Une partie des informations de ce site Web a été fournie par des sources externes. Le gouvernement du Canada n'assume aucune responsabilité concernant la précision, l'actualité ou la fiabilité des informations fournies par les sources externes. Les utilisateurs qui désirent employer cette information devraient consulter directement la source des informations. Le contenu fourni par les sources externes n'est pas assujetti aux exigences sur les langues officielles, la protection des renseignements personnels et l'accessibilité.
L'apparition de différences dans le texte et l'image des Revendications et de l'Abrégé dépend du moment auquel le document est publié. Les textes des Revendications et de l'Abrégé sont affichés :
| (12) Demande de brevet: | (11) CA 2127292 |
|---|---|
| (54) Titre français: | PROCEDE D'OBTENTION D'AMIDES D'ACIDE HYDROXYALCANECARBOXYLIQUE |
| (54) Titre anglais: | PROCESS FOR PRODUCING HYDROXYALKANE CARBOXYLIC ACID AMIDES |
| Statut: | Réputée abandonnée et au-delà du délai pour le rétablissement - en attente de la réponse à l’avis de communication rejetée |
| (51) Classification internationale des brevets (CIB): |
|
|---|---|
| (72) Inventeurs : |
|
| (73) Titulaires : |
|
| (71) Demandeurs : |
|
| (74) Agent: | MARKS & CLERK |
| (74) Co-agent: | |
| (45) Délivré: | |
| (86) Date de dépôt PCT: | 1993-05-05 |
| (87) Mise à la disponibilité du public: | 1993-11-11 |
| Licence disponible: | S.O. |
| Cédé au domaine public: | S.O. |
| (25) Langue des documents déposés: | Anglais |
| Traité de coopération en matière de brevets (PCT): | Oui |
|---|---|
| (86) Numéro de la demande PCT: | PCT/DE1993/000413 |
| (87) Numéro de publication internationale PCT: | DE1993000413 |
| (85) Entrée nationale: | 1994-07-08 |
| (30) Données de priorité de la demande: | ||||||
|---|---|---|---|---|---|---|
|
Abstract
A process is described for the production of hydroxyalkane
carboxylic acid amides of general formula I
<IMG> (I),
in which
n represents a whole number from 1 to 20 and
R1 and R2 each mean alkyl groups with a maximum of 8 carbon
atoms or together mean an alkylene group with 4 to 8 carbon atoms
optionally interrupted by an oxygen atom or a nitrogen atom, from
hydroxyalkane carboxylic acids of general formula II
HO-CnH2n-COOH (II),
in which
n has the above-mentioned meaning and amines of general
formula III
<IMG> (III),
in which
R1 and R2 have the above-mentioned meanings
that is characterized in that
the hydroxyalkane carboxylic acid is refluxed in a one-pot
reaction with 10 to 30 mol of acetyl chloride per mol of acid
each, the excess acetyl chloride is distilled off after
acetylation has taken place, the residue is refluxed with 1.5 to
5 mol of thionyl chloride per mol of acid, the thionyl chloride
is removed by distillation after acid chloride formation has
taken place, the residue is dissolved in a dialkyl ether with 4
to 8 carbon atoms, the solution obtained is placed
proportionately in an aqueous solution of the amine cooled to 0°C
to 10°C and the acetate group is cleaved off after amide
formation has taken place by treatment with aqueous sodium
hydroxide solution at a reaction temperature of 10°C to 30°C.
Note : Les revendications sont présentées dans la langue officielle dans laquelle elles ont été soumises.
Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
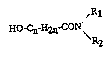
2024-08-01 : Dans le cadre de la transition vers les Brevets de nouvelle génération (BNG), la base de données sur les brevets canadiens (BDBC) contient désormais un Historique d'événement plus détaillé, qui reproduit le Journal des événements de notre nouvelle solution interne.
Veuillez noter que les événements débutant par « Inactive : » se réfèrent à des événements qui ne sont plus utilisés dans notre nouvelle solution interne.
Pour une meilleure compréhension de l'état de la demande ou brevet qui figure sur cette page, la rubrique Mise en garde , et les descriptions de Brevet , Historique d'événement , Taxes périodiques et Historique des paiements devraient être consultées.
| Description | Date |
|---|---|
| Inactive : CIB de MCD | 2006-03-11 |
| Le délai pour l'annulation est expiré | 1998-05-05 |
| Demande non rétablie avant l'échéance | 1998-05-05 |
| Réputée abandonnée - omission de répondre à un avis sur les taxes pour le maintien en état | 1997-05-05 |
| Demande publiée (accessible au public) | 1993-11-11 |
| Date d'abandonnement | Raison | Date de rétablissement |
|---|---|---|
| 1997-05-05 |
Les titulaires actuels et antérieures au dossier sont affichés en ordre alphabétique.
| Titulaires actuels au dossier |
|---|
| SCHERING AKTIENGESELLSCHAFT |
| Titulaires antérieures au dossier |
|---|
| GERALD KIRSCH |
| GUNTER NEEF |
| HANS-JORG VIDIC |
| MICHAEL HARRE |
| STEFAN SCHOLZ |