Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
21 38153
1
The present invention relates to a process for the
electrochemical preparation of dicarboxylic acids.
Salts of the dicarboxylic acids I are obtained in a
number of industrial processes, for example in the alkaline
hydrolysis of polyamides. To isolate the free acids the salts
are acidified, for example with hydrochloric acid according to
FR-A-926,873 (published on October 14, 1947) or with sulfuric
acid according to tfS-A-2,840,606 (published on April 15, 1955).
The disadvantage of this is the formation of salts such as
sodium chloride or sodium sulfate and their disposal.
The preparation of an organic acid by electrodialysis
is known, for example from DE-A-2,547,101 (published on May 6,
1976) which describes the electrodialysis of salt solutions of
glycine, diglycinE~, triglycine, citric acid, tartaric acid,
acetic acid, acrylic acid, malefic acid and ascorbic acid in a
four-compartment electrolysis cell.
SU-A-401,131 (published on October 5, 1976) describes
the electrochemical preparation of oxalic acid from sodium
oxalate.
It is an object of the present invention to provide
a process for preparing dicarboxylic acids in high purity and
yield without the known disadvantages.
We have found that this object is achieved by a
process for the electrochemical preparation of dicarboxylic
acids, which comprises subjecting an aqueous solution
containing essentially an alkali metal salt of a dicarboxylic
acid of the general formula I:
HOOC-(CH2)n-COOH I
where n is an integer from 1 to 8, or mixtures thereof, to an
electrochemical treatment.
The alkali metal salt used is in general the lithium,
21 38153
2
sodium or potassium salt. It is of course also possible to use
mixed salts, ie. salts with two different alkali metal rations,
or acid salts, ie. salts with a free acid group, or mixtures
thereof.
Examples are dilithium, disodium and dipotassium
malonate, succinat:e, glutarate, adipate, pimelate, octane-
dioate, nonanedioate and decanedioate, sodium potassium
adipate, preferably dilithium adipate, disodium adipate,
dipotassium adipate, sodium potassium adipate, dilithium
l0 octanedioate, disodium octanedioate and dipotassium
octanedioate, particularly preferably disodium adipate.
From obsE~rvatians to date the manner of the electro-
chemical treatment has in principle no bearing on the success
of the process of the invention.
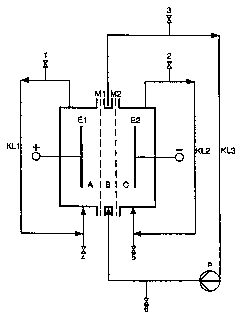
Fig. 1 i:~ a diagrammatical representation of a three
compartment electrolysis cell comprising three liquid cycles
20 KL1 to KL3.
Fig. 2 i.s a diagrammatical representation of a four
compartment electrolysis cell comprising four liquid cycles KL1
to KL4.
Fig. 3 is a diagrammatical representaiton of a
membrane stack cell comprising three liquid cycles KL1 to KL3.
The elecarochemical treatment may for example take
30 one of the following forms (a) to (f):
(a) In this version the splitting of the dicarboxylate
salt into tree corresponding dicarboxylic acid and the
corresponding base can be carried out in a two-part
electrodialysis cell using bipolar membranes. In general,
the electrodialysis cell has between the anode and the
cathode from 1 to 200, preferably from 20 to 70,
A
21 38153
2a
electrodialys.is units separated from one another by
bipolar membranes. The bipolar membranes are separated
from one another by ration exchange membranes, so that an
electrodialysi.s unit has the following structure: bipolar
membrane (anode side) -anolyte compartment - ration
exchange membrane - catolyte compartment - bipolar
membrane (cat.hode side). The individual electrodialysis
cells are preferably electrically connected in series.
In this version it is advantageous to feed the
aqueous dicarboxylate salt solution into the anolyte
compartment. In the electric field of an applied direct
voltage the a7_kali metal rations generally migrate through
the ration e~;change membrane into the catolyte compart
ment . The hydroxyl anions required for compensating the
~~,~~ra~orl nharrtoc arc
,
X1_38153
- 3 - O.Z. 0050/43332
formed by the dissociation of the water in the
bipolar :membranes on the cathode side. In this way
the corresponding alkali metal hydroxide solution
collects in the catolyte compartment. In the anolyte
compartment the dicarboxylate anion can combine with
the hydrogen ions from the bipolar membrane on the
anode side to form the free dicarboxylic acid.
It is advantageous to feed the dicarboxylate salt
solution into the anolyte compartments in parallel.
The product streams from the anolyte compartments,
containing the free acid and unconverted dicarboxyl-
ate salt, and the product streams from the catolyte
compartments are advantageously combined with one
another. The free dicarboxylic acid is in general
obtained by crystallization from the combined
product streams from the anolyte compartment without
coprecipitation of the corresponding dicarboxylate
salt, which is preferably subjected again to the
electrod.ialysis process.
The ealectrodialysis process can be carried out
not only continuously but also batchwise. A prefer-
red form of the continuous process involving a
plurality of electrodialysis cells comprises
splitting the total conversion between from 2 to 20,
preferably from 4 to 6, electrodialysis cells and
achieving only partial conversion in each electro-
dialysis; cell.
It is particularly advantageous here to guide the
flows in countercurrent. The outflow from an anolyte
compartment forms the inflow into the next anolyte
compartment, etc., so that the outflow from the last
anolyte compartment is rich in dicarboxylic acid and
lean in dicarboxylate salt. The outflow from the
last catolyte compartment, containing a low
concentration of alkali metal hydroxide, forms the
inflow into the last but one catolyte compartment,
etc., so that the first unit has a high
2138153
- 4 - O.Z. 0050/43332
concentration of dicarboxylate salt in the anolyte
compartment and a high concentration of alkali metal
hydroxide in the catolyte compartment. The result is
that the alkali metal hydroxide concentration
differences in the anolyte and catolyte compartments
are small within a unit. This ultimately leads in
general to an energy saving due to a higher current
yield and on average to lower cell voltages.
The current densities are in general within the
range from 0.1 to 1, preferably from 0.3 to 0.5,
kA/m'. Tree cell voltage is in general from 3 to 8, V
per electrodialysis unit.
The pH is in general within the range from 2 to
10 in the anolyte compartments and within the range
greater than 13 in the catolyte compartments.
The compartment width is in general from 0.2 to
5, preferably from 0.5 to 1, mm.
The e:lectrodialysis temperature is in general
within the range from 40 to 110°C, preferably from
65 to 90°C.
The inflow and outflow velocities are in general
within the range from 0.05 to 0.2 m/sec.
The concentration of dicarboxylate salt used is
in general from 5 to 40% by weight, preferably from
10 to 20% by weight.
If desired, the conductivity in the anolyte
system c:an be increased by adding salts or acids
such as sodium sulfate or sulfuric acid. Substances
of this type are in general added within the range
from 0.1. to 10% by weight, preferably from 1 to 6%
by weight, based on the total weight of the solution
present in the anolyte compartment.
To the catolyte compartment it is advantageous to
add the substances which are obtained in the course
of the operation, preferably the corresponding
alkali metal hydroxide such as sodium hydroxide or
potassium hydroxide, preferably sodium hydroxide.
_~138~53
- 5 - O.Z. 0050/43332
The inflow into the catolyte compartment gener-
ally comprises fully demineralized water, but at the
beginning it is preferable to employ the from 1 to
25, preferably from 5 to 10, % strength by weight
alkali metal hydroxide solution formed in the course
of the electrodialysis.
To increase the conductivity, the anolyte com
partment may have added to it for example an oxo
acid such as sulfuric acid, phosphoric acid and
nitric acid.
(b) A three-part electrodialysis cell with bipolar
membranes has the advantage over the procedure
described under (a) that the feed materials need not
be very pure. Furthermore, in general, significantly
lower salt contents are obtained not only in the
dicarboxylic acid solution obtained but also in the
corresponding alkali metal hydroxide solution.
The three-compartment system contains not only a
cation exchange membrane but also an anion exchange
membrane, so that the structure of an electrodialy
sis cell is as follows: bipolar membrane (anode
side) - anolyte compartment - anion exchange
membrane - center compartment - cation exchange
membrane - catolyte compartment - bipolar membrane
(cathode side).
The dicarboxylate salt solution is advantageously
introduced into the center compartment. Under the
influence of a direct current electric field the
dicarbox:ylate anions generally migrate through the
anion eacchange membrane into the anolyte compart-
ment, where they can combine with the hydrogen ions
present there to form the free acid. Apart from
selectivity losses of the anion exchange membrane
the free acid can be withdrawn from the anolyte
compartment devoid of salt. As in (a) the catolyte
compartment yields the alkali metal hydroxide
solution. The outflow from the center compartment,
2138153
- 6 - O.Z. 0050143332
still containing residual quantities of
dicarboxylate salt, can be disposed of or
advantageously added again to the feed for the
electrod:Lalysis process. Again as in (a) the flows
can be guided countercurrently in order to increase
the current yield.
To increase the conductivity the anolyte compart
ment can have added to it for example an oxoacid
such as sulfuric acid, phosphoric acid or nitric
acid.
The catolyte compartment can advantageously have
added to it the substances which are obtained in the
course o:E the operation, preferably the correspond-
ing alkali metal hydroxide such as sodium hydroxide
or potass;ium hydroxide, preferably sodium hydroxide.
As for the rest, the process of (b) can be
carried .out under the same conditions as described
under (a).
(c) In principle it is also possible to use electro
dialysis cells having four compartments. The layout
generalhy resembles that of an electrodialysis cell
with three compartments except that, to protect the
bipolar :membranes from possible fouling, a further
ion exchange membrane, preferably a cation exchange
membrane, is included. In general, an electrodialy
_ sis unit will have the following structure: bipolar
membrane ( anode side ) - anolyte compartment - cation
exchange membrane - anode-near center compartment
anion exchange membrane - cathode-near center
compartment - cation exchange membrane - catolyte
compartment - bipolar membrane (cathode side).
The d:icarboxylate salt solution is advantageously
introduced into the cathode-near center compartment
with the dicarboxylic acid solution being withdrawn
from the anode-near center compartment and the
alkali metal hydroxide solution from the cathode
compartment.
.. .~~38I53
- 7 - O.Z. 0050/43332
In other respects, the process of (c) can be
carried out under the same conditions as described
under (b).
( d ) The e7Lectrochemical cleavage of the dicarboxylate
salt into the dicarboxylic acid and the correspond
ing base can be carried out under a further embodi
ment in a two-part membrane electrolysis cell known
per se from chlor-alkali electrolysis. The membrane
electrolysis cell comprises in general from 1 to
100, preferably from 20 to 70, electrolysis units
grouped together in a block. In this block, the
individual electrolysis units can be electrically
connected in series by electrically connecting the
cathode of one unit to the anode of the next unit or
by using internally connected bipolar electrodes.
The products generally flow in and out via separate
collector lines for each compartment type. The two
part membrane electrolysis unit generally has the
following structure going from the anode to the
cathode:
anode - anolyte compartment - cation exchange mem-
brane - catolyte compartment - cathode.
The aqueous dicarboxylate salt solution is advan
tageously introduced into the anolyte compartment.
Under t:he electric field of the applied direct
_ voltage the alkali metal cations generally migrate
through the cation exchange membrane into the
catolyte; compartment, where they are converted into
alkali. The hydroxyl anions required for compensat-
ing the separated charges are released in the
cathode reaction. The cathode reaction can be for
example the cathodic evolution of hydrogen or a
cathodic reduction of oxygen. The anolyte compart-
ment generally retains the organic acid radical
which combines with the hydrogen ions or their
hydrated forms released in the course of the anode
reaction to form the corresponding free acid. An
_~138I53
- 8 - O.Z. 0050/43332
example of an anode reaction is the anodic evolution
of oxygen or the anodic oxidation of hydrogen. The
anode compartment will thus have in general become
leaner i.n the salt and richer in the free dicar
boxylic acid.
The membrane electrolysis process can be carried
out not only batchwise but also continuously. If it
is carried out over the continuous process, one
option is to divide the conversion between from 2 to
20, preferably from 4 to 6, cells and to guide the
flows countercurrently (see (a)).
The d.icarboxylate salt solution used, which may
contain a plurality of such salts, has in general a
concentration of from 1% by weight up to the satura-
tian limit of the salt(sj, preferably from 5 to 35,
particularly preferably from 15 to 30, % by weight.
The current densities are in general within the
range from 0.5 to 10, preferably from 1 to 4, kA/mz.
The cell voltage is in general from 3 to 10 V,
preferably from 4 to 6 V, per membrane electrolysis
unit.
The pH is in general within the range from 2 to
10 in th.e anolyte compartments and within the range
greater than 13 in the catolyte compartments.
The compartment width is in general from 0.5 to
10, preferably from 1 to 5, mm.
The temperature selected for carrying out the
membranes electrolysis process is in general within
the range from 50 to 110°C, preferably from 65 to
90°C.
To ensure mass transport, the compartment con-
tents are in general recirculated either by means of
pumps or through natural convection, ie. through the
mammoth pump effect due to gas evolution at the
electrodes. The flow velocities in the compartments
are in general within the range from 0.05 to 0.5,
preferably from 0.1 to 0.2, m/sec.
2138153
- 9 - O.Z. 0050/43332
(e) A particularly preferred embodiment is the
electrochemical cleavage of the dicarboxylate salts
into the corresponding dicarboxylic acids and bases
in a thrE~e-part membrane electrolysis cell.
The three-part membrane electrolysis unit has in
general i:he following structure:
anode - anolyte compartment - cation exchange
membrane - center compartment - cation exchange
membrane - catolyte compartment - cathode.
The aqueous dicarboxylate salt solution is in
general :introduced into the center compartment. To
increase the electric conductivity in the center
compartment, a mineral acid or a salt can be added
to the cE:nter compartment electrolyte. Examples are
sulfuric acid, nitric acid, sodium sulfate and
sodium nitrate.
The center compartment generally retains the
organic acid radical, which can react with the
hydrogen ions liberated in the course of the anode
reaction and which have migrated into the center
compartment through the anode-side cation exchange
membrane to fona the free acid. The acid is in
general removed from the center compartment system
together with unconverted salt . The anolyte used can
be an aqueous mineral acid such as sulfuric acid,
nitric acid or hydrochloric acid, preferably
sulfuric acid. The anolyte's essential function is,
together with the anode-side cation exchange mem
brane, t~o protect the organic dicarboxylic acid from
anodic oxidation.
As for the rest, the process of (e) can be
carried out under the conditions described at (d).
(f) The e:lectrachemical splitting of the dicarboxy-
late salts into the corresponding dicarboxylic acids
and basea can also be carried out in a four-part
membrane electrolysis cell.
The four-part membrane electrolysis unit
2.138153
- 10 - O.Z. 0050/43332
generall~,r has the following structure:
anode - anolyte compartment - cation exchange
membrane - anode-near center compartment - anion
exchange membrane - cathode-near center compartment
- cation exchange membrane - catolyte compartment -
cathode.
The aqueous dicarboxylate salt solution is
advantageously introduced into the cathode-near
center compartment.
To increase the electric conductivity in the
center compartment, a mineral acid or a salt such as
sulfuric acid, nitric acid, sodium sulfate or sodium
nitrate can be added to the center compartment
electrol:Yte .
The acid anion generally passes from the cathode-
near center compartment into the anode-near center
compartment, where it reacts with hydrogen ions,
which are evolved in the course of the anode reac-
tion and pass into the anode-near center compartment
through 'the anode-side cation exchange membrane, to
form the free acid. The acid is in general withdrawn
from the center compartment system in high purity.
The remaining salt solution is in general withdrawn
from the cathode-near center compartment and recir-
culated into the adipate dissolution stage as a
part-stream or disposed of.. The anolyte used is in
general an aqueous mineral acid, preferably sulfuric
acid. The anolyte's essential function, together
with the anode-side cation exchange membrane, is to
protect the organic acid from anodic oxidation.
As for the rest, the process of (f) can be
carried out under the conditions mentioned at (d).
In the above-described alternatives the cation
exchange membranes used are particularly preferably
polymers based on perfluorinated olefins or copolymers of
styrene and clivinylbenzene containing sulfonic acid and
if desired carboxyl groups as charge carriers. Very
. 21 3815 3
- 11 - O.Z. 0050/43332
particular preference is given to using membranes that
contain sulfonic acid groups only, since in general they
are more resiE;taut to fouling by multivalent cations than
other membranes. Membranes of this type are known (for
example Nafion~ membranes of type 324). They consist of
a copolymer o:E tetrafluoroethylene with a perfluorinated
monomer that contains sulfone groups. In general they
have a high chemical and thermal stability. The ion
exchange membrane can be reinforced with a Teflon~
support fabric. It is also possible to use copolymers
based on styrene and divinylbenzene.
Suitable anion exchange membranes are for example
the membranes described in detail in EP-A-449,071 (pu-
bushed on October 2, 1991 ) so no details will be given here.
The ellectrode materials used can be in general
perforated materials, for example in the form of nets,
lamellae, oval profile webs or round profile webs.
Theoxygen overvoltage at the anodes is in
general set at less than 400 mV within the current
density ranges according to the invention in order that
the formation of ozone or per-compounds may be prevented.
Suitable anode materials of low oxygen overvol
tage are for example titanium supports with electrically
conducting interlayers of borides and/or carbides and/or
silicides of subgroups IV to VI such has tantalum
borides, titanium borides or titanium suboxide, doped or
undoped tin oxides, or tantalum and/or niobium with or
without platinum metal doping, whose surface has in
general been doped with electrically conducting, non-
stoichiometri.c mixed oxides of subgroups IV to VI and
metals or metal oxides of the platinum group or platinum
metal compounds such as platinates. On top of these
interlayers i.s in general the active electrode material,
which preferably consists of mixed oxides of tantalum
with iridium,, platinum or rhodium and platinates of the
type Lio,3Pt,~0~. To enlarge the surface area it is
customary to use superficially roughened or macroporous
_ _138153
- 12 - O.Z. 0050/43332
titanium supports.
The cathodes are in general made of electrode
materials having a low hydrogen overvoltage in order to
avoid additional voltage losses in the membrane
electrolysis or electrodialysis cell. Suitable cathodes
are for examp:Le iron or nickel supports which have been
surface coatE:d with finely divided cobalt, nickel,
molybdenum, tungsten, manganese, Raney metal compounds of
nickel or of cobalt, nickel- or cobalt-aluminum alloys,
or nickel-iron alloys or cobalt-iron alloys containing
from 65 to 90% by weight of iron.
To i.a~prove selectivity and membrane life the
cathode side can be equipped with cation exchange mem-
branes containing hydroxyl ion blockers. The selectivity
can be further improved by keeping the level of calcium,
magnesium and aluminum ions and also the silica content
in each case lbelow 5 ppm.
The d:icarboxylic acid I obtained by the electro
chemical treatment is in general present as an aqueous
solution having a concentration within the range from 1
to 30, preferably from 4 to 30, % by weight. This solu-
tion can contain the conductivity salt, if present, in a
concentration within the range from 0.05 to 15, prefer-
ably from O.OIi to 6, % by weight and the mineral acid, if
present, in a concentration within the range from 0.05 to
15,_ preferably from 0 to 6, % by weight.
The alkali obtained according to the invention
generally contains an alkali metal hydroxide in a con
centration within the range from 5 to 35, preferably from
15 to 25, % by weight.
To obtain the dicarboxylic acid in pure form, it
is in general crystallized out of the solution obtained
according to the invention, then separated off, for
example by filtration, and dried.
The d.icarboxylic acid is preferably obtained from
the electrodi~alysis or membrane electrolysis solutions by
cooling or evaporation crystallization. Then the
_2138153
- 13 - O.Z. 0050/43332
dicarboxylic acids are in general separated from the
resulting suspensions, for example by filtration, decant-
ing or centrii:uging.
The cooling crystallization is customarily
carried out ait from 0 to 50C, preferably at from 15 to
40C, advantageously at pressures within the range from
1 to 100 kPa, preferably from 4 to 20 kPa.
The clicarboxylic acids separated off can be
preferably obtained in a pure form by washing, for
example with water or Cl-C,-alkanols, and if desired by
recrystallizai:ion. If a plurality of dicarboxylic acids
are present at: the same time, the individual dicarboxylic
acids can be isolated in pure form by utilizing the
solubility differences in a conventional manner such as
fractional crystallization.
The ac;ueous solutions obtained by crystallization
and washing can be concentrated in a conventional manner
and resubject:ed to a crystallization, for example by
adding them to as-electrodialyzed or as-electrolyzed
solutions that have still to be crystallized.
An essential advantage of the process of the
invention over known processes is that it eliminates the
f ormation and disposal of salts which are customarily
obtained when the dicarboxylic acids are freed from their
salts by acidification.
EXAMPLE 1
Hatchwise electrolysis in a three-compartment
electrolysis cell as per variant e)
The three-compartment electrolysis cell used was
that diagrammatically depicted in Figure 1 with three
liquid cycles (RL1 to RL3). All product-contacting parts
with the exception of the electrodes consisted of poly-
propylene, glass or quartz. The anode (E1) (in compart-
ment (A)) was a titanium expanded-mesh anode having an
area of 100 cm~ and a coating suitable for oxygen evolu-
tion. The cathode (E2) (in compartment (C)) likewise had
an area of 1.00 cm~. It consisted of a chromium-nickel
2138153
- 14 - O.Z. 0050/43332
stainless steel (1.4571) which had been coated with a
nickel network activated for hydrogen evolution. The two
membranes (M1 and M2) of the type NafionA324 were posi-
tioned directly on the electrodes (E1 and E2, respec-
tively) and were separated from each other by a 1 mm wide
center compartment (B) with a polypropylene spacer.
The anode (RL1) and cathode (RL2) cycles were
kept in natural circulation owing to the gas evolutions
at the electrodes. The cycle of the center compartment
(B), (RL3), w.as recirculated using a cycle pump (P). The
flow velocity in the center compartment (H) was 0.1 m/
sec.
The anolyte used comprised 1131 g of 5% strength
by weight sulfuric acid introduced at location (1), the
catolyte comprised 1161 g of 5% strength by weight sodium
hydroxide solution introduced at location (2), and the
center compas-tment electrolyte comprised 995 g of 27%
strength by weight sodium adipate solution to which 21 g
of 96% strength by weight sulfuric acid were added so
that 1015 g of a solution containing 22% by weight of
sodium adipate, 2.9% by weight of adipic acid and 2.8% by
weight of sodium sulfate were introduced at location (3).
A temperature of 80°C, atmospheric pressure, a
current density of 3.0 kA/m', a cell voltage of 4.0 V (at
the beginning) and 5.3 V (at the end of the run) produced
in a current 'yield of 83% and after a reaction time of 2h
26min the following electrolytes:
anolyte (removed at location (4)): 729 g of 6.9% strength
by weight sulfuric acid,
catolyte (re:moved at location (5)): 1294 g of 10.9%
strength by weight sodium hydroxide solution,
center compartment electrolyte (removed at location (6)):
904 g of a solution containing 20.4% strength by weight
adipic acid, 1.2% by weight of sodium adipate and 3.2% by
weight of sodium sulfate.
Batchwise crystallization
900 g of the center compartment electrolyte
213$I,~~
- 15 - O.Z. 0050/43332
solution thus obtained were introduced at 80°C into a
vacuum vessel with reflux condenser and then cooled down
over 100 min i,o 10°C by continuously reducing the inter-
nal pressure (absolute) from 1013 mbar to 12 mbar. The
resulting adipic acid crystals were then separated off by
means of a v<icuum nutsche at a filtration pressure of
450 mbar and washed with 700 g of water which had a
temperature close to 0°C. The crystalline product thus
washed was thE:n dissolved in 420 g of water, giving a 30%
strength by weight adipic acid solution. Then the
crystallization process was repeated. Drying the crystal
line product obtained in the second crystallization
process at 80°C and 100 mbar (absolute) left 175 g of
adipic acid having a purity of 99.8% and an ash content
of less than ~B ppm.
Continuous crystallization
Example 1 was repeated except that the adipic
acid was purii:ied by continuous crystallization. For this
two vacuum vessels (0.75 1 nominal capacity, with
stirrer) were connected in series. The absolute pressure
of the first atage (vessel 1) was 95 mbar (corresponding
to a boiling 'temperature of the adipate solution used of
45°C), the absolute pressure of the second stage was
12 mbar (corresponding to a boiling temperature of the
adipate solution used of 10°C). The liquid level was kept
constant in the two vessels by using a membrane metering
pump to pump 0.75 kg/h of adipate solution continuously
into the first vacuum vessel and decompressioning under
a blanket of liquid. A level control valve was used to
likewise introduce 0.75 kg/h of the solution contained in
the first vacuum vessel into the second vacuum vessel,
the solution transported from the first into the second
vacuum vessel likewise being decompressioned "dipped". A
charge (900 g) of the adipic acid crystallized out of the
second vesse7L was separated off by means of a vacuum
nutsche at a filtration pressure of 450 mbar and washed
with 700 g of: water which had a temperature of close to
2~38~~
- 16 - O.Z. 0050/43332
0°C. The crystalline product thus washed was then diss-
olved in 420 ~g of water, giving a 30% strength by weight
adipic acid solution. Then the crystallization process
was repeated. Drying the crystalline product obtained-in
the second crystallization process at 80°C and 100 mbar
(absolute) gave 175 g of adipic acid having a purity of
99.8% and an ash content of less than 8 ppm.
EXAMPLE 2
Batchwise electrolysis in a three-compartment electro-
lysis cell as per variant f)
The four-compartment electrolysis cell used is
diagrammatically depicted in Figure 2 with four liquid
cycles (RL1 i:o RL4). All product-contacting parts with
the exception, of the electrodes consisted of polypropy-
lene, glass or quartz. Anode (E1) (in compartment (A))
was a titanium expanded-mesh anode having an area of
100 cm2 and a caating suitable for oxygen evolution.
Cathode (E2) (in compartment (D)) likewise had an area of
100 cm~. It consisted of chromium-nickel stainless steel
(1.4571) which had been coated with a nickel network
activated for hydrogen evolution. The two electrode-near
cation exchange membranes (M1 and M3) of the type Nafion~
324 were positioned directly on the electrodes (E1 and E2
respectively) and were separated by two center compart-
meats, (B) and (C), each 1 mta in width, with a centrally
disposed anion exchange membrane.(M2) of the type Tokuy
ama Soda~ AMH. The center compartments, (B) and (C), were
provided with two polypropylene spacers which served to
keep the flow channel free and to prevent direct contact
between the membranes.
The anode (KL1) and cathode (RL4) cycles were
kept in natural circulation owing to the gas evolutions
at the electrodes. The cycles of the center compartments
(B) and (C), (KL2) and (RL3), were recirculated using the
cycle pumps (P1) and (P2). The flow velocities in the
center compartments (B) and (C) were in each case 0.1 m/
sec.
.~1~81~3
- 17 - O.Z. 0050/43332
The anolyte used comprised 1108 g of 5.1%
strength by weight sulfuric acid introduced at location
(1), the catolyte comprised 1101 g of 4% strength by
weight sodium hydroxide solution introduced at location
( 2 ) , the electrolyte of the anode-near center compartment
(B) comprised 1097 g of 2.1% strength by weight sulfuric
acid introduced at location (3), and the electrolyte of
the cathode-near center compartment (C) comprised 1505 g
of 27% strengi:h by weight sodium adipate solution intro
duced at locaition (4).
During the reaction a total of 900 g of water was
additionally introduced into the cathode-near center
compartment ( C ) .
A temperature of 80°C, atmospheric pressure, a
current densiity of 3 . 0 kA/ms, a cell voltage of 7 . 0 V ( at
the beginning) and 8.7 V (at the end of the run) produced
with a current yield of 75% and after a reaction time of
5 h, during which the pH in the cathode-near center
compartment (C) was within the range from 10 to 12, the
following electrolytes:
anolyte (removed at location (5)): 793 g of 7.1% strength
by weight sulfuric acid,
catolyte (removed at location (6)): 1584 g of 13.3%
strength by weight sodium hydroxide solution,
product of the anode-near center compartment (B) (removed
at location (7)): 2034 g of a solution containing 15.1%
strength by weight adipic acid, 1.1% by weight of
sulfuric acid,
product of the cathode-near center compartment (C)
(removed at location (8)): 1061 g of a 1.4% strength by
weight sodium. adipate solution.
EXAMPLE 3
eatchwise electrolysis in a membrane stack cell as per
variant b)
The membrane stack cell used is diagrammatically
depicted in :Figure 3 with three liquid cycles (KL1 to
RL3). All product-contacting parts with the exception of
2.1.38153
- 18 - O.Z. 0050/43332
the electrodEa consisted of polypropylene, glass or
polytetrafluo:roethylene. Anode (E1) (in compartment (A))
was a titanium expanded-mesh anode having an area of
320 cm' and a coating suitable for oxygen evolution.
Cathode (E2) (in compartment (D)) likewise had an area of
320 cm~. It consisted of a chromium-nickel stainless
steel (1.4571) which had been coated with a nickel
network activated for hydrogen evolution.
The compartments (A) and (B1), (D1) and (H2),
(D2) and (B3) and also (D3) and E were in each case
separated from each other by a bipolar membrane (from DE
A 40 26 154). The compartments (B1) and (C1), (B2) and
(C2) and also (H3) and (C3) were kept separated from each
other by anion exchange membranes (Tokuyama~ Soda AMX).
The compartments ( C 1 ) and ( D1 ) , ( C2 ) and ( D2 ) and also
(C3) and (D3) were kept separated from each other by
cation exchange membranes (TokuyamaA Soda CMX). The
membrane spacings were in each case 0.5 mm.
All liquid cycles with the exception of those of
the anode (A) and cathode (E) compartments were recircu
lated by means of cycle pumps, (P1) to (P3), the flow
velocity being in each case 0.1 m/sec.
The electrolyte used in the acid medium comprised
10000 g of 1.5% strength by weight sulfuric acid intro
duced at location (1), the electrolyte in the basic
medium_compri.sed 5000 g of 1% strength by weight sodium
hydroxide so7Lution introduced at location (2) and the
center compartment electrolyte comprised 5000 g of 20%
strength by weight sodium adipate solution introduced at
location (3).
A temperature of 55°C, atmospheric pressure, a
current density of 0.31 kA/ms, a cell voltage of 13 V (on
average) produced with a current yield of 70% and after
a reaction time of 13 h the following electrolytes:
dialysis product (removed at location (4)): 11555 g of
6.4% strength by weight adipic acid which additionally
contained 1.3% strength by weight of sulfuric acid,
. ~~38.~53
- 19 - O.Z. 0050/43332
"basic" electrolyte (removed at location (5)): 6597 g of
6.9% strength by weight sodium hydroxide solution,
"depleted" center compartment electrolyte (removed at
location (6)): a 1.8% strength by weight solution of
sodium adipate.
EXAMPLE 4
The apparatus used was an electrodialysis cell
having four compartments, the anode compartment being
separated from the anode-near center compartment ("acid
compartment" _ "SK") by a bipolar exchange membrane
(obtained by adhesively bonding a cation exchange mem-
brane of the type Selemion~ CMV to an anion exchange
membrane of tlhe type Selemion~ AMV as per EP-A 103 959),
the acid compartment from the cathode-near center com-
partment ("base compartment" _ "BK") by a cation exchange
membrane of the type Selemion~ CMV, and the base compart
ment from the cathode compartment by a bipolar exchange
membrane as used for separating the anode compartment
from the acid compartment. The electrodes used were
platinum electrodes.
The effective area of a membrane was 3.14 cm~,
and the membrane spacing 1 cm. Compartments SK and BK
were each connected to a cycle, while the anode and
cathode compartments were connected to a common cycle
consisting of reservoir vessel and pump. The temperature
setting was by heat exchangers integrated into the
cycles. The monitoring of the process was by pH and
conductivity measurement in the acid and base cycle.
The materials introduced were into the acid cycle
83.4 g of a solution containing as the solid sodium
adipate in a concentration of 0.68 eq/kg, into the base
cycle 24.2 g of a dilute NaOH solution having a concen
tration of 0.08 eq/kg, and into the electrode rinse cycle
dilute NaOH having a concentration of 0.1 eq/kg. The
solutions were recirculated and the electrodialysis was
carried out at 35°C with a constant current of 0.3 A. The
process was discontinued at a pH in the acid cycle of
_~138I53
- 20 - O.Z. 0050/43332
about 2.4. Toward the end of the electrodialysis the
adipic acid partially precipitated in the reservoir
vessel. The e:~cit stream from the acid cycle comprised a
total of 79.6 g of solution and solid containing 56.1 meq
of adipic acid and 0.4 meq of the bis-Na salt (material
yield: 99.6%, acid purity: 99.1% by weight, current
yield: 76%). 'The sodium hydroxide solution produced had
a concentration of 2.1 eq/kg.
EXAMPLE 5
Example 4 was repeated except that the materials
introduced were in the case of the acid cycle 82.7 g of
a sodium ad3.pate solution (0.49 eq/kg) and an NaOH
concentration of 0.09 eq/kg, in the case of the base
cycle 23.9 g of a dilute NaOH solution having a concen-
tration of 0.075 eq/kg and in the case of the electrode
rinse cycle dilute NaOH having a concentration of 0.1 eq/
kg . The solutions were recirculated and the electrodialy-
sis was carried out at 35°C with a constant current of
0.3 A. The process was discontinued at a pH in the acid
cycle of about 2.4. The exit stream from the acid cycle
comprised 78.5 g of a solution containing 39.9 meq of
adipic acid and 0.3 meq of the bis-Na salt (material
yield: 99.2%,, acid purity: 99.0% by weight, current
yield: 72%). The sodium hydroxide solution produced had
a concentration of 1.75 eq/kg.
EXAMPLE 6
Example 4 was repeated except that the materials
introduced were in the case of the acid cycle 98.2 g of
a solution containing 1.64 eq/kg sodium adipate, in the
case of the base cycle 39.3 g of a dilute NaOH solution
having a concentration of 0.08 eq/kg and in the case of
the electrode rinse cycle dilute NaOH having a concent-
ration of 0.1 eq/ kg. The solutions were recirculated and
the electrod:ialysis was carried out at 60°C with a
constant current of 0.3 A. The process was discontinued
at a pH in the acid cycle of about 2. The exit stream
from the acid cycle comprised 76.9 g of a solution
_2138153
- 21 - O.Z. 0050/43332
containing 2.07 eq/kg of adipic acid and < 0.01 eq/kg of
Na+ (material yield: 99.1%, acid purity: > 99.4% by
weight, current yield: 49%). The solution was cooled to
0°C, and the precipitated solid was filtered off, washed
with cold watE:r and dried. The crystallization yield was
82%, the acid content of the crystalline product > 99.96%
by weight (Nay content < 0.01% and bis-Na salt content
< 0.04% by weight). The sodium hydroxide solution prod-
uced had a concentration of 2.1 eq/kg.
EXAI~LE 7
The apparatus used was an electrodialysis cell
having five <:ompartments, the anode compartment being
separated from the anode-near center compartment ("acid
compartment" ~~ "SR") by a bipolar exchange membrane (see
Example 4 ) , t:he acid compartment from the diluate com-
partment ("DR") by an anion exchange membrane (Selemion~
AMV), the diluate compartment from the cathode-near
center compartment ("base compartment") by a cation
exchange membrane (SelemionA CMV), the base compartment
from the cai:hode compartment by a bipolar exchange
membrane (seE; Example 4). The electrodes used in the
anode and cathode compartments were in each case platinum
electrodes.
The effective area of a membrane was 3.14 cm=,
the membrane spacing was 1 cm. The compartments SR, DR
and_BR were each connected to a cycle, while the anodes
and cathodes compartments were connected to a common
cycle, consisting of reservoir vessel and pump. The
temperature fsetting was by heat exchangers integrated
into the cycles. The monitoring of the processes was by
pH and conductivity measurement in the acid, diluate and
base cycle.
The materials introduced were into the acid cycle
42.9 g of wager, in the diluate cycle 90.6 g of a solu
tion containing as solid sodium adipate in a concentra
tion of 1.60 eq/kg, into the base cycle 39.3 g of a
dilute NaOH solution having a concentration of
_ X1381 ~3
- 22 - O.Z. 0050/43332
0.08 eq/kg, and into the electrolyte rinse cycle dilute
NaOH having a concentration of 0.1 eq/kg. The solutions
were recirculated and the electrodialysis was carried out
at 60°C with a constant current of 0.3 A. The process was
discontinued a,t a conductivity value in the diluate cycle
of 0.2 mS/cm (starting value = 117). The exit mixture
from the acid cycle comprised 77.5 g of solution having
an adipic acid concentration of 1.82 eq/kg and an Na+
concentration of 0.03 eq/kg (material yield: 99.6%, acid
purity: 98.0% by weight, current yield: 50%). The solu-
tion was coolE:d down to 0°C, and the precipitated solid
was filtered off, washed with cold water and dried. The
crystallization yield was 86%, the acid content of the
crystalline product was > 99.96% by weight (Na content
< 0.01% and b:is-Na salt content < 0.04% by weight). The
sodium hydroxide solution produced had a concentration of
1.89 eq/kg. The degree of depletion in the diluate cycle
was 99.6%.
EXAMPLE 8
A solution having a temperature of 80°C and
containing d:isodium adipate in a concentration of
1.98 eq/kg and adipic acid in a concentration of
2.14 eq/kg ways cooled down to 20°C. The crystalline
product formed was filtered off, then washed with cold
water and dried. The crystalline product contained 0.05%
by weight of lVa+ (corresponds to.a purity of adipic acid
of 99.8% by weight), the aqueous phase 2.12 eq/kg of Na,
adipate and 1,.10 eq/kg of adipic acid (H* depletion about
50%).
EXAMPLE 9
150 g of a solution containing Nay adipate in a
concentration of 0.99 eq/kg and adipic acid in a concen-
tration of 1.00 eq/kg was extracted three times at 40°C
with 150 g of methyl tert-butyl ether and the extracts
were distilled. The following amounts of adipic acid were
obtained:
1st fraction
_ _~~~8158
- 23 - O.Z. 0050/43332
Amount = 3.10 g (corresponds to 28.3% of the
adipic acid contained)
Purity = 99.95% by weight
2nd fraction
Amount = 2.26 g (corresponds to 20.6% of the
adipic acid contained)
Purity = 99.79% by weight
3rd fraction
Amount = 1.86 g (corresponds to 17.0% of the
adipic acid contained)
Purity = 99.95% by weight
Examples 8 and 9 show that adipic acid can be
separated with high purity from a mixture of disodium
adipate and ,adipic acid. The solution which had been
depleted in ~adipic acid can be recirculated into the
electrodialysis.