Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
-`` 2108~21 RD0022079
METHOD AND APPARATUS FOR
DESTROYING SODIUM- CONTAINING
WASTE PRODUCTS
This invention relates to the destruction of
waste materials, and more particularly to the combustion
of waste streams which contain organic compounds and
which are high in sodium.
A major cost element in chemical processes
carried out on a commercial scale is the disposal of non-re-
cyclable by-products and wastes. These may include or-
ganic and inorganic compounds, many of which are toxic or
otherwise hazardous. Increased attention to environmental
hazards has made it imperative to devalop improved meth-
ods for disposing of such waste products. In particular,
many of such products must be destroyed so that they do not `
pass into the environment and cause harm. ~ -~
Among the industries demanding effective waste
disposal is the plastics industry. Millions of pounds of
plastic are produced per year, and almost every production
process also creates large amounts of-by-products and
wastes. .
A single example of a plastics production pro-
cess which produces wastes is the process of making
polyimides, and particularly polyetherimides. Many steps
are involved, only a few of which need be described here to
indicate the complexity of the waste materials which must
be dealt with.
In a typical polyetherimide preparation method,
an early step is the nitration of a phthalimide with nitric ;
acid, which produces as wastes excess nitric acid and vari~
ous nitro-substituted phthalic acid derivatives. The
nitrophthalimide is slurried with water and the slurry is
contacted with an organio solvent, whereupon the nitro-
RD0022079
2~08~21
phthalimide dissolves in the organic phase and leaves be-
hind an aqueous waste containing traces of various com-
pounds, organic and inorganic.
The nitrophthalimide in the organic solution un-
5 dergoes a displacement reaction with the disodium salt of a
dihydroxyaromatic compound such as 2,2-bis(4-hydroxy- -
phenyl)propane, commonly designated bisphenol A, with the
formation of a bisimide and sodium nitrite as a by-product.
This displacement reaction ordinarily takes place in the
10 presence of a phase transfer catalyst, traces of which may
also be found in the waste stream. Numerous other by-prod-
ucts, as well as further quantities of those already men-
tioned, are removed by further extraction of the organic re-
actant solutions with aqueous sodium hydroxide. The
15 bisimide subsequently undergoes further reactions to form
the polyetherimide.
The complexity of the combined aqueous waste
stream formed in this production process will be apparent
from the above description. It contains large quantities of
20 water, typically 60-80% and usually on the order of 70% by
weight. About 15-30%, usually about 20%, is ordinarily
nitrite ion (in the form of sodium nitrite) and about 5-10%,
usually about 7-8%, various organic compounds, and the re-
mainder includes sodium hydroxide. The sodium ion content
25 of the aqueous waste stream is substantial, often on the or-
der of 5-15% and usually about 7-8% by weight. pH values
may be in the range of about 2-12 but are usually on the :
alkaline side, typically about 10-12. i:
A typical waste disposal method for such by-
30 products involves feeding the aqueous waste stream into amulti-stage destruction apparatus including oxida~
tion/combustibn and reduction stages. In such an apparatus,
organic compounds are combusted and nitrogen compounds
converted to nitrogen oxides in one or more stages con-
~ -.
RD002207s
2~ 03421
ducted in an oxygen-rich atmosphere. At least one other
stage may be conducted in a reducing atmosphere created by
the injection of natural gas, the effect of which is to con-
vert nitrogen compounds to elemental nitrogen. All of these
5 stages are carried out at very high temperature, typically
on the order of 1000-1300C.
The effect of sodium compounds under such
conditions is profound and adverse. Even in concentrations
as low as 0.1% by weight, sodium can corrode metal vessels
10 so fast that such vessels, if unlined, are utterly useless as
containers. A typical way to minimize such corrosion is to
line the vessel with a refractory material. It has been
found, however, that sodium is also profoundly destructive
of most refractories. A majority of the sodium is converted
15 to sodium oxide which subsequently reacts with carbon
dioxide to form sodium carbonate. Any remaining sodium, ~ `
however, reacts with the refractory material to produce a
slag which spalls off the inside surface of the chamber in
varying quantities. The result is ultimate destruction of
20 the refractory, exposing the bare metal to the action of the
sodium and ultimately rupturing the vessel. As a side ef-
fect, large quantities of slag are produced and they compli- ~ ~
cate disposal of the treated waste stream. It has been es- ~;
timated that as much as 90% of the deterioration of refrac-
25 tories in these environments is the result of corrosion by
sodium compounds.
Various types of refractories have been evalu-
ated for use in a process such as that described above. They
are typically in the form of brick containing various pro-
30 portions of combined aluminum, generally in combinationwith combined silicon and other combined metals and non-
metals. For the most part, however, such brick has been
found to have a very short life when exposed to a waste
stream high in sodium.
i
,.. ~ . .. * .. , ~ . - . ..
RDoo2207s
....
2108~21
- 4 -
The present invention is based on the discovery
of certain types of refractory materials high in aluminum
which are particularly resistant to the corrosive effects of
sodium compounds. Combustion chambers lined with these
refractory materials are capable of undergoing hundreds of
hours of contact at high temperatures without failing.
Moreover, the slag produced when such refractories are used
is small in volume and relatively easy to remove and dis-
pose of.
Accordingly, the invention in one of its aspects
is a method for destroying waste materials which com-
prises feeding a stream comprising waste products, includ-
ing at least about 0.1% by weight combined sodium, to a
treatment vessel, combusting said organic waste products
at temperatures in the range of about 1000-1300C and re-
moving by-products including sodium compounds from said .
vessel,
the surface of said treatment vessel which
contacts said waste products comprising a refractory ma- -
terial which comprises at least about 85% by weight com-
bined aluminum calculated as aluminum oxide, and which, if
of sintered construction, comprises alumina grains and an
aluminosilicate binder and contains at least about 6.5%
combined silicon calculated as silicon dioxide. ~
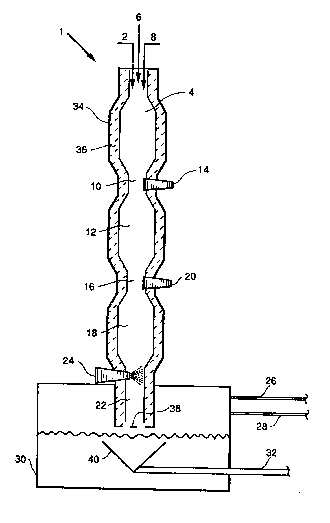
2 5 The drawing illustrates a typical combustion ;
apparatus for wastes which may be employed in accordance
with the invention.
The present invention is capable of employment
to destroy a wide variety of organic and inorganic waste
products. For the most part, it is advantageous in connec-
tion with waste streams containing organic chemicals and a
relatively high concentration of sodium compounds, the -~
latter being at least about 0.1% by weight and frequently at
least about 5% by weight. - -
RD002207s
21i3,~21
The vessel into which the waste stream is fed
may be made of any non-porous material which is capable of
withstandin~ the combustion temperatures of about
1000-1300C attained during the process. Metals such as
steel are typically used. It is necessary, however, that the
interior surface which contacts the waste products be a
suitable refractory material in order to prevent the rapid
corrosion of metal which occurs upon contact with sodium
compounds at the prevailing combustion temperatures.
Accordingly, the nature of the refractory lining
of the vessel is a key feature of the invention. It should
comprise at least about 85% and preferably at least about
90% combined aluminum by weight, calculated as aluminum
oxide (alumina).
Th~ fact that such materials effectively resist
attack by sodium compounds is extremely unexpected, par-
ticularly in view of observations made by Caprio and Wolfe
of Harbison-Walker Refractories in a paper entitled
"Refractories for Hazardous Waste Incineration--An
Overview", in which it is observed that alkalies attack :
high-alumina refractories and the rate of attack is s~vere
as the alumina content is increased to values on the order
of 70%.
In sintered refractories, the presence and nature
of a binder material are also critical. Said binder should
comprise a major proportion of aluminosilicate, and should
be present in sufficient amount to provide at least about
6.5% combined silicon, calculated as silicon dioxide (silica),
in the refractory. Mullite is a particularly effective form
of aluminosilicate for use as a binder.
If the refractory is of fused-cast construction,
the alumina therein essentially serves as its own binder. In
that case, the presence of a silicon-containing binder is not
required, although it may be present in small proportions.
RDoo2207s
2108~21
It is also generally important that the refrac-
tory be free from heavy metals, which may be environmen-
tally harmful if present in the slag. For example, some re-
fractories contain chromium which remains in the slag in
5 the hexavalent state, known to be an environmental hazard.
Such materials are unsatisfactory for the purposes of the
invention.
The bulk density of the refractory used accord- ~
ing to the present invention is generally at least about 2.9 ~-
g./cm.3. It is most often above 3.0 g./cm.3. -- ~
Various commercially available brick composi- ~ `
tions have been identified which are particularly valuable
for use according to the present invention. They include~
"Korundal XD" manufactured by Harbison-Walker ~; -
15 Refractories, "GreenAI-90" manufactured by A.P. Green
Industries, Inc., and "Monofrax Type M" and "Monofrax Type
H" of The Carborundum Company. The essential properties
of these materials are given in the following table.
_ Korundal XD GreenAI-90 Monofrax M Monofrax H
Type ~ S S F F
Percent by weight~
Al2O3 9 0 9 0 9 5 9 3 . 1
SiO2 9 9 0.8 0.3
MgO 0.1
Fe2o3 0.1 0.2 0.1 0.1
TiO2 0.1 0.1
CaO 0 . 1 - - 0 . 2 0 . 1 ::
NazO - - 0 . 2 4 . 0 6 . 0
P2O5 -~ 1.5
~S--sintered; F--fuse~cast.
Several things are noticeable in the above table.
In the first place, the proportions of sodium in the fused-
cast bricks are substantially higher than those in the sin-
tered bricks. No position is taken in connection with the
'~" RDo022079
` 2108~21
present invention on whether it is necessary to minimize
the proportion of sodium in sintered bricks.
In the second place, the silicon proportion is .
substantially higher in sintered than in fused-cast brick.
5 The reason for this is essentially the one described herein-
above; no binder, which is the aluminosilicate-containing
and therefore the predominant silicon-containing con-
stituent, is necessary in the fused~ast brick, but one is
necessary in the sintered brick.
In the third place, "GreenAI-90" differs from
the other bricks in containing 1.5% phosphorus as phos-
phorus pentoxide. In actual plant installations, "GreenAI-
90" has been found slightly superior to "Korundal XD" in
wear characteristics. Accordingly, in a preferred embodi-
ment of the invention the refractory also contains at least
about 1% combined phosphorus as P2Os.
The slag produced in the method of this inven-
tion may vary in nature from a uniformly granular material
to one which is thick and flowable near the refractory sur-
face and more granular at a distance from said surface. The
formation of a thick, flowable slag is frequently advanta-
geous in that less shearing of the pebbles from the refrac-
tory surface may occur than with a granular slag.
The organic constituents of the waste stream
are combusted in the aforementioned vessel, at tempera- ~ -
tures above about 700C and most often in the range of
about 1000-1300C. The precise nature of the combustion
step is not critical for the purposes of this invention. Most
often, however, it comprises at least one oxidizing and at
least one reducing step, conducted under fuel-lean and fuel-
rich conditions, respectively. The fuel is normally natural
gas and is employed in admixture with an oxygen-containing
gas, typically air.
RD002207s
2~0~421
A typical combustion operation according to the
invention utilizes the apparatus shown in the drawing The
waste stream is charged at 2 to the top zone 4 of a three-
zone combustion stack 1, where it is contacted with air and :--
5natural gas entering at 6 and 8, respectively. The stream
then passes downward via passage 10 into second zone 12,
and finally through passage 16 into third zone 18.
Additional natural gas and air are introduced via
nozzles 14 and 20, producing fuel-rich or fuel-lean zones as i
10desired to result in reduction of the nitrogen oxides to ele-
mental nitrogen or oxidation of nitrogen compounds to ni-
trogen oxides, respectively. -
From zone 18, the vapors of the waste stream -
are passed through downcomer 22 where they are quenched h
15and removed by contact with water introduced through wa-
ter jet 24. Volatile components are removed via at least
one gas conduit, with two being shown at 26 and 28, and -
may be passed to scrubbers for further treatment. Liquid
components, predominantly water, are removed from quench
20tank 30 through liquid conduit 32.
In terms of construction, combustion stack 1 ~ ~1
includes an external metal shell 34, typically of steel, and
an inner refractory lining 36, typically of brick as previ-
ously described. The thickness of lining 36 will vary with
the conditions but is generally on the order of 15-30 cm.,
with about 20-25 cm. often being preferred.
In the course of the waste treatment process in
stack 1, attack by sodium compounds on lining 36 often
causes spalling of said lining in the form of pieces of vari-
ous sizes, ranging from small particles to large chunks. ~
Most of the larger pieces are caught by grate 38, with -
smaller particles passing through said grate and being
caught in funnel 40.
RD002207s
21 ~421
g
Prior to the present invention, some pieces dis-
lodged by spalling were so large that they could penetrate
the wall of downcomer 22 and fall elsewhere in quanch tank
30. They were known on occasion to be so hot that they
caused vaporization of the liquid in the quench tank, occa- ~ -
sionally resulting in rupture of safety pressure disks in the
system. The present invention has materially reduced the
amount of spalling and alleviated this problem. -
Another aspect of the invention is apparatus, of
the type illustrated in the drawing, for destruction of
waste materials. Said apparatus comprises a plurality of
intercommunicating vertically disposed combustion stacks,
means for feeding waste and fuel materials to the upper-
most of said stacks, and a downcomer from the lowermost
of said stacks. Each of said stacks, and each passage con-
necting them, has its inner surface lined with the above-de-
scribed refractory. Said apparatus optionally further
comprises fuel-lean and fuel-rich zones and means for sup-
plying oxygen-containing gas and fuel, respectively, to said
zones, as described hereinabove.
Various advantageous resuits of the present in-
vention will be apparent to those skilled in the art. For ex-
ample, the treatment system can be operated for substan-
tially longer periods without deterioration of the interior
2 5 of the treatment vessel. Further, the essential elimination
of spalled refractory causes a substantial decrease or even
elimination of the operational and cost burdens of conveying
such material to a landfill.
The overall result, with particular reference to
the substitution of a 23~m. thickness of "GreenAI-90" for
a similar thickness of a refractory substantially lower in
aluminum and higher in silicon, has been to decrease down
time of the unit and increase waste stream capacity by 90%
and 36%, respectively. A waste destruction unit according
RD0022079
2108'12~
- 10 -
to the invention can operate without down time for periods
of at least 128 days.