Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
W093/~4 2 1 2 0 6 1 7 PCT/USg2/09330
MUSC~E CONTRO~ ~ND MONITORING 8YSTEN
CROSS REFERENCES TO CO-PENDING APP~ICATIONS
This application is a continuation-in-part of the
following commonly assigned U.S. Patent Applications:
Serial No. 07/446,593, filed~December 6, 1989, entitled
"Muscle Fitness Detection by Colorimetry"; Serial No.
07/446,594, filed December 6, 1989, entitled UMuscle
Stimulator with Variable Duty Cycle"; Seria} No. 07/446,592,
filed December 6, 1989, entitled "Muscle Output Monitor by
Intramuscular Temperature Variation Measurement"; and Serial
No. 07/446,811, filed December 6, 1989, entitled "Muscle
Contraction ~ontrol by Intramuscular Pressure Monitoring".
'
~ACXGRO~ND O~ ~B INVENTTON
1. Field of the i~ t~on - The present invention
relates generally to cardiac assist systems and more
particularly, relates to control and monitoring of cardiac
assist systems which are powered b~ skeletal muscle.
2. De-ori~ti:on of t~e P~iox Art - Cardiac assist
systems do not replace the human heart, but merely
supple~ent-it. ~any techniques have been proposed using a
variety~of mechanical power sources. Typically these
required~some form of percutaneous energy transfer because
of the difficulty in storing sufficient energy ~-
subcutaneously. Such systems are cumbersome and
incon~enient for the patient, and are prone~to infection
along the percutaneousienergy transfer path.
A technique holding a great deal of promise is to power
the.cardiac assist system from~a surgically modified
skeletal muscle.~ The cardiac assist system is thus powered
by normal bioche~ical processes. U.S. Patent No. 4,813,952
issued to Khalafalla teaches a number of configurations of a
skeletal muscle powered cardiac assist system.
WOg3/08874 PCT/USg2/09330
--2--
One problem peculiar to a skeletal muscle powered
cardiac assist system is that the skeletal muscle must be
conditioned to with stand the constant load of continuous
contraction/relaxation demanded of the myocardium. U.S.
Patent No. 4,411,268 issued to Cox teaches a technique for
conditioning the ske}etal muscle. Whereas the apparatus of
Cox is effective to accompli~h this conditioning, his system
has no provisions for feedback to permit the self-regulation
of the conditioning regimen or for chronically monitoring
the stability of the skeletal muscle following the
conditioning process. In practice this necessitates the
attention of highly skilled medical personnel to monitor the
operation of the skeletal muscle with sophisticated
instrumentation and to exercise manual control of the
stimulation parameters with pulse generator programming
equipment. Furthermore, neither Cox nor Xhalafalla teach a
real time monitoring mechanism, whereby adequate vascular
support to the skeletal muscle and accurate stimulation
timing can be chronically verified.
A second problem is basic monitoring of the skeletal
muscle contractions. This is important because it provides
a way to check and modify various pulse generator timing and
amplitude parameters. Currently, the prior art suggests no
effective means for performing this monitoring function.
Whereas the feasibility of a skeletal muscle powered
; cardiac assist system has been established in the literature
and the clinic, a practical system must address concerns
regarding efficiency and safety of operation. Of specific
concern is the tying of the rate of stimulation of the
skeletal muscle directly to t~e heart rate. This seems
appropriate in some instances, but care must be exercised
because of the wide range of possible rates. For example,
it may be quite inefficient to stimulate the skeletal muscle
at the cardiac rate when the patient is at rest and requires
only modest cardiac output. Similarly, it may be
W093/ ~ 74 2 ~ 2 0 ~ 1 7 PCT/US92/09330
inefficient and even dangerous to stimulate skeletal muscle
contraction at very high rates~ The nature of the skeleta~
muscle stimulation may also be changed to improve efficiency
over the range of available rates and cardiac demands.
811M~I~RY OF THE INVENTION
one embodiment of the present invention employs a
chronically implantable oximeter which is positioned within
the skeletal muscle of a cardiac assist system. It is
preferably a two wave length reflectance oximeter which
measures the relative oxygen level within the skeletal
muscle as it powers the cardiac assist system. The two
wave~ength reflectance signal is sent to be processed within
the implantable pulse generator of the cardiac assist
system.
lS Circuitry which is internal to the implantable pulse
generator determines the relative oxygen level and performs
a trend analysis concerning the chronic sufficiency of the
vascularization of and circulatory support to the skeletal
muscle. This data is stored in memory within the
implantable pulse generator. This memory may be
interrogated by medical personnel using telemetry to obtain -~
status and trend information concerning the cardiac assist
system.
The data may be analyzed by medical personnel to ;~
determine the effectiveness of conditioning, the sufficiency
of maintenance stimulation, the~adequacy of ~ascularization,
and the chronic prognosis for the cardiac assist system.
This enables the medical personnel to manually modify the
conditioning regimen, chanqe the maintenance stimulation,
institute various drug therapies, and plan for necessary
surgical intervention.
In a second embodiment, a chronically biocompatible
pressure transducer is implanted wit~in the skeletal muscle
tissue. This transducer produces electrical signals
W093/~4 PCT/US92/09330
sufficient to enable an implantable pulse ~enerator to
measure the timing and extent of contraction and relaxation
of the skeletal muscle in the performance of cardiac
assistance.
The timing indications are important because they
permit the implantable pulse generator to stimulate the
skeletal muscle at the appropriate time to optimize the
assist. For a confiquration wherein the skeletal muscle is
wrapped about the aorta, for example, contraction of the
skeletal muscle should be delayed until immediately
following contraction of the myocardium. Contraction of the
skeletal muscle during the contraction of the myocardium
will increase rather than decrease the load on the human
heart. For skeletal muscle wrapped directly about the human
heart, on the other hand, the stimulation should cause
simultaneous contraction to achieve maximum benefit.
Measurement of timing and extent of skeletal muscle
contractions permits the implantable pulse generator to
monitor and control the conditioning regimen. This is
important from a system viewpoint as it permits efficient
energy utilization, as various phases of the conditioning
process require the use of substantial stimulation energy.
Such monitoring and control are important medically, because
prior to complete conditioninq, the skeletal muscle will
2S readily fatigue, possibly resulting in excess loading of the
myocardium.
An additional embodiment of the present invention
employs a sensor to determine cardiac demand. Preferably
this is an activity sensor although other types of sensors
may be used, such as blood oxygen level. During periods of
low.demand, such as when the patient is at rest and the
patien~'s heart requires little assistance, the duty cycle
is lowered to improve overall efficiency. As cardiac demand
increases, the duty cycle is increased ensuring that the
patient's heart obtains greater assistance at hiqher loads.
W093/ ~ 74 2 1 2 0 6 1 7 PCT/US92/Og330
-5-
Above a very high rate, the duty cycle is again decreased to
improve overall hemodynamic efficiency and as a safety
measure.
The nature of the skeletal muscle stimulation is also
changed with cardiac demand. At low demand levels, the
number of pulses in a given burst and the amplitude are
decreased to improve efficiency. As demand is increased,
pulse count and amplitude are increased to increase the
amount of cardiac assistance. Pulse count and amplitude are
again decreased at excessively high cardiac rates as a
safety measure.
A further embodiment of the present invention employs a
chronically implantable temperature sensor which is
positioned within the skeletal muscle of a cardiac assist
system. The sensor preferably employs a thermoresistive
device, such as a thermistor, coupled to the implantable
pulse generator of the cardiac assist system.
A circuit in the implantable pulse generator senses the
changes in resistance of the thermistor which correspond to
temperature changes within the skeletal musc}e. The ~-~
implantable pulse generator is thus able to monitor the
efficiency of the work output of the skeletal muscle.
Circuitry within the imp}antable pulse generator
changes the timing and characteristics of the generated
pulses in relation to naturally occurring and paced heart
contractions to optimize muscle activity. This improves the
efficiency of the cardiac assist system by minimizing -
parasitic heat production. It also ensures that the
myocardium obtains maximum assistance from contractions of
the skeletal muscle.
Employing each of these embodiments of the present
invention substantially improves the efficiency of the
cardiac assist system through monitoring and control of the
conditioning activity. Such monitoring and control also
decreases the medical risk of the procedure.
WO 93/08874 ` rCr/US92/0g330
--6--
BRIEF DESC~IPTION OE' THE DRAWINGS
Other objects of the present invention and many O r the
attendant advantages of the present invention will be
readily appreciated as the same becomes better understood by
reference to the following detailed description when
considered in connection with the accompanying drawings, in
which like reference numerals designate like parts
throughout the figures thereof and wherein:
FIG. l is a first embodiment of the present invention
wherein the skeletal muscle is wrapped about the myocardium;
FIG. 2 islan alternative embodiment of the present
invention wherein the skeletal muscle-is wrapped about the
descending aorta;
FIG. 3 is an alternative embodiment of the present
lS invention w~erein the skeletal muscle performs counter
pulsation of the descending aorta;
FIG. ~ is a plan view of an oximetry probe;
FIG. S is a block diagram of a implantable pulse
generator;
FI6. 6 is a graphical representation of the oximetry
re.urn in an oxygen sufficient environment;
FSG. 7 is a graphical representation of the oximetry
return in an oxygen insufficient environment;
FIG. 8 is a plan view of an implantable pressure
'7 transducer;
FTG. 9 is a block diagram of an alternative embodiment
of the implantable pulse generator;
FIG. lOA is a graph of stimulation pulses applied to
the unconditioned muscle;
FIG. 109 is the contraction pattern resulting from the -~
- .stim~lation of Fig. 6A; :-
FIG. 1oC is the waveform of the contraction as viewed
by the pressure sensor;
FIG. lOD is the differentiated pressure sensor signal
showing that the skeletal muscle is unconditioned;
,
wO93/~n4 2 1 2 0 6 1 7 PCT/US92/Og330
FIG. llA is a graph of stimulatio~ signals applied to
the conditioned muscle;
FIG. llB is the contraction pattern resulting ~rom th~
stimulation of Fig. llA; .
S FIG. llC is the waveform of the contraction as viewed
by the pressure sensor;
FIG. llD is the differ~ntiated pressure sensor signal
showing that the skeletal muscle is fully conditioned;
FTG. 12 shows the timing relationship between the
cardiac pacing pulse and skeletal muscle stimulation signals
for the embodiments of Figs. 1, 2, and 3;
FIG. 13 is a block diagram of an alternative embodiment
of the implantable pulse generator;
FIG. 1~ is a graphical representation of pulse
S5 amplitude as a function of activity level;
FSG. lS is a graphical representation of pulses per
burst as a function of activity level;
FIG. 16 is a graphical representation of
synchronization ratio as a function of activity level;
FIG. 17 is a graphical representation of stimulation
rate as a function of cardiac rate with decreasing ~ ~
synchronization ratio; ;:
FSG. 18 iS a plan ~iew of a sensing lead with
temperature sensor attacbed;
25 . FIG. 19 is a block diagra~ of an alternative embodiment
of the implantable pulse generator;
FIG. ~0 is a graphical representation of the timing
relationship ~etween contractions of the human heart and the .
pulses produced by the implantable pulse generator;
PIG. 2~ is a graphical representation Or the force
produced by conditioned and unconditioned skeletal muscle;
FSG. 22 is a graphical representation of the
temperature sensed for unconditioned, conditioned but
improperly timed, and conditioned and properly timed
skeletal muscle contractions; and
wog3/~n4 PCT/US9~09330
-B-
FIG. 23 is a graphical representation of the change in
temperature over time for conditioned and unconditioned
skeletal muscle.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention employs one or more sensors
implanted within the skeletal muscle of a skeletal muscle-
powered caraiac assist system to chronically monitor the
adequacy of circulatory support. The cardiac assist system
may be configured in a variety of ways as described in U.S.
Patent No. 4,813,9S2 issued to Khalafalla, herein
- incorporated by reference. Several of these configurations
are discussed herein by way of i~lustration and are not
intended to limit the present invention.
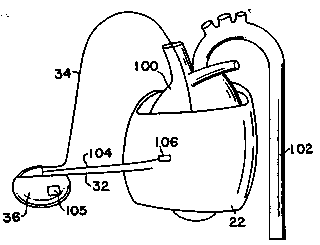
~IG. 1 is an embodiment o~ the present invention
wherein skeletal muscle 2Z is wrapped about human heart 100.
Skeletal muscle 22 is conditioned as a "slow twitch" muscle
as described by Cox in U.S. ~atent No. 4,411~268, herein
incorporated by reference. Tmpla:ntable pulse generator 36
is coupled to pacing }ead 34 to produce a demand pacemaker
- 20 as taught by Cox. In~addition, implantable pulse generator
36 stimulates skeletal mus~cle 22 to contract in synchrony
with human heart 10~0. Assistance to human heart 100 is
provided~by the siw ltaneous contraction of skeletal muscle
22~to ~increase systolic pressure in-descending aorta 102 and
elsewh~ere in~the circulatory system. -~
According to the present invention, a sensor 106 is ~
implanted upon or within skeletal muscle 22 to determine the
adequacy of chronic support. The data measured by sensor
106 is transferred to implantable pulse generator 36 via
lead~104 where it is processed, stored, and may be
telemetéred percutaneously using normal implantable pulse
generator telemetry circuitry for analysis by medical
personnel.
According to the present invention, implantable pulse
.,
W093/ ~ 74 2 1 2 ~ 6 1 7 PCT/US92/09330
generator 36 may also employ activity sensor 105 in ~ddition
to the other sensors. The activity sensor input is u~ed by
implantable pulse generator 36 to adjust the various
parameters of the skeletal muscle stimulation regimen a~
explained below. The parameters to be adjusted include duty
cycle, and pulse width, amplitude, count and interval.
FIG~ 2 is an alternative embodiment of the present
invention. In this embodiment skeletal muscle 22 is wrapped
about artificial chamber 20 inserted in series with
descending aorta 102. Unlike the embodiment of Fig. 1,
implantable pulse generator 36 stimulates skeletal muscle 22
to contract following evacuation of human heart 100. This
is accomplished by the insertion of a delay between a paced
or sensed beat of human heart 100 and the stimulation of
skeletal muscle 22. -
FS6. 3 is a further embodiment wherein artificial
chamber 20 is coupled external to descending aorta 102. In
this configuration skeletal muscle 22 is stimulated to
counter pulse human heart 100. This raises diastolic ~
pressure, thereby increasing perfusion of human heart 100. ~-
This is accomplished by the insertion by implantable pulse
generator 36 of a sufficient delay between a sensed or paced
contraction of human heart 100 and stimulation of skeletal
muscle 22 to cause the desired counter pulsation.
; FIG. ~ is a plan view of lead 104 wherein sensor 106
employs an oximeter for measuring adequacy of oxygen level
within skeletal muscle 22. U.5. Patent No. 4,813,421 issued
to Baudino, et al., herein incorporated by reference,
describes in greater detail the preferred embodiment of an
oximeter probe within sensor 106 and lead 104.
. Lead 104 is a typical chronically implantable lead. It
contains an insulated. bifurcated proximal connector
assembly 220 which sealingly plugs into implantable pulse
generator 36. ~he proximal end of connector assembly 220
contains terminal pins 224 and 226. A third conductor
,
wog3/0~n4 PCT/US92/0g330
'o--
within lead 104 is terminated at ring terminal 222. The
main body of lead 104 is covered with biocompatible outer
sheath 218 of silicone rubber or polyurethane. Anchoring
sleeve 228 facilitates securing of the proximal end of lead
104 in the manner well-known in the art.
The distal end 210 of lead 104 contains sensor 106
which is preferably a two wavelength reflectance oximeter as
taught by Baudino, et al. Maintenance of the position of
sensor 106 may be facilitated by tine members 212 which work
particularly well for positioning of transvenous pacing
leads as is well-known in the art. Oximetry structure 216
is positioned near distal end 210. Oximetry structure 216
is covered with synthetic sapphire as taught by Baudino, et
al.
FIG. S is an overall block diagram of the circuitry
within implantable pulse generator 36 for embodiments
employing an oxygen sensor. Demand pacer 300 is constructed
according to circuitry known in the art of pacing and
communicates with human heart 100 via lead 34. Demand pacer -~-
300 notifies delay logic 302 via line 360 of a contraction
of human heart 100. This may be the result ~of either a
sensed natural heart contraction or an artificially
generated pacing pulse. In either situation, delay logic
302 generates a delày appropriate to the particular
. embodiment (see above) and signals stimulation generator 304
by line 358 to stimulate skeletal muscle ~2 via lead 32.
Stimulation generator 304 may also contain muscle
conditioning circuitry, which is not shown for clarity.
U.S. Patent No. 4,411,268 issued to Cox should be consulted
for a more detailed description of skeletal muscle
con,ditioning. Delay logic 302 also provides timing 308 with
a begin sensing signal via line 338. ~his begin sensing
signal is synchronous with the contraction of human heart
100 and delayed from it so that motion artifacts are
minimized during the sensing process.
wOg3/~n4 2 1 2 0 ~ 1 7 PCT/US92/09330
Timing 308 notifies ~roltag~ drivor 306 via lines 3.~
and 336 when to energize infrared LE~ ~12 and red ~ 4,
respectively. Current driver 310, coupled via commcn ~ine
362 to voltage driver 306, maintains t~e illumination G~
each LED to enable photosensor 316 to measure the reflected
return. Infrared LED 312, red LED 314, and photosensor 316
are all }ocated within sens~r 106 and coupled to implantable
pulse generator 36 by lead lG4 as shown. Lines 364, 366,
and 368 comprise the three conductors of lead 104 (see also
Fig. 4).
The sensed return of photosensor 316 is transferred to
current mirror 318 via line 368 for pr.ocessing. After
processing, the resultant is transferred to IR sample and
hold 332 and red sample and hold 330 by line 346. The
signal is gated to the proper sample and hold circuit by
timing 308 using gating signals on lines 340 and 342.
IR/ M division network 328 compares the infrared and
red signals received via lines 344 and 348 to sense color
shifts. The periodic sensor outputs of IRIR division
networX 328 are sent by line 350 to memory 322 for storage
awaiting readout by medical personnel. Each measured signal
is time tagged by the output of real time clock 320 on line
352.
Medical personnel can access the time-tagged sensor
, data stored in memory 322 by telemetry techniques common in
the i-mplantable device field. Preferably this access is via
a radio frequency si~nal prepared by telemetry transmitter~
324 as modulated with data received on line 356 from memory
322. This radio frequency signal is transmitted by radio
frequency antenna 326. The signal is received outside of
the body by antenna 402, demodulated by telemetry receiver
404 and processed and presented to medical personnel by
programmer 400 in the manner ~nown in the art.
An alternative implementation of implantable pulse
generator 36 is through the use of a microprocessor
W093/ ~ 74 PCT/US92/09330
-12-
controlled general purpo~e implantable pulse gener~tor s~ch
as PrometheusSM pu~se genera'~r manufactured by Me~tr~r.io!
B.V. of the Netherlands. $he primary advanta~e of suc~ an
implementation is the ease with whi~h such a ~rogra~3ble
device can change modes of operation. This is part~cularly
usef~l when doing clinical research. A description of the
use of such a device may be~found in the pæper "Pulse
Generator for Biomechanical Cardiac Assistance by C~unter-
Pulsation Technique", by Grandjean, et 21., publihed in the
"Record of the Conference on Skeletal Muscle for Cardiac
Assist and Repair, Banff Sept. 28-Oct. 2, 1988", published
by Futura Editions (August 1989) and in "Transformed
Skeletal Muscle for Cardiac Assist and Repaîr", edited by R.
Chiu and I. Bourgeois, (August 1989).
lS FIG. 6 is a graphical representation 406 of the sensed
signals from a skeletal muscle 22 which is adequately
supported by the vascular system. The amplitude of the
reflected light 408 is relatively sharply peaked within the
region of visible red wavelengths 410. This indication when
read from memory 322 via telemetry indicates that skeletal
muscle 22 was receiving sufficient support for its workload
at the ti~e tag of the sensor reading. A complete series of
such signals stored within memory 322 ~erifies that skeletal
muscle 22 continues to be healthy.
FIG. 7 shows the response 412 of sensor 106 when
skeletal muscle 22 is not ade~uately supported by the
vascular system. As can be seen, the amplitude of reflected
light 414 is shifted to the blue wavelengths 416 and is not
sharply defined. Medical personnel upon seeing this
indication from me~ory 322 will conclude that skeletal
muscle 22 is not receiving sufficient oxygen for its
workload. Continuation of this state indicates a high risk
of ischemia to a portion or all of skeletal muscle 22.
Immediate medical action includes reduction of the
physical load on skeletal muscle 22 by reducing the duty
WOg3/Q~n4 2 1 20 6 1 7 PCT/US92/09330
-13-
cycle of stimulation pulses. Total cessation of stimulati~g
pulses will place skeletal muscle 22 at rest witho~ut any
load. Skeletal muscle 22 may respond to additional
conditioning as taught ~y Cox. In severe cases, surgical
intervention may be required.
FIG. 8 is a plan view of sensor 106A employing a
chronically implantable pressure transducer within sensor
106A. This pressure transducer is preferably of the type -~
disclosed in U.S. Patent No. 4,485,813 issued to Anderson,
et al., herein incorporated by re~erence. The pressure
transducer is piezoelectric. Piezoresistive pressure
sensors are disclosed in U.S. Patent No. 4,407,296 issued to
Anderson and U.S. Patent No. 4,432,372 issued to Monroe,
also incorporated by reference.
lS Pressure sensor 106A has a distal tip lO at the end of
hollow and rigid shank 12. Tines 11 are appended to aid in
attachment. These work particularly well with transvenous
pacing leads. However, different attachment means may be
more appropriate depending upon the exact nature of the
skeIetal muscle used. The pressure capsule 18 is
hermetically sealed. Bore 16 provides fluid communication
with pressure capsule 18. Because pressure~capsule 18 uses
a piezoelectric element, incident forces present produce a
voltage across terminals 420 and 422. This signal is
coupled to implantable pulse generator 36A via conductors S6
and 58 which run the length of lead 104.
FIG. 9 is a block diagram of implantable pulse
generator 36A incorporating circuitry for processing the
output of the pressure transducer. The implantabIe pulse
generator 36A contains two basic portions. The first of
these is primarily a demand pacemaker 110, which is readily
known in the art. Its components include terminal 114,
which couples transvenous lead 34 to sense amplifier 112 via
line llS and also directs artificial pacing pulses from
pulse generator 113 to the myocardial tissue. Sense
wOg3/~n4 PCT/US92/09330
amplifier 112 attempts tO dete~t nat-rally occurrin~
heartbeats. If one is foun~, the artificial pacing pu'se i5
inhibited.
Skeletal muscle 22 is coupled to implantable pulse
generator 36A via terminal 121 which couples to electrical
lead 32 to deliver the electrical stimulation energy. This
stimulation energy is supplied by pulse generator 120. The
signals used to condition skeletal muscle 22 are generated
by conditioning generator 122 and supplied to termina~ 121. -
The generation of such conditioning signals is discussed
more extensively in U.S. Patent No. 4,411,26~, issued to
Cox, which is incorporated herein by r,eference.
Feedback on the conditioning process is sensed by
, pressure sensor 106A and transferred to sensor processing
107 which processes the signal in a manner described below.
This processed sensor signal is transferred via line 108 to
sensor logic 109 which determines the degree of conditioning
yet required using the technique described below. When the
conditioning process is complete, sensor logic 109 notifies
conditioning generator 122 via line 124 to produce the
maintenance signals described below.
Sensor logic 109 also notifies logic 119 via line 125
of the timing of the actual contraction of skeletal muscle
22. This permits logic 119 to properly time the stimulation
, signal to skeletal muscle 22 as explained below.
,Trigger circuit 123 and OR-gate 118 function as
described by Cox to time the generation of the stimulation
pulse to skeletal muscle 22 in relation to the contraction
of human heart 100. A discussion of this timing for the
various embodiments may be found below.
FIG. 10~ shows the stimulation patterns used to perform
the conditioning. Skeletal muscle stimulation is different
from cardiac stimulation in that the skeletal muscle does
not have an all or nothing response to the electrical
stimulus as does the myocardium. The skeletal muscle
WOg3/~4 2 1 2 0 fi ~ 7 PCT/US92/09330
exhibits a gradual recruitment of fibers with increases in
2ulse amplitude and pulse width. Threshold fcr skeleta'
muscle 22 is the pulse amplitude/width needed to st~rt
muscle force recruitment. Pulse 602 i5 the stim~lation
pulse produced by pulse generator 120. It is generated to
occur at the correct time in relation to the contraction of
human heart 100. To be effective in causing contraction of
skeletal muscle 22, pulse 602 must have a voltage greater
than capture threshold 600. Pulses 604, 606, 608, and 610
are conditioning pulses produced by conditioning generator
122. The pulse rate is dependent upon the specific nature
of skeletal muscle 22 as taught by Cox, but it is typically
in a range of 20-30hz. To optimally perform conditioning,
pulses 604, 606, 608, and 610 have a voltage in excess of
capture threshold 600.
FI6. lOB shows the response of unconditioned skeletal
muscle 22 to receipt o~ pulses 602, 604, 606, 608, and 6~0.
Notice that each produces a contractile force 614, 616, 618,
620, and 622, respectively. This occurs with unconditioned
muscles which are known as "fast-twitch" muscles. A more
detailed explanation may be found in the Cox reference.
FIG. lOC~shows the response of pressure sensor 106A to
the contractions of Fig. lOB. These result in pressure
peaks 624, 626, 628, 630, and 632 ! respectively.
FIG. lOD shows the result of differentiation by sensor
processing 107 of the sensor signal of Fig. lOC. This
diiferentiation produces sharp peak pairs 634, 636, 638,
640, and 642, respectively; indicating the inflection
points. From this waveform, a simple analog filter and
detector known to those in the art could easily determine
that skeletal muscle 22 is unconditioned.
F~GS. llA 1~8, llC, and 1lD show the corresponding
waveforms for skeletal muscle 22 after complete
conditioning. When presented with the stimulation pattern
3S of Fig. lOA, the contractile response is shown in Fig. llB
W093~W~4 PCT/US92/09330
~6-
as waveform 646. Notice thal individual conditioning pu1s2s
no longer produce major contractile peaks~ This occurs
because skeletal muscle 22 hzs ~een condit.icned to zct as ?
"slow-twitch~ muscle, similar to myocardial tissue: ~en
S the conditioned response of Fig. llB is, sensed by pressuxe
sensor 106A, the resulting waveform 648 of Fig. llC is
produced. This results in the differentiated waveform of
~ig. llD after processing by sensor processing 107. This
represents but two inflection points as excursions 650 and
652. Again this becomes easily recognizable as a s~eletal
muscle 22 which is fully conditioned.
FIG. llA shows the stimulation pattern used after
skeletal muscle 22 is fully conditioned. Pulse 602 has a
voltage in excess of capture threshold 600. This pulse
15 which is produced by pulse generator 120, stimulates the
contraction of skeletal muscle 22. Conditioning pulses 604,
606, 608, and 610 (see also Fig. lOA) produced by
conditioning generator ~22 have been replaced by maintenance
pulses 603, 605, 607, and 609, respectively. The
maintenance pulses must yet ~ave a voltage greater than
capture threshold 600. However, because of the; smoother
contraction pattern of the conditioned skeletal muscler
pulse width, pulse amplitude, pulse spacing and pulse number
can be safely adjusted to save energ,y. Conditioning
generator 122 switches from conditioning pulses to
maintenance pulses in response to a notification of a
conditioning accomplished signal from sensor logic 109 via
line 124.
FIG. ~2 shows the timing relationship between
stimulation of the myocardium and stimulation of skeletal
mNscle 22 for the various embodiments of Figs. 1, 2, and 3.
For simplicity it is assumed that all myocardial
,contractions are artificially stimulated by pacing pulses
700, 702, 704, and 706 at a fixed rate. These might also be
natural contractions which inhibit the pacing pulse, but the
wO93~n4 PCT/US92/09330
2120617
-17-
rate would then not be constant.
For the embodiment of F` g. 1, it is desired th~t human
heart 100 and skeletal muscle ~ontract simultaneously.
Therefore, stimulating pulses 708, 712, 716, and ,ZO occur
at the same time as pacing pulses 700, 702, 704, and 706,
respectively. Maintenance pulse groups 710, 714, 718, and
722 occur as explained above. The timing for this
embodiment is easily accomplished for paced beats of human
heart 100, since the timing is coincident. For sensed beats
(i.e., the artificial pacing pulses are inhibited),
stimulating pulses 708, 712, 716, and 720 are generated
immediately upon sensing a naturally~ occurring R-wave.
Skeletal muscle 22 is stimulated by pulses 724, 728,
732, and 736 for the embodiment of Fig. 2. These zre
delayed for a period following the corresponding pacing
pulse (or sensed R-wave) sufficient to enable human heart
100 to empty. Contraction of skeletal muscle 22 too soon
will increase the load on human heart 100. A delay which is
too long will cause skeletal muscle 22 to pump less than the
optimal quantity of blood. The exact delay is easily
measure by pressure sensor 106A as explained above-. The
delay-may be made a function of rate, stroke volume, etc.
It may be determined empirically by medical personnel or
simply programmed to the nominal values known in the art.
- Stimulation pulses 740, 744, and 748 cause skeletal
muscle 22 to counterpulse the descending aorta. This
increases the total perfusion through the coronary system,
thereby assisting human heart 100. These pulses are timed
to occur approximately one-half heart cycle after
contraction of human heart 100.
~IG. 13 is a block diagram of implantable pulse
generator 36B having circuitry for processing the output of
activity sensor 105. It includes a pacing generator 754
which operates in the demand mode as is known in the art.
Basically, the electrical activity of the patient's heart is
WOg3~ 4 PCT/US92/o9330
-18-
monitored via pacing lead 34. Whenev~r a naturall~
occurring contraction of the hea t is f~und, sense a~pli~ier
756 detects it and notifies pacin~ ge~le~~tor 754. I~ this
naturally occurring contraction is ~ensed within th~
allotted time, the output of pacing genarator 754 is
inhibited. However, if pacing generator 754 determines that
sufficient time has elapse~ since the last c~ntraction of
the heart, it produces a pulse which is conveyed to the
heart via pacing lead 34 to artificially stimulate the
desired contraction.
The main purpose of stimulation generator 766 is to
produce a burst of pulses to cause contraction of skeletal
muscle 22 in the proper timing relation to the contraction
of the patient's heart. To do so, OR-gate 760 produces an
output whenever sense amplifier 756 senses a naturally
occurring contraction or pacing generator 754 supplies an
artificial pacing pulse. In either situation, timing logic
762 is started to generate the desired amount of delay.
This delay is nearly zero for the embodiment of Fig. 1,
because maximum assistance to the myocardium is provided
when skeletal muscle 22 contracts at the same time as the ~-
heart.
The embodiment of Fig. 2 requires a much longer delay.
This period is on the order of one-half of the cardiac cycle
(i.e. R-to-R interval). The embodiment of Fig. 3 requires
yet a slightly longer delay, being somewhat greater than
one-half of the cardiac cycle. This is necessary because
this embodiment is intended to increase diastolic pressure
in the aorta.
The output of timing logic 762 is a pulse which is
synchronous with the naturally sensed or artificially
stimulated contraction of the patient's heart but delayed in -~
time according to the specific embodiment 25 described
abovs. This puise is supplied to duty cycle timing circuit
764. This circuit is simply a variable digital counter -
wOg3/~n4 PCT/US92/09330
2120617
which produces an output corresponding to a ~ariable number
of pulses received from timing logic 76~. The nol~a' output
of duty cycle timing circuit 764 is one pulse for,each pulse
received from timing logic 762. This ~orrespond~ to the
S one-for-one sti~ulation mode cf skelet l muscle 22. A
lesser ratio of output pulses to input pulses is determined
by overall cardiac rate and anti~ipated cardiac demand.
Overall cardiac rate is determined by integrator 758.
It is A circuit which receives inputs from both sense
amplifier 756 and pacing generator 7S4, much as with OR-gate
760. In this way integrator 758 is notified of both
naturally occurring and artificially paced contractions of
the patient's heart. Integrator 7S8 simply integrates these
two signals to produce an average current heart rate. This -
signal is sent to duty cycle timer c~rcuit 764 to adjust the
variable rate counter in a manner which i5 described in more
detail below.
The anticipated cardiac demand may be deter,mined in a
number of ways known in the art of cardiac pacing. These
include, without limitation, measurement of venous blood
oxygen level, ~easurement of blood ph, determination of
respiratory rate, computation of minute volume, and
méasure ent of~stroke volume. The preferred mode of the ,
present invention uses an activity sensor such as found in ,~-
Medtronic ActivitraxR pacemakers. Those of skill in the art
will'readily be able to substitute yet other sensors to
determine anticipated cardiac demand.
In the preferred embodiment, an activity sensor 105 is
mounted permanently to the housing of implantable pulse
generator 36B. This activity sensor is preferably a piezo-
electric crystal which converts mechanical energy received
at the housing of implantable pulse generator 368 to
electrical energy. It has been shown in the literature that
activity sensing in this way is a very good means for
anticipating cardiac demand. The output of activity sensor
W093/~4 PCT/US92/09330
,~ ,
-20-
105 is amplified and integrated by signal processing circui.
752. The result i~ a signal indicative cf anticipat~d
cardiac demand which is transferred to duty cycle timing
circuit 764.
The output of duty cycle timing circuit 764 is a pulse
train which is a variable number of counts of the output of
timing logic 762. The exact relationship is described in
more detail below. Stimulation generator 766 receives the
output of duty cycle timing circuit 764 and generates an
I output burst of energy corresponding to each of the output
pulses of duty cycle timing circuit ~64. The number of
pulses in this burst is determined in part by the output of
signal processor 752 such that additional pulses are added
to the burst when the anticipated cardiac demand becomes
high. -
Conditioning generator 768 supplies conditioning pulses
as needed. The stimulation pulses of stimulation generator
766 are combined with the conditioning pulses of
conditioning generator 768 and supplied to skeletal muscle
0 22 by stimulation lead 32. -
FTG. ~ is a graphical representation of a relationship
between the pu}se amplitude and the anticipated cardiac
dem~nd~ In this case anticipated cardiac demand corresponds ~-
to the ~ppropriate cardiac rate which is determined from the
!5 output of activity sénsor 105. This is computed in the
manner known in the art from U.S. Patent No. 5,479,402
issued to Anderson, et al. As can be see, points 502 and
504 correspond to very low and low anticipated cardiac
demand, respectively. These are on the order of less than
70 beats per minute. At these rates, stimulation generator
766 supplies output pulses of mini~u~ amplitude. These
pu}ses must be greater than the stimulation threshold of
skeletal muscle 22. However, considerable energy is saved
through using an amplitude which is only slightly greater
than this threshold.
W093/~4 PCT/US92/093~
~ 2120617
--2 .L--
Points 506 and 508 correspond to aver~ge and high
anticipated cardiac demand, res~ective].y. These corresponQ
to rates in the range of 70 to 1~0 bea~s per minute althouah
the exact values are patient dependent. At this ~emand
level, the cardiac loading i5 sufficient to benefit from the
additional amplitude and therefore addi~ional assurance o~
capture. Point 510 is above 120 pulses per minute for most
patients. Again notice that this is the anticipated cardiac
demand and not the actual heart rate.
0 FIG. lS is a graphical representation of the number of
pulses in a given stimulation burst as a function of
anticipated cardiac demand. The ranges along the abscissa
are as explained above for most patients. Average and high
anticipated cardiac demand again requixe the greatest number
.5 of pulses per burst and t~herefore the highest energy demand.
Thé number of pulses per burst is decreased at ~ery high
anticipated demands because efficiency is impaired if the
individual pulses occur too frequently.
FIG. 16 is a graphical representation of the
synchronization ratio performed ~y the variable counter of
duty cycle timing circuit 764. A one-to-one synchronization
ratio is used for average anticipated cardiac demand. This
provides the greatest chronic assistance to the myocardium
with thé least battery consumption by implantable pulse
~ generator 36B. The synchronization ratio is greater for
less than average anticipated cardiac demand because less
assistance is actually required. The synchronization ratio
increases as the anticipated cardiac demand increases to -~
ensure ~he fatigue of skeletal muscle 22 is minimized.
FIG. 17 is a graphical representation of actual cardiac
rates on the a~scissa in relation to actual rates of
sti~ulation of skeletal muscle 22 along the ordinate. Shown
is the change in duty cycle with actual rate. The duty
cycle is one-for-one in the typical patient in the range of
50 to 100 ~eats per minute. At point 572, the actual
WO g3/08874 PC~/USg2/09330
!
-2?.-
cardiac rate is 1oo beats per minute and th~ rate ~f
stimulation of skeletal mus~l e ~2 i s lO0 beats per minute.
Above that rate, skeletal muscle ~2 i5 ~timulated ~nly onc--
for every two cardiac cy~les . At point 5~0 ( 140 beats pë~
minute), the duty cycle ~ecomes one stimula~ion of keletal
muscle 22 for every three cardiac cycles.
FIG. 18 is a plan view of lea~ 104 with temper~ture
sensor 106B attached to the distal end. The outer coveriny
of lead 104 is outer sheath 156 which is of a chronica~
implantable biocompatible material such as medical grade
silicone rubber or polyurethane. The proximal end of leaa -~
104 contains bifurcated connector 158 which sealingly
inserts into implantable pulse generator 36C. Sealins ring
pairs 160 and 162 complete the seal against the ingress of
i bodily fluids. Lead 104 contains two electrically separated
conductors which couple the thermistor of temperature sensor
1068 with implantable pulse generator 36C. These two
conductors are electrically coupled to te D inal pins 164 and
166.
3 The distal end of lead 104 contains temperature sensor
106B. It is a commonly available thermoresistive device
which is housed within rigid housing 154. Preferably rigid -~
housing 1~4 is a ti~anium cylinder which is insulated inside
and outside with medical grade silicone rubber. The two
terminals o the thermistor within rigid housing 154 are
coupled to the two conductors within the body of lead 104.
The thermistor is thermally coupled to distal tip 150 of
temperature sensor 106B which is preferably comprised of a
biocompatible material such as titanium. Distal tip 150 is
~0 not insulated to promote heat conduction to the thermistor
of temperature sensor 106B and therefore must be of a
biocompatible material. Tine structures 152 assist in the
chronic attachment of temperature sensor 106B within
sXeletal muscle 22.
FIG. 19 is a block diagram of the circuitry of
W093/~4 PCT/US92/093~
` 2120617
-23-
implantable pulse generator 36~, wniah processes ~he cutput
of temperature sensor 106B. P2~ 9 lead 34 electrically
couples implanta~le pulse generator 36C to human hear~ 10
2S is shown in Fig. 1. Pacing s-nerator 800 supplies
S artificial pacing pulses whenever it aetermines that a
naturally occurring pacing event has not transpired at the
correct time. Sensing for such a naturally occl~rring pacin~
event is facilitated by sense amplifier 802 which is coupled
to pacing lead 34 via line 814. The amplified signal is
.0 sent to pacing generator 800 by line 816.
The amplified naturally occurring pacing signal is also
sent to OR-gate 808 by line 818. OR-gate 808 also receives
an indication of an artificial pacing signal via line 820.
In either event the output of OR-gate 808 on line 824
L5 indicates the time at which a contraction of human heart 100 ~-~
has been stimulated, whether naturally or artificially.
Timing logic 806 provides a signal via line 826 to
notify stimulation generator 812 to produce a pulse to
stimulate contraction of skeletal muscle 22. This signal
occurs at a predétermined delay after t~e contraction of `~
human heart 100. ~he exact amcr.nt of this delay is based
upon ~wo factors. ,The first of these is the configuration
of the cardiac assist system. As explained above~, this
delay is necessary to provide the contraction of skeletal
muscle 22 at the proper time relative to human heart 100.
This delay is very short for the configuration of Fig. 1 and
is quite substantial for the,configuration of Fig. 3. The
second factor is an adjustment provided by signal processor
804 via line 822. This factor is explained in detail below.
Conditioning generator 810 provides the pulses used to
condition skeletal muscle 22 as a "slow twitch" muscle as
taught by Cox. These pulses are transferred to skeletal
musclç 22 by line 828 and lead 32, along with the
stimulation pulses of stimulation generator 812. After
skeletal muscle 22 has been fully conditioned as taught by
WOg3/ ~ 74 PCT/US92/09330
-~4-
Cox, the conditioning pulses may be replaced by maintenance
pulses which diff_r from conditioning pulses by their iower
amplitude and hence lower power requirements. The change to
maintenance pulses is triggered ~y signal processor 804 vi~
line 830 under the conditions ~s ~iscussed ~elow.
Signal processor 804 is coupled to temperature sensor
106B by the two conductors of lead 104 as explained above.
Signal processor 804 uses circuitry known in the art to
measure the resistance of the thermistor of temperature
sensor 106B, and therefore, the temperature of skeletal
muscle 22. Based upon the temperature sensed, signals are
sent via lines 822 and 830 to vary the delay of the
stimulation pulses and change to maintenance pulses, `~
respectively.
FIG. 20 is a graphical representation 900 of a single
cycle containing a pacing pulse 902 occurring at time tl and
the corresponding pulses transferred to skeletal muscle 22.
Stimulation pulse 904 is that pulse which i~ intended to
cause the primary contraction of skeletal muscle 22. It
occurs at time t2 following a delay 908. As explained
above, delay 908 is a part determined by the configuration
of the cardiac assist system and in part ~y signal processor
804. Following stimulation puls~ 904,
conditioning/maintenance pulses 906 are generated at times
2S t3a, t3b, t3c, and t3d. These pulses are produced by
conditioning generator 810 in accordance with the teaching
of Cox.
PIG. 21 is a graphical representation of the force of
contraction of skeletal muscle 22 for one unconditioned -;
cycle -918 and one conditioned cycle 910. The force curve
for the conditioned cycle 910 is smooth and continuous and
is representative of a slow twitch muscle. The force curve
for the unconditioned cycle 918 is discontinuous and is
characteristic of a fast twitch muscle. Force peaks 912,
914, 916, and 920 are secondary contractions corresponding
WOg3/08874 2 1 2 0 6 1 7 PCT/US92~09330
-25-
to the conditioning pulses, occurring at times t3a, t3b,
t3c, and t3d, respectively. These specific curves show
ideal responses. Actual measurement of these specific
curves using a thermistor would probably be very difficul~.
FIG. 22 is a graphical representation of the
temperature curves measured by temperature sensor 106~ unde-
three different situations. Temperature curve 919
corresponds to the ideal situation of a properly timed -
contraction of a fully conditioned skeletal muscle 22. The
two key characteristics of this curve are its smooth and
continuous nature and the relatively low peak temperature at
peak 922.
Temperature curve 921, on the other hand, although
smooth and con~inuous, is delayed somewhat and reaches a
much higher temperature peak 923. $his higher temperature
peak is readily sensed by signal processor 804 as an
improperly timed stimulation pulse. The higher temperature
results from the much larger component of isometric and much
smaller component of isotonic activity associated with the
- improperly timed contraction. Upon sensing this elevated
te~perature peak 923, signal processor 804 notifies timing
logic 806 via line 822 to shorten delay 908 (see also Figs.
19 and 20).
,
Temperature curve 924 is characteristic of an
2S unconditioned s~eletal muscle 22. This temperature curve
924 has a number of relative temperature peaks at 926, 928,
930, and 932. These re}ative temperature peaks correspond
to fast twitch response to the conditioning pulses. Because
the skeletal muscle 22 of temperature curve 924 is
unconditioned, signal processor 804 must so notify
conditioning generator 810 via line 830.
FIG. 23 is a graphical representation of the
differentiated temperature curves 919 and 924 wherein curve
934 corresponds to curve 919 and curve 938 corresponds to
curve 924. Through the use of the differentiated
W093/W~4 PCT/USg2/093
temperature curves, sign-~l processor ~ uc~ mor~
readily distinguish between conditioned ~nd unconditioned
skeletal muscle 22. Because curve 934 represents fuliy
conditioned skeletal muscle ~2, it has a smooth and
S continuous temperature cur-ve as explained a~ove, and ~he
corresponding differentiated curve has a single ~ero
crossing at point 936. Differential c~rve 938, Gn the other
hand, has zero crossings at ~0, 944, 948, and ~52. This is
easily detected by signal processor 804 either ~igitally or
by frequency discrimination using well ~nown techniques.
The foregoing muscle control and monitoring methods and
systems can also be used in various applications beyond
monitoring skeletal muscles. Exemplary applications include
stimulating and training particular muscles to regain
control of their deficient functions. For instance, the
foregoing methods can be used to stimulate the diaphragms,
and the upper and lower limb muscles. Other applications
include assisting deficient organs, such in cardiomyoplasty
or cardiac assist applications, and neo-sphincter
applications where a transferred muscle is stimulated to
augment or replace the sphincter function in incontinent
patients.
Having thus des~:ribed the preferred embodiments of the
present invention, t;hose of sXill in the art will be able to -~
readily apply these teachings to other embodiments without
deviating from the scope of the claims hereto attached.
I CLAIM: