Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
_,_ 21' 1661 l
BACKGROUND OF THE INVENTION
1. Field of the Invention
The present invention relates to novel esculetin
derivatives and pharmaceutical compositions, more particularly
an agent for protecting cartilage, i.e., a chondroprotective
agent.
2. Description of the Related Art
There are various types of arthropathy, for example,
rheumatoid arthritis, rheumatic fever, and osteoarthritis. Many
people particularly suffer from rheumatoid ari~hritis and
osteoarthritis, and these diseases are considE~red the major
types of arthropathy. There are congenital and secondary
osteoarthritis, and further primary osteoarthr_itis caused by
degeneration of the articular cartilage along with aging.
Patients suffering from primary osteoarthritis have recently
been increasing along with the increase in the population of the
aged.
Although there are considerable differences of the causes
and conditions between rheumatoid arthritis and osteoarthritis,
the articular function becomes eventually obstructed by the
destruction of the cartilage in both of rheumatoid arthritis and
osteoarthritis.
The first choice of medicines for treatment of rheumatic
diseases, such as rheumatoid arthritis, rheumatic fever,
systemic lupus erythematosus, or osteoarthritis, are analgesic
and anti-inflammatory agents, for example, aspirin or
indomethacin. Further, gold compounds (for example, Shiosol),
immunomodulators, steroids, or D-penicillaminE~ is used as the
medicine for treatment of rheumatoid arthritis.
The above conventional analgesic and anti-inflammatory
agents, however, were not effective against the destruction of
the articular cartilage, and in fact, sometimes exhibited
adverse effect in the experiments using chondrocytes. Further,
no function to suppress the destruction of art:icular cartilage
was found in the above medicines for treatment~ of rheumatoid
2116617
-2-
arthritis.
It was known that esculetin and 4-methylesculetin exhibit
the function to reduce cholesterol level, strengthening the
veins, and anti-oxidation (Japanese Examined :Patent Publication
No. 42-16626). Further, it was also known that diesters of 4-
methylesculetin with carboxylic acids having 6 to 25 carbon
atoms, particularly, diesters of caprylic, lauric or palmitic
acid exhibit an effective anti-inflammatory action in the
treatment of skin disease (FR 2276819). However, it was not
known that the above esculetin and esculetin derivatives exhibit
a function to suppress cartilage destruction.
SUMMARY OF THE INVENTION
The inventors found that esculetin compounds, namely,
esculetin and esculetin derivatives, showed significant
inhibition of the depletion of proteoglycan which is a major
component of the cartilage matrix, and therefore, are useful as
a chondroprotective agent for prohibiting the destruction of the
articular cartilage. Further, the above escu:Letin compounds
involve some novel compounds.
Accordingly, the object of the present invention is to
provide a pharmaceutical composition, particu:Larly, a
chondroprotective agent, comprising an esculetin compound as an
effective ingredient.
Another object of the present invention .is to provide a
novel esculetin derivative.
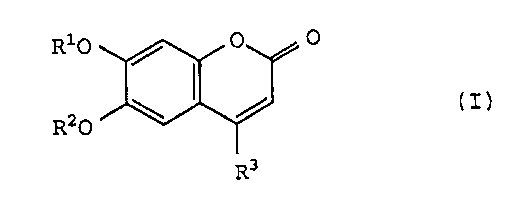
The present invention relates to a pharmaceutical
composition, particularly a chondroprotective agent, comprising
a compound of the general formula (I):
Ri0 / 0 O
\ / ( I ,I
R O
R3
wherein R1 and R2 are, independently, a hydrogen atom, a
saturated or unsaturated aliphatic acyl having 2 to 25 carbon
-3- 21 16617
atoms or benzoyl group and R3 is a hydrogen atom or alkyl group
[hereinafter referred to as the present compound (I)].
Further, the present invention relates to a compound of the
general formula (II):
8110 / 0 O
12 ~ ~ ~ (I7.)
R O
R13
wherein R11 and R12 are independently a hydrogen atom, pivaloyl,
capryloyl, lauroyl, palmitoyl, stearoyl, linoleoyl,
docosahexaenoyl, or benzoyl group and R13 is a hydrogen atom or
methyl group [hereinafter referred to as the present compound
(II) ] .
The present compound (II) is novel, and encompassed in the
scope of the present compound (I) exhibiting the cartilage
protecting function. Therefore, in the present specification,
the following explanation regarding the present compound (I) may
also be applied to the present compound (II), if applicable.
DESCRIPTION OF THE PREFERRED EMBODIMENTS
In the present compound (I), preferred examples of the
groups R1 and R2 are a hydrogen atom, acetyl, pivaloyl,
capryloyl, lauroyl, palmitoyl, stearoyl, lino:Leoyl,
docosahexaenoyl and benzoyl group, and more preferred examples
are the groups R11 and R12 defined in the present compound (II),
namely, a hydrogen atom, pivaloyl, capryloyl, lauroyl,
palmitoyl, stearoyl, linoleoyl, docosahexaenoyl, or benzoyl
group. Particularly preferred examples of the groups R1 and R2
or groups R11 and R12 in the present compounds (I) and (II) are
a hydrogen atom, pivaloyl, stearoyl, and benzoyl group. The
examples of the group R3 are preferably a hydrogen atom and a
lower alkyl group having 1 to 4 carbon atoms, more preferably
the group R13 in the present compound (II), namely, a hydrogen
atom or methyl group.
The examples of the present compounds (I) and (II) are as
?116617
follows:
esculetin,
4-methylesculetin,
esculetin 6,7-bis(acetate),
4-methylesculetin 6,7-bis(acetate),
esculetin 6,7-bis(pivalate),
4-methylesculetin 6,7-bis(pivalate),
esculetin 6-monopivalate,
4-methylesculetin 6-monopivalate,
esculetin 7-monopivalate,
4-methylesculetin 7-monopivalate,
esculetin 6,7-bis(caprylate),
4-methylesculetin 6,7-bis(caprylate),
esculetin 6,7-bis(laurate),
4-methylesculetin 6,7-bis(laurate),
esculetin 6,7-bis(palmitate),
4-methylesculetin 6,7-bis(palmitate),
esculetin 6,7-bis(stearate),
4-methylesculetin 6,7-bis(stearate),
esculetin 6,7-bis(linoleate),
4-methylesculetin 6,7-bis(linoleate),
esculetin 6,7-bis(docosahexaenoate),
4-methylesculetin 6,7-bis(docosahexaenoate),
esculetin 6,7-bis(benzoate),
4-methylesculetin 6,7-bis(benzoate)
Of the above-mentioned compounds, esculetin 6-monopivalate,
esculetin 6,7-bis(pivalate), esculetin 6,7-bi~s(stearate), 4-
methylesculetin 6,7-bis(stearate), 4-methylesculetin 6,7-
bis(linoleate), 4-methylesculetin 6,7-bis(docosahexaenoate), and
4-methylesculetin 6,7-bis(benzoate) are the typical examples of
the present compound (II) and are novel.
Esculetin and 4-methylesculetin are commercially available
as reagents. For example, esculetin is purchased from the Tokyo
Kasei Kogyo K.K., Japan, and 4-methylesculeti;n is purchased from
the Sigma Chemical Company, (St. Louis, MO).
The various mono or diesters of carboxylic acids with
esculetin or 4-alkylesculetin may be prepared by reacting
esculetin or 4-alkylesculetin and various carboxylic acids
SECTION 8 CORRECTION
SEE CERTIFICATE
CORRECTION - ARTICLE S
VOIR CERTIF1CAT
_5-~ 01 16617
according to the following processes:
1) Esculetin or 4-alkylesculetin and a carboxylic acid are
reacted in a suitable solvent in the presence of an acid
catalyst, for example, an inorganic acid, such as hydrochloric,
sulfuric or phosphoric acid or an organic acid, such as acetic
or p-toluene sulfonic acid.
2) Esculetin or 4-alkylesculetin and a carboxylic acid are
reacted in an organic solvent, for example, dimethylformamide,
acetone, dioxane, acetonitrile, chloroform, methylene chloride,
tetrahydrofuran, or pyridine in the presence of a condensing
agent, for example, dicyclohexylcarbodiimide, N,N'-carbonyldi(2-
methylimidazole), diphenylketene-N-cyclohexylimine,
alkoxyacetylene, polyphosphoric acid ethyl ester, thionyl
chloride, or oxalyl chloride, usually while cooling or at room
temperature.
3) Esculetin or 4-alkylesculetin and an acid anhydride are
reacted in the presence of a basic compound, for example,
triethylamine, pyridine, 4-(N,N-dimethylamino)pyridine, or
diethylmethylamine.
4) Esculetin or 4-alkylesculetin and an acid halide (acyl
halide, such as chloride or bromide) are reacted in a solvent to
which a basic compound, for example, triethylamine, pyridine, 4-
(N,N-dimethylamino)pyridine or diethylmethylamine, or in a basic
solvent, for example, pyridine.
In the above processes, the monoester or diester is formed
depending on the ratio of the starting materials used. When the
carboxylic acid, acid anhydride, or acid halide is used in an
equimolar amount or a small excess amount to 'that of esculetin
or 4-alkylesculetin used, the monoester may be prepared, whereas
when the carboxylic acid, acid anhydride, or acid halide is used
in a large excess amount, usually in double the molar ratio or
more, the diester may be prepared.
In the latter case, a mixture of the diester and monoester
may sometimes be obtained. In this case, it :is possible to
easily separate the resulting diester and monoester by a
conventional separation method such as chromatography.
As the method to purify the reaction product, extraction,
chromatography, recrystallization, or reprecipitation may be
2116617
-6-
used. The structure of the purified product may be confirmed by
the infrared absorption spectrum, ultraviolet absorption
spectrum, nuclear magnetic resonance absorption spectrum,
elemental analysis, or mass spectrum.
The toxicity of the present compound (I) was examined.
Typical examples of the present compound (I) were administered
intraperitoneally at a dose of 750 mg/kg (bod;y weight) to male
mice continuously for four days. No deaths and no remarkable
toxicity were observed. The present compound (I) is extremely
safe (see Example 2).
The present compound (I) exhibits, as a pharmacological
effect, the function to inhibit destruction of chondrocyte
matrix in cultured chondrocytes (derived from cartilage of
rabbit shoulder and knee joints) (see Example 3).
Accordingly, the present compound (I) is useful as a
chondroprotective agent for treating various types of
arthropathy accompanying the cartilage destruction of the
joints. Examples of such arthropathy include rheumatoid
arthritis, osteoarthritis, periarthritis hume:roscapularis,
shoulder-arm-neck syndrome, lumbago, etc.
The pharmaceutical composition, particularly the
chondroprotective agent containing the present compound (I) as
an effective ingredient may be in the form of any conventional
formulation. The pharmaceutical composition may contain the
present compound (I) alone, or a mixture of t:he present compound
(I) with any pharmaceutically acceptable carrier or diluent.
The pharmaceutical composition may contain the effective
ingredient in an amount of 0.01 to 100 percent by weight,
preferably 0.1 to 70 percent by weight.
The chondroprotective agent of the present invention may be
administered orally or parenterally.
The dose of the pharmaceutical composition, particularly
the chondroprotective agent according to the present invention
varies with the patient (animal or human), age, individual
differences, state of illness, and the like. Generally
speaking, however, when a human is treated, the dose of oral
administration of the present compound (I) is in the range of
0.1 to 500 mg/kg (body weight) per day, preferably 0.5 to 200
-~7~ 21 16617
mg/kg (body weight), which is usually divided into 1 to 4 dosage
in a day, although the dose outside the above range may
sometimes be administered.
MP
The present invention now will be further illustrated by,
but is by no means limited to, the following Examples. In the
following Examples, TLC means thin layer chromatography.
~~g~gle 1 ' Pre~2aration of the Present Compound ( I ) or ( I I )
(1) Preparation of esculetin 6,7-bis(acetate)
Esculetin (Tokyo Kasei; 890 mg, 5 mmol) .and 4-(N,N-
dimethylamino)pyridine (1.528 g, 12.5 mmol) were put into an
eggplant type flask (50 ml), and then, methylene chloride (10
ml) was added to give a suspension. To the resulting
suspension, acetyl chloride (Wako Pure Chemicals; 918 mg, 12.5
mmol) was added dropwise at 10°C. The reaction was exothermic.
After the reaction mixture was stirred at 10°C' for 2 hours,
white precipitates were obtained. Methylene chloride (25 ml)
was added to thereby completely dissolve the formed precipitate.
After the end of the reaction had been confirmed, distilled
water (40 ml) was added to the reaction solution. The resulting
solution was extracted with methylene chloride (25 ml x 2). The
collected organic layers were washed with distilled water (20 ml
x 1), and dried over sodium sulfate. Then, the solvent was
evaporated by a rotary evaporator to obtain a crystalline crude
product (1.265 g). The crude product was rec~_ystallized from
ethyl alcohol to obtain the above-titled compound (1.03 g,
yield=78.60 as a colorless needle shaped crystal.
Melting point: 133-133.5°C
TLC: Rf 0.33 (n-hexane/ethyl acetate 1:1)
1H-NMR (CDC13, 8 ppm):
2.32 (s, 3H, Ac), 2.33 (s, 3H, Ac), 6.43 (d, 1H, ,7 = 9.62
Hz, C3-H), 7.22 (s, 1H), 7.35 (s, 1H), 7.64 (d, 1H, J=9.62
Hz, C4-H)
IR (KBr, vmax):
1778s, 1738s, 1636m, 1570m, 1510m, 1436m, 1378m, 1218s,
1128s
(2) Preparation of 4-methylesculetin 6,7-bis(acetate)
.~ s- - 21 16 617
The procedure of Example 1(1) was repeated, except that 4-
methylesculetin (Sigma Chemical Company) was used as a starting
material. The above-titled compound was obtained as a yellow
crystal.
1H-NMR (CDC13, S ppm):
7.33 (s, 3H), 7.40 (s, 3H), 6.32 (s, 1H), 7.24 (s, 1H),
7.44 (s, 1H)
'ECTION 8 CORRECTION
SE~ CERTIFICATE
(3) Preparation of esculetin 6,7-bis(acetate)
CORRt=CTION ~ ARTICLE 8
Esculetin (890 mg, 5 mmol) and 4-(N,N-
VOIR CEflTIFICAT
dimethylamino)pyridine (1.528 g, 12.5 mmol) were put into' an
eggplant type flask (50 ml), and then, methylene chloride (20
ml) was added to give a suspension. To the resulting
suspension, stearoyl chloride (Tokyo Kasei; 3.787 g, 12.5 mmol)
was added dropwise at 10°C. The reaction mixture became turbid
to white, and then, was solidified. Thereafter, methylene
chloride (20 ml) was added to the resulting reaction mixture to
give a suspension. The resulting suspension was stirred at room
temperature for 5 hours. After the end of the reaction had been
confirmed, the reaction mixture was poured into ice water (20
ml). The resulting mixture was extracted with methylene
chloride (100 ml x 1). The separated organic layer was washed
with distilled water (20 ml x 1) and dried over sodium sulfate.
Then, the solvent was evaporated by a rotary evaporator to
obtain a white crude product (3.91 g). The crude product was
recrystallized from methylene chloride/n-hexane to obtain the
above-titled compound (2.773 g; yield=78.00 as a white powdery
crystal.
Melting point: 85-86°C
1H-NMR (CDC13, 8 ppm):
0.88 (t, 6H, CH3), 1.26 (m, 56H, CH2), 1.72 (m, 4H, CH2),
2.55 (q, 4H, CH2C0), 6.42 (d, 1H, C3-H), 7.21 (s, 1H,
aromatic), 7.33 (s, 1H, aromatic), 7.63 (d, 1H, C4-H)
(4) Preparation of 4-methylesculetin 6,7-bis(stearate)
The procedure of Example 1(3) was repeated, except that 4-
methylesculetin was used as a starting material. The above-
titled compound was obtained as a white crystal.
Melting point: 121-122°C
1H-NMR (DMSO, 8 ppm):
2116617
0.85 (t, 6H, CH3), 1.24 (m, 56H, CH2), 1.48 (m, 2H, CH2),
1.64 (m, 2H, CH2), 2.18 (m, 2H, CH2), 2.34 (s, 3H, C4-CH3),
2.57 (m, 2H, CH2), 6.18 (s, 1H, C3-H), 6.84 (s, 1H,
aromatic), 7.42 (s, 1H, aromatic)
(5) Preparation of 4-methylesculetin 6,7-bis(linoleate)
The procedure of Example 1(4) was repeated, except that
linoleoyl chloride (Tokyo Kasei) was used instead of stearoyl
chloride. The above-titled compound was obtained as a yellow
oil.
1H-NMR (CDC13, 8 ppm):
0.90 (t, 6H), 1.2 to 1.4 (m, 32H), 1.4 to 2.0 (m, 4H), 2.0
to 2.2 (m, 8H), 2.4 (s, 1H), 2.8 (t, 4H), 5.3 to 5.5 (m,
8H), 6.3 (s, 1H), 7.2 (s, 1H), 7.4 (s, 1H)
(6) Preparation of 4-methylesculetin 6,7-bis(docosahexaenoate)
The procedure of Example 1(4) was repeated, except that
docosahexaenoyl chloride (Tokyo Kasei; docosa-4,7,10,13,16,19-
hexaenoyl chloride) was used instead of stearoyl chloride. The
above-titled compound was obtained as a yellow oil.
1H-NMR (CDC13, b ppm):
0.95 (t, 6H), 2.10 (m, 4H), 2.2 (s, 3H), 2.4 to 2.7 (m,
8H), 2.7 to 3.0 (m, 12H), 5.3 to 5.7 (m, 24H), 6.3 (s, 1H),
7.2 (s, 1H), 7.4 (s, 1H)
(7) Preparation of esculetin 6,7-bis(benzoate)
Esculetin (890 mg, 5 mmol) and 4-(N,N-
dimethylamino)pyridine (1.528 g, 12.5 mmol) were put into an
eggplant type flask (50 ml), and then methylene chloride (10 ml)
was added to give a suspension. To the suspension, benzoyl
chloride (Tokyo Kasei, 1.757 g, 12.5 mmol) was slowly added
dropwise at 10°C. White precipitates were immediately formed.
The reaction mixture was stirred at room temperature for 4
hours.
After the reaction had been completed, t:he reaction mixture
was poured into ice water (20 ml), and then, extracted with
methylene chloride (20 ml x 3). The collected organic layers
were washed with distilled water (20 ml x 1), dried over sodium
sulfate, then the solvent was evaporated by a rotary evaporator
to obtain a crystalline crude product (2.136 g). The crude
product was recrystallized from methylene chloride/n-hexane to
-~Q- 21 1661 l
obtain the above-titled compound (1.899 g, yield=98.40 as a
white powdery crystal.
Melting point: 183-184.5°C
TLC: Rf 0.69 (n-hexane/ethyl acetate 1:1)
1H-NMR (CDC13, 8 ppm):
6.47 (d, 1H, J=9.62Hz), 7.38 (m, 6H, aromatic), 7.44 (s,
1H), 7.56 (s, 1H), 7.71 (d, 1H, J=9.62Hz), 8.04 (m, 4H,
aromatic)
IR (KBr, vmax):
1765s, 1745s, 1625w, 1605w, 1570w, 1510m, 1475m, 1430m,
1390m, 1325w, 1255s
(8) Preparation of 4-methylesculetin 6,7-bis(benzoate)
4-methylesculetin (960 mg, 5 mmol) and 4-(N,N-
dimethylamino)pyridine (2.445 g, 20 mmol) were put into an
eggplant type flask (50 ml), and then, methylene chloride (10
ml) was added to give a suspension. To the ra_sulting
suspension, benzoyl chloride (2.811 g, 20 mmo:L) was slowly
added dropwise at 10°C. White precipitates were immediately
formed. The reaction mixture was stirred at :room temperature
for 4 hours.
After the reaction had been completed, the reaction mixture
was poured into ice water (20 ml), and then extracted with
methylene chloride (20 ml x 3). The collected organic layers
were washed with distilled water (20 ml x 1), dried over sodium
sulfate, then the solvent was evaporated by a rotary evaporator
to obtain a crystalline crude product (2.250 g). The crude
product was recrystallized from methylene chloride/n-hexane to
obtain the above-titled compound (1.960 g, yield=98.00 as a
white powdery crystal.
Melting point: 146-152°C
1H-NMR (CDC13, 8 ppm):
2.41 (d, 3H, J=2.OHz), 6.34 (d, 1H, J=2.OHz), 7.33 to 7.64
(m, 8H), 8.00 (d, 2H, J=2.6Hz), 8.09 (d, 2H, J=2.3Hz).
(9) Preparation of esculetin 6,7-bis(pivalate) (I) and
esculetin 6-monopivalate (II)
Esculetin (200 mg, 1.12 mmol) and pyridine (3 ml) were put
into an eggplant type flask (50 ml). To the mixture, pivaloyl
chloride (283.6 mg, 2.35 mmol) was added at 0"C, and then the
~ir- 21 16 617
mixture was stirred at room temperature for 26 hours.
Thin layer chromatography was used to confirm the
disappearance of the starting material and the formation of the
two products (Rf=0.8 and 0.23, methylene chloride/methanol =
9:1). Then, the reaction mixture was poured into ice water (10
ml) and extracted with ether. The collected organic layers were
dried over sodium sulfate and concentrated under reduced
pressure to obtain a crude product.
The crude product was separated and purified by silica gel
chromatography. Methylene chloride was used to obtain the
above-titled compound (I) as the first effluent (colorless
crystal, yield=72~) and the above-titled compound (II) as the
second effluent (colorless crystal, yield=25~).
Above-titled compound (I)
Melting point: 148-149°C
1H-NMR (90 MHz, CDC13, S ppm):
1.35 (s, 18H), 6.40 (d, 1H, J=10.3Hz), 7.15 (s, 1H), 7.29
(s, 1H), 7.64 (d, 1H, J=10.3Hz)
Above-titled compound (II)
Melting point: 159-162°C
1H-NMR (90 MHz, CDC13, b ppm):
1.39 (s, 9H), 6.26 (d, 1H, J=9.5Hz), 6.98 (s, 1H), 7.18 (s,
1H), 7.60 (d, 1H, J=9.5Hz)
Example 2: Mice Toxicity Test via Intraperitoneal Administration
A suspension of esculetin or esculetin 6,7-bis(benzoate) in
0.5~ methylcellulose aqueous solution was int:raperitoneally
administered to 6 week-old Crj:CD-1 (ICR) male mice (five mice
in a group) once a day for four days at the dose of 750 mg/kg.
No deaths and no remarkable toxicity were observed in both of
the above compounds.
The same toxicity test was performed on 4-methylesculetin,
esculetin 6,7-bis(acetate), 4-methylesculetin 6,7-bis(acetate),
esculetin 6,7-bis(stearate), 4-methylesculetin 6,7-
bis(stearate), 4-methylesculetin 6,7-bis(linoleate), 4-
methylesculetin 6,7-bis(docosahexaenoate), 4-methylesculetin
6,7-bis(benzoate), esculetin 6,7-bis(pivalate), and esculetin 6-
-1a- 21 1661 l
monopivalate, but no deaths were observed.
Example 3: Effect of test compounds on Proteoglycan Depletion
(a) Preparation of cultured chondrocytes
Cartilages were taken from the shoulder and knee joints of
male rabbits (New Zealand White Rabbit) (body weight of 1 to 1.5
kg) under the sterile condition. The cartilages were thoroughly
washed with PBS(-) (Ca2+, Mg2+ free), Hanks' solution and
finally 0.1~ EDTA-PBS(-), and then, cut into small segments (1
mm x 1 mm x 1 mm). After PBS(-) containing 0.1$ EDTA was added,
the segments were incubated at 37°C for 30 minutes. Then, the
segments were treated with trypsin solution (~0.25~) at 37°C for
one hour to remove the connective tissues attached to the
cartilages. After the supernatant had been removed, the
cartilages were treated in a Ham F-12 medium ~~ontaining 10~
fetal bovine serum (FBS) and 0.2~ collagenase for approximately
2 to 2.5 hours. Thereafter, the collagenase solution was
removed by centrifugation (1500 r.p.m.), the :residue was washed
twice with Ham medium containing 10~ FBS (chondrocyte culture
medium). The resulting cell dispersion was adjusted so that the
chondrocytes were suspended in the concentration of 3 x 105
cells/ml in the chondrocyte culture medium. The chondrocytes
were seeded in an amount of 1 ml/well on 24-wall plates. The
chondrocytes became confluent after 4 days. 'rhe experiments
were performed within 2 weeks after reaching this stage.
(b) Addition of compounds to be tested and proteoglycan
depleting agents
The chondrocyte culture medium which had been used for
cultivating the chondrocytes was removed from each well, and 800
ail of fresh serum-free S-Clone (Sanko Junyaku, Tokyo, Japan)
medium containing 0.1~ human serum albumin was added. Further,
100 ~1 of S-Clone medium containing the compounds to be tested
in various concentrations (containing the compound in the
concentration of 10 folds the final concentration; DMSO = 2.5~)
was added. The chondrocytes were cultured in the presence of 5~
carbon dioxide and 95~ air for 2 hours. Then, the proteoglycan
depleting agent, PMA (phorbol myristate acetate) (final
-~ 3 - 21 16 b 1 l
concentration = 0.1 ~,g/ml) or interleukin-1a (IL-1a) (final
concentration = 20 u/ml) was added into the culture medium of
the chondrocytes.
The compounds to be tested were as follows:
Compound of the present invention: esculetin (Tokyo Kasei),
4-methylesculetin (Sigma Chemical Company), 4-methylesculetin
6,7-bis(acetate), 4-methylesculetin 6,7-bis(stearate), 4-
methylesculetin 6,7-bis(linoleate), 4-methylesculetin 6,7-
bis(docosahexaenoate), esculetin 6,7-bis(benzoate), 4-
methylesculetin 6,7-bis(benzoate), esculetin ~6,7-bis(acetate),
esculetin 6,7-bis(pivalate), and esculetin 6-monopivalate. All
of the above compounds were prepared in Example 1 except
esculetin and 4-methylesculetin.
Comparative substance: Indomethacin (Sig:ma Chemical
Company)
(c) Determination of proteoglycan
Proteoglycan depletion was determined by the measurement of
the glycosaminoglycan (major constituent of proteoglycan,
hereinafter referred to as GAG) content following digestion of
the chondrocyte matrix with papain.
After 2 days, the supernatant of the chondrocyte culture
was removed. Then, 1 ml of 0.03 papain solution was added to
the remaining chondrocyte matrix layer and a :reaction was
performed at 65°C for 1 hour to liberate the C~AG from the matrix
layer. The content of the GAG in the treated papain solution
was determined by the 1,9-dimethylmethylene blue method (refer
to R. W. Farndale, Biochim. Biophys. Acta., Vol. 883, pp. 173 to
177, 1986).
The GAG content in the chondrocyte matrix of the control
test wherein the proteoglycan depleting agent was not added was
shown as "100", and the relative amount of the GAG of each
experiment except the control test was calculated by the
following formula:
GAG relative amount (~) - (B/A) x 100
wherein A represents the GAG content of the control tests
wherein the proteoglycan depleting agent was not added, and B
represents the GAG content wherein the proteoglycan depleting
agents were added alone or the GAG content wherein the
-''~- 21 16 617
proteoglycan depleting agents and the compounds to be tested
were added.
The GAG contents of the control tests varied in a range of
10.9 to 99.9 ~g/ml, depending on the period from the time when
the chondrocytes became confluent until the time when the
chondrocytes were used in the above experiment.
The results are shown in Table 1. The content of the GAG
in the table is the value of the mean value + standard error
(n=3). For each of the compounds to be tested, the control test
and the proteoglycan depleting test wherein the proteoglycan
depleting agent was added were carried out and the results
thereof are also shown. The significance was determined by
Student's t-test with respect to the proteoglycan depleting test
wherein the proteoglycan depleting agent was added. The results
of the determination are shown as follows:
*: P < 0.05;
**: P < 0.01;
***: P < 0.001.
In comparison with the GAG content in the control tests
wherein the proteoglycan depleting agent was not added, the
addition of the proteoglycan depleting agents, PMA or IL-1a,
induced a loss of GAG content. Under these conditions, the
SECTIONBCOflRECT101~resent compound inhibited or reduced the loss of GAG
content,
SEf CERTIFICATE
~OflRECTION-ARTICLEf~nd showed a function to inhibit or suppress the
proteoglycan
VOIRCERTIFICAT depletion. To the contrary, indomethacin, a conventional
analgesic and anti-inflammatory agent, did not show the function
to inhibit or suppress the proteoglycan depletion, but showed a
function to exacerbate the cartilage destruction.
Table 1
Samples GAG content (Relative amount
(~g/ml) of GAG ) (~)
Control 72.1~3.20 *** (100)
IL-1a 37,8+2.21 (52.4)
+esculetin
100 ~.1M 63.9~3.80** (88.6)
-~5- 21 1661 l
Control 58.82. 60*** (100)
PMA 37.91. 37 (64.5)
+esculetin
100 ~1.M 78.02. 32*** (133)
Control 99.9+1.10*** (100)
PMA 59.10. 80 (59.2)
+4-methylesculetin
100 ~.~.M 72.10. 70*** (72.2)
Control 20.3+2.33*** (100)
zL-1a 14.32. 57 (70.4)
+4-methylesculetin
6,7-bis(acetate)
100 ~.lM 27.3+3.66* (134)
Control 39.70. 55** (100)
zL-1a 33.00. 55 (83.1)
+4-methylesculetin
6,7-bis(linoleate)
100 N,M 37.3+0.73** (94.0)
+4-methylesculetin
6,7-bis(stearate)
100 ~.M 43.70. 37*** (110)
+4-methylesculetin
6,7-bis(docosahexaenoate)
100 N.M 43.00. 12*** (108)
+4-methylesculetin
6,7-bislbenzoate)
100 ~1M 44.70. 93*** (113)
Control 10.90. 23** (100)
zL-1a 8.710. 23 (79.9)
+esculetin
6,7-bis(benzoate)
100 ~1M 9 . 15 * ( 87 . 5 )
540
.
-16- 21 16 617
Control 24.3+3.33*** (100)
PMA 11.31.76 (46.5)
+4-methylesculetin
6,7-bis(acetate)
100 ~.1M 25.43.84** (105)
Control 44.30.61*** (100)
PMA 37.91.67 (85.6)
+4-methylesculetin
6,7-bis(linoleate)
100 N.M 42.30.82* (95.5)
+4-methylesculetin
6,7-bis(stearate)
100 ELM 47.01.10** (106)
+4-methylesculetin
6,7-bis(docosahexaenoate)
100 ~,tM 47.6+0.49** (108)
+4-methylesculetin
6,7-bis(benzoate)
100 E,~M 48.11.56* (109)
Control 24.0+0.44 *** (100)
1L-1a 16.00.68 (66.7)
+esculetin
6,7-bis(acetate)
100 E.~M 20.91.86 (87.1)
+esculetin 6,7-bis(pivalate)
~tM 19.21.58 (80.0)
+esculetin 6-monopivalate
100 uM 21.8+2.19* (90.8)
Control 28.0~0.7*** (100)
PMA 15.4+0.5 (55.0)
+indomethacin
10 13.2~0.6* (47.1)
33 ~.M 11.7+0.8** (41.8)
Example 4: Formulation of Granule
The following ingredients were mixed homogeneously:
Esculetin 20 parts by weight
2116617
Lactose 68 parts by weight
Low-substituted
hydroxypropylcellulose 10 parts by weight
Hydroxypropylcellulose 2 parts by weight
mt~
The mixture was kneaded using 32 parts by weight of a wetting
a agent, ethanol. Then, the kneaded mixture was glanulated by wet
granulation and dried to obtain the granule.
As explained above, the present compound (I) strongly
inhibits proteoglycan depletion from the chondrocyte matrix and
exhibits a function to protect cartilage. Further, the present
compound (I) has low toxicity. Accordingly, the present
compound (I) is extremely effective for the treatment of
arthropathy, such as rheumatoid arthritis, osteoarthritis,
periarthritis humeroscapularis, shoulder-arm-neck syndrome,
lumbago, and so on.
Although the present invention has been described with
reference to specific embodiments, various changes and
modifications obvious to those skilled in the art are deemed to
be within the spirit, scope, and concept of the invention.