Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 93/OOg59 PCI`/US92/05587
. ~.
2 t l 23 6 6
METHOD AND APPARATUS FOR TREATING
TISSUE
s Field of the Invention
This invention relates to a novel method and
apparatus for treating tissue, including the application of an
electric field to tissue surfaces as well as the delivery of topical
drugs to an area of tissue to be treated using such electric
field. More specifically, the invention relates to methods of
delivering topical drugs, including proteins ~and nucleic acids,
to skin by electrically stimulating the skin. Further, the
invention relates to the transdérmal delivery of electrical
- stimuIation across the skin boundary to treat the underlying
. 25 tissue. Such treatment can include the delivery of a drug or
- other compound to such tissue.
, ~ ,
~ ,
.
Background
Iontophoresis is a well known technique for
- 30 delivering drugs through the skin which involves applying an
electric potential gradient at the skin surface to enhance the
permeability of the skin to the drug molecules. Research has
been conducted on iontophoretic transdermal delivery of
S~JBSTITU~E SHEET
WO g3/00959 PCI /US92/05587
~ 1 12 3 ~ ~ 2
peptide and protein drugs (Chien, Yie W., et al., ~1989) ~. of
Phann. ~ ~, 376-383); nonpeptide drugs (Sanderson, et al.,
(1989) J. of Pharm. ~, 361-364); and polypeptide drugs
(Srinivasan, V., et al., (1989) ~ of Pharm. Sci. Z~, ~70-375).
s Iontophoresis has proven effective for
transdermal delivery of local anesthesia, metallic and
nonmetallic ions, vasodilators, dermatological medications,
and steroids (Behl, et al. (1989) J. of Pharm. Sci. ~ 355-
360). One of the earliest uses of iontophoresis involved
introducing a sweat-inducing drug through the skin to
diagnose cystic fibrosis (Chien, Yie W., et al. (1989) J. of
Pharm. Sci. ~, 353-354). Other biomedical applications of
; iontophoresis include treatment for hyperhidrosis, edema
~' formation, calcium deposits, and hay fever (Behl, et al.,
(1989) J. of Pharm. Sci. 78, 355-360).
An iontophoretic drug delivery system generally
includes a power source, control circuitry, and two electrode
pads. One of the electrode pads contains a solution of an ionic
drug and the other electrode pad contains an electrolyte. To
effect delivery of the drug,~the electrode pads are placed on
the skin, and an electric current is supplied to the pads to
induce an electric field at the skin surface. The power supply
is connected to the electlode pads so that the charge of the pole
connected to the drug-containing electrode is the same as the
charge of the ionic drug.~ The application of an electric
potential at the drug-containing electrode will drive the drug
ions through the skin and into the body (Sanderson, John E. et
al. (1987) J. of Pharm. Sci. ~, 215-218; Phipps, J. B., et al.
(1989) J. of Pharm. ~i,~., 365-369).
,,~
One such iontophoretic drug delivery system,
marketed by Iomed, Inc. under the trademark TransQl,
includes a dispersive electrode and a drug delivery electrode.
The drug delivery electrode is mounted to a plastic holding
~ SU~STiTUrE SHEE~
WO g3/00959 2 1 1 2 3 ~ ~ PCI`/US92/05587
tray which includes an absorbent pad for receiving the drug.
Once the pad is fully hydrated with the drug, the drug delivery
electrode and absorbent pad are separated from the holding
tray and placed on the skin at the treatment site. The
dispersive electrode is placed on the skin spaced from the drug
delivery electrode, and leads are attached to the pair of
electrodes to generate an electric potential gradient at the
trea~ment site. This drug delivery system is primarily used to
deliver a water soluble medication, such as a local anesthetic
o and/or corticosteroid, through a patient's skin. A disadvantage
of this system is ~e excessive length of time required for
treatment.
Rogaine (Minoxidil, 2% topical solution) is a
topically applied drug, marketed by the Upjohn Company,
s which has proven effective in treating .nale pattern baldness
involving ~e top, or vertex, of the head. Although Rogaine
has successfillly stimulated hair growth on the top of ~e head,
no clinically significant effect has been seen in patients ~,vith
predominantly frontal hair loss.
Accordingly, it is therefore a general object of the
invention to provide methods of treating the surface of tissue
with an electric field.
It is a~ specific object of the invention to provide
; - - rnethods of applying a topical d~ug to a tissue which increases
` ~ 25~ the efficiency and effectiveness of the drug.
,
It is a further specific object of the invention to
provide a method of applying Rogaine to the frontal lobes of a
-~ user's head which stirnula~es detectable hair growth.
It is anod er object of the invention to provide an
- 30 electrode device for use with the subject method which is
sterile, disposable, noninvasive, pain free, easy to
manufacture, and inexpensive to mass produce.
SUBSlTIIII~ SHEET
WO 93/00959 PCI`/US92/OS587
,~.~
.
211.~6
Disclosure of the Invention
A preferred method of delivering topical drugs to
the skin which is intended to accomplish at least some of the
foregoing objects includes the step of applying the drug to be
delivered to one of an area of skin to be treated and an
electrode device. In a preferred embodiment, the drug is
applied to the electrode device. The electrode device, whic;h is
formed to generate a multiplicity of simultaneous and
directionally distinct electrical fields, is then placed in contact
with the area of skin to be treated. Finally, voltage is
delivered to the electrode device to generate simultaneous and
directionally distinct electric fields at the sldn surface.
Such methods~ and apparatus may be readily
applied to similar treatment of other tissue surfaces. Further,
such methods and apparatus may be readily adapted to apply
~ ~ ~unique electric f1elds to the~ surface of ~tiswe and to tissue
!~ ~ ullderlying~ such tissue surfaces to enhance circulation or the
d~elivery of drugs to suc~h dssue.
20 -
,;~ , - ~ , .
Brief ~Description of the ~Drawings
Other objects~and~advantagcs of the present
irlventi~on~will ;become~apparent~from the~following detailed
descriptio n o f a~ pref ed embodiment thereof taken in
. 25 conjunction with the accompanying drawings, wherein:
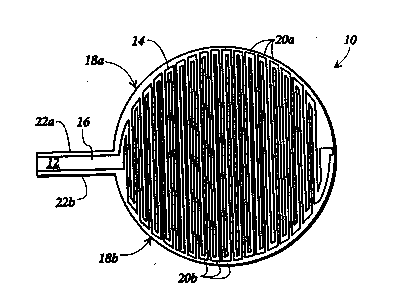
nauRE 1 is a~top~plan view of an electrode
- device for use with the subject method of delivering topical
drugstothe skin; ~ ~ ~
~ ~ FIGURE 2 is an enlarged top view of a section of
-~ 30 the electrode device of Flgure l; and ~
,-
:
~ SUBSTI~UIE SHEET
WO 93/OOgS9 PCI`/US92/05587
h ;1 ~ 2 ~ S ~
~:IGURES 3a-c are photographs of enhanced hair
growth using the methods and apparatus of the invention.
Best Mode of Carrying Out the Invention
s Before describing the subject methods of applying
novel electric fields and the delivery of drugs to a tissue
surface or underlying tissue, it may be helpful to describe the
electrode device for use with the subject method. Referring to
the drawings, wherein lilce numerals indicate like partsi and
o initially to FlGURE 1, there will be seen an electrode device
10 including a substantially planar support member 12
composed of a substantially non-conductive material. This
device in general is made of a flexible planar support member
to accommodate the contours of the tissue surface to be
: 15 ~treated. Suitable non-conductive materials for forming support
member 12 include mica, ~polyester, and polystyfene. Support
member ~12 has ~ ~a main portion~ 14~ preferably circular in
shape, and a~ tab~portion 16 ~extending from the~perimeter of
~ortion 14. ~ Tab~pôItion~ 16 facilitates connection of lead
~lines~to electrode d~evice lO. ~
A f~st~ electrode pattern 18a and a second
electrodé~-patt~em~ 18b ~are disposed on main portion l4 of
elec~rode~devicë 10.~ st electrode pattern 18a;and second
;el~ctfodè pattérn 1~8b~each~lude a~set of substandally
- ~ : 25 ~ pafalld~electrodes~20a~ and-~20b, respectively, which extend
~, across main~ portion 14 in an alternating arrangement.
;Electrodes~20a~;and-20b~-are uniformly spaced across main
portion 14;~to ensu~e~sn~even distribution of current density
- beneath elect~ode dèvice lO,~tbe eby reducing the likelihood of
skinbums. ~
A~ pair of eIectrode leads 22a and 22b extend
along opposite sides of tab portion 16 and halfway around the
' '
~, ' .
.
~ SU~ST~UTE SHEE~
r~
WO 93/OOgS9 PCI`/US92/05587
~, A ,~
i.,l ~/J~ 6
perimeter of main portion 14. Electrodes 20a are electrically
coupled together by electrode lead 22a, and electrodes 20b are
electrically interconnected by electrode lead 22b. Each set of
electrodes 20a and 2ûb are electrically connected to one
s another so that a voltage applied to the corresponding
electrode leads 22a and 22b is uniformly distributed across
each of the electrodes.
The alternating arrangement of ~lrst and second
electrode patterns 18a and 18b, respectively, generates
multiple directionally distinct electrical fields when opposite
charges are applied to electrode leads 22a and 22b. Turning to
FIGURE 2, the arrows represent electric field lines generated
by connecting lead lines of opposite polarity to electrode leads
æa and 22b.
s Al~ough a preferred embodimen~ of support
member 12 is circular in shape, support member 12 may be
fabricated in different shapes according to the area of tissue to
be`treated and according to the specific medical application.
Electrode patterns may be formed by screen
printing a conductive ink on support member 12. A technique
for printing a conductive ink on a flexible substrate is
disclosed by M. Martel in "The Basics of; Area Source IR",
SITe Magazine. pgs. 60-64. Suitable conductive inks for
- folminc the electrode pattems of the subject invention include
2~ E-1400 ink marketed by Ercon ~corporated and Electrodag
PTF inks marketed by ~cheson Colloids Company.
Alternatively, the electrode patterns may be
formed by selectively depositing a metallic material, such as
aluminum, on main portion 14. Or, the elec¢rode patterns
may be formed by using a mas~ing technique to deposit copper
vapor or other metallic vapor such as aluminum, gold,
platinum, and nickel, on main portion 14.
SU~STI~UrE SHEET
WO93/OOg59 f.,.. ~.~' 23~ PCl/US92/05587
. ~
The methods used to produce such vapor
deposited electrode devices and selectively etched electrode
devices are well known. Metal deposition may be carried out
according to the methods disclosed by P. Gise and R.
Blanchard in "Modern Semiconductor Pabrication
Technology", ~hapter 8 (Prentice-Hall, England Cliffs, New
Jersey, 1986), and A.B. Glaser and G.E. Subak-Sharpe in
"Integrated Circuit Engineering", Chapter S (Addison-Wesley,
Reading, Massachusetts, 1977) and selective etching of
o laminated material may be carried out according to the
methods disclosed by C.F. Coombs, Jr., ed., in "Printed
Circuit Handbook", Chapters 1 and 8 (McGraw-Hill, New
York, New York, 1976).
The electrode material preferably ranges in
s thickness from approximately .001 inches to 1 inch. Each
electrode has a width of approximately lO,um to 2mm,
preferably between O.lmm and Imm. The spacing between the
electrodes ranges from approximately lO~lm to 2mm,
preferably O.lmm to Imm.
Since these electrodes are positioned close
together, a user may apply a relatively low voltage across first
and second electrode leads 22a and 22b to generate an
appropriate voltage gladient or sufficient electrical current.
mos~ applications, 10 to 100 volts DC are applied across
2s ele de leads 22a and 2Zb; however, the applied voltage may
~nge from approxinutely I to 500 volts DC. It is preferable
to deliver a pulsed voltage, as opposed to a continuous voltage,
to electrode device 10 to ~prevent the build-up of charge on the
~; ski~
The subject method for delivering a topical drug
to tissue will now be ~described. According to the subject
method, a topical drug is applied to one of an area of tissue to
be treated and electrode device 10. Tissue includes but is not
- ~UBS~ITIJTE SHEET
WO 93/OOg59 PCI'/US92/05587
2 1 1 ~ 8
limited to skin, tendons, muscles, nerves, internal organs and
tissues of the oral cavity. ~ a preferred embodiment, the
drug is applied to a localized area of skin to ensure even
distribution of the drug across the skin surface. If the`drug is
s applied directly to electrode device 10, the drug should be
spread evenly across the surface of device 10 on which
electrode patterns 18a and 18b are disposed, or the conductive
surface of device 10- Once the drug has been applied, the
- conductive surface of device 10 is placed in contact with the
o area of skin to be treated. A voltage is then applied acr~ss
electrode leads 22a and 22b to generate simultaneous and
directionaUy distinct electric fields between electrodes 20a and
20b. The electric fields electrically stimulate the area of skin
being treated. Although the exact mechanism is unknown, it is
believed that 3pplication of electric fields to the skin surface
enhances the ~ermeability of the skin to the topical drug,
~ereby increasing the absorption of the drug through the skin.
The increased absorption contributes to an increase in the
efficiency and effectiveness of the drug delivery.
: . .
The subject method of the invention may be used
in combination with the topical hair growth drug Rogaine,
commercia11y marketed by The Upjohn Company. Previously,
Rogaine has only been successful in promoting hair growth on
- dle vertex of the head. However, when used in combination2s ~ with elec~ode device 10, Rogaine has been shown to stimulate
hair growd~ on the front of-the scalp. According to the subject
method, a user applies a full dose of Rogaine, specifically ImL,
to the front of the sc`alp per the directions found in the
literature accompanying a Rogaine prescription. After the full
dose has been applied to the user's scalp with the supplied rub-
ON applicator, the user places the conductive surface of
electrode device 10 in contact with the area of the scalp to be
treated. The user then tums on the power supply to electrode
device 10 which is preferably set to deliver approximately
fifty to one hundred 100 volt DC pulses of 1 ampere of
SUBST~TUT~ SHErT
WO 93/00959 PCI`/US92/05587
2 1 1,~3~1~
g
current over a period of 15 to 30 seconds, each pulse lasting
approximately 1 to 10 msec. As the pulses are being applied
to electrode device 10, the user may move electrode device 10
over the area of skin being treated. The user may selectively
apply pressure to electrode device 10 to adjust the contact of
electrode device 10 with the skin. During treatment, the user
may feel a sensation of mild tingling. After approximately
four months of treatment, hair growth becomes apparent. This
was repeated twice daily over 60 days.
o The results of applying Rogaine to the frontal
lobe of the scalp and treating the scalp with the electrode
device as described above over a 60 day period are shown in
FIGURES 3a-c. PIGURE 3a shows the condition of the scalp
on day 0 prior to the start of the experiment. FIGURES 3b
and 3c show the same scalp after 20 and 60 days, respectively.
3 The right side of the scalp (left side in the figures) is the area
treated with Rogaine and the electrode device 10. The left side
of the scalp (right side in the figures) is the control area, i.e.,
treated only with Rogaine. ~n FIGURE 3b, new hair grow~ is
seen at 20 days. As is also apparent in FIGURE 3c, hair
gro~h after just 60 days was substantial.
, , .~ .,
The subject dectrode device 10 may also be used
in combination with o~ier topical drugs to treat a variety of
~ ~ - skin maladies including: (i) Retin A ~retinoin, all, trans
: :~ 25 Retinoic ~Acid3, commercially marketed by OrthoPharmaceuticals,~for treatment of brown spots, acne, and
wrinkles, (ii) steroids to treat inflammation and psoriasis, (iii)
topical pain relievers for treatment of aberrations of the skin
- and underlying tissue, and (iv) S-fluorouracil to treat skin
~; 30 cancer and psoriasis.
addition to delivering drugs to a localized area
- of skin and skin tissue, electrode device 10 may also be used
without an accompanying topical drug. Electrical stimulation
SUE~TlllJTE SHEET
WO 93/00959 PCI`/US92/05587
3 6 ~ ,
alone bas therapeudc and diagnostic value. Electrode device 10
may be used to electrically stimulate the skin of a user to, for
example, increase blood flow, induce secretions, and. stimulate
nerves.
s As an example, tests were performed where the
electrode device 10 was applied to the inner forearm area of a
human. The same protocol as previously described was used
except no topical drug was applied to the skin or electrode
device 10. The test produced a region of redness indicatmg
o increased circulation to the area. The increased circulation is
' useful for enhancing the delivery of drugs to the localized skin
, area which are administered IV or orally.
An advantage of using the apparatus and method
of the invention lies in the fact that treatment can be effected
without significant discomfort of tissue damage (even after
twice daily treatment for almost six months). The only feeling
sensed by the user is a mere tingling of the scalp which is
believed to be evidence of subcutaneous nerve stimulation.
: .
Electr~de device 10 may also provide gene
therapy to skin or other tissue by transfecting nucleic acid into
the cells of the skin or tissue or cells underlying such surfaces
for treatmg~ gene deficiencies or inducing-such deficiencies in
laboratory ~animals. Nucleic acids which~ may be used for
transfection include DNA capable of e~ssing an antisenæ
RNA transcript which is capable of down modulating
translation of endogenous~RNA transcripts. Proteinaceous
gene products may also be delivered to the skin cells by the
subject method. ~Such proteins include Factor VIII, insulin,
and adenosine deaminase.
- In describing the invention, reference has been
~; made to a preferred embodiment and illustrative advantages of
the invention. lhose skilled in ~e art, however, and familiar
with the instant disclosure f the subject invention, will
SUBSTITUTE SHEET
WO 93/00959 2 1 1 .~ 3 ~i ~ PCI/US92/05587
~.
11
recognize additions, deletions, modifications, substitutions, and
other changes which will fall within the purview of the subject
invention and claims.
. ,j , ~
.
,
:
,
SUBSTITUTE SHEET