Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
_. z~z~~z~
72932-124D
TITLE
THERMOPLASTIC RESIN COMPOSITION
This is a divisional of Canadian Patent Application
Serial No. 2,059,389 filed January 15, 1992.
FIELD OF THE INVENTION
The present invention relates to a novel thermoplastic
resin composition, and more in detail to a thermoplastic resin
composition comprising a polymer comprising recurring units
derived from a polycyclic (meth)acrylate and a soft polymer.
BACKGROUND OF THE INVENTION
Amorphous resins such as polyester resins, ABS resins
and modified PPO resins are excellent in characteristics such
as rigidity, dimension accuracy and heat resistance, and
accordingly have heretofore been widely used for automobile
parts, electrical appliances, office automation instruments,
miscellaneous goods, etc.
However, thermoplastic resins used for products as
mentioned above have recently been increasingly employed under
severe conditions such as at high temperature. The severe
quality is required for the thermoplastic resins used under such
severe conditions depending upon the conditions of use. Few of
the conventionally used amorphous resins as described above
satisfy such requirements, and therefore the realization of
thermoplastic resins having still higher quality has been
desired.
OBJECT OF THE INVENTION
The present invention has been accomplished in view of
the prior art techniques as described above, and an object of the
1
_.
72932-124D
invention is to provide a thermoplastic resin composition
excellent in characteristics such as heat resistance, rigidity,
dimension accuracy, impact resistance and light resistance.
SUMMARY OF THE INVENTION
A thermoplastic resin composition according to the
present invention comprises (A) a polymer comprising recurring
units derived from a polycyclic (meth)acrylate represented by
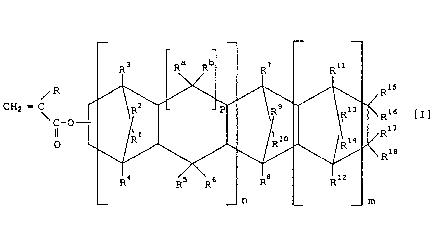
the following formula:
Ris
CH2 = C Ris [I]
\ C-O-
0
R1e
(wherein m is 0 or a positive integer, n is 0 or 1, R is hydrogen
or methyl, R1-R18 are each independently an atom or a group
selected from the group consisting of hydrogen, halogen and
h drocarbon rou s R15_R18
Y g p . , linked together, may form a mono-
cyclic or polycyclic group which may have a double bond, R15 and
R16, or R17 and R18 may form an alkylidene group, p is 0 or 1,
and Ra and Rb each independently represent a hydrogen atom or a
hydrocarbon group when p is 1, and a 5-membered ring is formed
as the result of forming a bond between the two corresponding
carbon atoms when p is 0) and (B) a soft polymer.
In this divisional application, aromatic vinyl hydro-
carbon/conjugated dime polymer or a hydrogenated or graft-
2
72932-124D
modified product (iv) or (meth)acrylic acid ester polymer or
copolymer (vi) mentioned more in detail hereinunder is employed
as the soft polymer (B) at a weight ratio of the polycyclic
(meth)acrylate polymer (A) to the soft polymer (B) of 93:7 to
60:40.
In the parent application, the other soft polymers
mentioned hereinunder are employed. However, it should be borne
in mind that the expression "the present invention" or the like
in this specification includes the subject matter of both this
divisional application and the parent application.
The thermoplastic resin composition of the invention
is excellent in characteristics such as rigidity, dimension
accuracy, impact resistance and light resistance. Accordingly,
molded articles excellent in characteristics such as heat
resistance, rigidity, impact resistance and light resistance
can be prepared from the resin composition of the invention.
DETAILED DESCRIPTION OF THE INVENTION
The thermoplastic resin composition according to the
present invention is more specifically illustrated hereinafter.
The thermoplastic resin composition of the invention
comprises a polymer comprising recurring units derived from a
specific polycyclic (meth)acrylate and a soft polymer.
The polymer comprising recurring units derived from a
specific polycyclic (meth)acrylate may be a (co)polymer of two
or more different polycyclic (meth)acrylates of the formula (I),
or a copolymer of a polycyclic (meth)acrylate of the formula (I)
and at least one other copolymerizable monomer.
3
_..
72932-124D
POLYCYCLIC (METH)ACRYLATE
Firstly, the polycyclic (meth)acrylate is illustrated.
The polycyclic (meth)acrylate can be represented by
the formula:
R is
CH2 = C R [ I ]
~C-0- Ris
II Rm
0
R1e
In the aforementioned formula [I], m is 0 or a
positive integer, preferably, 0, 1 or 2, and n is 0 or 1.
However, when the soft polymer is the aromatic vinyl hydro-
carbon/conjugated diene polymer or hydrogenated or graft-modified
product thereof (iv) or the (meth)acrylic acid ester polymer
or copolymer (vi), the total of m and n should be at least 1.
In the formula [I], R is hydrogen or methyl. That is,
of compounds represented by the above-mentioned formula [I],
those having a hydrogen atom as R are acrylate compounds, and
those having a methyl group as R are methacrylate compounds.
These acrylate compounds and methacrylate compounds can both be
used in the present invention.
In the formula [I], Rl-R18 are each independently
selected from the group consisting of hydrogen, halogen and
4
zm~~~~
72932-124D
hydrocarbon groups. Ra and Rb are each independently a hydrogen
atom or a hydrocarbon group. The hydrocarbon groups usually
have 1 to 15 carbon atoms, preferably 1 to 10 carbon atoms, and
are
4a
72932-124
linear or branched. Specific examples of the hydrocarbon group
include aliphatic hydrocarbon groups, preferably alkyl groups
having 1 to 10 carbon atoms, such as methyl, ethyl, propyl, n-
butyl, isobutyl, tert-butyl, pentyl, hexyl, heptyl, octyl, nonyl
and decyl; alicyclic hydrocarbon groups such as cyclohexyl; and
aromatic hydrocarbon groups, preferably those having 6 to 14
carbon atoms, such as phenyl, benzyl, tolyl, ethylphenyl,
isopropylphenyl, a-naphthyl and anthracenyl. Examples of the
halogen include fluorine, chlorine, bromine, and iodine. R1-R18
are not required to be the same, and may, of course, be groups or
atoms different from each other.
Still furthermore, R15-R18, linked together, may form a
monocyclic or polycyclic group. For example, R15 and R17, linked
together, may form as a whole, a cyclopentyl, or cyclohexyl ring,
or a ring structure in which a plural number of such rings are
bonded together. The monocyclic or polycyclic group may have a
double bond in the ring.
Moreover, R15 and R16, or R17 and R18 may form an
alkylidene group such as ethylidene and propylidene.
Furthermore, p represents 0 or 1 in the formula jI]. Ra
_.-.anal Rb_each independently represent a hydrogen atom or a
hydrocarbon group when p is 1, and when p is 0, the corresponding
two carbons are bonded together to form a 5-membered ring.
Among the polycyclic (meth]acrylates of the formula (I],
a group of preferred compounds are represented by the formula=
z~~~~z~
Sa 72932-124
R
RX
H2C=C-C-O
O RY
Rd R12
7 8 9 11 12
(wherein R , R , R , R , R and R13 are each hydrogen or lower
l0 alkyl,
RX and Ry are each hydrogen, halogen,lower alkyl, and
R is as defined above.
Specific examples of the polycyclic (meth)acrylate
represented by the aforementioned formula [IJ include the
compounds described below.
R~ R11
21 24923
6
H
Tetracyclo[4.9Ø12~5.1~~1~]dodecyl-.3-
CHZ = C
I acrylate
C-O -
II
O
CH3
Tetracyclo[4.4Ø12~5,1~~1~]dodecyl-3-
CH2 = C
I methacrylate
C-O -
II
O
2,10-Dimethyltetracyclo-
CH2 = i CH3 CHj [4.4Ø12~5.1~~1~)dodecyl-3-acrylate
C-O
II
O
iH3 2,10-Dimethyltetracyclo-
CH2 = i CH3 CH3 [9.4Ø12~5.1~~1~)dodecyl-3-
C-O - methacrylate
II
O
2,7-Dimethyltetracyclo-
CH2 = i CH3 [4 , q . 0 . 12~ S . 1~~ 1~ ] dodecyl-3-acrylate
C-O
II
O
CH3
2124923
iH3 2,7-Dimethyltetracyclo
CHZ = i CH3 [4.4Ø12~5.17~10]dodecyl-3
C-O - methacrylate
II
O
CH3
11,12-Dimethyltetracyclo-
CH2 = j [4.4Ø125.17.10]dodecyl-3-acrylate
C-O -
II CH3 CH3
O
iH3 11,12-Dimethyltetracyclo-
CHZ = i [ 4 . 4 . 0 . 12 ~ 5 . 17. 10 ] dodecyl-3 -
C-O methacrylate
I) CH3 CH3
O
9-Substituted tetracyclo-
CH2 = i x [4 . 4 Ø 12~ 5. 17~ 10] dodecyl-3-acrylate
R
C-O
II
O
iH3 9-Substituted tetracyclo-
CH2 = C [ 4 . 9 . 0 . 12 ~ 5 . 1.10 ~ dodecyl-3-
I Rx
C-O methacrylate
II
O
In the above-mentioned two formulas, RX reprESents an
aliphatic hydrocarbon group such as methyl, ethyl, propyl,
2124923
isobutyl, hexyl and stearyl, an alicyclic hydrocarbon group
such as cyclohexyl, or a halogen atom such as a bromine
atom and a fluorine atom.
8-Substituted tetracyclo-
CHz = i (4 . 4 Ø 12~5. 1~, 10] dodecyl-3-acrylate
C-O -
I)
O
Rx
iH3 8-Substituted tetracyclo-
CH2 = i (4 . 4 . 0 . 12, 5 . 17, 10~ dodecyl-3-
C O methacrylate
II
O
Rx
In the above-mentioned two formulas, Rx represents an
S aliphatic hydrocarbon group such as methyl, ethyl, propyl,
isobutyl, hexyl and stearyl, an alicyclic hydrocarbon group
such as cyclohexyl, or a halogen atom such as a bromine
atom and a fluorine atom.
8,9-Disubstituted tetracyclo-
CHz = i Rx [9 . 4 ~. 0. 12~ 5. 1~. 10~ dodecyl-3-acrylate
C-O _
II
O
Ry
iH3 8,9-Disubstituted tetracyclo-
CH2 = i x [ 4 . 4 . 0 . 12 ~ 5 .1.10 ~ dodecyl-3-
R
C-O methacrylate
Il
O
Ry
2124923
9
In the above-mentioned two formulas, RXand RY each
independently represent an aliphatic hydrocarbon group such
as methyl, ethyl, propyl, isobutyl, hexyl and stearyl, an
alicyclic hydrocarbon group such as cyclohexyl, or a
halogen atom such as a bromine atom and a fluorine atom.
H
Hexacyclo [ 6 . 6 . 1 . 13 6 . 110, 13 . 02, 7
CHZ = C 3 1 '
C-O 4 2 1q 13 12 09,14~heptadecyl-4-acrylate
U
7 9 11
6 B 10
CH3
Hexacyclo [ 6 . 6 . 1 . 13 6 . 110, 13 . 02, 7
CH2 = C
C-O O9'147heptadecyl-4-methacrylate
a
0
H
12-Methylhexacyclo-
CH2=C
C-O CH3 [6.6.1.13~6.110,13.02,7.09,14~-
heptadecyl-4-acrylate
CH3
12-Methylhexacyclo-
CH2 = i
C-O CH3 [ 6 . 6 . 1 . 13. 6 . 110, 13 . 02, 7 . 09, 14 ~ ..
heptadecyl-4-methacrylate
21 24923
10
H r
11-Methylhexacyclo-
CH2 = C
C-O [6.6.1.13~6.110,13.02,7.09,14~-
II heptadecyl-4-acrylate
O
CH3
CH3
11-Methylhexacyclo-
CH2 = C
( [6.6.1.13~6,110,13,02,7,0g,14~_
CO
I) heptadecyl-4-methacrylate
O
CH3
H
12-Ethylhexacyclo-
CH2 = C
C-O C2H5 [6.6.1.13.6.110,13.02.7.09,14]_
II heptadecyl-4-acrylate
O
CH3
12-Ethylhexacyclo-
CHz = I
C-0 CZHS [6.6.1.13~6.110.13.02.7.0g.14~_
II heptadecyl-4-methacrylate
O
H
11-Ethylhexacyclo-
CH2=C
C-O [6.6.1.13~6.110,13.~2,7.09,14~_
II ) ~ heptadecyl-4-acrylate
O
CzHs
2.1 24923
11
CH3
11-Ethylhexacyclo-
CHZ = C
[6.6.1.13~6.110,13.~2,7.
II O9~ 14] -heptadecyl-4-
O
C2Hs methacrylate
H
Octacyclo[8.8.1z~9.14~~.
CHz = i q 2 le is 111, 18 . 115, 16 . 0 . 03~ 8 . 012, 17 ] -
C-0 s 3 1 ~ 1 s
II docosyl-S-acrylate
O s 8 to lz lq
7 9 i 11 13
fH3 Octacyclo[8.8.12~9.14.7,
CH2 = i 111, 18 . 115. 16 . 0 . 03. 8 . 012, 17 ] _
C-O docosyl-5-methacrylate
(I
O
15-Methyloctacyclo-
CH2= i [8.8.12.9,14,7.111,18,
C-0 CH3 115,16Ø038.012.17
II )docosyl
O -S-acrylate
_iH3 . 15-Methyloctacyclo-
CHZ = i [8.8.12~9.14~7.111.18.
C-O CH3 15, 16 3 8 12 17
II 1 . 0 . 0 ~ . 0 ~ ] docosyl
O -S-methacrylate
Examples of the polycyclic (meth)acrylate in which
Rls-Rle together- form a single ring group represented by the
2124923
12
aforoementioned formula [IJ include the compounds described
below.
H
Pentacyclo[6.6.1.13~6.02~~,p~.l~~_
CH2 = C
I hexadecyl-4-acrylate
C-O -
II
0
CH3
Pentacyclo [ 6 . 6 . 1 . 13~ 6 , 02, ~ , p9, i4 J -
CH2 = C
I hexadecyl-4-methacrylate
C-O -
II
O
H
CH3 CH3 1,3-Dimethylpentacyclo-
CH2 = C
C-0 - [6.6.1.13.6.02.~.09,14Jhexadecyl-~-
II acrylate
0
CH3
CH3 CH3 1,3-Dimethylpentacyclo-
CH2 = C
[ 6 . 6 . 1 . 13~ 6 . 02~ ~ . 09.14 ] hexadecyl-:~-
II methacrylate
O
H
CH3 1,6-Dimethylpentacyclo-
CH2 = C
C-O- [ 6 . 6. 1 . 13~ 6 . 02~ ~ . O9~ 14 ] hexadecyl-:~-
II acrylate
O
CH3
2124923
13
CH3
CH3 1,6-Dimethylpentacyclo-
CHZ = C
I [6.6.1.13~6.02~7.09~14 A Y
C-O- ]h_xadec 1--~-
II methacrylate
O
CH3
H
15,16-Dimethylpentacyclo-
CHZ = C
( [6.6.1.13~6.02.x.09,14 y
C-O- )hexadec 1--=~-
IOI CHI CH3 acrylate
CH3
15,16-Dimethylpentacyclo-
CHz = C
C-O [ 6. 6. 1 . 13~ 6 . 02~ ~ . O9, 14 ] rlexadecyl--~-
II CH3 CH3 methacrylate
O
H
Pentacyclo[6.5.1.13~s,02.~,p9.13]-
CH2 = C
C-O pentadecyl-4-acrylate
II
O
CH3
Pentacyclo[6.5.1.13.6,02,~,p9,13~_
CHZ = C
I pentadeGyl-4-methacrylate
C-O -
II
O
.. 2 ~ 4923
14
H
CH3 CH3 1,3-dimethylpentacyclo-
CHZ = C
C-O - f6.5.1.13~6.02~~.09~13)pentadecyl-
4-acrylate
O
H3 .
CH3 CH3 1,3-dimethylpentacyclo-
CH2 = C
C-O C6.5.1.13~6.02~~.09,13)pentadecyl-
4-methacrylate
O
H
CH3 1,6-dimethylpentacyclo-
CH2 = C
C-O - f6.5.1.13~6.02.~,09,13~pentadecyl-
9-acrylate
O
CH3
H3 .
1,6-dimethylpentacyclo-
CH2 = C
C-O ~6.5.1.13~6.02~~.09.13~pentadecyl-
9-methacrylate
O
CH3
H
19,15-dimethylpentacyclo-
CHz = C
C-O - f6.5.1.13~6.02~7.09.13~pentadecyl-
CH3 CH3 4-acrylate
O
224923
CH3
1 14,15-dimethylpentacyclo-
CHZ = C
C-0 [ 6 . 5 . 1 . 13, 6 . 02 7 . 09. 131 _
II CH3 CH3 pentadecyl-4-methac:rylate
O
Heptacyclo[8.8.12,9.14,7.
CH2 = i 111, 16 . 0 . 03. 8 . 012, 171 _
C-O - heneicosyl-5-acrylate
Il
O
IH3 Heptacyclo[8.8.12,9.14~7.
CHz = i 111, 16 . 0 . 03. 8 . 012, 171 _
C-O heneicosyl-5-methacrylate
II
0
H
CH2 = C Heptacyclo [ 8 . 8 . 12~ 9 . 14, 7
I
II O 111, 16 . 0 . 03~ 8 . 012, 171 eicosyl-
O
5-acrylate
i H3
CH2 = i ~ Heptacyclo ( 8 . 8 . 12, 9 . 14, 7 .
111, 16 . 0 . 03, 8 . 012, 171 eicosyl-
O
5-methacrylate
The polycyclic (meth)acrylates as mentioned above can
be prepared, for example, by reacting a polycyclic alcohol,
Which has been prepared by reacting a cycloolefin having a
structure corresponding to the polycyclic (meth)acrylate
2124923
16
represented by the aforementioned formula [I] with formic
acid, with a (meth)acrylic acid derivative including
acrylic acid or methacrylic acid, or a (meth)acrylyl halide
such as an acrylyl halide or a methacrylyl halide.
The cycloolefin having a structure corresponding t.o
that of the polycyclic (meth)acrylate used in this method
can be represented by the following formula [II]
Ris
Ris
R17
(II]
Rie
wherein R1-R18, Ra, Rb, and m, n and p are as defined
in the aforementioned formula [I].
The polymer used in the thermoplastic resin
composition of the invention comprising the recurring units
derived from a polycyclic (meth)acrylate may be a
2 0 homopolymer of_the polycyclic (meth)acrylate as mentioned
above. The polymer may also be a copolymer of the
224923
17
polycyclic (meth)acrylates, which are different from each
other, as mentioned above. The polymer may also ba a
copolymer of the polycyclic (meth)acrylate as mentioned
above and other copolymerizable monomers. In the present
S invention, the term "polycyclic (meth)acrylate (co)polymer"
generally refers to these (co)polymers unless otherwise
noted.
These other monomers to be copolymerized with the
polycyclic (meth)acrylate include compounds having at least
one polymerizable double bond in the molecule.
Concrete examples of such a compound include scrylic
acids such as (meth)acrylic acid; acrylic acid derivatives
such as methyl (meth)acrylate, ethyl (meth)acrylate, propyl
(meth)acrylate, isopropyl (meth)acrylate, butyl
(meth)acrylate, pentyl (meth)acrylate, hexyl
(meth)acrylate, heptyl (meth)acrylate, octyl
(meth)acrylate, nonyl (meth)acrylate, decyl (meth)acrylate,
cyclohexyl (meth)acrylate and benzyl (meth)acrylat.e;
aromatic vinyl compounds such as styrene, a-methylstyrene
~2 0 and vinyltoluene; acrylonitrile; malefic acid derivatives
such as malefic anhydride, maleimide and phenylmaleimide;
and vinyl esters such as vinyl acetate and vinyl benzoate.
The polycyclic (meth)acrylate (co)polymer is a
(co)polymer prepared by polymerizing polycyclic
2 S (meth)acrylate(s) and.if necessary other monomers as
mentioned above.
2124923
18
When the polycyclic (meth)acrylate (co)polymer is a
copolymer of a polycyclic (meth)acrylate and other
monomers, the polycyclic (meth)acrylate (co)polymer
comprises recurring units derived from the polycyclic
(meth)acrylate in an amount of usually at least S mold,
preferably 10 to 99 mol=e and especially 30 to 95 mold. A
resin composition excellent in heat resistance and heat
stability can be obtained by using a copolymer comprising
recurring units derived from a polycyclic (meth)~crylata in
1 0 an amount in the range rnentioned above.
The polycyclic (meth)acrylate (co)polymer has an
intrinsic viscosity [~], as measured in toluene at 30°C, of
usually 0.002 to 20 dl/g, preferably 0.05 to~l0 dl/g and
especially 0.2 to 5 dl/g. The (co)polymer has a glass
transition temperature, as measured by a differential
scanning type calorimeter, of usually 10 to 200°C,
preferably 50 to 200°C and especially 105 to 200°C.
Furthermore, the polycyclic (meth)acrylate (co)polymer
has a molecular weight distribution (Mw/Mn) of usually not
__T_.20.greater than 10, preferably 1..0 to 3.0, as measured by gel
permeation chromatography, a crystallinity of usually not
greater than S~, preferably not greater than 1~, as
measured by X-ray diffraction, and a softening temperature
of usually 20 to 220°C, preferably 70 to 220°C and
2 5 especially 120 to 220°C, as measured by a thermal
mechanical analyzer (TMA) (manufactured by DuPont)..
.v 2 124923
19
When the polycyclic (meth)acrylate (co)polymer is a
copolymer, the copolymer has a substantially linear
molecular structure in which recurring units derived from
the polycyclic (meth)acrylate and recurring units derived
$ from other selected monomers are randomly arranged. The
fact that the copolymer has a substantially linear
molecular structure can be proved by observing dissolution
thereof in an organic solvent without leaving any insoluble
component. This can be proved, for example, by the fact
that the polycyclic (meth)acrylate (co)polymer completely
dissolves in toluene at 30°C during the measurement of the
intrinsic viscosity (T~] as described above.
The polycyclic (meth)acrylate (co)polymer can be
prepared by various polymerization methods. For exampla,
1$ the polycyclic (meth)acrylate and other monomers can be
copolymerized by polymerization methods such as suspension
polymerization, emulsion polymerization, solution
polymerization and bulk polymerization.
Examples of the bulk polymerization method include a
----.2.0 method wherein the polymerization is carried out at a
temperature of usually 60 to 250°C, preferably 150 to
230°C, and a method wherein the reaction temperature is
elevated, for example, from 80°C, with the progress of the
polymerization reaction, and the polymerization reaction is
2 $ terminated, for example, at a temperature of 180 to 230°C.
In these methods, radical initiators can be used. Examples
of the radical initiators include organic peroxides such a:;
2 1 24923
72932-124D
di-tert-butylperoxide, dicumyl peroxide, methyl ethyl ketone
peroxide, di-tert-butyl perphthalate, tert-butyl perbenzoate,
tert-butyl peracetate and tert-butyl perisobutyrate, and azo
compounds such as 1,1'-azobiscyclohexanecarbonitrile and 2-cyano-
2-propylazoformamide. These radical initiators are used in an
amount of usually not greater than 1 mol% based on the monomers.
During the (co)polymerization, there may also be used
chain transfer agents such as tert-butylmercaptan, n-butyl-
mercaptan, n-octylmercaptan and n-dodecylmercaptan in order to
control the molecular weight of resultant polycyclic (meth)-
acrylate (co)polymer. The chain transfer agents are used in an
amount of usually not greater than 1 molo based on the monomers.
Furthermore, the aforementioned (co)polymer may also
be prepared by photopolymerization using energy ray irradiation
with or without the use of the radical initiator as mentioned
above.
The procedures of polymerization as described above
are disclosed in detail, for example, in Japanese Unexamined
Patent Publication No. 243108/1988, etc., and can be utilized
in the present invention.
SOFT POLYMER
The thermoplastic resin composition of the invention
comprises the polycyclic (meth)acrylate (co)polymer mentioned
above and a soft polymer (an elastomer).
2124923
20a 72932-124
A group of preferred polymers among such soft polymers
[B] include=
(I) a copolymer selected from the group consisting of an
ethylene/C3_20 a-olefin copolymer having an ethylene content of 40
to 95 mol %, a propylene/C4_2pa-olefin copolymer having a
propylene content of 50 to 95 mol % and an ethylene/C3_20a
olefin/diene copolymer having an ethylene/C3_20or-olefin molar
ratio of 40/60 to 90/10 and a diene content of 1 to 20 mol %, and
(II) a graft-modification product of the copolymer defined
above in (I) with tetracyclo[4.4Ø11'2.17'10]decan-3-
yl(meth)acrylate along or together with styrene;
wherein the soft polymers (B] have a glass transition
temperature of not higher than O°C, an intrinsic viscosity [~] as
measured in decalin at 135°C of 0.01 to 10 dl/g and a
crystallinity index as measured by X-ray diffraction of 0 to 10%.
21
Examples of the soft polymer include
(i) a soft polymer comprising recurring units
derived from a cycloolefin,
(ii) an oc-olefin polymer,
(iii) an oc-olefin/diene copolymer, and a hydrogenated
product thereof,
(iv) an aromatic vinyl hydrocarbon/conjugated diene
soft copolymer, and a hydrogenated product thereof,
(v) a soft polymer or copolymer selected from
polyisobutylene, a polymer of a conjugated diene, or a
copolymer of isobutylene and a conjugated diene, and
(vi) a polymer (so-called acrylic rubber) coiaprising
recurring units derived from a (meth)acrylic acid ester.
Of these, the soft polymers (ii) and (vi) are
preferably used. The soft polymers (i) and (ii) may be
graft-modified polymers with OC,~-unsaturated carboxylic
acid or derivatives thereof.
Concrete examples of the soft polymer used in the
invention are illustrated below.
SOFT POLYMER f i ) COMPRTSTNC' RF('ttRtZTxrr 'vIpdTTS
DERIVED FROM A CYCLOOLFFT~
The soft polymer comprising recurring units derived
from a cycloolefin is a copolymer formed from ethylene, a
cycloolefin represented by the formula [II] and oc-olefin
2 S having 3 or more carbon atoms.
21 ~~9~3
22
A graft-modified soft polymer comprising recurring
units derived from the cycloolefin may also be used as the
soft polymer.
Ris
Ris
Rm
...... [ I I
Rie
wherein R1-R18, Ra, Rb, and m, n and p are as defined
in the above-mentioned formula [Ij.
Examples of the cycloolefin include
bicyclo[2.2.1]hept-2-ene derivatives,
1 0 tetracyclo[4.9Ø12~5.17,10]-3_dodecene derivatives,
hexacyclo[6.6.1.13~s,110,13_02,7.09,14j_q-heptadecene
derivatives,
octacyclo [8 . 8 . 0 . 12~ 9 . 14~ 7. 111, 18. 113, 16. 03, B . 012, 17 j -5_
w docosene derivatives, .
1 5 pentacyclo [ 6. 6. 1 . 13 6. 02~ 7 . 09,14 j _q_hexadecene
derivatives,
heptacyclo-5-eicosene derivatives,
heptacyclo-5-heneicosene derivatives,
tricyclo[4.3Ø12~5)-3-decene derivatives,
2 0 tricyclo[4.3Ø12 5]-3-undecene derivatives,
212923
23
pentacyclo[6.5.1.13,6.02,7.09,13]_4_pentadecene
derivatives,
pentacyclopentadecadiene derivatives,
pentacyclo[4.7Ø1',5.08,13.19,12]-3_pentadecene
S derivatives,
pentacyclo [ 7 . 8 . 0 . 13, 6. 02, ~ . 110, 17 , 011, 16 . 112, 15 ] _4_
eicosene derivatives,
nonacyclo[9.10.1.1.4.7.03,8.02,10.012,21.113,20.014,19.
115,18]_5-pentacosene derivatives,
1 0 phenylbicyclo(2.2.1]-2-ene derivatives,
benzylbicyclo[2.2.1]-2-ene derivatives,
1,4-methano-l,la,4,4a-tetrahydrofluorene derivatives,
1,4-methano-1,4,4a,5,10,10a-hexahydroanthracene
derivatives,
cyclopentadiene-acenaphthylene adducts,
pentacyclo [7 . 4 . 0'. 12, 5 . 08, 13 . 19,12 ] pentadecene-3-
derivatives,
heptacyclo [8 . 7 . 0 . 02~ 7. 13~ 6. 110, 17. 011, 16 _ 112, 15 ] eicosene-
4 derivatives and
2 ~ ' ~ ~ nonacyclo [ 10 . 9 . 1 . 02..10 . 43. 8 . 14~ 7 , p12, 21 , 113, 20
. 014, 19 .
115,18~pentacosene-5 derivatives.
Concrete examples of the compounds mentioned above are
described below.
Bicyclo (2.2.1]hept-2-ene derivatives such as those
2 S mentioned below.
2124923
24
Bicyclo[2.2.1]hept-2-ene
CH3 6-Methylbicyclo[2.2.1]kept-2-ene
CH3
5,6-Dimethylbicyclo[2.2.1]hept-2-ene
CH3
CH3
1-Metylbicyclo[2.2.1]kept-2-ene
C2H5 6-Ethylbicyclo[2.2.1]kept-2-ene
nCqHg
6-n-Butylbicyclo[2.2.1]kept-2-ene
_--. _. ~ . iCqHg 6-Isobutylbicyclo [2 . 2 .1 ] hept-2-ene
CH3
7-Methylbicyclo[2.2.1]kept-2-ene
21 X4923
?s
Tetracyclo[4.4Ø12~5.1~,10~_3-dodecene derivatives
such as those described below.
Tetracyclo(4.4Ø12~5.1~,10~_3_
dodecene
CH3
5,10-Dimethyltetracyclo-
(4 .4Ø 125. 1~.10~ _3_dodecene
CH3
H3 ~ H3
2,10-Dimethyltetracyclo-
(4 .4Ø12~5. 1~.10~ _3-dodecene
CH3 CH3
11,12-Dimethyltetracyclo-
(4.4Ø12~5.1~.i0~_3_dodecene
CH3
CH3 2,7,9-Trimethyltetracyclo-
[4.4Ø12~5.1~.10~_3_dodecene
CH3
2124923
26
CH3
C2H5 9-Ethyl-2,7-dimethyltetracyclo-
[4.4Ø12~5.1~~10]-3-dodecene
CH3
CH3 i H3
CH2iH 9-Isobutyl-2,7-dimethyltetracyclo-
CH3 [4.4Ø12~5.17~10]-3-dodecene
CHg
CH3 CH3
CH3 9,11,12-Trimethyltetracyclo-
[4.4Ø12~5.17.10]_3-dodecene
CH3 CH3
C2H5 9-Ethyl-11,12-dimethyltetracyclo-
[ 4 . 4 . 0 . 12 ~ 5 . 17 ~ 10 ] -3-dodecene
CH3 CH3 CH3
i
- ~ CH2iH 9-Isobutyl-11,12-dimethyltetracyclo-
CH3 [4.4Ø12~5.17.10]_3-dodecene
CH3
CH3 5,8,9,10-Tetramethyltetracyclo-
CH3 [4.4Ø12~5.17.10]_3_dodecene
CH3
21 ~~923
8-t~tethyltetracyclo [4 . 4 . 0. 12~ 5. 1.10]
3-c~odecene
CH3
8-Ethyltetracyclo[4.4Ø12~5.1~.10~-3-
dodecene
C2H5
8--Propyltetracyclo [4 . 4 . 0. 12~ 5. l~.lU] _
C3H~ 3-dodecene
8-Hexyltetracyclo[4.4Ø12~5,1~.10~_3_
dodecene
C6H13
8-Stearyltetracyclo[4.4Ø12~5.1~.10~_
3-dodecene
C18H37
CH3
8,9-Dimethyltetracy'clo-
___.. . _ . 2 5 7 10
CH3 [ 4 . 9 . 0 . 1 ~ .1 ~ ] -3-dodecene
CH3
8-Methyl-9-ethyltetracyclo-
C2H5 [4.4Ø12.5.1~.1o]-3-dodecene
8-Chlorotetracyclo[4.4Ø12~~.1~.10]_
3-dodecene
2' 124923
?s
8-Bromotetracyclo[4.4Ø12~5.1~~10]-3-
\Br dodecene
8-Fluorotetracyclo[4.4Ø12~5.1~.10]_
\F, 3-dodecene
CQ
8,9-Dichlorotetracyclo-
[9 .4 Ø 12~ 5. 1.10] _3_dodecene
8-Cyclohexyltetracyclo-
(4.4Ø12~5.1~.10~_3_dodecene
iH3 8-Isobutyltetracyclo-
CH CH
[4.4Ø12~5.1~~10]_3_dodecene
CH3
8-Butyltetracyclo[4.9Ø12~5,1,10]_3_
- CQH9 dodecene
8-Ethylidenetetracyclo-
CHCH3
[4.4Ø12~5.1~10]_3-dodecene
CH3 8-Ethylidene-9-methyltetracyclo-
CHCHg [4 . 4 , 0 . 12~ S . 1~. 10 ] _3_dodecene
2124923
29
C2H5
8-Ethylidene-9-ethyltetracyclo-
CHCH3 [ 4 . 4 . 0 . 12, 5 . 1~, 10 ] _3-dodecene
CH ( Cfi3 ) 2
8-Ethylidene-9-isopropyltetracyclo-
CHCH ' [4 . 4 . 0 . 12~ 5 , 1~. 10] _3-dodecene
3
CQH9
8-Ethylidene-9-butyltetracyclo-
CHCH
[ 4 . 9 . 0 . 12, 5 . 1~. 10 ] -3_dodecene
8-n-Propylidenetetracyclo-
CHCH2CH3 [4 . 4 . 0.12 5, 1~, 10] _3-dodecene
CH3
8-n-Propylidene-9-methyltetracyclo-
CHCH2CH3 [4 . 4 . 0 .12, 5. 1~~ 10 ] -3-dodecene
C2H5
- 8-n-Propylidene-9-ethyltetracyclo-
CHCH2CH3 [ 4 . 4 . 0 . 12~ 5 , 1~. 10 ] _3-dodecene
CH ( CHg ) 2
8-n-Propylidene-9-isopropyltetra-
CHCH2CH3 cyclo [4 . 4 . 0 .12~ 5. 1~,1o] _3-dodecene
2124923
C4H9 8-n-Propylidene-9-butyltetracyclo-
CHCH2CH3 (4 . 4 Ø 12~ 5. 17~ 10] -3-dodecene
8-Isopropylidenetetracyclo-
i CH3 [4.4Ø12~5.17,10]-3-dodecene
CH3
CH3
8-Isopropylidene-9-methyltetracyclo-
I 3
C-CH [ 4 . 4 . 0 . 12. 5 . 17, 10 ] -3-dodecene
CH3
C2H5
8-Isopropylidene-9-ethyltetracyclo-
i -CH3 [ 4 . 4 . 0 . 12~ 5 . 17. 10 ] _3-dodecene
CH3
CH ( CH3 ) 2
8-Isopropylidene-9-isopropyltetra-
i CH3 cyclo[9.4Ø12~5.17~10]-3-dodecene
_._.. .- .. _ _ CH3
. . C4H9
8-Isopropylidene-9-butyltetra-
i CH3 cyclo[9.4Ø12~5.17.10]_3-dodecene
CH3
Hexacyclo[6.6.1.13~6,110,13.02,7.09,14]_4-heptadecene
derivates such as those mentioned below.
W..
2124923
31
Hexacyclo [ 6 . 6 . 1 . 13~ 6 , 110, 13 , 02, r , 09,
14J-4-heptadecene
CH3
12-Metylhexacyclo[6.6.1.13~6.110,13
02~~.09~14J-4-heptadecene
C2H5
12-Ethylhexacyclo[6.6.1.13 6.110,13
02~7.09~14j-9-heptadecene
CH3
12-Isobutylhexacyclo(6.6.1.13~6
CH2CFf
110, 13 . 02, 7 . 09, 19 j _q-heptadecene
Cfi3
CH3 CH3
CH iff 1,6,10-Trimethyl-12-isobutyl-
2
Cfi3 hexacyclo [ 6 . 6 . 1 . 13~ 6 , 110, 13 . 02, 7 . 0 g,
CH3 CH3 14j_q-heptadecene
32~ 1 24923
Octacyclo[8.8Ø12~g.14~7,111,1B,113,16,03,8.012,17~_5_
docosene derivatives such as those mentioned below.
Octacyclo[8.8Ø12~9.1q~7,111,18
113,16,03,8,012,17_5-docosene
CH3 15-Methyloctacyclo(8.8Ø12~9.
14,7,111,18.113,16.~3,8.012,17~_5_
docosene
C2H5 15-Ethyloctacyclo(8.8Ø12~9.
. 1q,7,111,18.113,16.p3.8.012.17~_5_
docosene
Pentacyclo[6.6.1.13~6.02~7.09.14~_q_hexadecene derivatives
such as those mentioned below.
Pentacyclo [ 6 . 6 .1 .13~ 6 . 02, 7 . O9.14~ _q_
hexadecene
CH3 CH3
1,3-Dimethylpentacyclo(6.6.1.13~6.
02,7.p9,14~_q_hexadecene
_. 2124923
33
CH3
1,6-Dimethylpentacyclo[6.6.1.13,6.
02,x.09,14]-4-hexadecene
CH3
CH3 CH3
15,16-Dimethylpentacyclo[6.6.1.13~6
02~~.09,14]-4-hexadecene
Heptacyclo-S-eicosene derivatives or heptacyclo-5-
heneicosene derivatives such as those mentioned below.
Heptacyclo [ 8 . 7 . 0 . 12, 9 .14, ~ .111,1
03, 8 . 012, 16 ] _5-eicosene
Heptacyclo ( 8 . 7 . 0 . 12, 9 .19, ~ .111,18 .
03,8,p12,1~]_5-heneicosene
Tricyclo(4.3Ø12,5]-3-decene derivatives such as those
mentioned below.
Tricyclo[4.3Ø12,5]-3-decenP
v
2124923
34
CH3
2-Methyltricyclo[4.3Ø12,5]-3-
decene
5-Methyltricyclo[4.3Ø12,5]-3-
a decene
CH3
Tricyclo[4.4Ø12,5]-3-undecene derivatives such as those
mentioned below.
Tricyclo[4.9Ø12,5]-3-undecene
CH3
10-Methyltricycloj4.4Ø12,5]-3-
- undecene
_,___ _- .. _ : Pentacyclo [ 6 . S .1.13~ s . 02, ~ . 09,13 ] _4_pentadecene
derivatives such as those mentioned below.
Pentacyclo [ 6 . 5 .1 .13, s . 02, ~ . 09,13] _4_
pentadecene
2124923
72932-124
CH3 CH3
1,3-Dimethylpentacyclo(6,5.1.13,6
Uz' ~ . Oy~ 131 -4-pent~clecwv
CH3
1,6-Dlmettiylpentacyclo(6.5.1.13~6.
()2~ 7 .O9~ 13 J -4-pentadc:cene
CH3
CH3 CH3 ,
14,15-Dimethylpentacyclo(6.:i.1.
13~6.02~x.09.13)-q-pentadecene
Dlene compounds such as mentioned beloN.
t'e:ntacyclo(6.5.1.1~~6.Oz~~~.U'~~1~~-
4.10-pentadecadiene
Pentacyclo(4.7Ø12'5.0~~13.19,12~-3-Pentadecene
derivatives such as those. mentioned below.
.._ 2124923
72932- 1 2c,
Pentacyclo(4.7Ø12~5.08,13
19,12)-3-pentadecene
CHI
Methyl-substituted-pentacyclo
( 4 . 7 . 0 . 12, 5 . ~~. 13 . 1 9, 12 ~ _3_
pentadecene
Heptacyclo(7.8Ø13,6.02,7.110,17.011,16.112,15)_q-eicosene
derivatives such as those mentioned below.
Heptacyclo(7.8Ø13,6.02,~.
110, 17.11, 16. 112, 15) _q_eicosene
CHI CH3
Dimethyl-substituted
heptacyclo-(7.8Ø13,6.02,x.
110, 17.011, 16. 112, 15) _q_eicosene
Nonacyclo(9.10.1.14,~.03,8.02,10.012,21,113,20.014,19,115,18)
-S-pentacosene derivatives such as those mentioned below.
Nonacyclo(9.10.1.14.~.03~~.02~10
~12,21,113,20.~14,19,115,18)
-S-pentacosene
CHI CHI Trimethyl-substituted
nonacyclo-(9.10.1.14,~.03~~.
-. 02.,10.012,21.113,20.014,19.115,18)
-S-pentacosene
CHI
Further more, the cycloolefins used in the invention
include the compounds described below.
36
224923
7z~3z-, z<<
2 6
S-Phenylbicyclo(2.2.1)hept-2-ene
O
S-Methyl-S-pheny~lbicyclo(2.2.1)-kept
-2-ene
CHI
S-Benzylbicyclo(2.2.1)hept-2-ene
CHZ
S-Tolylbicyclo(2.2.1)hept-2-ene
CHI
5-(Ethylphenyl)bicyclo-(2.2.1]kept-2-ene
CH2CH~
CH3 5_(Isopropylphenyl)bicyclo-(2.2.1)hept-
2-ene
CH
CHI
1 10 y
l0a 9a
2 . O 8
1,4-Methano-1;4,9a,S,10,10a-
he:cahydroanthracene
4a Sa
S
___. _. ~ . qa Sa 6 ' _
1,4-Methano-1,4,4a,9a-tetrahydrofluorene
9a U 8a
1 9 8
1.
Cyclopentadiene-acenaphthylene adduct
37
2124923
72932-124
0
S-(CC-Naphthyl)bicyclo-(2.2.1)hept-2-ene
S-(Anthracenyl>bicyclo-(2.2.1)hept-2-ene
Furthermore, examples of the cycloolefins which can be
used in the present invention include the compounds described
below.
Pentacyclo ( 7 . 4 . 0 . 12~ 5 . 08, 13 _ 19, 12 )
pentadecene-3
Methyl-pentacyclo(7.4Ø12,5.00,13
19~12)pentadecene-3
cH,
Dimethyl-pentacyclo(7.4Ø12,5
pe,13.19,12)pentadecene-3
CH3 Cf~~
Trimethylpentacyclo(7.4Ø12~5
pe,13.19,12)pentadecene-3
CH,
CHI CHI
Heptacyclo ( 8 . 7 . 0 . 02~ 7 . 13~ 6. 110, m
011,16.112,15)-eicosene-4
3d
.~. ~ 124923
72932-124
htethyl-Ileptacyclo ( ~ . ~ .0 . 02~ 7 . 13. 6
1 10, 17 . nl 1. 16 , 1 17, 1 5 f _~. L~:~~.~7rrn~~.-~
CH,
Dimethyl-heptacyclo(E3.7Ø02~~.
13,6.110,17.011,16.112,15
-eicosene-4
Cfl~ Ctl,
Tr imetl~y 1-heptacyclo ( El . 7 . 0 . 02 ~ ~
13, 6 . 110, 17 . ~ 11, 16 . 112, 15 ~ _
eicosene-~1
CIf~ CfI.1 (.ff 1
'1'etrarnetllyl-heptacyclo ( d . 7 Ø02~ ~
13, 6 . 1 10, 17 . ~1 1 , 16 . 112, 15 ~ _
eicosene-4
cll, cll, cFl, cfl,
Nonacyclo(10.9.1.02.10.03,0.1~,~,
~12,21.113,20,~14,19.115,18~_
pentacosene-5
Useful a-olefins having 3 or more carbon atorns include
propylene, butene-1, 4-methylbutene-1, hexene-1, octene-1,
39
212 4 9 2 J 72932-124D
decene-1, dodecene-1, tetradecene-1, hexadecene-1, octadecene-1
and eicosene-1. Of these, preferred is a-olefin having 3 to 20
carbon atoms. Cycloolefins and cyclodienes such as norbornene,
ethylidenenorbornene and dicyclopentadiene may also be used.
The soft polymer (i) comprises recurring units derived
from ethylene in an amount of usually 40 to 98 mold, preferably
50 to 90 mol%, recurring units derived from a-olefin in an amount
of usually 2 to 50 mold, and recurring units derived from a
cycloolefin in an amount of usually 2 to 20 mold, preferably 2
to 15 mold.
The soft polymer (i) has a glass transition temperature
(Tg) of usually not higher than 0°C, preferably not higher than
-10°C, an intrinsic viscosity [n], as measured in decalin at
135°C, of usually 0.01 to 10 dl/g, preferably 0.8 to 7 dl/g, and
a crystallinity index, as measured by X-ray diffraction, of
usually 0 to 10~, preferably 0 to 7~ and especially 0 to 5~.
The soft polymer (i) as described above can be
manufactured according to the method proposed by the present
applicant in Japanese Unexamined Patent Publication Nos.
168708/1985, 120816/1986, 115912/1986, 115916/1986, 271308/1986,
272216/1986 and 252406/1987.
a-OLEFIN COPOLYMER (ii
2 4 9 2 ~ 72932-124D
The a-olefin copolymer (ii) used as a soft polymer is an
amorphous or low crystalline copolymer prepared frocn at least
two oc-olefins. Concrete examples of the a-olefin copolymer
(ii) include an ethylene/OC-olefin copolymer and a
propylene/a-olefin copolymer.
A graft-modified Oc-olefin copolymer (ii) may also be
used as a soft polymer.
The a-olefin from which the ethylene/a-olefin copolymer
is prepared has usually 3 to 20 carbon atoms. Concrete
examples of the oc-olefin include propylene, butene-1, 4-
methylbutene-1, hexene-1, octene-1, decene-1 and a mixture of
these oc-olefins. .Of these, preferred are a-olefins eacn
having 3 to 10 carbon atoms.
The molar ratio of the recurring units derived from
ethylene to those derived from a-olefin (ethylene/oc-olefin)
in the ethylene/ot-olefin copolymer is usually 40/60 to 95/5,
though it differs depending on the type of the a-olefin. The
molar ratio is preferably 40/60 to 90/10 when propylene is
used as the oc-olefin, and is also preferably 50/50 to 95/5
w 2 0' when an a-olefin having at lea-st 4 carbon atoms is used.
The a-olefin from which the propylene/a-olefin copolymer
is prepared has usually 4 to 20 carbon atoms. Concrete
examples of the oc-olefin include butene-1, 4-methylpentene-l,
hexene-1, octene-1, decene-1 and a mixture of these a-
72932-124D
2124923
olefins. Of these, particularly preferred are a-olefins each
having 4 to 10 carbon atoms.
The molar ratio of the recurring units derived from
propylene to those derived from a-olefin (propylene/a-olefin)
S in the propylene/a-olefin copolymer is usually 50/50 to 95/5,
though it differs depending on the type of the a-olefin.
i7C-OLEFIN/DIENE COPOLYMER AND
The a-olefin/diene copolymer (iii) used as a soft
polymer includes an ethylene/a-olefin/diene copolymer rubber
and a propylene/a-olefin/diene copolymer rubber.
An a-olefin having 3 to 20 carbon atoms is used for the
ethylene/a-olefin/diene copolymer. Concrete examples of the
a-olefin include propylene, butene-1, pentene-1, 4-
methylpentene-1, hexene-1, octene-1, decene-1 and a mixture
of these a-olefins. Of these, preferred are a-olefins each
having 3 to 10 carbon atoms. An a-olefin each having 4 to 20
carbon atoms is used for a propylene/a-olefin/diene
copolymer.
-- 2 0- ----- -Furthermore, the dienes from which these copolymer
rubbers are prepared include acyclic nonconjugated dienes
such as 1,4-hexadiene, 1,6-octadiene, 2-methyl-1,5~-hexadiene,
6-methyl-1,5-heptadiene and 7-methyl-1,6-octadiene, cyclic
nonconjugated dienes such as 1,4-cyclohexadiene,
2 5 dicyclopentadiene, methyltetrahydroindene, 5-vinylnorbornene,
-~ Z -
72932-124D
2124923
5-ethylidene-2-norbornene, 5-methylene-2-norbornene, 5-
isopropylidene-2-norbornene and 6-chloromethyl-S-isopropenyl-
2-norbornene, and 2,3-diisopropylidene-5-norbornene, 2-
ethylidene-3-isopropylidene-5-norbornene and 2-propenyl-2,2-
$ norbornadiene.
The molar ratio of the recurring units derived from
ethylene to those derived from oc-olefin (ethylene/et-olefin)
in the ethylene/a-olefin/diene copolymer rubber is usually
40/60 to 90/10, though it differs depending on the type of
the a-olefin.
The copolymer rubbers comprises recurring units derived
from the diene component in an amount of usually 1 to 20
mold, preferably 2 to 15 mold.
Furthermore, a hydrogenated product of the
1$ aforementioned a.-olefin/diene copolymer may also be used in
the present invention.
AROMATT . VINY . fiYDRO ARBON/ ON. 1 ,AT .D T .N .
SOFT C'.OPnT,YMER AND A HYDROGENATED PRODUCT THEREOF ( i_y)
The aromatic vinyl hydrocarbon/conjugated diene soir_
--2 0~ copolymer used as a soft polymer is a random copolymer or
block copolymer of an aromatic vinyl hydrocarbon and a diene
compound, or a hydrogenated product thereof. Concrete
examples include a styrene/butadiene block copolymer rubber,
a styrene/t5utadiene/styrene block copolymer rubber, a
2 $ styrene/isoprene block copolymer rubber, a
-.~ 3 -
72932-124D
2124923
styrene/isoprene/styrene block copolymer rubber, a
hydrogenated styrene/butadiene/styrene block copolymer
rubber, a hydrogenated styrene/isoprene/styrene block
copolymer rubber and a styrene/butadiene random copolymer
rubber.
The molar ratio of the recurring units derived from the
aromatic vinyl hydrocabon to those derived from the
conjugated diene (aromatic vinyl
hydrocarbon/conjugated~diene) in the copolymer rubbers is
usually 10/90 to 70/30.
In the present invention, there can be used a
hydrogenated product of the above-mentioned aromatic vinyl
hydrocarbon/conjugated diene soft copolymer may also re used.
The hydrogenated copolymer rubber is a copolymer rubber
wherein double bonds remaining in the above-mentioned
copolymer rubbers have been partially or entirely
hydrogenated..
SOFT POLYMRR OR COPnT YMFR f1F TSO$T1TYT FNF ON TTIrATFT) nT~~r.rr nn
ISO$ 1TY NF/ ON T 1GATFT) DI N (yl
2 0 ___ .Concrete examples of the ,isobutylene soft polymer or
copolymer (v) used as a soft polymer include a
polyisobutylene rubber, a polyisoprene rubber, a
polybutadiene rubber or an isobutylene/isoprene copolymer
rubber.
_ .4c
72932-124D
2 124923
The copolymers (ii) to (v) may have similar
characteristics to those of the cycloolefin soft polymer (i).
These soft polymers have an intrinsic viscosity [T]], as
measured in decalin at 135°C, of usually 0.01 to 10 d.~/g,
S preferably 0.08 to 7 dl/g, a glass transition temperature
(Tg) of usually not higher than 0°C, preferably not higher
than -10°C and especially not higher than -20°C, and a
crystallinity index, as measured by X-ray diffraction of 0 to
10~, preferably 0 to 7~ and especially 0 to 5~.
1 0 In addition to the polymers as exemplified in (i) to
(v), there may also be used as soft polymers copolymers (so-
called block copolymers) obtained by graft polymerizing the
soft polymers (i) to (v) with a polycyclic (meth)acrylata
represented by the above-mentioned formula [I] or other
15 monomers polymerizable with the polycyclic (meth)acrylate.
A resin composition having excellent impact resistance can be
obtained using such block copolymers.
POT.YMRR COMPRTSTN , R 1RRTN , WITS D RIVFn FRnM
--2 0-- ~ --- Concrete examples of a (meth)acrylic acid ester polymer
or copolymer (vi) used as a soft polymer include a
homopolymer or copolymer of such an alkyl (meth)acrylate
having 2 to 14 carbon atoms as ethyl acrylate, butyl
acrylate, hexyl acrylate, octyl acrylate, decyl acrylate and
2 $ octyl methacrylate, or a copolymer obtained by copolymerizing
_~tf~_
72932-124D
2124923
the above-mentioned monomers in a predominant amount with
other monomers such as 2-chloroethyl vinyl ether,
acrylonitrile, methoxyethyl acrylate, ethoxyethyl acrylate,
vinyl chloroacetate, allyl chloroacetate, glycidyl acrylate
S or glycidyl methacrylate.
These soft polymers may be incorporated singly or in
combination.
The thermoplastic resin composition of the invention
comprises the polycyclic (meth)acrylate (co)polymer and the
soft polymer as described above in the proportion by weight
of the (co)polymer to the soft polymer of 99/1 to 40/60. The
composition having the proportion in the range of 95/S to
SO/S0, preferably 93/7 to 60/90, is particularly excellent in
heat resistance, rigidity, dimension accuracy, impact
resistance and light resistance.
The thermoplastic resin composition of the invention as
described above has a melt flow rate (MFR, as measured
according to ASTM D 1238) of usually 0.1 to 100.
~-2 0~ ~~-~- The thermoplastic resin composition of the invention can
be prepared by mixing the polycyclic (meth)acrylate
(co)polymer and the soft polymer as described above in a
predetermined proportion, and kneading the mixture using, for
example, a melt kneading apparatus.
--~cd -
72932-124D
2124923
Furthermore, the thermoplastic resin composition of the
invention may be the one having a crosslinked structure among
the components of the composition. For example- a
crosslinked structure can be formed between the polycyclic
S (meth)acrylate (co)polymer and the soft polymer as mentioned
above by using an organic peroxide, etc. Moreover, in
forming the crosslinked structure by using such organic
peroxide, the crosslinking reaction may also be conducted by
incorporating such a compound having at least two
1 0 polymerizable functional groups in the molecule as
divinylbenzene, vinyl acrylate and vinyl methacrylate.
OTHER ADDITIyFS
The thermoplastic resin composition of the invention may
be incorporated with heat stabilizers, weathering
15 stabilizers, antistatic agents, slip agents, anti-blocking
agents, anti-haze agents, lubricants, dyes, pigments, natural
oil, synthetic oil, wax, organic or inorganic fillers, etc.
so long as the incorporation does not impair the object of
the invention.
---20-- --- . Stabilizers to be used as. optional components include,
for example, phenolic antioxidants such as
tetrakis[methylene-3-(3,5-di-tert-butyl-4-
hydroxyphenyl)propionate]methane, alkyl (3-(3,5-di-tert-butyl-
9-hydroxyphenyl)propionate and 2,2'-oxamidobis[ethyl-3-(3,5-
2 S di-tert-butyl-4-hydroxyphenyl)]propionate, aliphatic acid
72932-124D
2124923
metal salts such as zinc stearate, calcium stearate and
calcium 1,2-hydroxystearate, and aliphatic acid esters of
polyhydric alcohols such as glycerin monostearate, glycerin
distearate, pentaerythritol monostearate, pentaerythritol
distearate and pentaerythritol tristearate. These
stabilizers may be used singly or in combination. For
example, tetrakis[methylene-3-(3,5-di-tert-butyl-4-
hydroxyphenyl)propionate]methane, zinc stearate and glycerin
monostearate may be used in combination.
1 0 Useful organic or inorganic fillers include silica,
diatomaceous earth, alumina, titanium oxide, magnesium oxide,
pumice powder, pumice balloons, aluminum hydroxide, magnesium
hydroxide, basic magnesium carbonate, dolomite, calcium
sulfate, potassium titanate, barium sulfate, calcium sulfite,
talc, clay, mica, asbestos, glass fibers, glass flakes, glass
beads, calcium silicate, montmorillonite, bentonite,
graphite, aluminum powder, molybdenum sulfide, boron fibers,
silicon carbide fibers, polyethylene fibers, polypropylene
fibers, polyester fibers and polyamide fibers.
__ 2 0__ . __ _ .These other components can be mixed with the
thermoplastic resin composition of the invention by any known
method. For example, each of these other components can be
simultaneously mixed with the thermoplastic resin
composition.
~,f _
72932-124D
2124923
Furthermore, the thermoplastic resin composition of the
invention may be incorporated with other resins so long as
the incorporation does not impair the object of the
invention. Examples of these other resins include
S polyolefins, halogen-containing vinyl polymers,
poly(meth)acrylate, polyacrylamide, polyacrylonitrile,
acrylonitrile/butadiene/styrene copolymer, acrylonitrile
styrene copolymer, acrylonitrile/styrene/acrylic acid ester
copolymers, polyvinyl alcohol, polyvinyl acetate, polyvinyl
stearate, polyvinyl benzoate, polyvinyl butyral, polyallyl
phthalate, polyallylmelamine, ethylene/vinyl acetate
copolymer, polyethylene oxide, polyacetal, polyphenylene
oxide, polycarbonate, polysulfone, polyurethane, urea resins,
polyamides, polyethylene terephthalate, polybutylene
1$ terephthalate, poly-1,4-dimethylolcyclohexane terephthalate,
phenol/formaldehyde resin, urea/formaldehyde resin,
melamine/formaldehyde resin, unsaturated polyester resins and
natural polymers.
w 2 0- ---w Molded articles in variows forms can be prepared by _
using the thermoplastic resin composition of the invention,
and utilizing conventional molding methods such as injection
molding.
For example, molded articles in a film or sheet form can
2 $ be manufactured by extrusion molding, and refrigerator
_y
72932-124D
2124923
interior boxes and trays can be manufactured by vacuum
molding. Moreover, there can be manufactured containers for
chemicals, drinks, etc., air ducts, sun visors, consoles,
automobile interiors, various toys, floats, etc. by blow
$ molding.
Concrete examples of molded articles which can be
manufactured from the thermoplastic resin composition of the
invention include
(1) automobile parts:
instrument panels, console boxes, meter clusters,
column covers, grille door mirrors, bumpers, fenders, bonnets
and radiators;
(2) machine housings:
tools (e. g., electric tools), business machines
(e. g., word processors, personal computers, copying machines,
printers, FDD and CRT), precision instruments (e. g., cameras)
and electrical appliances (e. g., electric ovens, electric
rice cookers, pots and cleaners); and
(3) others:
2 0~ ~ . toys, miscellaneous goods, furniture and sports
goods.
FFFFC'.T OF TH TN NTT(~N
The thermoplastic resin composition of the present
2 $ invention is excellent in characteristics such as rigidity,
-~b-
72932-124D
2'124923
dimension accuracy, impact strength and light resistance.
Accordingly, molded articles excellent in characteristics
such as heat resistance, rigidity, impact strength and light
resistance can be prepared from the resin composition of the
$ invention.
The present invention is illustrated below with
reference to examples, but it should be construed that the
invention is in no way limited to these examples.
Methods for measuring and evaluating various physical
properties in the invention are described below.
( 1 ) Melt flow rate (MFRT)
The melt flow rate is measured according to ASTM D 1238
at a predetermined temperature of T°C under a load of 2.16
1 5 kg .
(2) Preparation of test pieces
Test pieces are molded under the following conditions
using an injection molding machine (trade'name of IS-55 EPN,
manufactured by Toshiba Kikai K.K.) and a mold for the test
---2 0-- pieces
a cylinder temperature of 270°C and a mold temperature
of 90°C,
primary/secondary injection pressures of 1000/800
kg/cm2, and
an injection speed (primary) of 30 mm/sec.
'/_
72932-124D
2124923
(3) Flexural test
The flexural test is carried out according to ASTM D 790
under the following conditions:
the shape of the test piece: a size of Sxl/2x1/8t inches
S and a span of 51 mm,
a test speed of 20 mm/min, and
test temperatures of 23°C, 80°C and 100°C.
(4) Heat Deflection Temperature (HDT)
The heat deflection temperature is measured according to
ASTM D 628 under the following conditions:
a test piece size of Sxl/4x1/2L inches, and
a load of 264 psi.
(5) Softening temperature (TMA)
The softening temperature is measured by observing the
1S heat deformation behavior of a sheet 1 mm thick using Thermo
Mechanical Analyzer (trade name, manufactured by DuPont).
That is, a quartz needle is placed on the sheet, and the
sheet is heated at a rate of 5°C/min while a load of 49 g is
applied to the needle. The TMA is a temperature at which the
-~ 20-~ needle penetrates the sheet to the depth of 0.635 mm.
(6) Glass transition temperature (Tg) and melting point (Tm)
The glass transition temperature and the melting point
are measured by using DSC 20 (trade name, manufactured by
SEIKO Denshi Kogyo K.K.) and heating the test piece at a rate
2 S of 10°C/min .
72932-124D
2124923
(7) Rockwell hardness
The Rockwell hardness is measured at 23°C according to
ASTM D 785.
(8) Izod impact test
$ The Izod impact test is carried out according to ASTM D
256 under the following conditions:
a test piece (notched) size of 5/2x1/8xl/2t inches, and
a test temperature of 23°C.
(9) Tensile test
The tensile test is carried out according to ASTM D 638
under the following conditions:
a test piece shape: type IV,
a test speed of 50 mm/min, and
a test temperature of 23°C.
1 $ Exam lr~ a 1
[Preparation of a polycyclic (meth)acrylate polymer]
In a nitrogen atmosphere were mixed 99 parts by weight
of tetracyclo [ 4 . 4 . 0 . 12~ 5 . 17~ 1° ] dodecyl-3-acrylate (TDAC)
, 0 . 05
part by weight of n-octylmercaptan (OM) and 0.05 part by
2 0 weight of 2,2'-azobisisobutyronitrile (AIBN), and
polymerization was carried out at 80°C for 24 hours. The
resultant polymer (PTDAC) had an intrinsic viscosity ['~] of
0.58 dl/g as measured in toluene at 30°C and a TMA of 138°C.
[Preparation of a resin composition of PTDAC and a soft
2 $ polymer]
72932-124D
Eighty-five parts by weight of the thus obtained PTDAC
pellets and 15 parts by weight of an ethylene/propylene
random copolymer (having an ethylene structural unit content
of 80 mol$, a Tg of -54°C and an intrinsic viscosity [1~] of
2.2 dl/g) were sufficiently premixed. The mixture was melt
blended by a twin screw extruder PCM 45,
manufactured by Ikegai Tekkosho K.K.) at a cylinder
temperature of 230°C to be pelletized. Test pieces were
prepared from the pellets by the above-mentioned method, and
physical properties thereof were evaluated.
The results are shown in Table 1.
Exam 1~, a 2
Example 1 was repeated except that a soft polymer
prepared by a procedure described below was used in place of
the soft polymer of Example 1 to obtain a resin composition.
Test pieces were prepared from the resin composition.
The results are shown in Table 1.
[Preparation of a soft polymer]
To 100 parts by weight of a xylene solution (solute
2 0 concentration of 100 g/liter xylene) of an ethylene/propylene
random copolymer (having an ethylene content of 80 mobs and
an intrinsic viscosity ['~] of 2.2 dl/g as measured in decalin
at 135°C) held at 80°C in a nitrogen atmosphere was added
dropwise a mixture of 30 parts by weight of TDAC, 1 part by
weight of OM and 1 part by weight of AIBN over a period of 8
- 54 -
72932-124D
2124923
hours. The reaction was carried out for additional 16 hours
to obtain a TDAC-grafted ethylene/propylene random copolymer.
[Preparation of a polycyclic (meth)acrylate polymer]
S The procedure of Example 1 was repeated except that
tetracyclo[4.4.O.lz~5.17~1°]dodecyl-3-methacrylate (TDMAC) was
used in place of TDAC to obtain a polymer of TDMAC (PTDMAC).
[Preparation of a soft polymer]
To 100 parts by weight of an
ethylene/propylene/ethylidenenorbornene random copolymer
(having an ethylene content of 75 mold, an
ethylidenenorbornene content of 3 mol$ and an intrinsic
viscosity ['~] of 2.4 dl/g as measured in decalin at 135°C)
were added 30 parts by weight of TDMAC, 1 part by weight of
1S OM and 1 part by weight of AIBN to obtain a latex. The latex
was heated to 80°C in a nitrogen atmosphere. The reaction
was carried out for additional 24 hours to obtain a TDMAC-
grafted ethylene/propylene/ethylidenenorbornene random
copolymer.
2 0 [Preparation of a resin composition]
A procedure similar to that in Example 1 was carried out
by using PTDMAC and the soft polymer prepared above to obtain
a resin composition.
Test pieces were prepared from the resin composition,
2 5 and physical properties thereof were measured.
72932-124D
2124923
The results are shown in Table 1.
[Preparation of a polycyclic (meth)acrylate polymer]
The procedure of Example 1 was repeated except that a
$ mixture of 66 parts by weight of TDAC and 33 parts by weight
of styrene was used in place of 99 parts by weight of TDAC to
obtain a copolymer of TDAC and styrene (PTDAC/St). The
resultant polymer had an intrinsic viscosity [1]] of 0.61 dl/g
as measured in toluene at 30°C and a TMA of 121°C.
[Preparation of a soft polymer]
The procedure of Example 2 was repeated except that a
mixture of 20 parts by weight of TDAC and 10 parts by weight
of styrene was used in place of 30 parts by weight of TDAC to
obtain a TDAC/styrene-grafted ethylene/propylene random
copolymer.
[Preparation of a resin composition]
The procedure of Example 1 was repeated by using
PTDAC/St and the soft polymer prepared above to obtain a
resin composition.
2 0 Test pieces were prepared from the resin composition,
and physical properties thereof were measured.
The results are shown in Table 1.
72932-124D
2124923
The procedure of Example 2 was repeated except that the
proportion of PTDAC to the soft polymer was altered to obtain
a resin composition.
Test pieces were prepared from the resin composition,
S and physical properties thereof were measured.
The results are shown in Table 1
72932-124D
2124923
TensilHeat
Izod
Exampl * FlexuralFlexuraTensileelongareflectRockwellim a
t
modulusstrengtstrengttion T hardnessc
(wt.parts) - ' omp' p
strength
2 2 Z (%) ( (R-scale)(k ~cm/cm)
(kg/cm (kg/cm (kg/cm C)
) ) )
1 85/15 22000 750 450 15 113 110 5
2 85/15 21500 760 460 15 115 110 18
~
3 85/15 21800 750 470 15 114 110 20
4 85/15 21000 740 460 13 97 105 20
90/10 26500 800 530 10 115 115 10
6 70/30 16000 600 400 25 103 98 45
*: Polycyclic (meth)acrylate/soft polymer
S Examples 7 to 10
The procedures of Example 1 was repeated except that
acrylic rubber was used as a soft polymer and the type and
amount thereof were altered.
The results are shown in Table 2.
72932-124D
2124923
TensilHeat
Izod
Exam TYPe * FlexuraFlexuralTensileelon ~eflecc.ROCkwell
1 of a
p
acrylic(wt. modulusstrengtstrengttion~ t hardness
omp'
strength
rubberparts)(kg/cm2)(kg/cmz)(kg/cm2) ( (R-scale)
C)
(~) (k -cm/cm
7 NIPOL~~$5/1523500 770 470 15 116 110 15
AR-51*1
$ NIPOL 85/1522000 750 460 15 114 110 17
AR-54*2
9 NIPOL 90/1027000 820 550 10 115 lI5 10
AR-54r2
NIPOL 70/3016000 580 410 25 102 96 40
AR-5 ~ ~
4'2
Note: *: Polycyclic (meth)acrylate/acrylic rubber
In Table 2, the acrylic rubbers, NIPOL AR-51*1 and
5 NIPOL AR-54*2, are both acrylic rubbers manufactured by
Nihon Zeon K.K.
The acrylic rubbers, NIPOL AR-51*1 and NIPOL AR-54*2,
are each a compolymer containing recurring units
derived from a lower alkyl (meth)acrylate.
1 ~ ~-=,~ Tya~'-,ma-~.~2