Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
12~18375
Cvclic amine derivatives
The invention relates to novel cyclic amine derivatives
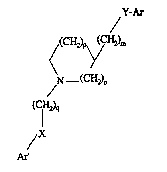
of the formula I
~(CH23y(cH2)m ~ :
N (CH2)~
(fH2)9
,x ~ .. ,."""`
in which
Ar and Ar' are each, independently of one another, phenyl
: whi~h is unsubstituted or substituted once or
twice by NO" NH2, 9al, CF3, A, N~S02A or ~HAc,
X and Y are each, independently of one another, O or ' ~ ~;
a bond,
,. ~'- '~
m i8 0 or 1,
n i~ 0, 1 or 2, ;~ .
p i8 0, 1, 2 or 3, . ~.
, ~, ,.
q is 2 or 3,
A is alkyl having 1-6 C atoms,
15 9al is F, Cl, Br or I and
Ac is alkanoyl having 1-8 C atoms, aralkanoyl
having 8-10 C atoms or aroyl having 7-11 C atoms,
-.
'' . ~' :"
J ~ ', ~ . , `,' ~, . :
; -` 21~3~5
- 2 -
and their physiologically acceptable salts.
The invention was based on the object of finding novel
compounds which can be used for the production of medica-
ments.
It has been found that the compounds of the formula I and
their physiologically acceptable acid addition salts have
valuable pharmacological properties while being well
tolerated. Thus, in particular, they show antiarrhythmic
effects and positive inotropic effects prolonging the
refractory period of the heart.
The cardiac action can be measured, for example, on
anaesthetized or conscious rats, guinea pigs, dogs, cats,
monkeys or minipigs, and the positive inotropic effect
can also be measured on isolated heart preparations (for
example atrium, papillary muscle or perfused whole heart)
of rats, guinea pigs, cats or dogs, for example by
methods as described in Arzneimittelforschung,
volume 31 (I) No. la (1981), pages 141 to 170, or by
Schliep et al. in the 9th International Congress of
Pharmacol., London (1984J, Abstracts of papers 9P.
The compounds can therefore be used as pharmaceutical
active substances in human and veterinary medicine. They
can furthermore be used as intermediates for preparing
other pharmaceutical active substances.
' ~ ''~ . . ' "
The invention accordingly relates to the compounds of the
formulà I, to their acid addition salts and to a process
for their preparation, characterized in that a compound
of the formula II
t ~(CH2)m
HN (CH2)n
in which
. '.,,' ' ' ''''~ . '' '''`' ~' ' ~ '
`' -`' 211~7~~f
Ar, Y, m, n and p have the stated meaning,
is reacted with a compound of the formula III,
Ar-X-(C~2)q~L III,
in which . ;;.
5 Ar, X and q have the stated meaning, and -~
L is 0~, Cl, Br or a reactive, functionally modified
0~ group, ~.
or in that, to prepare a compound of the formula I ; :~
according to Claim 1 in which Y i6 oxygen, a compound of
the formula IV
, ",,
(CH23y(CH2)m ,
N (ff~H2)D
(fH2)q ~ .
,X ' ', ~' ''~,' ''''.`'
;"':''.'' ~''''''";''~
in which ~ ~
Ar', X, L, m, n, p and q have the stated meanings, ~.:``.:`
' i " '~
i~ reacted with a compound of the formula V
Ar-Z V,
in which
Ar has the stated meaning, and
Z is 0~ or a reactive, functionally modified OB group, :
': :'`
211837~
-- 4 --
including a group with salt-like characteri~tics,
or in that a compound of the formula Va
Ar'-Z Va,
in which Ar~ and Z have the stated meanings,
5 i8 reacted with a compound of the formula Vl
~(CH2~, Y~
--(CH2)m
/ -- (CH2)
( ICH2)q
L ;~
.. .. ~ ; ....
in which
Ar, Y, L, m, n, p and q have the stated meanings,
or in that a compound which corre6ponds to the formula I
but h~s in place of one or more C~2 groups one or more
reducible groups i8 converted by reduction into a com~
pound of the formula I, and/or in that one or both groups
Ar and Ar~ in a compound of the formula I are converted
into other radicsls Ar and Ar', respectively, and/or in
that a basic compound of the formula I is converted by
treatment with an acid into one of its physiologically
acceptable acid addition salts.
" ' ~
~ereinbefore and hereinafter, Ar, Ar~, A, Hal, L, X, Y ~ e
and Z as well as the parameters m, n, p and ~ have the
meanings ~tated for the formulae I, III and V unless
20 expres~ly stated otherwise.
The radical A is alkyl having 1, 2, 3, 4, 5 or 6, in ~:
particular 1, 2 or 3, C atoms, preferably methyl, as well `~
as ethyl, n-propyl, isopropyl, n-butyl, isobutyl,
~'~';
211837~
sec-butyl or tert-butyl. A in NHSOiA is preferably
methyl.
The group Ac i8 preferably alkanoyl having 1-8 C atoms,
in particular having l, 2, 3, 4 or 5 C atoms;
specifically, Ac i6 preferably acetyl, furthermore
preferably formyl, propionyl, butyryl, isobutyryl,
valeryl, isovaleryl, pivaloyl (trimethylacetyl), further-
more preferably unsubstituted or optionally substituted
aroyl having 7-11 C atoms, suitable ~ubstituents being,
in particular, 1-3, preferably one, of the following
groups: alkyl, alkoxy, alkylthio, alkylsulfinyl or
alkylsulfonyl having, in each case, 1-3, preferably 1 or
2, C atoms, methylenedioxy, furthermore 0~, F, Cl, Br, I,
~2~ N~2, alkyl~mino or dialkylamino having, in each case,
1-3, preferably l or 2, C atoms in the alkyl group.
Individual preferred aroyl radicals are benzoyl, o-, m-
or p-toluyl, o-, m- or p-methoxybenzoyl, 2,3-, 2,4
2,5-, 2,6-, 3,4- or 3,5-dimethoxybenzoyl, 2,3,4-, 2,3,5-,
2,3,6-, 2,4,5-, 2,4,6- or 3,4,5-trimethoxybenzoyl, o-,
m- or p-methylthiobenzoyl, o-, m- or p-methylsulfinyl-
benzoyl, o-, m- or p-methylsulfonylbenzoyl, 2,3- or 3,4-
methylenedioxybenzoyl, 1- or 2-naphthoyl. Ac may further-
more be aralkanoyl having 1-10 C atoms such as, for
example, phenylacetyl, 2- or 3-phenylpropionyl or 2-,
3- or 4-phenylbutyryl.
N~Ac is particularly preferably acetamido.
If one of the compounds of the formulae I to VI contains
several groups A and/or Ac it i8 po6sible for
the groups of the same type in each case to be identical
to or different from one another.
The radicals Ar and Ar' are each, independently of one
another, unsubstituted or mono- or disubstituted phenyl,
but the two radicals preferably have the same substitu-
tion pattern. When they are monosubstituted, the appro-
priate substituent is preferably in the para position.
"~,'
':
2 ~ 7 ~
-- 6 --
Particularly preferred substituent~ on the phenyl radical
are -N~2, -NO2, -N~SO2C~3 or -NBCOC~3. ~:
Y i~ preferably oxygen, while X is preferably a bond. ~ -
~he variable m i8 preferably 1, q is preferably 2,
5 whereas n i8 particularly preferably 1 or 0 and p is 1, .~
2 or 3. ~ :
~ccordingly, the invention particularly relate~ to those
compounds of the formula I in which at least one of the
said radicals has one of the meanings mentioned above,
especially one of the preferred meanings mentioned above.
Some preferred groups of compounds can be expre3sed by
the following part-formulae Ia to Ig, which correspond to
the formula I and in which the undefined radicals and
parameters have the meaning stated for formula I but in i
15 which ;~
~. ~ ,'.'-.
în Ia Ar and Ar' are each p-methylsulfonamidophenyl; ~:
in Ib Ar and Ar~ are each p-nitrophenyl;
in Ic Ar and Ar~ are each p-a~; nophenyl;
in Id n ~ 2 and p ~ l 80 that the heterocyclic group i8
a piperidine radical;
in Ie n - 1 and p ~ 3 80 that the heterocyclic group i8'
a hexahydroazépine radical;
in If n ~ 0 and p ~ 2 80 that the heterocyclic group i8
a pyrrolidine radical;
5 in Ig q ~ 2 and m - 0, and n and p have the meaninge
stated in Id to If.
Further preferred compounds are those of the
.. ~ : .-
'! ' , . .
211837 ~
_ 7 _
part-formulae Ih and Iah to Igh which correspond to the
part-formulae I and Ia to Ig but in which additionally Y
is oxygen and X is a bond.
The compounds of the formula I are moreover prepared by
methods known per se, as described in the literature (for
example in the standard works such as ~ouben-Wey
Methoden der 0rganischen Chem~e [~ethods of Organic
Chemistry], Georg-Thieme-Verlag, Stuttgart or J. March,
Adv. Org. Chem., 3rd ~d., J. Wiley ~ Sons, N.Y. (1985)),
specifically under reaction conditions which are known
and suitable for the said reactions. It is m~reover
possible to make use of variants which are known per se
but which are not mentioned in detail here.
The starting materials for the claimed proce~s can, if
required, also be formed in situ in such a manner that
they are not isolated from the reaction mixture but
immediately reacted further to give the compounds of the
formula I.
. : . . ::
Some of the starting materials of the formula II and III
are known. Those which are unknown can be prepared by
methods known per se. The compounds of the formula II in
which Y i8 oxygen are prepared, for example, starting
from compounds which corre~pond to the formula II per se
but, in place of the radical -Y-Ar, contain, for example,
an OH group, a reactive functionally modified OH group,
Cl or Br and whose secondary N atom is blocked by a
protective group known per se, by reac~ion with phenol or
with a substituted derivative or with a corresponding
pheno}ate under conditions as are known per se for ether
~ynthesis, but especially under the condition~ of the
Mitsonubo reaction (J. Am. Chem. Soc. 104, 6876 (1982)).
Furthermore, certain compounds of the formula II (Y ~ a
bond) c~n also be prepared, for example, starting from
benzylpyrrolidines or -piperidines or phenylpyrrolidines
or -piperidines by substitution on the aromatic system,
in particular nitration, where appropriate with
.:
211837~
A ~ - 8 -
subsequent reduction and further derivatization.
The substituted phenyl compounds of the formula II are,
as a rule, known or can easily be prepared in analogy to
the known compounds.
S The reaction of the compounds II and III takes place by
methods as are known from the literature for the
alkylation of amines. It i~ possible for the components
to be melted together in the absence of a solvent, where
appropriate in a closed tube or in an autoclave. ~owever,
it is also possible to react the compound~ in the pres-
ence of an inert solvent. Examples of ~uitable solvents
- are hydrocarbons such as benzene, toluene, xylene;
ketones such as acetone, butanone; alcohols such as
methanol, ethanol, isopropanol, n-butanol; ethers such as
tetrahydrofuran (T~F) or dioxane; amides such as di-
methylformamide (DMF) or N-methylpyrrolidine; nitriles
such as acetonitrile, where appropriate also mixtures of
these ~olvents with one another or mixtures with water.
~he addition of an acid acceptor, for example an alkali
metal or alkaline earth metal hydroxide, carbonate or
bicarbonate or of another alkali metal or alkaline earth
metal salt of a weak acid, preferably of potassium,
sodium or calcium, or the addition of an organic base
such as triethylamine, dimethylaniline, pyridine or
quinoline or of an excess of the amine component, may be
beneficial. The reaction time depends on the condition~
employed and i5 between a few minutes and 14 days, and
the reaction temperature is between about 0 and 150
normally between 20 and 130.
It is furthermore possible for the preparation of a
compound of the formula I in which Y is oxygen to react
a compound of the formula IV with a benzene derivative of
the formula V.
Compounds of the formula IV ca~n be obtained, for example,
by reacting cyclic amines such as, for example,
~ "'''"
21183~S
g
piperidinols, pyrrolidinol~, prolinols or else cyclic
amines which are substituted by a hydroxymethyl or
halomethyl group, where halogen i8 preferably chlorine or
bromine, with a phenylalkyl bromide or chloride (alkyl
ethyl or propyl) or phenoxyalkyl bromide or chloride,
where the aromatic system can also be substituted by one
or two of the radical6 stated for Ar, so that N-
alkylation takes place. - ;
The benzene derivative~ of the formula V are known as a --
rule and can be prepared by the methods known per se for
aromatic substitution.
The reaction of the compounds of the formula IV with
those of the formula V takes place under condition~
typical for synthesizing ethers. Suitable solvents are
those mentioned above for the reaction of II with III.
Likewise, the same reaction times and temperatures are
suitable. Particularly preferred reaction conditions are
those of the Mitsonubo reaction, using azodicarboxylic
die6ter and triphenylphosphine if a compound of the
formula I with Y ~ oxygen is to be prepared.
It is noteworthy that in the reaction of IV with V under
"Mitsonubo conditions~ there may be a rearrangement with
ring contraction or ring expansion by in each case one
C atom of the cyclic A~;ne unit 80 that, for example, a
25 piperidine derivative may be produced from a pyrrolidine ~ ~;
derivative or a hexahydroazepine derivative may be
produced from a piperidine derivative, or else conver~ely
a pyrrolidine system'may be produced from a piperidine
system.
Resulting product mixture6 of five- and six-membered
rings or six- and seven-membered heterocycles can easily
be separated by preparative chromatography on ~ilica gel.
Furthermore, compounds of the formula I can also be
prepared by reacting benzene derivatives of the
': '~ ' :,'
211~37~
-- 10 --
formula Va with cyclic ~m;nes of the formula VI. The
compouDds of the formula Va are known per se or can be --
prepared in analogy to methods known per se, for example
by those of aromatic electrophilic substitution. ~ ~-
Compounds of the formula VI can be prepared, for example,
starting from the particular cyclic amine by
etherification of the free hydroxyl group on the side
chain as well a~ N-alkylation of the heterocycle, where ~ -
appropriate after elimination of a necessary proteGtive
group under the conditions stated above for the prepar-
ation of the compounds of the formula II using those of ~-
the formula III. ;~
The reaction of the compounds va with the substances of
the formula VI takes place in analogy to the reaction of
IV with V as stated above, although no rearrangements
take place in this case.
It is furthermore possible to obtain a compound of the
formula I by reducing a compound which corresponds to the
formula I but contain~, in place of one or more CB2
groups, one or more reducible groups, preferably at
temperatures between -80 and +250 in the presence of at
least one inert solvent.
Reducible (hydrogen-replaceable) groups are, in
particular, oxygen in a carbonyl group, hydroxyl, aryl-
sulfonyloxy (for example p-toluenesulfonyloxy),
N-benzenesulfonyl N-benzyl or O-benzyl.
It is possible in principle to convert compounts which
contain only one, or those which contain two or more of
the abovementioned groups side by side, by reduction into
a compound of the formula I. Preferably used for this
purpo6e is nascent hydrogen or complex metal hydrides,
furthermore reductions with gaseous hydrogen with
catalysis by transition metals.
.:, ~, -
211~3~5
11
If nascent hydrogen i8 used a8 reducing agent~ it can be
generated~ for example r by treating metals with weak
acids or with bases. Thus, for examPle, a mixture of zinc
with alkali metal hydroxide 801ution or of iron with
S acetic acid can be used. Also guitable is the use of
sodium of another alkali metal in an alcohol such as
ethanol, isopropanol, butanol, amyl or isoamyl alcohol or
phenol. It is furthermore poæsible to use an aluminium/
nickel alloy in alkaline aqueOus solution, where ap-
propriate with the addition of ethanol. Sodium or alu-
minium amalgam in aqueous/alcoholic or aqueous solution
i8 also suitable for generating nascent hydrogen. The
reaction can aI80 be carried out in heterogeneous phase,
in which case an aqueous and a benzene or toluene phase
is preferably used.
It is furthermore particularly advantageous to use
complex metal hydrides such a~ LiAl~" NaB~" diisobutyl-
aluminium hydride or NaAl(OC~2Q,OC~3)2H2 as well a~
diborane as reducing agents, if required with the addi-
tion of catalystc such as BF3, AlC13 or LiBr. Particularlysuitable solvents for this purpose are ethers such as
diethyl ether, di-n-butyl ether, ~EF, dioxane, diglyme or
1,2-dimethoxyethane as well as hydrocarbons such as
benzene. Solvents primarily suitable for a reduction with
NaB~, are alcohols such as methanol or ethanol, as well
as water and aqueous alcohols. Reducti~n by these methods
preferably takes place at temperatures between -80 and
~150, in particular between about O and about 100.
'.
The -CO- groups in the amides can be reduced to! C~z
groups particularly advantageously with LiAlH, in T~F at
temperatures between about O and 66.
It is furthermors possible to carry out certain reduc~
tions by using ~2 gas with the catalytic action of
transition metals such as, for example, Raney Ni or Pd.
It is possible in this way, for example, to replace Cl,
Br, I, S~ or, in certain case~, also OH groups by
, . -. .
:
:~.. 1' .~ .. .
21~7~
- 12 -
hydrogen. ~ikewise, nitro groups can be converted, for
exam~le, by catalytic hydrogenation with Pd/~2 in ~-
methanol into NH2 groups.
- ~.
Furthermore, one compound of the formula I can be con-
verted by methods known per se into a different compound
of the formula I.
The phenyl rings in the compounds of the formula I can,
if side reactions are to be precluded, for example be
chlorinated, brominated or alkylated under the conditions
of Friedel-Crafts reactions by reactinq the appropriate
halogen or àlkyl chloride or alkyl bromide with catalysis
by ~ewis acids such as, for example, AlC13, FeBr3 or Fe,
at temperatures between 30 and 150, preferably between
50 and 150, in an inert solvent such as, for example,
hydrocarbons, ~F or tetrachloromethane, with the com-
pound of the formula I which i8 to be derivatized.
It is furthermore possible to convert a compound of the
formula I in which Ar or Ar' is substituted by N~2 by
alkylation or acylation by methods which are generally
customary and known for amines into corresponding com-
pounds of the formula I in which Ar or Ar' is substituted
by N~A or NHAc.
, .: .
A resulting base of the formula I can be converted with
an acid into the relevant acid addition salt~ Acid~
suitable for this reaction are those which provide
physiologically acceptable salts. Thus, it is possible to.
use inorganic acid~, for example sulfuric acid, hydro-
halic acids such as hydrochloric acid or hydrobromic
acid, phosphoric acids such as orthophosphoric acid,
nitric acid, sulfamic acid, al30 organic acids, speci~
fically aliphatic, alicyclic, araliphatic, aromatic or
heterocyclic mono- or polybasic carboxylic, sulfonic or
sulfuric acids, ~uch as formic acid, acetic acid,
propionic acid, pivalic acid, diethylacetic acid, malonic
acid, succinic acid, pimelic acid, fumaric acid, maleic
,,
:
r
21~337
- 13 - ~
: ' .'
acid, lactic acid, tartaric acid, malic acid, benzoic
acid, salicylic acid, 2-phenylpropionic acid, citric
acid, gluconic acid, a~corbic acid, nicotinic acid,
isonicotinic acid, methane- or ethanesulfonic acid,
ethanedisulfonic acid, 2-hydroxyethanesulfonic acid,
benzenesulfonic acid, p-toluenesulfonic acid, naphthalene
mono- and -disulfonic acids and lauryl sulfuric acid.
The free bases of the formula I can, if required, be
liberated from their salts by treatment with strong base3
such as sodium or potas~ium hydroxide, sodium or
potassium carbonate, as long as no other acidic groups
are present in the molecule.
he compounds of the formula I may have a centre of
asymmetry. They may therefore result from their prepara~
tion as racemates or, if optically active starting
materials are used, also in optically active form.
Resulting racemates can, if required, be resolved into
their optical antipodes mechanically or chemically by
methods known per se. Preferably, diastereomer~ are
formed from the racemate by reaction with an optically
active resolving agent. ~:~
Examples of suitable resolvins agents are optically
active acids such a~ the D and L forms of tartaric acids,
dibenzoyltartaric acid, diacetyltartaric acid,
camphosulfonic acids, mandelic acid, malic acid or lactic
acid. The various forms of the diastereomers can be
separated in a manner known per se, for example by.
fractional crystallization, and the optically active
compounds of the formula I can be liberated from the
diastereomers in a manner known per se.
~ ~ -
It is furthermore possible to separate the enantiomers by
preparative chromatographic methods known per se. Silica
gel is preferred as stationary phase. Particularly ~ ~
preferred mobile phases are mixtures oi'ethyl acetate and ~ -
35 heptane or dichloromethane and methanol. ~
2118375
- 14 -
The invention furthermore relates to the use of the
compounds of the formula I and their physiologically
acceptable salts for the production of pharmaceutical
preparations, in particular by non-chemical means. For
this purpo~e they can be converted together with at least
one vehicle or ancillary substance and, where appro-
priate, in combination with one or more other active
substance(s) into a ~uitable dosage form.
The invention furthermore relates to compositions, in
particular pharmaceutical preparations, containing at
least one compound of the formula I and/or one of its ~s
physiologically acceptable salts. These preparations can
be used afi medicaments in human and veterinary medicine.
Suitable vehicles are organic or inorganic substances
which are suitable for enteral (for example oral),
parenteral or topical administration and which do not
react with the n~vel compounds, for example water,
vegetable oils, benzyl alcohols, polyethylene glycols, ~ -~
gelatin, carbohydrates such as lactose or starch,
magnesium stearate, talc, petrolatum. Tablets, coated
tsblets, capsules, syrups, ~olutions, drops or
suppositories are used in particular for enteral ~ :
administration, solutions, preferably oily or aqueous
solutions, as well as suspensions, emulsions or implants
25 are used for parenteral administration, and ointments, -
creams or powders are used for topical application. The
novel compounds can also be lyophilized and the resulting
lyophilizates u~ed, for example, to produce products for
injection. -
,, , ,:
The stated preparations can be sterilized and/or contain
ancillary ~ubstances such as lubricants, preservatives,
stabilizers and/or wetting agents, emulsifiers, salts to
influence the osmotic pres~ure, buffer substances,
colorants, flavourings and/or aromatizing substances.
They can, if required, also contain one or more other
active substances, for example one or more vitamins.
; . .~ ~:
: ~
.~",",~.
21~37~
~he compounds of the formula I and their physiologically
acceptable salts can be used for the therapeutic treat-
ment of the human or anLmal body and for controlling
diseases. They are particularly suitable for the treat-
ment of arrhythmias and tachycardias.
For this purpose the substances according to the inven-
tion are, as a rule, administered in analogy to known
substances with antiarrhythmic activity, such as
aprindine, flecainide or amiodarone, preferably in
dosages between about 1 and 100 mg, in particular between
2 and 20 mg per dosage unit.
The daily dosage i8 preferably between about 0.02 and
2 mg/kg of body weight. The specific doæe for each
particular patient depends, however, on a wide variety of
factors, for example on the efficacy of the specific
compound used, on the age, body weight, general state of
~ealth, sex, on the diet, on the time and route of
administration, on the rate of excretion, pharmaceutical
combination and severity of the particular disorder to
which the therapy applies. Oral a~inistration is
2referred.
In the following examples, Nusual working up" means~
If necessary, water or dilute sodium hydroxide solution
i~ added, extraction is carried out with an organic
solvent such as ethyl acetate, chloroform or dichloro~
methane, the organic phase is ~eparated off, dried over .
sodium sulfate, filteied and evaporated, and purificatibn ~;
is carried out by chromatography on silica gel and~or `~
crystallization. The optical rotations were measured in
~0 methanol (c ~ 1) unless ~tated otherwise. If the produc-
tion of two substances is de~cribed in the individual
examples, these are always separate from one another.
All temperatures are stated hereinbefore and hereinafter
in degrees Celsius. ~ ;
-,,
~11837 ;i
- 16 -
Bxample 1
4 mmol of 1-(2-p-nitrophenylethyl)-3-piperidinol
[prepared from 3-piperidinol by alkylation with p-nitro-
phenethyl bromide or p-nitrostyrene], 4 mmol of p-nitro-
phenol, 4 mmol of triphenylphosphine and 4 mmol ofdiethyl azodicarboxylate are dissolved in 100 ml of T~F
and stirred at room temperature for 48 h. After the usual
working up, the residue is chromatographed on silica gel
using dichloromethane/methanol (99:1~ and ethyl acetate/
methanol (99~ uccessively as mobile phases.
The following are obtained: -
,:,,
a) 1-(2-p-nitrophenylethyl)-2-(p-nitrophenoxymethyl)-
pyrrolidine;
b) 1-t2-p-nitrophenylethyl)-3-(p-nitrophenoxy)- ;
piperidine. ;- ;~
~ample 2
In analogy to Example 1, starting from (-)-1-(2-p-nitro~
phenylethyl)-2-prolinol~ the following are obtained after ~;
chromatography on silica ~el using ethyl acetate/heptane
(7:3) and subsequently dichloromethane/ methanol (99~
as mobile phases: `~ ; ;
a) (-)-1-(2-p-nitrophenylethyl)-2-(p-nitrophenoxy-
methyl)pyrrolidine ; `~
la]20~ -98.4; -
; 25 b) (+)-1-(2-p-nitrophenylethyl)-3-(p-nitrophenoxy)-
piperidine
[a]'~ l37.2
The following are obtained analogously
from (+)-1-(2-p-nitrophenylethyl)-2-prolinol:
':
,'';'' ~
-~ 2~1837S
- 17 -
a) (~t-1-(2-p-nitrophenylethyl)-2-(p-nitrophenoxy-
methyl)pyrrolidine
[a]20D +97.8;
b) (+)-1-(2-p-nitrophenylethyl)-3-(p-nitrophenoxy)-
piperidine
[~]20D -36.6 .
Example 3
In analogy to Example 1, starting from 1-(2-p-nitro-
phenylethyl)-2-hydroxymethylpiperidine, the following are ~ -
obtained by reaction with p-nitrophenol with a reaction
time of 60 h (purification: silica gel/methyl tert.-butyl
ether and subsequently heptane/acetone (7:3))
1-(2-p-nitrophenylethyl)-2-(p-nitrophenoxymethyl)- ~ ~
piperidine and ~ ;
1-(2-p-nitrophenylethyl)-3-(p-nitrophenoxy)-
hexahydroazepine.
Subsequent reaction with fumaric acid provides after
crystallization (ethyl acetate/diisopropyl ether)
a) 1-(2-p-nitrophenylethyl)-2-(p-nitrophenoxymethyl)-
piperidine -
m.p. (fumarate) 122;
b) l-(2-p-nitrophenylethyl)-3-(p-nitrophenoxy)-
hexahydroazepine
m.p. (fumarate) 137.
25 Esample 4 ; ;
.'~ ' '" .''''
In analogy to Bxample 1, the following are obtained by
resction of p-nitrophenol ~ ;~
with 1-(2-p-nitrophenylethyl)-3-hydroxymethylpiperidine
(purification: ~ilica gel/methyl tert.-butyl ether and
~,
211~375
- 18 -
subsequently heptane/acetone (7:3))
1-(2-p-nitrophenylethyl)-3-(p-nitrophenoxy-
methyl)piperidine;
with l-(2-p-nitrophenylethyl)-4-piperidinol
(purification: silica gel/heptane:acetone (7:3) and
subsequently ethyl acetate/methanol (19:1))
1-(2-p-nitrophenylethyl)-4-(p-nitrophenoxy)~
piperidine;
with (~ (2-p-nitrophenylethyl)-3-pyrrolidinol
(purification: silica gel/methyl tert.-butyl
ether:petroleum ether:methanol (25:24
(+)-1-(2-p-nitrophenylethyl)-3-(p-nitrophenoxy)~
pyrrolidine, m.p. 97,
[a]20D - +13.8 (dioxane).
.... " ~ .. ~.~ ..
. .~'-"''~i
Rxample 5
In analogy to Example 1, reaction of 1-benzhydryl-
azetidine tobtainable by reaction of benzhydrylamine with
1-chloro-2,3-epoxypropane] with N-(4-hydroxyphenyl)-
phthalimide results, after the usual working up, in
1-benzhydryl-3-[4-(1,3-dioxo-2-i~oindolinyl)phenoxy]-
azetidine
(purification: 8ilica gel~diisopropyl ether:methanol
(49
Elimination of the benzhydryl group by treatment with
~2 gas/Pd on carbon (Pd content 1 %) in toluene at room~
tempe~ature p~ovides 3-[4-(1,3-dioxo-2-isoindolinyl)-
phenoxy~azetidine.
Example 6
1 mmol of 3-[4-(1,3-dioxo-2-isoindolinyl)-phenoxy]-
azetidine is dissolved in 40 ml of dichloromethane,
1 equivalent of p-nitrophenethyl bromide is added, and
the mixture i8 stirred at room temperature for 6 h. The
:: ~ :..~: .
. ~ ~
19 - 21183~
usual working up results in 1-(2-p-nitrophenylethyl)-3-
14-(1,3-dioxo-2-i~oindolinyl)phenoxy]azetidine.
Example 7
0.9 g of 1-(2-p-nitrophenylethyl)-3-[4-(1,3-dioxo-2-
5 i80indolinyl)phenoxy]azetidine i8 dissolved in 50 ml of
THF, and 0.5 g of hydrazine i5 added. The mixture i8
boiled for 2 h and then worked up as usual. 1-(2-p-
Nitrophenylethyl)-3-(p-aminophenoxy~azetidine i~
obtained.
~xamDle 8
~ .. .: ~.
18 mmol of p-nitrophenol are dissolved in 15 ml of DMF,
22 mmol of Na~ are added, and the mixture is ~tirred at
40 for 30 min. Subsequently, 18 mmol of 1-(2-p-nitro~
phenylethyl)-2-chloromethylpiperidine tprepared from the
piperidinol derivative by halogenation with thionyl
chloride/DMF in dichloromethane] are dissolved in 20 ml
of DMF, added to the phenolate ~olution and stirred at
90 for 4 h. The solution i~ concentrated, the re6idue i8
taken up in toluene, and the solution is washed with 2 N
sodium hydroxide solution and extracted with 2 N hydro-
chloric acid. ~he usual working up and purification by
chromatography (silica gel/methyl tert.-butyl ether)
re~ults in
: . ,: ,. '`, ' .
a) 1-(2-p-nitrophenylethyl~-2-(p-nitrophenoxymethyl)-
piperidine;
., , j ,
b) 1-(2-p-nitrophenylethyl)-3-(p-nitrophenoxy)-
hexahydroazepine.
~xample 9
85 mmol of 1-(2-p-nitrophenylethyl)-2-(p-nitrophenoxy-
methyl)pyrrolidine are hydrogenated on 15 g of Raney Ni
in 400 ml of ethanol and 40 ml of T~F at 20 and 3 bar
~ 211837~
- 20 -
for 7 h. The solution i8 concentrated and worked up as
usual. 1-(2-p-Aminophenylethyl)-2-(p-aminophenoxymethyl)-
pyrrolidine i8 obtained.
The following are obtained analogously by catalytic
reduction with Raney Ni~
from 1-(2-p-nitrophenylethyl)-3-(p-nitrophenoxy)- -~
piperidine~
1-(2-p-aminophenylethyl)-3-~p-aminophenoxy)- ; ~ .
piperidine;
from (~ (2-p-nitrophenylethyl)-2-(p-nitrophenoxy-
methyl)pyrrolidine:
(-)-1-(2-p-aminophenylethyl)-2-(p-aminophenoxy- :~
methyl)pyrrolidine;
from (+)-1-(2-p-nitrophenylethyl)-3-(p-nitrophenoxy)-
piperidine:
(+)-1-(2-p-aminophenylethyl)-3-(p-aminophenoxy)-
piperidine;
from (+)-1-(2-p-nitrophenylethyl)-2-(p-nitrophenoxy- ;~ :~
methyl)pyrrolidine:
(+)-1-(2-p-aminophenylethyl)-2-(p-aminophenoxy-
methyl)pyrrolidine;
from (-)-1-(2-p-nitrophenylethyl)-3-(p-nitrophenoxy)-
piperidine: - ::
(-)-1-(2-p-aminophenylethyl)-3-(p-aminophenoxy)-. : ~ ~
piperidine;~ ' ' I `; :~''
from 1-(2-p-nitrophenylethyl)-2-(p-nitrophenoxymethyl)-
piperidine: .~
1-(2-p-aminophenylethyl)-2-( p-~mi nophenoxymethyl)- ~ `.
piperidine; ~ ~:
,,, :,
,,
211~37~
- 21 -
~ .
from l-(2-p-nitrophenylethyl)-3-~p-nitrophenoxy)-
hexahydroazepine: ~ ~
1-(2-p-aminophenylethyl)-3-(p-aminophenoxy)- :
hexahydroazepine;
' ' .
from 1-(2-p-nitrophenylethyl)-3-(p-nitrophenoxymethyl)-
piperidine: .
1-(2-p-aminophenylethyl)-3-(p-aminophenoxymethyl)- - -
piperidine;
from l-(2-p-nitrophenylethyl)-4-(p-nitrophenoxy~
piperidine~
1-(2-p-aminophenylethyl)-4-(p-aminophenoxy)-
piperidine;
from (+)-1-(2-p-nitrophenylethyl)-3-(p-nitrophenoxyl)- :
pyrrolidine~
(+)-1-(2-p-aminophenylethyll-3-(p-aminophenoxy)- ~: .
pyrrolidine.
. ~
~xample 10
13 mmol of 1-(2-p-aminophenylethyl)-2-(p-aminophenoxy- ~ :
methyl)pyrrolidine are dissolved in 40 ml of dry pyridine
20 and, while stirring under an N2 atmosphere and cooling in ~ :
ice, at a temperature of 5-10 39 mmol of mesyl chloride :~
are added dropwise and the mixture i8 6tirred at the same : :
temperature for 2 h. Ethyl acetate i~ added to the
solution. ~he resulting precipitate i8 separated off and
taken up in ethyl acetate~Na~C03 solution. The usual .
working up and~ purification by chromatography (silica
gel/dichloromethane/methanol (49:1)) results in 1-(2-p-
methylsulfonamidophenylethyl)-2-(p-methylsulfonamido~
phenoxymethyl)pyrrolidine, m.p. 61-63.
The following are obtainad analogou~ly by reaction with
m ~yl chloride~
.. ~ .:
. ~ ;,,,:,.
. :, . . .
- . ~ .
: ": ... .
7 i
. r~
- 22 -
from l-(2-p-aminophenylethyl)-3-(p-aminophenoxy)- ~ ~ .
piperidine: :
1-(2-p-methylsulfonamidophenylethyl)-3-(p-methyl-
sulfonamidophenoxy)piperidine, m.p. 148-149; ~ ~ ~
.,.:.
from (-)-1-(2-p-aminophenylethyl)-2-(p-aminophenoxy-
methyl)pyrrolidine:
(-)-1-(2-p-methylsulfonamidophe~ylethyl)-2-(p- :
methylsulfonamidophenoxymethyl)pyrrolidiner
[a]20 _73 go;
-: . '
10 from (+)-1-(2-p-aminophenylethyl)-3-(p-aminophenoxy)- ~:
piperidine~
(+)-1-(2-p-methylsulfonamidophenylethyl)-3-(p-
methylsulfonamidophenoxy)piperidine, m.p. 168, .
[a]20D +31.8; :~
from (+)-1-(2-p-aminophenylethyl)-2-(p-aminophenoxy~
methyl)pyrrolidine:
(+)-1-(2-p-methylsulfonamidophenylethyl)-2-(p-
methylsulfonamidophenoxymethyl)pyrrolidine, [a]~oD
-72.0; ~ ;;
from (-)-1-(2-p-aminophenylethyl)-3-(p-aminophenoxy)-
piperidine: `
(-)-1-(2-p-methylsulfonamidophenylethyl)-3-(p- ::
methylsulfonamidophenoxyJpiperidine, m.p. 168,
[a]20D -32.7; ~.
25 from 1-(2-p-aminophenylethyl)-2-(p- ~ nophenoxymethyl)- ~
piperidine: ! l ! ': "; ..
1-(2-p-methylsulfonamidophenylethyl)-2-(p-methyl-
sulfonamidophenoxymethyl)piperidine;
from 1-(2-p-aminophenylethyl)-3-(p-aminophenoxy)- ~ ~ a
hexahydroazepine~
1-(2-p-methylsulfonamidophenylethyl)-3-(p-
methylsulfonamidophenoxy)hexahydroazepine;
.. ~,,~;,, ~,
., ..~ i. -.". ,~.
. . ... . ~
21~37~
- 23 -
from l-(2-p-aminophenylethyl)-3-(p-aminophenoxymethyl)-
piperidine:
1-(2-p-methylsulfon~m;dophenylethyl)-3-(p-methyl-
6ulfonamidophenoxymethyl)piperidine, m.p. 199-200;
S from 1-(2-p-aminophenylethyl)-4-tp-aminophenoxy)-
piperidine~
1-(2-p-methylsulfon~;dophenylethyl)-4-(p-methyl- ~-
sulfonamidophenoxy)piperidine, m.p. 182-183~
from (~)-l-(2-p-aminophenylethyl)-3-(p-aminophenoxy)- --
pyrrolidine:
(+)-1-(2-p-methylsulfonamidophenylethyl)-3-(p- ~
methylsulfonamidophenoxy)pyrrolidine,m.p.l99-200, ~;
[a]20D ~7.3 (dioxane). ; -
Example 11
.
10 mmol of 1-(2-p-Pm;nophenylethyl)-3-(p-aminophenoxy)- ~ f
pyrrolidine are dissolved in 45 ml of dry pyridine and
45 ml of acetic anhydride and stirred at room temperature
for 24 h. Water i9 added to the suspension while cooling
in ice, the mixture is stirred at room temperature for
20 3 h, 2 N sodium hydroxide ~olution is added until ~;~
opalescent, and the usual working up is carried out.
Recrystallization from methanol results in 1-(2-p-
acetamidophenylethyl)-3-(p-acetamidophenoxy)-pyrrolidine,
m.p. 213-214. - ~ -~
The following examples relate to pharmaceutical prepara--
tions. ' ! i
.: ~
Esample A: Vi~
~. .........
A solution of 100 g of an active substance of the
formula I and 5 g of disodium hydrogen phosphate in 3 1 ~ ~
30 of double-distilled water is adjusted to p~ 6.5 with 2 N ~ ~`
hydrochlcric acid, sterilized by filtration, dispensed
into vials, lyophilized and sealed sterile. Each vial
~ ,....,,, ,-
, ...... .
:~r -
~ 211~37~
- 24 -
contains 5 m~ of active ~ubstance.
E~ample B: Suppo~itories
A mixture of 20 mg of an active substance of the
formula I with 100 g of soya lecithin and 1400 g of cocoa
butter is melted, poured into moulds and left to cool.
Each suppository contains 20 mg of active substance.
Bxample C: Solution ~-
A solution i8 prepared from 1 g of an active substance of ~ ;~
the formula I, 9.38 g of Na~,PO, x 2 H~O, 28.48 g of
Na2HPO~ x 12 ~2O and 0.1 g of benzalkonium chloride in
940 ml of double-distilled water. The p~ is adjusted to
6.8, the volume i8 made up to 1 1 and the solution is ~ ~`
sterilized by radiation. This solution can be used in the
form of eye drops. ~ -
Example D: Ointment
500 mg of an active substance of the formula I are mixed ~;
with 99.5 g of petrolatum under aseptic conditions. ~;
Bxample B: Tablets `~
A mixture of 1 kg of active substance of the formula I, ~ -
4 kg of lactose, 1.2 kg of potato starch, 0.2 kg of talc
and 0.1 kg of magnesium stearate i~ compressed to tablets
in a conventional way ~uch that each tablet contains- il;
10 mg of active substance. :
Example F: Coated tablets
,;. i , ~ ,, ~.
Tablets are compressed in analogy to Bxample E and are
then coated in a conventional way with a coating compo~ed
of sucrose, potato starch, talc, tragacanth and colorant.
.', ;'.','1-.
~ 25 21~37:~.
B~ample G: Capsules
2 kg of active substance of the formula I are packed into
hard gelatin capsules in a conventional way BO that each
capsule contains 20 mg of the active substance.
Bsample ~: Ampoules
A solution of 1 kg of active substance of the ,~ ~.
formula I in 60 1 of double-distilled water i8 dispensed ;;~
into ampoules, lyophilized under a~eptic conditions and :~
sealed sterile. Each ampoule contains 10 mq of active f~
10 substance. ;;
;;
~ ,. !
.. ' '''~
'' ~ ~" .~
"' ~: ,~ f.i, ".',,, ', .".; `'
'", " " .,