Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2121969
PRE-TREATMENT OF SULFITE PULP BEFORE
RT-T~'~G~TNG WITH OkY~--. CONTAINING CHEMICALS
FIELD OF THE INVENTION
This invention relates to the pre-treatment of
sulfite pulp. More particularly, this invention relates
to a novel process for caustic extraction of unbleached
sulfite pulp to reduce metallic impurities in the pulp.
BACKGROUND OF THE INVENTION
Although wood is primarily composed of organic
materials, it always contains some inorganic metallic
impurities, which adversely affect the bleachability and
bleach chemical consumption of the chemical pulp produced
from wood. This is particularly important where hydrogen
peroxide, oxygen or ozone is used as the bleaching chemi-
cal. Therefore, for effective and economic bleaching of
chemical pulp, it is sometimes necessary to remove the
metallic impurities from the pulp. This is normally
achieved by chelation and chelating compounds such as EDTA
or DTPA, which tie up with the metals and thereby remove
them from the pulp.
Even with the assistance of chelating compounds,
the removal of metallic impurities from pulp is sometimes
difficult. In fact, the pulp has to be treated with the
right amount of a chelating compound at an optimum pH and
temperature, and then thoroughly washed with water in order
to remove the metals effectively. An effective chelation
and peroxide stage combination to produce high brightness
pulp has been described in the literature as the Lignox
process.
A number of patents have been granted over the
years disclosing various proposals for treating unbleached
sulfite pulp. C~n~;an Patent No. 996,312, issued
September 7, 1976, ITT Industries, Inc. discloses a process
9~q
_ - 2
for producing refined and bleached wood pulp which com-
prises digesting wood in a sodium-base acid sulfite diges-
tion liquor to produce a completely digested, unwashed,
unbleached sodium-base acid sulfite pulp. The unwashed,
unbleached acid sulfite pulp is separated from the result-
ing spent sulfite digestion liquor. Without subjecting the
pulp to a water wash, the unwashed, unbleached acid sulfite
pulp is treated in a hot caustic extraction refining step
with sodium hydroxide in an amount of about 3-35~ (based on
the weight of oven dried pulp) at a temperature of about
40-150C for a period of about 15-90 minutes. This pro-
duces an unwashed, unbleached, chemically refined pulp.
The chemically refined pulp is separated from the hot
caustic extraction effluent resulting from said wash. The
refined pulp is then bleached. This patent does not dis-
close washed, unbleached pulp. The process focuses on the
recovery of caustic soda for a caustic soda basis process.
The process, being a caustic soda based process, does not
contemplate or relate to ammonia based processes. An
ammonia based process and a caustic soda based process are
re~l~n~nt relative to one another. This patent does not
disclose that caustic extraction can unexpectedly reduce
metal content in the washed pulp.
SUMMARY OF THE INVENTION
The invention is directed to a process of reduc-
ing metallic impurities in unbleached sulfite pulp which
comprises subjecting the pulp to caustic extraction. In
the process, the caustic extraction can be conducted under
pressurized extraction at a temperature between about 105C
to 145C and at above atmospheric pressure. The caustic
extraction can be conducted with caustic soda between 4 to
10~ wt. concentration. The caustic extraction of the
unbleached sulfite pulp can be followed by an oxygen
containing bleaching stage. The oxygen containing bleach-
ing stage can be conducted with hydrogen peroxide, oxygen
~.
2121~69
_ - 3 -
or ozone, individually, or in any combination. The pulp
can be washed unbleached pulp.
In the process, the caustic extraction of the
unbleached sulfite pulp can also be followed by an acidic
wash stage. The acid washed pulp can then be bleached by
an oxygen containing bleaching agent. The oxygen contain-
ing bleaching agent can be hydrogen peroxide, oxygen or
ozone, individually, or in any combination.
In the process, the caustic extraction of the
unbleached sulfite pulp can be followed by a chelation
stage. The resulting pulp can then be bleached by an
oxygen containing bleaching agent like hydrogen peroxide,
oxygen or ozone, individually, or in any combination.
DRAWINGS
In drawings which illustrate specific embodiments
of the invention, but which should not be construed as
restricting the spirit or scope of the invention in any
way:
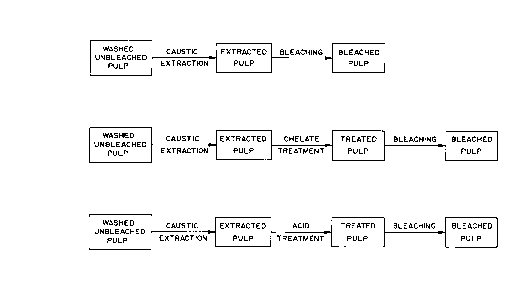
Figure la illustrates a schemtaic flow sheet of
a first alternative method of practicing the process of the
invention.
Figure lb illustrates a schematic flow sheet of
a second alternative method of practicing the process of
the invention.
Figure lc illustrates a schematic flow sheet of
a third alternative method of practicing the process of the
invention.
2121969
_ -- 4
DETAILED DESCRIPTION OF SPECIFIC EMBODIMENTS
OF THE INVENTION
Although wood is primarily composed of organic
materials, it always contains some inorganic metallic
impurities coming primarily from the soil where the tree
has grown. When wood is chemically pulped, it still
retains some of these metals in the unbleached pulp. Other
metals may also come from the cooking chemicals, water or
from corrosion of storage tanks or equipment. The pres-
ence of these metallic impurities, such as manganese, iron
and copper, particularly manganese, disadvantageously
affect the bleachability and bleach consumption of the
pulp, where oxygen containing bleaching chemicals such as
oxygen, ozone or hydrogen peroxide are used.
It has been argued that acid sulfite pulp should
not have metallic impurities, because the acid used for
pulping removes these metals during the cooking process.
However, unbleached acid sulfite pulp may contain suffi-
cient amounts of metals to substantially affect its bleach-
ing by oxygen containing bleaching agents. We have found
that the manganese content of acid sulfite pulp of pulp
mills located on the westcoast region of Canada can vary
between 20 to 40 ppm, where hemlock is used as the raw
material.
Both cellulose and lignin have been known to be
very good absorbents of heavy metals. Although metals may
be dissolved out in the cooking liquor during the pulping
process, the liquor associated with the blown pulp, due to
recycling of process water in the washing and screening
plant, can enrich the metal concentration and redeposit
these metals on the fibres. We have found that the ray
cells removed by side hill screens in the screening plant,
with their large surface to volume ratios, contain more
metals than the bulk of the pulp. This again shows the
q (D q
_- - 5
importance of the absorption phenomenon in binding the
metals. From a number of measurements carried out over
several days, we have noted an average manganese content of
49.3 ppm in the side hill screen rejects as compared with
26.5 ppm manganese in the washed and screened pulp.
It is necessary to remove these metallic impur-
ities from the unbleached pulp, whether made by kraft or
sulfite process, to effectively and economically bleach the
pulp by oxygen containing bleaching agents. Conventional-
ly, this can be achieved by acid washing which can desorb
the metals from the pulp, or by application of chelating
agents which tie up the metals and thereby remove them from
the pulp. The pulp must be treated with the right amount
of a chelating compound at an optimum pH and temperature
and then be thoroughly washed with water in order to remove
the metals effectively.
A large number of studies have been conducted on
the pretreatment of kraft pulps and the use of acidic wash
and chelates like EDTA or DTPA to reduce the metal contami-
nation of the unbleached pulp. It has been found that
either a low pH acid wash or a pH-optimized chelate treat-
ment will generally remove the metals adequately from a
kraft pulp. However, the manganese content could not be
completely removed by such methods. In one such example
(Tappi, 76, No. 8, page 194) superchelation by three
successive treatments of 10~ wt. DTPA only reduced the
manganese content of the kraft pulp to 3 ppm.
We have developed a pulp treating sequence
starting with a hot caustic extraction of washed unbleached
sulfite pulp, which is obtained from an ammonia-base acid
sulfite cooking process, and then bleaching the resultant
pulp with oxygen or alkali oxygen bleaching as the next
stage. We have discovered surprisingly that hot caustic
extraction provides good removal of metallic impurities
q~q
_~ - 6
from the unbleached pulp. This enhances the efficiency of
the following bleaching sequence and reduces the costs of
a chelation stage, if one is to be used.
Figure la illustrates a first way of practicing
the invention. Washed, unbleached pulp is subjected to
caustic extraction to yield extracted pulp. The extracted
pulp is subjected to oxygen bleaching to yield bleached
pulp. The bleaching agent is an oxygen bleaching agent
such as hydrogen peroxide, oxygen or ozone, individually,
or in any combination.
Figure lb illustrates a second alternative method
of practicing the invention. Washed, unbleached pulp is
subjected to caustic extraction to yield extracted pulp.
The extracted pulp is subjected to chelate treatment to
yield treated pulp which is then bleached to yield bleached
pulp.
Figure lc illustrates a third alternative method
of practicing the invention. Washed, unbleached pulp is
subjected to caustic extraction. The extracted pulp is
then given an acid treatment. The acid treated pulp is
then bleached to yield bleached pulp.
Unbleached pulp was obtained from the Port Alice
mill of Western Pulp Inc. Unlike kraft pulp, acid wash by
itself is not as effective in lowering metal content as the
chelate treatment. In the case of the pulp we tested, a
sulfuric acid wash removed 31% of manganese at pH 2.5, 60%
at pH 2 and 70% at pH 1.7. Even with 70% removal, the
manganese content was still 9.4 ppm. Therefore, acidic
wash as such is not satisfactory for sulfite pulp for
further bleaching by oxygen containing bleaching agents.
However, with sulfite pulp, we have found that a 0.2% wt.
application of the chelate DTPA at a neutral (pH 7) can
2121969
- 7
reduce the manganese content of pulp to 1 ppm or less. The
results of these tests are tabulated in Table 1 below.
TABLE 1
EFFECT OF CHELATE TREATMENT AND ACID WASH
Ot~ UNBLEACHED SU~FITE PUU AT DIFFERENT pH
~pl~Y -~ r~aooen~ ~$~
16.1 DTPA 02 2.0 9.8
.5 DTPA 0~ 7.0 0.25
33.5 DTPA 0.04 7.0 112
33.5 H2SO4 1.1 2.5 232
33.5 H2SO4 42 1 20 13.5
33.5 H2SO4 10.8 1-7 94
Specifically, in the course of our studies, we
have unexpectedly discovered that a caustic extraction of
the washed sulfite pulp can reduce the metal content
substantially. When hot caustic extraction was carried out
with 6% wt. caustic soda at 122C and a corresponding
elevated pressure for 40 minutes, we found that the manga-
nese content dropped to less than half of the originalvalue while the K.No. dropped by about 25%. Pulp metal
levels dropped with its lignin content.
To prove that lignin is an important factor in
binding of metals to pulp, we carried out complete deligni-
fication of the pulp by acid chlorite treatment. We found
that with complete delignification, the pulp K.No. was less
than 0.5 and the manganese content was reduced from 38 ppm
to 1 ppm or less. These results, compared with controls,
are shown in Table 2 below.
2121969
TABLE 2
REMOVAL OF MANGANESE FROM UNBLEACHED SULFITE PULP BY 100% DELIGNIFICATION
Two Sta~e Delignification by~e Chlorite l lo'oce"u'~e Method
Star~ng Mat~rial - Unbleached Sulfite Pulp wffll 33 5 ppm Manganes~
Chlorite 1 2 3 4
Treatments100% Delig~') Control~100~C Delig(') Control~
Time, min 90+60 90+60 90+60 90+60
Tel"pe,~ture, C 70 70 70 70
Pulp, o.d.g. 20 20 50 50
NaClO2g 100+9 0+0 250+22.5 0+0
Glacialaceticacid,mL 100+6 100+6 250+15 250+15
Dis~lled water, mL800+800 800+800 2000+2000 2000+2000
Fmal pH - Stage 1 3.4 1.7 4.0 2.5
Fmal pH - Stage 2 3.1 2.6 3.4 2.9
K.No. (% reduction)0.5 (9n18.4 (2) 0.3 (98) 17.7 (6)
Mn, ppm (~6 removal) 1.0 (9n 26.0 (22) 0-9 (9n 24.0 (28)
1: Chlorite tréatrnent was done in ~o sta~es wWl washin~ bet~en sta~es
2: Two - stage control w;~out any sodium chlorite added
We have also discovered that after a caustic
extraction stage, it is easier to remove the metals from
the resulting pulp by both chelate treatment and acid wash.
Chelating compounds are expensive and this invention
provides a way of saving the cost of chelants. An acidic
wash at a pH of 2 reduced the man~anese to 1.7 ppm, so a
simple acidic wash instead of a chelate treatment may be
sufficient in many cases. Table 3, which is a composite of
three sequences of tests, tabulates the data obtained from
conducting these tests, compared to a blank (control).
al~l9~q
- - - 9
TABLE 3
DTPA DOSAGE OPTIMIZATION FOR INITIAL AND POST E-STAGE CHELATION
r- Vc-~-, -U~chec ~ W~5 ~- V,~
B 2~ F~ ~ C ~ e
(i)(ii)
Dosage('), % -- 0.20.04 2.8
Temperature, C 20 20 20 30
10 Initial pH . 5.5 . 2.0
- Mn, ppm 33.40.2511.2 15.4
Alkaline Purification S-age: 12.5% Consi~,tency, 12'`C, 40 mil, 6.0% NaOH, 0.1% Antarox
Final pH -- -- -- -- 9 9
15 25mLKno. -- -- -- -- 12.6
Intrinsic Viscosity - ---- -- 13.3
- Mn, ppm -- -- -- -- 15.9
T or Acid Sta~e: 2% Consistenc~, 30 min
(i)(ii) (iii) (iv)
DTPA, % -- -- -- -- 0.040.04 0.02
HCI, % -- -- -- -- -- -- -- 2.6
Temperaturel C -- -- -- - 20 20 20 30
Inital pH -- -- -- -- 7.0 8.0 7.0 2.0
- Mn, ppm -- -- -- --0.81 1.2 9.2 1.6
25 Mn removal, % 0 99 67 54 98 96 73 95
The first sequence of four tests shown in Table
3 shows that the chelate DTPA per-forms best at the neutral
pH of 7 and that a 0.2~ wt. addition of DTPA to the pulp
reduces the Mn content to less than 1 ppm. The second
sequence in Table 3 shows that the Mn content is reduced to
less than half compared to the blank by caustic extraction
under the conditions shown in Table 3. The third four test
sequence in Table 3 shows that after caustic extraction the
Mn content can be reduced to 1.6 ppm by 2.6~ wt. HCl and as
low as 0.81 ppm by 0.04~ wt. DTPA.
~ l~l9 ~9
- 10 -
In certain situations, it is possible that a
caustic extracted pulp, even without further chelate
treatment or acid wash, can be bleached fairly satisfactor-
ily by oxygen containing bleaching chemicals in spite of
the presence of some minor amounts of residual metals.
As will be apparent to those skilled in the art
in the light of the foregoing disclosure, many alterations
and modifications are possible in the practice of this
invention without departing from the spirit or scope
thereof. Accordingly, the scope of the invention is to be
construed in accordance with the substance defined by the
following claims.