Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1 ~i37406
Process for Key Intermediates for HIV Protease
Inhibitors
Field of the Invention
This invention relates to HIV protease
inhibitors. More specifically, this invention
relates to a process for the stereospecific
synthesis of key intermediates for preparing
hydroxyethylamine isosteres.
Background of the Invention
HIV protease is an essential enzyme for the
replication of human immunodeficiency virus (HIV),
the causative agent of acquired immunodeficiency
syndrome (AIDS). Within the last decade, the
enzyme has become recognized as a virus-specific
therapeutic target. As a result, much attention
has been focused on the development of HIV
protease inhibitors for the treatment of AIDS.
For a recent review on the state of HIV protease
inhibitors, see S. Thaisrivongs, Annual Reports In
Medicinal Chemistry, 1994, 29, 133.
As noted in the latter review, potent HIV
protease inhibitors have been realized by the
CA 02137406 2003-10-07
2
placement of a hydroxyethylamine isostere (also
known as a hydroxyethylamine transition state
analog) in a peptide having the p 17/p24 substrate
cleavage site sequence.
5 Noteworthy reports describing HIV protease
inhibitors with hydroxyethylamine isosteres
include N.A. Roberts et al., Science, 1990, 248,
358; D.P. Getman et al., J. Med. Chem., 1993,
36, 288; and P.C. Anderson et al., European
10 patent application, publication no. 560 268,
September 15, 1993.
As exemplified in the last three references,
an often-used intermediate for the elaboration of
the hydroxyethylamine isostere-containing
15 inhibitors is an aminoepoxide intermediate
represented by the following general formula A:
R
x~ o lA)
Y/
20 wherein X is an N-protective group, Y is hydrogen
or an N-protective group, and R is a typical amino
acid side chain; for example, phenylmethyl derived
from phenylalanine, or protected amino acid side
~13~406
3
chain, for example, {4-(phenylmethoxy)phenyl}meth-
yl derived from tyrosine.
Note that the above general formula for the
aminoepoxide intermediate contains two asymmetric
carbon atoms.
Preferred enantiomerically pure amino epoxide
intermediates for the preparation of the
hydroxyethylamine isostere containing HIV protease
inhibitors are those in which the carbon atom
bearing the nitrogen atom and the carbon atom
bearing the oxygen atom both have the (S)
configuration.
Hence, in view of high profile of the
intermediates, a process for the preparation of
the enantiomerically pure (S, S)-aminoepoxide
intermediates, which meets the criteria of being
efficient, safe and amenable to scale-up, is most
desirable.
Paradoxically, the reported preparations of
the desired enantiomerically pure aminoepoxide
intermediates, or chemical equivalents thereof, do
not meet the aforementioned three criterea.
4
More explicitly, B.E. Evans et al., J. Org.
Chem., 1985, 50, 4615 reports the synthesis of
enantiomerically pure aminoepoxide intermediates
by reacting the corresponding Boc-oc-amino
aldehydes with dimethylsulfonium methylide and
separating the resulting mixture of
diastereoisomeric epoxides. This method suffers
from the lack of stereoselectivity, the use of a
hazardous combination of sodium hydride and
dimethylsulfoxide, as well as the use of
chromatography to separate diastereoisomers, a
step not easily amenable to scale-up.
M.T. Reetz and J. Binder, Tetrahedron
Letters, 1989, 30, 5425 describe a similar process
involving the reaction of N,N-(doubly protected)-
oc-aminoaldehydes with dimethylsulfonium
methylide. Although the N,N-(doubly protected)-
a-aminoaldehyde lends itself to a more
stereoselective conversion , the process suffers
from the aforementioned disadvantages of safety
and the need to separate the mixture of
diastereoisomeric products by chromatography. The
authors note on page 5428 (reference 8) that the
separation of the diastereoisomeric products
(aminoepoxide) is difficult and suggest the
separation of later oxirane-ring opened products
x~~7~~s
as a more practical method to diastereomerically
pure products.
Recently, A. Albeck and R. Persky,
5 Tetrahedron, 1994, 50, 6333 described a multistep
process for preparing aminoepoxide intermediates.
The process utilizes an oc-(chloromethyl)-y-N-
(benzyloxycarbonyl)aminoketone precursor which is
synthesized in turn by a step involving
diazomethane. The use of diazomethane, a
hazardous reagent, limits this process to small
scale preparations.
Again recently, J.S. Ng et al., PCT patent
application WO 93/23388, published November 25,
1993 reported a process for preparing the
aminoepoxide intermediate by reacting N,N-doubly
protected-y-amino aldehydes with a
halomethyllithium reagent generated in situ from
chloroiodomethane and butyllithium to give the
aminoepoxide intermediate as a mixture of
diastereoisomers. Although the isolation of two
diastereomerically pure aminoepoxide intermediates
by chromatography is described, like Reetz and
Binder, supra, Ng et al. recommend that the
diastereoisomeric mixture of the aminoepoxide
intermediate be used directly for further
6 ~13~406
elaboration of the ultimate end product, saving
the separation of diastereoisomers for a later
stage.
The Ng et al. process has the disadvantages
of (1) requiring chromatographic separation of the
diasteroisomeric mixture to obtain the desired
isomerically pure intermediate; (2) using
pyrophoric butyllithium; and (3) forming an
environmentally undesirable side product, namely
butyliodide.
Similarly to the Ng et al. patent
application, J. Barluenga et al., J. Chem. Soc.,
Chem. Commun., 1994, 969, disclose the preparation
of a N,N-doubly protected aminoepoxide
intermediate by reacting N,N-dibenzylalaninal with
chloromethyllithium generated in situ from
chloroiodomethane and methyllithium. This
preparation has disadvantages similar to those as
noted in the preceding paragraph.
The present process, on the other hand,
fulfills the need for a safe, efficient process
that can be worked on a large scale. The process
has the features of simplicity and expediency, and
it avoids the use of hazardous chemicals. The
~~~~~os
process efficiently and economically produces the
desired intermediate, or a chemical equivalent
thereof, with an enantiomeric and diastereomeric
purity of 95~ or greater.
Summary of the Invention
Disclosed herein is a process for preparing a
hydrochloric acid addition salt of an isomerically
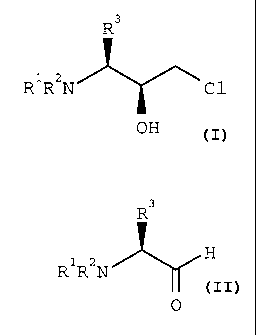
pure chlorohydrin of formula 1
R3
R1R2N ~ \ (1)
C1
OH
wherein R1 and Rz each independently is an N-
benzyl protective group selected from benzyl or
benzyl monosubstituted or disubstituted with (1-
4C)alkyl, (1-4C)alkoxy or halo, and R3 is an amino
acid side chain or a protected amino acid side
chain, which comprises the following steps:
(a) reacting an aldehyde of formula 2
8 ~1~~~~s
R3
R1R2N H (2)
0
wherein R1, Rz and R3 are as defined herein with
(chloromethyl)lithium in an inert solvent at
-76 °C to -20 °C to obtain a diastereoisomeric
mixture of lithium alcoholates of formula 3 and
formula 4, i.e.
Rs R3
R1RZN ~ \ C1 ( 3 ) + i z -
R R N C1 (4)
OLi OLi
wherein R1, Rz and R3 are as defined herein, the
(chloromethyl)lithium being generated in situ in
the reaction mixture by a metered series of tandem
additions thereto of portions of
bromochloromethane and of lithium metal so that
the temperature of the reaction mixture is
maintained at -20 °C or below;
(b) while maintaining the temperature of the
aforementioned reaction mixture at -20 °C or
below, separating unreacted lithium metal from the
reaction mixture to obtain a chilled solution of
the mixture of the lithium alcoholates in the
inert solvent;
(c) transforming the mixture of the lithium
alcoholates into a corresponding mixture of
hydrochloric acid addition salts by immediately
contacting the aforementioned chilled solution of
the mixture of the lithium alcoholates with
aqueous hydrochloric acid to obtain an inert
solvent/aqueous hydrochloric acid solution of the
hydrochloric acid addition salt of the
chlorohydrin of formula 1 in admixture with a
hydrochloric acid addition salt of a chlorohydrin
of formula 5
R3
R1R2N C1 (5)
off
wherein Rl, RZ and R3 are as defined herein;
(d) removing the inert solvent from the inert
solvent/aqueous hydrochloric acid solution to
obtain an aqueous phase and a water-insoluble
phase, the latter phase comprising the mixture of
the hydrochloric acid additions salts of the
chlorohydrins;
10
(e) separating the water-insoluble phase from the
aqueous phase;
(f) preparing a concentrated solution of the
water-insoluble phase in a lower alkanol; and
(g) selectively crystallizing the desired
hydrochloric acid addition salt of the
isomerically pure chlorohydrin of formula 1 from
the lower alkanol solution.
Another aspect of the present invention
involves the deprotection of the hydrochloric acid
addition of the isomerically pure chlorohydrin of
formula 1 by hydrogenolysis to give the
hydrochloric acid addition salt of the
isomerically pure compound of formula 6
R3
H2N ~ ~c 1 ( 6 )
OH
wherein R3 is as defined herein.
11
Details of the Invention
The term "isomerically pure" or "isomeric
purity" as used herein with reference to a
compound means that the compound has an
enantiomeric and diastereomeric purity of 95~ or
greater as determined by high performance liquid
chromatography analysis using a column with a
chiral support.
The term "amino acid" or "oc-amino acid" as
used herein means an a-amino acid having an
(S)configuration at the oc-carbon but excludes
glycine. The term is not meant to be limiting
other than it applies to Oc(S)-amino acids having
a side chain containing at least one carbon atom
attached to the a-carbon. The term therefore
would encompass 19 of the 20 primary protein amino
acids (i.e. those coded by DNA, except for
glycine), other naturally occurring oc-amino acids
and synthetic oc-amino acids. Examples of the
amino acids encompassed by this term include
phenylalanine, valine, leucine, isoleucine, oc-
aminocyclohexylpropionic acid, ornithine, serine,
tyrosine, asparagine, cysteine, arginine and the
like.
~I3~~06
12
The term "amino acid side chain" as used
herein means the radical of one or more carbon
atoms attached to the oc-carbon of an amino acid.
It is the characterizing portion of an amino acid
and is derived from a corresponding amino acid by
elimination of the NHZCHC(O)OH moiety. For
example, the amino acid side chain of
phenylalanine is the phenylmethyl radical. The
practicality of preparing the hydrochloric acid
addition salt of the isomerically pure halohydrin
of formula 1 having the latter amino acid side
chain (i.e. R3 is phenylmethyl) is demonstrated by
example 1 hereinafter. Examples of other amino
acid side chains (R3) for the compounds of
formulae 1 to 6 are 2-methylpropyl from leucine,
1-methylethyl from valine and methyl from alanine.
The term "protected amino acid side chain" as
used herein means an amino acid side chain as
described above which in addition bears a
protected functional group. Protecting groups for
functional groups are well known in the peptide
art and are intended to protect such functional
groups as amino, hydroxy, thio or carboxy against
undesirable reactions during synthetic procedures.
Examples of protected amino acid side chains (R3 )
for the compounds of formula 1 to 6 would
~i~7~o~
13
therefore include 3-(N,N-dibenzylamino)propyl
derived from ornithine, benzyloxymethyl derived
from serine, (4-methoxyphenyl)methyl derived from
tyrosine, and {4-(phenylmethoxy)phenyl}methyl also
derived form tyrosine.
The term "N-protecting group" or "N-
protected" as used herein refers to groups
intended to protect nitrogen atoms against
undesirable reactions during chemical synthesis.
Examples of N-protecting groups include tert-
butyloxycarbonyl (Boc), benzyloxycarbonyl (Z) and
benzyl (Bzl).
The term "(1-4C)alkyl" as used herein means
straight chain alkyl radicals containing one to
four carbon atoms and branched chain alkyl
radicals containing three to four carbon atoms and
includes methyl, ethyl, propyl, butyl, 1-
methylethyl, 1-methylpropyl, 2-methylpropyl and
1,1-dimethylethyl.
The term "(1-4C)alkoxy" as used herein means
straight chain alkoxy radicals containing one to
four carbon atoms and branched chain alkoxy
radicals containing three to four carbon atoms and
~13740~
14
includes methoxy, ethoxy, propoxy, 1-methylethoxy,
butoxy and 1,1-dimethylethoxy.
The term "halo" as used herein means a halo
radical selected from bromo, chloro, fluoro or
iodo.
The term "lower alkanol" as used herein means
straight chain alkanols containing from one to
four carbon atoms and branched chain alkanols
containing three to four carbon atoms and includes
methanol, ethanol, 1-methylethanol, 1,1-
dimethylethanol, propanol, 2-methylpropanol and
butanol.
The starting materials for the present
process, i.e. the aldehydes of formula 2, are
either known or can be prepared by standard
methods. One general method for the preparation
of the aldehydes has been described by M.T. Reetz
et al., Angew. Chem. Int. Ed. Engl., 1987, 26,
1141. An example of an aldehyde of formula 2
described in the literature is a(S)-
[bis(phenylmethyl)amino]benzenepropanal described
by M.T. Reetz and M.W. Drewes, US patent
4,990,669, issued February 5, 1991, and J.S. Ng et
z~37~os
al., PCT patent application w0 93/23388, published
November 25, 1993.
Process
5
The key step of the process of this invention
is the reaction of the aldehyde of formula 2 with
(chloromethyl)lithium in an inert solvent at a
temperature ranging from -76 °C to -20 °C,
10 preferably -76 °C to -60 °C, to give the
diastereoisomeric mixture of the lithium
alcoholates of formulae 3 and 4. A relevant
factor contributing to the efficacy of this step
is the metrical or portionwise in situ generation
15 of (chloromethyl)lithium from bromochloromethane
and lithium in the solution of the aldehyde of
formula 2 in the inert solvent. The reaction of
bromochloromethane with lithium is exothermic and
the product, (chloromethyl)lithium, is highly
prone to thermal instability; for example, see
K.M. Sadhu and D.S. Matteson, Tetrahedron
Letters,1986, 27, 795. It has now been found that
the disadvantages associated with the use of
(chloromethyl)lithium can be overcome by
generating the reagent in the reaction mixture in
a semi-continuous manner. Accordingly, the
(chloromethyl)lithium is generated in this
16
instance by subjecting the stirred solution of the
aldehyde of formula 2 to a series of tandem
additions, each tandem addition consisting of (1)
adding a portion of bromochloromethane and then a
portion of lithium in a molar equivalent ratio
ranging from 1:4 to 1:20, respectively. The
series of tandem additions are done at a rate
which allows the heat of reaction ensuing from the
in situ generation of the (chloromethyl)lithium to
be conducted away from the reaction mixture by
conventional cooling means. In this manner, the
temperature of the reaction can be maintained
within a range of -76 °C to -20 °C, preferably
-76°C to -60 °C.
More particularly with respect to the key
step of the process, a solution of the aldehyde of
formula 2 in an inert solvent is cooled to
between -76 °C and -20 °C, preferably between
-76 °C and -60 °C, in a suitable reaction vessel.
Suitable inert solvents include various ethers,
for example, tetrahydrofuran, dioxane, 1,2-
dimethoxyethane and diethyl ether.
Thereafter, (chloromethyl)lithium is
generated in situ in the cooled solution in the
following manner: Under an inert argon
1~ ~~~'4~6
atmosphere, the vigorously stirred, cooled
solution is subjected to a series of portionwise
additions of first bromochloromethane and then
lithium metal (shot or wire). (Note: the lithium
wire or shot should be ground briefly, prior to
their addition, to expose fresh metal surfaces.)
The series of additions are performed at a
rate which allows for sufficient removal of the
ensuing heat of reaction by external cooling of
the reaction vessel. In this manner, the
temperature of the reaction mixture is controlled
and maintained at -20 °C or below. Depending on
the size and scale of the reaction mixture, the
reaction time for this key step usually is from 30
minutes to five hours.
In total, about 1.1 to 1.5 molar equivalents
of bromochloromethane and about 5 to 20 molar
equivalents of lithium are added to one molar
equivalent of the aldehyde of formula 2.
The completion of the reaction can be
monitored by various analytical techniques
including thin layer chromatography.
X137106
18
At the completion of the reaction, i.e. the
key step, the stirring is stopped, the reaction
mixture is maintained at a temperature of -20 °C
or below, preferably at -60 °C or below, and the
unreacted lithium metal is allowed to float on the
surface of the reaction mixture. The chilled
liquid phase of the reaction mixture is
immediately separated from the lithium metal and
mixed with an aqueous solution of excess
hydrochloric acid. In practice, the liquid phase
can be conveniently separated by suction. A
laboratory suction system capable of transferring
the liquid phase away from the metal and into
contact with the aqueous solution of excess
hydrochloric acid is a convenient manner to
accomplish this part of the process.
Alternatively, the separation can be done by
gravity or by suction filtration by opening a
stop-cocked outlet system provided at the bottom
of the reaction vessel.
The resulting inert solvent/aqueous
hydrochloric acid solution is concentrated under
reduced pressure (i.e. the inert solvent is
removed from the latter solution by distillation
under reduced pressure) to give a clear aqueous
phase and a water insoluble oily residue. The
~1~4~6
19
aqueous phase is decanted from the oily residue.
Next, a concentrated solution of the oily residue
in a lower alkanol, preferably methanol or
ethanol, is prepared. Upon standing the desired
hydrochloric acid addition salt of the compound of
formula 1 selectively crystallizes from the
solution. Recrystallization of the hydrochloride
salt from a lower alkanol, preferably methanol or
ethanol, yields the desired chlorohydrin
hydrochloride salt in about a 40~ yield from the
aldehyde of formula 2 with a 95~ isomeric purity
or greater as determined by high performance
liquid chromatography analysis using a column with
a chiral support.
Thereafter, if desired, the hydrochloric acid
addition salt of the compound of formula 1 can be
deprotected to give the hydrochloric acid addition
salt of a-(chloromethyl)-a-aminoalcohol of formula
6 by subjecting the compound of formula 1 salt to
hydrogenolysis methods capable of converting a
benzyl-protected amino group to an amino group.
A convenient and practical method for
effecting the deprotection involves subjecting the
hydrochloric acid addition salt to hydrogenolysis
using gaseous hydrogen or using a hydrogen
20 X137406
transfer agent for example, ammonium formate, in
the presence of a noble metal catalyst, such as
platinum or palladium. The noble metal catalyst
can be employed in the form of oxides (e. g. Pt02)
or hydroxides [e. g. Pd(OH)2], on a suitable
support (e.g. charcoal or calcium carbonate in a
finely divided form).
If desired, the latter hydrochloric acid
addition salt of the compound of formula 6 can be
transformed to a corresponding N-(monoprotected)-
aminoepoxide intermediate of formula A. The
transformation can be effected by subjecting the
hydrochloric acid addition salt of the compound of
formula 6 to known methods for converting amino
groups to N-(monoprotected)-amino groups to obtain
the corresponding N-(monoprotected)-oc-(chloro-
methyl)-(3-aminoalcohol of formula 7
3
R
X-NH ~ C1
OH
wherein X is a N-protective group and R3 is as
defined herein; followed by reacting the N-
(monoprotected)-oc-(chloromethyl)-(3-aminoalcohol of
formula 7 with a base (e. g. sodium hydroxide,
potassium hydroxide or potassium carbonate) to
z~~~4~s
21
give the corresponding N-(monoprotected)-
aminoepoxide of formula A wherein X is a N-
protective group, Y is hydrogen and R is a amino
acid side chain or a protected amino acid side
chain and the carbon atom bearing the nitrogen
atom and the carbon atom bearing the oxygen atom
both have the (S) configuration.
Finally, it will be appreciated by those
familiar with the art that N-(monoprotected)-
aminoepoxide of formula 1, and the hydrochloric
acid addition salts of formula 1 and 6 are useful
intermediates for the elaboration of HIV protease
inhibitors. Also, it will likewise be appreciated
that the process described herein can be used to
prepare the corresponding enantiomers of the
chlorohydrin of formulae 1 and 6 in the form of
hydrochloric acid addition salts, i.e. the
corresponding (R,R) enantiomers, by using the (R)
enantiomer of the aldehyde of formula 2 as
starting material.
The following examples further illustrate
this invention. Solution percentages or ratios
express a volume to volume relationship. Nuclear
magnetic resonance (NMR) spectra were recorded on
a Bruker AMX400 spectrometer and referenced to
~1~?'4~6
22
trimethylsilane as the internal standard; the
chemical shifts (8) are reported in parts per
million. Optical rotations were recorded on a
Perkin Elmer 241 MC polarimeter at the D line of
sodium with a 1 dm path length, 1 mL cell; the
concentrations are expressed in grams of compound
per 100 mL of solution. High performance liquid
chromatography (HPLC) analyses were performed
using a C18 reversed phase column, aqueous
trifluoroacetic acid and acetonitrile gradients
and UV detection (230 nm). Abbreviations and
symbols used in the examples include Boc: tert-
butyloxycarbonyl; Et20: diethyl ether; MeOH:
methanol and THF: tetrahydrofuran.
Example 1
Preparation of (3(S)-[bis(phenylmethyl)amino]-oc(S)-
(chloromethyl)benzenepropanol hydrochloric acid
addition salt (the hydrochloric acid addition salt
of the chlorohydrin of formula 1 wherein R1, RZ
and R3 are phenylmethyl).
A solution of oc(S)-[bis(phenylmethyl)amino]-
benzenepropanal (2233 g, 6.77 mol) in reagent
grade THF (22.3 L) was placed in a 50 L three-
necked flask equipped with a mechanical stirrer
23
and a low temperature thermometer. The solution
was cooled to an internal temperature of -76 °C.
Under an argon atmosphere, the mixture was stirred
vigorously. Bromochloromethane (245 g, 123 mL,
1.893 mol) was added in one portion to the stirred
solution followed by the addition of lithium shot
(Aldrich Chemical Co., Milwaukee, WI, USA, catalog
# 22,849-4, 180g, 25.9 mol). (The lithium shot
was ground briefly in a mortar, just prior to
their addition, to expose fresh metal surfaces.)
The internal temperature rose to -67 °C over the
next 30 min. After an additional 15 min, the
temperature started to drop. A second portion of
the reagents, i.e. bromochloromethane (245 g) and
lithium shot (180 g), was added. After an initial
temperature rise to -64 °C, the reaction mixture
was allowed to cool down to -72 °C before a third
portion of the reagents was added at time 120 min
from the start of the reaction. The last portion
of the reagents was added at time 2.5 h and the
reaction mixture was stirred for one hour more.
In all, bromochloromethane (980 g, 7.57 mol) and
lithium shot (720 g, 104 mol) were added in four
equal portions while carefully keeping the
internal temperature of the reaction mixture below
-60 °C at all times. The total reaction time was
3.5 h from the first addition.
~137~~ti
24
After completion of the reaction, the
stirring was stopped and the unreacted lithium
metal was allowed to float to the surface of the
reaction mixture. The liquid phase (still at -72
°C) was suctioned from the bottom of the reaction
vessel into 7 L of aqueous 6N HC1. The lithium
shot remaining in the flask was washed with THF (1
L) and the washings were added to the hydrochloric
acid solution. (The lithium shot was recovered by
suction filtration, washed with THF and hexane,
and dried under reduced pressure for future use.)
The THF/aqueous hydrochloric acid solution
was concentrated under reduced pressure until a
clear aqueous phase separated from a brown gummy
residue. The aqueous layer was removed by
decantation. The residue was washed with H20 (3 X
1 L) and then dissolved in 3 L of warm MeOH. The
solution was allowed to stand at 5 °C for 18h.
The resulting precipitate was collected by suction
filtration and washed with 10~ MeOH in EtzO (2 X
500 mL). (The filtrate was discarded because it
contained very little material, but the washes
were set aside for recovery of a second crop.)
The precipitate was dried under reduced pressure
to give the crude title compound as a tan-colored
~137QOG
solid (1128 g, 77~ pure by HPLC). Concentration
of the aforementioned washes gave a second crop
(105 g, 73~ pure by HPLC).
5 The two crops (1233 g total) were combined
and dissolved in warm MeOH (4 L). After cooling
overnight at 5 °C, the crystalline material from
the solution was collected on a filter, washed
with 10~ MeOH in EtzO (500 mL) and dried under
10 reduced pressure to give the pure title compound
(750 g, 94~ pure by HPLC). The preceding mother
liquor and washes were combined. The resulting
solution was concentrated to 800 mL and cooled
overnight at 5 °C to give a second crop (262 g,
15 90~ pure by HPLC). From the latest mother liquor
and washings, a third crop was obtained (52 g, 81~
pure by HPLC). The total yield of recrystallized
material (3 crops) was 1064 g (38~ overall yield
from a(S)-[bis(phenylmethyl)amino]propanal) with
20 a HPLC purity of 89~, [a]D -7.3° (c = 1, MeOH), mp
172-174 °C (dec.). The 1H NMR (400 MHz, CD30D) of
the title compounds showed 8 2.69 (t, J = 10.3 Hz,
1H), 3.05 (dd, J - 11.0 Hz, 1H), 3.31 (m, 1H),
3.39 (dd, J - 13.7, 4.0 Hz, 1H), 3.53 (dd, J -
25 14.0, 9.9 Hz, 1H), 3.99 (broad d, J = 6.3 Hz, 1H),
4.5 (m, 3H), 4.72 (broad d, J = 14.0 Hz, 1H), 5.03
(broad d, J = 13.2 Hz, 1H), 7.13 (m, 2H), 7.28 (m,
~137~06
26
3H), 7.5 (m, 10H); FAB mass spectrum, m/z: 380
(M+H)+.
The isomeric purity of the title compound in
its free base form was determined by normal phase
HPLC on a Chiracel~ OD column from Daicel
Chemical Industries Limited, Tokyo, Japan (US
distributor: Chiral Technologies Inc., Exton PA,
USA). EtOH-hexane (1:19) was the eluent and UV
detection at 205 nm was employed. The sample of
the hydrochloric acid addition salt was
neutralized with NaHC03 or Na2C03, and extracted
into hexane prior to analysis. The isomeric
purity of the title compound was > 99.5.
By following the procedure of example 1 but
replacing oc(S)-[bis(phenylmethyl)amino]benzene-
propanal with an equivalent amount of 2(S)-
[bis(phenylmethyl)amino]-4-methylpentanal, the
hydrochloric acid addition salt of 2(S)-
[bis(phenylmethyl)amino]-1(S)-(chloromethyl)-4-
methylpentanol (1: R1 and RZ are phenylmethyl and
R3 is 2-methylpropyl) was obtained; [a]D - +1.5° (c
- 1, MeOH); mp 165-166 °C; FAB mass spectrum, m/z:
346 (M+H)+. The isomeric purity of the latter
compound was > 98~.
~137~Q6
27
Again, by following the procedure of example
1 but replacing a(S)-[bis(phenylmethyl)amino]ben-
zenepropanal with an equivalent amount of 2(S)-
[bis(phenylmethyl)amino]-3-methylbutanal, the
hydrochloric acid addition salt of 2(S)-
[bis(phenylmethyl)amino]-1(S)-(chloromethyl)-3-
methylbutanol (1; R1 and R2 are phenylmethyl and R3
is 1-methylethyl) was obtained; [a]D - -52.8° (c -
1, MeOH); mp 164-165 °C; FAB mass spectrum, m/z:
332 (M+H)+. The isomeric purity of the latter
compound was > 99~.
Again, by following the procedure of example
1 but replacing a(S)-[bis(phenylmethyl)amino]-
benzenepropanal with an equivalent amount of 2(S)-
[bis(phenylmethyl)amino]propanal, the hydrochloric
acid addition salt of 2(S)-[bis(phenyl-
methyl)amino]-1(S)-(chloromethyl)propanol (1; R1
and RZ are phenylmethyl and R3 is methyl) was
obtained; ~a~D - -21.3° (c = 1, MeOH); mp 170-173
°C; FAB mass spectrum, m/z: 304 (M+H)+. The
isomeric purity of the latter compound was > 98~.
Again, by following the procedure of example
1 but replacing a(S)-[bis(phenylmethyl)amino]ben-
zenepropanal with an equivalent amount of a(S)-
[bis(phenylmethyl)amino]-4-(phenylmethoxy)phenyl-
zm74os
28
propanal, the hydrochloric acid addition salt of
(3(S)-[bis(phenylmethyl)amino]-oc(S)-(chloromethyl)-
4-(phenylmethoxy)phenylpropanol {(1; R1 and R2 are
phenylmethyl and R3 is {4-(phenylmethoxy)phenyl}-
methyl was obtained; [a]D - +12.7° (c - 1, MeOH);
mp 178-180 °C; FAB mass spectrum, m/z: 486 (M+H)+.
The isomeric purity of the latter compounds was
>97~.
Example 2
Preparation of ~i(S)-amino-oc(S)-(chloromethyl)-
benzenepropanol hydrochloric acid addition salt
(the hydrochloric acid addition salt of the
compound of formula 6 wherein R3 is phenylmethyl).
The title compound of example 1 (53.6 g,
0.129 mol) and the catalyst 20~ Pd(OH)z on
charcoal (5.0 g) were suspended in MeOH (500 mL)
in a 1 L three-necked flask. The suspension was
stirred at 20-22 °C under an atmosphere of
hydrogen for 18 h. Thereafter, the catalyst was
removed by filtration through a glass filter. The
collected catalyst was washed with MeOH. The
combined filtrate and washings were concentrated
to dryness under reduced pressure. The solid
residue was triturated with Et20, collected on a
,~~~7~os
29
filter and dried under reduced pressure to give
the title compound (29.6 g, 97~ yield, 97~ purity
by HPLC) as a white solid; [a]D -42.5° (c - 1,
MeOH), mp 204-208 °C.
Exam le 3
(The following is an example of an N
(monoprotected)-aminoepoxide intermediate of
formula A.)
Preparation of 3(S)-(tert-butyloxycarbonylamino)-
1,2(S)-epoxy-4-phenylbutane (the compound of
formula A wherein X is Boc, Y is hydrogen and R is
phenylmethyl, and the carbon atom bearing the
nitrogen and the carbon atom bearing the oxygen
atom both have the (S) configuration).
A solution of di-tert-butyl dicarbonate (1320
g, 6.046 mol) and triethylamine (1.7 L, 12.18 mol)
in reagent grade THF (10 L) was cooled to 1 °C.
Under an atmosphere of nitrogen, the title
compound of example 2 (1418 g, 6.00 mol) was added
in portions (as a solid) to the stirred solution
over a 30 min period, keeping the temperature of
the reaction mixture below 15 °C. Thereafter, the
30
reaction mixture was stirred at ambient
temperature (20-22 °C) for 3h.
The reaction mixture was cooled to an
internal temperature of 5-7 °C. A solution of KOH
(1344 g, 24 mol) in MeOH (5.3 L) was added over a
min period, keeping the temperature of the
reaction mixture below 15 °C. Thereafter, the
reaction mixture was stirred at ambient
10 temperature for 75 min. (Thin layer
chromatography (Si02, hexane/ethyl acetate, 4:1)
indicated that the reaction was complete).
The reaction mixture was poured into H20 (60
15 L). The precipitate was collected on a filter by
suction, washed to neutral pH with H20 ( 60 L) and
air-dried by suction on the filter to give the
title compound as fine white needles (1530 g, 96~
yield, 90~ pure by HPLC). The material was
sufficiently pure for further use.
Recrystallization of a sample from MeOH gave the
pure title compound of this example; [oc]D + 6.9°
(c - 1, chloroform); mp 124-125 °C. The 1H NMR
(400, CDC13) of the title compound showed 8 1.38
(s, 9H), 2.7-3.0 (m, 5H), 3.7 (broad m, 1H), 4.53
(d, J = 8.3 Hz, 1H) , 7 .2-7 .3 (m, 3H) , 7.3-7.4 (m,
2H) .