to about 5.0 mm in depth and from about 0.1 to about 10.0
mm in width. A typical overall size for such an
agglutination reaction device having four chambers and a
sample receiving well is about 37.5 mm x 12.5 mm x 1.5 mm
(1 x w x h).
The aforesaid copending United States Patent
Application also generally discloses a means for
controlling the flow of fluid in an agglutination reaction
chamber involving the configuration of the channel or
geometric formations within the channel such as ridges,
particularly ridges formed in the channel which extend
across the entire width of the channel and for at least a
portion of the len~th of the channel. The aforesaid
copending United States Patent Application also discloses
SU8~3TITUTE SHEET
. .. ... . - ... `. . , . . - . . ,.. .. - . .. . . ., ., . . . ,. . . . . .:: , ~
WO9~/08973 PCT/~JS91/~8573
2~ ~363
another means for controlling the flow of fluid in the
chamber, namely utilization of a water-soluble material,
such as a water-soluble polymer, (e.g.,
polyvinylpyrrolidone, polyvinylalcohol, gelatin, or bovine
serum albumin) dried in portions of the channel.
However, it has been found that such expedients,
while useful in helping to control the overall rate of
liquid (fluid) flow in the channels, can be difficult to
employ so as to obtain consistently uniform results. For
example, where a water-soluble polymer such as
polyvinlypyrolidone is utilized, it has been found that it
can be difficult to obtain dried coatings of the
polyvinylpyrrolidone so as to obtain consistent stability
of overall flow of liquid in the channels. Also, there are
advantages with respect to the ease of manufacture of
devices utilizing a porous absorbent medium such as paper
compared to utili~ation of coatings such ae dried
polyvinylpyrrolidone.
The present invention is directed to devices for
performing agglutination reactions having impro~ed
properties including improved means for controlling the
overall rate of liquid flow through the agglutination
chamber. The present invention also is directed to such
devices constructed in the form of convenient, disposable
structures, such ~s disposable, laminated cards, optionally
mounted in disposable rigid containers.
BRIEF DESCRIPTION OF THE DR~WINGS
Figure 1 is a top perspective view of one
embodiment of the present agglutination reaction device
showing a rec~angular sample receiving well and four
reaction chambers.
Figure 2 is a top perspective view of one
SUE~STITUTE 5HIEE:T
W092/08973 PCT/US91/08573
~.~
'2~00363
embodiment of a bottom surface of the present agglutination
reaction device showing a rectangular sample receiving well
and four channels ~for chambers) having strips of porous
material at their distal ends.
Figure 3 is an exploded, top perspective view of
another embodiment showing a three layer structure
comprising a first or base layer, a second layer showing a
cutout for a round receiving well and a generally straight
agglutination chamber, a strip of porous absorbent
material, and a third or top layer.
Figure 4 is an exploded, top perspective view of
another embodiment showing a three layer structure
comprising a first or base layer, a second layer showing a
cutout for a round receiving well and an agglutination
chamber having a flared portion at its distal end, a strip
of porous absorbent material, and a third or top layer.
Figure 5 is an exploded, top perspective view of
another embodiment showing a three layer structure
comprising a first or base layer, a second layer showing a
cutout for a round receiving well and a generally straight
agglutination chamber with an integral porous absorbent
strip in the second layer at the distal end, and a third or
top layer.
Figure 6 is an exploded, top perspective view of
another embodiment showing a three layer structure
comprising a first or base layer, a second layer showing a
cutout for a round receiving well and an agglutination
chamber having a flared portion at its distal end with an
integral porous absorbent strip in the second layer at the
distal end of the chamber, and a third or top layer.
Figure 7 is a top plan view of another embodiment
showing the parts of a laminated structure comprising a
base layer, a second layer~having a cutout for a round
receiving well and multiple radiating agglutination
chambers having flared distal zones, an annular structure
SUBSTITUTE SHEET
- .
... ~.. . . . .... . .. . . .
WO9~/08973 PCT/US~1/08573
2~003~3 6 ,'
having alternating liquid absorbent regions (4) and liquid-
occlusive regions (26), and another round layer which in
cooperation with the annular structure forms the top layer.
Fi~ure 8 is a schematic diagram illustrating
regions of different flow rate per unit area outward from
the receiving well for an agglutination chamber having a
flared distal end, and illustratiny a band of agglutinated
particles in the flared distal end.
Figure 9 is a schematic diagram illustrating
regions of different flow rate per unit area outward from
the receiving well for an agglutination chamber having
semicircular, or bowl-shaped, distal end, and illustrating
a band of agglutinated particles in the semicircular end.
Figure 10 is a schematic diagram illustrating
regions of different flow rate per unit area outward from
the receiving well for an agglutination chamber having an
approximately rectangular shape, and illustrating a band of
agglutinated particles in the rectangular area of increased
width.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention is directed toward improved
devices suitable for performing agglutination reactions.
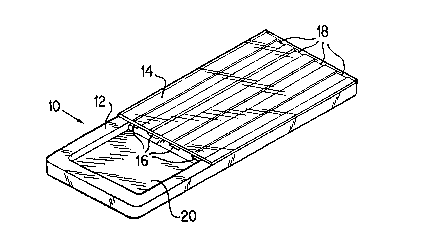
One embodiment of the invention is shown in Figure 1. The
device (10) as shown in Fig. 1 generally comprises a first
surface (12) and a parallel second surface (14) one of
which has channels (16) formed therein such that when the
two surfaces are placed together a chamber or microchamber
(18) is formed through which liquid will flow by capillary
action. In order to have capillary flow it i5 rsquired that
one of the channel surfaces be water wettable
(hydrophilic). Preferably, the surfaces are designed such
that they create a plurality of chambers or microchambers
(18) when assembled. Each of the channels (18) has a strip
5lJBSTlTUTE 5HEET
,
W092/~973 PC~/VS9t/08S~3
~ ~ . " ~
2100363
of porous, absorbent material, preferably a cellulosic
material, more preferably paper, at the distal end of each
channel for controlling the overall rate of flow of liquid
through the channel. The aforesaid porous material, for
example paper, is to be distinguished from water-soluble
materials such as dried coatings of water-solùble polymers
such as polyvinylpyrrolidone, polyvinylalcohol, gelatin, or
bovine serum albumin. The porous absorbent material
utilized in present invention is itself generally not
water-soluble.
The surface having channels grooved therein also
preferably has a sample receiving well (20) which is open
to each of the channels (16). The receiving well (20) is
positioned such that it is not covered by the second
surface (14) as shown in Fig. 1. A liquid sample can be
placed in the receiving well (20), such that it can be
wicked into the chambers (18) by capillary action. Thus it
is possible to form a device having multiple chambers and
one large sample receiving well such that a single drop of
sample can be placed in the well and wicked into the
multiple chambers. Each chamber can then perform a
different agglutination assay on a single sample, the
porous absorbent strip in the distal end of each channel
advantageously controlling the overall rate of liquid flow
through the channel.
It is a further objective of the present
invention to provide a device which is small enough to be
utilized in automated instrumentation and monitored by
automated means for the presence of agglutinated particles
in each of the chambers. Thus in another embodiment of the
agglutination device, the lower surface (12) will be opaque
and the upper surface (14) will be transparent to
transmitted or reflected light where an optical scanner can
be positioned above the device (10). Thus, the opaque
lower surface (12) gives a better background for detection
SUBSTITUTE SH~ET
: . ~ . . .. . . . ... . . . .... . . .
W092/08973 PCT/US91/08573
2~3~3 ~
of agglutinated particles in this embodiment.
For example, when a solution of cells is
introduced to one end of a chamber (18), containing
antibodies directed against antigens on the cells and dried
onto the interior of the channels (16), the solution will
migrate ~hrough the channel, mix with the antisera, and the
cells will aggregate. This will all occur without any
centrifugation or mixing steps. Control of the flow of the
liquid through the channel is necessary because the
agglutination reaction occurs preferably during the period
of liquid flow. Sufficient incubation time must be
incorporated into the period of liquid flow to achieve
optimum reaction of the reagents.
The rate of liquid flow through a capillary
chamber in the present embodiment is controlled by means of
a strip of porous, absorbent material (22) positioned in
the distal end of each channel (16) of the agglutination
reaction chamber. Preferably the porous material is a
cellulosic material, more preferably paper such as Whatman
filter paper. In this embodiment represented in Figure 1,
one can consider the reaction zone of the agglutination
chamber as ending just at the beginning of the strip of
paper (22). It has been found that porous material such as
paper utilized as the fluid flow control means provides
advantages in both manufacturing and performance over the
utiIization of coatings of water-soluble materials such as
polyvinylpyrrolidone (PVP).
Figures 3 and 4 represent other embodiments of a
device for performing agglutination reactions according to
the invention. These embodiments have, in adherent
relationship, a first wettable, but liquid-occlusive, layer
(1), a second liquid-occlusive layer (2) parallel to and
overlying ~he first layer (1), and a third liquid-
occlusive, preferably non-wettable, layer (3) parallel to
and overlying the second layer (2) and having a window, or
r~ s~EEr
.. . . . . .
... . ;. -.. - ~,. ~ . .... .. ... . . .
.. ~, . .... ~ .. .... - . 1 ~ ;.. ,.; .. .. . . .
W092/08973 ~CT/US91/08~73
,..
9 2~3
viewing area, for observing particles. The first layer (l)
is made of a liquid-occlusive material having a water-
wettable surface. In this embodinlent the third layer (3)
is made from a clear, liquid-occlusive, non-wettable, film,
such as a clear polycarbonate film, and therefore also
serves as a window, or viewing area, for observing
particles in the agglutination chamber. The second layer
(2) is interposed between, and is adhered to, the first
layer (l) and third layer (3), for example by means of an
adhesive on each side of layer (2) facing the topside of
the first layer (l) and the underside of the third layer
(3) respectively. The second layer (2) has a slot (25) cut
through its thickness defining a channel for directing
liquid for conduction by capillary action through the
chamber defined by the slot (25) in conjunction with the
first (l) and third (3) layers respectively.
In other words, when the first, second and third
layers are laminated together, a portion of each of the
first and third layers serve respectively as the floor and
roof of the agglutination chamber with part of the walls of
the slot (25) of the second layer (2) defining the walls
(9) of the chamber. The agglutination reaction chamber has
a proximate zone (6) and a distal zone (7), which proximate
zone (6) for ex~mple in Figure 4 is represented by the
génerally rectangular portion of the slot (25) of the
second layer (2) with the distal zone (7) being represented
by the deltoid or flared portion of the slot (25) of the
second layer (2).
Each of the embodiments illustrated by Figures 3
and 4 has a well-defining slot (8) in the third layer (3)
and a corresponding second well-defining slot (5) in the
second layer (2) of the same size and configuration as the
well~defining slot (8) in the third layer (3). The well-
defining slot (5) in the second layer (2) is positioned
directly below the well-defining slot (8) in the third
... .
SUBSTITWTE SHEET
WO92/Og973 PC~/US91/08573
~: .
21 0036~
layer (3) such that when all three layers are laminated
together, the second well-defining slot (5) in conjunction
with the well-defining slot (8) along with the
corresponding portion of the first layer define a well for
receiving liquid, the well being in liquid communication
with the proximate zone (6) of the chamber. The bottom of
the well is formed from a corresponding circular portion of
the first layer (1) which portion can be considered to be
the projection of the outline of slots t5) and (8) onto the
surface of layer ~1).
The overall rate of liquid flow through the
agglutination chamber in these embodiments is controlled by
means of a strip of porous absorbent material (4),
pLeferably filter paper, in liquid communication with the
chamber and positioned adjacent to the distal end of the
chamber, and preferably extending partially into the distal
end of the chamber, when the structures of Figures 3 and 4
are laminated respectively together. In a more preferred
embodiment, layer (3) as shown in Figures 3 and 4 has a
slot (28), of slightly larger dimensions as the strip of
porous paper (4), such that when the respective layers are
adhered together, the strip of porous absorbent material
(4) lies partially within the slot (28), more particularly
so that a front minor portion of the strip (4) lies within
the distal zone (7) of the slot (25) with the remaining
major portion of the strip lying within slot (28), so as to
prevent disadvantageous formation of microcapillary
channels at the sides of and along the length of the strip
(4).
The resulting laminated structure, can be thought
of as being in the form of a thin, disposable card with the
paper strip (4) being in liquid communication with the
distal zone (7) of the agglutination chamber.
Figures 5 and 6 represent other embodiments of a
device, in the form of a laminated card when the layers
SL)B5TITUTE 5~11EET
W092/08973 PCT/US91/08573
ll 21~ 3
shown in the Figures are adhered together, for performing
agglutination reactions. These embodiments have, in
adherent relationship, a first wettable, but liquid-
occlusive, layer (1), a second layer (2) parallel to and
overlying the first layer (1), and a third liquid-
occlusive, preferably non-wettable, layer (3) parallel to
and overlying the second layer (2) and having a window, or
viewing area, for observing particles. The first layer (1)
is made of a liquid-occlusive material having a water-
wettable surface. As in the embodiments represented by
Figures 3 and 4, these embodiments also utilize a third
layer (3) made from a clear, liquid-occlusive, preferably
non-wettable film, such as a clear polycarbonate film or a
non-wettable cellophane tape, which therefore also serves
as a window for observing particles in the agglutination
chamber. The second layer (2) is interposed between, and
is adhered to, the first layer (1) and third layer (3), for
example by means of an adhesive on each side of layer (2)
facing the topside of the first layer (1) and the underside
of the third layer (3) respectively. The second layer (2)
has a slot (25) cut through its thickness defining a
channel for directing liquid for conduction by capillary
action through the chamber defined by the slot (25) in
conjunction with the first (1) and third (3) layers
respectively. .
As in the embodiments represented by Figures 3
and 4, when the first, second and third layers axe
laminated together a portion of each of the first and third
layers serve respectively as the floor and roof of the
agglutination~chamber with part of the walls of the slot
(25) of the second layer (2) defining the walls (9) of the
chamber, the other part of the walls of slot (25) defining
the walls of the circular receiving well (5). The
agglutination reaction chamber has a proximate zone (6~ and
a distal zone (7), which proximate zone (6) for example in
SU13STITUTE SHEEJ
.
. ... .... . . ....... . ... . .
W092/0~973 ~C~`/US91/08573
21~3~ ~
Figure 6 is represented by the generally rectangular
portion of the slot (25) o~ the second layer (2) with the
distal zone (7) being represented by the deltoid or flared
portion of the slot (25) of the second layer (2).
Each of the embodiments illustrated by Figures 5
and 6 has a well-defining slot (8) in the thixd layer (3)
and a corresponding second well-defining slot (5) in the
second layer (2) of the same size and configuration as the
well-defining slot (8) in the third layer (3). The well-
defining slot (5) in the second layer (2) is positioned
directly below the well-defining slot (8) in the third
layer (3) such that when all three layers are laminated
together, the second well-defining slot (5) in conjunction
with the well-de~ining slot (8) along with the
corresponding portion of the first layer define a well for
receiving liquid, the well being in liquid communication
with the proximate zone (6) of the chamber. The bottom of
the well is formed from a corresponding circular portion of
the first layer (1) which portion can be considered to be
the projection of ~he outline of slots (S) and (6) onto the
surface of layer (1).
However, in the embodiments of Figures 5 and 6,
the second layer (2) is made of a liquid absorbent
material, such as absorbent paper, selectively impregnated
through its thickness with a substance, such as a water-
repellent ink, to form an impregnated region (26) and a
non-impregnated region (~). The non-impregnated region (4)
is liquid absorbent and the impregnated region (26) is
liquid-occlusive. In these embodiments, the non-
impregnated region (4) which is in liquid communication
with the distal zone (7) of the chamber serves as the means
for controlling the overall rate of liqui.d flow through the
agglutination chamber. The second la~er (2) also has a
slot (25) in the impregnated region (26) defining a channel
for directing liquid conducted by capillary action through
SlJB51~1TlL)T~ SHE:~;T
- .. . `.` . ` .. .. ~. . ` . . , ~ . . . .. . .. .. .
W092/08973 PCT/U~l/0~573
~,.'.
13 21~3~3
a chamber defined by the slot (25) in conjunction with the
first layer (1) and third layer (3). This chamber also has
a proximate zone (6) and a distal zone (7). It is within
this chamber that agglutination reactions can be performed.
As can be seen from Figures 5 and 6, the non-impregnated
region (4) is located adjacent to the distal end of the
agglutination chamber and is in liquid communication with
the chamber.
Also in the embodiments illustrated by Figures 5
and 6 there is a well-defining slot (8) in the third layer
(3) and a corresponding second well-defining slot (5) in
the second layer (2) of the same size and configuration as
the well-defining slot (8) in the third layer (3). The
well-defining slot (5) in the second layer (2) is
positioned directly below the well-defining slot (8) in the
third layer (3) such that when all three layers are
laminated together, the second well-defining slot (5) in
conjunction with the well-defining slot (8) along with the
corresponding portion of the first layer (l) define a
circular well for receiving liquid, the well being in
liquid communication with the proximate zone (6) of the
chamber. The bottom of the well is formed from a
corresponding circular portion of the first layer (l).
Figure 7 illustrates an exploded, plan view of a
preferred embodiment of the invention. This embodiment
provides for performing a plurality of agglutination
reactions utilizing a minimal amount of liquid sample. The -
device in assembled form can be thought of a relatively
thin, laminated, disposable structure having in this
particular illustration six agglutination chambers
radiating from a common liquid receiving well. The device
of Figure 7 comprises, in adherent relationship, an
approximately circular first wettable but liquid-occlusive
layer (1), an approximately circular second liquid-
occlusive layer (2) parallel to and overlying the first
,
SVBSTITUTE SHEET
:: '
.. . ,; . , . . . .. , , ,, ~ ~ ~
W092/08973 PCT/US91/~8573
2~0~363
14
layer (1), and a third liquid-occlusive layer (3) parallel
to and overlying the second layer (2). These respective
layers can be bonded together, for example, by means of an
adhesive between the respective layers. In this embodiment
the third layer (3) is made of a circular clear plastic
film, such as a polycarbonate film, thereby providing
windows, or viewing areas, ~or observing particles in the
six radiating agglutination chambers. The second layer
(2), interposed between and in adherent relationship to
the first and third layers has a slot (25) in the form of
a central, circular portion (5) having si~ radial, slotted
arms extending outward therefrom. ~hese radial arms of the
slot (25) define six channels for directing liquid
conducted by capillary action through chambers respectively
defined by the radial, slotted arms in conjunction with the
first layer (1) and the third layer ~3). Within the
resulting six chambers agglutination reactions can be
performed simultaneously. Each of the six chambers has a
generally rectangular proximate zone (6) and a generally
flared or deltoid shaped distal zone (7). The overall rate
of liquid flow through each agglutination chamber in this
embodiment is controlled by means of a strip of porous
absorbent material (4)r preferably filter paper, projecting
from a generally annular ring (27) of such porous material
having a hole (28), into the distal zone (7) of each of the
channels defined by the radial, slotted arms. The annular
ring (27) is selectively impregnated through its thickness
with a substance to provide alternating non-impregnated
liguid absorbent regions (4) and impregnated liquid-
occlusive regions (26). These non-impregnated strips (4)
of paper projecting from the annular ring (27) are in
liquid communication with the chambers and are positioned
adjacent to the distal ends of the chambers, pre~erably
positioned partially in the distaI ends, when the
structures of Figure 7 are laminated respectively together.
SUBSTITUTE SI~EE:T
.
.. -.. .... , .. , -.. . ... , , .. , . , ., ..... . . ,-, ~, .. .. ,.. ..
W092/08973 PCI/US9l/0~S73
2~3~3
The third layer (3) of the device represented by
Figure 7 has a circular well-defining slot (8), and the
second layer has a corresponding circular second well-
defining slot (5) of the same size and configuration as the
well-defining slot (8) in the third layer (3). The well-
defining slot (5) of the second layer (2) is positioned
directly below the well-defining slot in the third layer
(3) in the assembled configuration. Thus the second well-
defining slot (5) in conjunction with the well-defining
slot ~8) in the third layer (3) and the respective circular
portion of the first layer (l) define a well for receiving
liquid, the well being in liquid communication with the
proximate zone (6) of each of the chambers.
The resulting, generally circular laminated
structure, can be thought of as being in the form of a
relatively thin, disposable card with the fluid-absorbent
paper strip (4) being in liquid communication with the
distal zone (7) of the agglutination chamber. Thus, in all
of the embodiments of the present invention, the device of
the invention has means for controlling overall rate of
liquid flow through the reaction chamber which is a porous
absorbent material in liquid communication with the
chamber, which material is typically positioned adjacent
to, and usually extending partially into, the distal end of
the chamber. However, it has been found that the utility
of devices of the present invention, for performing
agglutination reactions, can be enhanced further by
utilizing an additional means for controlling the flow of
liquid through the reaction chamber of the device, by
modifying the geometric configuration of the chamber or the
internal shape of the chamber as illustrated in Figures 4,
6, 7 and 8.
For sxample, in Figures 4, 6, and 7, the general
slot ~25) in layer (2) defines at least approximately
parallel walls (9) in the proximate zone (6) of the chamber
SUBSTITUTE SHEc:T
` - . , . .... , ., " . .. ... ,..... ; . . ...... ..
WO9~/08973 PCr/US91/08573
2~ 3~3 ~,
16
thereby defining a first path of approximately constant
width. Looking in the direction toward the distal end of
the chamber, the general slot (25) defines walls in the
distal zone (7) which are spaced to define a second path of
increased width compared to the first path of the proximate
zone (6). It has been found that agglutination reactions
perfo~ed in such a chamber advantageously can result,
surprisingly, in the formation of one or more patterned
formations, such as, for example bands, of agglutinated
particles in the distal zone (7) of the chamber which
patterns are more easily observable through the window of
the third layer (3) than non patterned aggregates of
agglutinated particles which generally result in
agglutination chambers of the prior art. Figure 8 shows a
schematic representation of an approximately semicircular
band (27) of agglutinated particles in the zone of
increasing chamber width, namely in the flared (here
approximately deltoid-shaped) "second path" of the chamber
in the distal zone (7) of the chamber. As represented in
schematic form in Figure 8 through the use of arrows of
different length along the reaction path in the chamber,
the walls in the distal zone (7) are spaced to provide a
decreased liquid flow rate per unit area of liquid path
along this second path. In Figure 8, the shorter arrows
are, of course, intended to represent smaller flow rate per
unit area of path, compared to that represented by the
longer arrows.
While deltoid-shaped configurations of the second
path of the distal zone in the chambers is preferred, it
has been found that other geometric conigurations for this
so-called "second path" provide advantageous patterned
formations of agglutinated particles. For example, the
side walls in the second path can be formed to be convex
giving an approximately semicircular or bowl-shaped
configuration to the second path as illustrated in
SUBS~ E SH. -~ ~
-
WO92/08973 PCT/US~1/08573
.
2~ ~3~3
17
Figure 9. Alternatively, although less pxeferred, the side
walls of the second path can be formed to provide a second
path with an approximately rectangular shape as illustrated
in Figure 10.
Moreover, if desired, the flow rate per unit area
in the distal zone of the reaction chamber can be gradually
decreased along the general direction of flow by gradually
increasing the space between the floor and the roof of the
chamber along the direction of liquid flow, for example by
gradually bowing the roof of the chamber in the distal zone
upward and/or gradually bowing the floor of the chamber in
the distal zone downward. It has been found that such
modification of the space between the floor and the roof of
the chamber in the distal zone of the chamber can also
contribute to the formation of regular patterns of
agglutinated particles being formed in the distal zone of
the chamber. For example, the space between the floor and
the roof of the chamber can be gradually increased by
stamping a spherical dome-shaped or cylindrical dome-
shaped configuration in an area of the third layer (3) in
such manner that when the third layer is adhered to the
second layer (2) the dome in the third layer overlies the
distal zone of the reaction chamber. Another example or a
way to provide a gradually increasing space between the
floor and the roof of the distal zone of the reaction
chamber is to stamp a spherical bowl-shaped or cylindrical
bowl-shaped depression in the base or first layer (l) in
such manner that when the first layer (l) is adhered to the
second layer (2) the bowl_shaped depression occurs in the
floor of the distal zone of the reaction chamber.
All types of agglutination-based assays can be
accommodated with a device according to the present
invention. In some instances, a soluble reag~nt can be
dried as spots or strips in the reaction chamber, for
example, in blood typing. In other instances, a
.
:
SUBSTIT~ITE SHEE~
W092/08973 P~/VS91/08573
2~363 ~ ,
18
particulate reagent, such as a latex reagent, can be dried
in the chamber. In yet another approachr a reagent can be
dispersed in a solution which is placed in the chamber.
One preferred reagent solution is microparticulates in a
solution of dextran and sucrose. Preferably, the
microparticulate reagent is mixed in a solution of about
2.5 to about 5.0 percent by weight dextran and from about
to about 20 percent by weight sucrose. Another
preferred solution for mixing reagents is FICOLL (a
trademark by Sigma Chemical Co., St. Louis, MO for a
nonionic synthetic polymer of sucrose) from about 20 to
about 3~ percent by weight. Also, depending on the
requirements of the assay, the flow of the liquid through
the chamber can be controlled as described above to
accommodate any necessary incubation times and assay
sequences.
A particularly unique feature of the present
invention is that it protTides for the ability to
simultaneously perform multiple assays while utilizing a
very small amount of sample material, for instance, a
single drop. Also, the agglutination a~say is essentially
self-performing once the drop has been added to the
agglutination reaction device. Moreover, in those
embodiments of the invention utilizing an additional means
for controlling the flow of liquid through the reaction
chamber of the device, namely by modification of the
geometric configuration of the chamber or the internal
shape of the chamber as discussed above, additional
enhanced results can be obtained such as enhanced
observability of aggregates of agglutinated particles in
the distal zone of the reaction chamber.
A device of the invention is especially suitable
for use in an automated fashion where the agglutination
reaction can be monitored by an optical scanner. For
example, the construction of the agglutination reaction
5VE~STITIIJTE SH ET
- . ~ . ~ . . . . ............................... .
.
W092/08973 PCT~US91/~8573
~, .
2l~3~3
19
device enables one to use an image analysis system
available from Olympus (CUE-2, LaXe Success, N.Y.) to
determine the quantity and concentration of agglutinated
material. The agglutination reaction device is
illuminated, such ~hat transmitted or reflected light can
be read by the reader. The image is then compùter analyzed
to determine the quantity of agglutination which has
occurred and to enhance the image for very accurate and
sensitive determinations. By confining the sample to a
chamber such as formed in the agglutination reaction
device, there is no problem with curvatures of droplets or
water which could interfere with the image seen by the
reader. Thus, the uniformity of the reacted sample and
reagents achieved by the agglutination reaction device
provides an excellent imaging format for a reader or other
imaging devices. Besides being able to read the
transmission of light through the bottom of the
agglutination reaction device, it is also possible to read
reflected light because the sample and reacted reagents are
confined to capillary chambers formed by the agglutination
reaction device.
Again referring to Figure 1, one embodiment of
the present invention is shown. Figure 1 shows a
perspective view of device (10) constructed of two layers
of material, a bottom layer (12) covered by a top layer
(14). Layer (12) has a plurality of channels (16~ and a
sample well (20) formed into the surface. The sample well
(20) is contiguous with the ingress of each of the chambers
(16). In the construction of the device (10), either the
bottom layer (12) or the upper la~er (14) can be opaque.
Preferably the layer which is further from an optical
scanning device is opaque to enhance the background. It is
required that the bottom surface (12) be hydrophilic or
wettable such that the capillary flow is induced when a
sample is placed in contact with the ingress of a chamber
SUBS I ITUTE S~cT
.
W~92/08973 PCT/~S91~08~73
'~L0~3~
(18). This can be accomplished by using a hydrophilic
material for the surface (12); however, it is also possible
to chemically treat or coat otherwise non-wettable
(hydrophobic) materials such that they become wettable.
This preparation of a wettable surface can also be used to
influence the flow rate in the capillary chamber (18) so
formed.
Suitable materials for preparlngia wettable layer
for various embodiments of the invention include, for
example, cellulose acetate butyrate, or a wettable nylon
material, or a layer coated with an acrylic latex emulsion
to render the surface water-wettable. For example in one
embodiment of the invention, an agglutination reaction
device is prepared by molding a layer of cellulose acetate
butyrate (CAB), commercially available from Eastman Chem.
Prod. Inc., Kingsport, TN, to have a plurality of channels
(16) from about .010 to about 5 millimeters in depth and
from about 0.1 to about 10 millimeters in width. ~ach of
the channels (16) extends from a larger well (20) molded
into one end of the layer (12~ of CAB. Next, a piece of
transparent tape (14) sufficient to cover all the channels
(16~ molded into the CAB is applied to the surface (12) to
form the capillary chambers (18). A section of adhesive
cellophane tape can be used to provide the upper cover or
surface for the recessed surface (14) to form the capillary
chambers (18). Other non-wettable (hydrophobic) materials
can be used to form the upper surface (14) of the chambers
(18). The preferred overall dimensions of this embodiment
of the an agglutination reaction device of the in~ention is
from about 10 to about 75 mm in length, from about 5 to
about 20 mm in width and from about 0.5 to about 5.0 mm
thick. The dimensions of the channels in the wettable
surface are preferably from about 0.01 to about 5.0 mm in
depth and from about 0.1 to about 10.0 mm in width.
The very small size of the reaction devices of
SUBSTITUTE SHEE:T
W092/08973 PCT/US91/08573
.;;,~
21 21~363
the invention allows for the rapid and convenient handling
of a plurality of devices and therefore samples. A device
can then be loaded into an automated apparatus which
indexes and scans the individual channels for the assay
result and records this information for future access. The
~mall dimensions of the agglutination reaction device also
provide for efficient use of sample and reagents.
The following examples are provided to further
illustrate embodiments of the invention and should not be
construed as a limitation on the scope of the invention.
EXAMPLE 1
Laminate disposable cards were prepared by
assembling together a wettable base layer, a die cut
adhesive core layer, paper strip assemblies, and a clear
polycarbonate top assembly as shown in Figure 3. To
prepare the wettable base layer, 1 mil thick nylon film
(Capran Emblem 2500, Allied Signal, Morristown, New Jersey)
was first laminated onto a paperboard backing (Westvaco Hi
Yield PrintKote, 16 mil, New York, NY) through the use of
a two-sided adhesive layer (Fasson Fastape A, Fasson
Specialty Division, Avery, Painesville, OH). Base
subassemblies (3"X6", i.e., 3 inches X 6 inches) were cut
from this material, using care to keep the exposed nylon
surface clean. Steel rule dies were prepared to cut the
channel shapes as shown in Figure 3 from a second sheet of
two-sided adhesive (3.1 mil, Specialty Tapes, Division of
RSW Inc., Racine, WI) which has release liner on both
adhesive surfaces. One piece of release liner was removed
from the die-cut part and this adhesive layer was placed
onto the nylon surface of the base subassembly. Pieces of
filter paper (2.5Xl9 millimeter, 1 CHR, Whatman, Clifton,
New Jersey) which have a layer of one-sided adhesive
(ARCare 7S97, Adhesive Research, Glen RocX, PA) laminated
;SVBST~TUTE ~;HEET
W O 92/08973 P~r~US~1/08573
2iO~363
22
to one surface were positioned on the base/core
subassemblies with the one-sided adhesive away from the
card. Finally, a sheet of clear polycarbonate film (GE
Part 8040-112, Cadillac Plastics, Evansville, IN) was die~
cut as shown in it~m (3) of Figure 3, and laminated onto
the Base/core/paper subassembly using a mechanical
laminator set a' 50 psi and 0.2 ft/sec.
EXAMPLE 2
Laminate disposable cards were prepared using a
3--X6 piece of paperboard coated with a wettable acrylic
latex emulsion coat (Part 150HT(26-1), Daubert Coated
Products, Dixon, IL) in place of the nylon base
subassemblies described in Example 1. Die-cut core layers
were prepared using 3.1 mil two-sided adhesive (ARCare
7580, Adhesive Research, Glen Rock, PA). All other steps
in card assembly were identical to those of Example 1.
EXAMPLE 3
Fixed human erythrocytes (Duracytes TM, Abbott
Laboratories, North Chicago, IL) were coated with affinity
purified goat antibodies directed against Hepatitis s
surface antigen (HBsAg) at a ~inal concentration of 100
ug/ml (micrograms/milliliter) in the presence of 0.05%
(weight/volume) chromic chloride in 0.1 M (Molar) acetate
buffer at a pH of 4Ø These cells were overcoated with 1%
(weight to volume; w/v) human serum albumin (Sigma Chemical
Co., St. Louis, MO) in 25 mM (millimolar) Tris-HCl (pH =
7.4) buffer and then resuspended with 0.1% bovine serum
albumin (BSA)(Sigma Chemical Co., St. Louis, MO) in
phosphate buffered saline (pH=7.4) containing 5%
(volume/volume) normal goat serum at a final cell
concentration of 10% (volume/volume). Serum samples (20
SLJ~S7-lTllTE 511E~:ET
W ~ 92/08973 PC~r/US9~/08573
- 2 1 ~ 3
23
ul; i.e., 20 microliter) containing either 0, 6.25, or 25
ng/ml (nanograms/milliliter) of HBsAg were mixed with 10 ul
(microliter) aliquots of these coated Duracytes and the
solution was immediately added to the sample addition well
of laminate disposable cards prepared as described in
Example 1. The solutions flowed rapidly through the
capillary channel (1-2 seconds; sec) and then slowly flowed
into the paper strips. It took approximately 7 minutes for
the liquid to completely saturate the paper strip. After
the paper strips had completely wetted, agglutinated
reaction products of the Duracyte cells could be observed
within certain of the capillary channels of ~he laminate
disposable cards. As seen in Table 1, Duracytes which had
been mixed with samples containing HBsAg agsregated,
whereas the duracytes which were mixed with sera which did
not contain HBsAg, did not aggregate.
TABLE 1
HBsAq Concentration Aqqreqation
6.25 ng/ml +/-
25 ng/ml +
EXAMPLE 4
Laminate disposable cards were prepared as
described in Example 2 with a flared channel design as
shown in Figure 4. Duracytes coated with anti-HBsAg
(Example 3) were mixed with sera containing various
concentrations of HBsAg and were introduced into the
laminate disposable cards having flared channels. After 5
SUBS~lTllTE S~tEtT
-
,,, . ., . I . , .
--,
W O 92/08973 P~r/VS91/08573
21003~3
24
minutes, aggregated particles appeared and formed into an
easily visible band of agglutinates which stretched across
the flared portion of the channel as shown in Figure 8. In
channels where there was not any HBsAg present, the
Duracytes did not aggregate and no band of cells was
visible.
SUE3STITUTE SHEET
.