Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
wo 94/2543~ 213 91~ M PCT/GB94/00896
PEPTIDYL DERIYATIVES AND THE~R USE AS METALLOPROTEINASE INHIBITORS
5 FIELD QF THE ~NVENTIC)N
This invention relates to a novel class of peptidyl derivatives, to
processes for their preparation and to their use in medicine.
BAC:KGROUND TO THE ItlVENTlON
10 In normal tissues, cellular connective tissue synthesis is offset by
extracellular matrix degradation, the two opposing effects exis~ing in
dynamic equilibrium. Degradation ot the ma~rix is brought about by the
action of proteinases released from resident connective tissue cells and
invading inflammatory cells, and is due, in part, to the activity of at least
15 three groups of metalloproteinases. These are the collagenases, the
gelatinases (or type-lV collagenases~ and the stromelysins. Nom~ally
these catabolic enzyrnes a-e tightly regulated at the level of their
synthQsis and secretion and also at the level of their e~nracellular
activity, the latter through the action of specific inhibitors, such as a2-
20 macroglobulins and TIMP (tissue inhibitor o~ metalloproteinase), whichforrn inactive complexes with metalloproteinases.
The accelerated, uncontrolled breakdown of connective tissues by
- metalloproteinase catalysed resorption of the extracellular matrix is a
2~ feature of msny pathological conditions, such as rheum~toid arthritis,
comeal, epidennal or gastric ulceration; tumour metastasis or invasion;
periodontal disease and bone- disaase. ~ It can be expected tha~ the
pathogenesis of such diseases is likely to be modifiad in a beneficial
manner by the administration of metalloproteinase inhibitors and
30 numsrous compounds have been suggested for this purpose [for a
general review see Wahl, R.C. ~al A~m. Rep. Med. Chem. 25, 175-184,
Academic Press Inc., San DieQo (1990)l.
. .
Although numerous metalloproteinase inhibitors have been described,
35 many have not been suitable for further development as medicines
since they have lacked any useful actiYity when administered orally at
WO 94/2~435 PCT/GB94/00896
?.,~39~9 2
pharmaceutically acceptable doses. What is therefore needed is a
potent orally active compound with a good duration of action.
SUMMARY OF THE INVENT!ON
5 We have now found a new class of peptidyl derivatives, members of
which are metalloproteinase inhibitors. The compounds according to the
invention have surprisingly good oral bioavailability, and atter oral
administr~tion have an advantageously longer duration of action than
related known compounds, such as those described in International
10 Patent Specification No. W092/09564.
Thus according to one aspect of the invention we provide a compound
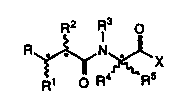
of formula (1)
R2 R3 o
~R~RsX
15 wherein R represents a -CONHOR~ lwhere R3 is a hydrogen atom or an
acyl groupl, carboxyl (-C02H), esterified carboxyl, -SR~ or -P(o)(X1R7)-
-X2R8 group, where X1 and X2, which may be the same or dfflerent, is
each an oxygen or sulphur atom and R7 and ~, which may be the same
or different each represents a hydrogen atom or an optionally substitued
20 alkyl, aryl, or aralkyl group;
.
R1 represents a hydrogen atom or an optionally substituted alkyl,
alkeny!, aryl, aralkyl, heteroaralkyl or heteroarylthioalkyl group;
25 R2 represents an optionally substituted alkyl, alkenyl, cycloalkyl,
cycloalkylalkyl, aryl, amino (-NH2), substituted amino, carboxyl (-CO2H),
or esterified carboxyl group;
,
R3 !epresents a hydrogen atom or an alkyl group;
R4 represents a hydrogen atom or an alkyl group;
WO 94t25435 PCT/GB94/00896
213912``~
R5 represents a group -C(R9)(R10)Het-R11, wherein R9 and R10 which
may be the same or different is each=an optionally substituted alkyl or
a~kenyl group optionally interrupted by one or more -O- or -S- atoms or
-N(R12)- groups (where R12 is a hydrogen atom or an optionally
substituted aikyl group), or an optionally substituted cyc~oalkyl, cyclo-
alkenyl, aryl or heteroaryl group, or Rg and R10 together with the carbon
atom to which they are attached are linked together to form an optionally
substituted cycloalkyl or cycloalkenyl group, Het is -O-, -S(O)p- ~where p
is zero, or an intager 1 or 2l or -N(R12)-, and R11 is a hydrogen atom or
an aliphatic, cycloaliphatic, heterocycloaliphatic, aromatic, or hetero-
aromatic group;
X is an amino ( Ntl2), substituted amino, hydroxyl or substituted hydroxyl
group, or is linked to the atom or group Het in R5 to form a chain -X-Alk-
R5- where X is -N(R12)-, Alk is an optionally substituted alkylene chain
and R5 iS-Het-c(R9)(R1o)-;
and the salts, solvates, hydrates and prodrugs thereof.
It will be appreciated that the compounds according to the invention can
contain one or more asymmetrically substituted carbon atoms, for
example those marked with an asterisk in formula (1). The presence of
one or more of these aysmmetric centres in a compound of formula (1)
can give rise to stereoisomers, and in each case the invention is to be
understood to extend to all such stereoisomers, including enantiomers
and diastewisomers~ and mktures, inc!uding racemic miktures, thereof.
In the formulae herein, the -line is used~at a potential asyrnmetric centre
to represent the possibility of R- and S- configurations, the _ line and
the - ~ line to represent an unique configuration at an asymmetric
centre.
.
In the compounds according to the in~ention, when the group R
represents an esterified carboxyl group, it may be for example a group of
- 35 formula -CO2R13 where R13 is a straight or branched, optionally
substituted C1.8alkyl group such as a methyl, ethyl, n~propyl, i-propyl,
WO 94/25435 PCTIGB94/00896
~39~29 4
n-butyl, i-butyl, s-butyl or t-butyl group; a C6 12arylCl.8alkyl group such
as an optionally substituted benzyl, phenylethyl, phenylpropyl,
a-naphthylmethyl or ~-naphthylmethyl group; a C6.~2aryl group such as
an optionally substituted phenyl, -naphthyl or ~-naphthyl group; a
5 C6.12aryloxyC1.~alkyl group such as an optionally substituted phenyl-
oxymethyl, phenyloxyethyl, a-naphthyloxymethyl or ~-naphthyloxy-
methyl group; an optionally substituted Cl.8alkanoyloxyCl.8alkyl group,
such as a pivaloyloxymethyl, propionyloxyethyl or propionyloxypropyl
group; or a C6.12aroyloxyC1.8alkyl group such as an optionally
10 substituted benzoyloxyethyl or benzoyloxypropyl group. Optional
substituents present on the groups R13 include for example one or more
halogen atoms such as fluorine, chlorine, bromine or iodine atoms, or
Cl.4alkyl, e.g. methyl or ethyl, or C1~alkoxy, e.g. methoxy or ethoxy,
groups.
In general, when the group R represents an esterified carboxyl group, it
may be a metabolically labile ester of a carboxylic acid.
When the group R~ in compounds of forrnula (1~ represents an acyl
20 group, it may b: for exarnple a group of formula R14C=X3 where X3 iS an
oxygen or~sulphur atom and F~14 represents a hydrogen atom or a group
selected from~ amine~ (-NH2), substituted amino (for example a group
-NR17P~18 as described below in relation to the group X), or an optionally
- - substituted G~allq~l, Cl~alkoxy,C1 ~Salkythio, C2~alkenyl, C2 ~Salkynyl,
25 C~.lyaryl,~ C~12aralkyl, C3.6cycloalkyl, C3.6heteroaryl or C3.6hetero-
aralkyl group. ~Particular groups of these types include optionally
- substi~utod~-methyl, e~hyl, n-propyl, i-propyl, methoxy, ethoxy, methylthio,
ethyl~io, e~nyl, 1-propenyl, ethynyl, 1-propynyl, phenyl, 1-naphthyl,
2-naphthyl, benzyl, phenethyl, 1-naphthylmethyl, 2-naphthylmethyl,
30 cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, furanyl, pyrrolyl, thienyl, furanylmethyl, pyrrolylmethyl or thienylmethyl groups. Optional
substituents which may be present on such R14 groups include one or
more-s~stituents selected from those described below in relation to the
group Rtor p~2 when such groups represent substitued alkyl, aryl or
35 heteroaryl groups.
,
WO 94n543s 21 3 9 2 2 ~ PCT/G11194/00896
The group R7 and/or F~8 in compounds of forrnula (1) may each be a
hydrogen atom or an optionally substituted straight or branched
Cl.6alkyl, e.g. methyl, ethyl, n-propyl, i-propyl, n-butyl or i-butyl,
C6.12aryl, e.g. phenyl, or C6 12arylC1.6alkyl, e.g. benzyl, phenylethyl or
5 phenylpropyl group. Optional substituents present on alkyl groups of
this type include one ormore C1~alkoxy, e.g. methoxyor ethoxy, or
C1.6alkylthio, e.g. methylthio or ethylthio groups or an optionally
substituted C6.12aryloxy e.g. phenyloxy, C6.12arylthio e.g. pheny~thio,
C6.12arylC1.6alkoxy e.g. benzyloxy or C6 l2arylC1.6alkylthio e.g.
10 benzylthio. Optional substituents present on the group R7 or R8 when it
is an aryl or aralkyl group or an alkyl group substituted by an aryloxy or
arylthio group include R16 groups as defined below.
When the group R1 in compounds of formula (1 ) represents an
15 optionally substituted alkyl or alkenyl group, it may be, for example, a
straight or branched C1~ alkyl or C2.Balkenyl group, such as a methyl,
ethyl, n-propyl, i-propyl, n-butyl, i-butyl, s-butyl, t-butyl, n-pen~yl, i-pentyl,
n-hexyl, ethenyl, 1-propenyl, 1-butenyl or 2-butenyl group optionally
substituted by one or more Cl~alkoxy, e.g. methoxy, ethoxy, propoxy,
20 C1~alkylthie, e.g. methylthio, ethylthio, propylthio, C~12arylCl~alkoxy,
e.g. phenylC1~alkoxy such as ber~yloxy, aralkylthio, e.g. phenyl-
C1.6alkylthio such as benzylthio, amino (-NH2), substttuted amino, lsuch
as -NHR1~, where R15 is a C1~alkyl e.g~ methyl or ethyl, C~12aryl-
C1~alkyl, e.g. phenylC1~alkyl, such as benzgl, Cff.12aryl, e.g. phenyl,
25 C3.8cyc!oalkyl, e.~. cyclohexyl,~ or Cj.8cycloalkylCl.6alkyl, ~.9.
cyclohexylmethyl groupl, carboxyl (-C02H) or -CO2~13 twhere R13 is as
defined above~ groups.
Aryl groups represented by R1 and/or R2-in compounds of formula (1)
30 include optionally substituted mono or bicyclic C~;.t2 aryl groups such
as phenyl or 1- or 2-naphthyl groups..- ~~ ~
Aralkyl ~roups represented by R1=nclu~e optionally substituted mon~
or bicyclic C6.12arylC1~alkyl groups-such as phenylCl~alkyl, or 1- or 2-
naphthylC1.6alkyl, for example benzyl, phenylethyl, phenylpropyl~ I
phenylbutyl, phenylpentyl, 1- or 2- naphthylmethyl, naphthylethyl,
wo 94/2s43s PCT/GBg4/00896
~3~S 6
naphthylpropyl, naphthylbutyl or naphthylpentyl groups.
When the group R1 in compounds of formula (1) is a heteroaralkyl
group, it may be for example an optionally substituted mono-or bicyclic
C3.9heteroarylC1.6alkyl group, such as an optionally substituted
pyrrolylmethyl, furanylmethyl, thienylmethyl, imidazolylmethyl, t
oxazolylmethyl, thiazolylmethyl, pyrazolylmethyl, pyridinylmethyl, or
pyrimidinylmethyl group.
Heteroarylthioalkyl groups represented by Rl include optionally
substituted mon~ or bicyclic C3 9heteroarylthioC ~.6alkyl groups such as
optionally substituted pyrrolylthiomethyl, turanylthiomethyl, oxazolyl-
thiomethyl, thiazolynhiomethyl, pyrazolylthiomethyl, pyridinylthiomethyl,
or pyrimidinylthiomethyl groups.
When ~he group R2 in compounds of fom ula (1) represents an alkyl or
alkenyl group it may be for example a straight or branched C1~alkyl, or
C2~alkenyl group, such as a me*yl, ethyl, n-propyl, i-propyl, n~butyl, i-
butyl, s-but~l, t-butyl, n-pentyl, i-pentyl, n-hexyl, e~henyl, 1-propenyl, 1-
butenyl, or 2 bu~ny1 group. Optional substituents whic~h may be present
on such groups include one or more C1~alkoxy, e.g. methoxy, ethoxy, 1;
propoxy, C1~alkylthio, e.g. methylthio, ethylthio, propy~thio, amino,
substhuted amino~such as NHR15 where~R1s is as defined abovel,
car~xyl or CO2F'~l3 Iwhere R13 is as defined above] groups.
- Cycloalkyl groups represen~d by the group R2 in compounds according
to th-e; invention inc!ude C3.8cycloalkyl groups such as cyclopentyl or
cyclohexyl groups.
When the group R2 in compounds of formula (1) is a substituted amino
- - group, this may be for example a group -NHR15 where R15 is as defined
above.
, .
When~ R2 is a cycloalkylalkyl group it may be for example a C3.8cyclo-
alky!C1~alkyl group such as a cyclopentylC1.6alkyl or cyclohexyl- !
C1.6alky! group, for example a cyclopentylmethyl, cyclopentylethyl,
WO 94/;!5435 21 ? 9 ~ 2 f! PCT/GB94/00896
cyclopentylpropyl, cyclopentylbutyl, cyclohexylmethyl, cyclohexylethyl,
cyclohexylpropy~, or cyclohexylbutyl group.
Optional substituents which may be present on aryl, aralkyl,
5 hetsroaralkyl or heteroarylthioalkyl groups represented by R1 or R2
include those R16 substituents discussed below.
The aryl, aralkyl, heteroaryl, heteroaralkyl or heteroarylthioalkyl groups
represented by R1 and/or R2 in compounds of formula (1) may each
10 optionally be substituted in the cyclic part of the group by one, two or
more substituents ~R16] selected from halogen atoms, e.g. fluorine,
chlorine, bromine or iodine atoms, or Cl.6alkyl, e.g. methyl or ethyl,
C 1.~alkoxy e.g. methoxy or ethoxy, C2.6alkylenedioxy, e.g.
ethylenedioxy, haloC1.6alkyl, e.g. trifluoromethyl, C1.6alkylamino, e.g.
15 methylamino or ethylamino, C1.6dialkylamino, e.g. dimethylamino or
diethylarnino, arnino (-NH2), nitro, c~sno, hydroxyl (-9H), carboxyl
(-C02H), -CO2Rl3, where R13 is as defined above, Cl~alkylcarbonyl,
e.g. acetyl, sulphonyl (-SO3H), Cl~alkylsulphonyl, e.g. methylsulphonyl,
aminosulphonyl (-:SO2NH2), Cl.8 alkylaminosulphonyl, e.g. methyl-
20 aminosulphonyl or ethyiamino~ulphonyl, Cl.~dialkylamino-sulphonyl
e.g. dimethylaminosulphonyl or diethylaminosulphonyl, c~rboxamido
(-CONH2), C1.6alkylaminocarbonyl, e.g. methylaminocarbonyl or
ethylaminocarbonyl, Cl.6dialkylaminocarbonyl, e.g. dimethylamino-
- carbonyl or diethylaminocarbon~l, sulphonylamino (-NHS02H),
25 C1~alkylsulphonylamino, e.g. methylsulphonylamino or ethylsulphonyl-
amino, or C1~6dialkylsulphonylamino, e.g. dimethylsulphonylamino or
diethylsulphonylamino groups. It will be appreciafed that where two or
more R16 substituents are present, these need not necessarily be the
same atoms andlor groups. The R16 substituenSs may be present at any
30 ring carbon atom away from ~hat attached te the rest of the molecule ef
formula (1). Thus, for example, in phenyl groups any substRuents may
be present at the 2-, 3-, 4-, S- or 6- positions relative to the ring carbon
atom attached to the remainder of the molecule.
35 When the groups P/3 and R4 in compounds of formula (1) are alkyl
groups, they may be for example straight or branched C1~alkyl groups
" ,.. .
WO 94/2S43~ - PCTIGB94/00896
? ~9~ 8
such as methyl or ethyl groups.
When the group R9 or R10 in compounds of forrnula (1) is an optionally
substRuted alkyl or alkenyl group it may be a straight or branched
5 C1~alkyl, e.g. methyl, ethyl, n-propyl i-propyl, n-butyl, i-butyl, n-pentyl or n-hexyl or C2.6alkenyl e.g. ethenyl or 1-propenyl group optionally
interrupted by one or more -O- or -S- atoms or -N(Rl2)- groups where
Rl2 is a hydrogen atom or an optionally substituted C1~alky group such
as a methyl, ethyl or propyl group.
: ~ ~
Optional substituents which may be present on such groups include one
Of mor* C1.6alkoxy, Cl.6alkylthio, C6.l2arylCl.6alkoxy, aralkylthio,
amino,-substituted amino, carboxyl, -C02Q13, aryl or heteroaryl groups
as defined above in connection with the group Rl, or an optionally
15 subst~tuted ~cyc!oalkyl or~ cyoloalkeny~ group as defined below in
connecbon wi~h~the~groups R9~and R10.
When-thé group R9, R10 or~ R9~;and R'0 togeUler with the~carbon atom to
ch they~are attached, is~ an op~ionally substituted cycloalkyl or
20 ~cycloalkenyl~ group, ~it may~ be tor ~example~ a C3.8cycloalkyl, e.g.
cycbp~ cycbp~yl or~cyclohe~yl, or ~C3.8cycloalkenyl e,g. cyclo
prope~l,~cyolop~or~cldlexenyl,gtoupoptionallysubstltuted by
one,~ two~or ~ more~ C1~alkyl,~ e.g. ~ melhyl or eth~, Cl~alkoxy, e.g.
m~qi or ~rorJ, C~alkyl~b, ~e.g. me~io, or hydro~yl groups.
The tenn Hét in ~ pounds ot tomwh~ (1 j may-rep~esent ~, -S-, -S(O)-,
S(O)~ or -N(~ ~*rè~Rl2~ b a hydrogen àtom or~a C1.6alkyl group
as defined~ at~e.
30 When R1 l-in- compounds ~ot formula (1 ) is an aliphatic group it may be tor
example an optionally substituted saturated or unsaturated straight or
branct~ 61~alkyl chain optionally interrupted by one or more -O- or
atoms or groups~ sel-cted from -N(R1~2)-, -CO-, -CON(R12)-, or
-N(Rl2)CO-. Particular groups include optionally substituted methyl,
3 5~ ethy', n~propyl, i-propyl, n-butyl, i-butyl, s-butyl, t-butyl, ethenyl,
1-propenyl, 1-buten~l or 2-butenyl groups. Optional substauents which
WO 94125435 PCT/GB94/00896
21~91~
may be present on groups of these types include one or more
amino (-NH2), substituted am~ro lfor example a group -NRl7R18 as
described below in relation to the group Xl, C6.l2aryl, e.g. optionally
substituted phenyl, C6 12aryloxy e.g. optionally substituted phenoxy, lthe
5 optional substituents in each case being R~6 groups as defined abovel
C3.8cycloalkyl, e.g. cyclopentyl or cyclohexyl, C3.8cycloalkoxy, e.g.
cyclopentyloxy or cyclohexyloxy, carboxyl (-CO2H) or -CO2P(l3 groups.
Cycloaliphatic groups represented by Rl1 in compounds of forrnula (1)
10 include optionally substituted C3.8cycloalkyl and C3.8cycloalkenyl
groups, for example optionally substituted cyclopropyi, cyclobutyl,
cyclopentyl, cyclohexyl, cyclopentenyl ~nd cyclohexenyl groups.
Optional substituents include those groups R16 described above.
1~ Heterocycloaliphatic groups represented by Rl1 in the compounds of
formula (1) include optionally substituted Cs 7heterocycloalkyl groups
containing one or two heteroatoms selected from -O- or -S-, or a group
-N(R12)-, for example optionally substituted piperazinyl, morpholinyl,
- pyrrolidinyl, tetrahydrofuranyl~ tetrahydropyranyl, piperidinyl, or N-
20 methy!piperidinyl groups. Optional substituents include those groups
R16 described above. The heterocycloalkyl groups represented by Rl1
may be attached to the remainder of the molecule through any ring
carbon atom.
25 When the group Rl1 in compounds of torrbula ~1) is an aromatic group R
may be for exasnple an optionally substituted mono- or bicyclic C~l2aryl
group, for example an optionally substitute~-phenyl or 1- or 2-naphthyl
group. Optional substituents which rnay be present on groups of this
type include those R16 substituents described above.
Heteroaromatic groups represented by'the group R11 include mono-or
bicyclic C5.9heteroaromatic groups containing one, two or three
heteroatoms selected from -O-~or--~, or-N(Q12) groups. Particular
examples include pyrrolyl, furanyl, thl'ënyl,' imidazolyl, oxazolyl, thiazolyl,
35 pyrazolyl, 1-indolyl, 2-indolyl, 1-quinolinyl or 2-quinolinyl groups. Such
groups may be optionally substituted, for example by one or more R16
WO 94125435 PCT/GB94/00896
,~ 9 ~ 1 o
substituents. The heteroaromatic group may be connected to the
remainder of the compound of formula (1) through any nng carbon atom,
or where appropriate through a heteroa~om or group -N(Rl2)-.
5 When X in the compounds of fonnula (1) represents a substituted amino
group it may be for example a group of formula -NR~7R18, where R17
and Rl8, which may be the same or different, is each a hydrogen atom
(with the proviso that when one of R17 or R18 is a hydrogen atom, the
other is not) or an optionally substituted straight or branched alkyl group,
10 optionally interrup~ed by one or more O- or -S- atoms or -N(R12)- or
aminocarbonyloxy ~-NHC(O)O-] groups or Rl7 and Rl8, together with the
nitrogen atom to wtiich they are attached, may form an optionally
substituted C3~cyclic amino group optionally possessing one or more
other heteroatoms sebcted from -~ or -S-, or -N(R12)- groups.
Whén R17 and/or R18 is an alkyl group it ma~ be for example a C1~alkyl
group such as a methyl, ethyl, n-propyl, i-propyl, n-butyl, i-butyl, s-butyl,
or t-butyl group, optionally interrupted by one or more -O- or S- atoms,
or -N(R13)- or aminocarbonyloxy groups and may be for example a
20 methoxyrnethyl, ethoxymethyl, ethoxyrnethyl, ethoxyethyl or ethylamino-
carbonyloxymethyl group. The optional substituents which may be
present on such groups include hydroxyl (-OH), carboxyl (-C02H),
esterified carboxyl (-CO2R13), carboxamido (-CONH2), substituted
carboxamido, e.g. a group -CONR17R18 where NR17R18 is as defined`
25 hereinj- ammo (-NH2), substituted amino, for example a group of formula
-NR17R18, aminosulphonylamino, for example -N(R12)SO2NH2 or
-N(Rt2~SO2NRt7R18 or aryl? e.g. C~12 aryl such as phenyl, optionally
substituted by onè, two or more R16 substituents selected from those
listed above.
- . -
Partic.ular examples of cyclic amino groups represented by ~NR17F~l8
include morpholinyl, imidazolyl, piperazinyl, pyrrolyl, oxazolyl, thiazolyl,
pyra~I,~pyrrolidinyl, pyridinyl and pyrimidinyl groups.
3~ When the group X is a ~ubstituted hydroxyl group it may be for example
a group -ORl1 where p/11 is as defined above, other than a hydrogen
WO 94/25435 2 1 ~ ~ I Z ~ PCT/GB94/~0896
atom.
When X is linked to the atom or group Het in R5 to forrn a chain -X-Alk-
R5, the optionally substituted alkylene chain represented by Alk may be
5 an optionally substituted straight or branched C2.g alkyl~ne chain, for
example an ethylene, propylene or butylene chain. Optional
substituents present on the alkylene chain inciude those described
above in relation to the alkyl group represented by p~2. In compounds of
this type, the group X is -N(R12)-, where R12 is as defined above. The
10 group P~5 iS -Het-C(R9)(R10)- where Het, R9 and R10 are as defined
above.
Salts of compounds of formula (1) include pharmaceutically accsptable
salts, for example acid addition salts derived from inorganic or organic
15 acids, sush as hydrochlorides, hydrobromides, hydroiodides, p-toluene
sulphonates, phosphates, sulphates, perchlorates, acetates, trifluoro-
acetates, propionates, citrates, malonates, succinates, lactates, oxalates,
tartarates and benzoates.
- 20 Salts may also be~ formed with bases. Such salts include salts derived
from inorganic or organic bases, for example alkali metal salts such as
sodium or potassium salts, alkaline earth metal salts such as
magnesium or calcium salts, and organic amine salts such as
morpholine, piperidine, dimethylarniae- or diethylarnine salts.
Prodrugs of compounds of formula (1) include those compounds, for
example esters, alcohols or amines,~which-are convertible, In viv~. by
metabolic means, e.g. by hydrolysist reduction, oxidation or
transesterification, to compounds o~ formula (1).
When the group R in compounds of- the invention is an esterified
carboxyl group, it may be a metabolicatly labile ester of formula -CO2Rl3
where Rl3 may be an ethyl, benzy!, phenylethyl, phenylpropyl, 1- or 2-
naphthyl, 2,4-dimethylphenyl, 4-t-butylphenyl, 2,2,2-trifluoroethyl,` 1-
(benzyloxy)benzyl, 1-(benzyloxy)ethyl, 2-methyl-1-propionyloxypropyl,
2,4,~trimethylbenzoyloxymethyl or pivaloyloxymethyl group.
~3~ ,~ PCTIGB94/00896
Wl~en the group R in compounds of formula (1) is a -P(O)(XlR7)X2R8
group it may in particular be a P(O)(OR7)0R8, e.g. a -P(O)(OH)OR8
group, or a ~P(O)(SH)OR8 or -P(O)(OH)SR~ group. Example-c of such
groups include -P(O)(OCH3)0CH3, -P(O)(OCH2CH3)0CH2CH3,
-P(O~(OH)OH, P(O)(OH)SH, -P(O)(SH)OH, -P(O)(OH)OCH3,
-P(O)(OH)SCH3,-P(O)(OH)OCH2CH3,-P(O)(OH~OPh,-P(O)(OH)SPh,
-P~O)(OH)OCH2Ph or -P(O)(OH)SCH2Ph, where Ph is a phenyl group
optionally substitued by one or more substituents R16.
In the compounds of formula (1) the group R1 may in particular be
a C1.6alkyl group such as a methyl group, an aralkyl group such as
benzyl group, an arylthioalkyl group such as a phenythiomethyl group or
a heteroarylthioalkyl group such as thienyUhiomethyl, pyridinyl~
thiomethyl or pynmidinylthiomethyl group or is especially a hydrogen
atom.
The group R2 in compounds of fo~ula (1) may be in particular an
optionally substituted C1.6alkyl, C~cycloalkyl, or C6.12aryl group.
Particular types of these groups are optionally substituted C3.6alkyl,
such as n-propyl, i-propyl, n-butyl, i-butyl, s-butyl, t-butyl, n-pentyl or i-
pentyl; cyclopentyl; cyclohexyl; phenyl; 1- or 2-naphthyl. Each of these
cycloalkyl or aryl groups may be substituted, by one, two or more
substituents R16 described above. -
- -
The groups R3 and R4 in compounds of formula (1) may each in
particular be~a -rnethyl group, or, especially, a hydrogen atom.
I
The group R5 in compounds of formula (1) may in particular be a group
-C(R9)(R10)Het-R11 where R9 and R10 are the same. Particular
.
compounds of this type are those wherein R9 and Rl is each the same
and is each an optionally substituted alkyl, alkenyl, cycloalkyl,
= _ ~
cycloalkenyl, aryl or heteroaryl group.
In another group of compounds of formula t1) the group R5 may be a
group -C(R9)(R10)Het-R~1 where Rl1 is an aliphatic, cycloaliphatic,
WO 9412~;435 PCT/GB94/00896
213g12 ~rl
13
heterocycloaliphatic, aromatic or heteroaromatic group as described
above for compounds of formula (1~;
The group X in compounds of formula (1) may be in particular an amino
5 (-NH2) or-NR17R18 group. Particular-NR17R18 groups are -NHR18
groups. Groups of this type include those where R18 is a C1.6alkyl
group, for example a methyl, ethyl, or n-propyl group, optionally
interrupted by one or more -O- or -S- atoms or -N(R12) ~e.g. -NH- or
N(CH3)-l or aminocarbonyloxy groups and optionally substituted by a
10 hydroxyl, carboxyl, carboxyalkyl, e.g. carboxymethyl, carboxamido,
amino, -NR1 7R 18, lfor example di-C1 -6alkylamino such as
dimethylamino, C1.6alkylamino such as methylamino, or C3.6 cyclic
amino such as morpholinyl, pyrrolidinyl or pyridinyll or phenyl optisnally
substituted by one, two or more Rl~ substituents.
- -
A particularly useful group of compounds according to the invention is
that of formula (1) wherein R5 is a group -C(R9)(R~0)Het-R11 where Het
is -S(O)p and R9, R10 and R11 are as defined for formula (1)
Compounds of this type wherein Het is -S- are particularly useful.
A further particularq~ useful group of compounds of formula (1 ) are those
wherein X is an amino or substituted amino group. Particularly useful
compounds of this type are those wherein X is -NHCH3 or, especially,
-NH2--
In general, in compounds of forrnula (1) the groups Rl, R3 and R4 is
each preferably a hydrogen atom. ~ ~
In a further preference, the group R in compounds according to the
30 invention is a -CONHOH or a -CO2H Qroup or a metabolically labile
ester thereof, or a group P~O)(OH)OR~. In a particular preference,
however, R is a -CO2H, -P(O)(OH)2 or,~ especially, a -CONHOH group.
, .
An especially useful group of compounds according to the invention has
35 the fonnula (1a)
WO 94/25435 PCT/GB94/00896
9~ 4
R2 H O
X
o Rs (1a)
wherein R, R2, R5 and X are as defined for forrnula tl ); and the salts,
solvatss, hydrates and prodrugs thereof.
A particularly useful group of compounds of ~orrnula (1a) are those
wherein R r~present a -CONHOH, -CQ2H or ~P(O)(OIt)2 group; p~2 and
R5 are as defined for formula ~1~; X is an amino (-NH2) or substitu~ed
amino group; and the satts, solvates, hydrates and prodrugs thereof.
Particularly useful compounds of forrnula (1a3 ars those wherein R5 is a
- group -C(R~)tRl0)S(O)pR1l. Compounds of this type in which R5 is a
-C(P~9~(R10)SR11 group are especially useful.
Other useful compounds of ~onnula ~1a) inelude those wherein R2
1~ represents a C3.~3alkyl group, partieularly an isobutyl or n-peniyl group,
or a cycloalkylC3 6alkyl ~roup, partieularly a cyelohQxylpropyl, cyclo
hea~lbutyl or cyclohexyipentyl ~roup.
In the compounds of formula (1a) X may be a -NH2 group or a group
-NR17Ftl8 as defined for compounds of formula (13, particularly a
-NHR18 group.
An especially us~ful group of compounds ac~ording to the invention has
the formul~ (1a) wherein R7 is a C3.~3alkyl group, R5 is a group
C(R9~(R10)SR~1 where R9 and Rl is each the same and is each an
optionally substituted Cl~ alkyl group, and Rll is as defined for formula
; and X is an amino ( NH2) or NHR18 group, particularly where R18 is
an optionally substituted C1.6 alkyl group. Compounds of this type
whe^roin- Q5 is a group -C(CH3)2SR11 are particularly useful, especially
where the group R11 is a hydrogen atom or an optionally substituted
saturatad Cl.6 alkyl chain. In compounds of this last type X is
preferably an amino (-NH2) group or a -NHCH3 group.
WO 94125435 213 9 ~ 2 `~ PCTIGB94/00896
In a still turther useful group of compounds of formula (1a), R is a
-CONHOH, -C02H or -P(0)(01~)2 group, R2 is an isobutyt group, R5 is a
group -C(CH3)2SR11 where R11 is a hydrogen atom or an optionally
substitued C1~ alkyl group, and X is an amino (-NH2) or -NHR18 group
5 where R18 is an optionally substituted C1 6 alkyl group Compounds of
this lype wherein R is a -CONHOH group are particularly useful; as are
those compounds wherein R11 is a hydrogen atom or a methyl group;
and~those compounds wherein R18 is a hydrogen atom or a methyl
group
One hrther group of compounds according te the invention has the
formula~ (1a) wh~ rein R and R2 are as defined~ for formula (1), Rs is a
~group~-C~CH3)25H o r -C(CH3)2SCH3 and X is -NH2 or -NHCH3
Particularly useful compounds of this type are those wherein R is a
~5 - group -CONHOH, -CO2H or -P(O)(OH)2 and R2 is a Cwalkyl group
P~articular~y useful compounds are~those wh-re R2 is an isobutyl group
. ~ -
In~compoun:ds~of~the above~described types, R5 is preferably a group
C(CH~3)2S~CH3 ~ 1n~;th-se ~compounds,~R is preferably -CO2H or
Z0 ~ P(O)(OH2) or~is ~blly -CONHOH X is prderably -NH2 or -NHCH3
*~ -impon~nt~ cornpoùnd according to th inv~nL;on is
4-(N-Hyd~nmo)-2(R~(2-methylpropyl)succinyl]-L IS-(methyl)
25 ~ ~nlbillaminel ~N-methylami~; and:. the~ salts,: solvat s, hydrates and
prodrugs~the~
me Gompounds~ ac~c~rdhg to the invention -may be ~prepared by the
following gen-ràl~ processes, more specifically described in the
3 0 ~ l~xamples hereinafter In the d scriptbn and formulae below the groups
R, R1, R2, R3, R4, R5 and X are as- defined above, except where
;otherwis indicated It will be appreciated that functional groups, such
as~ amino, hydroxyl or carb~xy~-groups, present in the various
- ~ - compounds described ~bebw, a~d ~ ch it is desired to retain, may need
: 35: to :be: in protect-d~ form ~before~ any reaction is initiated In such
instances, removal of the protecting group may be the final step in a
~':
~'
: .
?~ ~ PCTIGB94/00896
1 6
particular reaction. Suitable amino or hydroxyl protecting groups
-- include benzyl, benzyloxycarbonyl or t-butyloxycarbonyl groups. These
may be removed from a protected derivative by catalytic hydrogenation
using for example hydrogen in the presence of a metal catalyst, for
5 example palladium on a suppon such as carbon in a solvent such as an
alcohol e.g. methanol, or by treatment with trimethylsilyl iodide or
trifluoroacetic acid in an aqueous solvent. Suitable carboxyl protecting
groups include benzyl groups, which may be removed from a protected
derivative by the methods just discussed, or alkyl groups, such as a t-
10 butyl group which may be removed from a protected derivative bytreatment with trifluoroacetic acid in an aqueous solvent. Other suitable
protecting groups and methods for their use will be readily apparent.
The formation of the protected amino, hydro~l or carboxyl group may be
achieved using standard alkylation or esterification procedures, for
15 example as described bebw.
: l
Thus according to a further aspect of the invention a compound of
formulà~(1) may be prepared by coupling an acid of formula (2)
R2
R~
~1 o (2)
20 or an active derivative thereof, with an amine of formula (3)
- R3
- ' ~ ~ X
R4 (3)
followed by removal of any protecting groups.
: - .
.
~-25- Active derivatives of acids for formula (2) include for examp~e acid
;l anhydrides, or acid halides, such as acid chlorides. ~i~
. _ = .
The coupling reaction may be performed using standard conditions for
amination readions of this type. mus, for example the reaction may be
30 achieved in a solvent, for example an inert organic solv~nt such as an
I
WO 9412543~ PCT/GB94/00896
21~912~
17
ether, e.g. a cyclic ether such as tetrahydrofuran, an amide e.g. a
substituted amide such as dimethylformamide, or a halogenated
hydrocarbon such as dichloromethane at a low temperature, e.g. -30C
to ambient temperature, such as -20C to 0C, optionally in the
5 presence of a base, e.g. an organic base such as an amine, e.g.
triethylamine or a cyclic amine such as N-methylmorpholine. Where an
acid of forrnula (2) is used, the reaction may additionall~ be performed in
the presence of a condensing agent, for example a diimide such as
N,N'-dicyclohexylcarbodiimide, or 1-(3-dimethylaminopropyl)-3-ethyl-
10 car~odiimide, advantageously in the presence of a triazole such as2-hydroxybenzotriazole. Altematively, the acid may be reacted with a
chloroformate for example ethylchlorofonnate, prior to reaction with the
amine of formula (3).
15 Fr~e hydro~l or carboxy~ groups in the starting materials of formulae (2)
- lwhere R is -CONHOH or CO2Hl and (3) may need to b~ protected
- during the coupling reaction. Suitable proteoting groups and methods
for their removal may be those mentioned above. Where R in the
interrnediates ol fonnula (2) is a -P(O)(XlR7)X2R8 group, at least one of
20 P~7 or R8 is other than a hydrogen atom. Conveniently, each of R7 and
RB is an optionally substituted alkyl, aryl or aralkyl group. Such groups,
when present in compounds of the invention may be cleaved as
described below to yield other compounds of the invention wherein Q7
and~or R8 is each a hydrogen at-om.
It will be appreciated that where a particular stereoisomer of forrnula (1)
is required, this may be obtained by resolution of a mixture of isomers
following the coupling reaction of an acid of forrnula (2) and an amine of
formula (3). Conventional resolution techniques may be used, for
30 example separation of isomers by chromatography e.g. by use of high
performance liquid chrormatography. Where desired, however,
appropriate homochiral starting materials may be used in the coupling
reaction to yield a particular stereoisomer of formula (1). Thus, in
particular process a compound of formula (1a) may be preparad by
35 reaction of a compound of formula (2a)
WO 94125435 ~ PCT/GB94/00896
.
R~
o (2a)
with an amine of formula (3a)
~x
Rs (3a)
as described above
Intermediate acids of formula (2) wh~rein P~ is a carboxyl or esterified
carboxyl group or a group -P(O)(X~R7)X2R8 or-SP~8 may be prepared
10 from a corrsspanding ester of forrnula (4)
f~2
R~
- 11
R1 0 ~4)
where Rl9 is an alkyl group, for example a meth~ or t-buty~ group, using
for example tritluoroace~ic acid, or, when Rlg is an aralkyl group, such
- as a benzyl group, by hyqrogenolysis~ for example by r~action with
15 hydrogen in the presence of a metai catalyst, e.g. palladium, on a
support such as carbon in a solv~nt such as an alcohol, e.g. methanol
optionally at an eleYated pressllre and temperature. ~
.
Ar ester of fonnula (4) whQre R is a carboxyl or esterified carboxyl group
20 may be preparad by es~erification ot the corresponding~ acid of formula
(5)
R - (S)
~8~
WO 94/2~435 PCT/GB94/00896
2139~2.i,
1 9
using an appropriate acyl halide, for example an acyl chloride in a
solvent such as ~n alcohol, e.g. methanol at a low temp~rature, e.g.
around OC.
5 Acids of fsrmula (5) may be prepared by alkylation of a compound of
~orrnula (6)
O~ 12a~
Q1 0 (6)
with an appropriate halide, e.g. a compound R2Hal, where Hal is a
halogen atom such as a chlorine or bromine atom in the pres~nce of a
10 base, for example an alkoxide su~h as sodium ethoxide in a solve~t
such as an alcohol, e.g. ethanol at ambient temperature, followed by
decarboxylation using for example concentrated hydrochloric acid at an
elevated temperature,e.g. the reflux temperature.
15 Int~rrnediates of farmuia (6) are either known ccmpounds or may be
prepared by rnethods analogous to those used for the pr0paration of the
known compounds.
Intermediate esters of formula (4) where P( is a -P(O)(XlR7)X2R~ group
2û may be prepared by reaction ot an acrylate RlCHC(R2)CORl9 with a
phosphite:P(OR20~(X1R7)X2P48 lwhere R20 is a leaving group, for
ex~mple a sily~ group such as a trialkylsilyl group e.g. a trimethylsilyl
group~ at an elevated temperature. - -
25 Ac~ylatee of ~ormula RlCHC(R2)CORl9 may be prepared ~y reaction of a
mono-ester HOOCCH(R2)COOR19 with an aldehyde R1CHO or a
polymer thereof e.g. paraformaldehyde or paraldehyde in the presence
of a base, for example an organic base such as piperidine. The reaction
may be performed in a solvent, such as pyridine, optionally at an
30 elevated tempe~ature.
Mono-esters of formula HOOCCH(R2)COOR19 may b~ pr~par3d by
WO 94/25435 PCT/GB94/û0896
9~'~`3
hydrolysis of the corresponding di-ester R19OOCCH~R2)COOR19 using
a base, for example an alkali hydroxide, in an inert solvent such as
dioxane at a low temperature e.g. around 0C. The di-esters for use in
this reaction may be prepared by alkylation of the corresponding
5 malonates of formula R19OOCCH2COOR19 with a halide R2Hal [where
Hal is a halogen atom such as a chlorine or bromine atoml in the
presence of a base; e.g. a hydride such as sodium hydride in a solvent
such as tetrahydrofuran at ambient temperature. Malonates of formula
R19OOCCH2COOR19 are either known compounds or may be prepared
10 by methods analogous to those used for the preparation of the known
compounds.
i
Intermediate phosphites of formula P(OR20)(X1R7)X2R8 may be
prepared by reaction of a phosphite HP(O)(X1R7)X2R8 with an
15 appropriate amine (R2)2NH e.g. a silazane, at an elevated temperature,
e.g. the reflux temperature. Phosphites of formula HP(O)(X1R7)X2R8 are
either known compounds or may be prepared by methods analogous to
those used for the preparation of the known compounds.
20 Intermediates o f formula (4) where R is a -SR~ group are either knom
compounds or may be prepared from known starting materials of
formula R~SCH(R1)CH(CO2CH2CH3)2 by using a similar series of
reactions to those just described for the preparation of compounds of
- formula (4) whero R is a carboxyl group.
In another process, intermediate acids of formula (2) wherein R is a
-P(O)(X1R7)X2R8 group may be prepared by re ction-:of-an acid
- ~ R2CH2CO2H with a phosphonate P(O)(X1R7)(X2R8)CH2OR21 ~where
~21 iS a leaYing group, for example a trifluoromethylsulphonyloxy group
30 in the presence of a base such as n-butyllithium in a solvent such as
tetrahydrofuran. Phosphonates fo~ use in this reaction rnay be prepared
from the corresponding compound P(O)(X1R7)(X2R8)CH2oH by reaction
with paraformaldehyde in the presence of a base such-~s- triethylamine
at an elevated temperature followed by reaction with a halidè R21Hal in
35 the presence of a base such as sodium hydride in a so~ent such as an
sth~r. Phosphonatos P(O)(X1R7)(X2R8)CH20H and acids R2CH2CO2H
WO 94/25435 213 ~J 1 2 '~ PCT/GB94/00896
for use in the above reactions are either known compounds or may be
prepared by methods analogous to those used for the preparation of the
known compounds.
Interrnediate acids of fonnula (2) wherein R is a -CONHOR6 group or a
protected derivative thereof may be prepared by reaction of an
anhydride of formula (7)
R2~ l~
,1 ~
o (7)
with a hydroxylamine swch as O-benzylhydroxylamine or NH20R6
10 where R6 is an acyl group in a solvent such as tetrahydroturan at a low
temperature, e.g. around -20C, followed where desired by removal ot
the protecting group as described above.
~; The intermediate anhydrides of formula (7) may be prepared for
example~by heating for example at the reflux temperature, a diacid of
formula (5) where R is -CO2H with an acyl chloride such as acetyl
chloride.
.
The homochiral~ acids of formyla 12a) may be prepared according to
another feature of the invention by oxidation of an oxazolidinone of - -
~; formula (8)
:- ~ R2
R~ N~ ~0
- 1 0
- Ph (8)
(where Ph is a phenyl group)
~' ` -1,
- 25 using an oxidising agent such as peroxide, e.g. hydrogen peroxWe in a ~-~~
solvent such as an ether e.g.~ a cyclic ether such as tetrahydrofuran, a~ a
low temperature, e.g. around 0C followed by treatment with a base,
such as lithium hydroxide, at an elevated temperature.
WO 9~/25435 ~ PCT/GB94/00896
'l.~ 3~
22
The compounds of forrnula (8) may be prepared by reaction of an acyl
halide RCH2CH(R2)COHal (where Hal is a halogen atom such as a
chlorine, bromine or iodine atom) with a solution of (S)-4-(phenyl-
5 methyl)-2-oxazolidinone in the presence of a base such as n-butyl-
lithium in a solvent such as tetrahydrofuran at a low temperature. e.g.
around -78C.
Acyl halides PsCH2CH(R2)COHal may be prepared by treatment of the
10 corresponding known acids RCH2CH(R2)CO2H with conventional
halogenating agents for example thionyl halides under standard
reaction conditions.
Intermediates of formula (3) are either known compounds or may be
15 prepared from known amino acid starting materials using standard
methods, for example by employing a séries of substitution reactions to
manipulate the groups R5 and X as described in the Examples
hereinafter, or for example as described by Wessjohann .~L Chem.
Ber. 1992, ~2~, 867~882.
Compounds of formula (1) may also be prepared by interconversion of
other compounds of formula (1). Thus, for example, a compound of
formula (1) wherein R is a -CONHOR6 group may be prepared by
-reaction of a corresponding acid of forrnula (1) wherein R is ~ -CO2H
25 group or an active derivate thereof (for example an acid chloride or an
acid anhydride) with hydroxylamine or an O-protected derivative or a
salt thereof or a reagent R60NH2 where P/~ is an acyl group.- The
reaction may be performed using the reagents and conditions described
above in the preparation of compounds ot fomlula (1) from the starting
30 materials of fonnulae (2) and (3).
In another interconversion process, compounds of fomluia (1) wherein R
is -C02H andlor X contains a -CO2H group may-b~ prepared by
hydrolysis of the corresponding esterified compounds~(for example
35 where R is a-C02R13group and/or X contains a similar group) using
conventional procedures, for example by treatment with a base, e.g. an
WO 94/25435 21 3 912 3 PCT/GB94/00896
23
alkali metal hydroxide such as lithium hydroxide in a solvent such as an
aqueous alcohol, e.g. aqueous methanol, or by treatment with an acid
such as a mineral acid, e.g. hydrochloric acid in the presence of a
solvent, e.g. dioxane.
Similarly esters of formula (1), for example where R is a CO2P~13 group
andlor X contains a -CO2R13 group may be prepared by reaction of the
corresponding acids, where R is a -CO2H group and/or X contains a
-CO2W group or an active derivative thereof, with an alcohol R13OH
10 using standard conditions.
In another interconversion process, a compound of fonnula (1) wherein
R5 is a group -C(R9)(R10)S-R11 may be oxidised to a corresponding
compound where F15 iS a group -C(R9)(Rl0)SOR11 or
15 -C(R9)(R10)SO2R11 using an oxidising agent, for example a
peroxymonosulphate such as potassium peroxymonosulphate, in a
solvent such as an aqueous alcohol at ambient temperature or a
pero~yacid in a halogenated hydrocarbon solvent such as
dichloromethane at a low temperature, e.g. around -78G.
The compounds according to the invention are potent inhibitors of the
metalloprote~inases collagenase, stromelysin and gelatinase and
advantageously~have~ ~a long duration of action when administered
ora!ly.; The activity of the com-pounds may be detemlined by the use of
25 appropriat- enz~me inhibi~ion tests for example as described in
Exar:nple~ A~hereinafter or by oral administration to mice as described
hereinafter in~Exarinple B. In our tests using this approach, compounds
according to the invention have been shown to inhibit stromelysin, and,
!in particular, collagenase and gelatinase with Ki values in the
30 nanomolar range.
The compounds according to the invention can be expected to be of use
in the prophylaxis or treatment of diseases or disorders in which
- stromelysin, collagenase and gelatinase have a role. Thus for example
35 the compounds of formula (1) may be of use in the prophylaxis or
treatment of musculo-skeletal disorders, for example arthritic diseases
.
WO 94/25435 ~;,9 24 PCT/G119-100896
such as rheumatoid arthritis, osteoarthritis and septic arthritis, and to be
of usa to prevent tumour cell metastasis and inv~sion. The compounds
may therefore be o~ use in the treatment of cancer, partieularly in
conjunction with radiotherapy, chemotherapy or surgery, or in patients
5 presenting with primary tumours, to control the development of tumour
metastasis. Particular caneers may inelude breast, melanoma, iung,
head, neck or bladder caneers. Other uses to whieh the compounds of
the invention may be put, include use for prevention of myelin
degradation in the central and peripheral nervous system, for exampl~
10 in the treatment of multiple sclerosis, use for eontrolling peridontal
diseases sueh as gingivitis, and use in tissue remodelling.
The eompounds aeeording to the invention ean also be expeeted to be
of use in the prophylaxis or treatment of angiogenic diseases. Sueh
15 diseases may be characterised by the pathologieal growth or new
eapillaries ~see, for-example Folkman, J. and Klagsbrun, M. Scienee
~, 442-447 (1987) and Moses, M. ~ and Langer, R. Biorrechnology ~,
630-634 (1991~1. Particular angiogenesis dependent diseases include
solid tumours and arthritic diseases as described above, and,
20 additionally, psoriasis, eye diseases sueh as the proliferative
reinopathies, neovascular glaucome and ocular tumours,
angiofibromas, and hemangiomas.
- For use in the above applieations, the eompounds of formula (1) may be
25 formulated in a eonventional manner, optionally with one or more
physiologically aeeeptable earriers, diluents or excipients.
Thus according to a further aspeet of the invention-we provide a
pharmaeeutieal composition comprising a eompound of formula (1) and
30 a pharmaeeutieally aeceptable diluent, earrier or exeipient.
In a still further aspeet the invention provides a proeess for the
produetion of a pharmaeeutieal eomposition eom~rising bringing a
eompound of formula (1) into assoeiaiion with a pharmaeeutieally
35 aeeeptable diluent, earrier or exeipient.
WO 94125435 2 1 ~ 9 1 2 ~. PCTIGB94 00896
Compounds for use according to the present invention may be
forrnulated for oral, bucczl, parental or rectal administration or in a forrn
suitable for nasal administration or administration by inhalation or
insufflation.
For oral administra~ion, the pharmaceutical compositions may take the
form of, for example, tablets or capsules prepared by conventional
means with pharmaceutically acceptable excipients such as binding
agents (e.g. pregelatinised maize starch, polyvinylpyrrolidone or
10 hydroxypropl methylcel~lulose); fillers (e.g. Iactose, microcrystalline
cellulose or calcium hydrogen phosphate); lubricants (e.g. magnesium
stearate, talc or silica); disintegrants (e.g. potato starch or sodium
glycollate); or wetting agents (e.g. sodium lauryl sulphate). The tablets
may be coated by methods well known in the art. Uquid preparations for
15 oral administration may take the form~ of, for example, solutions, syrups
or suspensions, or they may be presented as a dry product for
constitution with water or other suitable vehicle before use. Such liquid
preparations may be prepared by conventional means with
pharmaceutically acceptable additives such as suspending agents,
20 emulsifying~agents, non-aqueous vehicles; and preservatives. The
prepara~ions may also contain buffer salts, flavouring, colouring and
sweetening agents as appropriate.
Preparations for oral administration may be suitably fomnulated to give
25 controlled r-base of the active compound.
For buccal administration the compositions may take the form of tablets
or lozen~es forrnulated in conventional manner.
30 The compounds of formula (1 ) may be formulated for parental
administration by injection e.g. by bolus injection or continuous infusion.
Formulations for injection may be presented in unit dosage forrn. The
compositions for injection may take such forms as suspensions,
solutions or emulsions in oil~ or aqueous vehicles, and may contain
35 formulatory agents such as suspending, stabilising and/or dispersing
agents. Altematively, the active ingredient may be in powder form for
WO 94/Z543~i ~3 9 ~? 3 PCTIGB94/00896
26
constitution with a suitable vehicle, e.g. sterile pyrogen-~ree water,
before use.
The compounds of formula ~1) may also be formulated in rectal
compositions such as suppositories or reten~ion enemas, e.g.
containing conventional suppository bases such as cocoa butter or other
glycerides.
In addition to the formulations described above the compounds of
formula (1) may also be formulated as a depot preparation. Such long
acting formulations may be administered by implantation or by
intramuscular inj~ction.
For nasal administration or administration by inhalation the compounds
1~ for use according to the present invention are conventiently delwered in
the form of an aerosol spray presentation for pressurised packs or a
nebuliser, with the use of suitable propellant, e.g. dichloro
difluoramethane, trichlorofluoromethane, dichlorotetrafluoroethane,
carbon dioxide or other sultable gas or mixture of gases.
The compositions may, if desired, be presented in a pack or dispenser
device which may contain one or more unit dosage forms containing the
active ingredient. The pack or dispenser device may be accompanied
- by instructions for administration.
f
The doses of compounds of formula (1) used in the above app!ications
wili vary depending on the disease or disorder and condition of the
patient to be troated but in general may be in the range~around 0.5mg to
100mgJkg body weight, particularly from about 1mg to 50mg/kg body
weight. Dosage units may be varied according to the route of
administration of the compound in accordance wit~ conventional
practice. - ~
_
DESCRIPTIONS OF SPECIFIC EMBODIME~I~
The invention is further illustrated in the following noFl-lirniting Examples.
WO 94/t~435 PCTIGB94/00896
21391~9
27
In the Examples, the following abbreviations are used:
~r - room temperature
DMF - dimethylformamide
THF - tetrahydrofuran
TFA - trifluoroacetic acid
EXAMPI.E~
10 Th~ activity of the compounds of the invention may be deterrnined as
described balow.
~11 enzyrne assays to determine Ki values were perforrned using the
peptide substra~e Dnp~Pr~Leu-Gly-Leu-Trp-Ala-D-Arg-NH2. [M. Sharon
15 Stock and Rob0rt D. Gray. JBC ~ 4277-81, 1989l. The enzyrnes
cleave ~t tha Gly-Leu bond which can be followed Sluorirnetrically by
measuring ~he increase in Trp fluorescsnce emission assvciated with
the removal of the quenching dinitrophenol ~Dnp) group.
20 Essentially, enzyrne (e.g. gelatinase, stromelysin, collagenase) at 0.08-
2nM; a range of inhibitor concentrations (0.1-50 x Ki) and substrate
(approx. 2011m~ are incubated ovemight in 0.1M Tris/HCI buffer, pH 7.~,
containing û.1M NaCI, 10mM CaCi2 and 0.05%. Brij 35 at either roorn
tempefature or 37C dependlng on the enzyme. ~he reaction is
25 stopped by adjusting the pH to 4 using 0.1M sodium acetate buffer and
the fluorescence read at an excitation wave~ength of 2~0nm and
emission wavelength of 346nm.
Kj val~Jes can be established using the equation for tight-binding
30 inhibition:-
Vj = vO /
~ ,pp) + [llz + 2 (~ ID~ + ~ (4pr lll - Iq)
where V0 is the initiai rate of reaction in the absence of inhibitor, Vj is ~he
WO 94/25435 PCTIGB94/00896
~ 9~ 28
initial rate in the presence of inhibitor, EE] is the total enzyme
concentration and ~I] the total~ inhibitor concentration in the reaction
mixture.
5 For stromelysin and collagenase, Kj (app) was assumed to approximate
to the true Kj as lS~ ~ Km for the substrate hydrolysis. For gelatinase
the Kj was determined by performing the analyses at several substrate
concentrations. A plot of Ki(app) ~/s. [S3 then gave the true Kj as the
value of the y-axis intercept.
The following results were obtained with the compound of Example 1c):
Kl(nUI)
Collageo~e Stromelysin-1 Ge6~tinas~72kD
2.9 90.0 1.55
EXAMPLE B
The oral activity of the compounds according to the imention may be
deterrnined using the mouse pleural cavity assay described below. ~his
assay measures the ability of compounds of the invention when
20 administered orally to inhibit a subsequent inoculation of ~elatinase into
the mouse pbural cavity.
A 2ml solution of the test compound (for example around 25~1m/kg) in an
appropriate sohent (e.g. 50% polyethylene glycol (PG~plus a variable
25 proportion of dimethyl sulphoxide (DMSO) (~ required) is`administered
orally. After an interval of up to 24 hrs, 0.4ml of a mixture of an equal
- volume (2.2ml) of the enzyme gelatinase A (72K foml at~a-c~centration
of 20nM) and radiolabelled [1 4C]~gelatin (at an approximate
concentration of 1011M i.e. 500 times molar excess) is injected into the
30 pleural cavity and maintained at 4C. A~ter 35 min mice are overdosed
with anaesthetic, the contents of the pleural cavity aspirated and the
aspirates cleared by centrifugation at 4C then diluted to t5% in
trichloroacetic acid (TCA) and lett ovemight at 4C. Tfie resuiting TCA
precipitate is then separated by centrifugation and radioactivity in each
35 supernatant measured by scintillation counting. Results are exprsssed
as a % inhibition of enzyme activity calculated by comparing the
wo s4ns43s PCTIGB94/00896
21391 2~
radioactivity measured for each test compound with a control value
obtained by performing the same assay in the absence of a gelatinase
inhibitor.
5 The ability of compounds of the invention to prevent tumour cell invasion
may be demonstrated in a standard, mouse model. Thus, briefly, nude
mice may be inoculated with a tumour cell line showing gelatinase -
dependent invasion and ~the ~ability of compounds according to the
invention to reduce subsequent lung tumour colonisation may be
10 evaluated in~ accordance wi~h standard~ procedures. In out tests,
compounds~ according to the invention, when administered orally in a
singb;,dose~at~ 10~kg to mice in the; above model have reduced lung
tumour~ cobnisa,tion~ to~ negligable levels ~for periods of twelve hours
duration or longer.
I n g éneral, ~comp~unds~ accolding to the inventbn are non-to~ac at
; pharmaceùtically u~ul~ doses. Thus, for example, when the I
~re;~admini~é~to mice at~the d~s d~ri~ above no
i~nvl-l~D_~
~mmol) in 10% wh aqueous
sodium~arbonàt,e ~olutbn~ was a~ d~-tert-butyl dicarbonate
',~':25~61mmol)~in~te~Pnol~(300inl).~Afterstirring~thereaction --
mi~re tor'-18 hr~at ~ `the;vo~m~ reduced by appro~amately one
half *~ rKi~t p~re an~t~the~ptl was~adjusted to 2 using 1N
H~ hydr~lo~ d.~ The~result~gs~urlywas~e~ra ed~seyeraltimeswith
die~yl~ether,~;the;` l~ rs~beinq combined,~d~ (MgS04) and
30 evaporated~to'give the-title com~ound (36.79) as actear gum- ~H
(CDCb) 8.65 (1;H, ~br s); S.50 (1H, d), 4.35 (1 H, d); 2.00 (1 H, br s); 1.60
(3H, s): 1.50 (9H, s); 1.45~ (3H, s).
~, ,
35 ~ INI E_~
t-i~ ~oll-c-rbonvl-L-D-nlolll-mlne~N~methv~'amlde
A solution in anhydrous~ DMF (250ml) of Intermediate 1 (11.699;
WO 94/25435 PCT/GB94/00896
~ ~ 9 ~ 30
33.5mmol), N-hydroxybenzotriazole (4.539; 33.5mmol), methylamine
hydrochloride (11.39; 167.5 mmol); N-methylmorpholine (20.6ml;
1 84mmol), 1 -(3-dimethylaminopropyl)-3-ethyl carbodiimide hydro-
chloride (7.19; 36.9mmol) and a trace of 4-dimethyiaminopyridine was
5 stirred at RT under an atmosphere of nitrogen for 18 hr. The reaction
mixture was poured into 10% w/v aq.citric acid (600ml) and extracted
into diethyl ether (600ml). The organic layer was s6parated, washed
with 10% w/v aq. NaHCO3 solution (500ml), dried (MgSO4) and
evaporated. Following chromatography on silica, eluting with 20-50%
10 ethyl acetate in hexane, the title comDolmd was obtained as a clear
glass (6.369). ~H (CDCI3) 6.~5 (1H, m); 5.75 (lH, d); 2.75 (3H, d); 2.50
(1 H, br s); 1.50 (3H, s); 1.45 (9H, s); 1.35 (3H, s).
INTERMEDIATE 3
15 N-tert-butvloxvcarbonvl~ S-(methyl)~enlclllamlne1-N-
methvlamld~
To a solution of Inte~ediate 2 (19; 3.82mmol) in 2N aq. NaOH/CH30H
(lOmll30ml)was added iodomethane (1.18ml; 19mmol) in CH30H
(4ml). After stirring at RT for 2 hr, the reaction mixture was concentrated
20 to one quarter volume, then partitioned between diethyl ether and brine.
The organic la~er was washed with 10% w/v aq. citric acid, dried
(MgSO4) and evaporated to give the title co~Qound (810mg) as a
colourless glass. ~H (CDCI3) 6.80 (1H, m); 5.65 (1H, d); 4.20 (1H, d);
- 2.80 (3H, s); 2.10 ~3H, s); 1.45 (9H, s); 1.40 (3H, s); t.30 ~3H, s).
- INTERMEDIATE 4
S~(M-thvl)D~nlcll!amlneJ-N~ thylamlde trl~oroa6et~te
A solution ot Intermediate 3 (810mg; 2.93mmol) in TFA/dichtoromethane
(10mU10ml) was stirred at RT for 2 hr. The solvent was then removed
30 under reduced pressure with the aid of a toluene/THF azeotrope. The
title comDound (855mg) was obtained as a ye!!ow tinged glass in
quantitative yield. ~H (CDCI3) 8.4 (3H, br s); 7.9 (1H, q); 4.20 (1 H, s);
2.80 (3H, d); 2.0 (3H, s); 1.45 (3H, s); 1.35 (3H, s~
35 EXAMPLE~ 1
a) [4-t-Butoxy-2(R) 3-(2-methvlprQeyl)succinvll L-~S
WO 94/25435 21 3 ~1 2 PCT/GB94100896
31
(methyl!~enicilLamioel-N-methyl~m~de
A solution in anhydrous DMF (30ml) of 2-~R)-(2-
methylpropy~)succinic acid~4-t-butyl monoester [2.9mmol;
prepared from t-butylbromoacetate, BuLi and (S)-4-
(phenylmethyl)-2-oxazolidinone according to the procedure of
Intermediate 4 in W093/24475], Intermediate 4 (2.93mmol), N-
hydroxybenzotriazole (2.93mmol); N~methylmorpholine
(8.79mmol~ (3-di-methylaminopropyl)~3-ethyl carbodiimide
hydrochloride (3. t 9 mmol) and a trace amount of 4-
dimeth~laminopyridine was stirred at RT under an atrnosphere of
nitrogen ~or 18 hr. The reaction mixture was poured into 10% w/v
aq.citric acid (100ml) and extracted into diethyl ether (100ml).
The organic layer was washed with 10% wlv aq. NaHCO3,
separated, dried (MgSO4) and evaporated. The residue was
chrornatographed on silica, eluting with 2-4% CH30H in CH2CI2,
to give the titie comDound.
b) l4-Hygrox~-2(R)-3-(2-methylQro~vll~u~ç~ -L-~
(methvl)~enlclllamlnel-N methylaml~
A solution of the compound of Example 1a (0.933mmol) in a
mixture of TFA (10ml) and wat~r (0.5ml) was lèft to stand at 4C
for 18 hr. The solvent was evaporated with the aid of a
toluenelTHF azeotrope to obtain ~he ~l~omQ~nd.
c) ~4-(N-Hvdroxv~ nQ~ 2(R~-3-(2 meth~lptQ~yl1~ clnvl1-
L~rs~(methyl)Den~ el~N~m~hv~ d~
To a solution in anhydrous THF of the compound of Example 1b
(0.933 mmol) at -20C was added N-methylmorpholine (1.87
mmol), and ethyl chloroformate (1.12 mmol). After 1 hr, 0-tri-
methylsilylhydroxylamine (3.75mmol) was added and the reaction
mixture was allowed to warm to RT ovemight. The solvent was
evaporated under reduced pressure and the residue was
partitioned between ethyl acetate and 10% w/v aq. citric acid
The organic layer was separated, dried (MgSO4) and evaporated
The residue was purified (SiO2; 5-10% CH30H in CH2C12) to give
the ~ H (CD30D) 4.50 (tH, s); 2.95 (lH, m);
WO 94/25435 PCT/GB94/00896
? ~39~q*~ 32
2.7~ (3H, s); 2.40 (1H, dd); 2.15 (1H, dd); 1.50 (2H, m); 1.40 (3H;
s); 1.35 ~3H, s); 1.20 (1H, m); 0.90 (6H, 2d). -=
,
_ .
,