Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 93/17112 ~ PCI`/USg3/01351
21~33-S` ~
BI~S OF MEIllICt~1E
U5~ A REDU~ 9a~::E OF
l~a~D OF qffE INV13~
widely as a food arxl feed ~l~t. It ~ ~~ally pc~ra~ ~y
D~thyl ~ptan, and ~nide a~ ~teri~l~. (H.H. 8z~nt, ~c
~ Blo~ of th~ n,_i~l y," pag~ 182, Jdm Wil~ & S~, N~
Yc~c, 1989.) q~re ar~ ulting pm~t:t f~: D,L~i~ ar~ it~
analogL ~like all ath~r ~o ~ thicnine is ~ted to
th~ ~ed ~fann in ~. A~ a re~t, ~1 ~r~ses,
~ypically re~lt in ~he D,L ~ix~, ~ feasibl~ arYl cost-effective in thi~
case.
~ever, f~tation E~n ~, ~hi~ are ca~on ~ds far ~-
(X. Aida, I. a~ata, K. N~, X. ~alcin~i, and H. Y~,
biolo~ 24, El~vl~r, 1986.) q~ ~r~ing given that t~
pnx~ ~6t-effec~ ely ~y f~ntation D~ds usi~ ~posive r~w
mat~rials su~h as mola~, ~ a. }~ly~tes, c~n ~ liq~ar, an~l s~y
}~olycate~. (See for e~le: P.L. P~, R.G. Cail, D~F. Midgley,
C. Fry~, ~e Pn~:ts for L-Lysine }~ction in A~trali~," ~
~ectnolo~v in Aus~alia 38, E~. 514--518, 1986; and S. }bn~, A. Oz~ci,
ar~ T. Nak~i~hi, ~ R~Qn by I~Aspartate- and L~rine- :
resi~ m~ of E~i~ia g21i," AFplied Micmibiolocly and
Bi~olocu 29, ~. 550-553, 1988.)
Varia~s micrabes have been used to ~e L-lysine and L~ni~. q~e
have been devel~ed ~h rlaQCi~l methods of ~agen~is and selection
as well as ~ic er~ineeri~. (K. Aida, ~a.) Greatest sxoess has
been realized histori~ly with the C~ryr~bact~ia and E~vibact~ia, ~t it
is also clear that okher micrcbes such as Esçherichia coli are viable.
There is a ne~d far Dekhods to reduce the metatolic c06t and ccmplexity of
methionine biosynthesis, ideally making it similar to that for lysine or
- WO 93/17112 p~ PCr/US93/01351
7~ 3~ ~ :
t~nire, ~ ff~at an eoon~nic~l fenaerltaticn E~:ion of ~ianine ~s
po6sible.
S~ OP ~E
~e are ~vi~ fea~;i.ble fen~ntati~n ~d~ rar D~line
ca~ri~ tl~ u~ of r~d ~ur a~ ins~d of 0ll~ate as the
fen~nta~ ~;ulfur ~a~rce and/cr Cal~i8i~1g IX~liÇ~nirlg ~d thereby
sillplifyir~ the biodlemical path~ay. Also pmvided are f~tian
far ba~ 8~the8is cQ~i~lg the u~e of redu~d ~uln~r
Q a ~ instead of sulfate a~ the fen ~ tia~ sul ~ ~a e and/or
comprising redesigning and thereby.si~plifying the biodhemical pathway. Ih .-
a preferred er}odi~ent of the present invent$on the reduced sulfur scurce i8 :
hydrogen sulfide, nekhyl escApt-n or salts thereof.
Ih ~ preferred erbcdi ent of the present invention there are pro~ided
improveimekhods for such f~ationFroc-sses comprising re-designing or
n~iy and ~reby silaplifying the bio~ic~l pathway.
~ , . . . ...
DE~CN OF q~E ~Ni6 . :
Figure la is th~ ca~n bi~c pat~y to ~sine, ~ionine ar~
..
Figure lb is the ql~ine bioynthetic patt~ay in E~eri~ia co~i. ;
.
Figure lc is the ~ysine bio8ynthetic pat~ay in E;~ri~ia.5æli- ~; -
", .,
Figure ld is the ~ionine bio~etic pat~ay in ~erl~i~ coli.
.
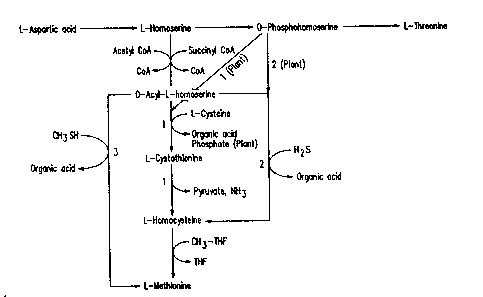
Figure 2. Variations in the pathways for MbthionIne biosynthesis: (1) .
Trznssulfurylation psthway; t2) Sulfhydrylation pathway; (3)
Methy~ f~ydrylation pathway.
. .
~FI:I E:~R~:C~Irl:0N OF TffE INVENTION
Ihe present invention relates to mekhods for the fermentation synthesis of
methiomne and hccccysteine. 1~ understard why a cost-effPr~ive
fe ~ tian method for methionine synkhesis dbe~ not exist, whereas such
me*hcds are available for lysine and theeonine, it is instructive to
consider in more ~Ft~il the differenLes a~Dng the methionine, lysine, and .~:
O 93/17112 PCT/US93/01351
21?~3~? 7
_Ire ~ e bi ~ etic pathways. All three amino.acids are bi ~ ically
derived from the same inbermediate metabolite, aspartic acid (Fig.l). In
fact, threcnine and methio mne also shEre additional biochemical steps and
the common intermed1ate homoser me. But ths ~yneheses diverge subst~ntillly
w.hen th~;~ specific pathway ~ranches ~re considered (Fig. l). These are
compared in Table I (J.L. Ingrabam, O. Mbaloe, and F.C. Nbidhardt, '~nowth
of the Bact~#rial Oell," pp. ~22-135, Sina~ Assoc., Inc., Sund~land,
~s., 1983. ) The pathways present in ~ 5~ are ~en as a ~ci~; of`
calparison, rex~nizing hawe~ that there i~ diver~ity in these path~y
amor~ micrcbes and plants and that thi~ cal4ar~scn shalld in no ~way be
interlreted as l~mitir~ ff~e present i ~ tion to pathways usir~ E. coli.
(K.M. I~onn and R.L. Sa~rville, C2~s 9-13 in "Am~ ids:
Biosynthesis and Genetic Pegu~tion," Adli~ Ebtlishing OD., 1983;
W.B. Jahoby and O.W. Griffith, ~icn m.D. in M~ds in Enzy ~loav 143,
A~iC ~ress, N~W Ya~, 1987. )
,
Table I
B'iod~emical ~ildina Blo~ ~d to ~yr~ize
sine. ql~nine. and ~icnine
Amino Acid A ~ te Pyruvate ~5~ NWDPH l-C S
Lysine 1 1 2 3 0 0
Ihreoni-~ 1 o 2 2 0 0
I~Ethicnine 1 0 7 8
It is evident from Table I that the biochemical energy requirements farmethionine biosynthesis, in t~rma of adenosine triphcsphate (ATP) and
reduoed niootinamide adenine dinucleotide ~hrcph~te (N~DPff), are about
three tImes higher than for lysine and threonine. This is due to the
reguirements of sulfate assimalatian (J.L. Ingraham, supra.) A tokal of
three moles of AIP and four les of NWDPH are reguirod to biochemically
r ~ e sulfate to ~lfide. Two additional moles of AIP are roguirel, one
each to transport sulfate into the cell and to inccrpor~te sulfide into
cysbeine. It is oyEteine, finally, that serves as the sulfur donor in the
biosynthesis of me*hiom ne (Fig. l). In addition, methiomne biosynthesis
uniquely roguires the 1noorporation of a ~ yl grcup (Fig. l, lable I).
This is derived as 5- m~hyl-tetrahydrofolate (CH3'IHF) from the conwersion
of serine to glycine. Clearly oonsidering the foregoing, the ne*aboli~ c06t
W O 93/17112 2~'3 ~ 3 ~ l P ~ /US93/01351
and complexity of synthesiz m g methi with sulfate as the sulfur sc~L~e
is much greater than that for lysine or threcnine.
There is natural diversity among micrcles and plants in the bicsynthe is of ~ `
nekhion m e. This is repre ented sdhematic~lly by Fi ~ 2 ~nd can be
summarized as follows (K.M. Herridnn, ~D5a; W.B. Jakoby, ~ ; F.C.
Neid ~ t, ~ r 27 in E~aYaCk~ sæ~Li ~nd S~uT~nella tyFtLb urlu~
American Society for N~crobiology, ~bY~L~ gtan, D.C., 1987; M. DiKan and E.C. -~
Wb~ib, "Eru~nnEs," 3rd editian, A~xY~smic Pr~#33~ New York, 1979; S. Yambgata, -:~ :
Bio~hi~ie 71 (1989) 112S-1143):.
1) In the methio m ne bicEqldlbetic patnh~yrBof all microbes, h~o~erine is
first activated either by Eu~x~yl-C~ oE, ool~ ~nd ~ tyllrlllrium) or
acetyl~ ~fungi, yeast, and bact~ia ~ as 8~i~cteriu~ and
Bacillus~ . q~e rou tiorts ar~ catalyzed by h~erine
succir~l~ansf~se (E~ 2~3.1.46) and h~erine a~r1trar~f~rase ~E~ :
2.3.1.31) r~ tlvely.
2) In the D~uonis~ bioep~c pat)~ of plants, ht ine is `:
acti~ated by A~R in a w:ti~ c~lyzed by h~rine kinase (E~
2.7.1.39). me h~erine kin~e reaction al~io 00~9i in micmbes,
h~t, the resulting ~_ is an inten~i~te in ~ir~,
h~t r~ methianine, bli~i~. mus in pl~nts o~rine
is thle ~a~oin~ be~een the DE~Iine d ~re pat~ys
whereas in micrabes the ~point is h~rine.
3) :~n thie micrcibial tr~s~:l~latian raIte to n~icnine,
acylhiamose~ine, in reactians catalyzed by O-succinylh~ine
(thiol)-lyase (EC 4.2.99.9) and c~ystathianine ~B-lyasie (EC 4.4.1.8),
aooepts r~oedi sulfur fr~ c:ystelre to give ~:yste~. (O-
Su~ciny~erine (thiol)-l~ase is also known asi cystathianine ~r-
~thase. )
4) In the micraibial sul~y~ylatian rc~te, ~st~ is pnxh~
dlrectly frn acyl~ierine ~ sulfidie by O~x:i~y~serine
(thiol)-lyase c~r O ~ oe ty ~ erine (thiol)-lyasie (EC 4.2.99.10). O-
a oe tyDhomoeerine (thiol)-lyase is alsio known as hYIrx~pdbeine s3r
and methionine sqrniYISe. ;
WO 93~17112 PCI'/US93/01351
3 ~ ~
5) In the microbial methylsul~ly~lati~n ra~te, methi~ ~s pr~d
directly frn ac~y~eerine and methyl ~n by O-
6) l~e transall~laticn and sulDy~ylaticn ra~ in plants are
~se i~ distinct f~ EIC 4.2.99.9 and i~
7) H~r~ne is a poar ~~ ,~ 0~ O~lha~ine (thiol)-lya~e,
e)oo~pt ~n the ~ of ~he enzy~re fra~ izoea~ar~oes ~ (S.
Yamagata, su~.
p
atic ReaG~icn ~ani~," W.H. Fr~ & Co., San Eh~isoD (1979).
of th~g~wp, ~yptc~ qntb~ a~rts ~rine ~ ~ulfide.at a
~ hi~h rate to ystei~ (~ ta, T. N~ra, M. Shi~da, a~Yl N.
Makigu*~i, "B~ratic PmhY~til of ~eire with q~ypt~an Synth~se of
~ic~hia coli," J. }~aentaticn ~nd Bice~in~q Ç7: 169--172, 1989).
n~is reactior is analogals with ~e reacticn of h~#erine and ~
ffle varia~s reactions relatin~ to sulfur ~atil ar~ D~thianine
bi~Y:y =is have yet to be oonsid~l in the design of a vi~le
~at~ method. nle use of ~llfide ar n~rl ~pt~ inste~d of
sulfate re~r~C the meta~olic c06t o~ 3 ~ hi ~ ~yr ~ is to the levals of
lysine and threonine. In the present invention two AIP and three N~DPH are
required s~nce the active transport of su}fate, reduction of sulfate, and
synthesis of cysteine are all eliminated.
Use of sulfide or ~ 1 DArc~ptan also reduces the metabolic ocmplexity of
methiomne blosynthesis since the bicsynthesis of cysteine and, in the case
of methyl mercapkan, CH3'rHF are eliminated. Further simplification is
pcssible and may be dRsirable by ~d~ptin; the plant bio~ynthetic pthway to
mlicrobes by methods known bo those skilled in the art. Since homoserine
kinase is already p ~ as an enzyme functioning in the microbial
threcnine pathway, this mcdification requires cnly introduction o plant
"
WO93/17112 '2.~L33~ ~ PCl/US93/01351 . ~ ~
c~ystathionine y-lyase activity. q~is aauld be ~pli~ed by struct~ally
n~dify~ microbial O-ac~ylh~serine (thiol)-lyase ar by expressing plant ~: -
modificaticns oould b~ ma~e in ~e enzy~ ce ather car~idate ~yrida~l :
pb~Aat:e enzylnes su~ as t~yptc~ ~ntha~ in ard~r 'co e~fecti~ely u~
h~erine directly as a sub6trate in sulh~
ao~tyl ~ serine (thiol)-lyase fra~.& ~ be c ~ ld be used witha~
ication. - :
~hile reduced forms of sul~ur wculd be preferred to m~nimize the requirement
for biochemical energy, okher mDre cxidized forms of sulfur are alsD :;
b~neficial. As described akove, an iIprcuerent t ~ ne*abolic
simplification results itenever sulfide, rather than cynteine, is -~
inocrpor~ted directly inko homoeerin~ or an ackivated derivative. Ihus more ::
oKidized forms subh as ~ulfate, sulfib~, and thio~ulfate may b~ prcvidea as :
scLroes and biodhemicslly rPduo~d to ~ulfide. q~lfite and
thiosulfate also di~dnish the need far bicche~ical energy relative bD
sulfate sinoe they are more redbo d foros, although th~ energy r quhrecent
is greater than for sulfide or ~cU-~l mesclpt m. : -
.. . ~.
By reducmg the complexity of the methionine bic~ynthetic pathway, the ~:
em agement of micrdb;a~ metabolism in ethionin cRec!lrcdbltion is less ::
extensive. Ihis rEduces the nuwber of genetic dhange~ that m~st be
introduoed into the prcc~oaing microke by cl~ssical or g ~ c engineEring
m-thods in order to dbrregulats methionine bdc6ynthes1s and l~mits the
disruption of microbial metabolism, in general. As used herEin, "de,
regulatel' neans any effect on the s~lf-regulation of the micrbbial
nekabolism for example, any effect on microbial self-regulaticn by feed-back
inhibition or reFression. ~Yih derregulation can be achieved through
~ s known to those skilled in the art such as for ~v~mple, classical ~ ;
mL~agenesis and selection or genekic efgineering.
i
The net result is to transform the methionine biosynthetic pathway to one
that c~mpores favarably with thoee for lysine and threorine m terms of
metabolic ccst and oomplexity. In this way, a feasible fermentation method
of methionine prcduction can be realized.
WO 93/17112 2 l ~ ~ ;3 4 7 PCl`/US93/01351
=~
me follawing disclos~e ~s intend~ to serva as a r~es0t~tion of
~di~nts ~n, and *x~uld nat be nstn~ as limitinq ~e sc~pe of
t~his application.
E. ~coli, C. ql~icum, and B. f~o ~ de-r~gulated far h~rine a~
~uction ~y classi~l cr gen~tic ~in~in3 ~s. ~e ~y~ti
route to me~i~ ~s irerc~l into these micrcbes ~y transf~ th~
(thio~)-lyase, and h ~ st ~ ~ ylase. qt~e parent and transfa ~ d
nL~3~1bes are cultivated individually in a f ~ ation nedium cfnt~ining
gluco6e, soy hydrolysate, and 1norganic nutrienks. m e med~um is
supplemented either with sulfate or sulf$de a~ a souroe of sulfur for
methionine pGodoction. Table I ind~cates the relative amount of nethionine
that is F~xxha~ed by each strain.
Table I -
~ onine
M~crcbe Sulfur Souroe Produced*
E. coli parent 8ulfate
E. ~ parent sulfide
E. coli transLformant ~lfate +
E. coli transformant sulfide ++
C glu~amicum parent sulfate
C. alutamdcum parent sulfide
C. glUt~liCIlIII trallSfC~lt S~ fate +
C. glu~amicum transformant sulfide ++
~lavum parent sulfate -
B. flavum parent ~lfide
B. flavum ~ formant ~lfate +
B. flavum transformant ~lfide ++
* l~w (-), ~um ~+), hi~ (~)
7 `"~
;:
.
WOg3/17112 ~ 30341 PCI'/US93/01351
1~2
(~) . ~ :'
~e pæent strains of Exa~le 1 are deleted fcr h~st~ ~rl~P activit~
me micrbbes are then trar~farmed wi~ pla~id(s) erf~dir~ h~er~e
acet~ltransferase and O-acet~ylh ~ erine (thiol)-lyase. Ihe ~ s
methylase negative parent and transformed microbes are ~ltivated as in EXamP1Q
1. Table II indicates the relative amount of hinlcysteine that is prcduoel ky : :
each strain.
Table II
H~c/s~ -
Micrcbe* Sulfur Source Produce~**
E~ S~ Pa~ fatQ
~. coli parent ~ ide
E. ooli transfcrmant sulfate +
E. ooli transformant ~lfide ~+ :~
C. alutamicum parent ~lfate -
C. alu~amicum parent ~llfide
C. alutamicum tranEformant sulfate +
C. alutamicum transformant sulfide ++
B. flavum parent sulfate
B. flavum parent sulfide
8. flavum transformant sulfate +
B. flavum transformant ~lfide +~
..:
*All s ~ lack hor wystelne methylase activity
**law (-), medium ~+), hi~ +)
Exam~le ~
Mbthi ~ Pr~duction via ~ey~omo6erine
n~Ethylsulfhydrylation Rcute~
The ~ of Example 2 are cultivated as in Example 1 except that
methylmercaptan is supplied as the s~pplemental sulfur source. Table III
indicates the relative amLunt of methionine that is produoed by each strain.
Methionine pr~duction is indicative of a functioning methyl~lfhydrylation
pa~ay.
. .
.- .-
.''' '- .~ :.
8 .
..:
``;
WO93/t7112 2~30~. i PCI/US93/01351
T~ble III
~ ~ne
MiC~* ~oed**
E. g21i parent --
C. alutamiQ~m pZ~nt .
C. qlut~Dian~ transfc~t +~
B. flau~n parent -
B. flavum t2msf~t H~
**law (-), high (~)
(~latit }~ .
~e pa~ s~s of Exz~ple l are transfan~d wi~h P~d(~) ~
indicates the relative a~ount of methicnine that i~ producel by eadh skrain.
.
Table IV
Mb*hionlne
M~crcbe Sulfur Souros ,Produoea*
.. -
ooli parent sulfate
ÇQli parent sulfide
E. çali traneformant sulfate +
E. çQ~ transformant sulfide ~+
C. g~y~amicum parent sulfate
C. alu~amicum parent sulfide
c~ ~u~amicum transfuLI~mt sulfate +
C. alutamicum transformant sulfide ++
B. flqyum parent sulfate
B. fla~um parent ~~1 fide
B. flavum transformant .sulfate +
B. flavum transformant sulfide ++
* law (-), medium (+), high (+~
, '.~
.
.
WO g3/17112 2 ~ 3 Q 3 ~ ~ PCI /US93/01351
Ex~ple 5
Methianine Pml4ç~via Tk~ine
Ihe dele~d parent strains of E~ple 2 are ~ansfc~d with plas~id(s)
of ~thi~nine that is p~ ~y e~ ~n.
,. . Tabl~ y ' ~ '
Micrcibe* ~8P* ...
E. QQli. paren~ -
E. .QQli transfanDar
B. flav~ ~
B. .f~av~ ttwfan~nt
- .
. .
Qp~ with ~ben~n ~ : .
**law (-), hi~h (~) ,
E~e 6 :`
`~''' .
qSe parent strains of F~le 1 are del~d far t~hsir h~rine - .
p~d(s) l~iir~ O~oety~serine (t~ol)-lyase fr~ S. ~ ~d
~e~ mE~ylase. lhe parent and trar~fc~d microbes are
alltivated as in Exanple l. Table VI ir~icates the relative an~nt a~ :
methi~ that is ~ by ea~ ~in.
-~' .~ ..
~. ,.-
~;' ' 10 :.'"' :
'~ `.;`"' '
WO 93/17112 PCr/US93/01351
Table VI
l~icrline
Microbe* Sulfur s~rce E~**
E. ooli parent sulfate
E. ooli parent sulfide
E. ooli transfa~mant sulfate +
E. ooli transf~t . sulfide ++
C. alut~ia~m parent sulfate
C. ' a~D~ parent sulf~e - '`
C. alut~iaml transfan~t sulfate +
C. alutania~n tw~fc~rlt sulfide ++
flav~ parent sulfate
fla~ parent su~ide
B. fla~n transf~ant: sulfate + ..
B. D~ t~nsfa~t sulfide ++
.
**l~r (-) ~ medium (+), high (++) .
. B~le 7
n~ ylsulhvdrylation ~ e)
The delete~ parent strains of Example 6 are transfaLw~d ~th a plasmid
encoding 0-aoetylhomoeerine (thiol)-lyase from S. EgEk~. me parent
and transformed mic ~ are cultivated as in Example 3. qable VII
indicates the relative amount of me*hi ~ that is prcduced by each
. '
lable VII
Mbthiomne
Micrcbe* Producod**
E..ooli parent
ooli transformant ~+
C. qlutamicum parent - ~?
C. qlutamicum transfonmant ++
B. flavum parent
. flavum transfnrm~t ++
*All strains lack homoserine acyltransferase activity
**low (-), high (++)
11 :