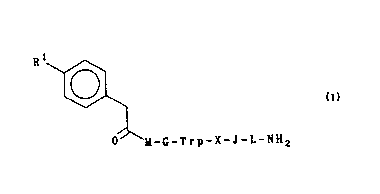Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~vo 93/13126 2 1 2 6 ~ 3 ~ PCI/GB92/02369
.. 1
Peptide compounds ha~in~ therapeutic acti~
This invention relates to peptide compounds having therapeutic activity (in particular
feeding inhibition), their use as pharmaceutical and cosmetic therapeutic agents, and
s formulations comprising them.
....
CCK-8 (a peptide having the structure As~Tyr(SO3H)-Met-Gly-Trp-Met-Asp-Phe-NH2)
is known to hav~ feeding inhibition properties [see for example J E Morley, Minireview:
'The ascent of cholecystokinin - from gut to brain', Life Sciences, vol 30 (6), pp479-493,
1982].
International Patent Application WO 91/08225 (to Fisons Corporation) discloses anumber of peptide compounds which are indicated for use in the inhibition of feeding.
A gr~up of peptide compounds have now been found which are particularly
advantageous.
According to the present invention, there is provided a compaund of formula I:
R l ~
ll-G-Trp-X-J -L-NH2
~s wherem
Rl is OH or OSO3H;
M is Met, Ahx or Ile:
G is Gly or Sar;
X is Met, Ahx, Ile, Phe or Lys(R2);
30 J is Asp, Asp(OBn), Dasp, MeAsp, or MeDAsp;
1 is Phe or MePhe;
R2 is a group of formula II,
wo 93/13126 pcr/GBs2/o2
2,~26 R3 R4
C(O~-E~Rs
R R
wherein E is NH, CH=CH or CH2CH2; and R3, R4, Rs, R6 and R' are independently
H, OH, halogen, alkyl C,.6 or OSO3H;
provided that when R' is OSO3H, G is Gly and:
(a) J is Asp, L is MePhe and M is Ahx, then X is not Ahx;
(b) J is Asp, L is MsPhe and M is Ile, then X is not Ile;
(c) J is Asp, L is MePhe and M is Ile, then X is not Ahx;
(d) J is Asp, L is Phe and M is Met, then X is no~ Met;
(e) J is Asp, L is MePhe and M is Met, then X is not Met;
(f) J is Dasp, L is MePhe and M is Met, then X is not Met;
~s or a pharmaceutically acceptable deri~rative thereof (hereinafter refelTed to en bloc as
"the compounds of the imendon").
.
The compounds of the inventdon, amino acids, pepddes and protecting groups are
represented by symbols commonly used in the art, for example those defined by IUPAC
~o and IUB.
All optically active amino acids have the L-configuration unless otherwise indicated.
Examples of symbals are given below:
Ahx 2-aminohexanoic acid
Asn asparagine
Asp aspartic acid
1 : ~sp(OBn) aspartic acid beta-benzyl ester
¦~ 30 Asp(OtBu) asparticacidbeta-tert-butylester
¦ Boc tert-butyloxycarbonyl
¦ ~ BrCH2-Pam 4-(bromomethyl)phenyl-acetamidomethyl
1~ DAsp D-aspartic acid
1 ~
~YO 93/13126 212 6 2 3 6 PCI`/GB92/02369
Fmoc 9-fluorenylmethyloxycarbonyl
Gly glycine
HOBt 1 hydroxybenzotriazolyl
Hpa 4-hydro~yphenylacetic acid
s Hpa(SO3H) O-sulpho-4-oxyphenylacetic acid
Ile isoleucine
Lys Iysine
MeAsp N-methylaspartic acid
MePhe N-methylphenylalanine
o MePhe-NH2 N methylphenylalanine amide
Met methionine
Nle norleucine (or 2-aminohexanoic acid)
OBt 1-benzotna~ulyl ester
OCH2-Pam ~oxymethylphenylace~amidomethyl
lS OSu succinimidylo7y ester
OtBu tert-but~lester
Phe phenylalanine
Phe-NH2 phenylalanine amide
resin pohfs~rrene
20 Sar sarcosine
Tbu tcrt-butyl
Tyr(SO3H) O-sulphoty~osiIle
.. , , , . "` I , !
Thus, Hpa(SO3H)-Met-Gly-Trp Met-Asp-MePhe-NH2 is the compound of formuia I in
25 which Rl is OH, M is Met, G is Gly, X is Met, J is Asp and L is MePhe:
~~ 3~S ~ oo~ 3
3~ 2
S`C113
WO 93/13126 PCr/GB92/023~
2,~,2,6~36
- By Lys(R2) we mean a Iysine residue in which the c-amino group forIsls an amide bond
with a group of ~ormula II, as defined above. Two palticular groups of formula II which
may be mentioned are ~he group in which E is NH, R3 is methyl and R'-7 are each
hydrogen, ie (2-methylphenyl)aminocarbonyl, also referred to herein as '~ac"; and the
c group in which E is trans-CH=CH, Rs is OH and R3, R~, R6 and R' are each hydrogen.
Pharmaceutically acceptable derivatives of the compounds of formula I include es~ers
and amides of any carboxylic acid groups which may be present, and pharmaceutically
acceptable salts. Pharmaceutically acceptable derivatives which may be mentionedo include unsubstituted amides of carboxylic acid groups (for example Asp may be present
as its unsubstituted amide derivative Asn) and alkyl Cl 6 (for example methyl) esters of
carboxylic acid groups. Pharrnaceutically acceptable salts which may be mentioned
include sodium and ammonium salts. Pharmaceutically acceptable derivatives of
compounds of formula I may be prepared from the corresponding compolmd of formula
15 I by conventional methods.
The term "pharmaceutically acceptable" used herein should be constmed to mean that
the compound, der~vative, salt or other substance to which it refers is suitable for
administration to the body as a pharmaceutical or cosmetic therapeutic agent. Similarly,
.o terms such as "use as a pharmaceutical" and "pharmaceutical formulation" include use
as a cosmetic therapeutic agent and a cosmetic therapeutic formulation respectively.
Preferably M is Ah~ or Ile; G is Glr, X is Ahc, Ile or Lys(R2~; J is Asp, Dasp, MeAsp
or MeDAsp; and 1~ is OSO3H.
~s
According to a further aspect of the invention, there is provided a process for the
preparation of a compound of formula I, or a pharmaceutically acceptable derivative
thereof, which comprises:
a) sulphating a compound of formula III,
.
~0 93/t3126 212 6 2 3 6 PCl'/GB92/02369
H0 ~
~ Trp-Xa-Ja-L-Za
wherein M, G and L are as defined above; Ja has the same definition as J above, except
tha~ the ,~-carbo~yl group of any Asp, Dasp, MeAsp or MeDAsp residue present is
optionally protected; Xa has the same definition as X above, except that it may
additionally represent Lys and any hydroxy or arnino group is present in protected form
o (except for any hydroxy groups to be conver~ed to a sulphate ester); and Za is NH2 or
a carboxyl protecting group;
b) removing one or more protecting groups from a compound of formula IV,
R~
15' ' ~ lV
O~U-G-T rp-Xa-J a-L-Za
wherein R', M, G, Xa, Ja, and Za are as defined above, and at least one of Xa, Ja9 and
Za comprises a protecting group;
~o c) reacting a compound of formula V,
HO
~ Y
2s O~l-G-Tr p-Xb- J -L-NH2
wherein M, G, J and L are as defined above, and Xb is Lys, with a compound of
formula VI,
R3 R4
O~C~N ~RS
R R6 Yl
2 ~2 6 23 Pcr/GBg2/023~
wherein R3, R4, Rs, R6 and R' are as defined above, to give a corresponding compound
of formula I in which X is Lys(R2~ and E is NH; or
d~ coupling a compound of formula V as defined above with a compound of
formula VII,
C R3 R4
~ ...
HO-C ~ O ) -E--<O~--R5
R~\R ~
,0 wherein E is CH2CH2 or CH=CH and R3, R~, Rs, R6 and R7 are as defined abov~, to
give a corresponding compound of formula I in which X is Lys(R2) and E is CH2CH2or CH=CH.
The invention provides intermediate compounds of formula rv per se.
In process (a~, the sulphating agent may be, for example, sulphur trio~ade or a complex
thereof, such as sulphur ~rio~ide pyridine. We particularly prefer to carIy out the
sulphation in a polar aprotic sohent, for example, dimethylfonnamide or p~ridine. The
reaction is preferably camed out using an excess of sulphating agent, for example a 1-40
20 molar excess, preferably a 5 molar excess.
In processes (a) and (b); protecting groups for peptides and methods for their removal
are well ~own in the ar~, see for example, T W Greene, Protecti~e Groups in Organic
Synthes~ Wiley-In~erscience (1981~. The choice of protecting groups ~td the methods
2s employed for their removal will depend, in-er alia, on the method of synthesis employed
for the preparation of the peptide and the a~ino acids in the peptide. Suitable amino
protecting groups include, for example, benzyloxycarbonyl, which may readily be
removed by hydrogenolysis or hydrogen bromide in acetic acid; t-butyloxycarbonyl,
(Boc), which is removed by standing the peptide in cold trifluoroacetic acid; Fmoc,
30 which may be removed by treatment v~ith dilute piperidine ~20% in DMF);
~4-methox~benzyl)oxycarbonyl and ~-nitrophenylsulphenyl. The Boc and Fmoc groupsare particularly preferred.
~o 93/13126 212 6 2 3 6 PCr/GBs2/02369
Suitable carboxyl protecting groups that Za may include are, for example, methyl,
tert-butyl, benzyl and ~methoxybenzyl. We particularly prefer benzyl, which may be
readily removed by treatment with alcoholic amine or ammonia to give the
corresponding amides. Similar groups may be used to protect the amino group in Iysine
s and the carboxyl group in aspartic acid.
When the peptide is prepared using solid phase techniques, for example those in which
th~ carboxyl end of the peptide is attached to a solid phase resin, linkage of the peptide
to the resin acts as a carboxyl protecting group. Cleavage of the peptidyl-resin linkage
o will deprotect the carboxyl terminus of the peptide. Since the peptide end produc~s of
this invention are carboxyl terminal amides, the chemical link which connects the
peptide chain to the resin must be such that its cleavage with suitable reagents readily
provides amides. Due to the lability of the sulphate ester group to strong acids (for
example, liquid hydrogen fluonde), the peptidyl-resin linkage may be cleavable with
15 either weaker acids (for example, brief trcatment with trifluoroacetic acid, TFA) and/or
nucleophiles (for example, amunonia, amines, hydroxide, and aLtcoxides).
Process (c) may be carried out in an inert solvent, for example DMF, in the presence
of a base such as N-methylmorpholine, and at a temperature of, for example~ from 0~-
20 SOC
Process (d) may be canied out using an activated ester derivative of the acid. Asuitablc activated ester derivative is the N-hydroxy succinimidyl ester. The reaction may
be carricd out irl the presence of a base such as N-mcthylmorpholine, under similar
2s conditions to those described ~or process (c) above.
Among suitable resin derivatives may be mentioned o~methyl-polys~qrene,
~(oxymethylphenyl)-(CH2),~-aminomethyl-polystyrene(n=0-3~and~(~methylphenyl)-
oxymethyl-polystyrene. Similarly substituted polyacrylamide resins are equallywell suited
30 as the abc~re polysn~rrene based resins. The term "polystyrene" includes copolymers with
minor amounts, usually 1%, of unsaturated monomers such as divinylbenzene.
WO 93/13126 PCr/GB92/û23~
2~264~ qmethylphenyl)-CH2CO aminomethyl polystyrene lherein referred to as
4-(oxymethylphenyl)-acetamidomethylpolystyrene or OCH2-Pam-resin~ is particularly
prefelTed for the generation of peptide amides. This linkage may readily be cleaved to
give the peptides of formula I by reaction with methanolic solutions of ammonia,s alkylamines or dialkylamines as required.
Another resin which may be mentioned is a polgstyrene resin (P) in which the backbone
linkage to the peptide is,
CH~
~ .
OCH3 ~ ~
Frnoc-NH-CH2--~CCH2)4-CO-Nle-NH-CH-~--~
OCH3
and herein referred to as ~5-[(4-Fmoc-aminomethyl)-3,5-
dimethoxyphenoxy]valeroyl]norleucyl]-4'-methylbenzhyd~rlamine divinylbenzene
polyst~rene or PAL resin. PAL resin is pa~ticularly preferred for the generation of
peptide amides in which X is not Lys. The li~kage between the assembled peptide and
this resin may be cleaved readily by reaction with the reagent formed by mixing TFA
20 (trifluoroacetic acid), phenol~ thioanisole, water and ethanedithiol in the proportions
8.5:0.5:0.5:0.5:0.2.
Another resin which may be substituted for the PAL resin is a polysty~ene resin (P) in
which the backbone linkage to the peptide is,
'~ O-P
Fmoc- NH~
CH30~
OCH3
2126236
,~0 93/13126 - PCI/GB92/0236g
andreferredtoas4-[(2,4-dimetho~yphenyl)(Pmoc-amino)methyl]pheno~ydivinylbenzene
polystyrene or Rink resin. The linkage may be readily cleaved in the same manner as
for PAL resin.
s The peptides of formulae III, IV and V may be prepared by methods well known to
those skilled in the art. For example, they may be prepared b~ ~ombining individual
amino acids on a solid phase resin on a step-by-step basis, or alternatively, by combining
groups of amino acids on a solid phase resin to yield the desired peptidyl-resin
intermediate. Such additions are accomplished by protecting the amino group of the
o amino acid or group of amino acids by converting it to, for example, its
tert-butyloxycarbonyl (Boc) or9-fluorenylmethyl-oxycarbonyl (Fmoc) derivative, and then
activating the carboxylic group of such amino acid or group of amino acids by converting
it, for example, to its l-hydroxybenzotriazole (HOBt) or N-hydroxysuccinimide (HOSu)
ester derivative. Such a protected-activated intermediate is then allowed to react with
s an amino acid rcsin or peptidyl-resin with a free amino group, thus extending the
pepdde chain to pro~ride the desired pcptidyl-resin.
Thc C-terminal amino acid of the peptide to bc prepared may be attached to the
OCH2-Pam-resin in several ways. For example, Boc-protectcd N-methylphenylalanine,
20 may be reacted with a suitable 4-(bromomethyl)-phenylacetate ester (for example,
~- phenacyl ester) and processed further to provide Boc-MePhe~ o~nethylphenyl)acctic
acid whicb~ may be coupled to aminomcthyl-polystyrene to provide Boc-MePhe-
~;~ (40~ethylphcnyl3acetamidomethylpolystyrene (Boc-McPhe-OCH2-Pam-resin).
Alternativcly, 4-(bromomethyl)phcnylacctic acid may be coupled to
s aminomcthylpolystyrene to provide 4-(bromomethyl)phenylacetamidomethylpolystyrene
(BrCH2-Pam-rcsin) which may be reacted with the caesium salt of Boc-MePhe-OH to
provide Boc-Phe-OCH2-Pam-resin.
The C-terminal amino acid may be attached to the PAL resin by removal of the Fmoc
30 protecting group with base, for example, piperidine, in a suitable solvent or mixture of
sohcnts, for emmple, DMF and toluene, and then coupling the protected activated
amino acid in the normal manner for solid phase synthesis. A particularly preferred
metbod of activating the carbo~l group is to form the N-hydroxvbenzotriazole (HOBt)
6~ lo PCI`~GB92/023fi~
ester in the presence of diisopropylcarbodiimide (DIPCDI). Suitable solvent systems,
for example, dimethylformamide (DMF) and dichloromethane (DCM) may be used for
this preactivation procedure.
s Among the suitable activating groups may be mentioned any combination of groups
which causes the acid function of the amino acid to become more reactive, such as acid
chlorides, mLxed and symmetrical anhydrides, reaction product with carbodiimide (for
example, dicyclohe~ylcarbodiimide, DCC), and active esters (for example, esters denved
from HOBt, HOSu, 2- or 4-nitrophenoL and 2,4,5-trichlorophenol). The use of DCC
o and esters of HOBt and HOSu is particularly preferred from the standpoint of ~ield,
lack of by-products, and consequent ease of purification.
An automatic peptide synthesizer may be used for the solid phase synthesis of the
sulphated peptide amides of this invention. The sulphate ester containing peptides of
15 formula I may be desalted and purified by the usual methods. For example, the product
may be puri~ed by ion-exchange chromatography with the use of Trisacryl M DEAE,
DEAE~ellulose or the li~e, partition chromatography with the use of Sephadex L~I-20,
Sephadex G-25 or the like, rcverse phase chromatography with the use of Amberlite
XAD-2 (or Biorad SM-2), ODS-silica gel or the like, normal phase chromatography with
20 the use of silica gel or the like, or high-performance liquid chromatography (HPLC).
The protocol of coupling onto an aminomethyl-resin or peptidyl-OCH2-Pam-resin (1- mmolc of available nitrogen), deprotecdon, sulphadon, cleavage, and product
purification is set forth in Table 1.
2s
Table 1
Protocol for Solid Phase Svnthesis of Sulphated Peptide Amides on Pam-resin (lmmole
~, scale!
!~
30 Step Reagent or Solvent Purpose ~Time
1 DCM Wash 1 min
2 Go to step 3, 5 or 8 ..... .....
3 Add filtered, pre- Pre- 2-15 hr
2126236
~o 93J13126 pcr/GBs2/o2369
11
activated (0C, 1 hr) activated
mixture of protected amino DCC/HOBt
acid (or protected coupling
dipeptide, 3mmole),
s HOBt (4.5mmole), and DCC
(3mmole) in 1:4 DMF/DCC
4 Go to step 10, 16, 21 ..... ~
or 26
Add protected amino acid In situ 2-15 hr
o (or protected dipeptide, activated
3mmole) in and HOBt DCC/HOBt
(4.5mmole) in 30ml coupling
1:2 DMFIDCM then DCC
(3mmole) in 20m1 DCM
lS 6 2-propanol Wash 1 min
7 Go to step lQ 16, 21 ..... .....
or ~6
8 Add activc ester or Non DCC/HOBt 2-15 hr
anhydride (3mmole) in DCM, acti~rated
DMF or a mixture thereof coupling
9 Go to step 10, 16, 21 ..... .....
or 26
10 DCM Wash 1 min
11 Treat with 49:1 TFAI Boc and 30 min
2s anisole/DCM tBu removal
12 DCM Wash 1 min
13 Treat with 1:19 DIEA /DCM Neutralise 1 min
14 DCM Wash 1 min
15 Go to step 10, 16, 21 ..... .....
30 or 26
16 DMF Wash 1 min
17 Treatwith 1:4 Fmoc removal 3 min
piperidine/DMF
:~ .
WO 93/13126 PCr/GB92/023~
~,~26 ~36 - 12
18 Treat with 1:4 Fmoc removal 7 min
piperidinç/DMF
19 DMF Wash 1 min
20 Go to step 10, 16, 21 ..... .....
s or 26
21 DMF Wash 1 min
22 1:2 pyridine/DMF Wash 1 min
23 Add sulphur trio~de Sulphation 20-24 hr
pyridine complex (40mmole)
o in 60ml 1:2 pyridine/DMF
24 DMF Wash 1 min
25 Go to step 10, 16, 21 ..... .....
or 26
.s 26 Methanol Wash 1 min
27 Ammonia saturated (-20~C) Resin 2-S
methanol or 20% cleavage days
methanolic amine (2SOml)
28 Methanol Wash 1 min
20 29 Combine and concentrate Isolation .....
Sltrates from steps 27-28
30 C~romatograph residue Purification .....
on column(s) of Ambcrlite
XAD-2 (Rohm and Haas,
2s 2.5 x 60 cm, methanol
gradient 0.1M in ammonia),
Trisaayl M DEAE (LKB Inc.,
æ5 x 47 cm, ammonium
bicarbonate gradient),
30 and/or P-40 ODS-3 (Whatman,
4.8 x SO cm, methanol
gradient 0.2~o in ammonium
acetate).
: . -
_ . ... ~ . . . . . .. . . ~ . . .
~o 93/13126 212 6 2 3 6 ~/FB92/02369
DIEA is diisopropylethyla=e
A general procedure for the synthesis of non-sulphated peptide amides on PAL-resin
~lg scale) is set out in Table 2.
Table 2
Step Reagent or Solvent Pu~pose MixTime
DCM wash 1 min
2 DCMIDMF (1:1) wash 1 min
3 30% Piperidine Fmoc 3 min
o 35% DMF removal
3S% Toluene
4 Repeat 3 Fmoc 7 min
removal
S DCM/DMF (1:1) wash (10x) 25 sec
s 6 Go to step 7 or 11 ..... .....
7 Preacti~ated-protected Pre- 10 min
amino acid Iroom activation
tcmperature, Fmoc-aa-OH, [Omit HOBt
DIPCDI (1 eq), HOBt (1 eq), if preceding
~o in DMF:DCM(1:4)] amino acid
is MeAs~, MeDAsp
or MePhe-]
8 Add to resin or resin- Coupling 1-2 hr.
pepdde
2s 9 DCM/DMP (1:1) Wash (4x) 1 min
10 Go to step 3 lRepeat ..... .....
sequence 3-10 as necessary
to complete peptide
sequence]
30 lI Preactivated Hpa-Osu Pre- 10 min
lHpa-OH (1 eq), HO~u ac~vation
(1 eq), DM~l
12 Add to resin-pep~ide Coupling 1-2 hr
W O 93/13126 PC~r/G B92/0 ~
212~23 6 14
- 13 DCM Wash (4x) 1 min
14 MeOH Wash (4x) 1 min
TFA/phenoVthioanisole/ Cleavage 2-3 hr.
water/ethanedithiol from resin
[8.5:0.5:0.~:0.5:0.2]
16 TFA Filter ..... -
resin and
wash
17 Combine and concentrate Isola~ion .....
filtrates from step 16 of peptide
18 Chromatograph residue on Purification .....
column(s) of Amberlite
XAD-2 (Biorad SM-2 20~400
mesh, MeOH gradient 0.1M
lS in ammonia) and/or C-18 (ODS-3
Amicon, MeOH gradient, 0.1%
in triethylamine, 0.1% in
glacial acetic acid) and/or
Amberlite XAD-2 (MeOH
~o gradient).
19 Combine and concentrate Isolation
purified frac~ions by
~eezc ~g from 0-lM - -
~I3
2s '
Modifications of the protocols in Tablès 1 and 2 which may be applicable may readily
be determined by experimentation.
Analogous procedures, wherein the reactions are carried out without the solid phase
30 component (resin), are well known in the art and are well suited to large scale
production ~see for example US Patent No 3,892,726).
~yO 93/13126 212 6 2 3 6 PCI/GB92/02369
The compounds of the invention inhibit feeding activity in mammals, and tO bind tO
cholecystokinin receptors. Distinct CCK receptors in peripheral and brain tissues have
been classified as CCK-A and CCK-B receptors respectively Differentiation between
agonist and antagonist interactions at CCK receptors can also be determined by
;~ 5 functional assays. Activation of CCK-A receptors in peripheral tissues plays an
important role in the control of pancreatic secretion, gut motility and gall bladder
contraction. Thus compounds with agonist activity at CCK-A receptors have utili~ in
the treatment of obesity and motili~r disorders ~nd compounds with antagonist activity
at CCK-A receptors may have utility in gastrointestinal disorders such as irritable bowel
~ Io syndrome, ulcers, excess pancreatic or gastric secretion, acute pancreatitis and motllity
'~ disorders. Therefore, compounds intended for use as therapeutic agents in the
inhibition of feeding are likely to lack unwanted side-effects if they bind selectively to
CCK-A receptors (rather than CCK-B receptors).
`' 15 Pharmacological activity of the compounds of the invention can be dcmonstrated in
Tests A-D below.
Test A
Feedin~ inhibition
~i 20 Male Sprague-Dawley rats (weighing 300-350g) are individually caged and maintained
on a 12 hour light/dark cyclc and trained for at least 14 days to feed during a three hour
period of the dark cycle but not thc 21 hours prcceding that three hour period. On the
- day of thc study, rats are dosed intrapcritoncallywith saline (controls) or test compound
7 (dissolved in salinc; usually at a concentration of 03 to 30Q~g of test compound per kg
2s of rat weight). Food is introduced 10 minutes after administradon of saline or test
compound. A test compound is deemed to be active if the test group consumes
significantly less food than thè saline controls during the feeding period, which ends
either 0.5 or three hours after presentation of the food.
30 Tes~ B
- CCK-A Bindin~
Evaluati~n of a test compound for its ability to bind to CCK-A receptors in rat
pancreatic membranes is measured against the binding of Bolton Hunter '2sI-CCK-8 and
i
WO 93/13126 pcr/GBs2/o23~
2~26~36 16 '
3H-L364718 to rat pancreas according to the procedures of Chang, Lotti, Chan andKunkel (Molecular Pharmacolgy, 30:212-216, 1986).
Test C
s CCK-B Bindin~
Evaluation of a test compound for its abiJity to bind to CCK-B receptors in rat cerebral
cortex membranes is measured against l2sI-CCK-8 according to the procedures of Chang
and Lotti (Proc, Natl. Acad. Sci. VoL 83, 4923-4926).
o Test D
Functional Assav For CCK-A Agonist/Antagonist Activitv
The evaluation of a test compound for its ability to inhibit or stimulate amylase release
by rat pancreatic tissue fragments (acinar cells) is measured according to the procedures
of Iin et al (J Pharm & Exper Therapeutics, 1986, 729-734) and Jung (Clinica Chema
15 Aeta, 1980,.~, 7-11).
Therefore, the invention also provides the use of a compound of the in~ention as a
pharmaceudcal.
.
20 Aeeording to a further aspect of the invention there is pro~ided the use of a eompound
of the imention in the manufacture of a medieament for the treatment of obesity.
,;~ ~ .
Aeeording~to the imention thero is also p~ovided a method of treatment of obesity
whieb co~ es administering a therapeutieally effeeti~e amount of a compound of the
s imendon to a Fatient in need of sueh treat nent; and also a method of improving the
bodi~ appearanee of a mammal whieh eomprises administering to that mammal a
eompound of!the imendon until a eosmetieaDy benefieial loss of body weight has
occwred. The mammals of greatest interest are human beings.
30 Aeeording to a further aspeet of the invention there is also pro~nded a pharrnaceutieal
:
formuladon eomprising (preferably less than 80%, and more preferably less than 50%
~ ~ ~y weight of) a eompound of the invention in combination with a pharmaceutieally
; ~ acceptaUe adjuvant, diluent or carrier.
~VO 93/13126 21 2 6 2 3 6 pcr/G~92/o236s
The compounds of the invention may be adminis~ered by a varie~ of routes, for
example, orally, intrapentoneally, intravenously, intramuscularly, subcutaneously or
intranasally. The dosage of the compounds of the invention will depend on several
factors, including the requirements of the recipient, but will typically be in the range
s 0.3~Lg to 3.0mg per kg of body weight per day, either in a single dose or divided among
two to four doses. ~
Examples of suitable adjuvants, diluents or carriers are:
for tablets and dragees; lactose, starch, talc or stearic acid;
10 for capsules; tartanc acid or lactose;
for injectable solutions; water, alcohols, glycerin or vegetable oils.
The compositions may also contain suitable preserving, stabilising and wetting agents,
solubilisers (eg a water-soluble cellulose polymer such as hydro~propyl methylcellulose,
S or a water-soluble glycol such as propylene glycol), sweetening and colouring agents and
flavounngs. The compositions may, if desired, be formulated in sustaincd release form.
The compounds of the invention haYe the advantage that they are more efficacious,
more potent, longer acting, more stable (particularly to en~ymatic degradation), more
20 selective, less toxic, give rise to fewer side ef~ects, are more readily absorbed, are
quicker acting, or have other advantageous pharmacological e~ects, in comparison with
the compounds of thc prior art.
The imcnffon is illustratcd by the following examples, in which an automatic pcptide
2s synthesizer was used for solid phase synthesis~
Example 1
Boc-MePhe-~ox~nethvlphenvl!acetic acid
To a solution of Boc-MePhe-OH (27.93g) and ~bromomethyl)phenylacetic acid
30 phenacyl ester (33.32g) in acetonitrile (11) was added potassium fluoride dihydrate
(18.28g). The suspension was stirred ~vernight, filtered and the filtrate evaporated to
d~yness. The residue, Boc-MePhe-(~o~methylphenyl)acetic acid phenacyl ester, wasdissolved in 85~ acetic acid (1.21), treated with zinc dust (128g~, and stirred for 2-4
WO 93/13126 PCI`/GB92/023~
~6~3~ 18 `
?J hours. Concentration of the filtered reaction mLxture to about 400ml and dilution with
3.2ml of water gave an oil which was dissolved in ethyl acetate and treated withdicvclohexylamine (DCHA) to ghe 41.31g of the DCHA salt of the title compound, mp
120-122C.
ample 2
H-MePhe-OCH2-Pam-resin
Boc-MePhe-(4-o~nethylphenyl)acetic acid (the product of Example 1, 1.82g, 3mmoleof its DCHA salt) and HOBt ~6.9g, 4.5mmole) in 40ml of 1:3 DMF/DC~
10 (dimethylformamide/dichloromethane) followed by DCC (1,3-dicyclohexylcarbodiiIffide,
0.62g, 3mmole) in 20ml of DCM were added to aminomethylpolystyrene resin (1.34g,lmmole available nitrogen) to give a suspension which was shaken for 2 to 15 hours.
Boc MePhe-OCH2-Pam-resin was isolated by filtration, washed with 2-propanol and
DCM, and treated according to Table 1 (steps 10-14) to give the title compound as the
5 free base.
ample 3
H-Phe~C~I~-Pam-resin
Boc-Phe~4-o~ymethylphenyl)acetic acid (prepared by the method of Example 1, 0.83g,
20 2mmole), l-hydroxybenzotriazole (HOBt, 0.46g, 3mmole) and DCC (0.41g, 2mmole)were dissolved in 50ml of 4:1 DCM/DMF and stirred at 0C for 1 hour.
Aminomethylpolystyrené resin (134g, lmmde available nitrogen) was suspended in the
filtered reaction mixture (precipitated DCU removed) and shalcen for 2-15 hours. The
product, 80c-Phe-OCH2-Pam-resin, was isohted b~r Sltration and treated according to
2s Table 1 (steps 10-14) to give the title compound.
~xample 4
Fmoc-Met-AsR~OtBu!-OH
Fmoc-Met-OSu was prepared in situ by the reaction of Fmoc-Met-OH (14.87g), HOSu
30 (5.S2g) and DCC (8.26g) in THF (tetrahydrofuran, 200ml) at 0C for 3.5 hours.Precipitated dicyclohe~ylurea (DCU) was removed by filtration and the THF filtrate was
added to a cold solution of H-Asp(OtBu)-OH in 220ml of 10:1 watertI~ to which had
been added 40ml of lN sodium hydrowde. After stirring the reaction mixture at room
~yO 93/13126 212 ~ 2 3 6 pcl/GB92/o236s
19
temperature overnight, solid citric acid (20g) was added along w~th ethyl acetate
(600m1~. The ethyl acetate layer was separated, washed with 10% citric acid and bnne,
then dried (MgSOI). Evaporation of the ethyl acetate solution gave a residue which was
dissolved in ethyl acetate (200ml) and treated with DC~HA (7.84ml) to precipitate 17.93g
of the DCHA salt of the ~itle compound, mp 159-162~C.
ample 5
Hpa(SO.H~ Gly-Trp-Ahx-Asp-Phe-NH2 (SEQ ID NO 1)
H-Phe-OCH2-Pam-resin (the product of Example 3) was sequentially coupled with
~o Fmoc-Asp(Ot~u)-OH, Fmoc~ OHt Fmoc-Trp-OH, Fmoc-Gly-OH, Fmoc-Ahx-t~H
according to Table 1 ~coupling steps 5-7 followed by Fmoc removal steps 1~20) toprovide H~ Gly-Trp Ahx-Asp(C)tBu)-Phe-OCH2-Pam-resin which was coupled with
Hpa-OSu according to Table 1 (coupling steps ~9~ to give Hpa-Ahx-Gly-Trp-Ahx-
Asp(OtBu)-Phe-OCH2-Pam-resin which was deprotected, sulphated and cleaved from
the resin according to Table 1 (steps 1~15, steps 21-2~ and then steps 2~29 withasnmonia) to give the title compound which was chromatographically purihed on SM-2
and ODS-3 columns sequcntially according to Tablc 2 (step 18). Amino acid analysis
following acid decomposition gave Asp 1.03(1~, Ciily 1.05tl), Ahx 1.94(2), Phe 0.99 (1),
Trp 0.74 (1), NH3 2.16. MS (FAB): m/e 961 (M-H~.
2~
Example 6
.
- Hpa-Met-Glv-T~Met-DAs~Phe-NH2
PAL-rcsin was deprotectcd and sequentially coupled according to Table 2 with Fmoc-
Phe-OH?Fmoc-DAsp(OtBu)-OH,Fmoc-Met^OH,Fmoc-Trp-OH,Fmoc-Gly-OH,Fmoc-
2s Met-OH and Hpa-OSu to provide the title compound. Amino acid analysis following
acid decomposition gave Asp 1.04(1), Gly 1.07(1), Met 1.79(2), Phe 1.10(t), Trp
0.71~1.0), NH3 1.40. MS (FAB): m/e 919 (M+H)~.
Example 7
30 Hpa-Ahx-Glv-T~p-AI~-As~Phe-NH2 (SEQ ID NO 2)
PAL-resin was deprotçcted and sequentially coupled according to Table 2 with ~moc-
Phe-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Ahx-OH, Fmoc-Trp-OH, Fmoc-Gly-QH, Fmoc-
Ahx-OH and Hpa^OSu to provide the title compound. Amino acid analysis following
WO 93/1~326 PCI`/GB92/023~
~623 20
acid decomposition gave Asp 1.d0(1), Gly 1.08(1.0), Ahx 1.85(2), Phe 1.10(1), Trp
0.68(1), NH3 1.00. MS (FAB): m/e 883 (M+H)+.
Example 8
s Hpa-Ahx-Glv-Trp-Ahx-MeAsp-Phe-NH2 (SEQ ID NO 3)
PAL-resin was deprotected and sequentially coupled according to Table 2 with Fmoc-
Phe-OH, Fmoc-MeAsp(OtBu)-OH, Fmoc-Ahx-OH, Fmoc-Trp-OH, Fmoc-Gly-OH,
Fmoc-Ahx-OH and Hpa-OSu, to give the title compound. Amino acid analysis following
acid decomposition gave Gly 0.89(1), MeA~p 1.04(1), Ahx 1.99(2~, Phe 1.09(1), Trp
to 0.71t1), NH3 a.sl. MS (FAB): m/e 897 (M+H) ' .
Example 9
Hpa-Ile-Glv-Trp-Ile-DAsp-MePhe-NH2
PAL-resin was deprotected and sequentially coupled according to Table 2 with Fmoc-
s MePhe-OH, Fmoc-DAsp(OtBu)-OH, Fmoc-Ile-OH, Fmoc-Trp-OH, Fmoc-Gly-OH,
Fmoc-Ile-OH and Hpa-OSu, to give the title compound. ~unino acid analysis f~llowing
acid decomposition gare Asp 1.00~1), Gly 1.14(1), Ile 1.91(2), MePhe 0.96(1), Trp
0.64(1), NH3 1.44. MS (FAB): m/e 897 (M+H)+.
20 E~mple 10
HpafSO )-Met-Glv-Trp-Met-DAsp-Phe-NH2
Hpa-Met-Gly-Trp-Met-DAsp-Phe-NH2 (the product of Ex~unple 6, 114mg) was dissolved
in pyridino (1.4ml) and sulphur trio~ade pyridine complex (130mg3 was added. Thereaction was stirred for 2 houss then an additional 80mg of sulphur trio~ade pyridine
2s complex was added. After 4 hours the reaction was diluted with 5% NH~OH (20ml)
~nd concentrated to d~yness. The crude residue was purified by chromatography onSM-2, ODS-3, SM-2 sequentially according to Table 2, step 18. The resulting product
was freeze dried from 0.1M NH3 to give the title compound (85~mg). Amino acid
analysis following acid decomposition gave Asp 1.03~1), Gly 1.11(1), Met 1.83(2), Phe
30 1.04(1), Trp 0.59(1), NH3 2.2. MS (FAB): m/e 997 (M-H)-.
ample 11
Hpa(SO~H)~ Glv-Trp Ahx-MeAsp-Phe-NH2 (SEQ ID NO 4)
,
~n~ 93/13126 2 I 2 6 2 3 6 PCr/GB92/02369
21
Hpa~ Gly-Trp-Ahx-MeAsp-Phe-NH2 (the product of Example 8, S5mg) was
sulphated essentially according tO the method of Example 10 to give purified title
compound (lSmg). Amino acid analysis following acid decomposition gave MeAsp
1.07(1), Gly 1.04(1), Ahx 1.90 (2), Phe 0.98 (1), Trp 0.61 (1), NH3 0.79. MS (FAB): m/e
5 975 (M-H)--
Example 12
Hpa(SO~H!-Ile-Glv-Trp-lle-DAs~MePhe-NH2
Hpa-Ile-Gly-Trp-Ile-DAsp-MePhe-NH2 (the product of Example 9, 58mg) was sulphated
.o essentially according to the method of Example 10 to give purified title comp~und
(16mg). Amino acid analysis following acid decomposition gave Asp 1.08(1), Gly
1.08(1), Ile 1.97(2), MePhe 0.87(1), Trp 0.75(1) NH3 1.39. MS ~FAB): m/e 97S (M-H)-.
Example 13
~s ~ ~ Glv-Trp-Met-Asp-MePhe-NH2 (SEQ ID NO S)
Thc title compound was prepared follo-~nng the method of Table 2, sequentially coupling
Fmoc~McPhe-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Met-OH, Fmoc-Trp-OH, Fmoc-Gly-
OH, Fmoc-Met-OH and Hpa-OSu. Amino acid ana~sis following acid dccomposidon
gave Asp 1.06 (1). Gly 1.04 (1), MePhe 1.00 (1), Met 1.90 (2), Trp 0.84 (1), NH3 0.51.
20 MS ~FAB): m/c 931 (M-H).
~plc
Hpa-Al~l~-Trp-AI~-DAsp-MePhe~
;~ PAL resin was sequentially coupled according to Table 2 with Fmoc-MePhe-OH, Fmoc-
2S DA9~R04H, Fmoc-AlL~-OH, Fmoc-Trp-OH, Pmoc-Gly-OH, Fmoc-Ahx-OH and
Hpa-OS4 to give thc title compound. Amino acid analysis following acid decomposidon
gave Asp 1.03(1), Gly 1.04(1), Ahx 1.95(2), MePhe 0.99(1), Trp 0.77(1), NH3 0.94. MS
(FAB): m/e 895 (M-H)-.
~; 30 Example 15
. ~ HFa-Alv~Glv-Trp-lle-DAsp-Phe-NH2
PAL resin was sequentially coupled according to Table 2 with Fmoc-Phe-OH, Fmoc-
DAsp(OtBu)-OH, Fmoc-Ile-OH. Fmoc-Trp OH, Fmoc-Gly-OH, Fmoc-A~-OH and
WO 93/l3126 PCI/GBs2/0~
~Su, to give the title compound. Amino acid analysis follow~ng acid decomposllion
gave Asp 1.03(1), Gly 1.04(1), Ahx 0.96(1), Ile 0.98(1), Phe 1.00(1), Trp 0.67(1), NH3
0.99. MS (FAB): m/e 881 (M-H)-.
s Example. 16
Hpa-Ahx-Glv-Trp-Ile-MeAsp-Phe-NH2 (SEQ ID NO 6) ...
PAL resin was sequentially coupled according to Table 2 with Fmoc-Phe-OH, Fmoc-
MeAsp(OtBu)-OH, Fmoc-Ile-OH, Fmoc-Trp-OH, Fmoc-Gly-C)H, Fmoc-Ahx-OH and
Hpa-OSu, to give the title compound. Amino acid analysis following acid decomposition
o gave MeAsp 1.19~1), Gly 1.00(1), Ahx 1.02(1), Phe 0~91(1), Ile 0.89(1), Trp 0.65(1), l~H3
0.60. MS (FAB): m/e ~95 (M-H)-.
Example 17
Hpa~ Glv-Trp-lle-Asp-MePhe-NH2 (SEQ ID NO 7)
15 PAI, resin was sequentially coupled according to Table 2 ~nth Fmoc-MePhe-OH, ~moc-
Asp(OtBu)-OH, Fmoc-Ile-OH, Fmoc-T~OH, Fmoc-Gly-OH, Fmoc-Ahx-OH and Hpa-
OSu, to give thc title compound. Amino acid ana~sis following acid dccomposition gave
Asp 1.10(1), Gly 1.09(1), Ahx 1.06(1), Ile 1.05(1), MePhe 0.71(1), Trp 0.76(1), NH3 0.80.
MS fjFAB): m/e 895 (M-H).
Example 18
Hpa(SO~H~ Glv-TrD-lle-DAsp-Phe-NH2 ..
Hpa-Ahl~-G~-Trp-Ile-DAs~Phe-NH2 (the product of Example 15, 41mg) prepared
according to Table 2 was sulphated essentially according to the method of ~xample 10
2s to give purified title compound (æmg). Amino acid analysis following acid
decomposition gave Asp 1.07(1), Gly 1.04(1), Ile 0.97(1), Phe 0.80(1), Trp 0.46(1) NH3
1.88. MS (FAB): m/e 961 (M-H)~.
Example 19
30 Hpa-Met-Glv-Trp Met-AspfOBn!-MePbe-NH2 (SEQ ID NC) 8)
By following essentially the procedures of Table 2 and sequentially coupling with Fmoc-
MePhe-OH, Fmoc-Asp(OBn)-OH7 Fmoc-Met-OH, Fmoc-Trp OH, Fmoc-Gly-OH,
;~093/13126 23 2126236 PCr/GB92/02369
Fmoc-Met-OH and Hpa-OSu, the title compound was prepared. MS (FAB): m/e 1023
(M+H)+.
Example 20
5 HpafSO3H!-Met-Ghr-Trp-Met-Asp(OBn!-MePhe-NH2 (SEQ ID NO 9)
Hpa-Met-Gly-Trp-Met-Asp(OBn)-MePhe-NH2 (the product of Example 19) was
sulphated essentially according to the procedures of Example 10 to give the purified title
compound. Amino acid analysis following acid decomposition gave Asp 1.07(1), Gly0.99(1), Met 1.91 (2), MePhe 1.02(1), Trp 0.47(1). MS (FAB): m/e 1101 (M-H)-.
Example 21
Hpa-Ahx-Glv-Trp-Ile-DAsp-MePhe-NH2
By following essentially thc procedure of Table 2 and sequentially coupling Fmoc-
MePhe-OH, Fmoc-DAsp(OtBu)-OH, Fmoc-Ile-OH, Fmoc-Trp OH, Fmoc-Gly-OH,
15 Fmoc~ OH and Hpa-OSu, the title compound was prepared~ An~ino Acid analysis
- following acid decomposition gavc Asp Q96 (1), Gly 1.04 (1), Ile Q96 (1), MePhe 1.07
(1), A~c 0.98 (1), Trp 0.61 (1), NH3 Q89. MS (FAB): m/e 89S (M-H).
E~amplc æ
20 Hpa(SO,H~-~-Glv-Trp-Ile-DAsp-MePhe-
~
Hpa-Ahx-Gly-Trp-Ik-DAsp-MePhc-NH2 (the product of Example 21) was sulphated
esscntiial~ according to the procedures of Example 10 to give the title compound.
o Acid anal~rsis follounng acid decomposition gave Asp 0.98 (1), Gly 1.07 (1), Ile
I Q95 (1), McPhc 1.41 (1), Ah~ 0.81 (1). MS (FAB): m/e 975 (M-H)-, m/e 895 (M-
,.~ C S03H);
1~ .
~xample 23
Hpa(SO~hx-Ghr-Trp-Ile-Asp-MePhe-NH? (SEQ ID NO 10)
1~ Hpu~ Gb-Trp-Ile-Asp-MePhc-NH2 (thc product of Example 17) was sulphatcd
I ~ 30 csscndally according to the proccdures of FYample 10 to givc the ti~le compound.
1~ ~ Amino Acid analysis following acid decomposition gave Asp 0.98 (1~, Gly 1.08 (1), Ile
¦ ~ Q96 (1), MePhe 1.55 (1), A}u~ 0.84 (1). MS (FAB): mJe 975 (M-H)-, m/e 895 (M-
~ SO3H)-.
WO 93/13l26 i pcr/GBs2/o23~
?,~.26~33 24
Hpa~SO~H~ Glv-Trp~ DAs~MePhe-NH?
Hpa-Ahx-Gly-Trp Ahx-DAsp-MePhe-NH2 (the product of Example 14) was sulphated
essentially according to the procedures of Example 10 to give the title compound.
Amino Acid analysis following acid decomposition ga~e Asp 1.00 (1), Gly 0.98 (1),
MePhe 1.10 (1), Ahx 1.93 (2), Trp 0.96 (1), NH3 0.30. MS (FA~): m/e 975 (M-H)-.
E~xample 25
Hpa-lle-Glv-Trp-Ile-DAsp-Phe-NH?
10 By following essentially the procedure of Table 2 and sequentially coupling Fmoc~Phe-
OH, Fmoc-DAsp(OtBu)-OH, Fmoc-Ile-OH, Fmoc-Trp-OH, Fmoc-Gly-OH, Fmoc-lle-
OH and Hpa-OSu, the title compound was prepared. Amino Acid analysis following
acid decomposition gave Asp 1.02 (1), Gly 1.03 (1), Phe 1.01 (1), Ile 1.94 (2), Trp 0.72
(1), NH3 0.85. MS (FAB): m/e 881 (M-H)-.
Example 26
Hpa(SO.H!-Ile-Glv-T~Ile-DAsp-Phe-NH2
Hpa-Ile-Gly-Tr~Ile-DAsp-Phe-NH2 (the product of Example 25) was sulphatcd
essentially according to the procedures of Example 10 to give the title compound.
20 Amino Acid analysis following acid decomposidon gave Asp 0.92 (1), Gly 1.03 (1), Phe
0.99 (1), Ile 1.93 (2). MS (FAB): m/e 961 (M-H)-, m/e 881 (M-SO3H)-.
~mpl~
Hpa-A}~-Glv~Trp-Phe-DAs~MePhe-~I2
2s By following essentially the procedure of Table 2 and sequentially coupling Fmoc-
MePhe~OH, Fmoc-DAsp(OtBu)-OH, Fmoc-Phe-OH, Fmoc-Trp-OH, Fmoc-Gly-OH,
Fmoc-Ahx-OH and Hpa-OSu, ehc title compound was preparcd. Amino Acid analysis
following acid decomposition gave Asp 1.01 (1), Gly 0.99 (1), MePhe 1.06 (1), Ahx 1.02
(1), Trp 1.06 (1), Phe 0.94 (1), NH3 0.82. MS (FAB): mle 931 (M-H)-.
Example 28
Hpa(SO~H~-Ahx-Glv-Trp-Phe-DAsp-MePhe-NH2
~0 93/13126 212 6 2 3 6 PCl/GB92/02369
Hpa~ Gly-Trp-Phe-DAsp-MePhe-NH2 (the product of Example 27) was sulphated
essentially according to the procedures of Example 10 to ~ve the title compound.Alnino Acid analysis following acid decomposition gave Asp 0.98 (1), Gly 1.06 (1),
MePhe 0.97 (1), Ahx 0.95 ~1), Trp 0.61 (1), Phe 1.04 (1), NH3 1.10. MS (FAB): m/e
s 1009 (M-H), m/e 929 (M-SO3H)-.
Example 29
Hpa(SO~!-A .hx-Glv-Trp-~-DAsp-Phe-NH2
Hpa-Ahx-Gly-Trp Ahx-DAs~Phe-~2 was prepared by the method of Table 2, and
o sulphated essentially according to the procedures of Example 10 to give the ~itle
compound. Amino Acid analysis following acid decomposition gave Asp 1.02 (1), Gly
1.05 (1), Phe 1.02 (1~, Ahx 1.92 (2), Trp 0.81 (1), NH3 1.48. MS (FAB): m/e 961 (M-H)-
, 881 (M-SO3H)-.
s ample 30
Hpa(SO~ Ghr-Trp lle-MeAsp-Me~he-NH2 (SEQ ID NO 113
Hpa-Ahx-Gly-Trp Ile-MeAsp-MePhe~NHz (SEQ ID NO 12) was prepared by the
method of TaSle 2, and sulphated cssentially according to the procedures of Example
10 to give the title compound. Amino Acid analysis following acid decomposition gave
2~ MeAsp 0.92 (1), Gly 1.00 (1), MePhe 1.29 (1), Ahx 0.77 (1), Ile 1.02 (1). MS (FAB):
m/e 989 tM-H)-, 909 (M-SO3H).
Examplc 31
Hpa(SO3~-lle-Glv-Trp-Ilc~MeAsp-Phe-NH2 (SEQ ID NO 13)
2s Hpa-Ile-Gly-Trp-Ile-MeAsp-Phe-NH2 (SEQ ID NO 14) was prepared by the method of
Table 2, and sulphated essentially according to the procedures of Example 10 to give
the title compound. Amino Acid analysis following acid decomposition gave MeAsp 1.11
(1), Gly 1.00 (1), Phe 1.06 (1), Ile 1.83 (2), Trp 0.73 (1), NH3 1.48. MS ~FAB): rn/e 975
- (M-H); 897 (M-SO3H).
; 30
Example 32
Hpa-A~-Glv-Trp~ MeDAsp-Phe-NH2
WO 93/13126 pcr/GBs2/o23~
623~ 26 '~ ~
By follo~ng essentially the procedure of Table 2~and sequentially coupling Fmoc-Phe-
OH, Fmoc-MeDAsp(OtBu)-OH, Fmoc-Ahx-OH, Fmoc-Trp-OH, Fmoc-Gly-OH, Fmoc-
Ahx-OH and Hpa-OSu, the title compound was prepared. Amino Acid analysis
following acid decomposition gave MeAsp 0.78 (1), Gly 1.10 (1), Phe 1.05 (1), Ahx 2.07
s (2), Trp 0.76 (1), NH3 0.71. MS (FAB): m/e 895 (M-H)-.
Example 33
Hpa(SO3H~ Glv-T~-Ahx-MeDAsp-Ph~ ~
Hpa-Ahx-Gly-Trp~ MeDAsp-Phe-NH2 (the product of Example 32) was sulphated
o essentially according to the procedures of Example 10 to give the title comp~und.
Amino Acid analysis following acid decomposition gave MeAsp 1.00 (1), Gly 1.02 (1),
Phe 1.06 (1), Ahx 1.91 (2), Trp 0.86 (1), NH3 1.48. MS (FAB): m/e 975 (M-H)-~ m/e 896
(M-SO3H)-.
Example 34
Hpa~ Glv-Trp-A~c-MeAs~MePhe-NH2 (SEQ ID NO 15)
By following essentially the procedure of Table 2 and sequentially coupling Fmoc-
MePhe-OH, Fmo~-MeAsp(OtBu)-OH, Fmoc~ OH, Fmoc-Trp OH, Fmoc-Gly-OH,
Fmoc-Ahx-OH and Hpa-OSu, the title compound was prepared. ~mino Acid analysis
20 following acid decomposition gave MeAsp 1.01(1), Gly 1.05 (1), MePhe 0.91(1), Ahx
2.02 (2), Trp 0.84 (1), NH3 0.82. MS (FAB): m/e S09 tM-H)-.
Example 35
Hpa-Ahx-Glv-Trp-L~rsfTac~ e~e-~H2 (SEQ ID NO 16)
2s Following the procedure of Table 2, Fmoc-MePhe-OH, Fmoc-Asp(OtBu)-OH, Fmoc-
Ahx-OH, Fmoc-T~OH, Fmoc-Gly-OH, Fmoc-Lys(Boc)-OH and Hpa-OSu were
sequentially coupled to the Rink amide resin. Following removal of the peptide from
the resin using standard procedures the 2-methylphenylacetamide group was added to
the -amino of Lys usîng o-tolyliso~anate. Amino Acid analysis following acid
30 decomposition gave Asp 1.01(1), Gly 1.03 (1), MePhe 0.98 (13, Lys Q94 (1), Ahx 1.04
(1), Trp 0.96 (1~. MS ~FAB): m/e 1046 (M-H)~.
Example 36
~yo 93/l3l26 2 1 2 6 2 3 6 PCI /GB92/02369
27
HparSO3H!-Ahx-Glv-Trp-Lvs(Tac!-Asp-MePhe-NH2 (SEQ ID NO 17)
Hpa-Ahx-Gly-Trp-Lys(Tac)-As~MePhe-NH2 (the product of Example 35) was sulphated
according to the procedures of Example 10 to give the title compound. Amino Acidanalysis ~ollowing acid decomposition gave Asp 1.01(1)~ Gly 1.04 (1), MePhe 0.92 (1),
5 Lys 0.93 (1), Ahx 1.10 (1), Trp 0.84 (1). MS (FAB): m/e 1123 (M-H)-.
Example 37
Hpa-Alul-Glv-Trp Ahx-Asp-MePhe-NH~ (SEQ ID NO 18)
Following the procedure of Table 2 and sequentially coupling Fmoc-MePhe-OH, Fmoc-
o Asp(OtBu)-OH, Fmoc-Ahx-OH, Fmoc-Trp-OH, Fmoc-Gly-OH, Fmoc-Ahx-OH ~nd
Hpa-OSu, to the resin, the dtle compound was prepared. Amino Acid analysis following
acid decomposition gave Asp 1.00 (1), Gly 1.02 (1), MePhe 1.05 (1), Ahx 2.1 (2), Trp
0.53 (1), NH3 0.69. MS (FAB): m/e 897 (M+H)~.
s ample 38
Hpa~ Sar-Trp~ Asp-MePhe-NH2 (SEQ ID NO 19)
FoDa~ing the procedure of Table 2 and sequcndal~ coupling Fmoc-MePhe-OH, Fmoc-
Asp(OtBu)-OH, Fmoc-A}uc-OH, Fmoc-Trp-OH, Fmoc~ar-OH, Fmoc-Ahx-OH and
Hpa-OSu, to the resin, the dtle compound ~,vas prepared. Amino Acid analysis following
20 acid decomposition gave Asp 0.93 (1), Sar 1.12 (1), MePhe 0.9S (1), Ahc 1.99 (2), Trp
-;~ 1.06 (1), HN3 0.88. MS (FAB): m/e 909 (M-H).
E~ample 39
Hpa(so~-A}vt-sar-Trp-Alvt-Asp-Mcphe-NH~ (SEQ ID NO 20)
Abl~T~A:~ Asp-MePhc-NH2 (the product of ~xample 38) was sulphatcd
according to the procedures of Example 10 to give the title compound. Amino Acidanalysis following acid decomposition Asp 1.04 (1), Sar Q87 (1), MePhe 0.86 (1), Ahx
- 2.23 (2), Trp Q89 (1), NH3 1.31, MS(FAB) m/e 989 (M-H).
:~
30 Example 40
The compound of P~mple 32 was tested in Test B abot~e, and found to bind to CCK-A
receptors with a binding constant (Kj) of 0.03nM.
: ~'
WO 93/13126 P~/GB921023~
.2623 28 ~ 1
r ~equenoe Li~t~na (Total of 20 sequences)
( 1 ) INFORMATION FOR SEQ ID NO l:
s ( i ) SE~2UENCE CHARACTERISTICS
tA) LENGTH: 6 residues
(B) TYPE: amino acid
(D) TOPOLOGY: linear
o ( ii ) MOLECULE TYPE: peptide
( ix ) FEATURE:
(A) NAME/~EY: misc-feature
( B~ LOCATION: 1
( D) OTHER INFORP~ATION: Xaa is N- ~ 2 - ( 0-sulpho-4 -
oxyphenyl ) ethanoyl ] -Nle
( ix) FEATURE:
(A) NA2qE/KEY: misc-feature
~o (B) LOCATION: 4
( D~ OTHER INFORMATION: Leu is Nle
( ix ) FEAl~
(A) NAMEfKEY: misc-feature
2s ( B) IJOCATION: 6
( D~ OTHER INFORMATION: Xaa is phenylalanine amide
(xi) SEQUENCE DESCRI~TION: SEQ ID NO 1
Xaa Gly Trp Leu Asp Xaa
( 2 ~ INFO~ATION FOR SEQ ID NO 2:
3s ( i) SEQUENCE CH~RACTERISTICS
(A) I.EN&TH: 6 residues
( B) 'rYPE: a~ino acid
(D) TOPOLOGY: linear
-~o (ii) MOI.ECUIE TYPE: peptide
(iX) FEATtJRE:
(A) NA~/KEY: misc-feature
(B) LOCATION: 1
~s ( D) OTHER INFORM~TION: Xaa is N- ~ 2 - ~ 4 -
hydr~sxyphenyl ) ethanoyl ] -Nle
ix ) FEATURE:
(A) NA~/REY: miæc-feature
so ( B) ~ L~0CATION: 4
( D) OTHER INFORMATION: Leu is Nle
( ix) FEATURE:
(A) NAME/KEY: misc-feature
ss (B) L~ ATION: 6
( D) OTHER INFO~TION: Xaa is phenylalanine amide
2126236
.~093/13126 ~ PCT/G~92/02~9
i 29
(xi) SEQUENCE DESCRIPTION: SEQ ID NO 2
Xaa Gly Trp Leu Asp Xaa
l 5
s
(3) INFORMATION FOR SEQ ID NO 3:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 6 residues
o (B) TYPE: amino acid
(D) TOPOLOGY: l inear
(ii) MOLECULE TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: misc-feature
(B) LOCATIO~: 1
(D) OTHER INFORMATION: Xaa is N-[2-(4-
hydroxyphenyl)ethanoyl]-Nle
(ix) FEATURE:
(A) NAME/KEY: misc-feature
(B) LOCATION: 4
2S (D) OTHER INFORMATION: Leu is Nle
(ix) FEATURE:
(A) NANE/KEY: misc-feature
(B) LOCATION: S
(D) OT~ER INFORMATION: Xaa is N-methyl aspartic acid
(ix) FEATURE:
(A) NAME~KEY: misc-feature
(B) LOCATION: 6
(D) OTHER $NFORMATION: Xaa is phenylalanine amide
txi) SEQUENCE DESCRIPTION: SEQ ID NO 3
- Xaa Gly Trp Leu Xaa Xaa
- 1 5
o
(4) INFORMATION FOR SEQ ID NO 4:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 6 residues
~s ` (B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLE~ULE TYPE: peptide
so (ix) FEATURE:
(A) NAME/KEY: misc-feature
: ~ (B) LOCATION: l
(D) OTHER INFORNATION: Xaa is N-[2-(0-sulpho-4-
ss oxyphenyl)ethanoyl]-Nle
(ix~ FEATURE:
(A) NAME/KEY: misc-feature
.
WO93/13~6 PCT/GB92/0236
- (B) LO~ATION: 4
(D) OTHER INFORMATION: Leu is Nle
(ix) FEATURE:
s (A) NAME/KEY: misc-feature
(B) LOCATION: S
(D) OTHER INFORMATION: Xaa is N-methyl-Asp
(ix) FEATURE:
o ~A) NAME/XEY: misc-feature
(B) LOCATION: 6
(D) OTHER INFORMATION: Xaa is phenylalanine amide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO 4
Xaa Gly Trp Leu Xaa Xaa
l 5
(5) INFO~MATION FOR SEQ ID NO 5:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 6 re~idues
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) ~OLECULE ~YPE: peptide
(ix) FEATURE:
(A) NAME/XEY: misc-feature
(B) ~OCATION: 1
(D) OTHER INFORNATION: X~a is N-t4-
hydroxyphenyl)ethanoyl]-Met
(ix3 FEATURE:
3s (A) NAME/KEY: misc-featuxe
(B) ~OCATION: 6
tD) O~HERINFORMATION:XaaisN-m~thyl-phenylalanine
2mide
xi) SEQUENCE DESCRIPTION: SEQ ID NO 5
Xaa Gly Trp Met Asp Xaa
S
~s (6) INFORMATION FOR SEQ ID NO 6:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 6 residues
(B) TYPE: amino acid
so (D) TOPOLOGY: linear
(ii) ~OLECULE TYPE: peptide
(ix) FEATURE:
ss (A) NAME/KEY: misc-feature
(B) LOCATION: 1
(D) OTHER INFORMATION: Xaa is N-~4-
-~093/13126 2 1 2 6 2 3 6 PCT/CB9Z/02369
hydroxyphenyl)ethanoyl]-Nle
(ix) FEATURE:
(A) NAME/ Æ Y: misc-feature
s (B) LOCATION: 5
(D) OTHER INFORMATION: Xaa is N-methyl-Asp
(ix) FEATURE:
(A) NAME/KEY: misc-feature
o (B) ~OCATION: 6
(D) OTHER INFORMATION: Xaa is phenylalanine amide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO 6
Xaa Gly ~rp Ile Xaa Xaa
(7) INFORMATION FOR SEQ ID NO 7:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTfH: 6 residues
(B) TYPE: amino acid
(D~ TOPOLOGY: lin~ar
(ii) MOLE~ULE TYPE: peptide
(iX3 FEATURE:
(A) NA~E/KEY: misc-feature
tB) LOf~ATION: 1
(D) OTHER INFOR~ATION: X~a is N-[~-
hydroxyphenyl)Qthanoyl~-Nle
(iX) FEATURE,:
(A) N~ME/KEY: misc-feature
3s (B) LOCATION: 6
(D) OTHERINFQRNATION: Xaa isN methyl-phenylalanine
am,ide
(xi~ SEQUENCE DESCRI~TION: SEQ ID NO 7
40 .. i . ~ . . `
Xaa Gly Trp Ile Asp Xaa
l 5
(8) INFORMaTION FOR SEQ ID NO 8:
' ~s
SEQUENCE fCHARACTERISTICS
(A) LENGrH: 6 residues
(B~ TYPE: a~ino acid
(D) TOPO~OGY: linear
so
', (ii3 MOLECU$E TYPE: peptide
~. (ix) FEATURE:
i (A) NAME/REY: misc-feature
I ss (B~ LOCATION: 1
(D) OTHER INFORMATION: Xaa is N-~2-(4-
hydroxyphenyl)ethanoy~ et
WO93/13126 PCT/GB92/023 ~
~6~ ( ix, FEA ~ : 32
_ (A) NAME/REY: misc-feature
(B) LOCATION: S
(D) OTHER INFORMATION: Xaa is Asp ~-benzyl ester
(ix) FEATURE:
(A) NAME/KE~: misc-feature
(B) LOCATION: 6
(D) OTHERINFORMATION:Xaa isN-methyl-phenylalanine
amide
(xi) SEQ~ENCE DESCRIPTION: SEQ ID NO 8
Xaa Gly Trp Met Xaa Xaa
s l 5
(9) INFORMATION FOR SEQ ID NO 9:
~i) SEQUENCE CHARACTER~STICS
(A) LENGTH: 6 residues
(B) TYPE: amino acid
(D) TOPOLOGY: lin~ar
(ii) MOLECUT~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: misc-feature
(B) L~CATION: 1
(D) OTHEX INFORMATION: Xaa is N-~2-(O-sulpho-4-
oxyphenyl)ethanoyl~-Met
(ix) FEA~URE:
(A) NANE/KEY: misc-feature
tB) LOCATION: S
35 (D) OTHER INFORMATION: Xaa is Asp ~-benzyl ester
(ix) FEATURE:.
(A) N~NE/XEY: misc-feature
(B) .-~OCATION: 6
~o (D) OTHERINFO~MATION: XaaisN-methyl-phenylalanine
2mide
(xi) SEQUENCE DESCRIPTTON: SEQ ID NO 9
~s Xaa Gly Trp Met Xaa Xaa
l 5
(10) INFORMATION FOR SEQ ID NO lO:
so (i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 6 residues
- ~ B) TYPE: amino acid
(D) TOPOLOGY: linear
ss (ii) ~OLECULE TYPE: peptide
(ix) FEATURE:
~126236
-~093/13126 PCT/GB92~0236g
33
(A) NAME/KEY: misc-feature
(B) LOCATIO~: 1
(D) OTHER INFO~MATION: Xaa i~ N-~O-sulpho-4-
oxyphenyl)ethanoyl~-Nle
(ix) FEATURE:
(A) NAME/KEY.: misc-featurs
(B) LOCATION: 6
(D) OTHERI~FORM~TION:Xaa isN-methyl~phenylalanine
amide
(xi) SEQUENCE DESCRIPTIO~: SEQ ID NO lO
Xaa Gly Trp Ile Asp Xaa
l 5
(11) INFORMATION FOR SEQ ID NO ll:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 6 residues
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
tiX) FEATURE
(A) NAME~KEY: misc-featur~
(B) LO~ATION: 1
(D) OTHER INFORMATION: Xaa is N-t2-(0-sulpho-4-
oxyphenyl)ethanoyl]~Nle
(ix) FEATURE:
(A) NAME/KEY: misc-feature
(B) LOCATION: 5
3s (D) OTHER INFORMATION: Xaa is N-~thyl-Asp
(ix) FE~TUgE:
a) NAN~/ Æ Y: misc-f~ture
~B) LOC~TION: 6
~o - - (D) OTHER IN~ORMATION:Xaa isN-methyl-ph~nylalanine
amide
(xi) SEQUENCE DESCRIPTION: 5EQ ID NO ll
~s Xaa Gly Trp Ile Xaa Xaa
l 5
(12) INFORMATION FOR SEQ ID NO 12:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 6 residues
(B) TYPE: amino acid
(D) TOPOLOGY: linear
ss ~ii) MOLE~ULE TYPE: peptide
(ix) FEATURE:
~ 6~ 34 PCT/GB92/02 ~
(A) NAME/KEY: misc-feature
(B) LOCATION: 1
(D) OT~ER INFORMATION: Xaa is N-[2-~4-
hydroxyphenyl)ethanoyl]-Nle
s
(ix) FEATURE:
(A) NAME/KEY: misc-feature
[8) LOCATION: 5
(D) OTHER INFORMATION: Xaa is N-methyl-Asp
...
(ix) FEATURE:
(A) NAME/KEY: misc-feature
(B) LOCATION: 6
(D) OTHER INFORMATION:Xaa isN-methyl-phenylalanine
amide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO 12
Xaa Gly Trp Ile Xaa Xaa
1 5
(13) INFORMATION FOR SEQ ID NO 13:
(i~ SEQUENCE CHARACTERISTICS
(A) LENGTH: ~ residues
(B) TYPE: amino acid
(D) TOPOLOGY: linear
( ii ) MOLECUI.E TYPE: peptide
(ix) FEATURE:
(A) NA~/KEY: misc-feature
( B) LOCATION:
(D) OT~ER INFORNATION: Xaa is N-~2-(0-sulpho-4-
3s oxyphenyl ) ethanoyl ] -Ile
(ix) FEATURE:
- (A) NA~/~OEY: misc-feature
( B) ~OCATION: 5
~o - - ( D) OT~3R IN~ORP~aTION: Xaa is N-methyl-Asp
( ix) FEATURE:
(A) NA~/KEY: misc-feature
(B) LOCATION: 6
~s ~ D) OTHE}~ INFORMATION: Xaa is phenylalanine amide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO 13
Xaa Gly Trp Ile Xaa Xaa
1 5
( 14 ) INFORMATION FOR S~:Q ID NO 14:
( i ) SEQUFNCE CE~RACTERISTICS
ss (A) LENGTH: 6 residues
( B) TYPE: amino acid
(D) TOPOLOGY: linear
t~ O93/13126 21 2 6 2 3 6 PCT/GB92/02369
(ii) MOLECULE TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: misc-featur~
s (B) LOCATION: 1
[D) OT~ER INFORMATION: Xaa is N-~2-(4-
hydroxyphenyl)ethanoyl]-Ile
(ix) FEATURE:
(A) NA~E/KEY: misc-feature
(B) LOCATION: 5
( D) OTHER INFORM~TION: Xaa is N-methyl-Asp
(ix) FEATURE:
(A) NAME/KEY: misc-~eature
(B) LOCATION: 6
( D) OTHER INFORNATION: Xaa is pherlylalanine amlde
( xi ) SEQUENCE DESCRIPTION: SEQ ID NO 14
Xaa Gly Trp Ile Xaa Xaa
(15) INFORM~TION FOR SEQ ID NO 15:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 6 residues
(B) TYPE: amino acid
(D) TO~OL~GY: linear
(ii) ~O~ECULE TYPE: peptide
(ix) F~ATURE:
(A) NAME/KEY: miæc-~eature
3s (B) LOCATION: 1
(D) OTHER INFORMATION: Xaa i5 N-[2-(4-
hydroxyphe~yl)ethanoyl]-Nle
- (ix) FEAIURE:
(A) NAME~KEY: misc-feature
(B) LOCATION: 4
( D) OTHER INFORN~TION: Leu is Nle
(ix~ FEATU~E:
(A) NANE/XEY: misc~eature
(B) LOCATION: 5
(D) OTHER INFORMATION: Xaa is N-~ethyl-Asp
(ix) FEATURE:
(A) . NAME~KEY: misc-feature
(B) LOCATION: 6
tD3 OTHERINFORMATION: Xaa isN-methyl-phenylalanine
amide
ss (xi~ SEQUENCE DESCRI~TION: SEQ ID NO lS
WOg3/l3l26 36 PCT/GE92/02
a Xaa Gly Trp Leu Xaa Xaa
1 5
(16) INFORMATION FOR SEQ ID NO 16:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 6 residues
(B) TYPE: amino acid
(D) TOPOLOGY: linear
,
(ii) MOLECULE TYPE: peptide
(ix) FEATURE:
(A) NAME/XEY: misc-feature
s (B) LOCATION: 1
(D) OTHER INFORMATION: Xaa is N-t2-(4-
hydroxyphenyl)ethanoyl]-Nle
(ix) FEATURE:
(A) NANE/KEY: misc-feature
(B) LOCATION: 4
(D) OTHER INFORMATION: Xaa is e-N-t(2-
methylphenyl)aminocarbonyl]-Lys
~- . (ix) FEATURE:
(A) NAME/KEY: misc-feature
(B) LOCATION: 6
(D) OT~ERINFORMATION: Xaa isN-methyl-phenylalanine
a~ de
- (xi) SEQUENCE DESCRIPTION: SEQ ID NO 16
Xaa Gly Trp Xaa Aæp Xaa
s
(17) INFORMATION FOR SEQ ID NO 17:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 6 residue~
(B) TYPE: amino a~id
(D) TOPO W Y: linear
(ii) MOLECULE TYPE: peptide
~s ~iX) F~ATURE:
~(A) NAME/KEY: misa-feature
(B) LOCATION: 1
(D) OTHER INFORMATION: Xaa is N-t2-tO-~ulpho-4-
oxyphenyl)ethanoyl]-Nle
so
: (ix) FEATURE:
:~ (A) NAME/KEY: misc-feature
(B) LOCATION: 4
(D) OTHER INFORMATION: Xaa is ~-N-t(2-
ss methylphenyl)aminocarbonyl]-Lys
(ix) FEATURE:
~ .
2126236
~NO93/13126 ~ PCT/GB92/02369
37
(A) NAME/KEY: misc-feature
(B) ~OCATION: 6
(D) OTHERINFORMATION:Xaa isN-methyl-phenylalanine
amide
s
(xi) SEQUENCE DESCRIPTION: SEQ ID NO 17
Xaa Gly Trp Xaa Asp Xaa
...
(18) INFORMATION FOR SEQ ID NO 18:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 6 residues
(B) TYPE:/amino acid
(D) TOPOLOGY: linear
(ii) NOLECUI~ TYPE: peptide
(ix) FEATURE:
(A) NAME/KEY: misc-f~ature
(B) LOCATION: l
(D) OTHER INFORMATION: Xaa is N-[~-(4-
hydroxyphenyl)ethanoyl~-Nle
( ix~ FEATURE:
(A) NAME/KEY: misc-feature
OC~TION: 4
(D) OIHER INFORMATION: Leu is Nle
(ix) FEATURE:
.(A) NAME/XEY: misc~feature
(B) LOCATION: 6
(D) OTHER INFORMATION:Xaa isN-methyl-phenylalanine
3s amide
(xi) SEQUEN OE DESCRIPTION: SEQ ID NO 18
Xa~ Gly Trp LQU Asp Xaa
~o 1 5
(19) INFORMATION FOR SEQ ID NO l9:
¦ (i) SEQUENCE CXARAC~ERISTICS
~s (A) LENG~H: 6 residues
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii~ NOLECULE TYPE: peptide
so
(ix) FEATURE:
(A) NANE/KEY: misc-feature
(8) LOC~TION: 1
(D) OTHER INFORMATION: Xaa is N-t2-(4-
¦ ss hydroxyphenyl)ethanoyl]-Nle
I (ix~ FEATURE:
~6~ 38 PCT/GB92/023 ~
(A) NAME/KEY: misc-feature
(B) LOCATION: 2
(D) OTHER INFORMATION: Gly is sarcosine
s (ix) FEATURE:
(A) NAME/KEY: misc-feature
(B) LOCATION: 4
(D) OTHER INFORMATION: Leu is Nle
~ix) FEATURE:
(A) NAME/REY: misc-feature
(B) LOCATION: 6
(D) OTHERINFORMATION:Xaa isN-m~thyl-phenylalanine
amide
1~
(xi) SEQUENCE DESCRIPTION: SEQ ID NO 19
Xaa Gly Trp Leu Asp Xaa
~o
(20) INFORMATION FOR 5EQ ID NO 20:
ti) SEQUENCE CHARACTERISTICS
(A) LENGTH: 6 residu~s
( B) TYPE: amino acid
(D) TOPOL4GY: linear
(ii) MOLECULE TYPE: peptide
(iX) FEATURE:
.(A~ NAMEJKEY: misc-feature
(B) ~OCATION: 1
(D) OTHER INFORMATION: Xaa i5 N-~2-(O-sulpho-4-
oxyphenyl)ethanoyl]-Nle
(ix) FE~TURE:
(A) NAME/XEY: misc-feature
(B) ~GCATION: 2
(D) OTHER INFORMATION: Gly is sarcosine
~o
(ix) FEATURE:
(A) ~AME/KEY: misc-feature
(B) L0CATION: 4
(D) OTHER INFO~MATION: Leu is Nle
~s
(ix) FEATURE:
(A) NAME/XEY: misc feature
(B) LO~ATION: 6
(D) OTHERINFORMATION:XaaisN-methyl-phenylalanine
amide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO 20
Xaa Gly Trp Leu Asp Xaa
ss 1 5