Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO ~3/17016 PCr/EP93/V0316
21~ 2~
Sulfonylureas as herb~cldes
The present invendon relates tO novel h~rbicidally active and plant-growth-~egulating
N-phenylsulfonyl-N'-pyrimidinyl- and N'-triazinylureas, to p~ocesses for their
preparation, to composi~ons containing them as acdve ing~dients, and to the use of these
compositions for controlling weeds, especially selec~vely in crops of useful plants, or for
regulating and inhibiting ~e growth of plants.
Urea compounds, triazine compounds and pyrimidine compounds which have herbicidal
activity are generally known. Such compounds are described, for exarnple, in European
Patent Applications Nos. 0 007 687, 0 030 138, 0 073 562 and 0 126 71 l.
Novel sulfonyluIeas which have herbicidal and plant-growth-regulating properties have
now been found. ;
The N-phenylsulfonykN'-pyrimidinyl- and N'-triazinylureas according to the invention
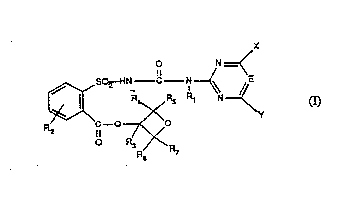
are those of the formula I
X -' '
--C--N~
O R3
R8
in which
Rl is hydrogen or methyl;
R2 is hydrogen, fluorine, chlorine, bromine, iodine, (W)n-R8, -NO2, -N(Rg)Rlo,
-- ~ GCRII or-CN;
Rl2
R3, R4, R5, R6 and R7 independently of one another are hydrogen or Cl-C4alkyl;
1~8 is Cl-C4alkyl, Cl-C4alkyl which is subsdtuted by l, 2, 3 or 4 halogen atoms,C~-C3aL~coxy or Cl-C3alkylthio, or is C2-C4aLcenyl or C2-C4aL~enyl which is substituted
WO 93/17016 Pcr/Eps3/~ ' 6
2128325 -2-
by 1, 2, 3 or 4 halogen atoms;
R9 is hydrogen, methoxy, ethoxy or Cl-C4aL~cyl;
Rlo is hydrogen or Cl-C4aL~cyl;
R1l is hydrogen, methyl or ethyl;
Rl2 is hydrogen or methyl;
E is -CH= or-N=;
X is Cl-C4aL~cyl, C~-C4alkoxy, Cl-C4haloaLkoxy, C~-C4haloalkyl, Cl-C4haloalkylthio,
Cl-C4aLkylthio, halogen, C2-C5aLkoxyaL~cyl, C2-CsaLkoxyaLlcoxy, amino, Cl-C3aL~cylasnino
or di-(CI-C3aL~cyl)arnino;
Y is Cl-C4alkyl, Cl-C4aLIcoxy, Cl-C4haloaLlcoxy, Cl-C4haloaL~cylthio, Cl-C4alky1thio,
C2-CsaLkoxyaL~cyl, C2-C5aLkoxyaLlcoxy, CrC5aL~cylthioaLlcyl, cyclopropyl or-OCHF2;
W is oxygen, sulfur, SO or SO2; and
n is the number 0 or 1;
and the salts of these compounds;
with the proviso that
a) at least one of the radicals R3, R4, R5, R6 and R7 is Cl-C4alkyl,
b) E is -CH= when X is halogen and
c) E is -CH= when X or Y are -OCHF2 or -SCHF2.
Suitable for X as halogen are: fluorine, chlorine, bromine and iodine, preferably fluorine,
chlorine and bromine.
Suitable for R3, R4, R5, R6, R7, R8, R9, Rlo, X and Y as C~-C4aL~cyl are: methyl, ethyl,
n-propyl, iso-propyl, n-butyl, sec-butyl, iso-butyl or tert-butyl. The aLkyl groups preferably
have 1-3 carbon atoms.
Suitable for R8 as Cl-C4aLlcyl which is subsdtuted by one to four halogen atoms is, in
particular, aLkyl which is substituted by fluorine, chlo~ine, bromine or iodine. Preferred
amongst these halogen-substituted Cl-C4aL~cyl groups are aLkyl groups which are mono- to - -
trisubstituted by halogen, in particular fluorine or chlorine, for example fluoromethyl,
difluoromethyl, trifluoromethyl, chloromethyl, dichloromethyl, trichloromethyl,
2,2,2-trifluoroéthyl, 2-fluoroethyl, 2-chloroethyl and 2,2,2-trichloroethyl; preferably
difluoromethyl and trifluoromethyl.
Suitable for R8 as C1-C4alkyl which is substituted by Cl-C3alkylthio are, for example: -
methylthioethyl, ethylthioethyl, propylthioethyl, isopropylthiomethyl, preferably
WO 93/17016 PCr/EP93/00316
21~832S
- 3 -
methylthiomethyl and ethylthioethyl.
Suitable for X as Cl-C4haloaLkyl is, in par~icular, aL~cyl which is substituted by fluonne,
chlonne, bromine or iodine. Preferred amongst these radicals are alkyl groups which are
mon~ to tlisubstituted by halogen, in particular fluorine or chlorine, for example
fluoromethyl, difluoromethyl, trifluoromethyl, chloromethyl, dichloromethyl, ;
trichloromethyl, 2,2,2-trifluoroethyl, 2-fluoroethyl, 2-chloroethyl and 2,2,2-trichloroethyl;
preferably difluoromethyl and trifluoromethyl.
Suitable for X and Y as Cl-C4alkoxy are, for example, methoxy, ethoxy, propyloxy,
i-propyloxy, n-butyloxy, iso-butyloxy, sec-butyloxy and tert-butyloxy; preferably methoxy
and ethoxy.
Suitable for X and Y as Cl-C4haloaLkoxy are, for example, difluoromethoxy,
trifluoromethoxy, 2,2,2-trifluoroethoxy, 1,1,2,2-tetrafluoroethoxy, 2-fluoroethoxy,
2-chloroethoxy and 2,2-difluoroethoxy; preferably difluoromethoxy and trifluoromethoxy.
Suitable for X and Y as C2-CsaLkoxyalkyl and for R8 as Cl-C3aL~coxy-Cl-C4allcyl are, for
example, methoxymethyl, methoxyethyl, ethoxymethyl, ethoxyethyi or propyloxymethyl.
Suitable for R8 as C2-C4alkenyl is straight-chain or branched aLkenyl, for example vinyl,
allyl, methallyl, 1-methylvinyl or but-2-en-1-yl; aLkenyl radicals having a chain length of
2 to 3 carbon atoms are preferably suitable.
Suitable for R8 as C2-C4aL~cenyl which is substituted by one to four halogen atoms is, for
example: 3,3-difluorobut-2-en-1-yl.
Suitable for X and Y as Cl-C4alkylthio are, for example: methylthio, ethylthio, propylthio,
isopropylthio, n-butylthio, iso-butylthio, sec-butylthio or tert-butylthio, preferably
methylthio and ethylthio.
- Suitable for X and Y as C~-C4haloalkylthio is, in particular, alkylthio which is substituted
by fluorine, chlorine, bromine or iodine. Preferred amongst these are alkylthio groups
which are mono- to trisubstituted by halogen, in particular fluorine or chlorine, for
example fluoromethylthio, difluoromethylthio, trifluoromethylthio, chloromethylthio,
dichloromethylthio or trichloromethylthio.
WO 93/17016 PCr/EP93/0~ ;
212~32~ ~
Suitable for Y as C2-C5aLIcylthioaL~yl are, for example: methylthioethyl. ethylthioethyl.
propylthioethyl or isopropylthiomethyl, preferably methylthiomedlyl and ethylthioe~hyl.
Suitable for X and Y as C2-Csalkoxyalkoxy are. for exarnple: methoxymethoxy,
methoxyethoxy, methoxypropyloxy, ethoxyrnethoxy, ethoxyethoxy and
propyloxymethoxy.
Suitable for X as Cl-C3alkylamino a~, for example, methylamino, ethylamino,
n-propylamino or iso-propylamino. Di(CI-C3al~rl)amino as radical X is, for cxample,
dimethylamino, methylc~ylamino, dicthylamino or n-propylmethylamino.
' ~:
The inven~on also embraces the salts which the compounds of the for nula I can form
with amines, alkali metal bases or ~ ne cartb metal bases or quateTnary ammoniumbases. -
Preferred alkali metal bydroxides and allcaline earth metal hydroxides as salt-forming
substances are lithium hydroxide, sodium hydro~cide, potassium hydr~xide. magnesium
hydroxide or calcium hydroxide, but in par~ticular sodium hydr~xide or potassiumhydroxide.
. .
Examples of amines which are suitable for salt formation are primary. secondary and
ter~ary aliphatic and aromatic amines such as methylamine. ethylamine, n-propylamine,
is~propylamine, the ~our isomeric butylamine radicals, n-amylamine, iso-amylamine,
hexylamine, heptylamine, octylamine, nonylamine, decylamine, pentadecylamine,
hexadecylamine, heptadecylamine, octadecylamine, methylethylamine,
methylisopropylaminc, methylhexylamine, methylnonylamine, methylpentadccylamine,methyloctadecylaminc, ethylbutylaminc, ethylheptylamine, ethyloctylamine,
hexylheptylamine, hcxyloctylaminc, dimethylaminc, diethylamine, di-n-propylamine, ~ `
di-iso-pr~pylaminej di-n' butylaminc, di-n-amylamine, di-is~amylamine. dihexylamine.
diheptylamine, dioctylamine, cthanolamine, n-propanolamine, iso-propanolamine,
N,N-diethylethanolamine, N-cthylpropanolamine, N-but~ lethanolamine, allylaminc,n-butenyl-2-amine, n-pentenyl-2-amine, 2,3-dimethylbutcnyl-2-aminc,
di-butenyl-2-amine, n-hexenyl-2-amine, propylenediamine, diethanolamine,
trimcthylamine, triethylamine, tri-n-propylamine, tri-iso propylarnine, tri-n-butylamine,
~i-iso-butylamine, tri-scc-butylarninc, tri-n-amylarnine; heterocyclic arnines, for example
pyridine, quinoline, iso quinoline, morpholine. piperidine, pyrrolidine, indoline, ~-
WO 93/17016 212 8 3 2 5 PCr/EP93/00316
quinuclidine and azepine; primary arylamines, for example anilines, methoxyanilines,
ethoxyanilines, o,m,p-toluidines, phenylenediamines, benzidines, naphthylamines and
o,m,p-chloroanilines; but in particular ethyl-, propyl-, diethyl- or triethylamine, but
especially iso-propylamine and diethanolamine.
As a rule, examples of quaternary ammonium bases are the cations of haloammoniumsalts, for example the tetramethylammonium cation, the trimethylbenzylammonium
cation, the triethylbenzylammonium cation, the tetraethylammonium cation, the
trimethylethylammonium cation, but also the ammonium cation.
PrefeITed compounds of the fonnula II
lN~H
O R3
are those in which Rl, R2, R3, R4, Rs, R6, R7, X and Y are as defined in formula I.
Other preferred compounds are those of the fo~ula III
O N =<
--N~
0 ~3
in which Rl, R2, R3, R4, Rs, R6, R7, X and Y æ as defined in formula I.
Particularly preferred compounds of the formulae I, II and m are those in which R2 is
hydrogen.
Other particularly preferred compounds of the formulae I, II and m are those in which R3
is hydrogen.
wo 93/170161 2 ~ 3 2 ~ PCl /EP93/0( ~ ~
Especially preferred compounds of the formulae I, II and III are those in which R3, R4, Rs
and R6 are hydrogen. :
Par~icularly important compounds of the formulae I, II and III are those in which Rl is :
hydrogen, in particular those in which R~ and R2, Rl and R3, R~, R2 and R3 or R~. R2, R3,
R4, Rs and R6 are hydrogen.
Other inseresting compounds of the formula I are those in which Rl and R2 are hydrogen,
R3, R4, Rs, R6 and R7 independently of one another are hydrogen or methyl, E is -CH= or
-N=, X is methyl, methoxy, methylamino, dimethylarnino, difluoromethoxy or chlorine
and Y is methyl, methoxy, difluoromethoxy, ethoxy or cyclopropyl, with the proviso that
a) at least one of the radicals R3, R4, Rs, R6 and R7 is methyl,
b) E is -CH- when X is chlorine and
c) E is -CH= when X or Y is -OCHF2.
The compounds of the formula I can be prepared either by
a) reacting a phenylsulfonamide of the formula IV
,~ S2 NH2 " "~,
R2 C--O7~0
3 R R7
in which R2, R3, R4, R5, R6 and R7 are as defined in formula I, with a pyrimidinvl
carbamate or tri~zinyl carbamate of the forrnula V
x :
R" O--C--N--~ ~ (V), ~:
in which R,, E~, X and Y are as;defined in formula I and R13 is phenyl or phenyl which is
substituted by Cl-C4aL~cyl or halogen, in the presence of a base, or
b) reacting a sulfonylcarbamate of the formula VI
WO 93/17016 PCr/EP93/00316
212332~
R C 07~o (Vl),
O R3 F~7
in which R2, R3, R4, Rs~ R6 and R7 are as defined in formula I and Kl3 is as defined in
formula V, with an amine of the formula VII
N =<X
HN--<~ ~ (V~
Y :~.
in which Rl, E, X and Y are as defined in foq~nula I, in the presence of a base, or
c) reacting a phenylsulfonamide of the formula IV
,~ Z~SO~NH2
R C--07~0 (IV)
~: O R3
in which R2, R3, R4, Rs~ R6 and R7 are as defined in fo~nula I, with a pyrimidinyl
isocyanate or tliazinyl isocyanate of the formula VIII
O = C = N ~
N
in which E, X and Y are as defined in formula I, in dle presence of a base.
Compounds of the formula I can also be prepared by reacting a compound of the fo~nula
:~ .
Rs
,; R6 R7
in which R2, R3, R4, R5, R6 and R7 are as defined in forrnula I with a compound of the
wO 93/17016 PCr/EP93/O~ >
2~ ~32S - 8-
fonnula VII in the presence of an amm~nium, phosphonium, sulfonium or aL~cali metal ~ -
cyanate salt of the foImula X
M~OCN- (X)
in which M is an alkali metal or the group R,SR,6Rl7Rl8Q, in which R~s, R16,R~7 and R18
independently of one another are Cl-Cl8al~1, benzyl or phenyl, thc total numbcr of C
atoms not being greater than 36; and Q is nitrogen, sulfur or phosphorus. Such reactions ~ -
are described in Swiss Patent Specification 662 348.
The reactions which give compounds of the formula I are advantageously carried out in
aprotic, inert organic solvents. Such solvents are hydrocarbons such as benzene, toluene,
xylene or cyclohexane, chlorinated hydrocarbons such as dichloromethane,
trichloromethane, tetrachloromethane or chlorobenzene, ethers such as diethyl cther,
ethylene glycol dimethyl ether, diethylene glycol dimethyl cther, tetrahydrofuran or
dioxane, nitriles such æ acetonitrile or propionitrile, amides such as di ncthy}fo..namide,
diethylformamide, or N-methylpyrrolidinone. The reaction temperatures are preferably
between -20 and +120C.
As a rlle, the reactions are slightly exothermic and can be carried out at room temperature.
To shonen the reacdon time or else to start up the reaction, it is expedient to heat the
reaction mKture briefly up to its boiling point. The reaction times can also be shortened
by adding a few drops of base as reaction catalyst. Suitable bases are, in par~cular, tertiary
amines such as trimethylamine, triethylamine, quinuclidine,
1,4 diazabicyclo-(2.2.2)-octane, 1,5-diazabicyclo(4.3.0)non-5-ene or
1,5-diazabicyclo(5A.O)undec-7-ene. Alternatively, inorganic bases such as hydrides, such
as sodium hydridle or calcium hydride, hydroxides such as sodium hydroxide and
potassium hydroxide, carbonates such as sodium carbonate and potassium carbonate or - ~ ~ -
hydrogen carbonates such as potassium hydrogen carbonate or sodium hydrogen
carbonate~ can also be used as bases
The end products of the formula I can be isolated by concentration and/or evaporation of
the solvent and purified by recrystallisation or trituration of the solid residue in solvents in
which they are not reely soluble, such as ethers, aromatic hydrocarbons or chlorinated
hydrocarbons.
. .
..
In the above-described preparation processes of the compounds of the fonnula I, Rl3-is ~
WO 93/l70l6 PCr/EP93/00316
212832~
preferably phenyl which can be subshtuted by Cl-C4aLlcyl or halogen, especially
preferably phenyl.
The phenylsulfonamides of the formula IV are novel compounds which were developed
and prepared specifically for the preparation of the active ingredients of the formula 1.
They are therefore also part of the present invention. The sarne preferred ranges for R2,
R3, R4, Rs, R6 and R7 which have been mentioned above in the case of compounds of the
formula I apply to the compounds of the formula IV. They can be prepared from the
corresponding phenylsulfonyl chlorides of the formula IX
~so~cl
7~$R
o R3
in which R2, R3, R4, Rs, R6 and R7 are as defincd in formula I, by rcaction with arnmonia
Such reactions are known and familiar to a person sldllcd in the art.
The phenylsulfonyl chlorides of the formula IX are novel compounds which were
developed and prcpared specificaUy for the preparation of the active ingredients of the
formula I. They are therefore also part of the present invention. The same preferred ranges
for R2, R3, R4, Rs, R6 and R7 which have been mentioned above in the case of the -
compounds of the formuia 1 apply to the intermedia~es of the for nula IX. The
phenylsulfonyl chlorides of the formula IX are prepared by reacting the suitablysubstituted 2-chlorosulfonylbenzoyl chlorides (sec, for cxample, D. Davis, Soc. 2042,
2044 (1932)) with a compound of dle formula XI "
R~R5 :~:
HO ~S< (XI)
F~6 R7
in which R3, R4, R5, R6 and R7 are as deffned in forznula I, in the presence of a base. Such
reactions are known and familiar to a person skilled in the art.
Phenylsulfonyl chlorides of the foImula ~ can also be prepared by reacting
2-isopropyl~hiobenzoic acid (see, for example, H. Gilman, F.J. Webb, Am. Soc. 71,
4062-4063) with thionyl chloride to give the corresponding benzoyl chloride, which is
wo 93/17016 pcr/Eps3/oî -
2 ,,2~æs
- 10-
subsequently converted with a compound of the foImula (XI) in ~e presence of a base to
give the coIresponding oxetan-3-yl 2-isopropyl~,iobenzoate, whereupon reaction with
chlorine finally gives the sulfonyl chloride of the formula IX. Such reac~ions are hlown
l~d familiar to a person sldlled in the art. ~ -
Compounds of the formula XI and the preparation thereof are known (see, for example, J.
Am. Chem. Soc. 112, 3535 - 3539 (1990); Bull. Chem. Soc. Japan ~, 2032 (1989); Acta
Chem. Scand. 28,701 (1974); Tetrahedron Letters~0, 2505 - 2508 (1969); J. Am. Chem.
Soc.77,4430 (1955)).
The sulfonylcarbamates of the formula VI are novel and pan of the present in~ren~on. The
preferred ranges for R2, R3, R4, Rs, R6 and R7 which have been mei~tioned above in the
case of the compounds of the formula I apply to the intermediates of the formula VI. They
can be obtained, for exarnple, by reacting the sulfonamides of the formula IV with
diphenyl carbamate in the presence of a base. Such reactions are known and familiar to a
per~on skilled in the ar~
The amines of the formula VII are described in European Patent Applications Nos.O007687,0030138,0073562and0126711andinUSPatent4579584.
Processes for the preparation of N-pynmidinyl- and N-triazinylcarbamates are descnbed,
for example, in EP-A-0 101 670.
As a rule, the active ingredients of tne formula I a~: applied successfully at application
rates OI 0.001 to ~ kg/ha, in particular O.OOS to 1 kg~a. The dosage rate required for the
desired act.on can be determined by experiments. It depends on the type of action, the
development stage of the crop plant and of the weed and on the application (location, time,
method) and, due to these parameters, can vary within wide ranges.
,
The compounds of the formula I are distinguished by growth-inhibiting and herbicidal
properties which make them outstandingly suitable for use in crops of useful plants, in
particular in cereals, cotton, soya beans, oilseed rape, maize and rice, their use in soya
bean crops and cereals being especially preferre~ Weeds in soya bean crops are preferably
controlled postemergence. The compounds of the formula I are particularly distinguished
by their good degradability.
WO 93/17016 PCI/EP93/00316
212g3~ ~
SuIpnsingly, it has emerged that the compounds of the formula 1 and further compounds
of the formula I, wherein R3~ R4, Rs, R5 and R~ simultaneously are hydrogen, respond well
to specific classes of safeners. These safeners or an~dotes are capable of protecnng the
crop plants against darnage caused by the herbicide (for example in the case of an
uninten~ional overdose). These an~dotes are furthermore capable of preventing damage to
crop plants when, in connection wi~h crop rotation, herbicide-resistant crop plants are
followed by other crop plants which have no, or only insufficient, resistance to the
herbicides. The present invention therefore also relates to a selective herbicidal
composition for controlling g~asses and weeds in crops of useful plants which is composed
of a herbicide of the formula I or a herbicide of the formula I, whe~ein R3, R4, R5, R6 and
R7 simultaneously are hydrogen, and a safener (antagonist, antidote) which protects the ~` `
crop plants, but not the weeds, against the phytotDxic action of the herbicide, and to the
use of this composition, or of the combination of herbicide and safener, for weed control
in crops of useful plants.
The safeners used in the composition according to the invention belong to the classes of --
the quinolin-8-oxyacetic acid derivatives as they are disclosed, for example, in EP-A-
0 492 366 and EP-A O 094 349, the diphenylcarboxylic acid derivatives as they are
disclosed, for example, in EP-A-O 268 554, and the sulfamoylphenylurea derivatives as
they are disclosed, for example, in EP-A-O 365 484. -~
The safeners of the formulae S 1 to S5 are particularly preferred for use in the composition `
according to the invention:
~'
Cl ;,',
~,!J (Sl);
.
O~CH2-COO-cH(CH3~C5Hl ~ -n ,
2i~8 PCr/EPs3/0 6
- 12- :
COOCH3
~J~
~ ~3,CI (S2);
CO--NH-SO2~ ,CH3
. OCH~
NH-SO2~NH--CO--N~
Cl
,.
~3 (S5).
O~H2~oo-cH(cH3~cH2ocH2cH=cH2 ,
;Par~cularly preferred compositions which comprise a safener of the formulae Sl, S2, S3,
S4 and SS are those in which the herbicide of the fo~nula I used is the compound of the
fonnula 2.01 1
;::
-WO 93/17016 212 8 3 2 5 PCr/EP93/00316 ~ ~
- 13 -
O N=~ :~
SO~HN C NH~
[~C 0~0 OCH,
r)epending on the intended use, a safener or an~dote can be used for pretreating the seed
of the crop plant (seed dressing or treatment of the cuttings) or introduced into the soil
before or after sowing. Alternatively, it can be applied as pure active ingredient or ` i
together with the herbicide before or after emergence of the plants. The plant or the seed
can therefore be treated with the safener essentially independently of the point in time of
applic~ion of the phytotoxic chemical. Alternatively, the plant can be treated by -
simultaneous application of phytotoxic chemical and safener (tank mix). Preemergence
~eatment includes treatment of the area under cultivation before sowing and treatmenl of
the areas under cultivation where seed has been sown but the plants are yet to emerge.
~.,
The application rate of safener to be applied relative to the herbicide depends largely on
the method of applicat on. As a rule, ~leld treatment, which is effected either using a tank
mix of a combination of safener and herbicide or by separate application of safener and
herbi~ide, requires a ratio of safener to herbicide of l:lQ0 to 1:1, preferably 1:20 to 1:1, in
particular 1:1. In contrast, seed dressing requires much smaller amounts of safener relative -
to the app!ication rate of herbicide per hectare of area under cultivation.
.
A~ a rule, 0~001 to 5.0 kg of safener~a, preferably 0.00~ to 0.5 kg of safener/ha, are
applied in the case of field treatment. --
The app}ication rates of herbicide are, as a rule, between 0.001 and 2 kg/ha, but preferably
between 0.001 and 0.5 kg/ha.
As a rule, 0.001 to 10 g of safener~cg of seed, preferably 0.05 to 2 g of safener/kg of soed,
are applied in the case of seed dressing. If the safener is applied in liquid form for seed
soaking briefly before sowing, it is expedient to use safener solutions which comprise ~he
active ingredient at a concentration of from 1 eo 10 000, preferably from 100 to 1000,
wo 93/17016 PCr/EP93/0~ ~
3æ5 -14-
ppm. ~ -
For application purposes, the safeners, or combination of safeners, used according to the
invention together with the herbicidcs to be antagonised are expediently employed
together wi~ the auxiliaries convendonally used in the art of formulation, which have
already been mentioned above in connection with application of the compounds of the ~-
fo~nula I.
The inven~on also relates to herbicidal and plant-growth-regulating compositions which
comprise a novel active ingredient of the fwmula I, and to methods for inhibiting the -
growth of plants. Plant growth regulators are substances which cause agronomically
desirable biochemical and physiological and/or morphological modifications in/on the
plant.
The active ingredients comprised in the compositions according to the invention affect
plan~ growth in many ways, dcpcnding on thc point in timc of application, thc dosage ratc,
the type of application and thc prcvailing cnviromnen~ For example, plant growthregulators of the forrnula I can inhibit the vegetative growth of plants. This type of action
is of interest on lawns, in the productio ~ of ornamentals, in fiuit plantations, on verges, on
sportsgrounds and industrial terrain, ~ also in the t~rgeted inhibition of secondary shoots,
such as in tobacco. In arable farming, inhibition of the vegetative growth in cereals by
strengthening the stems results in redllced lodging, and sirnilar agronomic effects are
achieved in oilseed rape, sunflowers, maize and other crop plants. Furthermore, inhibition
of the vegetative growth means that the number of plants per area can be increased.
, Another field in which growth inhibitors can be applied is the selective control of
ground-cover plants in plantations or crops with plenty of space between the rows, by
powerful growth inhibition without destroying these cover crops, so that competition with
the main crop is eliminated~ but the agronomically positive effects such as prevention of
~erosion, nitrogen fixation and loosening of the soil, are retained.
. .
A method for inhibiting plant growth is understood as meaning controlling the natural
development of the plant without altering the life cycle of the plant, which is determined
by its genedc make-up, in the sense of a mutadon. The method of growth regulation is
applied at a pardcular point in dme of the development of the plant, which is to be
determined in the particular case. The acdve ingredients of the fonnula I can be applied
before or after emergence of the plants, for example already to the seeds or seedlings, to
WO 93/17016 212 8 3 2 5 PCr/EPs3/00316
- 15 -
roots, tubers, staLlcs, leaves, flowers or other parts of the plant. This can be effected, for
example, by applying the active ingredient, as pure active ingredient or in the forrn of a
composition, to the plants and/or by treating ~e nutrient substrate of the plant (soil).
Various methods and techniques are suitable for using the compounds of the formula I or
compositions containing them for regulating plant growth, for example the following:
i) Seed dressin~
a) Dressing of the seeds with an active ingredient formulated as wettable powder by ~;
shaking in a container until the seed surface is uniformly covered (dry secd dressing). Up
to 4 g of active ingIedient of thc formula I are used per kg of seed (up to 8.0 g of wettable
powder in the case of a 50 % formulation).
b) Dressing of the seeds with an emulsion concentrate of the active ingredient or with an
aqueous solution of the active ingredient of the formula I formulated as a wettable powder,
using method a) (wct seed dressing).
c) Dressing by immersing the seeds in a liquor containing up to lO00 ppm of active
ingredient of the formula I for 1 to 72 hours, if desired followed by drying the seeds (seed
soaking).
Naturally, seed dressing or treatment of the germinated seedling are the prefe~red ;
application methods since the treatment with active ingredient is directed entirely at the
target crop. As a rule, O.OOl g to 4.0 g of active ingredient arc used per kg of seed, but it is
possible to deviate from the limit concentrations gi~ren in both directions, depending on
the method chosen, which also makes possible the addition of other active ingredients or
micronutrients (repeated seed treatment).
ii) n~rolled release of active iff~redient
The dissolved active ingredient is applied to mineral granule caniers or polymerised
granules (urea/formaldehyde) and allowed to dry. If desired, a coating can be applied
(coated granulcs), which permits slow release of the active ingredient over a certain ~
period. -
The compounds of the formula I are employed in unaltered form, as obtained from ~-
synthesis, or, preferably, together with the auxiliaries conventionally used in the art of ;
WO 93/17016 PCI/EP93/~ 6
2~2S~ 16-
formula~on, and they are therefore processed in a known manner to give, for example, -~
emulsifiable concentrates, directly sprayable or dilutable solutions, dilute emulsions,
wettable powders, soluble powders, dusts, granules and also encapsulations, for example
in polymeric substances. The application methods such as spraying, atomising, dusting,
wetting, scattering or pouring, as well as the type of the compositions are selected to SUit :
the intended aims and the prevailing circumstances. - -
The formulations, i.e. the cornpositions, preparations or combinations comp~ising the
active ingredient of the formula I and, if desired, one or more solid or liquid additives, are
prepared in a known manner, for example by intimately mixing andlor grinding the active ~ -
ingredients w~th extenders, for example solvents, solid ca~riers and, if desi~ed, - -
surface-active eompounds (surfactants).
The following are possible as solvents: aromatie hydrocarbons, in particular the fractions
C8 to C12, such as mixtures of alkylbenzenes, f~r exa nple xylene mixtures or alkylated
naphthalenes; aliphatic and eycloaliphatie hydroearbons sueh as paraffins, cyelohexane or
tetrahydronaphthalene; alcohols sueh as ethanol, propanol or butanol; glyeols as well as
their ethers and esters, sueh as propylene glyeol or dipropylene glycol ether, ketones sueh
as eyelohexanone, isophorone or diaeetone aleohol, strongly polar solvents sueh as
N-methyl 2-py~Tolidone, dimethyl sulfoxide or water, vegetable oils and esters thereof,
such as rapeseed oil, castor oil or soya oil silicone oils may also be suitable. -
Solid ea~iers whieh are used, for example for dusts and dispersible powders, are, as a rule,
- natural g~ound minerals such æ ealeite, tale, kaolin, montmorillonite or attapulgite. To
improve the physieal properties, it is also possible to add highly~isperse siliea or
highly-disperse absotptive polymers. Possible partieulate, adsorptive earriers for ~ranules ~-
are either-porous types, for example pumiee, briek grit, sepiolite or bentonite, or ~-
non-sorptive ea~ier materials, sueh æ calcite or sand. Moreover, a large number of
pregranulated;mate~ials of inorganic or organic natur~ can be used such æ, in p~icular,
dolomite or comminuted plant rcsidues.
Suitable surface-active compounds are non-ionic, cationic and/or anionic surfactants
having good emulsifying, dispersing and wetting properlies, depending on the nature of
the active ingredient of the formula I to be formulated. Surfactants are also to be
understood as meaning mixtures of surfactants.
WO 93/17016 PCr/EP93/00316
2128325 ~
- 17 -
Anionic surfactants which are suitable can be either s~called water-soluble soaps or
water-soluble synthetic surface-active compounds.
Suitable soaps which may be mentioned are the aLkali metal salts, aL~caline earth metal
salts or subs~ituted or unsubstituted arnmonium salts of higher fatty acids (Clo-C22), such
as, for example, the sodium salts or potassium salts of oleic or stearic acid, or of natural
mixtures of fatty acids which can be obtained, for example, from coconut oil or tallow oil.
Mention must also be made of the fatty acid methyltaurinates.
However, s~called synthetic surfactants are used more frequently, in particular fatty
alcohol sulfonates, fatty alcohol sulfates, sulfonated benzimidazole derivatives or
alkylarylsulfonates.
The fatty alcohol sulfonates or fatty alcohol sulfates are, as a rule, in the form of aLlcali
metal salts, alkalinc earth metal salts or unsubstituted or substituted ammonium salts, and
have an aLkyl radical having 8 to 22 carbon atoms, aL~yl also including the a~yl moiety of
acyl radicals, for example the sodium salt or calcium salt of ligr~insulfonic acid, of the
dodecylsulfuric ester or of a fatty alcohol sulfate mixture prepared from natural fatty
acids. This group also includes the salts of the sulfuric esters and sulfonic acids- of fatty
allcohoVethylene oxide adducts. The sulfonated benzimidazole derivatives preferably
contain 2 sulfonyl groups and one fatty acid radical having 8-22 carbon atoms. Examples
of alkylarylsulfonates are the sodium, calcium or triethanolamine salts of
dod~cylbenzenesulfonic acid, of dibutylnaphthalenesulfonic acid, or of a
naphthalenesulfonic acid/fc)rmaldehyde condensation product.
Other suitable compounds are the corresponding phosphates, such as the salts of the
phosphoric ester of p-nonylphenol/(4-14)-ethylene oxide adduct, or phospholipids.
Suitable non-ionic su~factants are mainly polyglycol ether derivatives of aliphatic or
cycloaliphatic alcohols, saturated or unsaturated fatty acids and alkylphenols which can
contain 3 to 30 glycol ether groups and 8 to 20 carbon atoms in the (aliphatic)
hydrocarbon radical and 6 to 18 carbon atoms in the aL~cyl radical of the alkylphenols.
Other non-ionic surfactan~s which are suitable are the water-soluble polyethylene oxide
adducts with polypropylene glycol, ethylenediaminopolypropylene glycol and
aL~cylpolypropylene glycol which have 1 to 10 carbon atoms in the alkyl chain and which
WO 93/17016 PCr/EP93/0~ 5
32~j '
- 18 -
contain 20 to 250 ethylene glycol ether groups and 10 to 100 propylene glycol ether
groups. The abovementioned compounds customarily contain 1 to S ethylene glycol units
per propylene glycol unit.
Examples of non-ionic surfactants which may be mentioned are
nonylphenolpolyethoxyethanols, castor oil polyglycol ethers, polypropylene/polycthylene
oxide adklucts, tributylphenoxypolyethoxyethanol, polycthylene glycol and
octylphenoxypolyethoxyethanol.
Other suitable substances are fatty acid csters of polyoxyethylenesorbitan, such as
polyoxyethylenesorbitan trioleate.
The cahonic surfactants are mainly quaternary ammoruum salts which contain at lcast one
alkyl radical having 8 to 22 carbon atoms as N subshtuents and which have lower -
halogenate~ or free aLkyl, bcnzyl or lower hydroxyaLlcyl radicals as funhcr substitucnts.
The salts are preferably in thc form of halides, methylsulfatcs or ethylsulfates, for cxample
stearyltrimethylammonium chloride or bcnzyldi(2-chloroethyl)cthylammonium bromide.
The surfactants conventionally used in the art of formulation are descIibed, inter alia, in
the following publications:
- "Mc Cutcheon's De~ergents and Emulsifiers Annual", Mc Publishing Corp.,
Glen Rock, New Jersey, 1988.
- M. and J. Ash, "Encyclopedia of Surfactants", Vol. I-m,Chemical PuWshing Co., -
New York, 198~1981.
- Dr. Helmut Stache "Tensid-Taschenbuch" ~Surfactant Guide], Carl Hanscr Verlag, ~ -
Munich/Vienna, 1981.
As a rule, the preparations comprise 0.1 to 99 %, in particular 0.1 to 95 %, of acti~e
ingredient of the fo~nula I, 1 to 99 % of the solid or liquid additive and 0 to 25 %, in
particular 0.1 to 25 %, of a surfactant.
While concentrated compositions are more preferred as commercial goods, the end user,
as a rule, uses dilute compositions.
The coinposi:ions can also comprise further additions such as stabilisers, for examplè
WO 93/17016 212 8 3 2 5 PCl~/EP93/00316
- 19-
epoxidised or unepoxidised vegetable oils (epoxidised coconut oil, rapeseed oil or soya
oil), defoamers, fi>r example silicone oil, preser~a~ives, viscosity regulators, binders,
tackifiers as well as fer~ilisers or other ac~ive ingredients for achieving specific effects.
Prefe~red formula~ions have in par~icular the following composi~ions:
(% = per cent by weight)
Emulsifiable concentrates:
Active ingredient: 1 to 20 %, preferably 5 to 10 % ~:
Surface-active agent: 5 to 30 %, preferably 10 to 20 %
Liquid carrier 15 to 94 %, preferably 70 to ~5 %
Dusts:
Active ingredient: 0.1 to 10 %, p~eferably 0.1 to 1 % -
Solid calTier: 99.9 to 90 %. preferably 99.9 to 99 %
;.,
Sus~ension concentrates: :
Acti~ ingredient: 5 to 75 %, preferably 10 to 50 % :
Water: 94 to 24 %, preferably ~8 to 30 %
Surface-ac~ve agent: 1 to 40 %, preferably 2 to 30 ~
Wettable powders: .
Active ingredient: 0.5 to 90 %, preferably 1 to ~0 %
Surface-active agent: O.5 to 20 %, pIeferably I to 15 3Co
Solid canier: S eo 95 %, preferably 15 to 90 % ;-
.............................. ......................................... :
Granules: - -
Active ingredient: 0.5 to 30 %, preferably 3 to 15 %
Solid calri~r: 99.5 to 70 %. pref~a~ly 971O 85 %
:-:
t
WO 93/17016 PCr/EP93/0~
C~ 37~i 20-
ePara~ion Examples:
Example Hl:
2-(3-Methvloxetan-3-oxvcarbonyl)phenv!sulfonvl chlonde
~ 7C
O H3C ~-;
-: '
A mixture of 6.25 g of 3-methyl-3-hydroxyoxetane, 16.1 g of 2-chlorosulfonylbenzoyl
chloride and 40 ml of absolute methylene chloqide is treated at a temperature from 0 ~o
5C with 5.6 g of pyridine (dissolved in 10 ml of absolute methylene chloride). The
reaction mixture is subsequently stiIIed for 2 hours at a temperature f~m 20 ~o 25C and
then poured into 100 rnl of ice-water. After the organic phase has bcen separated off and
dried over MgSO4, a methylene chloride solution of the dtle compound is obtained which
is employed in Examplc H2 without ~urther working-up.
Example H2:
2-(3-Methvloxetan-3~xycarbonvl)ph nylsulfonamide
,~S~2-NH2
11
~\
c--o 7~o
O H3C
1.5 g of ammonia aIe passed for 1 hour at a temperature from 0 to 5C into a methylené
solution obtained as in Example H1. The mixn~re is filtered and then treated with
ice-water, and the organic phase is separated off, washed with water and dried over
MgSO4. After concentra~on in vacuo, 2.1 g of the crystalline title compound with a
mel~ng point of 113-115C remain.
WO 93/l7016 212 8 3 2 ~ PCI/EP93/00316
- 21 -
Example H3:
N-~2-(3-Methvloxetan-3-oxvcarbonyl)phenvlsulfonvll N' (4,6 dimethvl- 1 ,3-~yrimidinyl)
urea CH3
o N c< ::
0~ ~CH
C--0~0 , .
A mixn~re of 1.36 g of 2-(3-methyloxetan-3-oxycarbonyl)phenylsulfonamide, 1.35 g of
4,6-dimethyl- 1 ,3-pyrimidinyl phenylcarbamate and 4 ml of absolute dimethylfonnamide
are treated dropwise at 20 to 25C with a mixture of 0.78 g of
diazabicyclo-~5.4.0]undec-7-ene and 1 ml of dimethylformamide and subsequently stirred
for 4 hours at a temperature from 20 to 25C. After thé mixture has been ~ansferred int~
water and 10 % hydrochloric acid have been added to bring the pH to 5, 1.72 g of the title ~ ~-
compound with a mel~ng point of 19~198C (decomposition) cTystallise ou~
The compounds of the formula I which are listed in the tables which follow and the
intermediates thereof are prepared analogously.
Table 1: Intermediates of the formula
~ R~XRs
Comp.
No. R3 R4 R5 R6 R7 M.p.
1~]
1.001 CH3 H H H H 113-115
1.002 CH3 CH3 H H H Oil -
1.003 H CH3 CH3 H H
1.004 H CH3 CH3 CH3 H
1.005 H CH3 CH3 CH3 CH3
1.006 CH3 CH3 CH3 CH3 CH
wo 93~l70l6 PCr/EP93/~ 6
'~9.S
~ - 22 -
Ta~le 2: Compounds of the fomlula I
~ O~N--C--N~
Comp.
No. R1 R2 R3 R4 Rs R6 R7 X Y E [M p C]
2.001 H H CH3 H H H H CH3 CH3 CH 196-198
2.002 H H CH3 H H H H CH3 OCH3 CH 183-185
Z.003 H H CH3 H H H H OCH3 OCH3 CH
2.004 H H C~3 H H H H OCH3 OCHE;2 CH
2.005 H H C~3 H H H H Cl OCH3 CH
2.006 H H CH3 H H H H C~H3 OCH3 N 13~132
2.0Q7 H H CH3 H H H H O~H3 ~ N
2.008 H H CH3 H H H H OCH3 oc~3 N
2.009 H H CH3 H H H H HNCH3 OC~Hs N
2.010 H H H CH3 H H H C~13 CH3 CH 120
2.011 H H H CH3 H H H CH3 OCH3 CH 173-175
2.012 H H H CH3 H H H O(~I3 OCH3 CH
2.013 H H H CH3 H H H OCH3 OCHF2 CH
, 2.014 H H H CH3 H H H CH3 OCHF2 CH
2.015 H H H - CH3 H H H OCH~2 OCHF2 CH
2.016 H H ~ CH3 H H H Cl OCH3 CH
2.017 H H H CH3 H H H CH3 OC~3 N 159-161
2.018 H H H CH3 H H H OCH3 ~ `
2.019 H H H CH3 H H H oc~3 OCH3 N
2.020 H H H CH3 H H H HNCH3 C)C~Hs N
2.021 H H H CH3 H H H N(CH3)2 OCH3 N
2.022 H H H C~13 CH3 H H CH3 CH3 CH
::
wo 93/17016 PCI/EP93/00316
212~325 -~
-23- :
Comp.
N~. Rl R2R3 R4 Rs R6 R7 X Y E lM.p.C]
, ~ ;.
2.023 H H H CH3 CH3 H H CH3 oc~3 CH
2.024 H H H CH3 CH3 H H OCH3 OCH3 CH
2.025 H H H CH3 CH3 H H OCH3 OCHF2 CH
2.026 H H H CH3 CH3 H H Cl OCH3 CH
2.027 H H H CH3 CH3 H H OCH3 CH3 N
2.028 H H H CH3 CH3 H H OC~3 ~
2.029 H H H CH3 CH3 H H OCH3 OCH3 N -2.030 H H H CH3 CH3 H H HNCH3 OC2H5 N
2.031 H H H CH3 CH3 CH3 H CH3 CH3 CH
2.032 H H H CH3 CH3 CH3 H CH3 OCH3 CH
2.033 H H H CH3 CH3 CH3 H OCH3 OCH3 CH
2.034 H H H CH3 CH3 CH3 H OCH3 OCHF2 CH
2.035 H H H CH3 CH3 CH3 H Cl OCH3 CH ~
2.036 H H H CH3 CH3 CH3 H OCH3 C~I3 N :~::~ / .. ,~
2.037 H H H CH3 CH3 CH3 H OCH3 ~ N :
2.038 H H H CH3 CH3 CH3 H OCH3 OCH3 N
2.039 H H H CH3 CH3 CH3 H HNCH3 OC2H5 N
2.040 H H H CH3 CH3 CH3 CH3 CH3 CH3 CH
`: 2.041 H H H . CH3 CH3 CH3 CH3 ~H3 OCH3 CH
2.042 H H H CH3 CH3 CH3 CH3 OCH3 OCH3 CH
~ 2.043 H H H CH3 CH3 CH3 CH3 OCH3 OCHF2 CH
: 2.0~4 H H H CH3 CH3 CH3 CH3 Cl OCH3 CH
2.045 H H H CH3 CH3 CH3 CH3 OCH3 CH3 N -~
2.a46 H H H CH3 CH3 CH3 CH3 OCH3 ~ N
:~ 2.047 H H H CH3 CH3 CH3 CH3 OCH3 OCH3 N
2.04~ H H H CH3 CH3 CH3 CH3 HNCH3 OC2Hs N
~: 2.049 H H CH3 CH3 CH3 CH3 CH3 CH3 CH3 CH
~:: 2.050 H H CH3 CH3 CH3 CH3 CH3 CH3 OCH3 CH
2.051 H H CH3 CH3 CH3 CH3 CH3 CH3 OCH3 N
2.052 H H CH3 CH3 CH3 CH3 CH3 OCH3 OCH3 N
2.053 H H CH3 CH3 CH3 CH3 CH3 OCH3 OCH3 CH
: :
.
wo 93/17016 pcr/Eps3/o~ ;
23 2~3?JS - 24 -
Comp.
No. ~l R2 R3 R4 Rs R6 R7 X Y E lM.p. C]
.
2.054 H H C~3 CH3 CH3 CH3 CH3OCH3 OCHF2 CH
2.055 H H CH3 CH3 C~3 C~H3 CH3 Cl OCH3 CH
2.056 H H CH3 CH3 CH3 CH3 CH3HNCH3 OC2Hs CH
2.057 H H CH3 CH3 CH3 C~3 CH3OCH3 ~1 CH
Formulation examples of active in~redienf~s of the fo~nula I
(% = per cent by wei~ht)
F1. Wettable powders a) b) c)
Active ingredient according to
Table2 20% 50% 0.5 %
Sodiumligninsulfonate 5 % 5 % 5 %Soa~um lauryl sulfate 3 % - -
Sodium diisobutylnapkthalenesulfonate - 6 % 6 %
Octylphenol polyetkylene glycnl ether
(7-8 moles of EO) - - 2 % 2 %
Highly-disperse silica 5 % 27 % 27 %
Kaolin 67 %
Sofdium chloride - - 59.5 %
The aceive ingredient is mixed thoroughly with the addif~ves and the mixture is fground
tho~oughly in a suitable mill. This gives wettable powders which can be diluted with water `~ -
to give suspensions of any desired concentration.
Wau~r- spersible Pranules a) b) :~
Active infgredient according to `-
Table2 75 % 5 %
Sodiumdibutylnaphthalenesulfonate 2% 0.5 %
Gum arabic 1 % 1 %
Sodium sulfate 5 % 3 %
Sodium ligninsulfonate 17 % 15 %
Kaolin ~ 75 5 %
.
wo s3/t7ol6 pcr/Ep93/oo3l6
21~32~
- 25 -
Emulsions of any desired ~oncentra~ion can be prepared from such concentrates bydilu~ion with water.
F3.Dusts a) b)
. .
Achve ingredient according to -~
Table2 O.l % 1 %
Talc 99-9 % -
Kaolin - 99 %
Ready-for-use dusts are obtained by in~nately mixing the camers with dle active
ingredient. -
::
F4. Extruder 2ranules a) b)
Active ingralient according to
Table2 10% l %
Sodium ligninsulfonate 2 % 2 %
Carboxymethylcellulose l % l %
Kaolin 87 % 96 %
The active ingredient is mixed with the addi~ives, and the mixture is ground and moistened
with water. This mixture is extruded and subsequently dried in a strearn of air.
F5. Coated ~ranules
Active ingredient according to Table 2 3 %
Polyethylene glycol (MW20()) 3 %
Kaolin 94 %
In a mixer, the kaolin which has been moistened with polyethylene glycol is coated
uniformly with the finely-gr~und active ingredient. Dust-free coated granules are obtained
in this manner.
F6. Suspensionconcentrate a) b)
Active ingredient according to
Table2 5 % 40 %
Ethylene glycol 10 % 10 %
WO 93/17016 PCl~EP93fC 6
,83~ i 26-
Nonylphenol polyethylene glycol ether
(15 moles of EO) 1% 6 %
Sodium ligninsulfonate 5 % 10 %
Carboxymethylcellulose 1% 1%
37 % aqueous formaldehyde solution - 0.2 % 0.2 %
Silicone oil in the form of a 75 %
aqueous emulsion 0.8 %0.8 %
Water 77 % 32 %
The fimely ground active ingr~iicnt is mixed intimately with the additives. This gives a
suspension concentrate from which suspensions of any desired concentration can be
prepared by dilution with water.
F7. Salt solution
Active ingredient according to Table 2 5 ~o - -
Isopropylamine 1% -
Octylphenol polyethylene glycol ether
(78 moles of EO) 3 %
Water 91 %
WO 93/17016 PCr/EP93/00316
~12832~
-27-
Biolo~ical Examples
Exarnple B 1: Herbicidal action bef~re emer~ence of the plants
Plastic pots are filled with expanded vermiculite (density: 0.135 g/cm3, water adsorption
capacity: 0.565 Vl). The non-adsorptive vermiculite is saturated with an aqueous active
ingredient emulsion in deionised water which comprises the active ingredients at a
concentration of 70 ppm, and seeds of the following plants are then sown onto the surface:
Nastur~um officinalis, Agrostis tenuis, Stellaria media and Digitaria sanguinalis. The test --
containers are then kept in a controlled-environment cabinet at a temperature of 20C, an
illumination of approx. 20 kLux and a relativc atmospheric humidiq of 70 %. Dunng a
germination phase of 4 to 5 days, the pots are covered with translucent material to increase
the local atmospheric humidity and watered with deionised water. After day 5, 0.5 % of a
commercially available liquid fertiliser is added to the irrigation water. 12 days after
sowing, the test is evaluated and the effect on the test plants is assessed using the
follow,ing key:
: plant not germinated or completely dead
2-3 : very powerful action
4-6 : medium action
7-8 : poor action
9 : no ac~ion (like untreated con~ol)
Table B1: Preemer,~ence action
Concentration of active ingredient emulsion: 70 ppm
Test plant: Nasturnum Stellaria Agrostis Digitaria
Active
Ingredient No. --
;.
2.001 1 2 1 2 ~
2.002 3 3 1 2 ```
2.006 1 2 2
2.010 2 2 2 2
2.011 2 2 1 2
2.017 2 2 2 2
wo 93/17016 Pcr/Eps3/~ 6
2~ 5
- 28 -
Example ES2: Post-emergence herbicidal action (contact herbicide)
A number of monocotyledon and dicotyledon weeds were sprayed after emergence ~in the
4- to ~leaf stage) with an aqueous dispersion of active ingredient according to Example
F6 at a dosage rate of 8-500 g of achve ingredient per hectare, and the plants were kept at -
24-26C and a relative atmospheric humidity of 45-60 %. The test is evaluated 15 days
after the treatment.
After 3 weeks, the herbicidal action is assessed using a 9-step (l = complete damage, 9 =
no action) score key in comparison with an untreated control group. Score figures from 1
to 4 (in particular l to 3) suggest good to very good herbicidal action. Score figures from 6
to 9 (in particular from 7 to 9) suggest a good tolerance (in particular in crop plants).
In this test, the compounds of the formula I show a powerful herbicidal action. Identical
results are obtained when the compounds of the formula I are fonnulated according to
Examples Fl to P5 and F7.
Example B3: Post-emer~ence phvtotoxic effects of herbicide No. 2.011 and of the
mixtures herbicide with safeners of the formula Sl and S2 in cereals
;.
Wheat is grown in plastic pots under greenhouse conditions up to the 3-leaf stage. At this
stage, a herbicide of the fonnula I as pure active ingredient as well as a mixture of the
herbicide with the safeners are applied to the test plants. Application is effected in the
fonn of an aqueous suspension of the test substances using 5001 of water/ha The
application rates of the herbicide are 30/lS/8 g/ha, the application rates of the safeners
60 g~a 28 days after application, the test is evaluated using a percentage key. 100 %
means that the test plant has died, 0 % means no phytotoxic effect. The results
demonstrate that the safeners employed are capable of noticeably reducing the herbicide -
damage in wheat. Examples of the good protective action of the safeners are listed in
Table B3.
WO93/17016 2l~32s PCl/EP93/00316
- 29 -
Table B3:
Dosage rate of herbicide in g/ha
Herbicide Safener, g/ha 30 15 8
Comp. No.
2.011 - 80 7û 60
2.01 1 S1 60 60 20 15
2.01 1 S2 oO 60 25 10
Example B4: Post-emergence phYtotoxic effects of herbicide No. 2.011 and of the
mixtures herbicide with safeners of the fonmlla S3 in maize
Maize is grown in plastic pots under greenhousc condidons up to the 2.5-lcaf stage. At dlis
stage, a herbicide of the formula I as pure active ingredient as well as a mLl~ture of the ~-
herbicide with the safener of the fo$mula S3 are applied to the test plants. Applieation is
effected in the form of an aqueous suspension of the test substances using 5001 of
water/ha. The applieaaon rates of thè herbicide a~e 30/1518 g/ha, th~ application rates of - `
the safener 60 gtha. 12 d~ys after applica~on, the test is evaluated using a percentage key.
100 % means that the test plant has died, 0 % means no phytotoxic effect. The ~sults
demonstrate that the safener employed is capable of noticeably reducing d~e herbicide -
damage in maize. Examples of the good protective action of the safener are listed in Table
B4.
Table B4: ~
Herbicide Safener, g/ha 30 15 8 -
(~omp. No.
. . .
2.011 ~ 80 7S
2.011 S3 60 65 50 30
WO 93/17016 PCl/EP93/0~ S
212~ ~25
- 30-
Example BS: Use of a mixture of herbicide No. 2.011 with safener of the formula S4 for
seed dressin~ in maize
Maize seed is dressed with the safener of the formula S4 at an equivalent of a dosage rate
of 1 g/kg of seed. The maize is subsequendy grown in plastdc pots under greenhouse
conditdons until it has reached the 2.5-leaf stage. Untreated maize is grown parallel with
treated maize up to the same stage. At dlis stage, the herbicidc of thc formula I is applicd
to treated and untreated test plants. Applicadon is effccted in the fo¢m of an aqucous
suspension of the herbicide at 5001 of water/ha The application rate of the herbicide is 30
or 15 gJha, and the application ratc of the seed-dressing safcner of thc formula S4 is 1 gJkg
of secd. I days after application, the tcst is cvaluated using a perccntagc kcy. 100 %
means that the test plant has died, O % means no phytotoxic effect. The rcsults
demonstrate that the safener used as seed-dressing agent noticeably ~educes the damaged
caused by post-emergence application of the herbicide. Similar results arc obtained when
the herbicide is applicd precmergence. Exanples o~ the good action of the safener of thc
fonnula S4 are shown in Table B5:
Table B5
Herbicide Safener, glha 30 15 8
Comp. No.
2.01 1 - 90 80 75
2.011 S4 60 20 10 OS
.