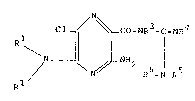Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~; ~
,~ FIL!E~, J~ THIS AM~NDED
T~:~ TRANSLATION 21~ ~ 7 ~ S
-- 1 --
New pyrazine derivatives the preparation and
use thereof
The invention relates to new tetra-substituted pyrazine
derivatives, the preparation thereof by conventional
methods and their use in the production of
pharmaceutical substances or drugs.
The new compounds correspond to the formula
N
Cl_~ / ~ Co-NR3-c=NR4
N~ ~LNH2 ~ ~
/ N
R
' ,~ '
and may occur in the form of bases or salts with acids.
.
In formula I, independently of one another:
R1 represents H or a (Cl~)alkyl group,
R2 represents a 1-morpholinyl group,
a straight-chained or branched (C18)alkyl chain
which may be substituted,
a) by a substituted or unsubstituted N,O,S-
heterocyclic group,
b) by the phenyl group which is optionally mono-
or polysubstituted by a tCl 4) alkoxy group,
which may in turn be functionalised by a
hydroxyl or alkylamino group of formula
.. .. .
!: ` ' '~ ~ ' '` ' ` ' '
- -```` 2 ~ 7 3 ~
, .
-N (II),
wherein R7 and R8 independently of each other
denote hydrogen or a (C14)alkyl group;
c) by an aminocarbonyl group of the formula
R7 ~ :
I
O l8 (III),
R :
wherein R7 and R8 are defined as hereinbefore;
d) independently of one another by one or more
(C14~alkoxy groups, the hydroxyl or a ~ ~`
hydroxyl(C14)-alkyl group or a phenoxy group
which may in turn be mono- or polysubstituted
by halogen (F, Cl), a cyano group, (C14)alkyl,
(cl4)alkoxy or (C1.4)alkoxy-(Cl.4)alkyl;
e) by an amino group of the formula
-N \ (IV),
R10
wherein the groups R9 and R10 independently of
each other denote hydrogen, a mono- or
polynuclear N,O,S-heterocyclic group, a mono~
or polysubstituted phenyl group substituted
either homogeneously or by a mixture of :
halogen (F, Cl), (C14)alkyl or a sulphonamide ~ :~
group, an N,O-heterocyclic acyl ~roup, a di-
or trichlorophenylsulphonyl group substituted
,,.. ~ 2~1 j7
-- 3
by an amine group, or a (C14)alkyl chain,
which may be substituted independently of each
other by the hydroxy group, or by a phenyl
group which is optionally mono- or
polysubstituted by halogen (F, cl), hydroxy,
(C~ 4) alkyl or (C14)alkoxy-(C14)alkyl, or R9 and
R10 form a morpholine ring together with the
nitrogen atom to which they are bound and an
oxygen atom;
a 4-piperidinyl group which may be substituted in
the 1-position ~ -
a) by the acyl group of an aliphatic, alicyclic,
aromatic or heteroaromatic carboxylic acid;
b) by a (C18)alkyl chain which in turn is
substituted, independently of each other, by
the hydroxy group or by a (C14)alkoxy- or
(C14)alkoxy-(C14)alkyl-substituted phenoxy
group or by an ~-naphthoxy group;
and amidino group of the formula
-C-NHRll (V), . .
NH
wherein R11 represents a phenyl group which is
mono- or polysubstituted by halogen ~F, Cl) or
a (Cl 4) alkyl group;
Rl and R2 together denote, together with the nitrogen
atom to which they are bound, a piperazine ring
which may be substituted
a) by an N,O,S-heterocyclic group,
,
211~7~
- 4
b) by the acyl group of an aliphatic, alicyclic,
aromatic or heterocyclic carboxylic acid,
c) by a (C18)alkyl chain, which may in turn be
substituted indep~ndently of one another, by
the hydroxy group or by a (C14~alkoxy- or
(C14)alkoxy-(Cl4)alkyl-subs-tituted phenoxy
group or an ~-naphthoxy group;
R3, R4, Rs and R6 have identical or different meanings and
represent hydrogen, (C18)alkyl or benzyl.
The phrase "N,O,S-heterocyclic group" denotes those ring
systems which contain one or more identical or different
heteroatoms of the type specified. The term
"polynuclear heterocyclic groups" also refers to those
ring systems which are made up of heterocyclic and
carbocyclic rings, such as quinoline and quinazoline.
These ring systems may contain one or more substituents
such as C14-alkyl, C16-alkoxy and/or amino. Similarly,
"N,O-heterocyclic group" encompasses both pure N-
heterocyclic groups such as piperidine, piperazine and
also morpholine. If the alkyl groups in the above
definitions may contain up to 8 carbon atoms, those
containing up to ~ carbon atoms are preferred; of the
(C1~4)alkyl or alkoxy groups, those which are preferred
are the ones which contain up to three carbon atoms.
The preferred substituents in phenyl or phenoxy groups
are alkyl or alkoxy groups having one or two carbon
atoms as well as F and Cl. R3 and Rs preferably denote
hydrogen, R4 and R6 preferably denote hydrogen, methyl or
ethyl, and one of the two groups also denotes butyl or
benzyl. The acyl groups of the aliphatic carboxylic -~
acids contain up to 18 carbon atoms, those of the
alicyclic carboxylic acid contain up to 8 carbon atoms.
The short-chained aliphatic carboxylic acids may also be
substituted by phenyl or heteroaryl, the aromatic
.,. ;,,"~ , ,,,, .,," ~,~" ","~ ~ ,".,,,, ~ ",; ~ , ",,~
2 1 1 5 7 5 r
. -~ .3
carboxylic acids optionally contain substituted phenyl,
and the heteroaromatic carboxylic acids are derived from
monocyclic heterocyclic groups which contain N, O and/or
S. Whereas Rl is preferably hydrogen or methyl, R2
preferably represents larger groups, e.g. those
contained in the following Tables, for example:
Cl ~\~ o-c~l2-cH(oH)-cH2-N(cH3)-(cH2)3
Cl
<~NH-C- .
R
CH3 NH
:: ~
j 2
CH30~ 7
3 ~ ~_NH-CH2-CH2-
N
21~ ~7~
-- 6
or R1 and R2 form a substituted heterocyclic group with
the nitrogen to which they are bound, e.g.
NH2
CH30 \~ N~N N
3 2
3 ( 2 ) 14 \~
CH30-CH2-CH2~ < ~ o-CH2-CH(OH)-ICH2
~ Methods of preparing the new compounds are known to
: those skilled in the art:
1) a lower alkylester of 3-amino-5,6-dichloropyrazine~
2-carboxylic acid is reacted under anhydrous
conditions with an amine of formula
7 ~ ~
HNR1R2 (VI)
wherein R1 and R2 are as hereinbefore defined, and
the resulting compound of formula
Cl__ f ~ _ C02-alkyl
R R N ~ NH2 (VII)
(alkyl = (C14)alkyl)
is converted with a guanidine of formula
HNR3 C=NR4
NRSR6 (VIII)
-:
wherein R3 to R6 are as hereinbefore defined, into
the desired end product.
The reaction of the 3-amino-5,6-dichloropyrazine-2-
carboxylic acid ester with the amine component VI
to obtain the intermediate compounds of general
formula VII is carried out using methods known from
the literature in an inert solvent at elevated
temperature in the presence of an acid acceptor.
The amine component is generally used in equimolar
amounts. However, it is also possible to use the
amine component as the acid acceptor and solvent.
Preferably, however, the solvent used is
dimethylformamide or dimethylsulphoxide or mixtures
thereof~ The reaction temperature is not crucial.
As a rule, the reaction is carried out at a
~ 21157~
8 --
temperature in the range from 80 - 100C depending
on the reactivity of the amine used. Under these
conditions the reaction is finished after about l
to 2 hours. Organic and inoryanic bases may be
used as acid acceptors. Preferably, tertiary
amines such as pyridine, N-methylpiperidine,
dimethylaniline or triethylamine are used.
The 3,5-diaminopyrazine carboxylic acid esters of
formula VII prepared in this way may, if desired,
be subjected to subsequent treatment, e.g. a
reaction of acylation or alkylation.
Acylation is carried out by known methods, e.g. by
reacting a diamine of formula VII with a reactive
carboxylic acid derivative.
The alkylation is used, for example, in the
preparation of corresponding phenoxypropanolamine
derivatives. For this purpose, a diamino compound
of the substructure IX of formula VII:
.
H\ Cl ~ ~ _ COzalkyl
N-alk-N ~ ~ _ NH2 (IX)
RS / ¦ \N
,., ~ .
(alk = (Cl~B)alkylene, alkyl, Rs as above)
is reacted in a suitable solvent e.g. with a
phenoxypropyleneoxide of general formula ~;
~; 2 1 ~ 5 7 ~ ~
~-CH2 -cH-cH2
~(=~ \ / (X)
Z
z = ~c14)alkoxy,
(C14)alkoxy- (C1 4) alkyl
at ambient temperature or slightly elevated
temperature. Generally, the reaction is complete
in about 0.5 to 1 hour.
However, the direct method of synthesis described
above is preferred.
In order to prepare the end products of formula I
the 3,5-diaminopyrazine carboxylic acid esters of
formula VII are reacted with a guanidine of formula
VIII in a suitable solvent with heating. Simple
alcohols are particularly suitable as the solvent.
Preferably, the reaction is carried out in methanol
at boiling temperature. Under these conditions the
reaction is generally complete after about 30 to 90
minutes.
2~ Reaction of a pyrazine derivative of formula
' ~'.:''.
Cl~ CO-NR -C=NR
~ ~ (XI),
\N R N-R5 .
211~ 7 5 ~
-- 10 --
wherein R3 to R6 are as hereinbefore defined,
with an amine of formula
~NR1R2 (VI),
wherein Rl and R2 are as hereinbefore defined.
The reaction is preferably carried out at elevated
temperature in a polar solvent, if possible
anhydrous, e.g. dimethylformamide,
dimethylsulphoxide.
The starting compounds of formula XI are obtained
by conventional methods. They may for example be
obtained according to process 1) if pyrazine
carboxylic acid esters are used which are of
similar construction to the esters of formula VII
but contain a chlorine atom instead of NR1R2 in the
5-position. ;~
,: :
The compounds of formula I may be used as active
substances in pharmaceutical preparations or may be used
as intermediate products for preparing such active
substances. The new compounds inhibit the Na~/H~- and
Na~/Li~-exchange, inter alia. The active substances~ ~ -
aceording to the invention may be used as
antihypertensives, mucolytics, diuretics and
caneerostatics; they may also be used in diseases
connected with ischaemia (for example: cardiae,
cerebral, gastrointestinal, pulmonary and renal
isc~aemia, ischaemia of the liver, ischaemia of the
skeletal musculature). Corresponding diseases include,
for example, coronary heart disease, angina pectoris, ;
embolisms in the circulation of the lungs, acute or
chronic kidney failure, chronic kidney insufficiency,
cerebral infarct, chronic circulatory ~isorders of the
brain. During reperfusion of the ischaemic heart (e.g.
``` 2~1~7~
-- 11 --
after an attack of angina pectoris or a cardiac infarct)
irreversible damage may occur to cardiomyocytes in the
affected region. The compounds according to the
invention may be used in such cases for
cardioprotection.
The prevention of damage which may occur as a result of
reduced circulation during transplants should also be
included in the field of ischaemia.
The active substances may be administered in
conventional forms, e.g. plain or coated tablets,
capsules, granules, injectable solutions, and possibly
nasally administered preparations, the active substance
generally being present in an amount of 1 to 200 mg,
preferably 20 to 100 mg per dosage unit. These
pharmaceutical preparations are produced in a manner
known per se.
2~ 1~rl~r~
- 12 -
Examples
l. Tablets (Composition)
Compound according to Example 40.0 mg
Corn starch 144.0 mg
Sec. calcium phosphate115.0 mg
Magnesium stearate l.0 mq
300.0 mg
2. Gelatine capsules
The contents of a capsule consist of 50.0 mg of a
compound according to the invention and 150.0 mg of
corn starch.
Note: Under the headings "Form" the Tables which follow
indicate whether the preparation is a salt (HCl = . :~
hydrochloride, 2.HCl = dihydrochloride, etc.) or the
base (BS).
',: . ~ ,.
: `~
2 ~ 1 3 7 ~ ~
- 13 -
Exam~le 1
a) Methyl 3-amino-6-chloro-5-(2-[1-(1,6-dimethyl-
phenoxy)]propylamino)-pyrazine-2-carboxylate
4.44 g (20 mmol) of methyl 3-amino-5,6-
dichloropyrazine-2-carboxylate, 3.6 g (20 mmol) of
2-amino-1-(2,6-dimethylphenoxy)propane and 2.2 g -
(22 mmol) of triethylamine are heated to 9~ - 100C
in 40 ml of anhydrous dimethylformamide for 1~
hours. After the solvent has been distilled off ln
vacuo the residue is purified on silica gel
(eluant: ethyl acetate/isopropanol/NH3 (70:30:1).
Yield 7.3 g
b) N-amidino-3-amino-6-chloro-5-(2-[1-(2,6-
dimethylphenoxy)~propylamino)pyrazine-2-
carboxamide-hydrochloride
7.3 g (0.02 mmol) of pyrazine carboxylic acid ester
from Example la) are dissolved in 50 ml of methanol
and heated with 80 ml of a 1 molar methanolic
guanidine solution for 45 minutes over a boiling
water bath. The solvent is distilled off in vacuo
and the residue is purified over a silica gel
column (eluant: ethyl acetate/isopropanol/NH3
(70:30:5) and the hydrochloride of the end product
is prepared.
Yield 4.9 g; melting point 267 - 270C.
2 ~ 7 ~ ~
- 14 -
Example 2
,~
a) Methyl 3-amino-6-chloro-~-[N'-(5-fluoro-2-
methyl)phenyl]-guanidino-pyrazine-2-carboxylate
13.3 g ~60 mmol) of methyl 3-amino-5,6-
dichloropyrazine-2-carboxylate, 15 g (90 mmol) o~
(5-fluoro-2-methyl)-phenylguanidine and 6 g of
triethylamine are stirred into 100 ml of DMS0 for 2
hours at 90C. After cooling, 100 ml of CH2Cl2 are
added. 100 ml of water are added dropwise, with
cooling, the CH2Cl2 phase is separated off and the
aqueous phase is extracted with 150 ml of CH2Cl2.
The CH2Cl2 phases collected are washed with a little
water, dried over MgSO4 and concentrated by
evaporation. The residue is pu~ified over silica
gel (eluant: ethyl acetate/isopropanol (95:5).
Yield: 12.6 g
/?
b) N-Amidino-3-amino-6-chloro- ~ '-(5-fluoro-2-
methyl)-phenyl]guanidino-pyrazine-2-carboxamide ~
:: ~ :~:
5.5 g of 2-methylpyrazinecarboxylate from Example
2a) are dissolved in 25 ml of anhydrous
dimethylformamide, 50 ml of a 1.2 molar methanolic
guanidine solution are added and the resulting
mixture is refluxed for 30 minutes. Then the
methanol is distilled off and the residue is
purified on silica gel (eluant: ethyl
acetate/isopropanol/NH3 (70:30:5)) and the reaction
product is converted into the hydrochloride.
Yield: 8.7 g; m.p. 242C.
::
211~7~
- 15 -
Example 3
N-Amidino-3-amino-6-chloro-5-(4-[2-(3-methoxy-
phenoxy)ethyl~l-piperazinyl)-pyrazine-2-
carboxamide-dihydrobromide
858 mg (3 mmol) of N-amidino-3-amino-5,6-
dichloropyrazine-2-carboxamide, 924 (3 mmol) of N-
[2-(3~methoxyphenoxy)ethyl]piperazine and 1.2 g
(12 mmol) of triethylamine are heated to 95 - 100C
for 75 minutes. The solvent is distilled off in
vacuo, the residue is purified over silica gel
(eluant: ethyl acetate/isopropanol/NH3 (7:3:1)) and
the reaction product obtained is converted into the
dihydrobromide.
Yield: 1.13 g; m.p.: 159 - 162~C.
~::
_ample 4
a) Methyl 3-amino-6-chloro-5~[N-(2-pyrrolocarbonyl-
amino)ethyl-N-methylamino]-pyrazine-2-carboxylate
2.73 g (10 mmol) of ethyl 3-amino-6-chloro-5-[N-(2-
amino)ethyl-N-methyl]amino-pyrazine-2-carboxylate,
1.35 g (10 mmol) of l-hydroxy-lH-benzotriazole-
hydrate and 1.11 g (10 mmol) of pyrrolo-2-
carboxylic acid are dissolved in 50 ml of anhydrous
tetrahydrofuran and stirred with 2.06 g (10 mmol)
of dicyclohexylcarbodiimide for 15 hours at ambient
temperature, whilst cooling with ice. The reaction
product is isolated in the usual way and purified
over silica gel (eluant: ethyl acetate/isopropanol
(7:3).
Yield: 3.09 g
21~7.~
- 16 -
b) N-Amidino-3-amino-6-chloro-5-[N-[2-(pyrrolyl-
carbonylamino)ethyl]-N-methyl]aminopyrazine-2-
carboxamide hydrochloride
Prepared analogously to Example 1.
3.09 g (10 mmol) of methyl pyrazine-2-carboxylate
from Example 3 yield 1.65 g of the title compound;
m.p. 181 - 184C.
Example 5
N-Amidino-3-amino-6-chloro-5-[4-[(4-amino-6,7-
dimethoxy)-2-quinazolinyl]-1-piperazinyl]-pyrazi~e-2-
carboxamide-dihydrochloride
13.1 g (45.3 mmol) of N-[(4-amino-6,7-dimethoxy)-2-
quinazolinyl]-piperazine, 10 g (45.3 mmol) of methyl 3-
amino-5,6-dichloropyrazine-2-carboxylate and 7 ml of
triethylamine are reacted in 60 ml of dimethylsulphoxide
for 2 hours at 80C. After cooling, the reaction
product is precipitated by the addition of 100 ml of
water, suction filtered, dried and converted into the
end product without any further purification using the
method described in Example la) by reacting with
guanidine.
Yield: 10.5 g; m.p. > 290C.
The compounds listed in the Tables which follow may be
obtained analogously to the Examples and in accordance
with the remarks in the specification. If the compounds
take the form of the base the abbreviation BS appears in
the column "Form", otherwise the acid with which the
salt is formed is specified~
2 ~ 7 ~ ~
- 17 -
Table 1
Compounds of formula
>~ NH2
Nr. Rl ~2 Form m p [Cl ~ ~:
~ .
I CH3 CH3 HCl >280
H CH2-C~2 ~ 188-189
3 H CH2-CH2-Cll-N(cH3)2 HCl 262-264
4 H CH2 ~ CH2-CH(QH)-CH2-N(CH3)2 2 HCl amorph
H CH2 ~ CCH3 2- HCl
6 H 2 ~ ~ ~ 2 HCl 252-253
7 H CH(CH3)-CH2 ~ 3 HCl 250-2S3
H3C
8 H CH(cH3)-cH2 ~ HCl 278
H3C
9 CH3 CH(CH3)-CH2 ~ HCl 232-235
H3C
~ `" 211 ~7~
- 18 -
Nr. Rl R2 Form mp. L C]
==== === ==== ==== = ================_=========_= ===== = .
IH CH2-~i2 ~ HCl 145-148
~`
O~i3 : : :
II H CH2-CH2 ~ 2-CH2-C~3 HCl - 117-119
l2 H CH2-CH(CH2OH) ~ i2-CH2-C-CH3 HCl 197-200
l3 H CH2-CH2 ~ 1 2 HCl 122-125
l4 C~i3 CH2-CH2 ~ i~Cl 141-143
CC~i3
H CH2-CH(OcH3)2 205-207
l6 CH3 C~i2-cH(o~ {~i2 ~ 2-CH2-c-cH3 HCl amorph
l7 H CHz-CH(C~I)-C~i2 ~ CH2-c*i2-c-{~l3 HCl 116-119
l8 CH3 CH2-CH(OH)-CH2 ~ l HCl 112-115
Cl
l9 CH3 Ci-i2-{~i(Oil)-C~i2 ~ 207-209
,
.
2 ~ 7 ~ ~
-- 19 -- .
Form mp,lC]
______ _ _ _ _==================================================
Cl
.20 H CH2-CH2-NH-sO2 ~ 1 HCl187-191
H ~
~ 3
21 H CH2-CH2-N(CH3)-C$I2-CH(c~) CH2 ~ 2- HCl118-120
Cl
( 22 H CH2--CH2-N(CH3)--CH(OH)--CH2 ~ ~S 83-87
Cl
23 H CH2-CH2-N~_~,0 200-202
24 CH3 CH2-CH2-N(CH3)-cH2-cH2 ~ 2-CH2-cCH3 BS 95-97
H3C
H CH2-CH2-NH ~ BS 210-215
3C
26 H CH2-CH2-NH- ~ 1 225-228
~ OCH3
27 H CH2-c*l2-NH ~N ~ cx~i3 2 HCl 230-235
Cl
28 H CH2-{~2-NH ~ NH2 2 HCl ~250
SD2NH2
29 CH3 CH2-C~I2-NH-ICI ~ BS 140-142
O : :~
CH3 CH2-CH2-NI~-c ~ HCl 183-186
O H
~\
~ 2 ~ ~57~
- 20 -
Nr. R R2 Form mplC~
H3C~__
31 H CH2-CH2-NH-C~l-cH(cH3)-cH2 ~ 2 HCl amorph
3C
. .,
( 32 H CH2-CH2-NH2 2 HCl >270
33 H CH2-CH2-N~ J 3 - HCl 195-200
H
34 H CH2-CH2-NH-502 ~ 2 HCl 168-171 ;
Cl
3 5 H CH2-CH2-CH2-N~-S02 ~ 1 HCl 250-252
Cl
H CH2-CH2-C~12-NH ~ 502NH2 HCl 211-215
H2~25
37 H CH2-{~12-{~l2-N(CH3)2 2 HCl 268-270
: :~
~:: : .: ::
38 H CH2-cH2-cH2-N(cH3)-cH2-cH(oH)-cH2 ~ 1 BS amorph :~
Cl ~ :
39 H CH2-CH2-CH2-N(CH3)-CH2-CH(OH)-CH2 ~ 2-CH2-OCH3 ~;
2 HCl amorph
: `\
211~7~
Tabl e 2
Compounds of formula
Cl ~ N CC~-C-~I
~ ,1~ ,~ NH
R-~V~ N ~12
Nr . R Form mp. [ C]
(
I CH2 - CH (0~) -CH2~2-cH2-ocH3 BS 184-187
2 CH2-CH(OH)-CH2~ BS 163-167
OCH3
3 CH2-CH (Ol~) -{~2~ BS amorph
4 C~
o 187-190 .
S ~Cl-CH(cH3)2 ~5 21~-220
O ~ ';
: ~ :
`- 21~57~
- 22 -
Table 3
Compounds of formula
~ N ~ CcNH-c-~H2
RN-C N N NH2
H ~
Nr. R Form _mp.lC]
================================================== === ===========_= :
Cl .
'I ~ - ': '.
~=J 2 HCl 237-240
H3C~
2 ~ Cl
Cl ~ 2 ~ICl 264-267
2 HCl 239-242
Cl Cl
F -~
4 ~ 2 HCl 242
H3C
C ~ 177-180 '~
,': ~ ~ :.:-~
- 23 -
Table 4
Compounds of formula
<IMG>
21 1 ~75~
- 24 -
Nr . R Form mp. l Cl
===================================================================
OCH
I O CH2 - CH2~ 2 HBr 159-162
I I C~2--CH(OH)-C~I2~2-cH2--ocH3BS --180-182
12 CH2 - CHtoH)-c~l2~ 2 HCl 268-270
13 CH2 - CH(OH)-CH2~ 2 - HCl 287-290
~. :
111 Cl~ BS196-198 - ~
O '~
: ~'
^' ~'~''