Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 92/17775 ~ fi 6~ ~ Pcr/EP92/oo769
ELEC~RODE CALIBRATION
This invention relates to electro-analytical
sensing instruments having sensor probe electrodes,
and is particularly concerned with the calibration and
use of the electrodes.
When measuring an active electrochemical species
potentiometrically, that is by measurement of the
voltage between electrodes in an elec$rolyte (the
analyte), it is first essential to calibrate the
instrument in standard solutions or buffers of known
activity to allow the slope and offset voltage to be
determined and used in subsequent measurements. These
values are either held by analogue potentiometers in
the measurement instrument circuits, or stored in
memory, if the instrument is digital. Once calibrated,
the measuring electrodes - either a separable
indicator electrode and reference electrode, or a
combined pair - cannot be disconnected from that
instrument and connected to another, perhaps in a
different area, without re-calibration.
. . .
.. ~
,' ,~ ,
,
WO 92/1777~ 2 1~ 6 ~ & ~ PCT/EP92/00769
- 2 -
The present invention is concerned therefore with
the proble~ of allowing the calibration and measurement
of such sensors to be carried out at one site or in one
instrument and used in another instrument or at another
site. -.
The invention is further concerned with the problem -~
of providing a device which can b2 used for any typ2 : . .
of sensor which requires r~-c21ibration.
' ' ' ' ' '''
It is known that the volLage m~asur~d ~h~n usins
a potentiometric electrochemical sensor is mainly .-~
dependant on the analyte, and is given by the . .
following Nernst equation: - ;
::.
E(measured) E(indicator electrode half cell) Eref
where
E(~n~c~ )electrode = E(stan~a~ ) species)
and
Eref E(reference half cell) E(junction potentials)
Aspeciesis the activity of species A and is approximately
equal to the concentration of A in dilute solutions, ,~
~';
wo 92/1777~ PCT/EP92/0~769
- 3 ~ J~ ~
In normal circumstances the reference half cell
and junction potentials are not predictable to more
than a few millivolts and may vary considerably
hetween different reference electrodes. If it iis i -
S necessary to obtain a direct instrument read out in
either concentration or In(concentration) it is
necessary to first convert from the millivolts
measured by calibrating the system on a number of
standard solutions. The difference between
theoretical millivolts and those measured is the
offset voltage of that particular system. It is
stored in the measuring instrument for later use,
either by using a potentiometer to offset the
millivolts measured back to the theoretical reading or
digitally by using a micro-processor and memory.
In a perfect system the millivolts measured are
proportional to the logarithm of the activity of the
analyte. From the Nernst equation this can be seen to
be:
(RT/nF)- In(Aspecies)
or
2.303 . (RTlnF) log(Aspecies)
At a temperature of 25C this is 59.13mV per r
decade increase in concentration.
- . . ,
2 1 ~
- 4 -
In practice this theoretical factor is rarely
obkained and an empiral factor calculated from the
calibration data obtained ~y ~easurin~ standard
solutions is used instead. This factor is obtained -
and stored for later use in an analogue instrument by
setting the gain of the input circuits when meas~ring
the standard solutions. In a micro-processor
instrument this is stored directly as a factor in
memory. This factor is defined as the slope of the ~ ;
system and may be presented as a percentage.
As stated above when measuring an active
electrochemical species potentiometrically, it is
first essential to calibrate in standard solutions or
buffers of known activity to allow the slope and -
offset voltage to be determined and used in subsequent
measurements. `
EP-A-0,074,498 discloses a system in which a
calibration unit includes a memory which includes
calibration data, however calibration involves
processing at a remote site and therefore there is a
risk of inaccuracies arising.
. '' ''
.' '
L~.aÇ~6~
-- 5 --
According to the invention the calibration data
is stored in a calibration unit in close physical
assoc ation with the electrode or electrcde pair
assembly rather than in the broader confines of the
5 instrument itself and the unit includes it Gwn ~ -.
microprocessor where calibration can take place in
situ. This means that the electrode assembly and
calibration unit can ~e disconnected from the
instrument and taken to a remote site. A new
instrument can then read and process this information
and be ready to measure without the need for
re-calibration and without the need to feed the data
to a remote site along circuitry of unkncwn
parameters. . .
Accordingly the invention provides a
measurinq instrument for connection in a sensing
instrument which measuring instrument has measuring
sensors which require calibration, and located in
close association with said sensors a calibration unit
including a memory means which is arranged to store
calibration data to enable the assembly to be used
with more than one sensing instrument, characterised
in that the calibration unit further comprises a
microprocessor which enables the sensors to be
calibrated in situ.
~la~
- 5a- .
This approach means that one indicator and
reference electrode pair can be calibrated in a ~. ~
laboratory or clean area and can then be taken into a ~ .
factory environment, where buffer or standard solution -~
S might be contaminated, for solution measurements.
~ '
Preferably the assembly in accordance with the :
invention comprises a small memory and communications ~. -
integrated circuit sealed into either electrode or `
probe or holder assembly together with a micro-
processor instrument. The electrode pair can be -
calibrated in the normal process with buffers or
standard solutions and the calibration data such as
the slope and the offset voltage, can then be stored
',.-.,: .
""''
~,q~ ET ::
WO 92/1777~ ~ 1 0 fi ~ 6 i~ PCT/EP92/00769
- 6
in the memory means in the electrode pair via a serial
communications link. This information may then be
stored in non-volatile memory, for example in battery-
backed RAM, EPROM or EEPROM for future use. The
electrodes may then be disconnected, taken into a new -
area and connected to a different instrument. The new
lnstrument will then retrieve this calibration data
and be ready to carry out measurements.
Other information regarding time of calibration,
safe time available before re-calibration is
necessary, identification numbers and electrode typ2
can also be stored.
The memory means is conveniently incorporated in
the lead assembly of an existing electrode system. ;
The memory means may also include line drivers or
impedance converters if the electrodes are of high
impedance or the measuring instrument is likely to be
located at a large distance from the electrodes.
The electrode assembly according to the invention
may also be made intrinsically safe for areas where
inflammable solvents are used. Calibration can then
- take place in a normal area where water baths and
~ .
.
WO 92/1777~ PCT/EP92/0~769
_ 7 _ 21~
associated equipment can be safely used. Connection ~ :
of the assembly to the measurement instrument is then
preferably made via a shunt-diode safety barrier, ~ :
S Several embodLments of the invention will now be
described by way of example with reference to the -::.
accompanying drawings in which:
Figure 1 is a schematic diagram of an indicator
electrode with the calibration unit installed;
Figure 2 is a schematic diagram of an electrode .:
combination pair with the ca~ibration unit installed;
` .: - .
Figure 3 is a schematic diagram of a sensor probe
with the calibration unit installed in a corner unit -
separate from the sensors;
:
Fi~ure 4 shows a schematic diagram of an electxode :::
system with the calibration unit installed in a lead; .; .
Fiqure S is a block circuit diaqram of the ~.s:~-
calibration unit; and
:: '~.:. `'
-, .;
WO 92/1777~ PCT/EP92/00769
6~ 8 -
Figures 6a to 6e show various known electrodes
which can incorporate the invention.
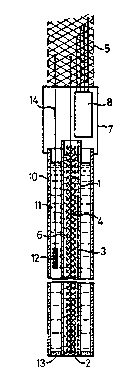
Figure 1 shows an indicator electrode
incorporating a calibration unit. A cylindrical glass
container 1 carries at its lower end an electrode
membrane 2 and encloses an electrolyte 3. The membrane
2 is connected by an electrically conducting lead 4 to
a screened multi strand output lead 5. The lead 4 is
screened by a metallic screening sheath 6. This is
shown schematically but in practice can be located
between inner and outer glass concentric walls rather
than within the electrolyte 3.
The glass container 1 is carried by a carrier 7
at the top of the electrode assembly which as well as
supporting the container 1 encloses wiring which feeds
out into the multicore cable 5. The carrier 7 also
encloses a calibration unit 8 whose output leads are
connected into the multi strand cable 5.
The electrode is used in conjunction with a reference
electrode, which then forms a part of the calibrated
pair. The significant difference in the reference
electrode is that instead of an electrode membrane 2
across which a voltage will be developed, a porous
wo 92/~777~ PCT/EP92/00769
- - ?~ ~ ~ 6 ~
wall i9 employed at its lower end so that the
reference electrode is retained at a potential derived
from the solution within the electrode and is
therefore constant. While the calibration data is -
retained in the unit associated with the indicator
electrode, the calibration also takes account of
variations within the reference electrode. Therefore
a particular reference electrode always has to be
identlfied and used together with the same calibrated
indicator electrode.
Figure 2 shows a combined electrode consis~ing
essentially of a measuring electrode of the kind sho~n
in Figure 1 combined with a reference electrode
surrounding it.
The same reference numbers are used for the same
component features as in Figure 1 example and these
components are essentially the same. However
surrounding the measuring electrode is an enclosing
glass jacket lO thus defining within an annular
compartment a reservoir for a reference solution 11.
Within the reference solution ll is a reference
element 12, and the lower face of the circular jacket
10 is closed by a porous annular plug 13. Thus, the
~,
~ ' .;
, ~, .
WO 92/l777~ PCT/EP92/00769
6;.) - lo-
reference element can be maintained at a constant
potential determined by the reference solution.
This contrasts with the measuring electrode where a
glass membrane 2 allows a potential to develop across
that membrane in order to sense the difference in
value between the liquids on either side of the
sensing element 2 (for measurement of Ph etc). The
reference element 12 is then connected via a
conducting line 14 into the multi strand output cable
5.
Figure 3 shows a further arrangement. In this
arrangement, a measuring electrode 20 and a reference
electrode 21 (substantially of the form shown in
Figure 1) are carried out in a connecting carrier 22 ;
which connects to the main carrier 7. The two
electrodes 20 and 21 and an earthing terminal 22 are
connected by conductors 23 via plugs and sockets 24
to an output amplifier 25 and thence to the multi
s~rand cable 5 for feeding to the instrument (nat
shown).
Figure 4 shows a further form of the invention
where a combined electrode 31 is fed by conductors 32
via the calibration unit 8 to the instrument (not
wo 92/1777~ PCT/EP92/00769
11- 2~ o
shown), i.e the calibration unit is incorporated in
the electrode connecting lead.
In each of these described examples, the
calibration unit 8 is always associated with one or
both electrodes whereby when the or each electrode
is unplugged from the instrument and taken to another
location, it will always have its correct calibration.
', '
Figure 5 sho~s a schematlc of a partlcular form
of calibration unit. This com~rises a pair of high
Lmpedance operational amplifiers 41 and 42 which are
fed from the sensors via input leads at the right
- hand side of the Figure and thence are fed to a pair
of output leads on the left hand side of Figure 5.
The unit also incorporates a microprocessor with
encapsulated battery and memory unit 43 fed from multi-
strand cable S via serial line drives 44 and 45.
,.
Thus in practice a pair of electrodes can be
calibrated and the calibration stored in the memory
unit 43 and the electrodes will then be available for
use when plugged into other inslruments at other
, ,
sites.
The invention can be applied to various forms of
,'' ' :'
' ." '
. ~
WO 92/l777~ PCT/EP92/00769
~ 2 -
known measuring electrode, A unit of the kind shown
in Figure 5 for example can readily be incorporated in
the output lead or in the carrier head for the ,
electrode.
Thus, Figure 6a to Figure 6e show various
electrodes to which the invention may be applied.
These electrodes are as follows ~
Fisure 6a shows a metal electrode,
Fioure 6b shows a coated metal electrode,
Fi~re 6c shows a solid s~ate ion selective
electrode,
Figure 6d shows a liquid iron exchange ion
selective electrode,
Figure 6e is a pH electrode.
In these electrodes the components are as
follows:-
In Figures 6a and 6b, 51 denotes the insulation
and 52 is the electrode proper while in Figure 6b
53 denotes a sensing coating.
WO 92/17775 PCT/EP92/00769
i~3~ ~ C ~ a~O
In regard to Figure 6c~ the solid state ion :`
selectlve electrode, 55 denotes an electrically
conductive contact for a solid membrane 54.
.' '.
In Figure 6d, the liquid ion exchange ion ~ . -.
selective electrode 57 is an internal
reference solution 58 is an internal reference .;
element and 56 is a sensing membrane across
which a volta~e can be developed.
1 0
Finally, Figure 6e den3tes a conventional pH
electrode incorporating 2 glass membrane 59 and ;.;
extending from a glass bcdy 60 which encloses an :-
internal reference element 61 within an internal
reference solution 62.
','~.''`.' ~
'',.. ,,' ' .
:
: .:
''' " "'. .
" ',
' ~ .