Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
\
W093J20885 ~ PCT/US93/03371
CLOSED DRUG DELIVERY ~Y~
S P E C I F I C A T I O N
Background of the Invention
This is a Continuation In Part Application of Serial No.
07/870,553 filed April 17, 1992 which is a Continuation In Part
of Serial No. 07/513,917 filed April 24, 1990, now U. S. Patent
No. 5,122,116.
Field of the Invention -
The present invention relates generally to fluid
mixing and delivery systems. More particularly, the invention
concerns an apparatus for intermixing selected medicaments to
form a flowable substance and for then infusing the substance
into a patient at a precisely controlled rate.
Discussion of the Invention
Medicament delivering systems that can separately
store and then controllably intermix a selected medicament with
a diluent for infusion into a patient at a controlled rate have
come into wide use. In the prior art systems the diluent is
generally packaged in flexible plastic containers having admin-
istration ports for connection to an administration set which
delivers the container contents from the container to the pa-
tient. The drug is often packaged in a separate, closed con-
tainer and is mixed with the diluent shortly before infusion of
the medicament in the patient.
Drugs are typically packaged separately from the
diluent for a number of reasons. One important reason is that
certain drugs do not retain their efficacy when mixed with a
diluent and, therefore, the mixture cannot be stored for any
appreciable length of time. Another reason is that many drug
manufacturers do not produce medical fluids in containers for
SU~STITlJTE SHEET
W O 93/20885 ~ PC~r/US93/03371
intravenous delivery. As a general rule, drugs are packaged in
powder form in small, closed containers, or vials, for later
mixing with a suitable diluent. In many instances it is neces-
sary to mix the drug with the diluent immediately prior to
delivery to the patient to insure that the drug will not sepa-
rate from the diluent prior to or during infusion.
Infusion of medicaments is most often accomplished in
a hospital environment and the nurse, doctor or other medical
personnel mixes the drug and diluent shortly before administra-
tion of the drug to the patient. This mixing step can be time
consuming and hazardous, as for example, when toxic drugs are
used. Additionally, many of the prior art mixing devices are
crude and imprecise making accurate, sterile and thorough
mixing of the drug and the diluent difficult, time consuming
and not well suited for use in the home environment.
Several types of closed drug delivery systems are
presently in use. These systems typically comprise a flexible
container such as a plastic bag to which a drug vial can be
coupled. The flexible container usually contains a liquid
diluent and often includes a frangible member that allows fluid
passage only when broken. When the drug vial is coupled with
the flexible container, the stopper of the drug vial is pierced
and the frangible member ruptured so as to allow sterile commu-
nication between the drug vial and the liquid diluent contents
of the flexible container. Mixing of the drug with the diluent
is accomplished by manipulating the flexible container. Exem-
plary of prior art systems of the aforementioned character are
those disclosed in U.S. Patent No. 4,583,971 issued to Bocquet,
et al. and in U.S. Patent No. 4,606,734 issued to Larkin.
Another prior art closed delivery and mixing system
is disclosed in U.S. Patent No. 4,458,733 issued to Lyons. The
SUBSTITUTE SHEEt
W093/20885 ~ PCT/US93~0337~
Lyons apparatus includes a compressible chamber with a liquid
component therein, the compressible chamber including gas-
trapping and reservoir compartments in open communication. The
gas trapping compartment can be connected to a container such
as a drug vial having a mixing component therein. After a
pathway between the vial and the gas trapping compartment is
opened, mixing is accomplished through manipulation of the
compressible chamber.
Another very successful prior art, dual container
system is described in U.S. Patent Nos. 4,614,267 issued to
Larkin and 4,614,515 issued to Tripp and Larkin. In this
system, a flexible diluent container includes a tubular port
which provides means for securing thereto a stoppered medica-
ment vial as well as a stopper removal means. The stopper
removal means includes an engagement element, or extractor,
which is attached to a removable cover and seals the inner end
of the port. In use, as the vial is advanced into the tubular
port, the vial stopper moves into engagement with the extractor
which grips the stopper enabling it to be pulled from the vial
as the cover is pulled from the port. Once the stopper has
been removed from the vial, the contents of the vial can be
dumped into the diluent in the bag and mixed therewith through
manipulation of the bag.
The prior art devices of the character described in
the preceding paragraphs typically use the traditional gravity
flow method for infusion of the medicament mixture into the
patient. Such a method is cumbersome, imprecise and typically
requires bed confinement of the patient. Also, the flexible
bag must be maintained in a substantially elevated position and
periodic monitoring of the apparatus by the nurse or doctor is
required to detect malfunctions of the infusion apparatus.
SUBSTITUTE SHEET
W O 93/20885 ~ O ~ 1 PC~r/US93/03371
The apparatus of the present invention overcomes the
drawbacks of the prior art by totally eliminating the need for
a flexible bag, the cumbersome manipulative mixing of the
medicaments using the flexible bag and the undesirable gravity
infusion method which is typically followed when the flexible
bag is used. As will be described in the paragraphs which
follow, the apparatus of the present invention makes use of
recently developed gas permeable elastomeric films and similar
materials, which, in cooperation with a plate-like base define
a fluid chamber that initially contains the first component,
such as a diluent. Adjacent the base and in communication with
the fluid chamber is a sterile coupling means for operably
interconnecting a container such as a drug vial containing the
second component. To enable controlled, sterile intermixing of
the first and second components, the apparatus includes flow
control means for controlling the flow of fluid through inter-
nal passageways which interconnect the fluid chamber and the
drug vial.
The apparatus of the present invention is small, com-
pact, easy to use and inexpensive to manufacture. The appara-
tus provides a sterile, closed delivery system which can readi-
ly be used by ambulatory patients and in home care environment.
Connector elements are provided on the housing of the device
which permits the apparatus to be conveniently affixed to the
patient's clothing or to be strapped to the patients body.
The apparatus of the invention can be used with
minimal professional assistance in an alternate health care
environment, such as the home. By way of example, devices of
the invention can be used for intermixing numerous medicaments
with suitable diluents and for the continuous infusion of
SuBsT~ruTE ~HEET
W093/20885 ~ PCT/US93/03371
medicament mixtures such as antibiotics, analgesics, hormonal,
anticoagulants, clot dissolvers, immuno suppressants, and like
medicinal agents. Similarly, the apparatus can be used for I-V
chemotherapy and can accurately deliver fluids to the patient
in precisely the correct quantities and at extended microinfu-
sion rates over time.
Summary of the Invention
It is an object of the present invention to provide a
compact, lightweight, low-profile apparatus for control-
lably intermixing two or more components in a closed environ-
ment to produce a flowable substance and then for expelling the
flowable substance at a precisely controlled rate. More par-
ticularly, it is an object of the invention to provide such an
apparatus for medical applications which can be used in either
a home care or hospital environment for the precise mixing and
infusion of diluents and selected medicaments to an ambulatory
patient at controlled rates over extended periods of time.
It is another object of the invention to provide an
apparatus of the aforementioned character which includes a dis-
penser portion with its own stored energy means and coupling
means for operably interconnecting a drug vial to the dispenser
portion for controlled mixing of the medicament within the drug
vial with a diluent stored within the dispenser portion via a
sterile pathway.
Another object of the invention is to provide an
apparatus of the class described which permits extremely
accurate fluid mixing and delivering, and one which is highly
reliable and easy to use by lay persons in a non-hospital
environment.
Another object of the invention is to provide an
apparatus which includes an internal fluid reservoir storage
SUBSTITUTE SHEET
W093/20885 PCT/US93/03371
J'~
chamber that can be factory prefilled with a diluent or one
which can readily be filled in the field shortly prior to use.
Another object of the invention is to provide an
apparatus of the character described in the preceding paragraph
in which the reservoir is provided with an elastomeric energy
source that can be subjected to gamma sterilization and extend-
ed thermal sterilization temperatures without degradation of
integrity and performance.
Another object of the invention is to provide an
apparatus in which intermixed fluids can be delivered to the
patient either at a fixed rate or at precisely metered variable
rates and one which is operational in all attitudes and al-
titudes.
Still another object of the invention is to provide
an apparatus of the class described which includes means for
securely interlocking the drug vial with the dispenser portion
of the apparatus.
Yet another object of the invention is to provide an
apparatus as described in the preceding paragraph which is pro-
vided with means for attaching the apparatus to the clothing of
the patient or to the patients body.
A further object of the invention is to provide a low
profile, fluid delivery device of laminate construction which
can be manufactured inexpensively in large volume by automated
machinery.
Another object of the invention is to provide a
device of the character described in which fluid is dispelled
from the apparatus through a cooperating infusion set by a
thin, distendable membrane cooperatively associated with a
thin, plate-like base.
Another object of the invention is to provide an
SUBSTITUTE SHEET
W093/20885 ~ PCT/US93/03371
apparatus of the aforementioned character in which the distend-
able membrane can be a single elastomeric film, a laminate
construction or a composite that is permeable to gases at least
in one direction, whereby gases within the intermixed fluids
can be released from the fluid chamber and not injected into
the patient.
Yet another object of the invention is to provide an
apparatus of the class described in which a thin, planar filter
element is disposed within the fluid chamber for filtering the
reservoir outflow to the patient.
Brie~ Description of the Drawings
Figure 1 is a generally perspective view of one form
of the drug delivery system of the present invention.
Figure 2 is an exploded perspective view of the device
shown in Figure 1.
Figure 3 is a top plan view of the apparatus shown in
Figure 1 partly broken away to show internal construction.
Figure 4 is a cross-sectional view taken along lines
4-4 of Figure 3.
Figure 5 is a cross-sectional view taken along lines
5-5 of Figure 3.
Figure 6 is an enlarged cross-sectional view of the
lower right hand portion of the apparatus as viewed in Figure 3
illustrating construction of the shut-off and metering valve of
the apparatus.
Figure 7 is an enlarged cross-sectional view taken
along lines 7-7 of Figure 3.
Figure 8 is a fragmentary cross-sectional view illus-
trating the first step involved in interconnecting the drug
vial with the device of the invention.
Figure 9 is an enlarged fragmentary view illustrating
SUBSTITUTE SHEFT
W O 93/20885 ~ PC~r/US93/03371
the check valve of the apparatus in a closed position.
Figure 10 is a cross-sectional view similar to Figure
8 illustrating the second step in the interconnection of the
drug vial with the device of the invention.
Figure 11 is a cross-sectional view similar to Figure
10 illustrating the next step in the interconnection of the
drug vial with the apparatus and showing the intermixing of
fluids contained within the infusion portion of the device with
the medicament contained within the vial which has been inter-
connected with the infusion portion of the device.
Figure 12 is a cross-sectional view similar to Figure
11 but illustrating the further step of transferring the inter-
mixed fluids contained within the drug vial to the reservoir of
the infusion portion of the device of the invention.
Figure 13 is a cross-sectional view taken along lines
13-13 of Figure 12.
Figure 14 is an enlarged fragmentary view partly in
cross-section illustrating the construction of the valving
mechanism of the drug vial.
Figure 15 is a fragmentary cross-sectional view simi-
lar to Figure 10 but showing an alternate embodiment of a drug
vial usable with the apparatus of the invention.
Figure 16 is a fragmentary cross-sectional view simi-
lar to Figure 11 and illustrating the intermixing of fluids
contained within the infusion portion of the device with the
medicament contained within the second form of the drug vial
shown in Figure 15.
Figure 17 is a generally perspective view of another
alternate form of the apparatus of the invention.
Figure 18 is fragmentary plan view of the apparatus
shown in Figure 17 partly broken away to show internal construc-
SU~3S~ITUTE SHEEr
W093/20885 ~7 ~ PCT/US93/03371
tion of the infusion portion of the apparatus prior to the cou-
pling therewith of another form of the drug container or vial.
Figure 19 is an enlarged cross-sectional view illus-
trating the initial step in mating the drug container shown in
Figure 18 with the infusion portion of the device.
Figure 20 is a cross-sectional view similar to Figure
11 showing the intermixing of the diluent contained within the
infusion portion of the device with the medicament contained
within this latest form of drug vial.
Figure 21 is a cross-sectional view similar to Figure
12 showing the transfer step wherein the intermixed fluids are
transferred from the drug vial to the reservoir of the infusion
portion of the device.
Figure 22 is a fragmentary cross-sectional view taken
along lines 22-22 of Figure 21 showing the means for interlock-
ing the drug vial with the infusion portion of the device.
Figure 23 is a rear perspective view of still another
embodiment of the invention.
Figure 24 is a generally perspective front view of the
apparatus of the embodiment shown in Figure 23.
Figure 25 is a generally perspective, exploded view of
the apparatus of this latest form of the invention.
Figure 26 is a bottom view of the apparatus.
Figure 27 is a cross-sectional view taken along lines
27-27 of Figure 26.
Figure 28 is a cross-sectional view taken along lines
28-28 of Figure 26.
Figure 29 is a cross-sectional view illustrating the
initial step in the coupling of the drug container with the
infusion portion of the device.
Figure 30 is a cross-sectional view similar to Figure
8UBSTITUTE SH~ET
W 0 93/20885 ~J ~ PC~r/US93/03371
11 showing the intermixing of the diluent contained within the
infusion portion of the device with the medicament contained
within the drug vial.
Figure 31 is a cross-sectional view showing the
transfer step wherein the intermixed fluids are transferred to
the reservoir of the infusion portion of the device.
Figure 32 is a cross-sectional view taken along lines
32-32 of Figure 30.
Figure 33 is a cross-sectional view taken along lines
33-33 of Figure 32.
Figure 34 is a plan view partly broken away to show
internal construction of this further form of closed drug
delivery apparatus of the present invention.
Figure 35 is a fragmentary cross-sectional view taken
along lines 35-35 of Figure 34.
Figure 36 is a side view, partly in cross-section of
the drug vial of this last embodiment of the invention.
Figure 37 is a cross-sectional view illustrating the
drug vial coupled with the delivery portion of the device to
accomplish the initial mixing step.
Figure 38 is a cross-sectional view showing the trans-
fer of the intermixed fluids within the drug vial to the reser-
voir of the infusion portion of the device.
Figure 39 is a plan view partly broken away to show
internal construction, of yet another embodiment of the drug
delivery system of the invention.
Figure 40 is a cross-sectional view taken along lines
40-40 of Figure 39.
Figure 41 is a plan view of the drug container of
this form of the invention.
Figure 42 is an enlarged cross-sectional view taken
SUBSTITUTE SH~ET
PCT/US93~03371
W~93J208~5
along lines 42-42 of Figure 43.
Figure 43 is an enlarged cross-sectional view showing
the drug vial assembly in position to be mated with the cou-
pling means.
Figure 44 is an enlarged cross-sectional view illus-
trating the initial mating of the drug vial assembly with the
infusion portion of the device.
Figure 45 is an enlarged cross-sectional view similar
to Figure 46 illustrating the intermixing of the diluent with
the drug upon the rotation of the drug vial relative to the
coupler means to permit fluid to flow from the fluid reservoir
of the apparatus toward the mixing chamber.
Figure 46 is a cross-sectional view taken along lines
46-46 of Figure 44.
Figure 47 is a cross-sectional view taken along lines
47-47 of Figure 45.
Figure 48 is an enlarged cross-sectional view similar
to Figure 45 showing further rotation of the drug vial in a
manner to throttle down the flow of fluid toward the mixing
chamber of the drug vial.
Figure 49 is a cross-sectional view taken along lines
49-49 of Figure 48.
Figure 50 is a fragmentary, generally ~erspective
view of the fine control valving means of the drug vial of this
further embodiment.
Figure 51 is an enlarged, generally perspective
exploded view of the drug vial illustrating the construction of
the fine flow control adjustment means of the invention.
Figure 52 is a plan view of another form of the drug
vial assembly of the present invention.
Figure 53 is an enlarged cross-sectional view of the
SUBSTITUTE SHE~T
-
~= ~
W093/20885 PCT/US93/03371
Q~ --
12
vial shown in Figure 54.
Figure 54 is an enlarged cross-sectional view showing
the new drug vial in position to be mated with the coupling
means of the apparatus.
Figure 55 is an enlarged cross-sectional view showing
the initial mating of the drug vial with the infusion portion
of the apparatus.
Figure 56 is an enlarged cross-sectional view showing
the initial intermixing of the diluent with the beneficial
agent contained within the alternate form of the drug vial.
Figure 57 is a fragmentary cross-sectional view taken
along lines 57-57 of Figure 56.
Figure 58 is an enlarged cross-sectional view similar
to Figure 56 showing the throttling down of the flow of fluid
toward the mixing chamber of the drug vial.
Figure 59 is a cross-sectional view taken along lines
59-59 of Figure 58.
Figure 60 is a cross-sectional view taken along lines
60-60 of Figure 58.
Figure 61 is a generally perspective view of the fine
control valving means of the container assembly of this last
form of the invention.
Figure 62 is a fragmentary cross-sectional view of
the portion of the mixing chamber of the device showing the
medicament in a tablet form.
Figure 63 is a plan view of yet another form of the
drug vial assembly of the present invention.
Figure 64 is a cross-sectional view taken along lines
64-64 of Figure 63.
Figure 65 is a generally perspective, exploded view
of the container assembly of this last form of the invention.
SUBSTITUTE SHE{~T
W093/20885 ~ PCT/US93~337
13
Figure 66 is a a generally perspective view of vari-
ous forms of adding means, or substrate assemblies, of this
later form of the invention
Figures 66A, 66B, 66C, and 66D are general diagram-
matic views illustrating various means for affinity attachment
of ligands, protein molecules and enzymes to the substrates.
Figure 67 is an enlarged, generally perspective
exploded view of still another embodiment of the invention.
Figure 68 is an enlarged cross-sectional view of the
embodiment shown in Figure 67 in an assembled configuration
ready for use with the fluid reservoir in an uncharged configu-
ration.
Figure 69 is an enlarged cross-sectional view similar
to Figure 68 but showing the fluid reservoir in a changed
configuration.
Figure 70 is an enlarged, generally perspective view
of one of the blunt-end cannulas of the apparatus.
Figure 71 is a generally perspective, exploded view
of yet another form of the invention.
Figure 72 is an end view of the diluent container of
the apparatus.
Figure 73 is a cross-sectional view taken along lines
73-73 of Figure 72.
Figure 74 is an enlarged cross-sectional view of the
drug vial of the apparatus prior to being inserted into the
coupling assembly of the apparatus.
Figure 75 is an enlarged cross-sectional view of the
embodiment shown in figure 71 as it appears in an assembled
configuration.
SUBSTITUTE SHEET
W093/20885 ~ PCT/US93/0337t
14
Description of the Invention
Referring to the drawings and particularly to Figures
1 and 2, the apparatus of one form of the present invention,
generally designated by the numeral 12, is used for intermixing
a first component contained within a separate container, such
as a drug vial 14, with a second component contained within a
storage reservoir disposed internally of the infusion portion
of the apparatus to form an injectable fluid and then for
infusing the fluid into a patient at a controlled rate. In
this first embodiment of the invention, shown in Figures 1
through 11, the apparatus comprises a housing 16 having a first
cylindrical portion 18 and a second infusion device portion
20.
As best seen by referring to Figure 2, first portion
18 includes coupling means for operably coupling container 14
with the infusion portion of the device. Second portion 20 of
housing 16, the construction of which will be described in
greater detail hereinafter, comprises the infusion device
portion of the apparatus and includes a base assembly 21 having
a generally planner, plate-like base 22. Base 22 includes a
fluid inlet 24 and a fluid outlet 26 which are in communication
via a multi-legged fluid passageway 28. Fluid passageway 28
includes a first transversely extending leg 28a which is in
communication with fluid inlet 24, a second, spaced-apart
transversely extending leg 28b and a pair of longitudinally
extending legs, or conduits, 28c which interconnect legs 28a
and 28b. Transversely extending leg 28b is in communication
with fluid outlet 26 in the manner shown in Figure 2.
Turning also to Figures 4 and 5, the apparatus of
this form of the invention further includes a distendable
membrane 30 constructed of an elastic material. Membrane 30 is
SUBSTITUTE SHEET
W093/20885 PCT/US93~03371
adapted to fit over base 22 and cooperate therewith to define
one or more diluent storage reservoirs, or chambers 32. Mem-
brane 30 is distendable by fluid introduced under pressure into
- chamber 32 through a sealable inlet port 33 provided in base
22. The elastic character of membrane 30 is such that the
membrane, after being distended has a tendency to return to its
original less distended configuration. This causes the fluid
to flow outwardly of the apparatus through fluid outlet 26 upon
opening the flow control means of the invention. The details
of construction of both the flow control means and of membrane
30 will be discussed in the paragraphs which follow.
Disposed intermediate distendable membrane 30 and the
upper planner surface 22a of base 22 is means for creating an
ullage within chamber 32. This means is here provided in the
form of a pair of spaced apart outwardly extending protuberanc-
es 34. Each of the protuberances 34 is provided with a longi-
tudinally extending first passageway or conduit 36. When the
apparatus is assembled in the manner illustrated in Figures 4
and 5, passageways 36 are superimposed directly over spaced-
apart fluid conduits 28c and the membrane engaging means, shown
here as protuberances 34, extend upwardly into fluid chambers
32 defining ullage therewith. In operation of the device, as
distendable membrane 30 attempts to return to its less distend-
ed configuration, it will move toward engagement with the upper
surfaces of protuberances 34 and, in so doing, will efficient-
ly force the fluid contained within chambers 32 into conduits
28c through passageways 36 (Figure 5). The configuration of
protuberances 34 ensure that substantially all of the fluid
within chambers 32 will be controllably dispelled therefrom as
the membrane returns toward its original planar configuration.
Superimposed over distendable membrane 30 is a porous
SUBSTITUTE SHEET
W O 93/2088S PC~r/US93/03371
16
plastic, free venting, structural filler member 40. As best
seen by referring to Figure 5, member 40 is provided with a
pair of longitudinally extending, concave channels 42 having
interior walls 43 against which membrane 30 initially engages
when it is outwardly distended by fluid flowing from an inlet
33 provided in base assembly 21 into chambers 32 under pres-
sure.
Superimposed over and sealably enclosing base 22 and
member 40 is a cover means shown here as a hard plastic cover
44. Cover 44 includes a first portion 44a which comprises the
upper segment of cylindrical portion 18 of housing 16 (Figure
2). As will be described further hereinafter, cylindrical
portion 18 houses the coupling means of the apparatus, which
includes first flow control means, for operably coupling the
drug vial with the infusion portion of the device. Cover 44
also includes gas venting means here provided as a plurality of
apertures 46 formed within the upper wall of cover member 44.
When distendable membrane 30 is constructed of a gas permeable
material, gas venting means, including apertures 46, permit
any gases contained within the fluids introduced into chambers
32 to pass through the gas permeable membrane, through filler
40 and to atmosphere through the gas venting means. A medica-
ment label 48, which may also be permeable to gases, covers
vent apertures 46. Forming still another part of cover assem-
bly 44 is a removable belt clip 50 provided with a dovetailed
mortise 52 adapted to be slidably receivable over an up-stand-
ing mating tenon 54 formed on the upper surface of cover member
44.
Base assembly 21 also includes an outlet port 56
which is normally closed, by a removable cover member 58.
Outlet port 56 is in communication with fluid outlet 26 via a
SUBSTITUTE SHEET
= W093~20885 PCT/US93/03371
0 ~ 1
conduit 57. Outlet port 56 is also in communication with a
transversely extending passageway 60 which terminates at its
outer end in an opening 62 (Figure 2). Receivable within
opening 62 is an outlet flow control means shown here as a
shut-off and fluid metering means 63 which comprises a needle
valve of standard construction having an elongated valve stem
or member 64 which is closely receivable within passageway 60
(Figures 2 and 6). Provided at one end of stem 64 is a control
knob 66. Provided at the opposite end is a tapered portion 68
adapted to cooperate with a valve seat 69 provided on base 22
for either substantially blocking or for controllably restrict-
ing the flow of fluid outwardly of the device through conduit
57 and outlet port 56. As best seen in Figure 6, passageway 60
is internally threaded to threadably receive external threads
70 formed on stem 64. With this construction, by rotating
control knob 66, valve member 64 can be moved axially of passa-
geway 60 to controllably move tapered portion 68 of the valve
relative to passageway 57 and into engagement with valve seat
69 so as to control fluid flow through passageway 57. An O ring
67 is provided to seal stem 64 relative to passageway 60.
Turning now to Figure 8, the construction of the con-
tainer, or drug vial portion 14 of the apparatus of the present
invention, is there illustrated. In this form of the inven-
tion, the container includes second flow control means for
controlling the flow of fluid into and out of an internal
chamber 75 of a vial 76, Closely received over vial 79 is a
plastic cover, or overpackage 78 which is provided with vial
interlocking means shown here as a pair of spaced apart, cir-
cumferentially extending safety interlocks 80 and 82, the
purpose of which will presently be described. Each of the
circumferentially extending interlocks 80 and 82 is provided
SU~3STITUTE SHEET
W093/20885 ~1~ 8 0 ~ ~ PCT/US93/03371
18
with a radially outwardly extending flat surface 83 which is
adapted to lockably engage one of a pair of spaced apart annu-
lar stops 18a and 18b provided internally of cylindrical por-
tion 18 (see also Figure 2). Annular stops 18a and 18b com-
prise novel stop means which are adapted to interengage the
vial interlocking means provided on the drug vial assembly to
prevent removal of the drug vial from cylindrical portion 18
after it has been introduced and mated therewith.
The second flow control means of this first form of
the invention comprises a plunger 86 which is substantially
sealably receivable within vial 76. Vial 76 is movable relative
to plunger 86 between a first position shown in Figures 8 and
10 and a second position shown in Figure 11 and from the second
position to a third position shown in Figure 12. Plunger 86 is
generally cylindrical in shape having a skirt portion 86a
adapted to substantially sealably engage the inner walls of
vial 76. Plunger 86 also includes first connector means, or
interengagement means, shown here as threads 86b, for intercon-
nection with the coupling means of the apparatus. Disposed
within a central passageway 86c formed interiorly of the plung-
er is a plunger valve means here provided as a valve assembly
88. Referring also to Figure 14, valve assembly 88 includes a
cylindrically-shaped central portion 90 closed at one end by a
disk-shaped member 92. Fluid passageways 94 are provided
through the cylindrical wall of central portion 90 proximate
member 92, which member is preferably integrally formed with
portion 90. Provided at the opposite end of central portion 90
is an annular shaped member 96.
As best seen in Figures 8 and 10, plunger 86 is
provided with a central portion 87 which includes spaced-apart,
radially, inwardly extending seats or shoulders 100 and 102.
SUBSTITUTE SHEET
W093/20885 PCT/US93JQ3371
When the valve assembly 88 is in the closed position shown in
Figure 8, the periphery of member 92 is substantially sealably
seated against shoulder 100 and annular portion 96 is spaced
apart from shoulder 102. On the other hand, when the valve is
in the open position shown in Figure 10, the periphery of
member 92 is spaced apart from shoulder 100 and annular portion
96 of the valve member is in engagement with shoulder 102. If
desired, central portion 90 can be constructed to provide
support to a stem 106 as stem 106 moves axially of the central
body portion. With the valve in the open position shown in
Figure 10, fluid can flow from the central passageway 103 of
the valve through radially extending passageways 94 and into
the vial in the manner indicated by the arrows of Figure 10.
Valve assembly 88 is moved from the closed position
into the open position by operating means, here comprising a
plunger stem portion 106 which is integrally formed with disk-
shaped member 92 and, as shown in Figures 8 and 14, extends
axially of valve passageway 103. As will be presently de-
scribed, the operating means functions to operate the first and
second control means of the invention, including valve assembly
88, for controlling the flow of fluid into and out of drug vial
14.
Before discussing the mode of operation of the oper-
ating means, the previously identified coupling means for
coupling the container 14 with portion 18 of the housing will
be discussed. As best seen by referring to Figures 2, 3, and
8, the coupling means here comprises a sterile coupling assem-
bly 110 which is supported centrally of cylindrical housing
portion 18 by a rigid coupling support 111 which extends trans-
versely of housing portion 18. Coupling assembly 110 comprises
an outer cylindrical portion 112 having second connector means
SUBSTITUTE SHEET
W O 93/20885 PC~r/US93/03371
O d~ ~
or internal threads 113 and a co-axially aligned, inner cylin-
drical portion 114. Inner portion 114 is held rigidly in
position within outer portion 112 by means of a circular shaped
end wall 116 (Figure 8). As best seen by also referring to
Figure 2, end wall 116 is closely received within a recess or
socket 118 formed in coupling support 111. Also forming a part
of coupling support 111, is a radially extending connector
element 120 having an internal fluid passageway 122 which is
adapted to communicate with inlet 24 of base 22 when support
111 is positioned within cylindrical portion 18 in the manner
shown in Figures 3 and 8. Passageway 122 communicates with a
passageway 124 which is defined by the interior walls of cylin-
drical portion 114. A smaller diameter fluid passageway 126
joins passageway 124 at a value seat defining, tapered wall
portion 128 (Figure 8).
Turning also to Figure 9, a coupling valve means,
generally designated by the numeral 136, which also forms a
part of the coupling means of the present invention, is recip-
rocally movable within passageway 124 and functions to control
the flow of fluid through passageway 126 in a manner presently
to be described. As indicated in Figures 8 and 9, coupling
valve means 136 includes a valve element 137 having a body
portion 138 and a coupling stem portion 140. At the junction
of portions 138 and 140 is a tapered wall 142 which is adapted
to substantially sealably engage the valve seat defined by
tapered wall portion 128 when the valve is in the closed con-
figuration shown in Figure 8. When the apparatus of the
invention is in a storage mode, the open end of coupling 110 is
closed by a removable sealing cap 130 which is provided with a
pull tab 132 for use in removing the cap from the sterile
coupling (Figure 2).
SUBSTITUTE SHFET
-
WO 93/20885 ~ O ~ ~" PCI/US93/03371
In operating the apparatus of the invention, the drug
vial closure cap 146 (Figure 2) is first removed from the drug
vial 14. This done the closure cap 130, which closes the
passageway of the sterile closure element llo, is removed and
the open end of the drug vial 14 is inserted through open end
148 of cylindrical portion 18 (Figure 8). As the drug vial 14
is received within open end 148, locking member 80 on the
overpackage will slip past stop member 18a on cylindrical
portion 18 and threads 86b will move into mating engagement
with threads 113 provided on coupler member 112. Rotation of
the drug vial in a clockwise direction will cause the plunger
to couple with coupling member 112 in the manner shown in
Figure 10. As the parts are coupled together, stem 106 of the
container valve will engage stem 140 of valve means 136 simul-
taneously axially moving both valve member 137 of the first
flow control means and valve member 92 of the second flow
control means into the open position shown in Figure 10. With
the valves of the flow control means in this position, distend-
able membrane 30 will cause the fluid contained within chambers
32 to flow under pressure past the valve seat 128 into fluid
passageway 126 of the coupler means and then into passageway
103 of the container valve means. The fluid under pressure
will next flow through radially extending passageways 94 of the
container valve and rapidly into the interior of container 76
in the manner shown by the arrows in Figure 10. This rush of
fluid under pressure into the drug vial initiates the mixing or
reconstitution process.
As illustrated in Figure 11, the fluid flowing into
the drug vial will mix with the medicament M contained within
the vial in the manner shown to form a flowable substance
SUBSTITUTE SHEET
~ ~ =
W O 93/20885 PC~r/US93/03371
comprising a mixture of the liquid which was stored within
chambers 32 and the medicament M which was stored within the
drug vial. It is important to note that, as the fluid under
pressure rushes into the drug vial, the drug vial will move
outwardly into the position shown in Figure 11 wherein surface
83 of the vial locking means or locking member 80 provided on
the plastic overpackage will engage first stop means or member
18a provided interiorly of cylindrical housing portion 18. It
is to be noted that in this position, the plunger 86 has trav-
eled from an intermediate position within vial 76, as shown in
Figure 8, to an outward position shown in Figure 11 wherein
plunger 76 is located proximate the open mouth of the glass
container 76. Contained air, if any, within vial 76 assists in
the turbulent mixing process.
The reconstituted mixture, the medicament M stored
within container 14, and residual air if any is next trans-
ferred back into the infusion device reservoir by exerting an
inward pressure on the drug vial in the direction of the arrow
150 of Figure 12. As the drug vial 14 is reinserted into
cylindrical portion 18, the reconstituted mixture contained in
the drug vial is directed through radial passageways 94, of the
drug vial valve into passageway 103 of the valve, into passage
126, passed valve seat 128 and into passageway 124 of the
coupling means. The fluid will then flow into chambers 32 via
passageways 122 and 28 (Figure 5). Entrained air, if any, will
vent to atmosphere through gas permeable elastomeric membrane
30 by the permeation transport process. As illustrated in
Figure 13, the interior wall of inner cylindrical member 114 of
the coupling means is provided with a plurality of circumferen-
tially spaced fluid passageways 152 to facilitate flow of fluid
to and from the chambers 32 provided within the drug infusion
SUBST~TUT~ SHEET
W093/20885 ~ PCT/US93J0~371
portion of the apparatus.
7 It is to be observed from Figure 12 that continued
inward pressure exerted on the drug vial 14 will cause locking
member 80 provided on overpackage 78 to slip past and lockably
engage second stop member 18b provided internally of cylindri-
cal chamber 18. Similarly locking member 82 will slip past
first stop member 18a of cylindrical portion 18 and lock
against locking member 18a. With the parts of the apparatus in
the configuration shown in Figure 12, the drug vial 14 is non-
removably locked in position within cylindrical chamber 18 of
housing 16.
The flow of the reconstituted mixture of the first
and second components contained within the vial 14 into cham-
bers 32 due to the telescopic movement of the drug vial into
cylindrical portion 18 will urge the partially distended mem-
brane 30 into the distended configuration shown in Figure 5.
Once distended, membrane 30 will continuously exert a pressure
on the now fully intermixed fluid contained within chambers 32
so that upon the removal of cap 58 and the opening of needle
valve 64, the newly reconstituted drug and diluent comprising
the combined intermixed fluid components will be infused into
the patient at a controllable rate through any suitable inter-
connection means such as an infusion needle connected to the
conduit shown in dotted lines in Figure 1 and designated by the
numeral 154. As previously discussed, the rate of infusion of
the liquid from the apparatus of the invention into the patient
can be precisely controlled through the manipulation of the
needle valve 64.
Contributing to the superior performance of the
apparatus of the invention are the several state of-the art
materials used in the construction of the apparatus. These
SUBSTITUTE SHEET
W093/20885 ~ PCT/US93/03371
materials markedly contribute to the reliability, accuracy and
manufacturability of the apparatus. Before discussing the
alternate forms of the invention shown in the drawings, a brief
review of the materials used in constructing the apparatus of
the invention is in order.
With respect to the base 22 and cover 44, a wide
variety of materials can be used, including; metals, rubber or
plastics that are compatible with the liquids they contact.
Examples of such materials are stainless steel, aluminum, latex
rubber, butyl rubber, nitrile rubber, polyisiprene, styrene-
butadiene copolymer, silicones, polyolefins such as polypropy-
lene and polyethylene, polyesters, polyurethane, polyamides and
polycarbonates.
Considering next the elastic distendable membrane 30,
this important component can be manufactured from several
alternate materials including rubbers, plastics and other
thermoplastic elastomers. These include latex rubber, polyiso-
prene (natural rubber), butyl rubber, nitrile rubber, polyur-
ethanes, Ethylene-Butadiene-Styrene Copolymers, Silicone modi-
fied Polyurethanes, fluorocarbon elastomers, fluorosilicones,
fluoralkoxyphosphazene ploymers and other polymer multicompon-
ent systems including copolymers (random, alternating, block,
graft, crosslink and starblock), mechanical poly-blends and
interpenetrating polymer networks.
Examples of materials found particularly well suited
for this application include; silicone polymers (polysiloxanes)
and high per~ormance silicone elastomers made from high molecu-
lar weight polymers with appropriate fillers added. These
materials are castable into thin film membranes and have high
permeability (which allows maximum transport of vapor and gas),
high bond and tear strength and excellent low temperature
SUBSTITUTE SH~ET
W093~20885 ~ ~8 ~ ~ ~ PCT/US93/03371
flexibility and radiation resistance. Additionally, silicone
elastomers retain their properties over a wide range of temper-
ature (-80~ to zoo~ C) are stable at high temperatures, and
exhibit tensile strengths up to 2,000 lb./in2 elongation up to
600%.
Further, silicone (polyorganosiloxanes) are thermally
stable, hydrophobic organometallic polymers with the lowest P-P
interaction (of all commercially available polymers. This fact
coupled with the flexibility of the backbone results in a low
Tg (-80~C) and an amorphous rubbery structure for the high MW
(polydimethylsiloxanes). Silicone rubber membranes are con-
siderable more permeable to gases than membranes of any other
polymer. Depending on the medicinal fluid used and the filling
of the storage mode, which will determine the desired mass
transport characteristics of the membrane (permeability and
selectivity), other materials of choice include polyurethane-
polysiloxane copolymers, ~lends and IPN's. By example, polydi-
methylsiloxane (PDMS) and polyurethane (PU) multicomponent IPN
containing 10%-20% weight of PU shows enhanced initial modulus
relative to that of PDMS itself.
Interpenetrating polymer networks (IPNS) are unique
blends of cross-linked polymers containing essentially no cova-
lent bonds, or grafts between them. True IPNS are also homoge-
neous mixtures of component polymers. Further examples of an
additional candidate materials would be a polyurethane-polysi-
loxane (IPN) bilaminated with a polyparaxylene or alternately
bilamination of polydimethylsiloxane (PDMS) and polyparaxylene.
Coextruded laminates of this type can be selected according to
the desired gas permeability for vapor and ~2~ N2 and C02
diffusion and their specific selectivity requirements as well
as for direction of gas migration when appropriately layered.
SUBSTITUTE SHEET
W O 93/2~885 ~ Q4 1 PC'r/U593/03371
26
Additionally, interfacial surface layers of various materials
of on the order of 5 to 20 angstroms thick can be provided on
the membrane to establish a biocompatible interface without
substantially effecting the membrane permeation rate.
With respect to the structural filter 40, many types
of porous plastic materials can be used. In certain embodi-
ments of the invention, this component can be produced from one
of several polymer groups. The plastic structure of this
component typically contains an intricate network of open
celled omni directional pores. The pores can be made in aver-
age sizes for 0.8 micron to 2,000 micron and, gives the porous
plastic a unique combination of venting and structural
strength. Further, the material is strong, lightweight, has a
high degree of chemical resistance and, depending on the
particular configuration of the apparatus, can be flexible.
The degree of hardness can range from soft, resilient or rigid,
and depending on the specific micro diameter range desired, the
following polymers can be employed: Polypropylene(PP), Ultra
high molecular weight polyethylene (UHMW PE), High density
polyethylene (HDPE), Polyvinylidene Fluoride (PVDF), Ethylene-
vinyl acetate (EVA), Styrene Acrylonitrile (SAN), Polytetra-
fluoroethylene (PTFF).
An alternate material for use in constructing the
cover 40 and base 22 so as to serve as a non-permeable gas
barrier, is a material sold by B-P Chemicals International of
Cleveland, Ohio, under the name and style "Barex". This mate-
rial, is a clear rubber modified Acrylonitrile Copolymer which
has wide application in the packaging industry because of its
superior gas barrier, chemical resistance and extrusion (ther-
moforming) and injection molding capabilities. Structures
using this or similar barrier materials can be manufactured in
SUBSTITUTE SHEET
-
=
W093J20885 ~ ~CT/US93/03371
either monolayer or coextrusion (with such other materials as
polyethylene, polypropylene, polystyrene and other modified
styrenes). Combinations of different materials can be used to
enhance the desired physical properties of the thermoformed
part.
Turning now to Figures 15 and 16, a second embodi-
ment of the present invention is there shown. In this form of
the invention, the first and second portions of the housing,
and the infusion device portion of the apparatus are identical
in construction and operation to those of the first embodiment
just described and like numbers are used to identify like
components. However, the container assembly~ generally desig-
nated in Figures 15 and 16 by the numeral 200, is somewhat
different.
Container assembly 200 comprises a glass vial 202
having a chamber 204 for containing a medicament M. A plastic
cover or overpackage 206 is closely received over vial 202 and
includes first and second locking members 208 and ~10 which are
identical to locking members 80 and 82 as previously described.
Housed within vial 202 is the second flow control means of this
form of the invention for controlling the flow of fluid into
and out of chamber 204. Here the second flow control means
comprises a plunger 212 substantially sealably received within
vial 202. Plunger 212 is of generally similar construction to
plunger 86 being cylindrical in shape and having a skirt por-
tion 214 adapted to substantially sealably engage the inner
wall, of vial 202. Plunger 212 also includes similar connector
means shown here as threads 216 for interconnection with
threads 113 provided on coupling number 112.
In this second form of the invention, however, plung-
er 212 has an internal passageway 218 which is normally blocked
SUBSTITUTE SHEET
W O 93/20885 PC~r/US93/03371
28
by a transversely extending, frangible or pierceable diaphragm
220. A first valve means, here provided as a valve assembly
222, is disposed within passageway 218 and, in cooperation with
diaphragm 220, controls fluid flow through passageway 218.
Plunger 212 includes an inwardly extending flange 224 against
which a flange 226 provided on valve assembly 222 normally
seats (Figure 15). Valve assembly 222 also includes a stem
228, which, in this form of the invention, comprises a part of
the operating means for operating the coupling valve and the
plunger valve. A fluid passageway 230 surrounds stem 228.
Stem 228 is integrally formed with the plunger body which
terminates in a point 232. As indicated in Figure 15, when
valve assembly 222 is in the normal position shown in Figure
15, point 232 is in engagement with diaphragm 220.
In operating the apparatus of this second form of the
invention, when plunger 214 is threadably connected to coupler
member 112 in the manner shown in Figure 16, valve assembly 222
will be moved to the right by stem 140 of the coupling valve
136 and diaphragm 220 will be ruptured. At the same time,
valve 136 will be axially moved into the open position permit-
ting the fluid contained within the reservoir of the delivery
portion of the device to flow through passageways 230 into
chamber 204 of the vial 202 and to mix with the medicament M.
The fluid under pressure flowing from the reservoir of the
delivery portion of the device forces the container assembly
outwardly to the position shown in Figure 16 with locking
member 208 engaging stop member 18a provided on the first
portion 18 of housing 16. After this reconstitution process,
the reconstituted fluid is forced into the reservoir of the
delivering portion of the device in the manner previously
described by pushing the container assembly to the left as
SUBSTITUTE SHEET
WO 93/20885 PCI'/US93/03371
~ 8 Q ~ ~
shown in Figure 16 and into a locked position similar to that
shown in Figure 12 and earlier described.
Turning now to Figures 17 through 22, still another
form of the invention is there illustrated. The infusion
device portion of this embodiment of the invention is substan-
tially identical in construction and operation to that of the
first two forms of the invention, and like numerals are used to
identify like component parts. However, the coupling portion
of the device is slightly different, as is the construction of
the drug vial assembly identified here by the numeral 300.
The infusion device portion of this third embodiment
of the invention also includes a base assembly 21 having a
generally planner base 22. Base 22 has a fluid inlet 24 and a
fluid outlet 26 (not shown) which are in communication via a
multi-legged fluid passageway 28. As before, fluid passageway
28 includes a first transversely extending leg 28a which is in
communication with fluid inlet 24, a second, spaced-apart,
transversely-extending leg 28b (not shown) and a pair of longi-
tudinally extending legs, or conduits, 28c which interconnect
legs 28a and 28b. Transversely extending leg 28b is in commu-
nication with fluid outlet 26 in the manner shown in Figure 2.
The apparatus of this form of the invention also in-
cludes a distendable membrane 30 constructed of an elastic
material. Membrane 30 is adapted to fit over base 22 in the
manner previously described and cooperates therewith to define
one or more diluent storage reservoirs, or chambers 32 of the
character shown in Figure 5. Membrane 30 is distendable by
fluid introduced under pressure into chambers 32 through a
sealable inlet port 33 provided in base 22 (Figure 18). As in
the previously described embodiments, the elastic character of
membrane 30 is such that the membrane, after being distended
8UBSTITUTE SHEET
W O 93/2088S ~ Q ~ ~ PC~r/US93/03371
has a tendency to return to its original less distended config-
uration. This causes the fluid to flow outwardly of the appar-
atus through the fluid outlet port upon opening the flow con-
trol means of the invention.
Disposed intermediate distendable membrane 30 and the
upper planner surface 22a of base 22 is means for creating an
ullage within chambers 32. This means is once again provided
in the form of a pair of spaced-apart, outwardly extending
protuberances 34. Each of the protuberances 34 is provided
with a longitudinally extending first passageway or conduit 36.
When the apparatus is assembled in the manner illustrated in
Figures 4 and 5, passageways 36 are superimposed directly over
spaced-apart fluid conduits 28c and membrane engaging means,
shown here as protuberances 34, extend upwardly into fluid
chambers 32 defining ullage therewithin. The operation of the
distendable membrane 30 to efficiently force the fluid con-
tained within chambers 32 outwardly of the device through
outlet 56 is as previously described. The construction and
operation of the outlet flow control means, or shut off and
fluid metering means 63 is also as previously described.
Superimposed over distendable membrane 30 is a porous
plastic, free venting, structural filler member 40 (not shown
in Figure 17), which, in turn, is covered by a cover 44 of the
character previously described.
The first portion of the housing, designated in
Figure 17 by the numeral 302, is of a slightly different con-
struction than first housing portion 18. Rather than being
provided with stop members 18a and 18b of the character shown
in Figure 2, first housing portion 302 is here provided with
internal threads 304, the purpose of which will presently be
described. Portion 302 is also provided with container locking
SUBSTITUTE SHEET
WO g3/20885 PCI'/US93/03371
t ~
means shown here as resilient ratchet teeth 306 which interface
and interlock with mating ratchet teeth 308 provided on the
drug vial container assembly.
The coupling means of this third form of the inven-
tion is substantially identical to the coupling means of the
earlier described embodiments and comprises a sterile coupling
assembly 110 which is supported centrally of cylindrical hous-
ing portion 302 by a rigid coupling support 111 which extends
transversely of housing portion 302. Coupling assembly llo
comprises an outer cylindrical portion 112 having internal
threads 113 (Figure 19) and a coaxially aligned inner cylindri-
cal portion 114. As before, inner portion 114 is rigidly held
in position within outer portion 112 by means of a circular
shaped end wall 116 (Figure 19). As best seen by also refer-
ring to Figure 19, end wall 116 is closely received within a
recess or socket 118 formed in coupling support 111. Also
forming a part of coupling support 111, is a radially extending
connector element 120 having an internal fluid passageway 122
which is adapted to communicate with inlet 24 of base 22 when
support 111 is positioned within cylindrical portion 302 in the
manner shown in Figures 17 and 18.
Turning to Figure 19, passageway 122 communicates
with a passageway 124 which is defined by the interior walls of
cylindrical portion 114. A smaller diameter fluid passageway
126 joins passage 124 at a valve seat defining tapered wall
portion 128. A valve means, generally designed by the numeral
136, which is of the character previously described, is recip-
rocally movable within passageway 124 and functions to control
the flow of fluid through passage 126 in a manner presently to
be described. As indicated in Figures 8 and 9, valve means 136
includes a valve element 137 having a body portion 138 and a
SUBSTITUTE SHEET
W093/20885 ' PCT/US93/03371
32
stem portion 140. At the junction of portions 138 and 140 is a
tapered wall 142 which is adapted to substantially sealably
engage the valve seat defined by tapered wall portion 128 when
the valve is in the closed configuration shown in Figure 19.
When the apparatus of the invention is in a storage mode, the
open end of coupling 112 is preferably closed by a removable
sealing cap 130 of the character shown in Figure 1.
In this form of the invention shown in Figures 17
through 21, container assembly 300 includes second flow control
means for controlling the flow of fluid into and out of an
internal chamber 309 of a glass vial 310 (Figure 19) which
contains the medicament M. The second flow control means of
this form of the invention is similar in construction and
operation to that previously described and includes a plunger
86 which is substantially sealably receivable within vial 310.
Plunger 86 also includes connector means, shown as threads 86b,
for interconnection with the coupling means of the apparatus.
As before, valve assembly 88 controls fluid flow through passa-
geway 86c formed within plunger 86 and is operated by operating
means of the character previously described. However, as seen
in Figures 19 and 20 plunger 86 includes circumferentially
extending, annular channel portions 89 and 91 which substan-
tially sealably engage members 92 and 96 respectively of member
90 .
Glass vial 310 is enclosed in a multi-part cover, or
overpackage 312 which surrounds vial 310 and includes the pre-
viously identified, circumferentially extending ratchet teeth
308. Provided proximate the open end of cover 312 are external
threads 314 which are adapted to mate with internal threads 304
provided within cylindrical portion 302 of the apparatus hous-
ing. Vial 310 (Figure 18) is closed by a tear-away removable
SUBSTITUTE SHEFT
=
W093/20885 PCT/US93/0337~
~ 0 ~ 1
33
closure cap such as 311 which is integrally formed with the
forward part 312 f of overpackage 312 .
In operating the apparatus of this third form of the
invention, vial closure cap 311 is first removed from the drug
vial assembly 300. This done closure member 130, which closes
the passageway of the sterile closure element 110, is also re-
moved and the open end of the drug vial assembly 300 is insert-
ed into open end 148 of cylindrical portion 302 (Figure 19).
As the drug vial 310 is received within open end 148, threads
314 will move toward a first internal thread 304a provided
within cylindrical portion 302 (Figure 19). Simultaneously
threads 86b will move toward mating engagement with threads 113
provided on coupler member 112. Rotation of the drug vial in a
clockwise direction will cause threads 314 to mate with first
internal thread 304a and will cause threads 86b on plunger 86
to mate with threads 113 on coupling member 112 in the manner
shown in Figure 2 0 . Teeth 308 provided on overpackage 312 will
also move to a location proximate ratchet teeth 306. As the
plunger couples with member 112, stem 106 of the container
valve will engage stem 140 of valve means 136 simultaneously
moving both valve member 137 of the first flow control means
and valve member 92 of the second flow control means into the
open position shown in Figure 20. With the valves of the flow
control means in this open position, distendable membrane 3 0
will cause the fluid contained within chambers 32 to flow under
pressure past the valve seat 128 into fluid passageway 126 of
the coupler means and then into passageway 103 of the container
valve means. The fluid under pressure will next flow through
radially extending passageways 94 of the container valve and
rapidly into the interior 3 09 of the glass container 310 in the
manner shown by the arrows in Figure 20. This flow of fluid
SUBSTITUTE SH~ET
W093/20885 . ~g~O ~ PCT/US93/03371
34
under pressure into the drug vial initiates the mixing or
reconstitution process.
As depicted by the arrows in Figure 20, the fluid
flowing into the drug vial will thoroughly mix with the medica-
ment M contained within the vial in the manner shown to form a
drug active flowable substance comprising a mixture of the
diluent stored within chambers 32 and the medicament M which
was stored within the drug vial.
After the medicament M is mixed with the diluent, the
drug vial assembly is once again rotated in a clockwise direc-
tion. During this further rotation, threads 314 on the drug
vial will move through a circumferentially extending space 316
provided within cylindrical portion 302. As shown in Figure
20, space 316 functions as a dwell space and is located inter-
mediate first thread 304a and threads 304. Continued clockwise
rotation of the drug vial assembly will cause threads 314 to
mate with threads 304 moving the drug vial assembly from the
position shown in Figure 20 to the seated position shown in
Figure 21. Ratchet teeth 308 on the overpackage will also mate
with resilient ratchet teeth 306 provided within housing por-
tion 302. As best seen in Figure 22, ratchet teeth 306 are
constructed so that they are yieldably deformable in a manner
to permit the drug vial assembly to be freely rotated in a
clockwise direction, but are designed to engage teeth 308 in
the manner shown in Figure 22 to preclude block counter-clock-
wise rotation of the vial assembly. With this construction,
once the drug vial assembly is mated with cylindrical housing
portion 302 it cannot be easily removed.
Movement of the drug vial assembly into the position
shown in Figure 21 causes the reconstituted mixture to be
transferred back into the infusion device via passageways 94,
SUBSTITUTE SHEET
W093/2088~ PCTJUS93/03371
103, 126, 124, 122 and 28 for introduction into chamber 30 and
further mixing with the diluent and for later infusion into the
patient in the manner previously described.
A label covering the peripheral surface of overpack-
age 312 and joining the forward and rear portions 312f and
312r, can be provided with indicia in the form of numbers,
color codes, or the like, to indicate the interconnection,
reconstitution and transfer location function of the vial
assembly with respect to cylindrical housing 302. Such indicia
are useful in training lay persons in the operation of the
apparatus.
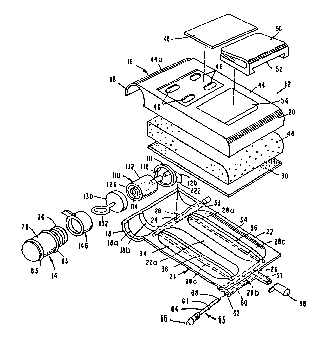
Referring to Figures 23 through 33, another embodi-
ment of the invention is there illustrated. This fourth form
of the invention is quite different in overall appearance, but
similarly includes a drug vial or container assembly of identi-
cal construction and operation to that of container assembly
300 of the third form of the invention. Accordingly, like
numerals are used in Figures 23 through 33 to identify like
container assembly component parts. The coupling members of
the coupling means of the present form of the invention are
also identical to those previously described in connection with
Figures 17 through 22. However, the cylindrically shaped first
portion of the apparatus housing which houses the coupling
means, here identified by the numeral 400, is of slightly
different construction, as is the second housing portion that
houses'the infusion portion of the device. The device of this
fourth form of the invention is generally larger than the
devices of the earlier described embodiment and is designed to
dispense larger volumes of medicaments.
Turning particularly to Figures 23, 24 and 25, the
second or infusion portion, generally designated by the numeral
SUBSTITUTE SHEET
W 093/20885 ~ PC~r/US93/03371
36
402, comprises a base assembly 404 which includes a curved base
member 406 having front and back surfaces 408 and 410. The
central portion 407 of base member 406 is provided with a
multiplicity of small, crossing fluid flow micro-channels 412
which communicate with a longitudinally extending, central
collection fluid passageway 414 having spaced-apart portions
413 and 416 (Figure 29). The function of these channels and
parts will be described presently. The side portions 418 of
base member 406 are provided with spaced-apart apertures 420
which can be used to grip the device during handling or can
accept straps for use in connecting the device to the patients
body.
A thin, generally planar distendable elastomeric mem-
brane, or member, 430 is termally bound and cooperates with
central portion 407 of base 406 to form a chamber 432 (Figure
27) . Member 430 is distendable out of plane in the manner
shown in Figure 27 by the introduction of fluid into the cham-
ber under pressure. As the distendable member 430 is distended
by fluid pressure, internal stresses are formed in the member
which continuously urge it to return to its original less
distended configuration.
Forming an important aspect of this latest form of
the apparatus of the invention is the provision of filter means
which is disposed internally of chamber 432 for filtering
fluids flowing from chamber 432 into fluid passageways 412
formed in base member 406. The filter means also functions as
an interfacial bubble trap. In the embodiment of the invention
here shown, the filter means is provided in the from of a thin,
micro-porous film, laminate or composite membrane 434 which is
fitted over the front surface 408 of base 406 in the manner
shown in Figure 27. Front surface 408 provides support means
SU8STITUTE SHEET
WOg3/20885 ~ PCT/US93/03371
for filter 434. Membrane 434 can be constructed from a wide
variety of filtering materials of a character well understood
by those skilled in the art, including Cellouous Acetate,
Polytetraflouroethylene, Polypropylene, Polyvinylidene Flouride
and Polyurethane/Polyethylene Composite.
Superimposed over distendable membrane 430 is a
porous plastic, free venting, structural filler member 436. As
best seen by referring to Figure 27, member 436 is provided
with a centrally disposed, longitudinally extending, concave
channel 438 having an interior wall 440 against which membrane
430 initially engages when it is maximally, outwardly distended
by fluid flowing into chamber 432 under pressure. Member 436
can be constructed of the same materials as previously de-
scribed in connection with member 40.
Extending over and sealably enclosing member 436 is a
cover means shown here as a hard plastic cover 442. Cover 442
includes gas venting means here provided as a plurality of
apertures 444 formed within the upper wall of the cover member.
When distendable member 430 is constructed of a material of
high gas permeability, gas venting means, including apertures
444, permit gases contained within the fluids, if any, then
introduced into chamber 432 to pass through the gas permeable
membrane, through filler 436 and to atmosphere throuyh the gas
venting means. A medicament label 446 having a removable
portion, covers vent apertures 444. In certain applications,
the cover and base can be constructed of similar materials of
the character previously described.
Base assembly 404 also includes an outlet or delivery
port 448, which is normally closed, by a removable cover member
450. Outlet port 448 is in communication with fluid passageway
414 and an outlet 417 via a conduit 452 (Figure 28). Outlet
SUBSTITUTE SHEET
W O 93/20885 ~ PC~r/US93/03371
port 448 and passageway 452 are also in communication with a
transversely extending passageway 454 formed in base 406 which
terminates at its outer end in an opening 456 (Figure 28).
Receivable within opening 456 (Figure 23) is an outlet flow
control means shown here as a shut-off and fluid metering means
of the character previously discussed herein and identified in
Figures 2 and 6 by the numeral 63. In the manner previously
described, needle valve means 63 functions to either substan-
tially close or to controllably restrict the flow of fluid
outwardly of the device through passageway 414 and outlet port
448. As seen in Figure 28, passageway 454 is internally
threaded to threadably receive external threads 458 formed on a
stem 460. With this construction, by rotating a control knob
462 attached to stem 460, the valve member can be moved axially
of passageway 454 to controllably move tapered portion 464
provided on stem 460 proximate its inner end relative to passa-
geway 454 and into engagement with a valve seat 464a provided
in base 406 (Figure 28). An 0-ring 463 is provided to seal
stem 460 relative to passageway 454. Alternatively to, or in
conjunction with, the needle valve, passageway 452 can be
initially sealed by an internal structural septum 465 (Figure
23) which can be pierced by an I-V administration set piercing
spike. This type of recipient port a septum structure is well
known in the art.
Turning to Figures 23 and 29, the construction of the
container assembly 300 can be seen to be of similar construc-
tion to that shown in Figures 17 through 22. The container
assembly, the details of construction of which will not be
repeated here, is receivable within cylindrical housing portion
400 and the plunger 86 is initially mated with the coupling
member 112 in the manner previously described (See also Figures
SUBSTITUTE SHEET
W093/20885 PCT/US93/0337t
39
32 and 33). In this latest form of the invention, cylindrical
portion 400 is integrally connected to the back or concave
surface 410 of base member 406 by means of a connector flange
470 (Figure 23). Portion 400 also includes a transversing
extending base wall 471 having a socket 473 which supports
coupling member 112 in a manner best seen in Figure 30. Base
wall 471 is provided with a passageway 477 which communicates
with passageway 124 of coupling member 114 and with passageway
414 of base 402 via port 415. Similarly, an outlet passageway
housing 472 and a needle valve housing 474 extend angularly
outwardly from back surface 410 (Figures 26 and 28). It is to
be noted that the front surface 408 of base member 406 is
provided with an upstanding mounting boss 475 which surrounds
port 415 and to which filter 434 is bonded. Filter 434 is
provided with an aperture 434a which peripherally receives boss
475 so that fluid can flow freely through port 415 between
channel 414 and chamber 432. (Figure 25).
In operating the apparatus of this fourth form of the
invention, the device is held by one of the side portions 418
and, with the vial closure cap and the cap which closes the
passageway of the sterile coupling element 110, removed, the
open end of the drug vial assembly 300 is inserted into open
end 401 of cylindrical portion 400 (Figure 23). As best seen
by referring to Figures 29 and 30, as the drug vial 310 is
received within open end 401, threads 314 will move toward a
first internal thread 403a provided within cylindrical portion
400. Simultaneously threads 86b will move toward mating en-
gagement with threads 113 provided on coupler member 112.
Rotation of the drug vial in a clockwise direction will cause
threads 314 to mate with first internal thread 403a and will
cause threads 86b on plunger 86 to mate with threads 113 on
SUBSTITUTE SHEET
W093/20885 PCT/US93/0337t
coupling member 112 in the manner shown in Figure 30. Teeth
308 provided on overpackage 312 will also move to a location
proximate ratchet teeth 405 provided on cylindrical housing
portion 400. As the plunger couples with member 112, stem 106
of the container valve will engage stem 140 of valve means 136
simultaneously moving both valve member 137 of the coupling
flow control means and valve member 92 of the container flow
control means into the open position shown in Figures 30 and
33. With the valves of the flow control means in this open
position, distendable membrane 430 will cause the fluid con-
tained within chamber 432 to flow under pressure through port
415 (Figures 25 and 30) into passageway 477, into passageway
124, past the valve seat 128 into fluid passageway 126 of the
coupler means and then into passageway 103 of the container
valve means. The fluid under pressure will next flow through
radially extending passageways 94 of the container valve and
rapidly into the interior 309 of the glass container 310 in the
manner shown by the arrows in Figure 30. This flow of fluid
under pressure into the drug vial initiates the mixing or
reconstitution process.
As depicted by the arrows in Figure 30, the fluid
flowing into the drug vial will thoroughly mix with the medica-
ment M contained within the vial in the manner shown to form a
flowable substance of drug active concentrate comprising a mix-
ture of the diluent stored within changer 432 and the medica-
ment M which was stored within the drug vial.
After the medicament M is mixed with the diluent, the
drug vial assembly is once again rotated in a clockwise direc-
tion in the manner shown in Figure 31. During this further
rotation, the vial will move through a circumferentially ex-
tending space 479 proved within cylindrical portion 400. As
SUBSTITUTE SHEET
W093/20885 ~g Q ~ ~ PCT/US93/03371
before, space 479 functions as a dwell space and is located
intermediate first thread 403a and threads 403. Dwell space
479 provides momentary residence time allowing system back-
filling and drug reconstitution. Continued clockwise rotation
of the drug vial assembly will cause threads 314 to mate with
threads 403 moving the drug vial assembly from the position
shown in Figure 30 to the position shown in Figure 31. Ratchet
teeth 308 on the overpackage will also mate with resilient
ratchet teeth 405 provided within housing portion 400 so as to
substantially lock the vial in position within ho~sing portion
400.
Continued movement of the drug vial assembly into the
final position shown in Figure 31 causes the reconstituted mix-
ture to be subs~antlally Lransferred back inio cnamber 432 of
the infusion device via passageways 94, 103, 126, 124, 477 and
414 and through port 415 for later controlled infusion of the
reconstituted drug active medicament into the patient via the
filter 434 and the multiplicity of fluid collection passageways
412, into passageway 414 through port 417 and outwardly of the
device through passageway 452 and outlet 448. As previously
discussed, the rate of flow fluid through outlet 448 is con-
trolled by the needle valve means 63.
once again, a label covering the peripheral surface
of overpackage 312 is preferably provided with indicia in the
form of numbers, color codes or the like to indicate the inter-
connection, reconstitution and transfer functions of the vial
assembly with respect to cylindrical housing 400.
Turning to Figures 34 through 38, the latest embodi-
ment of the present invention is shown. This final embodiment
is similar in many respects to the embodiment of Figures 23
through 33 and like numbers are used to identify like component
SUBSTITUTE SHEET
W093/20885 ~ 4 1 PCT/US93/03371
42
parts. More particularly, the infusion container portion of
the device along with coupling members 112 and 114 are identi-
cal to those previously described as is the coupling valve
means 136 and the operating means. However, the cylindrical
housing portion 500, while mounted on the back surface 410 of
the base 406 is of a slightly different construction as is the
drug vial assembly 502. The details of construction of these
different elements and the method of operation of this last
form of the invention will be described in the paragraphs which
follow.
Turning first to Figures 34 and 35, the base assembly
can be seen to be quite similar to that shown in Figure 25
having a curved base member 406 provided with a multiplicity of
flow micro-channels 412 which communicate with a central passa-
geway 414 having spaced-apart portions 413 and 416. Side
portion 418 having apertures 420 are as previously described.
The apparatus also includes a distendable elastic membrane 430
and filter means 434 which function as before. Turning to
Figure 36, the drug vial or container assembly 502 of this form
of the invention, includes second flow control means for con-
trolling the flow of fluid into and out of an internal chamber
509 of a vial 510 which contains the medicament M. The second
flow control means of this form of the invention is identical
in construction and operation to that previously described and
includes a lower durometer plunger 86 which is substantially
sealably receivable within vial 510. Plunger 86 also includes
connector means, shown as threads 86b, for interconnection with
the coupling means of the apparatus. As before, valve assembly
88 controls fluid flow through flow passageways formed within
plunger 86 and is operated by operating means of the character
previously described.
SUBSTITUTE SHEET
W093/20885 PCT/US93/03371
43
Vial 510 is enclosed in a multi-part cover, or over-
package member 512 which surrounds vial 510. Overpackage
member 512 is, in turn, telescopically received within a collar
512a which includes system interlock stops 5110 Provided
proximate the lower end of collar 512a are external threads 514
which are adapted to mate with internal threads 504 provided
within cylindrical portion 503 of the apparatus housing. Vial
510 is closed by an integral tear-off type closure such as 508
(Figure 36). Collar 512a is also provided with circumferen-
tially spaced finger grips 507, the purpose of which will
presently be described. Overpackage member 512 includes lock-
ing means for locking the cover assembly to the cylindrical
portion 503. This locking means is here provided in the form
of an annular member 515 located proximate the lower end of
member 512.
Turning to Figure 37, cylindrical portion 502 of the
housing of this latest form of the invention has an enlarged
diameter mouth 517 which is adapted to receive stops 511 and
which defines a radially extending annular surface 519 against
which stops 511 engage. Longitudinally spaced apart from
surface 519 is a circumferentially extending, inwardly protrud-
ing annular stop member 521 which, in a manner presently to be
described, is adapted at the completion of the cycle, to lock-
ably engage annular member 515 provided on overpackage member
512.
In operating the apparatus of this final form of the
invention, vial closure 508 is first removed from the drug vial
assembly 300. This done, the open end of the drug vial assem-
bly 502 is inserted into sterile mouth 517 of cylindrical
portion 503 (Figure 37). Using finger grips 507 from control,
SUBSTITUTE SHEET
W O 93/20885 PC~r/US93/03371
44
threads 514 are threadably mated with internal threads 504
provided within cylindrical portion 503. Vial assembly 502 is
then pushed forward to move threads 86b on plunger 86 into
proximity with threads 113 provided on coupler member 112.
Further rotation of the drug vial in a clockwise direction,
using grips 507, will then cause threads 86b on plunger 86 to
mate with threads 113 on coupling member 112 in the manner
shown in Figure 37. As the plunger couples with member 112,
stem 106 of the container valve will engage stem 140 of valve
means 136 simultaneously axially moving both valve element 137
of the first flow control means and valve member 88 of the
second flow control means into the open position shown in
Figure 37. With the valves of the flow control means in this
open position, distendable elastic membrane 30 will cause the
fluid contained within chamber 432 to flow under pressure
through port 415, into passageway 477, into passageway 124,
past the valve seat 128 into fluid passageway 126 of the cou-
pler means and then into passageway 103 of the container valve
means. The fluid under pressure will next flow through radial-
ly extending passageways 94 of the container valve and rapidly
into the interior of the container 510 in the manner shown by
the arrows in Figure 37. This flow of fluid under pressure
into the drug vial initiates the mixing or reconstitution
process and causes the container assembly to move outwardly
(upwardly as shown in Figure 37).
In all forms of the invention previously described,
the plunger of the container valve is preferably constructed
from a rubber or silicon material. The valve member which
reciprocates within the plunger is preferably constructed of
higher durometer rubber or silicon, or from glass or plastic
materials such as polypropylene, polycarbonate, polystyrene,
~UBSTITUTE SHEE~
W~~ 93~20885 PCr/US9310337-
~ ~ p, ~
ABS, PTFE or high density teflon or nylon. Similarly, valve
member 137 is preferably constructed from silicon rubber,
rubber, flexible PVC, polyurethane, PTFE, or fluorsilicon
elastomers.
Referring now to Figures 39 through 53, still another
embodiment of the invention is shown. In this embodiment, the
construction of the cylindrical housing portion 600 and the
base assembly 604 are of similar construction to housing por-
tion 400 and base assembly 406 shown in Figures 23 through 28
and like numbers are used to designate like components. Howev-
er, the container assembly, the details of construction of
which will presently be described, is of a somewhat different
construction. More particularly, the apparatus of this new
form of the invention uniquely permits controlled intermixing
of the diluent or other parenteral fluid with the medicament by
providing flow rate control means for precisely controlling the
rate of fluid flow between the storage reservoir and the medi-
cament mixing chambers.
In this latest form of the invention, cylindrical portion
600 is integrally connected to the back or concave surface 410
of the base member by means of a connector flanges 470 (Figure
23). Portion 600 also includes a transversing extending base
wall 607 having a socket 609 which supports a coupling member
612 in a manner best seen in Figure 40. Base 406 is provided
with longitudinally extending passageways 614 and 616. Passa-
geway 614 communicates with storage reservoir 432 and with port
415. Passageway 616 communicates with the outlet port of the
device formed in outlet passageway housing 472. Similarly, a
needle valve housing 474 of the character previously described
extends angularly outwardly from back surface 410 (See also
Figures 26 and 28). It is to be noted that the front surface
SUBSTITUTE SHEET
W O 93/20885 PC~r/US93/0337t V ~ l
46
of the base member is provided with crossing micro flow chan-
nels 412 and with an upstanding mounting boss 475 which sur-
rounds port 415 and to which a filter membrance 434 is bonded
(Figure 39). Filter means and micro flow channels 412 function
in the same manner to accomplish the same result as previously
described herein.
Referring now to Figures 41 and 42, the container
assembly 620 of this form of the invention comprises a glass
vial 622 having a chamber 624 for containing a medicament which
may be an extended time release medicament, which is here shown
as capsules or tablets 625. A plastic cover or overpackage 626
is closely received over vial 622 and includes a metering
thread 627 (Figure 53), the purpose of which will presently be
described. Surrounding cover 626 is a collar 628 having exter-
nal threads 630 and system interlock stops 632. Threads 630
are adapted to mate with internal threads 634 provided on
cylindrical portion 600. Housed within vial 622 is the flow
control means of this form of the invention for substantially
controlling the flow of fluid into and out of mixing chamber
624. Here the flow control means comprises a plunger 636
sealably received within vial 622. Plunger 636 is of generally
similar construction to previously described plunger 86 being
cylindrical in shape and having a skirt portion 638 adapted to
sealably engage the inner wall of vial 622 (Figure 44).
Plunger 636 is provided with a centrally disposed
bore 639 and a plurality of circumferentially spaced fluid
passageways 640. An inlet or fine flow control valve member
642 also forms a part of the flow control means and includes a
centrally disposed inlet passageway 643. Member 642 is recip-
rocally movable within bore 639 and is provided with a first
flange portion 641 which functions to control fluid flow
~3UE3STITUTE SHEET
~ W093/20885 2 1 ~ 8 0 ~ ~ P~T~US93/03371
47
through passageways 640. Member 642 also includes a second or
inboard flange 645 which guides the travel of the valve member
within an annular channel 636a provided within plunger 636.
Valve member 642 further includes a stem 647 which, in a manner
presently to be described, cooperates with a check valve 646
carried within an internal passageway 649 provided in coupling
member 612 (Figure 43). Stem 647 forms an integral part of the
valve member 642 and ter~inates at an inboard end 647a. Prox-
imate the opposite end 647b of stem 647 is a transverse fluid
passageway 650 which permits fluid flowing past check valve 646
to enter inlet passageway 643 (Figure 50).
In operating the apparatus of this latest form of the
invention, the vial closure cap 651 and the cap 652 which
closes the passageway of the drug vial (Figures 43 and 44) are
first removed. The open end 653 of the drug vial assembly is
then ready to be inserted into end 601 of cylindrical portion
600 (Figure 45) which in some instances may be closed began
sterile peal away closure seal (not shown). As best seen by
referring to both Figures 43 and 44, as the drug vial assembly
620 is received within open end 601 of the infusion portion of
the apparatus, threads 630 will move toward engagement with a
first internal thread 634a provided within cylindrical portion
600. By griping finger grips 654 of collar 628 the drug vial
can be rotated in a clockwise direction causing the drug vial
to advance within cylindrical portion 600 to the position shown
in Figure 45. At this secured position, system interlock steps
632 will move toward locking engagement with an internal shoul-
der 655 provided on cylindrical housing portion 600. As the
drug vial thus mates with cylindrical portion 600, stem 647 of
valve member 642 will move into proximity with check valve 646.
Turning now to Figure 45, it is to be observed that
SUBSTITUTE SHEEt
W O 93/20885 ~ P~r/US93/03371
48
clockwise rotation of vial 620 relative to collar 628 will
cause stem 644 to engage check valve 646. Because of the
pressure being exerted on valve 646 by the fluid in the reser-
voir, movement of the check valve to the open position will be
resisted. Accordingly operating valve 642 will move to the
left to the position shown in Figure 45. However, when flange
645 seats against shoulder 636a on plunger 636, continued
clockwise rotation of the vial within collar 628 will cause
stem 644 to move the check valve 646 to the left into the open
position shown in Figures 45. With the check valve of the flow
control means in this open position, distendable membrane 430
will cause the fluid contained within chamber 432 to flow under
pressure through port 415 (Figures 40 and 45) into passageway
658, then into passageway 660, past the valve seat 662 into
fluid passageway 664 of the coupling member and then into
central passageway 643 of the inlet valve member 642. The
fluid under pressure will next flow rapidly into the medicament
chamber 624 of the glass container in the manner shown by the
arrows in Figure 45. This flow of fluid under pressure into
chamber 624 causes controlled diluent flow around capsules 625
and initiates the controlled mixing or reformulation process to
produce the beneficial agent to be infused into the patient.
Referring particularly to Figure 45, it is to be
noted that rotation of vial 620 relative to collar 628 not only
opened check valve 640 but also caused flange 641, including
protuberances 641a of member 642 to move from the position
shown in Figure 44 wherein fluid flow through passageways 640
of plunger 636 was blocked to the open flow position shown in
Figure 45. This movement of valve 642 to the right as shown in
figure 45 permits fluid to flow rearwardly from medicament
chambers 624 into passageways 640 of plunger 636 in the manner
SUBSTITUTE SHEET
W093/20885 ~ ~ CTJUS93J03371
49
shown by the arrows 665 in Figure 4S.
With valve member 642 in the position shown in Figure
45, the mixed solution, or beneficial agent, will continue to
flow under pressure through passageways 640, into passageways
668 formed in coupling member 612, into annular collector
passageway 670 (Figure 47) into passageway 672 formed in cylin-
drical portion 600 and thence to the outlet port via passageway
452. A filter member 674 is provided within medicament cham-
bers 624 to filter out particulate matter prior to the dispens-
ing of the reformulated beneficial agent from the device.
After commencement of the controlled mixing of the
medicament with the diluent, the rate of flow of the diluent
from the reservoir 432 toward the drug vial and back toward the
reservoir can be precisely regulated by counter clockwise
= rotation the drug vial relative to metering threads 677 provid-
ed on collar 628 in the manner illustrated in Figure 48. This
counter clockwise rotation of the vial as indicated in Figure
48 causes the check valve 646 to move toward the closed posi-
tion shown in Figure 48 tending to throttle fluid flow toward
the mixing chamber. In this way fluid flow toward the mixing
chamber 624 can be precisely controlled. Referring to Figures
47 and 51, it is to be noted that vial overpackage 626 is
provided with a plurality of circumferentially spaced grooves
680 while collar 628 is provided with a radially inwardly
extending rib 682. As the vial is rotated relative to the
collar, the rib 682 will sequentially engage the grooves caus-
ing a clicking sound and permitting very fine rotational ad-
justments to be made. As shown in Figure 41, overpackage 628
is provided with a pointer 684 which indexes with indicia 686
which may be color coded to show fluid flow rate bused on the
position of the vial relative to the collar. Ratchet teeth can
SIJBSTITUTE SHEET
W O 93/20885 . PC~r/US93/03371 ~
~8~4~ -
also be provided on the overpackage to mate with resilient
ratchet teeth provided within housing portion 600 so as to sub-
stantially lock collar 628 in position within housing portion
600 as the flow rate adjustments are made.
Referring now to Figures 52 through 62, yet another
embodiment of the invention is shown. In this embodiment, the
construction of the infusion portion of the device including
the cylindrical housing portion and the base assembly are of
identical construction to that of the embodiment of the inven-
tion shown in Figures 39 through 51 and like numbers are used
to designate like components. However, once again the contain-
er assembly, the details of construction of which will present-
ly be described, is of a somewhat different construction. As
was the case with the embodiment of Figures 39 through 51, the
apparatus of this latest form of the invention also uniquely
permits controlled inter ;x;ng of the diluent or other pareter-
al fluid with the medicament by providing flow rate control
means for precisely controlling the rate of fluid flow between
the storage reservoir and the medicament mixing chambers.
Referring particularly to Figures 52 and 53, the con-
tainer assembly 720 of this form of the invention comprises a
glass vial 722 having a chamber 724 for containing a medicament
which is here shown as dissolvable drug compounds 725. A
plastic cover or overpackage 726 is closely received over vial
722 and includes a metering thread 727 (Figure 53), the purpose
of which will presently be described. Surrounding cover 726 is
a collar 728 having external threads 730 and system interlock
stops 732. Threads 730 are adapted to mate with internal
threads 634 provided on cylindrical portion 600 (Figure 55).
Housed within vial 722 is the slightly differently configured
flow control means of this latest form of the invention for
SVBSTITUTE SHEET
W093/20885 PCT/US93~0~371
~ 0 4 1
51
controlling the flow of fluid into and out of mixing chamber
724. Here the flow control means comprises a plunger 736
sealably received within vial 722. Plunger 736 is of generally
similar construction to previously described plunger 86 being
cylindrical in shape and having a skirt portion 738 adapted to
sealably engage the inner wall of vial 722 (Figure 55).
Plunger 736 is provided with a centrally disposed
bore 739 and a plurality of circumferentially spaced fluid
passageways 740. An inlet or fine flow control valve member
742 of slightly different construction also forms a part of the
flow control means. Member 742 is reciprocally movable within
bore 739 and is provided with a first flange portion 741 which
functions to control fluid flow through passageways 740.
Member 742 also includes a second or inboard flange 745 which
guides the travel of the valve member within an annular channel
736a provided within plunger 736. Valve member 742 also in-
cludes a stem 747 which is disposed within a central flow
passageway 742a and which, in a manner presently to be de-
scribed, cooperates with a check valve 746 carried within an
internal passageway 749 provided in coupling member 612 (Figure
55). Stem 747 forms an integral part of the valve member 742
and terminates at an inboard end 747a (see also Figure 61).
Proximate the opposite end of stem 747 is an enlarged diameter
portion 747b which terminates in the previously identified
flange 741. Extending angularly through portion 747b are
circumferentially spaced fluid passageways 750 which, in a
manner presently to be described, permit fluid flowing past
check valve 746 to enter chamber 724. (Figure 56). Enlarged
diameter portion 747b is also provided with circumferentially
spaced, radially extending flow passageway 742b.
In operating the apparatus of this latest form of the
SUBSTITUTE SH~Et
W093/20885 PCT/US93/03371 ~
.
invention, the vial closure cap 651 and the cap 652 which
closes the passageway of the drug vial (Figures 54 and 55) are
first removed. The open end 753 of the drug vial assembly is
then ready to be inserted into open end 601 of cylindrical
portion 600 which may also have been previously sealed by a
removable cover (not shown) (Figure 54). As best seen by
referring to both Figures 54 and 55, as the drug vial assembly
is received within open end 601 of the infusion portion of the
apparatus, threads 730 will move toward engagement with a first
internal thread 634a provided within cylindrical portion 600.
By griping finger grips 754 of collar 728 the drug vial can be
rotated in a clockwise direction causing the drug vial to
advance within cylindrical portion 600 to the position shown in
Figure 55. At this position, system interlock steps 632 will
move toward locking engagement with an internal shoulder 655
provided on cylindrical housing portion 600. As the drug vial
thus mates with cylindrical portion 600, stem 747 of valve
member 742 will move into proximity with check valve 746.
Turning now to Figure 56, it is to be observed that
clockwise rotation of vial 720 relative to collar 728 will
cause stem 747 to engage check valve 746. Because fluid under
pressure within the reservoir resists movement of the check
valve, operating valve 742 will move to the left to the posi-
tion shown in Figure 56. However, when flange 745 seats
against internal shoulder 736a of plunger 736, continued clock-
wise rotation of vial 720 within collar 728 will cause stem 747
to move check valve 746 to the left into the open position
shown in Figure 56. With the check valve in this open posi-
tion, distendable membrane 430 will cause the fluid contained
within chamber 432 to flow under pressure through port 415
(Figures 40 and 46) into passageway 658, then into passageway
SUBSTITUTE SHEET
W093/20~85 ~ 1$~ P~T/US93/03371
660, past the valve seat 762 into fluid passageway 664 of the
coupling member and then into central passageway 742a of the
inlet or operating valve member 742. The fluid will then flow
into passageways 742b and 750 and then vigorously into mixing
chamber 724. (See also Figures 59 and 60). This flow of fluid
under pressure into chamber 724 causes controlled diluent flow
through and around drug compound 725 as shown in Figure 56 and
initiates the mixing or reformulation process to produce the
beneficial agent to be infused into the patient.
Referring particularly to Figure 55, it is to be
noted that so long as valve member 742 is in the position there
shown, flange 741, including protuberances 741a as provided
thereon, will block fluid flow through passageways 740 of
plunger 736. However, as previously mentioned, clockwise
rotation of the vial assembly 720 within the overpackage 726
will cause valve member 742 will move to the right due to the
urging of check valve 746. So long as member 742 is in the
position shown in Figure 56, The mixed solution, or beneficial
agent, will continue to flow under pressure in a reverse direc-
tion toward reservoir 432 through passageways 740, into passa-
geways 668 formed in coupling member 612, into annular passage-
way 670 (Figure 49) into passageway 672 formed in cylindrical
portion 600 and thence to reservoir 432. As before a filter
member 774 is provided within medicament chambers 724 to
filter out particulate matter prior to the beneficial agent
being dispensed from the device.
After commencement of the mixing of the medicament or
drug 725 with the diluent, the rate of flow of the diluent from
the reservoir 432 toward the mixing chambers of the drug vial
= and outwardly of the device can be precisely regulated by
rotating the drug vial in a counter clockwise direction rela-
SUBSTITUTE SHEEl'
W O 93/20885 PC~r/US93/03371 ~
g ~
tive to metering threads 777 provided on collar 728 in the
manner previously described and as illustrated in Figure 58.
Referring to Figure 62, the mixing chamber 747 is
shown to contain a medicament in the form of a dissolvable
tablet 725a. This tablet is comprised of a beneficial agent
such as a muscle relaxant.
Turning now to Figures 63 through 67, still another
embodiment of the invention is shown. In this embodiment, the
construction of the cylindrical housing portion 800 and the
base assembly 804 are of similar construction to housing por-
tion 400 and base assembly 406 shown in Figures 23 through 28
and like numbers are used to designate like components. Howev-
er, the container assembly, the details of construction of
which will presently be described, is of a somewhat different
construction. More particularly, the apparatus of this latest
form of the invention uniquely permits controlled intermixing
of a first component such as a diluent, solvent or other paren-
teral fluid with an additive such as a medicament or other
beneficial agent which is presented to the first component by a
unique adding means, the character of which will presently be
described.
In the paragraphs which follow, wherein the details
of this unique intermixing process will be discussed, the
following terms will have the following meanings:
Element - any of the fundamental substances that consist
of atoms of only one kind and that singly or in combina-
tion constitute all matter.
Additive - the element, compound, substance, agent,
biologically active material, or other material which is
to be added, all or in part, to the fluid introduced into
the device of the invention.
SUBSTITUTE~ EET
~ W093/20885 PCT/US93/0337~
û 4 ~
Polymer - a chemical compound or mixture of compounds
formed by polymerization and consisting essentially of
repeating structural units.
Parenteral Fluid - any solution which may be delivered to
a patient other than by way of the intestines, including
water, saline solutions, alkalizing solutions, dextrose
solutions acidifying solutions, electrolyte solutions,
reagents, solvents and like acquous solutions~
Beneficial Agents - any drug, medicament, pharmaceutical,
medical polymer, enzyme, hormone, antibody, element,
chemical compound or other material useful in the diagno-
sis, cure, mitigation, treatment or prevention of disease
and for the maintenance of the good health of the patient.
BiologicallY Active Material - a substance which is bio-
chemically, Immunochemically, physiologically, or pharma-
ceutically active or reactive. Biologically active mate-
rial includes at least one or more of the following:
biochemical compounds (such as amino acids, carbohydrates,
lipids, nucleic acids, proteins, and other biochemicals
and substances which may complex or interact with biochem-
ical compounds), such biochemical compounds biologically
functioning as antibodies, antigenic substances, enzymes,
co-factors, inhibitors, lectins, hormones, hormone produc-
ing cells, receptors, coagulation factors, growth enhanc-
ers, histones, peptides, vitamins, drugs, cell surface
markers and toxins, among others known to those skilled in
the art. Of the group of biologically active materials
described, proteins are of utmost current interest because
of the large molecule genetically engineered biopharmaceu-
ticals as those species to be immobilized and congregated
SUBSTITUTE SHEET
W093/20885 PCT/US93/03371
on the additive carriers hereinafter to be described. A
discussion of the use of biomosaic polymers as carriers
for biologically active materials is set forth in European
Patent Application 0,430,517 A2.
Addinq Means - an additive and any means for presenting
the additive to the fluid flowing through the fluid passa-
geways of the fluid delivery device of the invention in a
manner such that all or any part of the additive will be
added to the fluid. The adding means comprises the addi-
tive and the additive presentation means which may take
the form of a functional support, or carrier, an anchor-
age, a deposition or reaction site or an element holder
with or without some type of intermediate matrix or other
release composition.
Additive Presentation Means - Any means such as a func-
tional support or substrate for presenting the additive to
the fluid flowing through the device. The functional
substrate can comprise a polymer, copolymer, an inter-
polymer, a ceramic, a crystal sponge, a carbon based
matrix, a celullosic, glass, plastic, biomosaic polymers,
azlactone-functional polymer beads, adduct beads, carbox-
ylate-functional polymer beads, gums, gells, filaments and
like carriers.
The adding means of the invention can take several
different forms such as those illustrated in Figure 66. Howev-
er, in its preferred form, the adding means comprises a cylin-
drically shaped, microporous polymeric functional support
structure which is disposed within the mixing chamber of the
container assembly and to which various additives, including
beneficial agents such as drugs, biologically active materials,
and chemical elements and compounds can be releasably connect-
SUBSTITUTE SHEET
W093/20885 PCT~US93/03371
57
ed. These additives are carried by the structure in a mannersuch that, as the liquid, such as a diluent, reagent, or other
aqueous solvent flows around, about and through the support
assembly in the manner shown by the arrows in Figure 64, the
additives will be presented to the liquid flow and efficiently
released and added to the liquid as it flows through the cham-
ber which houses the adding means.
The additives themselves can also take various physi-
cal forms including liquid, solid, granular, powder, particle,
gel, wax, hydrocolloid carrier, a gum, film, tablet, crystal-
line, emulsions, microcrystalline, microspherical, spray dried
compounds and lypohilized compounds and saturantsO The addi-
~ives can be removably connected to, immobilized on, impregnat-
ed within or supported by the support means in a number of
ways. The additives can be chemically or mechanically at-
tached, affixed, or bound directly or indirectly through coop-
eration with an intermediate matrix. They can be captured,
affixed, linked or cross linked, anchored to the surfaces of
the support, or surface active agent, or they can be absorbed,
reaction catalyzed, electrostatically encapsulated, attached by
chemical modification in to the carrier surface, polymerized on
or through the carrier, localized, entrapped, deposited, sus-
pended or occluded within voids, cells, tubules, and interstic-
es formed in the support. One important method for removably
affixing the additive to the functional support means includes
treating the functional support means with a compound having
reactive functional groups such as azlactone functional com-
pounds with their high binding capacity. In certain applica-
tions, the biologically active material can be bound at the
surfaces of biomosaic polymers in the manner described in EPO
Patent No. 0 430 517 A2. Similarly, graft copol~rmers can be
SVBSTITUTE SHEET
W O 93/20885 PC~r/US93/03371
58
used in the manner described in U.S. Patent No. 5,013,795
issued to Coleman, et al. In this way complexing agents,
catalysts and biological materials such as enzymes or other
proteins as well as biomacromolecules can be attached to the
carrier .
Similarly, the additives can be immediately separated
from the functional support and added to or intermixed with the
liquid flowing through the device by one or more of various
mechanisms, including chemical reaction, dissolution, debind-
ing, delinking, displacement, bioseparation, diffusion, wash-
ing, disintegration, erosion, disassociation, desorbsion,
solubilization, leeching, enzymatic cleavage, biological reac-
tion, osmosis, separation from ring opening materials by a ring
opening reaction and like separation means.
Turning particularly to Figure 66 various forms of
adding means, or additive carriers are there illustrated.
These additive carriers are disposed within the container
assembly of the invention, the construction of which will now
be described.
As best seen in Figures 63 and 64, in this latest
form of the invention, cylindrical portion 800 is integrally
connected to the back or concave surface 410 of the base member
by means of connector flanges 470 (Figure 23). Portion 800
also includes a transversing extending base wall 807 having a
socket 809 which supports a coupling member 812 in a manner
best seen in Figures 40 and 64. Base 806 is provided with
longitudinally extending passageways 814 and 816. Passageway
814 communicates with storage reservoir 832 and with port 815.
Passageway 816 communicates with the outlet port of the device
formed in the outlet passageway housing 872. This passageway
can also be used to aseptically prefill the reservoir during
SUBSTITUTE SHEl~T
W093/20885 PCT/US93/03371
59
the manufacturing process. Similarly, a needle valve housing
874 of the character previously described extends angularly
outwardly from back surface 410 (See also Figur~s 26 and 28)
and carries the second flow control means of the invention for
controlling the flow of fluid through the fluid o~tlet of the
base assembly. It is to be noted that the front surface of the
base member is provided with crossing micro flow channels 812
and with an upstanding mounting boss 875 which surrounds port
815 to which a filter membrane 834 is bonded (Figure 63).
Filter means and micro-flow channels 812 function in the same
manner to accomplish the same result as previously described
herein.
Referring particularly now to Figure 64, the container
assembly 820 of this form of the invention is there shown and
comprises a glass vial 822 having a fluid flow passageway
therethrough and walls defining a mixing chamber 824 in commu-
nication with the fluid flow passageway. Chamber 824 functions
to contain the adding means of this latest form of the inven-
tion. A two-part plastic cover or overpackage 826 is closely
received over vial 822. Cover 826 includes first and second
portions 826a and 826b. Portion 826a is provided with external
threads 830 and system interlock stops 831. Threads 830 are
adapted to mate with internal threads 834 provided on cylindri-
cal portion 800 (Figure 64). Housed within vial 822 is the
first flow control means of this form of the invention for
substantially controlling the flow of fluid into and out of
mixing chamber 824. Here the first flow control means compris-
es a plunger 836 sealably received within vial 822. Plunger
836 is of generally similar construction to previously de-
scribed plunger 86 being cylindrical in shape and having a
skirt portion 838 adapted to sealably engage the inner wall of
SUBSTITUTE SHEET
W O 93/20885 ~ 4; PC~r/US93/03371
vial 822 (Figure 64).
Plunger 836 is provided with a centrally disposed
bore 839 and a plurality of circumferentially spaced fluid
passageways 840. An inlet or fine flow control valve member
842 also forms a part of the flow control means. Member 842 is
reciprocally movable within bore 839 and is provided with a
first flange portion 841 which functions to control fluid flow
through passageways 840. Member 842 also includes a second or
inboard flange 845 which guides the travel of the valve member
within an annular channel 836a provided within plunger 836.
Valve member 842 also includes a stem 847 which is disposed
within a central flow passageway 842a and which, in a manner
presently to be described, cooperates with a check valve 846
carried within an internal passageway 849 provided in coupling
member 812 (Figure 64). Stem 847 forms an integral part of the
valve member 842 and terminates at an inboard end 847a (see
also Figure 65). Proximate the opposite end of stem 847 is an
enlarged diameter portion 847b which terminates in the pre-
viously identified flange 841. Extending angularly through
portion 847b are circumferentially spaced fluid passageways 850
which, in a manner presently to be described, permit fluid
flowing past check valve 846 to enter chamber 824 via passage-
way 850a.
In operating the apparatus of this latest form of the
invention, the vial closure end cap 851 and the cap which
closes the socket or open end 809 of cylindrical portion 800
(not shown) are first removed. The open end of the drug vial
assembly is then ready to be inserted into open end 809 of
cylindrical portion 800. As the drug vial assembly is received
within open end 809 of the infusion portion of the apparatus,
threads 830 will move toward engagement with a first internal
SUBSTITUTE SHEET
W093/20885 PCT/US93/03371
thread 834a provided within cylindrical portion 800. Rotation
of the drug vial in a clockwise direction will cause the vial
to advance within cylindrical portion 800 causing stem 847 to
engage check valve 846. Because fluid under pressure within
the reservoir resists movement of the check valve, operating
valve 842 will move to the left. However, when flange 845
seats against internal shoulder 836a of plunger 836, continued
clockwise rotation of vial 820 will cause stem 847 to move
check valve 846 to the left into the open position shown in
Figure 64. With the check valve in this open position, dis-
tendable elastomeric membrane 430 will cause the fluid con-
tained within chamber 832 to flow under pressure through port
858a into passageway 858, then into circumferentially spaced
passageways 849, past the valve seat 862 and into fluid passa-
geway 846b of the coupling member. The fluid will then flow
into passageway 850 and then vigorously into mixing chamber 824
via a central fluid passageway 850a formed in the adding means.
This flow of fluid under pressure into chamber 824 causes con-
trolled flow of the first component, such as a diluent,
through, around and about the adding means.
Referring particularly to Figure 64, it is to be
noted that so long as valve member 842 is in the position there
shown, the mixed or dosed solution will continue to flow under
pressure in a reverse direction through passageways 840, into
passageways 868 formed in coupling member 812, and into annular
passageway 870. From passageway 870 the fluid flows into
passageway 87 formed in cylindrical portion 800 and thence
toward the outlet port of the device for controlled delivery.
It is important to recognize that in the adding means
of this form of the invention, affinity matrix supports can be
provided that are capable of binding capacity at a level that
SUBSTITUTE SHEET
W093/20885 PCT/US93/03371 ~
Q 4 ~
62
enables highly efficient, biospecific attachment to the support
of additives in substantial amounts. A substantial portion of
the additives can subsequently be efficiently released imme-
diately or, alternatively, over an extended period of time
thereby permitting controlled infusion in a manner which is
most therapeutically efficacious to a patient. Alternate
disassociation reaction kinetics for desorption of the absorbed
component from the affinity matrix can be established in a
manner to critically determine the optimal conditions for
elution over time of the bound additives. The appropriate
elution diluent acts as the desorption agent to break the
affinity attachment bond as a function of time. In this em-
bodiment, by providing different drug vial assemblies with
alternate extended drug release rates, a diluent flow rate can
be established independent of the dosing rate, (disassociation,
debonding or displacement of the additive from the activated
substrate support). In this way selective dosing and flow rate
opportunities can be optimized for individual patient physiolo-
gY-
As indicated in Figure 64, the adding means disposed
within mixing chamber 824 is here provided in the form of a
cylindrically shaped, functional support structure or scaffold
to which various additives including beneficial agents, such as
drugs, biologically active materials, and chemical elements and
compounds can be releasably connected. These additives are
carried by the structure in a manner such that, as the liquid
flows through chamber 824 and circulates through the scaffold
in the manner shown by the arrows in Figure 64 the additives
will be presented to the liquid flow and efficiently added over
time to the liquid as it flows through the mixing chamber.
Disposed on either side of scaffold 877 are glass flow distri-
SIJBSTITUTE SHEET
W093/20885 PCT/US93/03371
63
_ bution frits 879 (Figure 65) which function to evenly distrib-
ute the fluid flow into and out of the chamber.
In addition to the additive presentation means pre-
viously discussed, a polymer can also be used as the carrier or
support for the additive. Three classes of polymeric supports
can be used, namely polymeric reagents, polymeric catalysts and
polymeric substrates. A discussion of polymers as carriers or
supports is contained in Principles of Polvmerization, Second
Edition by George Odian. Microporous polymers usable as carri-
ers are also fully described in U.S. Patent 4,519,909 issued to
Castro.
The second component, such as a parenteral fluid, or
elution agent which flows into chamber 824 can include, by way
of example, a reagent, a solvent, a sterile diluent, various
electrolytes, aqueous solutions such as aqueous solutions of
dextrose, saline solutions, alkalinizing solutions, acidifying
solutions, polar solutions and any other liquids that can serve
as an appropriate vehicle for the administration of therapeutic
or beneficial agents which are desirable to administer to the
patient by infusion.
Turning now to Figure 66, various other forms of
adding means are there illustrated. For example, numeral 880
identifies an assembly comprising an insoluble, polystyrene
porous substrate having an axially extending fluid passageway
881 and interconnecting voids 880a interstitially of which one
or more additives are releasably carried. The selected addi-
tives such as elements, chemical compounds, drugs and function-
al intermediates are provided on or within the voids by tech-
niques well known to those skilled in the art. The additives
are, of course, introduced into the elution agent such as a
sterile diluent as the diluent flows around, about and through
SUBSTITUTE SHEET
W093/20885 PCT/US93/03371 ~
~ A '
64
substrate 880.
Another form of additive assembly designated in
Figure 66 by the numeral 882, comprises a plugged pore sub-
strate having an internal, axially extending fluid passageway
883. The pores of this alternate sized substrate releasably
carry the additives such as time released chemical compounds
and beneficial agents, or medicaments.
Still another form of additive assembly is identified
in Figure 66 by the numeral 884. This assembly comprises a
cylindrical, porous plug like member made up of a multiplicity
of fused together microspheres or beads 884a, each of which is
coated with a separation or reactive coating upon which is
deposited an additive such as a biologically active material or
other beneficial agent. The microspheres which embody internal
microchannels can be formed of glass, plastic or other suitable
materials.
The numeral 886 of Figure 66 identifies yet another
form of the adding means of the invention. In this form of the
invention a generally cylindrically shaped functional support
serves as an affinity attachment for attachment and subsequent
release of the additive. Support 886 has an axial fluid passa-
geway 887 and is formed from a multiplicity of microporous
polymers presenting a multiplicity of reactive sites over a
wide area for species immobilization.
The additive assembly designated in Figure 66 by the
numeral 888 comprises a solid tubular member having an axial
fluid passageway 889. The exterior surface of the member is
coated with a selected additive by any suitable means including
interfacial polymerization means with the use of an interpolym-
er.
Alternatively, member 888 can be constructed of an
SUBSTITUTE SHEEt
,
W093/20885 al~ ~a4~ P~/US93/a3371
ion exchange resin material to which the additive, such as a
~ drug can be bound, to provide a drug-resin complex of a charac-
ter that permits the drug to later be controllably released
over time when exposed to an appropriate elution fluid.
Another slightly more complex additive assembly is
identified by the numeral 890. This assembly is made up of a
plurality of spaced apart, porous bung wafers 890a, 890b, 890c,
and 890d, each wafer being of the same or different construc-
tion and porosity and each having reactive sites presenting to
the liquid flow specially selected additives such as beneficial
agents, elements or compounds so that multiple reactivities
and selectivities can be achieved. With this construction, a
wide variety of liquid flow rates, and complex sequential
separations and priority staged substance introduction into the
system output can be achieved by specially designing each of
the wafers that cooperate to make up the structural support.
Still another form of activating assembly is desig-
nated in Figure 66 by the numeral 892. This assembly comprises
a cylindrically shaped structure made up of a plurality of
elongated fibrous members 892a at least some of which are
coated, plugged or impregnated with selected additi~es and, as
necessary, functional intermediate materials.
The functional support member identified by the
numeral 894 exemplifies yet another form of adding means of the
invention. This member, which is also of a generally cylindri-
cally shaped configuration, is constructed from a plurality of
discrete layers such as polymer films 894a onto which selected
additives and intermediate compounds have been removably af-
fixed.
Functional support member 896 is constructed from a
multiplicity of glass spaghetti-like strands 896a forming open
SUBSTITUTE SHEEt
- ~=
W093/20885 ~k ~ ~ ~ 0 4 ~ PCT/US93/03371 ~
66
cell, sponge-like construction, the cells of which are inter-
connected by tortious interstitial flow paths. Some or all of
the open cells carry the selected additive or additives de-
sired.
Functional support member 898 is constructed from a
polymer foam which efficiently functions as the additive carri-
er.
It is to be understood that other forms of supports
such as gells, biomosaic polymers and other porous forms of
polymer reactive supports can be emplaced within mixing chamber
824 including joined azlactone-functional materials such as
foams or polymer beads suitable for the attachment of function-
al materials.
Assemblies 880 through 898, which may be soluble or
insoluble, hydrophillic or hydrophobic, are intended to merely
exemplify, not to limit, the wide variety of materials and con-
structions that can be used to present the desired additives to
the liquid flow introduced into the mixing chamber of the
device.
As previously mentioned, the additives can be remov-
ably affixed to the scaffolds, matrices or functional support
means in various ways including the process of chemical modifi-
cation of the matrix. One important manner of removably affix-
ing the additives enables the use of special separation tech-
niques broadly defined by the term chromotography. Chromotog-
raphy as used herein refers to a group of separation techni~ues
which are characterized by a distribution of the molecules to
be separated between two phases, one stationary and the other
mobile. Affinity chromotography involves the use of biological
interactions and contemplates the use of affinity chromotogra-
phy supports through which the eluting fluid flows. In the
SUBSTITUTE SHEEt
W093/20885 P~/US93103371
present embodiment of the invention, the various additive
- presentation means, as described herein, can assume the charac-
ter of an affinity chromotography support to which various
ligands are attached. In the practice of affinity chromotogra-
phy techniques, one of the members of the pair in the interac-
tion, the ligand, is immobilized on a solid phase, while the
other, the counterligand (most often a protein), is absorbed
from the extract that is passing the substrate during the
manufacturing process. Importantly, affinity chromotography
techniques can include the use of highly versatile azlactone
functional compounds, such as azlactone functional beads, as
well as the use of a wide variety of other media ~or activation
and coupling chemistry. Examples of ligands that can be at-
taGhed to th~ affin~ty ~upports ~ncluAe ant bodles, enzy~es,
lectins, nucleic acids hormones and vitamins. Examples of
important counterligands include antigens, virus, cells, cell
surface receptors and the like. Chromotography and affinity
chromotography techniques are described in detail in Protein
Purification by Janson and Ryden, Copyright 1989 and reference
should be made to this work to provide a working understanding
of the techniques.
Polymeric azlactones are well known in the prior art.
Their use in the production of homopolymers and copolymers has
been described in a number of patents. See for example, U.S.
Patent No. 3,488,327 (issued Jan. 6, 1970 to F. Kollinsky et
al.); U.S. Patent No. 3,583,950 (issued June 8, 1971 to F. Kol-
linsky et al.); U.S. Patent No. 4,304,705 (issued December 8,
1981 to S. M. Heilmann et al.); and U.S. Patent No. 4,737,560
(issued April 12, 1988 to S. M. Heilmann et al.); and U.S.
Patent No. 5,013,795 issued May 7, 1991 to Coleman, et al.
Azlactones, or oxazolones, are cyclic anhydrides of
SI~BSTIT~JTE ~3HEET
W O 93/20885 ~ Q ~1 PC~r/US93/03371
N-acylamino acids and have been used extensively in organic
synthesis. The formation of a five-membered azlactone of
particularly useful functionality for immobilization purposes
can be accomplished through the reaction of a carboxylate group
with a-methyl alanine using a two-step process. (See Immo-
bilized Affinity Liqand Techniques-Hermanson, Mallia and Smith,
Copyright 1992). One method of forming azlatone beads, the use
of which has been previously mentioned herein, makes use of
this process in the polymerization of monomers to first yield a
carboxyl group on the matrix. In the second step, the azlac-
tone ring is formed in anhydrous conditions through the use of
a cyclization catalyst. Suitable cyclization agents that will
drive this reaction include acetic anhydride, alkyl chlorofor-
mates, and carbondiimides. The process of forming these active
groups and of making beaded polymeric supports containing them
has been thoroughly described in patents assigned to 3M Corpo-
ration (U.S. Patent No. 4,871,824 and 4,737,560). These sup-
port materials are now available under the tradename "Emphase".
U.S. Patent Nos. 5,045,615 and 5,013,795 which have been as-
signed to 3M Corporation also describe recent advances in this
technology.
As pointed out in the 3M Corporation Patent No.
4,737,560, azlactone-functional polymer beads are useful reac-
tive supports for the attachment of functional materials to
provide novel adduct beads. The adduct beads are useful as
complexing agents, catalysts, reagents, and as enzyme or other
protein-bearing supports. The term "support" or "affinity
support" as used in this sense is usually understood to refer
to a combination of (1) a ligand (usually of some known molecu-
lar configuration), that is firmly attached (e.g.,
immobilized), often by covalent means, and (2) a matrix (usual-
SUBSTITUTE SHEET
W O 93/2088S ~ PC~r~US93J03371
69
ly a solid insoluble substance). Azlactone support matrixmaterials and coupling chemistry is also of special interest
because of its accessible matrix surface area and effective
ligand density that can be attached to that surface.
U.S. Patent No. 4,072,566 issued to Lynn on February
7, 1978, and entitled "Immobilized Biologically Active Pro-
teins" discloses a method of bonding enzymes or other biologi-
cally active proteins to an inorganic support material using p-
phenylenediamine. The support materials disclosed as useful in
the invention include siliceous materials, stannic oxide, tita-
nia, manganese dioxide, and zirconia.
The functional support structure 877 of the present
embodiment of the invention can take on the character of an
affinity support and is uniquely constructed to permit enzymes
or other biologically active proteins to be bound thereto for
later removal. This is accomplished by treating functional
support 877 in the manner disclosed in the prior art patents
identified in the preceding paragraphs with a compound having
selected reactive functional groups such as azlactone function-
al compounds. In this way complexing agents, catalysts and
biological materials such enzymes, proteins or other affinity
absorbants, as well as biomacromolecules can be attached to the
carrier for later removal and recovering.
When attaching certain biologically active proteins
and other macro molecules, the use of spacer arms or leashes
have been found to be very beneficial. Spacer arms or leashes
are low-molecular-weight molecules that are used as intermedi-
ary linkers between a support material and an affinity ligand.
Usually spacers consist of linear hydrocarbon chains with func-
tionalities on both ends for each coupling to the support and
ligand. First, one end of the spacer is attached chemically to
SUBSTITUTE SH~ET
W O 93/20885 PC~r/US93/03371
the matrix using traditional immobilization chemistries; the
other end is connected subsequently to the ligand using a
secondary coupling procedure. The result is an immobilized
ligand that sticks out from the matrix backbone by a distance
equal to the length of the spacer arm chosen.
Referring to Figure 66A, 66B, 66C, and 66D, the use
of spacer arms to attach proteins and enzymes to the substrate
is there schematically illustrated. The principal advantage of
using a spacer arm is that it provides ligand accessibility to
the binding site of a target molecule. When the target mol-
ecule is a protein with a binding site somewhat beneath its
outer surface, a spacer is essential to extend the ligand out
far enough from the matrix to allow interaction. As indicated
in Figure 66A, when the ligand binding site S is buried or
disposed in a pocket located just below the surface of the
protein P, a ligand L that is either below the surface of the
support material (upper portion) or a ligand L-l that is at-
tached directly to the surface (middle portion) cannot reach
the level of the binding site S on an approaching protein
molecule. The result may be weakened interaction or no binding
at all. Accordingly, in these instances, spacer arm 899 is
required to provide the ligand L-2 accessibility to the binding
site of the protein molecule (lower portion of Figure 66A).
The details covering the use of spacer arms are fully set forth
in Section 3.1.1 of the previously referred to work entitled
Immobilized Affinity Liqand Techniques. This Section 3.1.1 is
incorporated herein by reference.
Turning now to Figures 66B, 66C, and 66D, it is to be
noted that immobilized protein A can be used to immobilize an
antibody molecule by taking advantage of the natural affinity
of protein A for immunoglobulins. Incubation of a specific
SU8STITUTE SHEET
W093/20885 ~ P~TlUS93/0~371
antibody with protein A matrix will bind the antibody in the Fe
region, away from the antigen binding sites. Subsequent cross-
linking of this complex with DMP (dimethyl pimelimidate) yields
a covalently attached an~ibody with the antigen binding sites
facing outward and free to interact with antigen.
With rigid support materials, a spacer molecule may
also provide greater flexibility, allowing the immobilized
ligand to move into position to establish the correct binding
orientation with a protein. The degrees of freedom that a
hydrocarbon extender can provide are much greater than the
movement possible within the polymeric backbone of a matrix.
The choice of spacer molecule can affect the relative
hydrophilicity of the immediate environment of an immobilized
ligand. Molecules containing long hydrocarbon chains may in-
crease the potential for nonspecific hydrophobic interactions,
especially when the affinity ligand is small and of low molecu-
lar weight. Selecting spacers that have more polar constitu-
ents, such as secondary amines, amide linkages, ether groups or
hydroxyls will help keep hydrophobic effects at a m; n; um.
It is also important to consider the ionic effects a
spacer molecule may impart to a gel. Spacers with terminal
primary amine groups should be completely coupled with ligand
or blocked by a nonrelevant molecule (e.g., acetic anhydride;
see Section 3.l.l.9 of Immobilized Affinity Ligand Techniques)
to eliminate the potential for creating a positive charge on
the support. With small ligands, these residual charges can
form a secondary environment that may cause considerable non-
specific interactions with proteins. The same holds true for
spacers with terminal carboxylic groups. In general, a nega-
tively charged spacer will cause less nonspecific protein
binding than a positively charged one, but blocking excess
SU~S 111 ~JTE SHE~ l
W O 93/20885 PC~r/US93/03371 ~
4 ~
remaining groups is still a good idea. A good blocking agent
for use with carboxylic residues is ethanolamine, which leaves
a terminal hydroxyl group (See Immobilized Affinity Liqand
Techniques for an expanded discussion of types of spacers and
various immobilization and coupling protocols.)
As pointed out in Protein Purification, Janson and
Ryden, Copyright 1989 which describes some alternate form of
protein immobilization at Page 310:
"Ligand-protein interaction is often based on a
combination of electrostatic, hydrophobic and hydrogen
bonds. Agents which weaken such interactions might be
expected to function as effective non-specific eluants."
This work provides further teaching of the techniques described
herein.
The three important modes of chromatographic develop-
ment applicable to the present invention, namely isocratic elu-
tion, gradient elution and displacement chromatography, are
also discussed in the above-noted work.
Referring to Figures 67 through 70, another embodi-
ment of the invention is shown. In this embodiment, the con-
struction of the cylindrical housing portion 900 and the base
assembly 901 are of similar construction to those of the last
discussed embodiment and like numbers are used to designate
like components. However, the latest form of the invention is
unique in that the immobilized drug is contained in its own
vial assembly 902 and the liquid component, or parenteral
fluid, is contained in a separate assembly 904 that can be
mated with the vial assembly and with the cylindrical housing
portion to mix the drug with the liquid component and then to
charge the reservoir by distending the energy source or dis-
tendable membrane 430 in a manner presently to be described in
SUBSTITUTE SHEET
-
W O 93/20885 PC~r/US93~03371
~ Q ~ l
detail. Like the apparatus of the form of the invention just
described, this embodiment also uniquely permits controlled
intermixing of the first liquid component with the second
component or additive such as a medicament or other beneficial
agent which is presented to the first component by a unique
additive presentation means, of the character defined in the
preceding section of this specification.
As best seen in Figure 67, in this latest form of the
invention, cylindrical portion 900 is integrally connected to
the back or concave surface 410 of the base member by means of
connector flanges 410a. Portion 900 also includes a trans-
versely extending base wall 906 having a socket 907 within
which assemblies 902 and 904 are supported in a manner best
seen in Figures 68 and 69. Base portion 908 (Figure 68) is
provided with a longitudinally extending passageway 909 which
communicates with storage reservoir 910 in the manner shown in
Figure 69. The outlet port of the device is in communication
with reservoir 910 via passageway 872 which is of a dissimilar
construction and arrangement to that previously described
herein.
As seen in Figure 67, a needle valve housing 874 of
the character previously described extends angularly outwardly
from back surface 410 (See also Figures 26 and 28). Needle
valve 874a is carried by housing 874 and functions in the
manner previously described, with cap 874b securing the adjust-
ment end of the valve. It is to be noted that the front sur-
face of the base member is also provided with crossing micro
flow channels 812 which communicate with port 815 to which a
filter membrane 834 is bonded. Filter means and micro-flow
channels 812 function in the same manner to accomplish the same
result as previously described herein.
SUBSTITUTE SHEEt
W 0 93/20885 ~ 4 1 PC~r/US93/03371
74
Turning now particularly to Figures 68 and 69, the
liquid vial assembly 904 comprises a vial 912 having a chamber
914 for containing the liquid component such as a diluent D. A
plastic cover or overpackage 915 is closely received over vial
912. Cover 915 is provided with external threads 916 and
system interlock stops 917 (Figure 67). Threads 916 are adapt-
ed to mate with first and second sets of longitudinally spaced
internal threads 918a and 918b provided on cylindrical housing
portion 900. The manner of interconnection of the liquid vial
and the housing will presently be discussed.
The open end of vial 912 is closed by a sealing
member or stopper 920 which is provided with a centrally dis-
posed, sealably interconnected blunt cannula 921 that provides
communication between chamber 914, which contains the liquid
component, or diluent D, and the exterior of the vial assembly.
For a purpose that will presently become apparent, stopper 920
is movable within chamber 914 from a first position proximate
the open end of the vial to a second position proximate the
closed end. Prior to use, the open end of vial 912 and cannula
921 are sealed by a tear-off cap 912a of standard design
(Figure 67).
The drug vial assembly 902 includes a glass vial 922
having internal threads 923 and a chamber 924. Chamber 924 is
sealed at one end by an elastomeric seal 925 that is held in
place by a crimp cap 922a which fits closely over vial 922
(Figure 68). The opposite, internally threaded end of chamber
924 is also sealed by an elastomeric sealing member 926 which
is adapted to be pierced by a centrally disposed, blunt end
cannula 927 that is carried by a check valve housing 930
disposed within a chamber 931 provided in base 908. Cannula
SUBSTITUTE SHEET
W093/20885 ~ CTIUS93/03371
927 provides communication between chamber 924 and a fluid
passageway 928 that extends through the check valve housing and
communicates with reservoir slo via passageway 9o9 when a check
valve 932 is in an open position. Check valve 932 is guided by
circumferentially spaced splines or ribs 912a. The operation
of check valve 932 during the charging of reservoir slo will
presently be described.
Disposed proximate seals 925 and 926 are porous glass
distribution frits 933 and 934 which function to properly
control and distribute the elution diluent flowing through the
adding means of the invention which is disposed intermediate
the distribution frits. The adding means of the embodiment of
the invention shown in Figures 68, and 69 is provided in the
form of a cylindrically shaped, porous functional support
structure 937 which is carried within chamber 924 and to which
various additives including beneficial agents such as drugs,
biologically active materials and chemical elements and com-
pounds can be releasably connected in the manner previously
described herein. These additives are carried by the structure
in a manner such that, as the liquid flows through chamber 924
and circulates around, about and through the support assembly
the additives will efficiently removed from the support and
mixed with the liquid. It is to be understood that the adding
means disposed within chamber 924 can be of the general charac-
ter of any one, or a combination of, those previously described
herein and illustrated in Figure 66.
In operating the apparatus of this latest form of the
invention, the sterile vial closure caps 938 and 938a, which
are provided the ends of the vial 922 as well as a sterile
closure cap which closes the open end of cylindrical portion
gOo are first removed and discarded. As the drug vial assembly
SlJBSTITlJTE SHE~T
W O 93/20885 PC~r/~S93/03371
0 ~ ~ ~
is received within open end 907Of portion 900, threads 923 will
move toward engagement with threads 930a provided on housing
body 908. Rotation of the drug vial in a clockwise direction
will cause the vial to advance within cylindrical portion 900
causing cannula 927 to pierce elastomeric sealing member 926.
After the drug vial assembly is fully seated, scalloped cap
938a, which is used to rotate the vial, is removed and discard-
ed.
With the drug vial assembly in place within cylindri-
cal portion 900, the open end of liquid vial assembly 904 is
inserted into an annular space 900a and threads 916 provided on
cover or overpackage 915 are mated with internal threads 918a
provided proximate the mouth of cylindrical portion 900.
Rotation of assembly 904 will cause cannula 921 to pierce drug
vial sealing member 925 in the manner shown in Figure 69 there-
by opening fluid communication between chamber 914 of the
diluent vial assembly and chamber 924 of the drug vial assem-
bly.
Continued rotation of the diluent vial assembly will
cause threads 916 to move into an open annular space 942 there-
by permitting the vial assembly to be forcibly pushed to the
right from the position shown in Figure 68 to the position
shown in Figure 69. This movement causes elastomeric member
920 to move to the left within chamber 914 thereby forcing the
diluent D through cannula 921 and into the drug vial assembly
under substantial pressure. The elution diluent will flow
through glass frit 933 and then forcibly around, about and
through support structure 937 of the adding means. The diluent
flowing through glass frit 934 and into cannula 927 under
pressure will cause check valve member 932 to move to the right
into the open position shown in Figure 69 thereby permitting
SUBSTITUTE SHEET
W093/20885 PCT/US93/03371
~ O ~
the fluid mixture to flow toward the reservoir 910 via passage-
way 909. This fluid mixture flowing toward the reservoir will
cause distendable membrane 430 to distend outwardly into the
position shown in Figure 69. In its distended con~iguration,
membrane 430 provides the energy source for controllably expel-
ling the fluid mixture from the device in the manner previously
described.
Once the reservoir is charged, and the diluent vial
assembly has moved into engagement with the second set of
threads 918b, continued rotation of the assembly will cause
threads 912a on the overpackage to engage threads 918b and
securely lock the vial and drug assemblage in position within
cylindrical portion 900 pending infusion of the beneficial
agent into the patient.
Turning to Figures 71 through 75, still another
embodiment of the invention is shown. In this embodiment, the
construction of the cylindrical housing portion 950 and the
base assembly 951 are once again of similar construction to
those last discussed and like numbers are used to designate
like components. However, the latest form of the invention is
different from the embodiment just described in that while the
immobilized drug is contained in its own vial assembly 952 and
a first portion of the liquid component, or parenteral fluid,
is contained in a separate assembly 954, the reservoir is
prefilled with a second portion o~ the liquid component.
As before, vial assembly 954 can be mated with vial
assembly 952 and with the cylindrical housing to mix the drug
with the liquid component. With the unique construction of
this last form of the invention, the liquid already within the
prefilled reservoir can be controllably intermixed with the
fluid within assembly 954 and with the addititive contained
SUBSTITUTE SHE~T
W093/20885 ~ PCT/US93/0337t
78
within assembly 952 to form an infusible mixture which can be
later discharged by the energy source or distendable membrane
430 of the invention. Stated another way, like the apparatus
of the form of the invention just described, this embodiment
permits controlled intermixing of the portion of the first
liquid component or carrier component, contained within vial
954 with the second component or additive contained within vial
952 to produce a first mixture. This mixture of the carrier
liquid and the additive can then be introduced into the reser-
voir which has been previously filled with a diluent or other
liquid to produce a second, injectable mixture for later infu-
sion into a patient.
As best seen in Figures 71 and 75, in this latest
form of the invention cylindrical portion 950 is integrally
connected to the back or concave surface 410 of the base member
by means of connector flanges 410a. Portion 950 also includes
a base wall 956 having a socket 957 which receives assemblies
952 and 954 in a manner illustrated in Figure 75. As best seen
in Figure 75, base 958 is provided with a passageway 959 which
communicates with storage reservoir 960. The outlet port of
the device is in communication with reservoir 960 via passage-
way 872 which is of similar construction and arrangement as
previously described herein.
Similarly, a needle valve housing 874 of the charac-
ter previously described which houses a needle valve assembly
874a extends angularly outwardly from back surface 410 (See
also Figures 26 and 28). Valve 874a comprises the second flow
control means of the invention for controlling fluid flow
outwardly of the device. It is to be noted that the front
surface of the base member is also provided with crossing micro
flow channels 812 which communicate with port 815 to which a
SUBSTITUTE SHEET
WO 93/20X85 ~ PCT/US93~a337l
79
filter membrane 834 is bonded. Filter means and micro-flow
channels 812 function in the same manner to accomplish the same
result as previously described herein.
Turning now particularly to Figure 73, the liquid
vial assembly 954 comprises a vial 962 having a chamber 964 for
containing the carrier liquid component such as a diluent. A
two-part plastic cover or overpackage 965 is closely received
over vial 962. Cover 965 is provided with external threads 966
which are adapted to mate with the coupling means of the inven-
tion shown here as internal threads 968 provided on cylindrical
housing portion 950 (Figure 75). The manner of interconnection
of the liquid vial and the housing will presently be discussed.
The open end of vial 962 is closed by a sealing
member or stopper 970 which is proYided with a centrally dis-
posed, sealably interconnected blunt cannula 971. Cannula 971
provides communication between chamber 964, which contains the
carrier liquid component, and the exterior of the vial assem-
bly. For a purpose that will presently become apparent, stop-
per 970 is movable longitudinally of chamber 964 from a first
position proximate the open end of the vial to a second posi-
tion proximate the closed end. Prior to use, the open end of
vial 962 and cannula 971 are substantially sealed by a tear-off
cap 972 of st~n~rd design.
Turning now to Figure 74, the drug vial assembly 952
includes a glass vial 974 having internal threads 975 and a
chamber 976. Chamber 976 is sealed at one end by an elastomer-
ic seal 977a that is held in place by a crimp cap 778 which
fits closely over vial 974. The opposite, internally threaded
end of chamber 976 is also sealed by an elastomeric sealing
member 977b which is adapted to be pierced by a centrally
disposed blunt cannula 971 that is carried by a check valve
SUBSTITUTE SHEET
-
W O 93/20885 PC~r/US93/03371
housing 972 disposed within a chamber 973 provided in base 958.
Cannula 971 provides communication between chamber 976 and a
fluid passageway 978 that extends through the check valve
housing and communicates with reservoir 960 via passageway 959
when a check valve 962 is in an open position. The operation
of check valve 962 during the fluid mixture transfer reservoir
960 will presently be described.
Disposed proximate seals 977a and 977b are porous
glass distribution frits 979 and 980 which function, as before,
to properly control and distribute the liquid flowing through
the chamber containing the adding means of the invention which
is disposed intermediate the distribution frits. The adding
means of the embodiment of the invention shown in Figures 74
and 75 is provided in the form of a cylindrically shaped porous
scaffold 982 which is carried within chamber 976 and to which
any one or a combination of the various additives previously
described herein can be releasably connected. As before, these
additives are carried by the scaffold matrix in a manner such
that, as the liquid flows through chamber 976 and circulates
around, about and through the scaffold in the manner previously
discussed herein the additive will be released from the scaf-
fold and thoroughly intermixed with the liquid. It is to be
understood that the adding means disposed within chamber 976
can be of the general character of any one of those previously
described herein and illustrated in Figures 66 and 67.
In operating the apparatus of this latest form of the
invention, the sterile vial closure caps 972 and 983b which
close the ends of the vials 962 and 974 as well as a sterile
closure cap which closes the open end of cylindrical portion
950 are first removed and discarded. As the drug vial assembly
is received within open end 957 of portion 950, threads 975
SUBSTITUTE SHE-~T
W093/20885 PCT/US93/0337t
~ f ~
will move toward engagement with threads 958a provided in base
958 proximate check valve housing 972. Rotation of the drug
vial in a clockwise direction will cause the vial to advance
~ within cylindrical portion 950 causing blunt end cannula 971
to pierce elastomeric sealing member 977b.
With the drug vial assembly in place within cylindri-
cal portion 950, cap 983a is removed and the open end of liquid
vial assembly 954 is inserted into annular space 958a. Next,
threads 966 provided on cover or overpackage 965 are mated with
internal threads 968 provided proximate the mouth of cylindri-
cal portion 950. Rotation of assembly 954 will cause cannula
971 to pierce drug vial sealing member 977a thereby opening
fluid communication between chamber 964 of the liquid carrier
diluent vial assembly and chamber 976 of the drug vial assem-
bly.
Continued rotation of the li~uid carrier vial assem-
bly will cause it to move to the right as viewed in Figure 75.
This causes movement of elastomeric member 970 to the left
within chamber 964 thereby forcing the carrier liquid diluent D
through cannula 971 into the drug vial assembly under substan-
tial pressure. The diluent will flow through glass frit 979
and then forcibly around, about and through scaffold matricies
982 of the adding means. The elution diluent flowing under
pressure through glass frit 980 and into cannula 971 will cause
check valve member 962 to move to the right into the open
position shown in Figure 75 permitting the fluid mixture to
flow toward the reservoir 960 via passageway 959. This fluid
mixture flowing toward the reservoir will controllably intermix
with the liquid component contained within the prefilled reser-
voir to form the infusible liquid mixture. In its distended
configuration, elastomeric membrane 430 provides the energy
SUBSTITUTE SH~ET
W O 93/20885 PC~r/US93/03371 ~
~ ~$~
source for controllably expelling the infusible mixture from
the device in the manner previously described.
Having now described the invention in detail in accordance
with the requirements of the patent statutes, those skilled in
this art will have no difficulty in making changes and modifi-
cations in the individual parts or their relative assembly in
order to meet specific requirements or conditions. Such chang-
es and modifications may be made without departing from the
scope and spirit of the invention, as set forth in the follow-
ing claims.
SUBSTITUTE SHEET