Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~.
` 2~99~0
..
.,
The invention relates to novel aryl triazinones and aryl
triazinophthalazines, processes for their preparation and the
;~ use of the compounds of the invention for the treatment of
bronchial asthma, allergies of the most varied origin,
inflammatory processes, cardiac insufficiency and
hypertension.
The compounds of the invention are for example characterized
as potent and selective inhibitors of 5-lipoxigenase and can
therefore be used in illnesses in which 5-lipoxigenase or
leucotriene ar~ involved in the pathophysiolo~ical course.
Sayed et al. (Chinese Journal of Chemistry, No. 1, (1991), p.
45) describe the synthesis o~ substituted phthalazinone
derivatives by the reaction of phthalidene or substituted
benzoylbenzoic aci~ esters with hydrazine. Further reaction
leads to 7-tp-ethylphenyl)-2H-1,2,4-triazinone [3,4-a]
phthalazine-3(4H~-ones as by-product. Camparini et al (J.
Heterocyclic Chem. 15, 1271 (1978) describe the reaction of
imino esters and substituted hydrazines to 2,50dihydro-1,2,4-
trlazine 6(1H)-ones. The reaction produces a good yiald.
European patent 052 422 describes triazinones that are
substituted in 6-position by a substituted aromatic ring.
The compounds have cardiotonic properties and are also
suitable for the treatment of Morbus Parkinson.
¦ European published Patent Application 80 296 describes
oxadiazinones, triazinones and thiadiazinones substituted by
aromatic radicals in the 4-position that have cardiotonic ~nd
antihypertensive properties.
-- 1 --
.;,,: ~ .. . .. .. ....... ....... .
`s~: ~
~`
~6` 21~99~0
,~..................................................... .
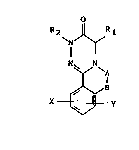
The compounds o~ the invention may be described by formula I:
o
R 2 J~R 1
~ - l .
~Z 5 N N
X~Y
where:
R1 and R2 may be the same or different and represent
hydrogen or alkyl groups with chain lengths ~rom Cl-C4,
` which may also be branched, benzyl groups that are
substituted by halides, such as for example fluorine,
chlorine or bromine, other substituents of the benzyl
group may be -CH3, -N02, amino and substituted amino
gruups as well as -CF3; if R2 = benzyl or is substituted
benzyl R2 may also be present in annealed form,
A-B may represent
- N - f ~ or - HC = ~ CH 5 N -
R4 R4 R~
. where: -
R4 represents alkyl, branched alkyl with a chain length of .
Cl-C4, phenyl substituted once or several times by
halide and/or nitro groups, phenyl substituted by alkyl
~5 groups with 3-6 carbon atoms which may be straight-chain
or branched, phenyl substituted by alkoxy gr~ups, cy~lic
alkoxy, thiaalkyl-quinuclidyl, by aryl groups or
heteroarylmethoxy groups.
'i`
~99~
,;,.
A may repre~ent alkyl straight-chain or branched, with a
chain length from 1-6 carbon atoms or benzyl or benzyl
substitutad by halogen,
B may represent halogen if there is no bond between A and
B.
X may represent aryl or heteroaryl, aryl or
heteroarylm~thoxy, heteroarylmethylamino or
heteroarylmethylthio
Y may represent halogen, hydroxy, O-alkyl, with a chain
length of 1-6 carbon atoms, N02, NR5R6, where R5 and R6
may be the same or different and may be hydrogen, alkyl,
branched alkyl with 1-6 carbon atoms.
X and Y may also be present several times.
The compounds of the invention may also be prasent in
tautom~ric form.
An asymmetric carbon atom in the molecule leads to the
occurrence of the compounds of the invention in
enantiomorphic form. The compounds of the invention may be
enantiomerically pure and present in the ~R)- or (S)- form or
in the form of the racemic mixture or in mixtures of any
composition.
: Synthesis of the compounds
- 2a -
~, ~! ' ' ' . . : ~ _
- ~ 0~900
.
~ All three variants of the proce s described lead to compounds
:l of general formula 1.
.,
Pro~ess
7-Phenyl-2H-triazino~3!4,a)phthalazine-3-(4H)one
The phenyltriazinophthalazinones of the invention in
accordance with formula IV may be prepared according to
diagram I. Th~ meanings of X and Y corre~pon~ to the above
definit~ons.
Based on methods mainly known from the literature, the
primary steps prepared are ~ arylphthalazinones of formula I
- 2b -
210990~
-- 3
(prepared according to J. Pharmaceutical Soc. Jpn 86, 576 (1966)
or USP 3,694,442) and by alkylation (according to J. Organic
Chem. 50, 1677 (1985)) of the alk~l-4-arylphthalazinone-acetate
of formula II. Solvents that may be considered in the step from
compound II to III in diagram I may be aromatic hydrocarbons
that are liquid and that may be substituted by one or several
alkyl groups such as for example benzene, toluene and isomeric
xylols.
After conversion into the thioamides of formula III (with
P2S5 or Lawesson reagent) the mixture is cyclised with
hydrazine or substituted hydrazine derivatives in alcohols at
50C - 80C.
Lawesson reagent is
2,4-(4-methoxyphenyl)-1,3-dithia-2,4-diphosphetane-2,4-disulfide
that may be prepared according to the synthesis on page 941
(1979) or according to T.P. Anderson et al, Tetrahedron, 39
(20), 2419 (1983).
Alcohols that may be used are aliphatic alcohols having one or
several hydroxyl groups and a chain length of 1-4 carbon atoms.
Methanol, ethanol, propanol, isopropanol and isomeric butanols
are for example suitable.
r,,
; :
"~ 2109900
-- 4 --
Diagram
Synthesis of ar~triazino-Phthalazines
O o . ~'
~Y ~, ~ ZCH2CO2Et y,~ N-CH2CO2E[
X~Y X~l Y
r ~awesso~toluene
~: reagent
E~l . or P7S5
,N~O
y~N Y~ CH2Co2
'~ X~ X~3Y
rv
21~9~0
-- 5 --
Example I
7-Phenyl-2H-triazino~3,4a)phthalazine-3(4H)-one
(D-19509~
7 g (0.032 Mol~ 4-Phenylphthalazinone (MP: 232-234C) are
prepared in 100 ml toluene and 0.8 g (0.036 Mol) sodium hydride
added thereto in portions. The mixture is then refluxed for 1
hour and mixed with 5 4 g (0.032 Mol) bromoacetic acid ethyl
ester after cooling to 50C. The mixture is refluxed for S h,
the precipitated precipitate is separated and the solution
concentrated. The precipitated crystals are taken up in 200 ml
toluene and the solution is mixed with 9 g (0.~22 Mol) Lawesson
reagent ~2,4-bis(4-methoxyphenyl)-2,4-dithiooxo-1,3,2,4-
dithiophosphetane). After 24 h at 80C the mixture is
concentrate~ in a vacuum and the precipitated thioamide
isolated. 8 g (0.025 Mol) thioamide are dissolved in 100 ml
ethanol, 1.5 g (0.03 Mol~ hydrazine hydrate are added and the
mixture xefluxed for 18 hours. ~he precipitate is suctioned
filtered, suspended in water and dried.
Melting point: 251-252C.
DC (chloroform/methanol/2S % ammonia solution 9S/S/1)
Rf = 0.69
" 2~99~0
.
The following compounds of Table 1 o~ ~ormula IV wer~
prepared according to example 1.
~ ;
I . I
Ex.No. Comp. ~ormula IV Cryst.from MP
X Y Rl Yl
______._______________________0__________________________ ____
2 3-Methoxy 4-Methoxy H H Methanol 249-252C
3 3-~ethoxy 4-Methoxy H 7-Methyl Methanol 237-239C
4 3-Oxethylene 4-Oxethylen H H glacial 266C
acetic acid
3-Fluorine 4-Fluor H H Ethanol 269C
6 - 4-Fluorine H H H Butanol 257-259C
7 4-~ethoxy H H H Dioxan 266C
8 4-OBzl ~ H H Dioxan 272C
9 N(cH3)2 H H H Dioxan 268C
4-S-CH3 H H H glacial 282C
acetic acid
11 3-C1 4-OH H H Methanol 275-277C
12 4-OH H H H Butanol 308C
13 4-(2-Pyridylmathyl)oxy ``~
Cl H H glacial 263-264C
acetic acid
- 6 -
2~990~
.
Table l cont.
Ex.No. Comp. fo~mula IV Cryst.from MP
. X Y Rl Yl
, .__________.________________________~______._________________
:!' 5 14 4-~2-Pyridylmethyl)oxy
J 3-Cl H H glacial >300C
acetic acid
15 4-~2-Quinolylmethyl)oxy
3-Cl H H glacial >300C
acetic acid
~0
i
- 6a -
` 2 1 ~990~
, -- 7
Process 2
"
3-Phenyl-4,5-dihydro-1,2,4-triazine-6-one
The compounds of the invention may be prepared according to
diagram II.
Substituted 4-hydroxy- or 4-amino-benzyolamino acid esters of
formula V (prepared according to the literature, for example
European patent 422191 or Bull. Chem. Soc. Chim. Belg., 92 (11),
1029 (1983)) serve as primary steps.
The benzoyl~mino acid esters y are converted into the~-
corresponding Type VI arylalkyl- or heteroalkylethers-,
-thioethers or -amines in conventional manner with aryl or
heteroarylalkylhalides in organic solvents such as toluene or
DMA or mixtures thereof using an auxiliary base.
Auxiliary bases that may be used are alkali metal carbonates,
alkaline earth metal carbonates and aliphatic amines, for
example sodium carbonate, potassium carbonate or triethylamineO
Intermediate steps VII are obtained by activating the amide
formation in VI using the formation of imide chlorides Susing
POCl3/PCl5 or SOCl2 as reagent~ or preparation of the
thioamides (using P2S5 or Lawesson reagent).
The compounds of the invention of type VII are obtained at
temperatures of 40C - 80C with hydrazine or substituted
hydrazine derivatives in C1-C4-alcohols, preferably ethanol
or butanol. Substituted hydrazine derivatives that may be
considered are alkylhydr~zines, such-as for example methyl-,
ethyl hydrazine and also benzyl hydrazine.
- 8 - 2~09900
~xample 16
3-~3-chloro-4-[~2-quinolyl)methoxy]phenyl}-4-methyl-4,5
-dihydro-1,2,4-triazine-6-one (D-~0783~
a) N-(3-Chloro-4-hydroxy)-benzoyl-sarcosine ethyl ester
Example for a compound of formula V
.
172.6 g (1 Mol) 3-Chloro-4-hydroxybenzoic acid, 153.6 g (1
Mol) sarcosine ethyl ester hydrochloride and 3 Mol
triethylamine are prepared in 1.5 1 dichloro~ethane and
cooled to -10C. 60 g (0.4 Mol) 1-hydroxybenzotriazol
hydrate and 211 g ~1.1 Mol)
N-(3-dimethylaminopropyl)-N'-ethyl carbodiimide
hydrochloride are added and post-stirred ~or 1 h at -10C.
Post-stirring continues at room temperature until no more
starting products are visible in the thin layer
chromatogram. The organic solution is then poured onto 4 l
water and the separated aqueous phase is extracted twice
more with in each case 300 ml dichloromethane. The organic
phases are combined, dried over sodium sulfate and
concentrated.
The product obtained (yellow oil) is dried using an oil pump
and then further reacted as a raw product.
b) N-[3-chloro-4-~2-quinolyl)methoxy]
benzoyl-sarcosine ethyl ester (2) x
Example for a compound of formula VI
271.7 g (1 Mol) (1~ and 195.4 g (1.1 Mol) chloromethyl
- ~uinoline are prepared in 600 ml toluene and 200 ml
dimethylacetamide. 1.2 Mol potassium carbonate powder are
added and the suspension heated under reflux for 6 hours.
When reaction has finished the residue is suction filtered
and post-washed with toluene. The filtrate is concentrated
` ` ` `
i
21~99~0
and a dark oil is obtained.
c) N-l3-chloro-4-(~-quinolyl)-methoxy]phenyl-thioxo-
sarcosine ethyl ester (3)
Example for a compound of the general ~ormula VII.
412.8 g (1 Mol~ (2) are prepared in 1 l toluene abs. and
reacted ~ith 202.2 g (0.5 Mol) Lawesson reagent ( =
2,4-bis-(4-methoxyphenyl)-2,4-dithiooxo-1,3,2,4-
dithiadiphosphetane). The mixture is heated for 4 hours at
80C. The solution is concentrated in a rotary evaporator. A
brown viscous oil is obtained that is further converted as a
raw product.
d) 3-t3-chloro-4-((2-quinolyl)methoxy)phenyl]-4-methyl-
4,5~dihydro-1,2,4-triazine-6-one (D-20783)
428.9 g (1 Mol) (3) are prepared in 1 l ethanol and mixed
-with 65 g (1.3 Mol) hydrazine hydrate. The mixture is then
heated under reflux for 6 to 10 hours. The reaction solution
is concentrated in a rotary evaporator. A brown crystal
paste is obtained that is mixed with 1.5 l acetone and
stirred at room temperature for 1 hour. The raw product is
suction filtered, washed with acetone and dried.
Yield: 39.89 g (12%), melting point: 205-209C
NMR data (500 MH~, D6-DMSO~: (ppm)
10.45 (s, lH, NH), 7.6-8.5 (m, GH, quinolyl radical)
7.55 (s, lH, 2H-aromat), 7.4 (d, lH, GH-aromat)
7,35 (a, lH, 5H-aromat), 5.55 (S, 2H, OCH2)
3,8 (s, 2H, N-CH2), 2.75 (s, 3H, N-CH3~
Compounds VII of the following Table 2 may be prepared according
to example 16.
? . ~
" ~ ~ ' ' ' ': . :
_ : ' ' ~ :
- lO- 2~09900
The compounds of the invention of type VII of
3-aryl-4,5-dihydro-1,2/4-triazine-6-ones may also be prepared
using alternative routes of synthesis. These are set out in
diagrams IIb and IIc and should be regarded as examples.
Diagram IIa
SYnthesis of 3-arvl-4,5-dihYdro-1,2,4-triazine-6-one
O R2 0 R2
~ ~Het~-Ar-alkylhalide ~N
X = OH, SH, ~nH2 X = Het-~kyVA~-a~yl-S/O~
S R2
Law~s~P2~5 ~ ~N~ H~N~R3~H20/.alcohol
X~ Rl O
N'
1RZ
vIIr
- ~1 2109900
Diaqram IIb
O , , O
Y ~ H~-A~- ~ ~ Y ~ o ~ Et
W = OH, SH, NH~ X = Het-all;yVAr-alkyl - S/O/NH
1.) Bzs~ o
2)E~ ~o~\coupling reage
1 , 1 I condensation
~1 agent
S R1 R~
Y~ ,o L~w~501~1P255 ~N~ \/
VII
\~NNHR2
alcohol ~ N ~ ~R~ ~ O
Y~NlR,
V~I
A = All;yl Cl-C4, Benzyl
Rl= H, Allcyl, Benzyl
- R~ = H, All~yl, Benzyl
~r~
-`` 2~990~
- 12 -
Dia~ram IIc
~V~J~~ Benzy:L halide BzlZ ~o~OR
R - AL~yl Z = SIO/NH
. . _ 1.) ~c
HN~ ~
. ..
S Rl O R1
y~J~ 0~ L~so~55 ~N~ ~/
X A O A O
VII ~1l
H~ 2
r ~
,NR2~0 ~NR.,2~S~;O
lkyl/Helcro3~ 11 f
~Y~ Rl ~ ~f \N ~R
A = Al~yl Cl-C4, Benzyl
Rl= H, Alkyl, Benzyl
R2 = H, All~yl, Benzyl
~2~09~0
Table 2. Compounds of formula VII
}
1 R~
' ~ ~ J~ ~
10Ex.No. Comp, ~ormula VIII Solvent MP
X Y A R1 R2
________________________________________________________
: 17 OH N02 H H H Glacial 242-244-C
acetic acid
18 OH NH2 H H H Aceton 213-215C
19 OH H H H H Methanol 249-251C
~H Cl CH3 H H Acetone 228-231C
21 OH Cl H H H Isopropanol/ 236-239C
Methanol
22 OH OCH3 H H H Isopropanol/ 256-258C
Methanol
23 OH OCH3 H H H Isopropanol/ 261-264C
Methanol
24 OH OCH3 H CH3 H Isopropanol 244-247C
Bzl-O H H H H ~ethanol 199-202C
26 3-~1-S2-Methoxyphenyl)-
pipera2inyl]-propyloxy
H H H H Isopropanol/ 133-135C
Methanol
. - 13 -
``` 21~99~
,
Table 2 Cont.
Ex.No. Comp. formula VIII Solvent MP
X Y A Rl R2
_ _______~_ __________________~________ ________________
S 27 3-[1-(2-Nethoxyphenyl)
-piperazinyl3-ethoxy
H H H H Isopropanol/ 139 141C
Methanol
28 (2-Pyridylmethoxy~
H H ~ H Isopropanol 178-182~C
29 Bzl-0 H CH3 ~ H Isopropanol/ 168-171C
Methanol
31 3-[1-(2-Methoxyphenyl)
piperazinyl]-ethoxy
Cl CH3 H H Isopropanol 113-115C
- 13a -
2 ~L O 9 9 O
-- ~4 --
Ex . No . CaT p. fonnula VIII Solvent MP
x A Rl R2 `
32 8zl-O OCH3H H Isopropanol/Melhanol 231-233 C
33 B~l-O Cl CH3 H Isopropanol 252-254 C
34 8zl-O Cl H H Isopropanol 241-243 C
Bzi-o OGH3CH3 H IsopropanoUMelhanol 212-214 ~C
36 3-Pyridylrnethoxy Cl H H IsopropanoVMethanol 246-249 C
37 3-Pyndylmethoxy OCH3 H H Methanol 243-245 C
38 3-Pyridylmethoxy Cl, CH3 H ; Isopropanol ~58-162 C
39 3-Pyndylmethoxy OCH3H H !sopropanol 186-188 C
(4-Fluor)-Bzl-O OC~13H H ~ Isopropanol 189-191 C
41 E~zl-O OCH3H CH3 - IsopropanoVMe~hanol 228-231 C
42 8zl-S F H H - IsopropanoUMethanol 244-247 C
43 E3zl-S H H H Isopropanol 250-252 C
44 Bzl-NH OCH3CH3 H Isopropanol 18l-183 C
8zl-NH H H H Methanol ,250 C
46 Bzl-S F CH3 H Isopropanol 138 C
47 Bz!-S NH2 CH3 H Isopropanol/Me~hanol 245-247 C
48 Bzl-NH OCH3CH3 H lsopropanol 252-254 C
49 3-Pyridylme~hylamino 3 Isopropanol 238-241 C
50 3-Pyridylmelhylamino 3 3 Isopropanol/Me~hanol 2?9-232 C
109900
- 15 -
~x.No ~omp. formula VIII Solvent MP
A R1 R2 J
.
51 (4-Methoxy)benzyloxy OCH3H H Isopropanol 198-20t C
52 (4-Melhoxy)benzyloxy Cl H H Isnpropanol 181-183 C
53 3-Pyridylme(hylamino OCH3H H Me~hanol 7~-75 C
54 3-Pyndylmelhylamino H H tl Isop-opanol 231-233 C
55 (2,3,4-Tr;methoxy) Cl H H Isop~opanoUMe~hanol 194-197 C
-benzyloxy
56 2-Chinolylmethoxy Cl CH3 H Isop~opanoVMethanol 205-209 C
~7 2-Chinolylmelhoxy OCH3CH3 H 238-241 C
58 2-Chinolylmeltloxy Cl H H IsopropanoUMethanol 203-205 C
~` 2~9~0
Process 3
Example 59
1~-Methyl-3-oxo-3,4-dihydro-2H-[1,2,4]triazino[4,3-C-~-
quinazoline (D-19749)
Starting from known quinazoline-4-one-3-yl acetic acid ethyl
ester derivatives of formula IX ~see M. Suisse, S. Johme, J.
Prakt. Chem. (2), 326, 342 (1984) and R.H. Clark, E.C Wagner J.
Org. Chem. 9, 55 (1944), the corresponding
quinoline-4-thione-3-yl acetic acid ethyl esters of ormula X
are obtained by fumigation with P2S5 or with Lawesson
reagent.
To present the tria~inoquinazolines of formula XI, the
thioamides of formula X were converted to XI with hydrazine
hydrate in alcohols.
Alcohols that ~ay be used are all C1-C4-aliphatic alcohols,
for example methanol, ethanol, propanol, isopropanol and
isomeric butanols.
a) 6-Methylquinazoline-4-thione-3-yl-acetic acid ethyl e~ter
A suspension of 20 g (0.081 Mol~
6-methylquinazoline-4-one-3-yl acetic acid ethyl ester and
17.1 g (0.042 Mol3 Law~sson reagent in 300 ml toluene is
stirred for 33 h at 80 - 105C, an orange to dark red
solution qradually being formed. When reaction is completed
the solution is cooled, concentrated and the residue
post-washed with a little cold toluene and then with
petroleum ether and recrystallised from
dichloromethane/methanol.
MP: 130-132C
.. . . , . _ . . . .
!,', ': . . ` ,~ .: . ` ' '
;"' '.' ~' '.. " ~ ,, ` " '~ ' ':
,,,,:. :,. - `- :, ,~ ' . ' . : ~ '
; :
2109~0
,, ~
. - 17 -
i b) ~0-Methyl-3-oxo-3,4-dihydro-2H-[1,2,4]-triazino-
.~ ~4,3-c]quinazoline
~,
8.7 g (0~033 Mol) o~ the thioamide are suspended in 200 ml
ethanol and mixed with 10.1 (0.2 Mol) hydrazine hydrate and
the mixture heated to reflux temperature. After four hours,
the precipitate is suction filtered, washed with ethanol and
the product recrystallised from ethanol/glacial acetic acid.
MP: 298-304C - decomposition.
The following compounds of the table were obtained according
to example 59:
Diagram 3
i
'~ o '1 s
~X ~ C 2 2 awcps2oSn5 R 5g(:nz X ~ N CII,CO Et
L~ X
R2N-NH2 ~ , N ~ O
X ~JN~ R
Xl
, ;. ~
2~9~
. - 18 -
,,
.
`
22
'Table 3, formula XII
~ Ex.~o, Comp. formula XII Solvent MP
X Y Q,1 R~
i 60 3 H H EthanoU ~ glacial298-3o4oc
¦ acetic acid
61 3 3 Ethano~glacial 265-269C
. acetic acid
62 OCH3 OCH3 OCH3 H acetic acid
63 Cl H H H Dichlo~methanlMe~hanol ~300 C
64 Cl H Cl H -E(hano~glacial >300C
acetic acid
Cl H H CH3Ethano~ glacial >300C
acetic acid
66 Cl H Cl CHElhano~ glacial ~300 C
3acetic acid
67 H H H HE~hanol >300C
` `` 2109~
_ 19 -
The compounds of the invention have an anti-inflammatory
(lipoxigenase inhibition) and bronchorelaxing effect (model of
guinea pig trachea precontracted with carbachol).
The compounds also have an action on the circulation (raisin~
let ventricular contractility, reducing blood pressure).
The compounds also inhibit histamine-induced rhinitis in the xat
and histamine release from the mast cells.
? ~
~' ..
~,'.,~