Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
W 0 94/08239 ~ e ;~ ~1 5 8 j PCT/GB93/02047
DETECTION OF ANTIGENS AND NUCLEIC ACIDS.
The present invention relates to a method for isolating and/or
identifying foreign antigens or nucleic acids associated with
leucocytes in fluids derived from a human or animal body, and to kits
for carrying this out. More particularly to such a method and kits as
specifically adapted to be capable of use 'in the field' in so far as
an electric power supply means is not essential to their operation.
The method and apparatus of the present invention enable antigens to
be derived from a human or animal body and identified without the need
for complex laboratory bound equipment. Such method has application,
inter alia, in field testing for infection of humans and animals with
various infective agents having known antigenic or nucleic acid
characteristics. Such testing may be carried out by a physician or
veterinarian in their surgery rather than in a laboratory.
In blood processing and therapeutics arts there is a requirement to
remove leucocytes from blood in or~er to render it suitable for
transfusions or for the purpose of ~reating those with conditions
where leucocytes are in excess. such as leukemia. There exists a
large and competitive market in leucocyte 'depletion' or 'removal'
filters that are capable of selective removal of leucocytes from blood
for these purposes. A great number of sources of such filters will be
known to those in the blood product and Sherapeutic arts.
The processing and therapeutic benefits of using these filters are
numerous, but include, inter alia, reduction in platelet
refractoriness, prevention af transmission of viruses such as
cytomegalovirus, prevention of non-haemolytic febrile transfusion
reactions and reduction of transfusion associated thrombocytopenia.
Direct use to filter a patients blood is helpful in allowing more
aggressive anticancer therapy and application to donor tissue fluids
and perfusates extends organ preservation time for transplantation.
W O 94/08239 PCT/GB93/02047
It is known that leucocytes may be analysed to determine the identity
of antigens which they have immobilised in the course of their
physiological function. Fenton et al (19~1) described an antigen
detection test for diagnosis of Border disease in sheep using
leucocytes as a source of viral antigen while Mignon et al (1991)
described a similar test for cattle using a monoclonal antibody for
capture and signalling of presence of antigen. Such methods have
focused upon analysis of leucocyte 'buffy coat' derived by methods
using flash lysis and centrifugstion, buffy coat separation or
gradient centrifugation either alone or in combination. It will be
immediately recognised that these ethods require laboratory equipment
that is not available in a surgery or on a farm.
Where one is assaying blood samples for organisms which produce low
amounts of antigen it has proved very difficult to attain the
sensitivity required to avoid false negative results. For example,
diagnostics testing for Bovine Viral Diarrhoea (BVD) infection has
been made difficult by the conservative nature of the virus, which is
efficient in replication, and although growing in high titre, rarely
produces sufficient antigen to be detected by conventional systems.
Serum is the natural first choice as a sample, being readily
accessible with the virus being potentially easily isolated from it.
However the large variation in titre of the viraemia makes serum a
potentially unreliable vector for antigen detection.
The present inventors have now provided a novel application for
leucocyte retention filters in the form of a method and apparatus for
isolating and/or identifying foreign antigens and/or nucleic acids
associated with leucocytes in fluids derived from a human or animal
body. The method and apparatus provides a simple to obtain yet rapid
determination of foreign antigens and/or nucleic acids associated with
the leucocytes, and thus of the likely presence of organisms of which
these antigens are characteristic. One significant advantage of this
method is that it offers the preparation of a sample outside the
W O 94/08239 PCT/GB93/02047
laboratory~using a few reagents, a syringe and a filter. Using this
method the inventors have assembled a pen-side test that can detect
animals persistently infected with BVD virus that t~kes only 1.5
hours, compared to the 7 days required for a virus isolation test.
The present invention provides a method for identifying foreign
antigens and/or nucleic acids associated with leucocytes in fluids
derived from a human or animal body. and/or isolating them in cell
free form, comprising
(a) passing a sample of the fluid through a filter material having the
ability to retain leucocytes thereon,
(b) treating the filter from (a) with an agent capable of acting upon
leucocytes to provide foreign antigens and/or nucleic acids therefrom
in elutable form,
(c) eluting antigens and/or nucleic acids from the filter,
(d) using the eluant as a source of isolated cell free foreign
antigens and/or nucleic acids for performance of identification and/or
further isolation steps.
The step (d) using eluant as foreign antigen and/or nucleic acid
source may be carried out using standard methods well known to those
skilled in the art, as will be exemplified further below.
Step (a) is conveniently carried out using any of the commercially
available leucocyte retaining filter materials or filter units. These
are normally comprised of many layers and trap leucocytes by both size
exclusion and adsorption with those capable of retaining greater than
99.999Z of leucocytes being readily available. Suitable examples are
available from Bellhouse Biosciences Ltd (Abingdon-UK), Biotest (UK)
Ltd (Shirley, W. Midl~nds (LK)), Kimal Scientific Products Ltd
~,
.
W O 94/08239 PCT/GB93/02047
~ ~ 2 .~
(UxbridgeoUK) and Pall Biomedical Ltd (Portsmouth-UK). Some of these
are capable of retaining platelets as well as leucocytes but this
should not affect the method, particularly the identification step.
The fluid to be filtered may be any bodily fluid or its derivative.
As absolute filtration is not necessary it is possible to use single
layer filter material or filters, eg. such as those sold by Pall
Process Filtration Ltd under the trademark 'Leukosorb L4'. This
material is available in sheet form but is particularly conveniently
available as 25mm disks in encapsulated form with a syringe-end
fitting. The use of such encapsulated form allows facile use of the ~-
filter with a Leur syringe whereby use outside a laboratory becomes
rapid and convenient.
Where the fluid to be filtered is whole blood an anti-clotting agent
is added in an effective amount prior to subjecting the mixture to the
filtration step. This agent is conveniently that such as EDTA,
Na-citrate or heparin, but may be any of the other well known
anti-clotting aFents that will not significantly damage leucocytes.
Step (b) is carried out using any agent capable of acting upon whole
leucocytes to provide the foreign antigens and/or nucleic acids in an
elutable form suitable for the intended end use and is conveniently
carried out after the filter has been rinsed through with a rinse
liquid, eg. water, saline or buffer, eg. phosphate buffered saline.
The agent is preferably a detergent and most preferably is a detergent
which is capable of providing the foreign antigens associated with the
leucocytes in a form whereby they are provided on the surface of
micelles in the eluant.
Any detergent capable of liberating the required elutable forms from
the leucocytes, and preferably acting upon the cell membranes to
solubilise them, may be used but preferably non-ionic detersents
will be selected. Where detergents are used which are known to
:
-:
W O 94/08239 ~ 2 ~ !J ~ ~ PCT/GB93/02047
interfere with step (d) they may be removed prior to such step using
adsorbents such as Bio-beads (available from Bio-Rad). A suitable
range of detergents are available from suppliers such as Sigma and
include the glucopyranoside range of non-ionic detergents and Nonidet
P-40. Use of an optimsl micelle forming amount of Nonidet takes about
1 hour contact with the f~lter to achieve a suitable solubilising
effect. Use of preferred detergent, eg. n-octyl-~-D-glucopyranoside,
achieves suitable solubilisation in about 15 minutes.
m e concentration of the detergent needs to be optimised if the
desired optimal micellular formation i~ to be achieved. For the
preferred n-octyl-~.-D-glucopyranoside detergent the optimal
concentration is about a 2% solution, eg. in water or commercial
preparation, but other suitable concentrations for this and other ;
detergents will be derivable by those skilled in the art by simple
bench experimentation using the optimised system as a measure of what
can be achieved.
The step (b) is conveniently carried out by adding the detergent to
the filter, eg. by passing it onto it and through its thickness, and
leaving the two in contact for a period suitable for transformation of
the foreign antigens and/or leucocytes to elutable form, preferably by
solubilisation of the leucocytes. Using an encapsulated filter such
as the Leukosorb L4 Leur syringe-end fitting filter, it is convenient
to add for example 0.1 to 2 mls. preferably 0.5 mls, of a 2% n-octyl-
~-D-glucopyranoside solution (eg. in water) by taking it up into a
Leur syringe and using that to inject the solution onto the filter
material. The syringe is conveniently that used for the taking
up and applying the sample blood and rinse solution.
Step (c) is carried out after the selected time for step (b) has
passed whereby the detergent solution and its contents are
conveniently expelled with a rinse solution or, air, preferably with
air, eg. by operation of the empty syringe, application of an
W O 94/08239 PCT/GB93/02047
8 j 6
:
air-line or by manual blowing. The expelled solution is preferably
collected in a container for further processing.
Step (d) will be common to many other methods of detecting antigens or
nucleic acids, or methods for further isolating these, and will be
carried out after step (c) with or without an optionsl detergent
removal step as required.
For example, for further isolation of foreign antigens~ native
antigens may be removed by adsorption with antibodies directed at
them. Nucleic acid removal will be carried out before or after this
step by methods utilising the very different properties of the
materials. Alternatively, where one is trying to isolate a known
antigen the eluted material may be passed down an affinity column on
which are immobilised antibodies which will specifically bind the
material. Techniques for isolating nucleic acids are known to those
skilled in the art, eg. SDS gel chromatography against known standards.
However, particularly advantageous application of the present method
is found where it is used for detection of specific foreign antigens
and/or nucleic acids associated with leucocytes. In this application
step (d) may be carried out a variety of methods, some of which are
particularly adapted for use in the field.
For detection of specific nucleic acid the current method of choice
would involve use of specifically targeted nucleic acid hybridization
probes of labelled nature, these being used on their own or in
combination with polymerase chain reaction primers which can be used
to amplify a target sequence prior to specific probing. Such method
usually involves use of radioactively labelled probes which renders it
dependent upon laboratory equipment for detection of positive results.
However, use of biologically labelled probes makes it possible to
; produce a colour forming end point on successful probing by removal of
any non-hybridized material before activating the label reaction.
~ .. . .
W 0 94/08239 ~ ~ 2 ~ PCT/CB93/02047 -`
.:
For detecti~n of antigens the options for step (d) are again various,
includins radio immuno-sssay (RIA) and enzyme linked immuno-assay
(ELISA) techniques and their derivatives. More suitable for field
assays however are simple slide agglutination methods utilising
antibodies targeted at the antigen of interest or use of specific
antibodies in immunostick 'dipstick' immobilised form.
The test kits of the present invention are those comprising
components specifically associated with the method of the invention
and thus comprise
(8) a filter material capable of retaining leucocytes from a fluid
when that fluid is passed through it,
(b) an agent capable of acting upon filter immobilised leucocytes such
that antigens and/or nucleic acids associated with them are provided
in elutable form. Such combinations clearly have application in the
method of the invent~on but not in previously noted prior art uses of
leucocyte retentive filters.
Optionally provided in the kit will also be any one or more of the
following components;
(c) reagents for preventing the clotting of whole blood,
(d) reagents for the specific assay of antigens andlor nucleic acids
for which the kit is targeted,
(e) affinity reagents for immobilising target antigens and/or nucleic
acids and optionally liberating them from their immobilised state
once the fluid from which they have been separated has been removed,
~f) reagents necessary for the removal of detergent from a solution
of detergent and antigen in the form of micelles.
(g) reagents necessary for the rinsing of test fluids from the filter.
For general use the reagents (a) to (g) will be those designated as
preferable in the description of the method as outlined above. For
field use in the preferred detection of antigens the reagents (d) are
;::
W O 94/08239 PCT/GB93/02047
2 ~
most conven-iently those of a colour forming immunoassay targeted at a
specific antigen associated with an organism for which infection with
the test kit is intended to detect. Most preferably the reagents (e)
are those of a dipstick assay suitable for obtaining rapid deeection
of a target antigen in the field with a colour forming end-point.
It will be realised by those skilled in the art that virtually any
foreign antigen or nucleic acid for which a suitably specific
detection method, eg. binding assay, exists will be detectable by use
of the present method and kits. 'Foreign' in the present context
refers to material that it is not native to the human or animal whose
fluids are being investigated.
The method and test kits of the invention will now be described by
way of illustration only by reference to the following examples;
further embodiments of the invention will occur to those skilled in
the art in the light of these and the general description above.
.
FIGURES
Figure 1 shows a graph plotting Optical Density (OD) v Reciprocal
dilution for colour assay of antigen prepared by the method of the
invention (---) as compared to flash lysed and centrifuged derived
antigen ( ). Virus titre at neat is 104 18 TCID50/ml. Assay was
carried out as detailed in Example 2.
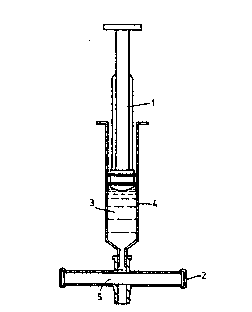
Figure 2 shows a Leur syringe (1) fitted with an encapsulated
Leukosorb filter (2) in a configuration for passing blood (3)
from its barrel (4) onto the filter material (5).
,
EXAMPLE 1. Test kit for detection of Bovine Viral Diarrhoea (BVD) in
cattle whole blood.
. .
~ A field adapted test kit was provided consisting of:
W O 94/08239 c~ 1,?'~ PCT/GB93/02047
1 x Leur s~ringe plus sampling needle
1 x 25mm encapsulated Leukosorb L4 filter with Leur syringe-end fitting
Phosphate Buffered Saline
2% n-octyl-~-D-glucopyranoside solution
EDTA solution or Vacutainer-EDTA K3 15% net (o.34M) lOml tubes or Li
Heparin 143 USP Unit 10 ml tubes (both tubes by Becton Dickinson).
The basic kit according to the invention was augmented by a separate
set of reagents making up a dip-stick assay for BVD; such reagents
optionally being provided within the same packaging as the reagents
above. These reagents consisted of:
Standard solution of streptavidin-biotin-horseradish peroxidase
suitable for biotin based assay.
Standard solution of tetra-methyl benzidine suitable for biotin based
assay,
2 x non-competitive monoclonal antibodies (mAbs) having specificity
for the p80/125 protein of BVD-prepared by conventional mAb raising
techniques as ascites fluid in Balb C ice. These shown in previous
work to react with high affinity to all pestivîruses tested to date.
These were: i
mAb WB112- on immunosticks for capture
mAb W~103- in solution in biotinylated form for signalling
The test system was first configured using polypropylene plates and
then adapted to use with Nunc Maxisorb immunosticks; mAb was bound
to the dipstick in carbonate buffer for 18 hours minimum. Suitable
test tubes and measuring/dispensing pipettes were also provided as
optional extras.
EXAMPLE 2. Method for-use of test kit of EXamDle 1 .
Samples of blood were taken from cattle and treated with an amount of
EDTA suitable for prevention of blood clotting. Aliquots of 5mls of
the EDTA treated blood were passed through the filter using the
W O 94/08239 P ~ /GB93/02047
! 5 8~i lo
syringe, th-e syringe was used to pass a similar amount of phosphate
buffered saline through the filter for rinsing purposes. The syringe
was then used to pass 0.5ml of the 2X n-octyl-~-D-glucopyranoside
solution onto the filter where it was left for 15 minutes before being
expelled into a test tube by air. The air was provided by operation
of the empty syringe.
The treated sample could be used for further isolation steps directly
from the test tube but in the present example the test tube was that
already contsining the monoclonal antibody WB103 in biotinylated form.
The dipstick from the kit was rinsed in tap water and placed into the
mixture of the sample and mAb solution, as is conventional in the art,
and after 30 minutes removed, again rinsed with tap water and then
placed into the solution of streptavidin-biotin-horseradish peroxidase
and left for a further 30 minutes. Finally the dipstick was washed
again and placed in a solution of tetra-methyl benzidine wherein the
colour reaction was allowed to develop for 15 minutes after which the
tube was ex~mined. Presence of a blue colour indicated the presence
of the BVD characteristic antigen.
Positive and negative trials were carried out in microplates to
demonstrate the reliability of the method for detecting persistent
infection. A small trial of the dipstick method was carried out using
EDTA blood from three persistently infected cattle and eight normal
cattle. A non-dipstick ELISA carried out on the antigen produced by
the filter method was carried out to assess the efficacy of the field
test.
EDTA or heparin was added to blood by use of Vacutainer tubes as
described in the alternatives for the kit in Example 1. Thus lOmls of
blood was added to such tube direct from the sampling syringe whereby
it contacts EDTA or heparin that has been coated onto the tube wall.
The EDTA or heparin anti-clotting agent is provided in such an
amount as to produce o.34M EDTA or 143 USP Units per lOmls, and the
:
~,
; : ~
.
W 0 94/08239 ,~ t2~ `3 PCr/GB93/02047
resultant treated blood was then taken up into the sampling syringe
and passed onto and through the filter. ~-
Results were obtained showing that the filter method of sample
preparation successfully provided a detectable amount of antigen in
blood derived from infected animals. See Figure 1 where detectability
of antigen in blood derived by flash lysed snd centrifugation is
compared with thst derived from the method of the present invention.
Further investigation into the storability of the dipstick reagent
W8S carried out whereby it wss shown that the test worked with
dipsticks prepared at weekly intervals for nine weeks and kept at 4C
and room temperature in the dark. At the end of the period the
performance of tests using them was sssessed and found to be
unimpared in all cases.
: ' .