Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
210~9~5
The present invention relates to the field of equipment and prothesis as a
circulatory
device aid for assisting or replacing the right or the left ventricle. More
particularly, this
invention is concerned with an electrohydraulic ventricular assist device
where the major
pumping and controlling functions are integrated into a package implantable
into the human
thorax.
Cardiac transplantation using a natural heart taken from a donor and grafting
it into a
recipient is now a relatively routine surgical technique. Recent advances have
resulted in an
appreciable reduction of rejection. However, practical transplantation is
limited to the
availability of natural heart donors and effective immunosuppressive drugs.
Clinical experience has shown that the cardiovascular circulation of patients
in severe or
total heart failure can be sustained with proper right and left ventricular
assist devices (RVAD
and LVAD).
For these and other reasons, a number of mechanical circulatory devices have
been
designed to replace and/or assist the diseased natural heart. Total artificial
hearts (TAIL and
ventricular assist devices (VAD) have been valuable clinical tools in recent
years with their
primary benefit as a bridge to transplantation following acute cardiac
failure.
For serious cases of heart failure, one should aim for long farm support
rather than
support limited in weeks, days or hours. In addition, it is preferable to have
an assisting device
which can be used for both left and/or right ventricular support. Typically,
devices for assisting
the left ventricle are located in the abdominal cavity or outside the chest.
Traditional artificial
hearts fit less than ideally inside the chest.
Generally, the requirements for a ventricular assist device are multiple and
not easy to
satisfy. It is desirable that such device be implanted in the thoracic cavity.
The intrathoracic
blood pumping components of the TCP must be similar in size and weight to the
natural heart.
The artificial heart life must be sufficiently long and the reliability
sufficiently high to avoid the
risk of sudden prosthesis failure. The formation of adherent thrombus must be
prevented.
Thromboemboli and extensive blood damage must also be prevented. The device
must not
-1-
210935
,,,...w.
damage adjacent tissue or traumatize adjacent organs by compression or by
excessive local
temperatures. The artificial hearts must also avoid skin penetration by
connections to the
exterior to prevent infections. As well, shortening of the length of the
artificial blood vessels
or cannulae employed for connecting the device to the natural heart or
circulatory system is
another desirable requirement for the device.
There has been a great deal of development activity in the area of artificial
hearts, and
especially for devices to assist the left ventricle (LVADs). Generally, a
ventricular assist device
comprises an elastomeric blood chamber or diaphragm capped cavity. The blood
chamber is
provided with an inflow and an outflow valve for cannulation to the
circulatory system.
Generally an artificial heart device operates as follows, the left pump
receives blood from the
pulmonary vein and impels blood into the main circulatory system via the
aorta. The right pump
receives blood from the inferior and superior versa cava and impels it into
the pulmonary artery.
The blood chamber volume is controlled with the elastomeric diaphragm which is
actuated to
oscillate between a systolic and a diastolic position.
To transform electrical triggering signals generated outside the body into the
rhythmic
movement of the diaphragm while obtaining suitable values for the above
parameters and
hemodynamics, various types of energy convertors have been tested.
The displacement of the diaphragm is obtained by various methods. Among these,
electrohydraulic operation of the diaphragm proves to be a dependable and
reliable method.
Electmhydraulic ventricular assist devices are provided with a pump which
displaces a hydraulic
fluid (oil) between a fluid reservoir and a fluid chamber, which is adjacent
with the blood
chamber so as to share the elastomeric diaphragm. The rhythmic fill and drain
of the oil, in and
out of the fluid chamber, displaces the diaphragm which in turn moves the
blood in and out the
blood chamber.
Canadian Patent No. 1,188,852 (Robinson) discloses a hydraulically actuated
cardiac
prosthesis with a hydraulic fluid reservoir, a hydraulic fluid pumping means
and a blood
pumping chamber with a flexible diaphragm. United States Patient No. 4,222,127
(Donachy et
al.) discloses the Fierce-Donachy artificial heart including a blood pump,
flexible diaphragm and
an inflow and an outflow valves which can be used paracorporeally or
intrathoracically. United
States Patents Nos. 4,588,404 (Lapeyre) and 5,089,018 (Lapeyre et al.)
disclose a biventricular
-2-
210593
cardiac prothesis. The device is a sealed case with a dual membrane system for
pumping the
systemic and pulmonary circulations.
However, a major limitation of these past devices is their physical shape and
complexity
which increase the surgical difficulty of implanting the device in either the
thorax or abdomen
of a patient. Power and information transfer requirements of the past devices
have also required
percutaneous access to the implantation site with its associated risk of
infection. These
limitations have also had an effect on the length of time that these devices
can be implanted.
Recently, devices for establishing communication of electrical signals between
the
implanted device and an external power source and electronics controller have
been developed
as disclosed in Canadian Patent application No. 2,007,439 {2,074,150} (Miller)
and U.K Patent
application #9009713.0 (Miller). Such transcutaneous energy transformers
employ
electromagnetic induction using a pair of coupled coils, one outside and one
inside the patient
body. The ventricular assist device then can have an internal control
mechanism for adjusting
the frequency of oscillation of the diaphragm. To maintain maximum power
transfer as the coils
move relative to one another, a phase locked loop system in the external power
converter
maintains a constant phase relationship between voltage and current in the
primary coil, thus
minimizing voltage fluctuations. This was disclosed by Mussivand et al.
(Performance
evaluation of a transcutaneous energy transfer system, ASAIO Abstr 21:39,
1992).
The bidirectional communication of information between the implanted device
and its
portable external control unit is achieved by an infrared data link. Data is
transmitted across
the skin without perforating it using a standard synchronous data transmission
protocol. This
protocol awards hardware compatibility with any computes having an RS232 type
interface. It
was disclosed by Miller J. et al. (Performance Evaluation of a transcutaneous
infrared telemetry
system, ASAIO Abstr 21:39, 1992). ,
An ideal artificial heart device should integrate the major pumping and
controlling
components into a single unified system for reducing the length of the
electrical connections and
of fluid conduits. There are also requirements for a material that should be
accepted by the
human body. Fluid dynamics of the device should not cause thrombus formations
and the
potential for thromboemboli.
Anatomical fit has a significant impact on the ease of surgical implantation,
organ
-3-
210593
compression, patient comfort and postoperative complications. The internal
body cavity
dimensions have been recognized as a prime limitation in the design of
implantable, mechanical
circulatory devices.
The present invention is based on the concept proposed by Dr. T. Mussivand in
1989
which forms the base for a copending patent application entitled "Integrated
System" .
It is an object of the present invention to provide a totally implantable
mechanical
circulatory device which integrates the major components of the system into a
single unified
system which is small enough to implant in the patient's chest cavity without
giving rise to any
serious clinical disadvantage or inconvenience to the recipient's organs. The
device of the
present invention takes into account the best location for implantation in the
patient and the best
shape which is compatible with this location.
It is another object of the present invention to provide a ventricular assist
device which
can be fixed into the human chest in the proximity of the natural heart
thereby shortening the
length of the artificial blood vessels or cannulae employed for connecting the
device to the
natural heart. This in turn reduces the surgical complexity associated with
implanting other
cardiac protheses.
It is another object of the present invention to provide an electrohydraulic
ventricular
assist device with a reduced fluid conduit length, which further allows for
the hydraulic fluid to
be used not only to actuate the device and act as a bearing lubricant, but
also to remove heat
from the device and to disperse it via the lungs and the circulatory system.
It is still another object of the present invention to provide an
edectrohydraulic ventricular
assist device having a design which allows for shorter electrical connections
due to the
integration of the major components.
It is a further object of the present invention to provide a totally
implantable mechanical
circulatory device that may be used to assist or replace both the left and/or
the right heart, with
a relatively simple surgical procedure.
It is a further object of the present invention to provide an electrohydraulic
ventricular
assist device with a portion of the control electronics integrated into the
back of the unified
system and the remainder of the control electronics ~~ p 9 ~d~ on a small
portable unit to
be worn on a belt thus eliminating the requirement for tethering the patient
to a large bulky
external console to drive the device.
In accordance with the present invention, there is provided an unified system
for an
electrohydraulic ventricular assist device adapted for implantation in the
thorax and for
cannulation to the blood circulatory system comprising: an internal electronic
controller for
receiving both an AC and DC supply voltages, an external communication channel
data stream
and generating an actuating signal, communication channel data stream and
internal battery
recharging signals; an actuating means for converting said actuating signal
into a back and forth
rhythmic displacement of a fluid; a blood pumping chamber having an inflow
blood port and an
outflow blood port for converting said back and forth displacement of said
fluid into a rhythmic
unidirectional displacement of blood through said inflow and outflow ports; a
volume
displacement chamber acting as a reservoir for said back and forth fluid
displacement; a hermetic
coupling means for connecting said controller by conductors carrying said
AC/DC supply
voltages, said communication channel data streams and internal battery
recharging signal; a
detecting means for generating said actuating signal in response to the status
of said blood
pumping chamber; and a support with a surface curvature compatible with the
internal human
sagittal and transverse chest wall curvatures for supporting said electronic
controller, said
actuating means, said blood pumping chamber, said hermetic coupling means and
said detecting
means in a compact structure with said blood pumping chamber arranged with
said inflow and
outflow ports (and one way valves) oriented away from said support and said
structure with an
overall size that when the unified system is placed within the human thorax
with said support
surface adjacent the chest wall, said structure does not adversely compress
adjacent organs.
According to another aspect of the present invention, there is provided an
unified system
wherein said support is a base cover having a longitudinal radius of curvature
of (11 t 0.5 cm)
and a transversal radius of curvature of (9.4 t 0.5 cm).
According to still another aspect of the present invention, there is provided
an unified
system further comprising a first cannula connected to said inflow port for
carmulation with the
blood circulatory system. Said inflow port is oriented for cannulation to the
systemic circulation
when the unified system is implanted in the thorax for assisting or replacing
a left ventricle.
-5-
210935
,,,....
The length of said first cannula is between 5 and 14 cm and can be adjusted to
fit the patient.
A second cannula connected to said outflow port is oriented for cannulation to
the systemic
circulation, when the unified system is implanted in the thorax for assisting
or replacing a left
ventricle. Said inflow port is oriented for cannulation with the pulmonary
circulation when said
unified system is implanted for assisting or replacing a right ventricle. Said
outflow port is
oriented for cannulation with the pulmonary circulation when said unified
system is implanted
for assisting or replacing a right ventricle.
According to still another aspect of the preset invention, there is provided a
unified
system having an overall thickness of less than 4 cm, an overall length less
than 18 cm and an
overall width less than 12 cm.
The device according to another aspect of the present invention is adapted for
cannulation
to the blood circulatory system and comprises: of said unified system for
implantation in a
human thorax proximal to the human heart to replace or assist a ventricle; a
rechargeable
internal battery for subcutaneous implantation to supply said unified system
with an internal DC
supply voltage; an external battery for providing a DC voltage to said
external controller; an
external controller for converting DC voltage received from the external
battery and/or external
power supply to AC voltage for transfer by a transcutaneous energy transformer
to the said
unified system, for recharging the external battery; a computer interface for
connecting said
device to a computer for control and monitoring of the device; a display means
for control status
and alarm display; a transcutaneous energy transformer for transmitting said
AC voltage across
the skin to said unified system; a transcutaneous information telemetry system
for bidirectional
transmitting said communication channel data streams between said external
controller and said
unified system; a connector for connecting said internal battery to said
unified system and an in-
line connector for connecting said transcutaneous energy transformer to said
unified system.
Brief Description of the Drawines
These and other features of the invention will become more apparent from the
following
description in which reference is made to the appended drawings, wherein:
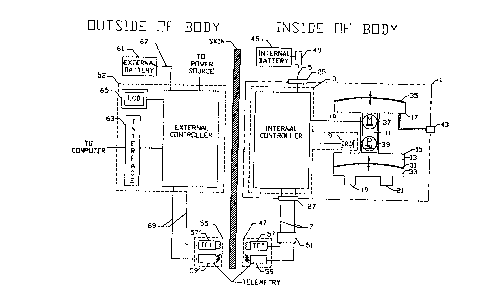
Figure 1 illustrates a block diagram of the electrohydraulic ventricular
assist device of
the present invention;
-6-
210935
Figure 2 illustrates a schematic front view (from the heart) of the unified
system; and
Figure 3 shows a schematic back view of the unified system (from the chest
cavity), back
cover removed.
Figure 4 illustrates a back view of the back cover;
Figure 5 shows transversal cross-section view along lines A-A of Figure 2.
Figure 6 shows longitudinal cross section view along lines B-B of Figure 2.
Referring to the drawings, a preferred embodiment of an electrohydraulic
ventricular
assist device in accordance with the present invention is described.
Figures 1 - 6 show the preferred embodiment of the device of the present
invention.
A unified system 1 incorporates the major units of an electmhydraulic
ventricular assist
device and is sized for convenient implantation into the thorax and for facile
cannulation to the
blood circulatory system. The unified system 1 comprises an internal
electronic controller 3
which receives a DC supply voltage on conductor set 5, an AC supply voltage
and
communication channel data stream on conductor set 7 and signals from
detecting means 9. The
controller 3 processes these signals and accordingly generates an actuating
signal on conductor
10. Actuating means il converts the actuating signal into a back and forth
rhythmic
displacement of a fluid, causing a rhythmic fill and drain of a fluid
compartment 13 of a blood
pumping chamber 15 with a fluid. A volume displacement chamber 17 acts as a
reservoir for
the fluid as it is displaced into and out of the fluid compartment 13. The
blood pumping
chamber 15 has an inflow blood port 19 and an outflow blood port 21. The blood
pumping
chamber converts the back and forth displacement of the fluid into a rhythmic
unidirectional
displacement of blood through the inflow and outflow ports. The ports are
equipped with
unidirectional valves (not shown) and cannulation means (not shown) for
connection to the
circulatory system. Hermetic coupling means 23 houses electrical feedthmughs
25, 27 which
connect to the conductors sets carrying the DC supply voltage, AC supply
voltage and
communication channel data streams to the internal electronic controller 3 and
units outside the
unified system 1.
210~93~
A support 29 with a surface curvature compatible with the internal human
sagittal and
transverse chest wall curvatures supports the internal electronic controller
3, the actuating means
11, the blood pumping chamber 15, the volume displacement chamber 17 and the
hermetic
coupling means 23 in a compact structure. The entire structure has an overall
size and shape
such that when the unified system 1 is placed within the human thorax with the
support 29
adjacent the chest wall, the unified system does not adversely compress
adjacent organs. The
blood pumping chamber is awanged with the inflow and outflow ports 19 and 21
oriented away
from the support surface such that the cannulae (not shown) are oriented
towards their respective
destinations of the blood circulatory system.
The shape of the blood pumping chamber 15 is flat and low ellipsoidal, as a
sac defining
a back face and a front face, the back face being placed in contact with the
feedthrough cover,
and the inflow and outflow ports protruding through the front face.
In the preferred embodiment represented in Figures 5 and 6, the blood pumping
chamber
has an elastomeric membrane 31 which converts the back and forth displacement
of the fluid,
15 driven by the actuating means 11, into the rhythmic displacement of the
blood. Membrane 31
divides the sac longitudinally in a plane normal to the axis of the blood
pumping chamber 15 so
that the height of the membrane oscillation is low. Membrane 31 defines a
blood compartment
33 and a fluid compartment 13. The blood compartment 33 is comprised between
the membrane
31 and the front face of blood pumping chamber 15. The fluid compartment 13 is
comprised
between membrane 31 and the back face of blood pumping chamber 15. Membrane 31
oscillates
between a systolic position, where blood is displaced from the blood
compartment and a diastolic
position, where the blood compartment is filled with blood. The shape of the
blood pumping
chamber allows the membrane to travel a short distance between the systolic
and diastolic
positions, this short distance improves the mechanical properties of the
membrane, thus
increasing long term reliability. The systolic and diastolic positions of the
membrane 31 and
VDC membrane 35 are shown in Figure 6.
The actuating means 11 converts the actuating signal received from the
internal electronic
controller 3 into a back and forth rhythmic displacement of the fluid. It
comprises a brushless
DC motor 37 and a reversible axial pump 39. Whenever motor 37 receives the
actuating signal
_g_
2105935
it reverses its rotation and accordingly the direction of fluid displacement
by pump 39,
converting this alternating rotational movement of the motor into a back and
forth displacement
of the fluid. The motor 37 and pump 39 form a so called energy convertor 11.
The energy
convertor 11 transfers fluid from the volume displacement chamber through the
energy convertor
11 and through oil conduit 41 to the fluid compartment 13. As a result of this
alternating fluid
dislodgement the membrane 31 and VDC membrane 35 oscillate alternately between
the systolic
(blood being ejected from the device) and diastolic (blood entering the
device) positions. The
brushless motor 37 has bearings which have an axial preload to decrease ball
skidding and
thereby improve bearing life. The motor stator is a toothless design and the
permanent magnet
material is made of neodium iron boron which creates high magnetic field
density.
The energy convertor 11 is arranged in the hydraulic fluid such that the
hydraulic fluid
is both a lubricant for the bearings in the energy convertor il and a heat
sink. In the
embodiment shown in Figures 1 to 6 the energy converter 11 is preferably
placed towards the
lower side of the unified system, beside the blood chamber 15 to reduce the
overall length of
the fluid path. The oil conduit 41 provides a fluid pathway from one end of
the energy
convertor 11 to the fluid compartment 13 of the blood chamber 15. The other
end of the energy
convertor opens directly into the volume displacement chamber 17.
The internal electronic controller 3 comprises a microprocessor which gives
the actuating
signal for reversing the direction of rotation of motor 37. Detecting means 9
detects the position
of the flexible membrane 31 and generates a signal to the microprocessor to
indicate when
membrane 31 has arrived at the end systolic or end diastolic positions.
Preferably, an infrared
sensor is used as detector 9, which is provided directly on the internal
controller and adjacent
to the blood pumping chamber 15.
The unified system 1 is connected to the pulmonary circulation (when it is
designed to
assist the right ventricle) or to the systemic circulation (when it is
designed to assist the left
ventricle). The connection is effected through two unidirectional valves (not
shown) arranged
between the blood chamber inflow and outflow ports 19 and 21, and the
respective cannulation.
The outputs from the cannulation are connected to the corresponding blood
vessels.
It is important that the dimensions of the human chest cavity be known for
designing the
2105935
shape and size of the unified system. The :pacx available for intratlwracic
pla~oanont of the
device is very limited. The implantation utc for the unified system of the
prma><t invention is
in the letl and/or right hemithoiax anchored to the chest wall bexweat< the
4th and 9th ribs.
Advents;es of this placemadt include limited device nation whey fixed to the
ribs, cosmetic
acc~ability, shorter cannulae lemgtha and thec~fore less hydraulic loses, less
potential for
kinldng of the c~nnulae, no need to pate~atc the diaphragm a~ decreawd risk of
possible
pressure necrosis of abdominal organs.
Aa shov~m m Figures 4, 5 sad 6, a back cave forms the support ?9 whereon the
implantable utrified system is fixed. The size and shape of the back cover 29
are selected to
snugly fit the geography of the ribs against which the dtvioe is arranged when
implanted.
A prime consideration in the back cover deaiga is the chest wall curvah><re.
Any pocloets
formed bexwear< the device sad chest wall would create dead spaces and a
potentially i>nc~sod
risk of infection. The transverse r~lius of curvature of the intrathoracic
wall at the 5th rib was
de~nined to be 9. 4 t 0.5 cm (n =19) as disclosed by Mussivand T. et al.
(Critical Anatomic
Dimensions for Intrathoracic Circulatory Assist Devices, Journal of Artificial
Organs, June
1992, Volume 16 ~4).
Another critical measuremoat in the design of the unified rystem is the
sagittal radius of
curvature at the Sth rib. This was mearu~ed to be 11.1 t 0.5. Knowledge of
this dimension
is oecxssary for the des;go of the longibidinal curvature of the device.
Back coves x9 has a l~gid>Idinal and transversal curvat<ue compleascatary to
the
sagittal and tran:varsc internal curvatures.
A feodthrough cover 23 is placed on top of internal electronic oontrolla 3. As
shown
is Figure 3, two hoc e~le~ic~1 feedthrough comectioas 2S and Z7 are provided
for
electrical conductor sets s and 7. An oil filling port 43 is also mounted on
fexdthrough cover
23 and is usod for filling the unifiod system 1 with the actuating fluid, the
port uses a push-pull
mechanism to opal and close the filling port. Once the system is filled the
port is closed and
a hermetic seal is provided by a compression O-ring.
The unified system of the presa>tt invention has an overall thickness of leis
than 4 cm,
an overall la~gth less than 16 cm and as overall e~tanal width less than 11
cm. It gives a
s~cnoN s cc~t~ECTtow
8EE CERTtRCATE
ECi101~ - ARTK;LE ~t -lO-
~OtR CEflTiFO~".~I1'~
,~. 210593
cardiac output of greater than 6 litreslminute with a mean preload pressure of
between 5 to 10
mmHg and a mean afterload pressure of 150 mmHg. With the unified system of
this invention,
the stroke volume of the blood pumping chamber is between 55 and 70 ml.
As shown in Figures 2, 5 and 6, the blood pumping chamber 15 and the volume
displacement chamber 17 are placed side by side. These chambers define a space
at the back
thereof to accommodate the internal electronic controller 3 which is placed
directly beside the
energy convertor 11 and connected thereto. This placement of the energy
convertor 11 close
to controller 3 allows short electrical connections, reduces electrical losses
and increases the
reliability and efficiency of the unified system.
The implantable components of the electmhydraulic ventricular assist device of
the
present invention consist of the unified system 1, inflow and outflow cannulae
(not shown), an
internal battery 45 and an internal TET/Telemetry coil 47.
The internal battery is connected to the unified system 1 via hermetic
connector 49,
electrical conductor set 5 and electrical feedthrough 25. The internal battery
45 is preferably
implanted subcutaneously in the abdomen. In the preferred embodiment the
internal battery
package 45 uses rectangular prismatic nickel/cadmium cells, however other
battery chemistries
could be utilized. These cells are housed in a custom designed laser welded
titanium enclosure
with a hermetic connector 49 suitable for implantation. Preferably, the
hermetic connector 49
is an internal connector, used for allowing an easy replacement of battery 45.
In this way, the
part of conductor set 5 from connector 49 through feedthrough 25 to unified
system 1, should
not be changed when internal battery 45 is replaced periodically. The
electrical conductor set
5 contains electrical connections for the supply of DC voltage to the internal
electronic controller
3, for the recharging of the internal battery and for activation of an audible
warning alarm
housed in the battery enclosure.
The internal TET/Telemetry coil 47 is connected to the unified system 1 via
the in-line
hermetic connector 51, electrical conductor set 7 and the electrical
feedthrough 27. The internal
TET/Telemetry coil 47 is implanted subclavicularly. The transcutaneous energy
transformer
('TET) and transcutaneous information telemetry (Telemetry) systems consist of
electronic
circuitry on both the internal electronic controller 3 and external electronic
controller 53, the
-11-
r.. 2105935
internal TET/Telemetry coil 47 and the external TET/Telemetry coil 55.
The TET uses wire coils 57,57 to electromagnetically couple power into the
body
without perforation of the skin. The Telemetry uses infrared components 59,59'
embedded in
the internal TET/Telemetry coil 47 and external TET/Telemetry coil 55 to
transfer the
communication channel data streams into and out of the body without
perforation of the skin.
The external components of the electrohydraulic ventricular assist device of
the present
invention consist of the external electronic controller 53, an external
battery 61 and an external
TET/Telemetry coil 55.
The external electronic controller 53 contains a portion of the circuitry for
both the TET
and Telemetry systems. External electronic controller 53 also produces and
receives the
communication channel data stream for control and monitoring of the unified
system 1 and
generates recharging signals for recharging the external battery 61 at
predetermined intervals.
External electronic controller 53 is connected to the external TET/Telemetry
coil 55.
Additionally, the external electronic controller of the device in the present
invention
further comprises a computer interface for connecting the device with a
computer for control and
monitoring of the device and a display means 65 which consists of a liquid
crystal display (I,CD)
screen for display of control status and alarms. The external electronics is a
compact unit
intended to be worn on a belt similar to a pager.
The external battery 61 is connected to the external electronic controller 53
via electrical
conductor set 67. In the preferred embodiment the external battery uses
silver/zinc cells,
however other battery chemistries could be utilized. The electrical conductor
set 67 contains
electrical connections for the supply of DC voltage to the external electronic
controller 53 and
for the recharging of the external battery 61.
The external TET/Telemetry coil 55 is connected to the external electronic
controller 53
via electrical conductor set 69.
The electrohydraulic ventricular assist device of the present invention
comprises the
unified system for implantation in a human thorax proximal to the human heart
as described
above.
The operation of the device is now described with reference to Figure 1.
-12-
.. 210593
The system is actuated by the hydraulic fluid that is pumped between the
volume
displacement chamber 17 and the fluid compartment 13 of blood pumping chamber
15, by the
reversing axial flow pump 39 driven by the brushless DC motor. Whenever the
motor 37
receives an actuating signal from the internal electronic controller 3, it
reverses its direction of
rotation which causes a reversal in the flow direction of the actuating fluid.
Actuating fluid is pumped into the fluid compartment 13 of the blood chamber
15 and
the membrane 31 displaces the blood during the systole phase of the cardiac
cycle thmugh the
outflow port 21. When the axial flow pump 39 is reversed, the hydraulic fluid
is pumped away
from the fluid compartment 13 of the volume displacement chamber 17, causing
outward
displacement of the VDC membrane 35, the membrane 31 is pulled away from the
blood
compartment 33 causing active filling of blood to occur thmugh the inflow port
19. This action
is further illustrated in Figures 5 and 6. Reversal points of the axial pump
37 are determined
by the membrane position detector 9 which sends the status signal to the
external electronic
controller 3 for reversing the axial pump flow when the membrane 31 reaches
the end points of
systole and diastole.
In vitro
Testing was conducted in vitro to verify that the system operated satisfactory
prior to any
in vivo experiments. Tests have been conducted on mock circulation with the
device in air and
completely submerged in saline solution to simulate expected chest pressures.
In vitro flow rates
of over 8 litres per minute with a preload of 10 and a mean afterload of 100
mmhg have been
obtained. The transcutaneous energy transformer has demonstrated a power
transfer efficiency
of over 809b at power levels of 10-35 Watts. The internal and the external
batteries have been
cycled tested to determine optimum charge and discharge requirements as well
as to determine
operating times and cycle lives.
In vivo
The complete unified system has been implanted in 6 bovine for in vivo testing
and has
-13-
~.... 210 ~ 9 3 5
maintained circulation from 3 to 27 hours. The performance of the complete
unified system has
proven satisfactory in acute experimentation. 1fie overall configuration of
the device has also
proven to be satisfactory, over a wide range of operating conditions.
-14-