Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2132997
MANUFACTURE OF HIGH PURITY BENZENE AND
PARA-RICH XYLENES BY COMBINING AROMATIZATION
AND SELECTIVE DISPROPORTIONATION OF IMPURE TOLUENE
Technical Field
The invention described herein contemplates
production of high purity benzene and para-xylene rich
xylenes, and if desired of chemically pure toluene as
well, from an impure toluene stream containing
nonaromatic impurities boiling in the benzene-toluene-
xylene (BTX) range by using an acidic para-selective
catalyst. Also contemplated is an aromatization step
using a substantially nonacidic catalyst followed by
use of an acidic para-selective catalyst on the
product stream from the aromatization or on the
toluene containing fraction therefrom to produce high
purity benzene and a para-xylene enriched xylene
stream which is substantially free of nonaromatic
impurities, and, if desired, chemically pure toluene.
Background of the Invention
The disproportionation of pure toluene
feedstocks over molecular sieve catalysts to produce
xylenes and benzene is a known phenomenon. The use of
para-selective catalysts which make a xylene product
HED/CVRN/C: 1995. 001
2132947
containing a greater than thermodynamic equilibrium
ratio of para-xylene to ortho- and meta-xylene is
described, for example, in U.S. Patent~ 4,117,026 and
4,097,543 of Haag and Olson.
It has been the practice to specify and use
only high purity toluene as the feed for both
conventional and para-selective toluene
disproportionation processes. High purity toluene is
usually made by extracting the aromatic compounds from
a reformate or pyrolysis gas fraction and then
distilling the aromatics in several steps to recover
substantially pure benzene, toluene and C8 aromatics.
Toluene recovered directly from reformate or
pyrolysis gasoline by distillation without prior
extraction typically contains several percent close
boiling nonaromatics. Nonextracted toluene has not
been considered as an acceptable feed for the toluene
disproportionation process because in the prior art
processes the nonaromatic impurities have led to
decreased benzene and xylene yields and to decreased
purity for the products. Thus, the production of
benzene and xylene via toluene disproportionation has
required a prior aromatics extraction step which has
added significantly to the cost of the final benzene
and xylene products.
Catalysts can be used to selectively remove
nonaromatics from reformates. For example, U.S.
Patent No. 3,849,290 describes a multi-step process to
upgrade the octane rating of a naphtha gasoline
blending stock. In a first step, the stock is
reformed over a nonacidic platinum-type catalyst,
producing a reformate contAining aromatics and
paraffins. The reformate is then contacted under mild
hydrocracking conditions with an intermediate pore
HEO/CVRN/C: 1995. 001
2132947
zeolite to selectively crack high boiling, low octane
paraffins, e.g., C71. The effluent from this
hydrocracking step is contacted with a small pore
catalyst to selectively hydrocrack low boiling, low
octane C6- paraffins. This three step, three catalyst
processing seguence yields a high octane product after
the preferential removal of low octane species, but
the product purity is insufficiently high for
petrochemical applications.
U.S. Patent No. 4,795,550 describes a low
temperature catalytic process for removing olefinic
impurities, but not paraffins or naphthenes, from an
aromatic stream having a bromine index between 50 and
2000.
U.S. Patent No. 4,150,061 describes a process
whereby a fractionated pyrolysis gasoline comprising
toluene, xylenes, ethylbenzenes, C7 - C10 paraffins,
olefins and naphthenes are selectively
hydrodealkylated and transalkylated to give
ethylbenzene-lean xylenes and benzene in the presence
of a catalyst comprising a tungsten/molybdenum
component (WO3-MoO9) and an acidic component of 60 wt%
mordenite and 40 wt% catalytically active alumina.
The product is then distilled to provide benzene and
xylene streams of unknown purity and a toluene stream
- for recycle.
U.S. Patent No. 4,861,932 describes a process
for producing gasoline blending stocks in which
nonaromatic C2-C~2 paraffins are converted to a mixture
of higher octane aromatics and alkylaromatics by first
contacting the paraffins with a noble metal/low
acidity catalyst. The effluent is then contacted with
an acidic catalyst based on a zeolite such as ZSM-5
with a metal such as gallium (Ga). Although the
HED/CVRN/C: 1995. 001
2132997
Ga/ZSM-5 catalyst is known to have a high aromatic
selectivity, the product purity is in6ufficiently high
for petrochemical applications.
A large number of molecular sieves are known
to have u6e as catalysts in various hydrocarbon
conversion reactions such as disproportionation,
aromatization including reforming, catalytic cracking,
hydrocracking, dehydrocyclization, isomerization and
dewaxing. Typical intermediate pore size molecular
sieve6 of this nature include ZSM-5, sllicalite,
generally considered to be a high silica to alumina
ratio form of ZSM-5, ZSM-11, ZSM-22, ZSM-23, ZSM-35,
SSZ-32, SAPO-11, SAPO-31, SAPO-41, and the like.
Zeolites such as ZSM-5, ZSM-11, ZSM-12, ZSM-23,
ZSM-35 and ZSM-38 are described in U.S. Patents Nos.
3,700,585; 3,894,938; 3,849,290; 3,950,241; 4,032,431;
4,141,859 4,176,050; 4,181,598; 4,222,855; 4,229,282;
and 4,247,388 and in British Patent 1,469,345.
However, the use of such catalysts, particularly
acidic and para-selective forms of such catalysts, for
the production of high purity benzene and para-xylene
enriched xylene products from a C7-rich aromatization
product, i8 not known and has generally not been
contemplated.
It would be highly advantageous if one could
use refinery streams, for example, aromatizer product
streams which had at most been distilled 80 as to have
a reasonably high toluene content, but which still had
a significant amount of impurities which boiled in the
toluene range, in a process which would selectively
produce para-xylene enriched xylene along with
benzene, and if desired chemically pure toluene as
well and would at the same time convert the impurities
to products which could be readily separated from the
HED/CVRN/C 1995. 001
2132947
para-xylene enriched xylene and the benzene and from
toluene, if desired, by simple distillation.
Disclosure of Invention
The present invention i8 directed to
overcoming one or more of the problems as set forth
above.
In accordance with an embodiment of the
invention a process is provided for the selective
production of a benzene and para-xylene-rich product
which is substantially free of close-boiling
nonaromatics. The product is produced from a feed
which comprises at least about 70% toluene and between
at least about 0.2 wt% and S wt% close-boiling
nonaromatics. The process comprises contacting the
feed with an acidic para-selective catalyst to produce
the desired product.
In accordance with other embodiments of the
invention a predominantly paraffinic feed is
aromatized over a substantially nonacidic catalyst. A
stream having at least 70% toluene and at least about
0.2 wt% but no more than about S wt% close-boiling
nonaromatics is obtained. The stream is used as the
toluene-containing feed. Alternatively, an aromatizer
product stream which may not have sufficient toluene
can be distilled to produce the toluene-containing
feed. In either case the feed is contacted with an
acidic para-selective catalyst to produce the product.
It has been surprisingly discovered that
para-selective catalysts, which selectivation would be
expected to have reduced cracking and other
activities, can be prepared and utilized'under
conditions such that they will, along with selectively
producing para-xylene, also convert close-boiling
HED/CVRN/C: 1995. 001
- 21329 17
-- 6
nonaromatics to products which are not close-boiling
to benzene or to the xylene isomers or to toluene
whereby simple distillation can be utilized to provide
chemical grade benzene and xylene (and toluene)
fractions. It is also surprising that a ~ product
stream or fraction from an aromatizer unit has a low
enough concentration of close boiling nonaromatics
such that an acidic para-selective catalyst can
convert sufficient of the close boiling nonaromatics
to compounds boiling outside the BTX range readily
separable by distillation and at the same time
selectively convert the toluene to a para-xylene
enriched ~tream and benzene.
Brief Description of Drawings
The invention will be better understood by
reference to the figures of the drawings wherein like
number6 denote like parts throughout and wherein:
Figure 1 illustrates, schematically, a
process in accordance with an embodiment of the
invention;
Figure 2 illustrate6, schematically, a
process in accordance with another embodiment of the
invention; and
Figure 3 illustrates, schematically, a
process in accordance with still another embodiment of-
the invention.
Detailed De~cription of The Invention
In one embodiment of the present invention,
high purity benzene and para-xylene-rich xylenes can
be made from an impure toluene stream by using an
intermediate pore size acidic para-selective catalyst
with high cracking activity, such as HZSM-5 which has
HED/CVRN/C 199 5 . 001
213294 7
been treated to make it para-selective. Under toluene
disproportionation conditions close-boiling
nonaromatic impurities comprising up to 5 wt% of the
toluene feedstream are converted, enabling the
recovery by distillation of benzene and any remaining
toluene (which can be recycled), both of which are
99.5 wt%, more preferably 99.8 wt% and stlll more
preferably 99.9 wt% pure. (Terms such as of high
purity, close-boiling nonaromatics-substantially free,
chemically pure or of chemical grade are sometimes
used herein to indicate 99.S wt% or better purity of
the benzene and toluene products.) In this manner, a
toluene containing aromatizer product or a
distillation fraction thereof, can be directed to a
reactor where a process according to the present
invention is carried out, bypassing an aromatics
extraction step. The impure toluene stream, in vapor
phase, can be reacted over the acidic para-selective
catalyst to ultimately yield high purity benzene and
xylene streams. The xylenes stream produced by
disproportionation in accordance with the invention
followed by distillation, has no more than about 0.5
wt%, preferably no more than about 0.2 wt% and more
preferably no more than about 0.1 wt% of close-boiling
nonaromatics. It will generally comprise a Ca,
fraction containing ethylbenzene. Such a stream is
sometimes referred to herein as a high purity xylene
fraction which i~ para-xylene enriched.
The principal aim of the present invention is
to produce high purity benzene and a high purity
xylene fraction which iB para-xylene enriched by
selectively reacting out nonaromatic impurities having
boiling point-s in the benzene and xylene boiling
ranges while producing the desired products from an
HED/CVRN/C: 1995. 001
? 213299 7
impure toluene stream. The separation of the product
is accomplished by non-extractive, e.g., distillative,
technique6.
The benzene to xylene (BTX) boiling range
generally covers temperature~ between about 140-F and
350-F (about 60 C and 180 C). Nonaromatics
(paraffins, olefins and naphthenes) boiling in this
temperature range are referred to hereln as close-
boiling nonaromatics.
The process in general involves cracking the
close-boiling nonaromatics by their selective reaction
over an acidic cataly6t during the selective
production of para-xylene. The close-boiling
nonaromatics are converted to light paraffins, olefins
and other compounds boiling substantially outside the
BTX boiling range whereby they can be readily
separated by simple distillation.
The term "nonextractive" is used herein to
mean that separation of hydrocarbon species is
achieved on the basis of differences in boiling point
as opposed to reliance on a solvent. As a result of
these nonextractive techniques, the purity of the
benzene and toluene produced is preferably at least
about 99.5 wt%, more preferably at least about 99.8
wt% and most preferably at least about 99.9 wt%. The
high purity xylene fraction which is para-xylene
enriched has no more than about 0.5 wt%, preferably no
more than about 0.2 wt% and more preferably no more
than about 0.1 wt% of close-boiling nonaromatics.
These aromatic products are referred to herein as
substantially free of nonaromatic impurities.
Catalyst acidity is essential to the
conversion of the nonaromatic impurities in the
toluene disproportionation step. As is known in the
HED/CVRN/C: 1995. 001
213299 7
art, one measure of catalyæt acidity is n-hexane
cracking activity. For example, according to the
I'alpha" test, ~ represents the relative n-hexane
cracking activity of a catalyst compared with a
st~n~Prd catalyst. The test is described more fully
in U.S. Patent No. 3,354,078, the Jollr~Al of
C~talysis, Vol. 6, p. 522-529, (Aug. 1965) and the
Jollrn~l of Catalys;s, Vol. 61, p. 395 (1980), each of
which are incorporated herein by reference.
According to the test, a catalyst is ~acidic"
if ~ is greater than about 10, or more preferably, is
greater than about 50. Highly acidic cataly6ts have
values greater than about 100. Acidic catalysts that
are useful in the present invention are characterized
by this definition of acidity, i.e., ~ greater than
about 10, more preferably greater than about 50 and
still more preferably greater than about 100.
Zeolites can be characterized by their
absorption characteristics at relatively low
temperatures, for example from 77-K to 420 K, as
described in , for example, Zeolite Molecular Sieves,
Donald W. Breck, First Edition, John Wiley & Sons,
1974. These absorption characteristics are a function
of the kinetic diameters of the particular molecule
being absorbed and of the channel dimensions of the
molecular sieve. Intermediate pore size molecular
sieves useful in the practice of the invention in the
H-form will typically admit molecules having kinetic
diameters of 5.0 to 6. oA with little hindrance.
Examples of such compounds (and their kinetic
diameters in A ) are: n-hexane (4.3),
3-methylpentane (5.5), benzene (5.8), and toluene
( s . 8 ) . Compound6 having kinetic diameters of about
6.0 to 6. sA can be admitted into the pores, depending
HED/CVRN/C: 1995. 001
2132947
-- 10 --
on the particular sieve, but do not penetrate as
quickly and in some cases are effectively excluded.
Compounds having kinetic diameters in the range of 6.0
to 6. 8A include: cyclohexane (6.0), 2,3-dimethylbutane
(6.1), 2,2-dimethylbutane (6.2), m-xylene (6.1)
1,2,3,4-tetramethylbenzene (6.4) and o-xylene (6.8).
Generally, compounds having kinetic diameters of
greater than about 6.8A do not penetrate the pore
apertures and thus are not absorbed into the interior
of the molecular sieve lattice. Examples of such
larger compounds include: hexamethylbenzene (7.1),
1,3,5-trimethylbenzene (7.5), and
tributylamine (8.1).
Specific molecular sieves which are useful in
the process of the present invention include the
zeolites ZSM-5, ZSM-11, ZSM-12, ZSM-21, ZSM-22,
ZSM-23, ZSM-35, ZSM-38, ZSM-48, ZSM-57, SSZ-23,
SSZ-25, SSZ-32, and other molecular sieve materials
based upon aluminum and/or magnesium phosphates such
as SAPO-11, SAPO-31, SAPO-41, MAPO-11 and MAPO-31.
Such molecular sieves are described in the following
publications, each of which is incorporated herein by
reference: U.S. Patents Nos. 3,702,886; 3,709,979;
3,832,449; 3,950,496; 3,972,983; 4,076,842; 4,016,245;
4,046,859; 4,234,231; 4,440,871 and U.S. Patent
Applications Serial Nos. 172,730 filed March 23, 1988
and 433,382, filed October 24, 1989. In most cases,
these molecular sieves must be further treated to
impart para-selectivity as discussed below.
The sieves are preferably bound with any of a
variety of well-known inorganic oxide binders.
Appropriate binders include inorganic compositions
with which the molecular sieve can be combined,
dispersed or otherwise intimately admixed. Preferred
HED/CVRN/C: 1995. 001
~` 2132997
oxide binders include alumina, silica, naturally
occurring and conventionally proce~sed clays, for
example, bentonite, kaolin, sepiolite, attapulgite and
halloysite.
The selective conversion of nonaromatics in
an aromatics-rich stream is performed in the vapor
state. In general, reaction conditions should be such
as to promote the preferential catalytic conversion of
the nonaromatics while still accomplishing the desired
conversion of toluene to benzene and a xylene fraction
enriched in para-xylene. Generally, the pressure
should be between about 30 and 1000 psig, preferably
from about 100 to about 600 psig. Suitably the
temperature is between about 700-F and 1,200-F,
preferably between about 800-F and about 1,OOO F. The
feed weight hourly space velocity may suitably be from
about 0.1 to about 10, preferably from about 1 to
about 8, more preferably from about 2 to about 6. The
process is carried out in the presence of hydrogen to
inhibit fouling. The molar ratio of hydrogen to
hydrocarbon feed is typically between about 0.5 and
about 10.0 and is preferably between about 2 and about
5. The Examples that follow illustrate suitable
operating parameters.
The processes of the present invention may be
used to make high purity aromatics streams from a
variety of toluene containing feeds which have a
toluene content of 70 wt% or more, preferably 80 wt%
or more and more preferably at least about 90 wt%.
This serves to minimize production of light gases and
enhance both the cost-effectiveness of the process of
the present invention and its suitability for use with
the processes described herein. Typical feeds for
various embodiments of the invention include
HED/CVRN/C: 1995. 001
- 2132947
aromatizer product streams, and fractions and
mixtures, generally made from C5-C1~ feedstocks (to the
aromatizer), C6-C~ feedstocks, C6-C7 feedstocks and C7
feedstocks. The aromatizer product streams (or the
toluene distillation cuts from them) will include at
least about 0.2 wt%, usually at least about 0.3 wt%
and may include up to but not more than about 5 wt%,
of close-boiling nonaromatics. The aromatizer product
streams (or the toluene distillation cuts from them)
can also include up to about 25 wt% of aromatics other
than toluene, e.g., benzene and ethylbenzene. Indeed,
one advantage of the present invention is that a
portion of the ethylbenzene is converted to benzene
and/or xylenes during toluene disproportionation.
In one embodiment, a toluene fraction of a
reformate or aromatizer product stream obtained by
reforming or aromatizing a C6-C7 feed is purified and
simultaneously disproportionated to produce benzene
and a xylene fraction which is enriched in para-xylene
by reaction over a acidic para-selective catalyst.
Such a feed could be a light naphtha feed, for
example, one rich in C6 and/or C7 components, reformed
or aromatized over any of a variety of conventional
reforming or aromatization catalysts to produce a
product stream containing benzene, toluene and
close-boiling nonaromatics. Exemplary reforming and
aromatizing process conditions include: feed rate of
0.1-10 WHSV, pressures between about 0 psig and about
200 psig, preferably between about 40 psig and about
100 psig, temperatures between about 800-F and llOO F,
and a hydrogen:feed molar ratio of between about
0.1-10. The reformate or aromatizer product stream is
suitably distilled to produce the feedstock to the
selective para-xylene production process.
HED/CVRN/C: 1995. 001
2132947
Preferably the process used on the naphtha
feed is aromatization u6ing a nonacidic catalyst. The
~ values of the non-acidic catalyst used in the
aromatization step should be less than 0.1. Catalyst
acidity in aromatics generation is undesirable because
it promotes cracking that in turn results in lower
aromatic selectivity. Also, the amounts of close
boiling nonaromatics is kept to acceptable limits as
defined above if the acidity of the reforming or
aromatization catalyst is kept sufficiently low. To
reduce acidity, the catalyst may contain an alkali
metal and/or an alkaline earth metal. The alkali or
alkaline earth metals are preferably incorporated into
the catalysts during or after synthesis according to
conventional methods. In addition, at least 90% of
the acid sites are desirably neutralized by
introduction of these metals, more preferably at least
95%, most preferably substantially 100%.
In addition, the catalyst for aromatics
generation may be based on alumina or molecular
sieves, such as L-zeolite or silicalite, with an
inorganic binder. Preferred catalysts for
aromatization include catalysts comprising platinum on
nonacidic forms of beta-zeolite, ZSM-5, silicalite and
L-zeolite. Other well-known aromatization catalysts
typically contain a catalytic metal such as platinum
disposed on any of a plethora of natural and man-made
crystalline aluminosilicates. Metallic promoters such
as platinum or other Group VIII metals also may be
included, as can other promoter metals. The preferred
aromatization catalyst is L-zeolite.
Examples of methods of manufacture of ZSM-5,
and particularly of a ZSM-5 catalyst having high
silica-alumina (SiO2:Al203) molar ratio, sometimes
HED/CVRN/C: 1995. 001
213294 7
- 14 -
referred to as silicalite, are shown in: Dwyer, et
al., U.S. Patent No. 3,941,871, issued March 2, 1976
and U.S. Patent No. 4,441,991, issued April 10, 1984;
and Derouane, et al., EPO Application No. 186,479,
published February 7, 1986, all of which are
incorporated by reference in their entireties.
Examples of the preparation of nonacidic
platinum on silicalite or L-zeolite catalysts may be
found in U.S. Patent Nos. 4,830,732 and 5,073,250,
both of which are incorporated herein in their
entireties by reference.
Low sulfur feed6 to the aromatics generation
step may be particularly attractive in order to avoid
poisoning the catalyst. In the case of
L-zeolite, it i8 preferable that the feed to the
aromatizer has a sulfur content of less than 50 ppbw,
and more preferably less than 5 ppbw.
As a specific example of the employment of
such an aromatics generation procedure with the
process of the present invention, valuable C6-C8
aromatics may be produced from relatively inexpensive
hydrocarbon feeds such as paraffinic naphthas. The
products are streams containing ethylbenzene, benzene,
toluene, and the three xylene isomers. A distillation
step may be carried out to produce a fraction having a
desired toluene content, e.g., 70 wt% or higher. The
aromatics generation procedure converts the
hydrocarbon feedstocks into very clean aromatic
mixtures that, however, include a limited amount of-
close-boiling nonaromatic impurities boiling in the
toluene boiling range. The amount of nonaromatic
impurities boiling in the toluene boiling range in a
C7+ cut of product must fall within the range from 0.2
wt% to 5 wt~, preferably from 0.2 wt% to 2 wt%. The
HED/CVRN/C: 1995. 001
21329g7
- 15 -
process according to the present invention then
simultaneously produces benzene and a para-rich xylene
stream, and converts the close-boiling nonaromatic
impurities to yield products that are substantially
free of close-boiling nonaromatlc6. Thus, in some
embodiments the process uses two separate catalysts: a
nonacidic catalyst to generate aromatics and an acidic
catalyst to purify the aromatics generated and to
simultaneously convert the toluene present into a
chemically pure xylene fraction which is enriched in
para-xylene and into chemically pure benzene.
More particularly, such a process could
involve passing an inexpensive hydrocarbon feed over
a nonacidic catalyst such as platinum on L-zeolite or
on silicalite (which is generally considered to be a
ZSM-5 zeolite with a low alumina content, e.g., ZSM-5
with a silica to alumina ratio of at least 1,000).
Such catalysts are especially effective at aromatizing
the feed, but, as described above, produce a complex
mixture containing a limited amount of nonaromatics
that are difficult to separate by distillation from
the desired aromatics.
Next, an acidic para-selective catalyst
cleans up the mixture by converting the close-boiling
nonaromatic components into both lighter and heavier
components that can be easily separated from the
aromatics, for example, by distillation, while
selectively converting the toluene to chemically pure
benzene and to a C8 aromatics fraction having an
enhanced para-xylene content and which is
substantially free of nonaromatics. This beneficial
combination of two different catalysts serves to
facilitate the production of high purity benzene and
xylenes.
HED/CVRN/C: 1995. 001
2132947
- 16 -
Some of the ethylbenzene present in the feed
to the toluene disproportionation 6tep is converted to
benzene and/or xylenes, while the remaining (i.e.,
unconverted) fraction can be conventionally handled by
feeding the C~ fraction to a para-xylene plant wherein
para-xylene i6 separated from the other C8 aromatics by
crystallization or by 6elective adsorption.
Substantially any catalyst which is
para-selective can be used in the process of the
present invention 80 long as it has enough acidity.
Examples of such catalysts are found in previously
mentioned U.S. Patents 4,117,026 and 4,097,543 of Haag
and Olson, both of which are incorporated herein in
their entireties by reference. In general, the term
"para-selective" as used herein means that the
catalyst, when used in accordance with the present
invention will produce a xylene fraction in which at
least 40% of the xylenes are para-xylene. Indeed, as
is shown in the examples well over 60% of the xylenes
produced can be para-xylene. Attaining such a higher
percentage of para-xylene is generally desirable.
The use of large crystal size zeolites having
a minimum crystal dimension of greater than about 0.5
micron, generally in the approximate range of 1-20
microns and particularly 1-6 microns is generally
preferred as adding to the para-selectivity. In a
preferred embodiment, the specified large crystal size
zeolite modified by precoking or combination therewith
of one or more difficulty reducible oxides is
employed.
In assessment of zeolite crystal size,
conventional scanning electron microscopy (SEM)
techniques can be used, the minimum crystal dimension
of a given crystal being taken as the dimension of
HED/CVRN/C: 1995. 001
2132g97
reference. The cryætalline aluminosilicate zeolites
used in the present invention in substantial
proportion are essentially characterized by a minimum
crystal dimension of greater than about 0.5 micron.
It is contemplated that the amount of zeolite of such
crystal size will be such as to exert a directive
influence in the desired selective production of
paradialkyl substituted benzenes. Generally, the
amount of zeolite of such crystal size will be present
in predominate proportion, i.e., in an amount
exceeding 50 weight percent, and preferably may
constitute up to 100 weight percent of the total
zeolite employed.
In the preferred embodiment, the crystalline
aluminosilicate zeolites employedj particularly those
having a minimum crystal dimension of greater than
about 0.5 micron, may have undergone modification
prior to use by selective precoking thereof to deposit
at least about 1 weight percent and generally between
about 2 and about 40 weight percent of coke thereon,
based on the weight of total catalyst. If the zeolite
is employed in substantially pure form or in
combination with a low coking binder, such as silica,
then the weight percent of coke is generally in the
range of 2 to 20 weight percent. When the zeolite is
combined with a binder of high coking tendencies, such
as alumina, coke content of the total catalyst is in
the approximate range of 10 to 40 weight percent.
Precoking can be accomplished by contacting the
catalyst with a hydrocarbon charge, e.g., toluene,
under high severity conditions at a reduced hydrogen
to hydrocarbon concentration, i.e., 0 to 1 mole ratio
of hydrogen to hydrocarbon for a sufficient time to
deposit the desired amount of coke thereon. Prior
HED/CVRN/C: 1995. 001
2132947
- 18 -
modification of the zeolite may also be suitably
effected by combining therewith an amount, generally
in the range of about 2 to about 30 weight percent, of
a difficulty reducible oxide such as an oxide of
antimony, phosphorous, boron and/or magnesium.
Combination of the desired oxide with the zeolite can
readily be effected by contacting the zeolite with a
solution of an appropriate compound of the element to
be introduced, followed by drying and calcining to
convert the compound to its oxide form.
ZSM-5 is the preferred catalyst for the
disproportionation reaction. The ZSM-5 preferably has
a silica to alumina mole ratio of between 10 and 100.
The conversion process described herein is
generally carried out as a semi-continuous or
continuous operation utilizing a fixed or moving bed
catalyst system.
The present invention will be more clearly
understood by reference to the following examples.
PT.~ 1
Comparison Example With Non-selectivated Catalyst
Debutanized reformate prepared by reforming
of a full boiling range naphtha feedstock over a
catalyst comprising platinum on alumina and was
distilled to obtain light and heavy fractions. The
heavy reformate was further distilled to a 30~ cut
point. The overhead product of the second
distillation, comprising about 92 wt% toluene and
about 5 wt% nonaromatics, was vaporized, blended with
hydrogen, and passed through a tubular fixed-bed
reactor charged with acidic but not selectivated
ZSM-5 catalyst. The reaction was carried out at
HED/CVRN/C: 1995. 001
2132947
-- 19 --
lOOO F (538 C), 150 psig, and 5.7 liquid toluene feed
weight hourly 6pace velocity (WHSV). The added
hydrogen:toluene molar ratio was about 3:1.
- Feed and product analyses were obtained by
capillary gas-liquid chromatography after nine hours
on stream, as shown in Table 1. The ga6 chromatograph
was equipped with a flame ionization detector and a
polar column that eluted nonaromatics before
aromatics.
HEO/CVRN/C: 1995. 001
21329~7
- 20 -
Feed Product
ComponentsArea % Area %Difference
Nonaromaticc
C1-C5 0.008 5.536 5.536
isohexanes0.002 0 -0.002
n-hexane 0.001 0 -0.001
C6-C7
nonaromatics0.092 0 -0.092
n-heptane 0.334 0 -0.334
C7-C8
nonaromatics2.044 0.009 -2.035
n-octane 0.933 0 -0.933
C8-C9
nonaromatics1.423 0.116 -1.307
C9 + nonaromatics 0.431 0.037 -0.394
Aromatics:
Benzene 0.05 25.25 25.20
Toluene 91.69 42.03 -49.66
Ethylbenzene1.38 1.07 -0.31
p-Xylene 0.53 4.94 4.41
m-Xylene 0.97 10.75 9.78
0-Xylene 0.11 5.05 4.94
C9 + aromatics0 5.21 5.21
Total99.998 99.998 0.008
30 p-Xylene % = p-Xylene x 100%
p-Xylene + m-Xylene + o-Xylene
= 4.94 x 100% = 24%
4.94 + 10.75 + S.OS
HED/CVRN/C: 1995. 001
21329~7
- 21 -
The analyses show that the toluene
disproportionated to make benzene and xylenes, while
nonaromatic impurities in the same boiling range were
sub6tantially eliminated by cracking to form light
ends. Para-xylene comprised 24 wt~ of the xylenes
produced.
This example demonstrates the elimination of
close boiling nonaromatics and the preparation of
chemically pure benzene and xylene using a non-para-
selective catalyst whereby only 24 wt% of the xylenefraction was para-xylene.
PT.P! 2
Aromatization Followed By
Selectivated Para-Xylene Production
A C6-C7 naphtha was aromatized over a
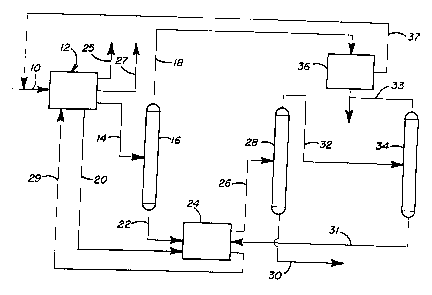
non-acidic platinum L-zeolite catalyst by being fed
via line 10 (refer to Figure 1) to an aromatization
unit 12. The aromatization was run to maximize yield
of benzene whereby the resulting liguid product
contained only a small amount of unreacted C~+ naphtha
components. The C6+ product stream was collected. It
was then taken, as represented by line 14 and was
separated in a distillation column 16 into a benzene-
rich fraction (removed as represented by line 18) and
a C7+ bottoms fraction, both having close-boiling
nonaromatics. Most of the unreacted paraffins,
olefins and naphthenes were found in the benzene
overhead fraction rather than the toluene and heavy
aromatics C7+ fraction after distillation. As a result
the feed (bottoms fraction) to the toluene
disproportionation step which followed contained about
0.4 wt% close boiling non-aromatics. The benzene
HED/CVRN/C: 1995. 001
2132947
- 22 -
fraction was sent as represented by line 37 to
aromatics extraction unit 36.
The conditions during the aromatization
process were as follows:
Naphtha WHSV = 1.0, feed molar ratio H2/naphtha = 5.0,
temperature = 890-F, pressure = 70 psig.
The C7, bottoms fraction, along with some of
the hydrogen from the aromatization unit 12 introduced
as represented by line 20, was fed via line 22 to a
disproportionation zone 24 wherein it was contacted
with a selectivated intermediate pore size zeolite
catalyst whereby para-xylene was selectively produced
and close boiling non-aromatics were converted to
materials which boil generally outside of the benzene
and xylene ranges. Excess hydrogen was removed from
the aromatization unit 12 via line 25 and light
hydrocarbons were removed via line 27. The toluene
fraction was reacted over an acidic, large crystallite
size (0.5 - 2.0) HZSM-5 (Silica to alumina ratio of
70:1) catalyst, which had previously been selectivated
by passing toluene and nitrogen over the catalyst at
llOO F and 150 psig for 27 hours. 12mL/h of toluene
feed and 75 cc - STP of hydrogen were passed over 2
grams of catalyst at 850-F and 150 psig. Table 2
shows the analysis of the toluene fraction of the
aromatization product and of the product of the
selectivated conversion of toluene to benzene and
para-xylene.
HED/CVRN/C: 1995. 001
2132947
Table 2. Reaction of C7 + Aromatization
Product over Para-Selective Catalyst
Composition, Wt% Feed ProductDifference
Light Ends 0.00 0.71 0.71
C6 non-aromatics 0.00 0.00 0.00
Benzene 1.01 10.35 9.33
C7 non-aromatics 0.11 0.02 -0.09
Toluene 83.79 68.36 -15.42
C8 + non-aromatics0.32 0.06 -0.26
Ethylbenzene 1.57 0.32 -1.25
p-Xylene 1.90 9.78 7.88
m-Xylene 1.86 3.02 1.16
o-Xylene 2.41 1.51 -0.90
Heavy aromatics 7.02 5.86 -1.16
Total 99.99 100.00 0.01
Catalyst: 'Selectivated Large Crystal HZSM-5'
Conditions: Catalyst Weight = 2.0 Grams
Liquid Feed Rate = 12mL/h
H2 Feed Rate = 75 cc/min
Temperature = 850-F
Pressure = 150 psig
p-Xylene % = p-Xylene x 100%
p-Xylene + m-Xylene + o-Xylene
= 9.78 x 100 68.3%
9.78 + 3.02 + 1.51
Better than 80~ of the close boiling
non-aromatic material in the toluene fraction was
converted to light or heavy ends in the toluene
disproportionation reactor leaving only 0.02 wt.%
C7 non-aromatics in the final product relative to
HED/CYRN/C: 1995. 001
2132997
- 24 -
10.35 wt~ benzene and 0.06 wt.~ C~+ non-aromatics
relative to 14.31 wt% xylenes. Thus the benzene
stream has a purity of 99.8 wt%. About 80% of the
ethylbenzene and a proportion of the ortho-xylene and
heavy aromatics in the original feed were converted
yielding even more benzene and para-xylene. The
para-xylene proportion of the xylene fraction was
68.3 wt~.
Hydrogen and light hydrocarbons from the
disproportionation zone 24 are recycled via line 29 to
the aromatization unit 12. The rest of the product
from the disproportionation zone 24 is conducted via
line 26 to distillation column 28 where it is
distilled to provide a bottom fraction having C8+
aromatics and being enriched in para-xylene which is
removed via line 30 and an overhead fraction
containing C6 and C7 aromatics. The C8+ aromatics
stream is sent to a para-xylene plant for the recovery
of para-xylene and the isomerization of ortho- and
meta-xylenes to para-xylene. Operating costs in the
para-xylene plant are significantly reduced because
the toluene disproportionation unit produces a xylene
stream which 1) is substantially free of nonaromatics,
2) is rich in the para-xylene isomer and 3) contains a
reduced amount of ethylbenzene.
The C6/C7 aromatics fraction is conducted via
line 32 to a further distillation column 34 where it
is further distilled to provide toluene for 6ending
via line 31 to the di6proportionation zone 24 (or for
recovery as a chemically pure product) and to provide
high purity benzene as an overhead. This benzene is
combined via line 33 with the high purity benzene
obtained from the aromatics extraction unit 36. The
benzene product is chemically pure and the xylene
HED/CVRN/C: 1995. 001
-
2132947
product is substantially free of non-aromatics. The
xylene product is enriched in para-xylene. The
para-xylene proportion of the xylene fraction is 60 to
80 wt%.
This example demonstrates the production of
high purity benzene and a para-enriched xylenes stream
from a C6-C7 naphtha. The process comprises
aromatization of a C6-C7 naphtha followed by
para-selective disproportionation of impure toluene.
PT.~! 3
Process Starting Wi~h C7 Na~htha
A C7 naphtha is fed via line 38 (see Figure 2)
to an aromatization reactor 40 along with hydrogen gas
which may be fed via line 42. The aromatics and
close-boiling non-aromatics contained in the product
from the reactor are fed via line 44 to a
disproportionation zone 46 containing an acidic
intermediate pore size zeolite catalyst which has been
selectivated for para-xylene production. Hydrogen is
separated from the product of the disproportionation
zone 46 in flash drum 48 and removed via line 47. All
or a portion of the hydrogen can be recycled, for
example, via compressor 49, to the aromatization
reactor 40 via line 42. The remainder of the product
is fed via line 50 to a stabilizer column 52 wherein
light hydrocarbons are stripped off and removed via
line S1. The bottoms fraction from the stabilizer
column is fed via line 54 to a benzene recovery column
56 wherein an overhead fraction of chemically pure
benzene is removed via line 58. The bottoms fraction
from the benzene recovery column contains C7 and C8+
aromatics and is substantially free of close-boiling
HED/CVRII/C: 1995. 001
2132g47
- 26 -
nonaromatics. The bottoms fraction is fed via line 60
to a toluene recycle column 62 wherein toluene is
removed as the overhead via line 64 and sent to the
disproportionation zone 46 (or recovered as a
chemically pure product). The bottom fraction from
the toluene recycle column contains chemical grade C8+
aromatics enriched in para-xylene. The para-xylene
proportion of the xylene fraction is 60 to 80 wt%.
This example demonstrates high para-xylene
yield with the product being of chemical grade quality
starting with a C7 naphtha.
~!X;~IPT~l;! 4
Process Startin~ With C5-C~1 Naphtha
Figure 3 illustrates this example which is
similar to the embodiment of Figure 1. A C5-C~ full
boiling range naphtha is fed to aromatization unit 12
via line 10. Hydrogen and light hydrocarbons are
removed via lines 25 and 27. The remainder of the
aromatization product is fed via line 14 to
distillation column 16. The overhead from column 16
contains benzene and lighter components. It is fed to
benzene extractor 36. The raffinate from extractor 36
is cycled to the aromatization unit 12 via line 37. A
chemically pure benzene product is removed via line
57.
The bottoms fraction from column 16 is
delivered via line 22 to a distillation column 60.
Cg, is removed from column 60 via line 62 and can be
used as a heavy gasoline blending stock. The C6-C8
overhead from column 60 is led via line 64 to a
further distillation column 66.
HED/CVRN/C: 1995. 001
2132947
Distillation column 66 separates the C6-C8
overhead from column 60 into a C8 product which is
removed via line 30 and into a C6-C7 overhead which is
further separated in distillation column 34 into
chemically pure benzene and into toluene which i8 sent
to disproportionation unit 24 via line 31 (or is
recovered as chemically pure toluene). The chemically
pure benzene from unit 34 is combined with the
chemically pure benzene from unit ~. The toluene
stream is disproportionated to yield chemically pure
benzene, toluene and para-rich-xylenes. The toluene
disproportionation product then proceeds to the
distillation train via line 26 and high purity
benzene, toluene and C8 aromatics are removed as
described above.
The above examples demonstrate the wide
applicability of the process of the present invention
for the production of para-xylene enriched xylenes
along with high purity benzene from full boiling range
naphthas and from toluene-rich feedstocks which can be
prepared from such naphthas.
Industrial Applicability
The pre6ent invention provides the capability
of synthesizing chemical grade benzene and para-xylene
enriched xylenes which are substantially free of
close-boiling nonaromatics from toluene streams which
include close-boiling non-aromatic impurities without
the necessity for solvent extraction steps.
While the invention has been described in
connection with specific embodiments thereof, it will
be understood that it is capable of further
modification, and this application is intended to
cover any variations, uses, or adaptations of the
HED/CVRN/C: 1995. 001
2132947
- 28 -
invention following, in general, the principles of the
invention and including such departures from the
present disclosure as come within known or cu6tomary
practice in the art to which the invention pertains
and as may be applied to the es6ential features
hereinbefore set forth, and as fall within the scope
of the invention and the limits of the appended
claims.
.
HEOtCVRN/C: 1995. OOI