Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
- 2~ 757
- ACTIV~TED CA~HOD~ FOR ~CTROLY~I~ CEL~S A~D~ HOD ~OR
PREPARING ~HE ~AM~
~TA~ OF T~ ~RT
Chlor-alkali electrolysis is certainly the electrolytic
process of greatest industrial interest. In general terms,
said electrolys~s process may be illustrated as the
splitting of a startlng reactant, which is an aqueous
solution of sodium chloride (hereinafter defined as brine),
to form gaseous chlorine, sodium hydroxide in an agueous
solution and hydrogen. This splitting is made possible by
the application of electrical energy which may be seen as a
further reactant. Chlor-alkali electrolysis is carried out
resorting to three technologies: with mercury cathodes
cells, with porous diaphragms cells or with ion exchange
membranes cells. This latter represents the most modern
development and is charactPrized by low energy consumptions
and by the absence of environmental or health drawbacks. Of
the others, the mercury cathodes cells are probably;destined
for a sharp decline in use because of the severe
restrictions adopted by most countries as regards the
-release of mercury to the atmosphere and soil. In fact, the
most modern cell designs allow one to meet the severe
, ", , ~ .. . ... - , . -, -~. . .. .. . . . ~ .
-" 2 1 1 ~
. ;:
requirements of the present regulations, but the public
opinion rejects l'a priori" any process which could lead to
the possible release of heavy metals ~n the environment.
~, ;-
The diaphragm process has illso problems as the maincomponent of the diaphragm is asbestos fibers, which is
recognized to be a mutagenic agent. The most advanced
technology foresees a diaphragm made by depositing a layer
of asbe~tos fibers mixed with certain polymeric blnders onto
cathodes made of iron meshes. The structure thus obtained is
then heated whereby the fusion of the polymeric particles
permits the mechanical stabilization of the agglomerate of
asbestos fibers. As a consequence, the release of fibers
during operation (particularly in the drain liquids of the
plant~ is minimized, as well as the release to the
atmosphere due to various expedients adopted during
::: :-
~ manipulation of the asbestos in the depo~ition step.
.,~,
,,
~ However, this appears to be only sufficient to prolong ~` -
; ~ the life of the diaphragm technology, in view of the ever
increasing difficulty in the supply of asbestos fibers due
to the progressive closing of the mines. For this reason, ; ;~
porous diaphragms have been developed where the asbestos
fibers are substituted by fibers of inorganic materials
considered to be completely safe, such as zirconium oxide,
mechanically stabilized by polymeric binders. The deposition ;~
' ~ ' '',:
-,:
2~1~7~ - 3 -
and the stabilization by heating in oven are carried out
following the same procedure adopted for asbestos
diaphragms.
. . . ;
In the last few years, graphite anodes have been nearly
completely substituted by dimensionally stahle anodes made
of a titanium substrate coated by an electrocatalytic film
based on noble metal oxides. In the plants using the most
advanced technologies, the dimensionally stable anodes are
of the expandable or non-expandable type. Expandable anodes
as ~escribed for example in U.S. patent 3,674,676, which
permit one to minimize the gap between the anode and the
cathode, with the consequent reduction of the cell voltage,
have the shape of a box with a rectangular cross-section,
rather flat, the electrode surfaces of which are kept in a
contracted position by means of suitable retainers while the
anode is inserted between the cathodes during assembling of
the cell. ~efore start-up, the anode electrode surfaces are
..
released and are moved towards the surfaces of the
diaphragms by suitable spreading means or extenders. Spacers
may be introduced between said electrode surfaces and the
diaphragms. ~hese technological improvements brought the
cost of production of chlorine and caustic obtained by the
diaphragm technology guite close, even if somewhat higher,
to those obtained by the membrane technology.
.
-- 21~ 7 - 4 -
It is therefore the current opinion of industry that
diaphragm cells plan~s may still remain in operation for a
long time and the future of these plants could be even more
promising if the following inconveniences still penalizing
the technology are overcome~
- cell voltages higher than that theoretically obtained by
the expansion of the anodes. It is well known that the
cell voltage linearly decreases with the decrease of the
anode-cathode gap. Said result ic connected to the lower
ohmic drop ln the brine layer between the diaphragm and
the anode. However, for anode-cathode distances below a
certain limit, usually 3.5-4 mm, the cell voltage remain
: . ~
more or less constant or even increases (see Winings et
al. in Modern Chlor-Alkali Technology, 1980, pages
30-32). "
This negative behaviour, quite unsatisfactory, is
commonly attributed to the chlorine bubbles which are
entrapped in the thin brine layer between the anode and ~;
the diaphragm. The problem is partially solved by
resorting to the use of internal hydrodynamic means as ;-
described in US patent 5,066,378. Said means are directed
to promote a strong circulation of brine capable of
removing the chlorine bubbles;
increase o~ the cell voltage in the electrolysis which
increase is commonly ascribed to gas antrapping inside ~-
the pores, favoured by insufficient hydrophilic
~ .
: ;~ ,'
æ~1 ~7 ~7 ~ 5 -
propertie~ of the material ~orming the diaphragm, in
- particular in the case of diaphragms containing polymeric
binders, as suggested by Hine in Electrochemical Acta
Vol. 22, page 429 (1979). The increase of cell voltage
may also be due to precipitation of impurities contained
in the brine inside the diaphragms;
- deposition of metallic iron or electrically conductive
compounds of iron, such as magnetite, formed by reduction
at the cathode, with growth of dendrites in the diaphragm
: and evolution of hydrogen in the anode compartment
(hydrogen in the chlorine which is explosive). This
problem is most liXely to occur with diaphragms
characterized by a scarcely tortuous porosity, as
discussed by Florkiewicz et al. at the 35th Seminar of
the Chlorine Institute, New Orleans, Louisiana, USA,
March 18, 1992;
- decrease of the faradic efficiency in the electrolysis
run;
- reduced life of the diaphragm.
OBJEC~S O~ IHE INV~NTION
It is an object of the invention to provide an improved
cathode assembly for use in a diaphragm chlor-alkali
electrolysis cell which permits the substantial elimination
. 6
211~7~7 ~ ~
.
of the inconveniences of the prior art and a method for
preparing the same.
It is another object of the invention to provide an
improved diaphragm electrolysis cell and the method for .
operating the same.
These and other objects and advantages of the invention :~
will become obvious from the following description. - .
SUMMARY OP ~HE INVEN~IO~
..- ;' '~:
The novel cathode assembly of the invention for use in ~:~
monopolar or bipolar diaphragm or membrane electrolysis ; ~-
cells comprises pairs of interleaved anodes and cathodes
having openings, said cathodes being provided with a :
corrosion resistant ion exchange membrane or a porous ~ ~
diaphragm, said cells being provided with inle~s for feeding ~.
brine and outlets for the withdrawal of chlorine, hydrogen ~ ~
and caustic. Said cathode assembly comprises a thin and ~ `.
flexible corrosion resistant, perforated or expanded sheet
ox mesh provided with an electrocatalytic coating for ~:
. :
hydrogen evolution in an alkaline environment, applied
between each of said cathodes and said diaphragm, the --
cathode and the sheet or mesh being in electrical and
, ~ ' `` . ~
2~47~7 - 7 ~
'
mechanical contact, preferably by a plurality of contact
points.
Preferred embodiments of the present invention will be
now described making reference to the drawings.
BRIEF DE~CRIPTION OF THE DRAWING~
,
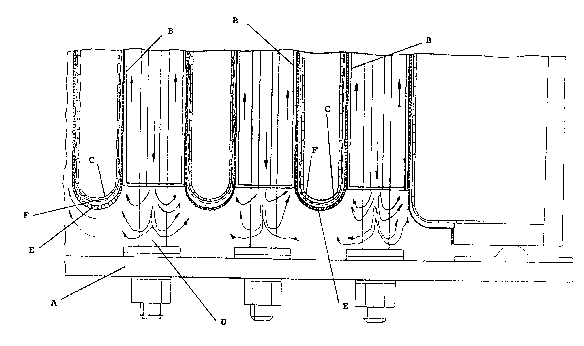
Flg. 1 is a cross sectional longitudinal ~iew of a
conventional diaphragm cell for chlor-alkali electrolysis
comprising anodes of the expandable type and the cathode
assemblies of the present invention.
Flg. 2 a cross sectional view of a detail of fig. 1
illustrating the cathode assemblies of the present
invention.
DESCRIPTION OF ~HE INVE~TION
In fig. 1, the diaphragm electrolysis cell comprises a
base (A) on which expandable anodes tB) are secured by
means of conductor bars (D). The cathode (C) is made of a
:
perforated or expanded sheet or mesh of interwoven iron
wire so shapled as to form a multiplicity of ra~her
; ~
2~7~7 - 8 -
flat parallelepipeds (so called fingers). The thickness of
. . .
the sheet or mesh is such as to ensure a sufficient rigidity
to the cathode structure. Further, the dimensions of the
openings in the sheet or mesh have suitable values so as
to permit easy deposition of the diaphragm starting from a
suspension of fibers and possib:Ly of a polymeric binder as
well as good adhesion of the diaphragm after deposition. The
anodes, usually of the dimensionally stable type, are
interleaved with the fingers.
Said fingers (C) are provided with a diaphragm (not shown in
the figure). Spacers (not shown in the figure) may be
optionally inserted between the surfaces of said anodes and
the diaphragms. The cover (G) is made of corrosion resistant
material with outlets (H) for chlorine and brine inlets (not
shown). Hydrogen and caustics are released through ~I) and ~ `
(L) respectively.
Referring to fig. 2, the fingers (C) are provided with
the thin, foraminous sheet or mesh (F) of the present
invention, coated with the diaphragm (E) constitu~ed by
fibers and possibly by a polymeric binder.
The cathode assembly of the invention is provided with
catalytic properties to ensure a decrease of the cell
voltage to 3.10-3.15 V and allows for utilizing the cathode
struc ures of existing cells made of interwoven iron wires
or of a perforated sheet, thus minimizing the financial
investment cost. The application to the fingers (C) of the
:, :',
'~'~'''
'
~ 7 ~ 7
cathode structure of conventional chlor-alkali cells of a
fine and flexible, foraminous screen (F) made of a mesh or
expanded or perforated sheet provided with an
electrocatalytic coa~ing for hydrogen evolution in an
alkaline environment gives unexpected advantages.
The improved cathode of the invention is characterized by a
composite layer structure, wherein the more internal layer
is formed by the fingers (C) of the conventional cells,
while the more external layer is formed by the mesh or sheet
(F), provided with the electrocatalytic coating, which is
mechanically and electrically connected to the fingers. ~he
fingers therefore act both as supports and current
distributors.
.
The mesh or sheet (F) of the present invention, provided
with the electrocatalytic coating, may be made of iron,
chromium, nickel, copper and alloys thereof. ~he materials
most commonly used, due to their availability on the market,
are iron, stainless steel and nickel. When the first two are
selected, preferably before the catalytic activation a thin
layer of nickel, some microns thick, is applied by galvanic
deposition. The dimensions of the openings are not critical
but must be suitably selected in order not to lnterfere with
the deposition of the diaphragm or not to spoil the adhesion ~`
of the diaphragm to the cathode. The mesh or sheet of the
., !
-- ~ 2~ 7~7 - lo -
present invention must be thin to permit the greatest
flexibility, which i~ nece~sary during fixing to the cathode
s~ructures (fingers) of conventional cells.
The electrocataly~ic coating must be advantageously capable
of resisting current reversals and to minlmize the
deposition of metallic iron or electrocondutive compounds of
iron, such as magnetite when the fresh brine is polluted by
some parts per million of iron. The current reversals, as is
well known, occur whenever a monopolar cell of a production
line mu~t be excluded from operation. The exclusion i~
carried out by short-circuiting the cell with a suitable
jumper switch means and the cell is then removed from the
production line and sent to the service area, while copper
rods are inserted in its place. In this way the production
is not interrupted. During short-circuiting, the cell is
crossed by high reverse current which may easily damage the
cathode coating. In order to avoid this problem, suitable
short-circuiting devices have been developed which minimize
the intensity of the reverse current but are extremely
expensive. An alternative method consists in providing the
cathodes with coatings capable of undergoing even strong
çurrents reversals.
Among the types having these characteristics, most suitable
coatings are made of a substrate of nickel metal containiny
a dispersion of electrocatalytic particles as described in
BE 848458, obtained by galvanic deposition from a bath
:::
. ':
, . ,,, . . . . .. . ~ .. . ,... . ~. .. . . -
211~7'a7
containing suitable nickel salts and particles of
electrocatalytic material held in ~uspension by mechanical
stirring with the po~sible addition of suitable sufipending
agents. A similar coatlng obta:lned by galvanic deposition
compri~es a nickel matrix havlng ~uspended thereln par~icles
of electrocatalytic material and particles of ~ material
capable of absorbing high quantities of hydrogen in the form
of hydrides, as described in U5 5,035,790.
The said coatings are al30 capable o~ resistlng the
aggressive attack by the active chlorine dissolved in the
brine which flows and diPfuses through the diaphragm during
the first minutes of the shut-down of the cells.
As regards the problem connected to the presence of
remarkable quantities of iron in the fresh feed brine, this
practically brings about the formation of dendrites of :~
metallic iron or electroconductive iron oxide such as
magnetite, capable of crossing the diaphragm and causlng
hydrogen discharge directly i~ the anodic compartment with
the formation of dangerous hydrogen/chlorine mixtures. A
first obvious alternative ~olution is represented by ~ :
sub~ecting the fresh brlne to a suitable pre-treatment and
excluding from ths circuit any steel component which with
time and with the formatlon of defects may become a
continuou~ source of poisoning of the brine. It is eviden~
:'' ''
~,
' ~,''.
'''' ' '
- - 12 -
2 1~ 7
thst these countermeasures involve high C03tS. A 3econd
alternative is to resort to very active electrocatalytic
coatings, which operate at such a potential that the
formation of metal iron or magnetite, if not impossible, is
at least strongly slowed down. This alternative,
particularly if combined with a geometrical ~hape of the
substrate capable of degrading the adhesion of the dendrites
favouring the detachment by the hydrogen bubbles, is
extremely efficient and permits one to eliminate the need
for additional investment costs for the installation of
equipments and filtering systems for brine purification.
Coatings of this type are described in US patent No.
4,724,052.
The method of preparation of the cathode assembly of the
present invention comprises preparation of the perforated or
expanded sheet or fine mesh screen provided with the
electrocatalytic coating, and the pre-treatment of the
cathode structure of conventional cells. In the case of used
cathodes, the original diaphragms must be carefully removed
to eliminate even the minimum residue of fibers and
polymeric binder. This removal may be efficiently carried
out in compliance wi~h the current health regulations by
using strong water ~ets under pressure and collecting the
liquid which is to be sent to a treatment
section. The polished structure thus obtained must be free
.
- 13 -
211~7~7
of any rust or deposits of any nature. This may be obtained
by hydrosand-blasting or chemical pickling using acid
solutions added with suitable filming corrosion inhibitors.
In the case of new cathode struc:tures, the pre-treatment is
limited only to hydrosand-blasting or chemical pickling.
After careful final washing and drying under forced air
circulation, the fine electrocatalytic expanded sheet or
mesh must be readily applied. The mesh or sheet is cut into
strips of suitable dimensions and the strips are then
pressed carefully onto the surface of the fingers of the
cathode structure. The strips are then mechanically flxed so
as to make the electrical continuity between the
electrocatalytic sheet or mesh and the cathode structure as
extended as possible. To attain this goal, the activated
sheet or mesh must be particularly flexible and capable of
conforming to the profile of the cathode structure, which
may certainly present distorsions of various kinds. Further,
the number of contact points must be very high. As a
consequence, the most advantageous fixing method is
electrical spot welding. It must be noted that the weldings
spots are only to ensure electrical continuity and a
particular mechanical resistance is not required. In fact,
the activated sheet or screen applied to the external of the
cathode structure, are subjected to a hydraulic head during
operation which tends to keep them pressed against the
cathode struc~ure themselves. The composite cathode assembly
^ - 14 -
211 ~7~7
thus obtained is ~hen subjected to deposition of the
diaphragm, which i8 carried out according to the
conventional technigues, without particular variations, if
the dimensions of the openings in the sheet or mesh are
suitably selected.
In the following Examples, there are described several
preferred embodiments to illustrate the pre~ent invention.
However, it should be understood that the invention is not
intended to be limited to the specific embodiments. For
example, it is evident to one skilled in the art that the
cathode assembly of the present invention may apply also for
membrane cell~ of the so called bag cell type, which are
obtained from existing chlor-alkali diaphragm cells using
ion exchange membranes in the form of a bag capable of
enveloping the cathode fingers. ~ ;~
. ~
EXAMPLE 1
Two MDC55 electrolysis cells from a chlorine production
line were shu~ down and disassembled. The diaphragms were
removed from the fingers by washing with water jets under
pressure and a subsequent pickling in inhibited 6~
hydrochloric acid at 70C, for about one hour. The
structures were then carefully washed with industrial water
.. ..
211~7~7 - 15 -
until a pH 5 was obtained, then w$th water alkalinized by 1%
by weight of sodium carbonate and then with demineral~zed
water, followed by drying under forced hot air clrculation.
The fingers of the two cells were readily provided
respectively with an activated nickel mesh and an activated
expanded sheet prepared according to the teachings of
Example 1 of US patent 4,724,052. -
The mesh was made of nickel wire having a diameter of 0.3 mm
and forming square openin~s of 2 x 2 mm and the expanded,
flattened sheet had square openings having dimensions of 5 x
5 mm. After flattenlng, the thickness of the sheet was 0.5
mm. The application was carried out by maintaining the
activated mesh and the sheet pressed against the surfaces of .
the fingers and then spot welding with a portable welding -`
machine. The welding points formed a square reticulate with
a distance among the points of 30 mm. :-:
: . . . -:
.- ' ..-
~: The two composite cathode structures were then coated
with a diaphragm comprislng asbestos fibers and a suitable
: fluorinated polymeric b~nder of the type MS2~ forming up to
a thickness of 3 ~m.
The coated cathode structures were then treated in oven
according to the conventional technique to obtain mechanical
stabilization of the fibers by the polymeric binder.
. ~, :",.
- ' '`:,
21147~7 - 16 -
The two cells were re-insexted in the production lin~,
with the following average parameters:
- cell voltage: 3.35 Volts - -~
- current density: 2200 Ampere/square meter
- fresh feed brine: 315 grams/liter, flow rate: 1.6 cubic
meters/hour
- outlet liquid: 125 grams/liter of caustic, l90
grams/liter of sodium chloride at 95C
- oxygen content in chlorine: 3.2%
- current efficiency: 93%
- dimensionally stable expandable anodes provided with 3 mm
spacer~.
The voltage of the two cells equipped with the composi~e
cathode assembly of the present invention, detected once
stationary operating conditions were reached, was about 3.07
Volts, that is 0.28 Volts lower than the average value
typical of the production line. The voltage then slowly
increased to 3.10 V in 15 days and remained thereafter ~-~
constant. No noticeable variations in current efficiency or
oxygen content in chlorine were detected.
After 47 days of operation, the cell equipped with the
. . :
activa~ed mesh made of nickel wire was subjected to 12 daily
short-circuitings. Upon reaching stationary operating
condition, the voltage had only negligibly increased to 3.12
2~7 ~7 - 17 - ~
` ~:
Volts. Similar results were obtained using activated meshes
made of a wire having a diamete:r of 0.5 mm foxming openings
of 5 x 10 mm. The above data demonstrate that the selection ~ ~ `
of the mesh geometry may be made over a wide range and it is
possible to operate chlor-alkali diaphragm cells equipped
with activated cathodes even in the event of severe
anomalies, as it is the case during short-circuiting,
without experiencing appreciable voltage increases.
': . `^ ',''.
. '. '. :-
EXAMP~E 2 ` -
~ .. ~. -
The cell of Example 1, provided with the same activatedmesh made of a nickel wire, was fed with fresh brine
containing 0.01 grams~liter of iron. For comparison
purposes, also a reference cell from the production line ~-
havinq an operating lifetime of 50 days, was also fed with
the same polluted brine. The reference cell was shut down
after 28 days of opera~ion when the hydrogen content in the ~-
chlorine reached 0.8%. The cell equipped with the cathode
assembly of the invention showed a hydrogen content in
chlorine of 0.2% substantially unvaried during the whole
operation.
~ ''
Various modifications of the structures and cells of the
invention may be made without departing from the spirit or
:. '. ~
- ~' "' ~', '
211~7~7 - 18 -
scope thereof and it l~s to be understood that the invention
- is intended to be limited only as defined in the appended
cl~ims.
: ', ' ,
~ ~ :- .;.. .:
: .,
:.:. ~.
,
.: ~ ,
'', "~:
~'' ',
~ . . ~'.