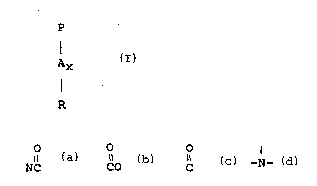Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 94/~2580 2 1 ~ 2 8 3 PCI/US93tO6223
~IRFACTANT-CO~AINING DYE T~ FE~
IBITING COMPO8ITION~3
Field of the_Invention
The present invention relates to a composition and a process
for inhibiting dye transfer between fabrics during washing.
More in particular, this invention relates to dye transfer
inhibiting comp~sitions comprising polyamine N-oxide containing
polymers and surfactants.
Backaround of the Invention
Detergent compositions useful for cleaning purposes, such as
laundering of fabrics, have commonly utilized a variety of
surfactants.
The ability of deter~ent compositions to clean a large
variety of soils and stains from other fabrics present in the
typical load of laundry is of high importance in the evaluation
of detergent performance. Each surfactant has both strenghts
and weaknesses.
SU~TUTE SHEEr
W094/02580 ,~40~3 2 PcTJus93/o6223,
Consequently, detergent compositions are formulated with more
than one surfactant active in order to maximize advantages and t
minimize disadvantages.
The relative ability of each surfactant to meet various
performance criteria is among others depending an the presence
of adjunct detergent ingredients.
One of the types of adjunct detergent ingredients that is
added to detergent compositions are dye transfer inhibiting
polymers.
Said polymers are added to detergent compositions in order to
-inhibit the transfer of dyes from colored fabrics onto other
fabrics ~washed therewith. These polymers have the ability to
complex or adsorb the fugitive dyes washed out of dyed fabrics
before the dyes have the opportunity to become attached to
other articles in the wash.
Polymers have been used within detergent compositions to
inhîbit~ dye~transfer. Copending European~Patent Application N
92202168.8~ describes polyamine~ N-oxide containing polymers
which~are very efficient~in e1iminating transfer of solubilized
or~s`uspended dyeæ~
It;~has~now been;found that polyàmine N-oxide containing
polymers~ are very compatible with surfactant systems. In
addition, it has been found that the dye trahsfer inhibiting
performan~ce ~has been increased in the presence of certain
surfactants. ~ ~
This~ finding~a11Ows us to formulate detergent compositions
which~have~both~ excel~lent dye transfer inhibiting properties
and~oYera11~detergency~performance.
- ~According to another embodiment of this invention a process
is also provided for laundèring operations involving colored
fabrics.
Summarv of the Invention ; ~J
-~
The present invention relates to inhibiting dye transfer
compositions comprising a polymer selected from polyamine N-
oxide containing polymers which contain units having the
following structure formula (I) :
i, -~
.- .
~094/02580 2 ~ 8 3 ` PCT/US93/06223
AX ~I)
I
R
wherein P is a polymerisable unit, whereto the N-O group can be
attached to or wherein the N-O group forms part of the
polymerisable unit or a combination of bo~h.
O O
Il \~ 11 1
A is NC, CO, C, -O-, -S-, -N- ; x is O or 1;
R are aliphatic, ethoxylated aliphatics, aromatic,
heterocyclic or alicyclic groups or any com~ination
thereof whereto the nitrogen of the N-O group can be
attached or wherein the nitrogen of the N-O group form
part of these groups
and a surfactant system.
Detailed descri~tion of the_invention
The compositions of the present invention comprise as an
essential element polyamine~N-oxide containing polymers which
contain units having the following structure formula :
Ax
R
wherein P is a polymerisable unit, whereto the R-N-O group can
be attached to or wherein the R-N-O group forms part of
the polymerisable unit or a combination of both.
O O' O
A is NC, CO, C, -O~,-S-, -N- ; x is O or l;
SUBSlTrllTE SHEET
W094/~2s80 2~ 4 PCT/VS93/06223~
R are aliphatic, ethoxylated aliphatics, aromatic,
heterocyclic or alicyclic groups or any combination
thereof whereto the nitrogen of the N-O group can be
attached or wherein the nitrogen of the N-O group is
part of these groups.
The N-O group can be represented by the following general
structures :
O O
(Rl)x -N- (R2)y =N- (Rl)x
(R3)z
wherein Rl, R2, R3 are aliphatic groups, aroma_ic, heterocyclic
or alicyclic groups or combinations thereof, x or/and y
or/and z is O or 1 and wherein the nitrogen of the N-O
group can be attached or wherein the nitrogen of the N-
O group forms part of these groups.
The N-O group can be part of the polymerisable unit (P) or
can be attached to the polymeric backbone or a combination of
both.
5uitable polyamine N-oxides wherein the N-O group forms part
of the polymerisable unit comprise polyamine N-oxides wherein
R is selected from aliphatic, aromatic, alicyclic or
heterocyclic groups.
One class of said polyamine N-oxides comprises the group of
polyamine N-oxides wherein the nitrogen of the N-O group forms
part of the R-group. Preferred polyamine N-oxides are those
wherein R is a heterocyclic group such as pyridine, pyrrole,
imidazole, pyrrolidine, piperidine, quinoline, acridine and
derivatives thereof.
Another class of said polyamine N-oxides comprises the group
of polyamine N-oxides wherein the nitrogen o~ the N-O group is
attached to the R-group.
: -
- ~: suBsmuTE SHEEl~
. ~ .
214~283
~VO 94/02580 5 PC~r/US93/06~23
Other suitable polyamine N-oxides are the polyamine oxides
whereto the N-O group is attached to the polymerisable unit.
Preferred class of these polyamine N-oxides are the polyamine
N-oxides having the general formula (I) wherein R is an
aromatic, heterocyclic or alicyclic group~ wherein the nitrogen
of the N-0 functional group is part of said R group.
- Examples of these classes are polyamine oxides wherein R is a
heterocyclic compound such as pyridine, pyrrole, imidazole and
derivatives thereof.
Another preferred class of polyamine N-oxides are the poIyamine
oxides having the general formula (I) wherein R are aromatic,
heterocyclic or alicyclic groups wherein the nitrogen of the N-
0 functional group is attached to said R groups.
Examples of these classes are polyamine oxides wherein R groups
can be aromatic such as phenyl.
Any polymer backbone can be used as long as the amine oxide
polymer formed is water-soluble and has dye transfer inhibiting
properties. Examples of suitable polymeric backbones are
polyvinyls, polyalkylenes, polyesters, polyethers, polyamide,
polyimides, polyacrylates and mixtures thereof.
The amine N-oxide polymers of the present invention typically
have a ratio of amine to the amine N-oxide of 10:1 to
1:1000000. However the amount of amine oxide groups present in
the polyamine N-oxide containing polymer can be varied by
appropriate copolymerization or by appropriate degree of N-
oxidation. Preferably, the ratio of amine to amine N-oxide is
from 2:3 to 1:1000000. More preferably from 1:4 to 1:1000000,
most preferably from 1:7 to 1:1000000. The polymers of the
present invention actually encompass random or block copolymers
where one monomer type is an amine N-oxide and the other
monomer type is either an amine N-oxide or not. The amine oxide
unit of the polyamine N-oxides has a PKa < 10, preferably PKa <
7, more preferred PKa < 6.
The polyamine N-oxide containing polymers can be obtained in
almost any degree of polymerisation. The degree of
SlJBSmUTE SHEET
W094/0~80 2 ~ 40 28 V 6 PCT/US93/06223 r ,
'
polymerisation is not critical provided the material has the
desired water-solubility and dye-suspending power.
Typically, the average molecular weight of the polyamine N-
oxide containing polymers is within the range of S00 to
.
1000,000; preferably from 1,000 to,50,000, more preferably from
2,000 to 30,000, most preferably from 3,000 to 20,000.
The polyamine N-oxide containing polymers of the present
invention are typically present from 0.001% to 10% , more
preferably from 0.01% to 2%, most preferred from 0.05% to 1% by
weight of the dye transfer inhibiting composition.
The~;present compositions are conveniently used as additives to
co m entional detergent compositions for use in laundry
operations. The present învention also en~ompasses dye transfer
inhibiting compositions which will contain detergent
ingredients and thus serve as detergent compositions. ~;
Méthods Tor~ma~inq Dolvamlne N-oxides :
The production~of~ the polyamine N-oxide contaihing polymers
may~ be~accomplished by~polymerizing the amine monomer and
oxidizing~ the~-resultant~;polymer with a ~suitable oxidizing
agent,~ or~the~'amine oxide~-monomer may itself be polymerized to
obtain the~ polyàmine N-oxide. ~,-,
The synthesis of polyaminè~N-oxide ~containing polymers can be
èxemplified~by~the~synthesis~of~polyvinyl-pyridine N-oxide.
Poly-4-vinylpyridine ~ex~ Polysciences (mw. S0 000, S.0 g.,
0.0475`mole~ was~predisolved~in~ 50~ml~àcetic acid and treated
with a peracetic acid~so'lution (25 g~of glacial acetic acid,
6~.4~g of a 30% vol. solution of H202, and a few drops of H2504
give 0.0523 mols of peracetic acid) via a pipette. The mixture
was~stirred over~30 minutes at ambient temperature (32 C). The
mixture was then heated to 80-85 C using an oil bath for 3 ~,'
-hours before allowing to stand overnight. The polymer solution
then;obtained is~;mixed with 11 of acetone under agitation. The
resulting ~yellow brown viscous syrup formed on the bottom is
washed ~again with 11 of aceton to yield a pale crystalline
.. , ~
,~"~ solid.
, . - " ~
~. ?~
SUBSTITUTE SHEET
21~02~3
W O 94/02580 7 P~r/US93/06223
The solid was filtered off by gravity, washed with acetone and
then dried over P205.
The amine : amine N-oxide ratio of this polymer is 1:4
(determined by NMR).
SURFACTANT SYSTEM :
The compositions according to the present invention comprise
in addition to the polyamine-N-oxide containing polymers a
surfactant system wherein the surfactant can be selected from
nonionic and/or anionic and/or cationic and/or ampholytic
and/or zwitterionic and/or semi-polar surfactants.
Preferred surfactant systems to be used accarding to the
present invention comprise as a surfactant one or more of the
nonionic surfactants described herein. These nonionic
~- surfactants have found to be very useful in that the dye
transfer inhibiting performance of the polyamine N-oxide
containing polymers has been increased in the presence of said
surfactants.
NONIONIC8 :
Polyethylene, polypropylene, and polybutylene oxide
condensates of alkyl phenols are suitable for use as the
nonionic surfactant of the surfactant systems of the present
invention, with the polyethylene oxide condensates being
preferred. These compounds include the condensation products of
alkyl~phenols having an alkyl group containing from about 6 to
about 14 carbon atoms, preferably from about 8 to about 14
carbon atoms, in either a straight-chain or branched-chain
configuration with the alkylene oxide. In a preferred
embodiment, the ethylene oxide is present in an amount equal to
from about 5 to about 25 moles, more preferably from about 3 to
about 15 moles, of ethylene oxide per mole of alkyl phenol.
Commercially available nonionic surfactants of this type
include IgepalTM C0-630, marketed by the GAF Corporation: and
SUBSmUTE SHEET
094/02~80 2 ~ 4i~ia3 8 PiCT/US93/06223,
TritonTM X-45, X-114, X-100 and X-102, all marketed by the Rohm
& Haas Company. These surfactants are commonly referred to as
alkylphenol alkoxylates (e.g., alkyl phenol ethoxylates).
The condensation products of primary and secondary aliphatic
alcohols with from about 1 to about 25 moles of ethylene oxide
are suitable for use as the nonionic surfactant of the nonionic
surfactant systems of the present invention. The alkyl chain of
the aliphatic alcohol can either be straight or branched,
primary or secondary, and generally contains from about 8 to
about 22 carbon atoms. Preferred are the condensation products
of alcohols having an alkyl group containing from about 8 to
about 20 carbon atoms, more preferably from about 10 to about
18 carbon atoms, with from about 2 to about 10 moles of
ethylene oxide per mole of alcohol. Examples of commercially
available nonionic surfactants of this type include TergitolTM
15-S-9 (the condensation product of C11-C15 linear alcohol with
9 moles ethylene oxide), TergitolTM 24-L-6 NMW (the
condensation product of CI2-C14 primary alcohol with 6 moles
ethylene oxide with a narrow molecular weight distribution),
both marketed by Union Carbide Corporation; NeodolTM 45-9 (the
condensation product of C14-C15 linear alcohQl with 9 moles of
~ethylene oxide), NeodolTM 23-6.5 (the condensation product of
C12-C13 linear alcohol with 6.5 moles of ethylene oxide),
NeodolTM 45-7 (the condensation product of C14-C15 linear i-
alcohol with 7 moles of ethylene oxide), NeodolTM 45-4 (the
condensation~product of C14-C15 linear alcohol with 4 moles of
ethylene oxide~ marketed by Shell Chemical Company, and Kyro~M
EOB (the condensation product of C13-C15 alcohol with 9 moles
ethylene oxide), marketed by The Procter & Gamble Company.
Also useful as the nonionic surfactant of the surfactant
systems of the present invention are the alkylpolysaccharides
disclosed in U.S. Patent 4,565,647, Llenado, issued January 21,
,~ ~
1986, having a hydrophobic group containing from about 6 to
about 30 carbon atoms, preferably from about 10 to about 16
^ ~ carbon atoms and a poIysaccharide, e.g. a polyglycoside,
~ ~ hydrophilic group containing from about 1.3 to about 10,
-~ preferably from about 1.3 to about 3, most preferably from
~ about 1.3 to about 2.7 saccharide units. Any reducing
:
SUBSmUTE SHEET
21~02~3
`~094/02580 9 PCT/US93/06223
saccharide containing 5 or 6 carbon atoms can be used, e.g.,
glucose, galactose and galactosyl moieties can be substituted
for the glucosyl moieties (optionally the hydrophobic group is
attached at the 2-, 3-, 4-, etc. positions thus giving a
glucose or galactose as opposed to a glucoside or galactoside).
The intersaccharide bonds can be, e.g., between the one
position of the additional saccharide units and the 2-, 3-, 4-,
and~or 6- positions on the preceding saccharide units.
Optionally, and less desirably, there can be a polyalkylene-
oxide chain joining the hydrophobic moiety and the
polysaccharide moiety. The preferred alkyleneoxide is ethylene
oxide. Typical hydrophobic groups include alkyl groups, either
saturated or unsaturated, branched or unbranched containing
from about 8 to about 18, preferably from about 10 to about 16,
carbon atoms. Preferably, the alkyl group is a straight chain
saturated alkyl group. The alkyl group can contain up to about
3 hydroxy groups and/or the polyalkyleneoxide chain can contain
up to about 10, preferably less than 5, alkyleneoxide moieties.
Suitable alkyl polysaccharides are octyl, nonyldecyl,
undecyldodecyl, tridecyl, tetradecyl, pentadecyl, hexadecyl,
heptadecyl, ~and octadecyl, di-, tri-, tetra-, penta-, and
hexaglucosides, galactosides, lactosides, glucoses,
fructosides, fructoses and/or galactoses. Suitable mixtures
.~ ,
include coconut alkyl, di-, tri-, tetra-, and pentaglucosides
and tallow alkyl tetra-,~penta-, and hexaglucosides.
The preferred alkylpolyglycosides have the formula
~ .
R20(CnH2nO)t(glycosyl)x
wherein R2 is selected from the group consisting of alkyl,
alkylphenyl, hydroxyalkyl, hydroxyalkylphenyl, and mixtures
thereof in which the alkyl groups contain from about 10 to
about 18, preferably from about 12 to about 14, carbon atoms; n
is 2 or 3, preferably 2; t is from 0 to about 10, preferably 0;
and x is from about 1.3 to about 10, preferably from about 1.3
to about 3, most preferably from about 1.3 to about 2.7. The
glycosyl is prèferably derived from glucose. To prepare these
compounds, the alcohol or alkylpolyethoxy alcohol is formed
SUBSTITUTE SHEET
wog4/02580 ~4~3 lo PCT/US93/06223 !'
first and then reacted with glucose, or a source of glucose, to
form the glucoside (attachment at the 1-position). The
additional glycosyl units can then be attached between their 1-
position and the preceding glycosyl units 2-, 3-, 4- and/or 6-
position, preferably predominately the 2-position.
Although not preferred, the condensation products of ethylene
oxide with a hydrophobic base formed by the condensation of
propylene oxide with propylene glycol are also suitable for use
as the additional nonionic surfactant of ;the nonionic
surfactant systems of the present invention. The hydrophobic
portion of these compounds will preferably have a molecular
weight of from about 1500 to about 1800 and will exhibit water
insolubility. The addition of polyoxyethylene moieties to this
hydrophobic portion tends to increase the water solubility of
the molecule as a whole, and the liquid character of the
product is retained up to the point where the polyoxyethylene
content is about 50% of the total weight of the condensation
product, which corresponds to condensation with up to about 40
moles ~of ethylene oxide. Examples of compounds of this type
include certain of the commercially-available PluronicTM
surfactants, marketed by BASF.
;Also suitable for use as the nonionic surfactant of the
nonionic surfactant system of the present invention, are the
condensation products of ethylene oxide with the product
. ~ ~
resulting ~ from the reaction of propylene oxide and
ethylenediamine. The hydrophobic moiety of these products
~ consists of the reaction product of ethylenediamine and excess
- ~propylene oxide, and generally has a molecular weight of from
-~ about 2500 to about 3000. This hydrophobic moiety is condensed
~, with ethylene oxide to the extent that the condensation product
contains from about 40% to about 80% by weight of
polyoxyethylene and has a molecular weight of from about 5,000
to about 11,000. Examples of this type of nonionic surfactant
nclude certain of the commercially available TetronicTM
~ compounds, marketed by BASF.
-~ Preferred for use as the nonionic surfactant of the
surfactant systems of the present invention are polyethylene
oxide condensates of alkyl phenols, condensation-products of
SuBs~ F~T
`~094/02580 11 ~ ~ 4 2 8 3 PCT/US93/06223
primary and secondary aliphatic alcohols with from about 1 to
about 25 moles of ethylene oxide, alkylpolysaccharides, and
mixtures thereof. Most preferred are C8-Cl4 alkyl phenol
ethoxylates having from 3 to 15 ethoxy groups and C8-C18
alcohol ethoxylates (preferably C10 avg.) having from 2 to 10
ethoxy groups, and mixtures thereof.
Highly preferred nonionic surfactants are polyhydoxy fatty
acid amide surfactants.
Also suitable as nonionic surfactants are poly hydroxy fatty
acid amide surfactants of the formula
R2 - C - N - Z,
11
O Rl
wherein R1 is H, or Rl is C1_4 hydrocarbyl, 2-hydroxy ethyl, 2-
hydroxy propyl or a mixturé thereof, R2 is C5_31 hydrocarbyl,
~and Z is a polyhydroxyhydrocarbyl having a linear hydrocarbyl
chain with at least 3 hydroxyls directly connected to the
chain, or~an alkoxylated derivative thereof. Preferably, Rl is
methyl, R2 is a straight Cll_l5 alkyl or alkenyl chain such as
coconut alkyl or mixtures thereof, and Z is derived from a
reducing sugar such as glucose, fructose, maltose, lactose, in
a reductive amination reaction.
,
When~ included~ in such laundry d:tergent compositions, the
nonionic surfactant systems of the present invention act to
improve the greasy/oily stain removal properties of such
laundry detergent compositions across a broad range of laundry
, conditions.
.: ~
~ ANIONIC ~RFACTANT~ ~
.
Suitable anionic surfactants include alkyl alkoxylated
sulfate surfactants hereof are water soluble salts or acids of
the formula RO(A)mS03M wherein R is an unsubstituted C10-C24
~ alkyl or hydroxyalkyl group having a ClQ-C24 alkyl component,
`:
SUBSTITUTE SHEET
?,~40?.~3 ~:
W094/02580 12 PCT/US93/06223 -
preferably a C12-C20 alkyl or hydroxyalkyl, more preferably
C12-C18 alkyl or hydroxyalkyl, A is an ethoxy or propoxy unit,
m is greater than zero, typically between about 0.5 and about
6, more preferably between about 0.5 and about 3, and M is H or
a cation which can be, for example, a metal cation (e.g.,
sodium, potassium, lithium, calcium, magnesium, etc.), ammonium
or substituted-ammonium cation. Alkyl ethoxylated sulfates as
well as alkyl propoxylated sulfates are contemplated herein.
Specific examples of substituted ammonium cations include
methyl-, dimethyl, trimethyl-ammonium cations and quaternary
ammonium cations such as tetramethyl-ammonium and dimethyl
piperdinium cations and those derived from alkylamines such as
ethylamine, diethylamine, triethylamine, mixtures thereof, and
~he like. Exemplary surfactants are C12-Cl8 alkyl
polyethoxylate (1.0) sulfate (C12-C18E(1.0)M), C12-C18 alkyl
polyethoxylate (2.25) sulfate (C12-C18E(2 25)M)~ C12-C18 alkyl
polyethoxylate (3.0) sulfate (C12-C18E(3.0~M), and C12-C18
alkyl polyethoxylate (4.0) su}fate (C12-C18E(4.0)M), wherein M
i5 conveniently selected from sodium and potassium.
,
; Suitable anionic surfactants to be used are alkyl ester
sulfonate surfactants including linear esters of C8-C20
carboxylic acids (i.e., fatty acids) which are sulfonated with
gaseous SO3 according to "The Journal of the American Oil i`
Chemists Society", S2 (1975), pp. 323-329. Suitable starting
materials would include natural fatty substances as derived
from tallow, palm oil, etc.
The preferred alkyl ester sulfonate surfactant, especially
for laundry applications, comprise alkyl ester sulfonate
; surfactants of the structural formula :
O
R3 - CH - C - oR4
SO3M
wherein R3 is a C8-C20 hydrocarbyl, preferably an alkyl, or
combination thereof, R4 is a Cl-C6 hydrocarbyl, preferably an
alkyl, or combination thereof, and M is a cation which forms a
-~ SUBSTITVTE SHEET
~V094/02~80 2 1 4 0 2 8 3 PCT/US93,~6223
water soluble salt with the alkyl ester sulfonate. Suitable
salt-forming cations include metals such as sodium, potassium,
and lithium, and substituted or unsubstituted ammonium cations,
such as monoethanolamine, diethanolamine, and triethanolamine.
Preferably, R3 is C10-C16 alkyl, and R4 is methyl, ethyl or
isopropyl. Especially preferred are the methyl ester sulfonates
wherein R3 is C10-C16 alkyl.
Other suitable anionic surfactants include the alkyl sulfate
surfactants hereof are water soluble salts or acids of the
formula ROSO3M wherein R preferably is a C10-C24 hydrocarbyl,
preferably an alkyl or hydroxyalkyl having a C10-C20 alkyl
component, more preferably a C12-Cl~ alkyl or hydroxyalkyl, and
M is H or a cation, e.g., an alkali metal cation (e.g. sodium,
potassium, lithium), or ammonium or substituted ammonium (e.g.
methyl-, dimethyl-, and trimethyl ammonium cations and
quaternary ammonium cations such as tetramethyl-ammonium and
dimethyl piperdinium cations and quaternary ammonium cations
derived from alkylamines such as ethylamine, diethylamine,
triethylamine, and mixtures thereof, and the like). Typically,
alkyl chains of C12-C16 are preferred for lower wash
temperatures (e.g. below about 50C) and C16_18 alkyl chains
are preferred for higher wash temperatures (e.g. above about
50C) .
Other anionic curfactants useful for detersive purposes can
also be included in the laundry detergent compositions of the
present invention. These can include salts (including, for
example, sodium, potassium, ammonium, and substituted ammonium
salts such as mono-, di- and triethanolamine salts) of soap,
C9-C20 linear alkylbenzenesulfonates, C8-C22 primary of
secondary alkanesulfonates, C8-C24 olefinsulfonates, sulfonated
polycarboxylic acids prepared by sulfonation of the pyrolyzed
product of alkaline earth metal citrates, e.g., as described in
British patent specification No. 1,082,179, Cg-C24
alkylpolyglycolethersulfates (containing up to 10 moles of
ethylene oxide); alkyl glycerol sulfonates, fatty acyl glycerol
sulfonates, fatty oleyl glycerol sulfates, alkyl phenol
SUBSTITUTE SHEET
W094/0~80 ~49~ 14 PCT/US93/06223,--
ethylene oxide ether sulfates, paraffin sulfonates, alkyl
phosphates, isethionates such as the acyl isethionates, N-acyl
taurates, alkyl succinamates and sulfosuccinates, monoesters of
sulfosuccinates (especially saturated and unsaturated Cl2-Cl8
monoesters) and diesters :of sulfosuccinates (especially
saturated and unsaturated C6-Cl2 diesters), acyl sarcosinates,
sulfates of alkylpolysaccharldes such as the sulfates of
alkylpolyglucoside~ (the nonionic: nonsulfated compounds being
de~scribed~below), branched: primary~ alkyl sulfates, and alkyl
polyethoxy carboxylates such as those of the formula
RO~(~CH2CH20)~k-CH2C00-M+ wherein R; is a C8-C22 alkyl, k is an
integer~from~:-0 to~lO,~and`M is a ~soluble salt-forming cation.
Res~i:n:~ acids~:and~ hydrogenated resin~ acids are also suitable,
such ~as ~r~osin,: hydrogenated ~rosin, and resin acids and
hydrogenated resin acids present in or derived from tall oil.
Further~examples are described in~ "Surface~ Artive Agents and
Detèrgents~ Vol.~ I and ~II by Schwartz, Perry~and Berch). A
;of~ such~ surfactants are~ also generally~ disclosed in
U.:S. Patent -3,~9~29,678~,: issued December 30, 1975~to Laughlin, et
al.`~ ~Col ~ ~:2~3~ 1ine~58~through~Column 29, line 23 (herein
i ~ orated~by~ reference;~
Whe.n~ included:~thërèin,:~the laundry~detergent compositions of
t.he~:~`prese~nt;~inv-ntion~typl Qlly comprise:~from about l~ to about
4Q%:, preferàbly from.~about~3% to :about 20% by weight of such
anionic`:~æùrfactants~
he laundry~detergent~ compositions~of; the present invention
~ a:lso~co ~ in'cat~ionic,`~ampholytic~, zwitterionic~, and semi-
polàr~surfàctants,~`as~;we11~as nonionic surfactants other than
those~already~described.~:herein.:~
Preferred ~cationic ~surfactant systems include nonionic and
a~mpholytic surfactants. Cationic detersive surfactants suitable
fQr ~use:: in~the'~làundry;~deterg-nt compositions of the present
i m ention-~are~ those~having one long-chain hydrocarbyl group.
Examples of::such: cationic surfactants include the ammonium
surfactants:~such :as alkyldimethyIammonium halogenides, and
'those~-surfactants:having the:formula :
[R2(oR3)y]~R4(0R3)y]2RSN+X-
SUBSmUTE SHEET
iO ~ r,J.
'~094/02580 15 PCT/US93/06223
wherein R2 is an alkyl or alkyl benzyl group having from about
8 to about 18 carbon atoms in the alkyl chain, each R3 is
selected from the group consisting of -CH2CH2-, -CH2CH(CH3)-,
-CH2CH(CH2OH)-, -CH2CH2CH2-, and mixtures thereof; each R4 is
selected from the group consisting of C1-C4 alkyl, Cl-C4
hydroxyalkyl, benzyl ring structures formed by joining the two
R4 groups, -CH2CHOH-CHOHCOR6CHOHCH2OH wherein R6 is any hexose
or hexose polymer having a molecular weight less than about
lOOO, and hydrogen when y is not 0; R5 is the same as R4 or is
an alkyl chain wherein the total number of carbon atoms of R2
plus R5 is not more than about 18; each y is from 0 to about lO
and the sum of the y values is from 0 to about 15; and X is any
compatible anion.
-
Preferred cationic surfactants are the water-soluble
quaternary ammonium compounds useful in the present composition
having the formula :
:
' RlR2R3R4N+x ( i )
wherein R1 is C8-Cl6 alkyl, each of R2, R3 and R4 is
independently C1-C4 alkyl, Cl-C4 hydroxy alkyl, benzyl, and
(C2H40)XH where x has a value from 2 to 5, and X is an anion.
Not more than one of R2, R3 or ~4 should be benzyl. ~;
The preferred alkyl chain length for R1 is C12-C15 particularly
where the alkyl group is a mixture of chain lengths derived
from coconut or palm kernel fat or is derived synthetically by
olefin build up or OXO alcohols synthesis. Preferred groups for
R2R3 and R4 are methyl and hydroxyethyl groups and the anion X
, may be selected from halide, methosulphate, acetate and
phosphate ions.
Examples of suitable quaternary ammonium compounds of
formulae (i) for use herein are :
coconut trimethyl ammonium chloride or bromide;
coconut methyl dihydroxyethyl ammonium chloride or bromide;
decyl triethyl ammonium chloride;
decyl dimethyl hydroxyethyl ammonium chloride or bromide;
SUBSTITUTE SHEET
W 0 94/02580 2~4Q~3 16 PC~r/US93/06223 ,.-
C12_15 dimethyl hydroxyethyl ammonium chloride or bromide;
coconut dimethyl hydroxyethyl ammonium chloride or bromide;
myristyl trimethyl ammonium methyl sulphate;
lauryl dimethyl benzyl ammonium chloride or bromide;
lauryl dimethyl (ethenoxy)4 ammonium chloride or bromide;
choline esters (compounds of formula (i) wherein R1 is -CH~-
-Il-c12-l4 alkyl and R2R3R4 are methyl).
o
di-alkyl imidazolines [compounds of formula (i)].
Other cationic surfactants useful herein are also described
in U.S. Patent 4,228,044, Cambre, issued October 14, 1980.
When included therein, the laundry detergent compositions of
the present invention typically comprise from 0% to about 25~,
preferably from about 3% to about 15% by weight of such
cationic surfactants.
Ampholytic surfactants are also suitable for use in the
laundry ~detergent compositions of the present invention. These
surfactants can be broadly described as aliphatic derivatives
of secondary or~tertiary amines, or allphatic derivatives of
heterocyclic secondary and tertiary amines in which the
aliphatic radical can be straight- or branched-chain. One of
the aliphatic substituents contains at least about 8 carbon
atoms, typically from about 8 to about 18 carbon atoms, and at
least one~contains an anionic water-solubilizing group, e.g.
carboxy, sulfonate, sulfate. See U.S. Patent No. 3,929,678 to
Laughlin et al., issued December 30, 1975 at column 19, lines
18-35, for examples of ampholytic surfactants.
When included therein, the laundry detergent compositions of
the present invention typically comprise from 0% to about 15%,
preferably from about 1% to about 10% by weight of such
ampholytic surfactants.
Zwitterionic surfactants are also suitable for use in laundry
detergent compositions. These surfactants can be broadly
described as derivatives of secondary and tertiary amines,
derivatives of heterocyclic secondary and tertiary amines, or
derivatives of quaternary ammonium, quaternary phosphonium or
tertiary sulfonium compounds. See U.S. Patent No. 3,929,678 to
SUBSTITIJTE SHEET
--~094/02580 17 ~ I ~ 0 2 8 3 pCT/US93/06223
Laughlin et al., issued December 30, 1g75 at column 19, line 38
through column 22, line 48, for examples of zwitterionic
surfactants.
When included therein, the laundry detergent compositions of
the present invention typically comprise from O% to about 15%,
preferably from about 1~ to about 10% by weight of such
zwitterionic surfactants.
Semi-polar nonionic surfactants are a special category of
nonionic surfactants which include water-soluble amine oxides
containing one alkyl moiety of from about 10 to about 18 carbon
atoms and 2 moieties selected from the group consisting of
alkyl groups and hydroxyalkyl groups containing from about 1 to
about 3 carbon atoms; water-soluble phosphine oxides containing
one alkyl moiety of from about lO to about 18 carbon atoms and
2 moieties selected from the group consisting of alkyl groups
and hydroxyalkyl groups containing from about 1 to about 3
carbon atoms; and water-soluble sulfoxides containing one alkyl
moiety of from about lO to ab~ut 18 carbon atoms and a moiety
selected from the group consisting of alkyl and hydroxyalkyl
moieties of from about 1 to about 3 carbon atoms.
Semi-polar nonionic detergent su,factants include the amine
oxide surfactants having the formula
R3(oR4)xN(R5)2
wherein R3 is an alkyl, hydroxyalkyl, or alkyl phenyl group or
mixtures therof containing from about 8 to about 22 carbon
atoms; R4 is an alkylene or hydroxyalkylene group containing
from about 2 to about 3 carbon atoms or mixtures thereof; x is
from;0 to about 3; and each R5 is an alkyl or hydroxyalkyl
group containing from about 1 to about 3 carbon atoms or a
polyethylene oxide group containing from about 1 to about 3
ethylene oxide groups. The R5 groups can be attached to each
other, e.g., through an oxygen or nitrogen atom, to form a ring
structure.
These amine oxide surfactants in particular include C1O-C18
alkyl dimethyl amine oxides and C8-C12 alkoxy ethyl dihydroxy
ethyl amine oxides.
SUBSTITUTE SHEEl-
W094t02580 ~3 18 PCT/US93/06223
When included therein, the laundry detergent compositions of
the present invention typically comprise from 0% to about 15%,
preferably from about 1% to about 10% by weight of such semi-
polar nonionic surfactants.
The present invention further provides laundry detergent
compositions comprising at least 1% by weight, preferably from
about 3% to about 65%, more prPferably from about 10~ to about
25% by weight of total surfactants.
DETERGENT ADJUNCT8
The compositions according to the present invention may
further comprise a builder system. Any conventional builder
system is suitable for use herein including aluminosilicate
materials, silicates, polycarboxylates and fatty acids,
materials such as ethylenediamine tetraacetate, metal ion
sequestrants such as aminopolyphosphonates, particularly
ethylenediamine tetramethylene phosphonic acid and diethylene
triamine pentamethylenephosphonic acid. Though less preferred
for obvious environmental reasons, phosphate builders can also
be used herein.
Suitable builders can be an inorganic ion exchange material,
commonly an inorganic hydrated aluminosilicate material, more
particularly a hydrated synthetic zeolite such as hydrated
zeolite A, X, B or HS.
Another suitable inorganic builder material is layered
silicate, e.g. SKS-6 (Hoechst). SKS-6 is a crystalline layered
silicate consisting of sodium silicate (Na2Si205).
Suitable polycarboxylates containing one carboxy group
include lactic acid, glycolic acid and ether derivatives
thereof as disclosed in Belgian Patent Nos. 831,368, 821,369
and 821,370. Polycarboxylates containing two carboxy groups
-include the water-soluble salts of succinic acid, malonic acid,
(ethylenedioxy) diacetic acid, maleic acid, diglycollic acid,
tartaric acid, tartronic acid and fumaric acid, as well as the
ether carboxylates described in German Offenlegenschrift
:: SUBSTITUTE SHEET
~094/02580 19 2 1 ~ o ~ ~ ~ PCT/US93~06223
2,446,686, and 2,446,687 and U.S. Patent No. 3,935,257 and the
sulfinyl carboxylates described in Belgian Patent No. 840,623.
Polycarboxylates containing three carboxy groups include, in
particular, water-soluble citrates, aconitrates and
citraconates as well as succinate derivatives such as the
carboxymethyloxysuccinates described in British Patent No.
1,379,241, lactoxysuccinates described in Netherlands
Application 7205873, and the oxypolycarboxylate materials such
as 2-oxa-1,1,3-propane tricarboxylates described in British
Patent No. 1,387,447.
Polycarboxylates containing four carboxy groups include
oxydisuccinates disclosed in British Patent No. 1,261,829,
1,1,2,2-ethane tetracarboxylates, 1,1,3,3-propane
tetracarboxylates and 1,1,2,3-propane tetracarboxylates.
Polycarboxylates containing sulfo substituents include the
sulfosuccinate derivatives disclosed in British Patent Nos.
1,398,421 and 1,398,422 and in U.S. Patent No. 3,936,448, and
the sulfonated pyrolysed citrates described in British Patent
No. 1,082,179, while polycarboxylates containing phosphone
substituents are disclosed in British Patent No. 1,439,000.
Alicyclic and heterocyclic polycarboxylates include
cyclopentane-cis,cis,cis-tetracarboxylates, cyclopentadienide
pentacarboxylates, 2,3,4,5-tetrahydrofuran - cis, cis, cis-
tetracarboxylates, 2,5-tetrahydrofuran -cis - dicarboxylates,
2,2,5,5-tetrahydrofuran - tetracarboxylates, 1,2,3,4,5,6-hexane
-hexacarboxylates and and carboxymethyl derivatives of
polyhydric alcohols such as sorbitol, mannitol and xylitol.
Aromatic polycarboxylates include mellitic acid, pyromellitic
acid and the phtalic acid derivatives disclosed in British
Patent No. 1,425,343.
Of the above, the preferred polycarboxylates are
hydroxycarboxylates containing up to three carboxy groups per
molecule, more particularly citrates.
Preferred builder systems for use in the present compositions
include a mixture of a water-insoluble aluminosilicate builder
SUBSmUTE SHEET
W094/0258~ ~ ~0 PCTIUS93/06223
such as zeolite A or of a layered silicate (sks/6), and a
water-soluble carboxylate chelating agent such as citric acid.
A suitable chelant for inclusion in the detergent
compositions in accordance with the invention is
ethylenediamine-N,N'-disuccinic acid (EDDS) or the alkali
metal, alkaline earth metal, ammonium, or substituted ammonium
salts thereof, or mixtures thereof. Preferred EDDS compounds
are the free acid form and the sodium or magnesium salt
thereof. Examples of such preferred sodium salts of EDDS
include Na2EDDS and Na4EDDS. Examples of such preferred
magnesium salts of EDDS include MgEDDS and Mg2EDDS. The
magnesium salts are the most preferred for inclusion in
compositions in accordance with the invention.
Especially for the liquid execution herein, suitable fatty
acid builders for use herein are saturated or unsaturated C10-
18 fatty acids, as well as well as the corresponding soaps.
Preferred saturated species have from 12 to 16 carbon atoms in
the alkyl chain. The preferred unsaturated fatty acid is oleic
acid.
Preferred builder systems for use in granular compositions
include a mixture of a water-insoluble aluminosilicate builder
such as zeolite A, and a watersoluble carboxylate chelating
agent such as citric acid.
Other builder materials that can form part of the builder
system for use in granular compositions the purposes of the
invention include inorganic materials such as alkali metal
carbonates, bicarbonates, silicates, and organic materials such
as the organic phosphonates, amiono polyalkylene phosphonates
and amino polycarboxylates.
Other suitable water-soluble organic salts are the homo- or co-
polymeric acids or their salts, in which the polycarboxylic
acid comprises at least two carboxyl radicals separated from
each other by not more than two carbon atoms.
Polymers of this type are disclosed in GB-A-1,596,756.
Examples of such salts are polyacrylates of MW 2000-5000 and
their copolymers with maleic anhydride, such copolymers ha~ing
~.'
SUBSTITUTE SHEET
- ~094/02~80 2 1 1 ~ 2 ~ ~ PCT/~S93/06223
a molecular weight of from 20,000 to 70,000, especially about
40,000.
Detergency builder salts are normally included in amounts of
from 10% to 80% by weight of the composition preferably from
20~ to 70% and most usually from 30~ to 60% by weight.
Detergent ingredients that can be included in the detergent
compositions of the present invention include bleaching agents.
These bleaching agent components can include one or more oxygen
bleaching agents and, depending upon the bleaching agent
chosen, one or more bleach activators. When present bleaching
compounds will typically be present at levels of from about 1%
to about 10%, of the detergent composition. In general,
bleaching compounds are optional components in non-liquid
formulations, e.g. granular detergents. If present, the amount
of bleach activators will typically be from about 0.1% to about
60%, more typically from about 0.5% to about 40% of the
bleaching composition.
The bleaching agent component for use herein can be any of
the bleaching agents useful for detergent compositions
including oxygen bleaches as well as others known in the art.
In a method aspect, this invention further provides a
method for cleaning fabrics, fibers, textiles, at temperatures
below about 50~C, especially below about 40C, with a detergent
composition containing polyamine N-oxide containing polymers,
optional auxiliary detersive surfactants, optional detersive
adjunct ingredients, and a bleaching agent.
The bleaching agent suitable for the present invention can be
an activated or non-activated bleaching agent.
One category of oxygen bleaching agent that can be used
encompasses percarboxylic acid bleaching agents and salts
thereof. Suitable examples of this class of agents include
magnesium monoperoxyphthalate hexahydrate, the magnesium salt
of meta-chloro perbenzoic acid, 4-nonylamino-4-oxoperoxybutyric
acid and diperoxydodecanedioic acid. Such bleaching agents are
disclosed in U.S. Patent 4,483,781, U.S. Patent Application
740,446, European Patent Application 0,133,354 and U.S. Patent
4,412,934. Highly preferred bleaching agents also include 6-
SUBSTITUTE SHEET
94/02580 2 ~ 4 0 ~ 3 3 22 PC~r/US93/06223 ~
nonylamino-6-oxoperoxycaproic acid as described in U.S. Patent
4,634,551.
Another category of bleaching agents that can be used
encompasses the halogen bleaching agents. Examples of
hypohalite bleaching agents, for example, include trichloro
isocyanuric acid and the sodium and potassium
dichloroisocyanurates and N-chloro and N-bromo alkane
sulphonamides. Such materials are normally added at 0.5-10% by
weight of the finished product, preferably 1-5% by weight.
Preferably, the bleaches suitable for the present
invention include peroxygen bleaches. Examples of suitable
water-soluble solid peroxygen bleaches include hydrogen
peroxide releasing agents such as hydrogen peroxide,
perborates, e.g. perborate monohydrate, perborate tetrahydrate,
persulfates, percarbonates, peroxydisulfates, perphosphates and
p-roxyhydrates. Preferred bleaches are percarbonates and
perborates.
The hydrogen peroxide releasing agents can be used in
combination with bleach activators such as
tetraacetylethylenediamine (TAED), nonanoyloxybenzenesulfonate
(NOBS, described in US 4,412,934), 3,5,-
trimethylhexancloxybenzenesulfonate (ISONOBS, described in EP
120,591) or pentaacetylglucose (PAG), which are perhydrolyzed
to form a peracid as~the active bleaching species, leading to
improved ~bleaching effect. Also suitable activators are
acylated citrate esters such as disclosed in Copending European
Patent Application No. 91870207.7.
The hydrogen peroxide may also be present by adding an
enzymatic system (i.e. an enzyme and a substrate therefore)
which is capable of generating hydrogen .peroxide at the
beginning or during the washing and/or rinsing process. Such
enzymatic systems are disclosed in EP Patent Application
9120265S.6 filed October 9, 1991.
SUBSTITUTE SHEET
,~
,-,. .
`~94/02580 2 1 ~ 0 2 8 3 PCT/US93/06223
Other peroxygen bleaches suitable for the present
invention include organic peroxyacids such as percarboxylic
acids.
Bleaching agents other than oxygen bleaching agents are
also known in the art and can be utilized herein. One type of
non-oxygen bleaching agent of particular interest includes
photoactivated bleaching agents such as the sulfonated zinc
andJor aluminum phthalocyanines. These materials can be
deposited upon the substrate during the washing process. Upon
irradiation with light, in the presence of oxygen, such as by
hanging rlothes out to dry in the daylight, the sulfonated zinc
phthalocyanine is activated and, consequently, the substrate is
bleached. Preferred zinc phthalocyanine and a photoactivated
bleaching process are described in U.S. Patent 4,033,718.
Typically, detergent compositions will contain about 0.025% to
about 1.2S%, by weight, of sulfonated zinc phthalocyanine.
Other detergent ingredients that can be included are
detersive enzymes which can be included in the detergent
formulations for a wide variety of purposes including removal
of protein-based, carbohydrate-based, or triglyceride-based
stains, for example, and prevention of refugee dye transfer.
The enzymes to be incorporated include proteases, amylases,
lipases, cellulases, and peroxidases, as well as mixtures
thereof. Other types of enzymes may also be included. They may
be of any suitable origin, such as vegetable, animal,
bacterial, fungal and yeast origin.
Enzymes are normally incorporated at levels sufficient to
provide up to about 5 mg by weight, more typically about 0.05
mg to about 3 mg, of active enzyme per gram of the composition.
Suitable examples of proteases are the subtilisins which are
obtained from particular strains of B.subtilis and
B.licheniforms. Proteolytic enzymes suitable for removing
protein-based stains that are commercially available include
those sold under the tradenames Alcalase , Savinase and
Esperase by Novo Industries A/S (Denmark) and Maxatase by
SUBSTITUTE SHE~
~ ~W~ ZC ~ <n
O 94/02580 2~ 40~3 24 PC~r/US93/06223 .-
International Bio-Synthetics, Inc. (The Netherlands) and FN-
base by Genencor, Optimase and opticlean by MKC~
Of interest in the category of proteolytic enzymes,
especially for liquid detergent compositions, are enzymes
referred to herein as Protease A and Protease B. Protease A is
described in European Patent Application 130,756. Protease B is
described in European Patent Application Serial No. 87303761.8.
Amylases include, for example, -amylases obtained from a
special strain of B.licheniforms, described in more detail in
British Patent Specification No. 1,296,839 tNovo). Amylolytic
proteins include, for example, Rapidase, Maxamyl (International
Bio-Synthetics, Inc.) and Termamyl,tNo~ro Industries).
The cellulases usable in the present invention include both
bacterial or fungal cellulase. Preferably, they will have a pH
optimum of between 5 and 9.5. Suitable cellulases are disclosed
in U.S. Patent 4,435,307, Barbesgoard et al, which discloses
fungal cellulase produced from Humicola insolens. 5uitable
cellulases are also disclosed in GB-A-2.075.028 ; GB-A-
2.095.275 and DE-OS-2.247.832.
Examples ~of such cellulases are cellulases produced by a
strain of Humicola insolens (Humicola grisea var. thermoidea),
particularly the Humicola strain DSM 1800, and cellulases
produced by a fungus of Bacillus N or a cellulase 212-producing
fungus belonging to the genus Aeromonas, and cellulase
extracted from the hepatopancreas of a marine mollusc
(Dolabella Auricula Solander).
Other suitable cellulases are cellulases originated from
Humicola Insulens having a molecular weight of about 50KDa, an
isoelectric point of 5.S and containing 415 amino acids. Such
cellulase are described in Copending European patent
application No. 93200811.3, filed March 19, 1993.
Especially suitable cellulase are the cellulase having color
care benefits. Examples of such cellulases are cellulase
described in European patent application No. 91202879.2, filed
November 6, 1991 Carezyme (Novo).
Suitable lipase enzymes for detergent usage include those
produced by microorganisms of the Pseudomonas group, such as
SUBSmVTE SHEET
~VO 94/02580 2 1 ~ ~ 2 8 3 PC~r/US93/06223
Pseudomonas stutzeri ATCC 19.154, as disclosed in British
Patent 1,372,034. Suitable lipases include those which show a
positive immunoligical cross-reaction with the antibody of the
lipase, produced by the microorganism Pseudomonas fluorescent
IAM 1057. This lipase is available from Amano Pharmaceutical
Co. Ltd., Nagoya, Japan, under the trade name Lipase P "Amano,"
hereinafter referred to as "Amano-P".
Especially suitable Lipase are lipase such as M1 Lipase (Ibis)
and Lipolase (Novo).
Peroxidase enzymes are used in combination with oxygen
sources, e.g. percarbonate, perborate, persulfate, hydrogen
peroxide, etc. They are used for "solution bleaching", i.e. to
prevent transfer of dyes of pigments removed from substrates
during wash operations to other substrates~ in the wash
solution. Peroxidase enzymes are known in the art, and include,
for example, horseradish peroxidase, ligninase, and
haloperoxidase such as chloro- and bromo-peroxidase.
Peroxidase-containing detergent compositions are disclosed, for
example, in PCT Internation Application WO 89/099813 and in
European Patent application EP No. 91202882.6, filed on
November 6, 1991.
In~ liquid formuIations, an enzyme stabilization system is
pre~erably utilized. Enzyme stabilization techniques for
aqueous detergent compositions are well known in the art. For
example, one technigue for enzyme stabilization in aqueous
solutions involves the use of free calcium ions from sources
such as calcium~ acetate, calcium formate and calcium
propionate. Calcium ions can be used in combination with short
chain carboxylic acid salts, preferably formates. See, for
example, U.S. patent 4,318,818. It has also been proposed to
use polyols like glycerol and sorbitol. Alkoxy-alcohols,
dialkylglycoethers, mixtures of polyvalent alcohols with
polyfunctional aliphatic amines (e.g., such as diethanolamine,
triethanolamine, di-isopropanolamime, etc.), and boric acid or
alkali metal borate. Enzyme stabilization techniques are
additionally disclosed and exemplified in U.S. patent
4,261,868, U.S. Patent 3,600,319, and European Patent
Application Publication No. 0 199 405, Application No.
SU8STITUTE SHEET
W094/02580 ~ ~ ~ ~ 26 PCT/US93tO62~3~-
86200586.5. Non-boric acid and borate stabilizers are
preferred. Enzyme stabilization systems are also described, for
example, in U.S. Patents 4,261,868, 3,600,319 and 3,519,570.
Other suitable detergent ingredients that can be added are
enzyme oxidation scavengers which are described in Copending
European Patent aplication N 92870018.6 filed on January 31,
1992. Examples of such enzyme oxidation scavengers are
ethoxylated tetraethylene polyamines.
Especially preferred detergent ingredients are combinations
with technologies which also provide a type of color care
bene~it. Examples of these technologies are cellulase and/or
peroxidases and/or metallo catalysts for color maintance
rejuvenation. Such metallo catalysts are described in copending
European Patent Application No. 92870181.2.
In addition, it has been found that the polyamine-N-oxide
containing polymers eliminate or reduce the deposition of the
metallo-catalyst onto the fabrics resulting in improved
whiteness benefit.
Another optional ingredient is a suds suppressor, exemplified
by silicones, and silica-silicone mixtures. Silicones can be
generally represented by alkylated polysiloxane materials while
silica is normally used in finely divided forms exemplified by
silica aerogels and xerogels and hydrophobic silicas of various
types. These materia}s can be incorporated as particulates in
which the suds suppressor is advantageously releasably
incorporated in a water-soluble or water-dispersible,
substantially non-surface-active detergent impermeable carrier.
Alternatively the suds suppressor can be dissolved or dispersed
in a liquid carrier and applied by spraying on to one or more
of the other components.
A preferred silicone suds controlling agent is disclosed in
Bartollota et al. U.S. Patent 3 933 672. Other particularly
useful suds suppressors are the self-emulsifying silicone suds
suppressors, described in German Patent Application DTOS 2 646
126 published April 28, 1977. An example of such a compound is
DC-544, commercially available from Dow Corning, which is a
siloxane-glycol copolymer. Especially preferred suds
SUBSTITUTE SHEET
~.... , . .. '
`V0~4/02580 27 2 I ~ Q ~ 8 3 PCT/U593/06~23
controlling agent are the suds suppressor system comprising a
mixture of silicone oils and 2 alkyl-alcanols. Suitable 2- ~
alkyl-alcanols are 2-butyl-octanol which are commercially 3
available under the trade name Isofol 12 R.
Such suds suppressor system are described in Copending European
Patent application N 92870174.7 filed 10 November, 1992.
Especially preferred silicone suds controlling agents are
described in Copending European Patent application N92201649.8
Said compositions can comprise a silicone/silica mixture in
combination with fumed nonporous silica such as AerosilR.
The suds suppressors described above are normally employed at
levels of from 0.001% to 2% by weight of the composition,
preferably from 0.01% to 1% by weight.
Other components used in detergent compositions may be
employed, such as soil-suspending agents soil-release agents,
optical brighteners, abrasives, bactericides, tarnish
inhibitors, coloring agents and/or encapsulated or more
encapsulated perfumes.
Antiredeposition and soil suspension agents suitable herein
include cellulose derivatives such as methylcellulose,
carboxymethylcellulose and hydroxyethylcellulose, and homo- or
co-polymeric polycarboxylic acids or their salts. Polymers of
this type include the polyacrylates and maleic anhydride-
acrylic acid copolymers previously mentioned as builders, as
well as copolymers of maleic anhydride with ethylene,
methyl~inyl ether or methacrylic acid, the maleic anhydride
constituting at least 20 mole percent of the copolymer. These
materials are normally used at levels of from 0.5% to 10% by
weight, more preferably from 0.75% to 8%, most preferably from
1% to 6% ~y weight o~ the composition.
Preferred optical brighteners are anionic in character,
examples of which are disodium 4,41-bis-(2-diethanolamino-4-
anilino -s- triazin-6-ylamino)stilbene-2:2l disulphonate,
disodium 4, - 41-bis-(2-morpholino-4-anilino-s-triazin- 6-
SUBSTITUTE SHEET
094/02580 ~3 28 PCT/US93/06223,
ylaminost~ ene-2:21 - disulphonate, disodium 4,41 - bis-t2,4-
dianilino-s-triazin-6-ylamino)stilbene-2:21 - disulphonate,
monosodium 41,411 -bis-(2,4-dianilino-s-triazin-6
ylamino)stilbene-2-sulphonate, disodium 4,41 -bis-(2-anilino-4-
(N-methyl-N-2-hydroxyethylamino)-s-triazin-6-ylamino)stilbene-
2,21 - disulphonate, disodium 4,41 -bis-(4-phenyl-2,1,3-
triazol-2-yl)-stilbene-2,21 disulphonate, disodium 4,41bis(2-
anilino-4-(1-methyl-2-hydroxyethylamino)~s-triazin-6-
ylamino)stilbene-2,21disulphonate and sodium 2(stilbyl-411_
(naphtho-11,21:4,5)-1,2,3 - triazole-211-sulphonate.
Other useful polymeric materials are the polyethylene
glycols, particularly those of molecular weight 1000-10000,
more particularly 2000 to 8000 and most preferably about 4000.
These are used at levels of from 0.20% to 5% more preferably
from 0.25% to 2.5% by weight. These polymers and the previously
mentioned homo- or co-polymeric polycarboxylate salts are
valuable for improving whiteness maintenance, fabric ash
deposition, and cleaning performance on clay, proteinaceous and
oxidizable soils in the presence of transition metal
impurities.
Soil release agents useful in compositions of the present
invention are conventionally copolymers or terpolymers of
terephthalic acid with ethylene gly~ol and/or propylene glycol
units in various arrangements. Examples of such polymers are
disclosed in the commonly assigned US Patent Nos. 4116885 and
4711730 and European Published Patent Application No. 0 272
033. A particular preferred polymer in accordance with EP-A-0
272 033 has the formula
(CH3(pEG)43)o.7s(poH)o.2s~T-po)2.8(~-pEG)o.4]T(
H)o.2s((pEG)43cH3)o.75
where PEG is -(OC2H4)O-,PO is (OC3H6O) and T is (pcOC6H4CO).
Also very useful are modified polyesters as random copolymers
of dimethyl terephtalate, dimethyl sulfoisophtalate, ethylene
SUBSTITUTE SHEET
'~094/02580 2 1 ~ ~ 2 8 3 PCT/US93/06223
glycol and 1-2 propane diol, the end groups consisting
primarily of sulphobenzoate and secondarily of mono esters of
ethylene glycol and/or propane-diol. The target is to obtain a
polymer capped at both end by sulphobenzoate groups,
"primarily", in the present context most of said copolymers
herein will be end-capped by sulphobenzoate groups. However,
some copolymers will be less than fully capped, and therefore
their end groups may consist of monoester of ethylene glycol
and/or propane 1-2 diol, thereof consist "secondarily" of such
species.
The selected polyesters herein contain about 46% by weight of
dimethyl terephtalic acid, about 16% by weight of propane -1.2
diol, about 10% by weight ethylene glycol about 13% by weight
of dimethyl sulfobenzoid acid and about 15% by weight of
sulfoisophtalic acid, and have a molecular weight of about
3.000. The polyesters and their method of preparation are
descFibed in detail in EPA 311 342.
The detergent compositions according to the invention can be
in liquid, paste, gels or granular forms. Granular compositions
according to the present invention can also be in "compact
form", i.e. they may have a relatively higher density than
conventional gr~nular detergents, i.e. from 550 to 950 g/l; in
such case, the granular detergent compositions according to the
present invention will contain a lower amount of "inorganic
filler salt", compared to conventional granular detergents;
typical filler salts are alkaline earth metal salts of
sulphates and chlorides, typically sodium sulphate; "compact"
deter~ents typically comprise not more than 10% filler salt.
The liquid compositions according to the present invention can
also be in "concentrated form", in such case, the liquid
detergent compositions according to the present invention will
contain a lower amount of water,compared to conventional liquid
detergents. Typically, the water content of the concentrated
liquid detergent is less than 30%j more preferably less than
20%, most preferably less than 10% by weight of the detergent
SUBSTITUTE SHEET
094/02~80 2 ~ ~02~ 3 30 PCT/US93/06223,
compositions. Other examples of liquid compositions are
anhydrous compositions containing substantially no water.
Both aqueous and non-aqueous liquid compositions can be
structured or non-structured.
The present invention also relates to a process for
inhibiting dye transfer from one fabric to another of
solubilized and suspended dyes encountered during fabric
laundering operations involving colored fabrics.
The process comprises contacting fabrics with a laundering
solution as hereinbefore described.
The process of the invention is conveniently carried out in
the course of the washing process. The washing process is
preferably carried out at 5 C to 75 C, especially 20 to 60,
but the polymers are effective at up to 95C and higher
temperatures. The pH of the treatment solution is preferably
from 7 to 11, especially from 7.5 to 10.5.
The process and compositions of the invention can also be
used as~detergent additive products.
Such additive products are intended to supplement or boost the
performance of conventional detergent compositions.
The detergent compositions according to the present invention
include compositions which are to be used for cleaning
substrates, such as fabrics, fibers, hard surfaces, skin etc.,
for example hard surface cleaning compositions (with or without
abrasives), laundry detergent compositions, automatic and non
automatic dishwashing compositions.
The following examples are meant to exemplify compositions of
the present invention, but are not necessarily meant to limit
or otherwise define the scope of the invention, said scope
being determined according to claims which follow.
A liquid detergent composition according to the present
invention is prepared, having the following compositions :
SUBSTITUTE SHEET
`~0~4/02580 ~ 8 3 PCT/US93/06223
31
% by weight of the total detergent compo~ition
Fatty acid 10
Oleic acid 4
Citric acid
NaOH 3.4
Propanediol 1.5
Ethanol 10
Tabl~ I
EXAMPLE I :
The extent of dye transfer from different colored fabrics
was studied using a launder-o-meter test that simulates a 30
min wash cycle. The launder-o-metsr beaker contains 200 ml of a
detergent solution, a 10cmxl0cm piece of the colored fabric and
a multifiber swatch which is used as a pick-up tracer for the
bleeding dye. The multifiber swatch consists of 6 pieces
(1.5cmx5cm each~ of different material (polyacetate, cotton,
polyamide, polyester, wool and orlon) which are sewn together.
The extent of dye transfer is assessed by a Hunter Colour
measurement. The Hunter Colour system evaluates the colour of a
fabric sample in terms of the ~E value which represents the
change in the Hunter L, a, b, values which are determined by
reflecting spectrometrie. The ~E value is defined by the
following equation:
E {(af -ai)2 + (bf-bi)2 + (Lf-Li) } /
where the subscripts i and f refer to the Hunter value before
and after washing in the presence of the bleeding fabric,
respectively. The least significant difference is 1 at 95
confidence level.
Example I demonstrates the enhanced dye transfer inhibiting
performance of the nonionic surfactants in combination with the
polyamine N-oxide containing polymers.
SUBSTITUTE SHEET
og4/o2s8o'~ ~~ 3 32 PCT~US93/06223,-
The surfactant that is used is a nonionic surfactantmanufactured by Shell and sold under the Tradename Dobanol.
The dye transfer inhibiting performance was determined by
measuring the whiteness of textile items washed with
compositions containing the nonionic and/or the polyamine N-
oxide containing polymers.
ExPerimental conditions:
pH =7.8
Washing temperature 40 C
A. A detergent composition according to Table I which contains
.,
no nonionic and no PVN0 (poly(4-vinylpyridine-N-oxide).
B. A detergent composition according to Table I which contains
nonionic (Dobanol 45/11) (270 ppm) and no PVN0 (poly(4-
vinylpyridine-N-oxide)).
C:~ A detergent composition according to Table I containing 6
ppm ~ of PYN0 (poly(4-vinylpyridine-N-oxide)) which has an
- ~average molecular weight of about 10,000 and an~amine to amine
H N-oxide~ratio~of 1: 10 (determined by NMR).
D :~ A ~detergent composition according to Table I containing 6
ppm of PVN0 (poly(4-vinylpyridine-N-oxide)) which has an
average molecular weight of about 10,000 and an amine to amine
N-oxide ratio of 1:10 and 270 ppm nonionic (Dobanol 45/11).
,~
~: .
Results: ~E values for the cotton pick-up tracer.
Bleeding fabric Bleeding fabric A B C D
, ;composition color
100% cotton Direct blue 9013.1 12 9.4 5.1
. ~ ~
,
- ,
BXAMPLE SI ~A/B/C)
A liquid detergent composition according to the present
invention is prepared, having the following compositions :
~, . .
SUBSTlTlJTE SHEET
21~02~ -
, -v094/~2580 33 PCT/US93/062~3
% by weight of the total detergent compositio~
jA B C
Linear alkylbenzene sulfonate 10 - -
Alkyl alkoxylated sulfate - 9
Polyhydroxy fatty acid - - 9 t
Trimethyl ammonium chloride C12-C14 - - 4
Alkyl sulphate 4 4 4
Fatty alcohol (C12-Cls) ethoxylate 12 12 12
Fatty acid 10 10 10
Oleic acid 4 4 4
Citric acid
:~ Diethylenetriaminepentamethylene 1.5 1.5 1.5
Phosphonic acid
NaOH 3.4 3.4 3.4
Propanediol 1.5 1.5 1.5
,:
Ethanol 10 10 10
Ethoxylated tetraethylene pentamine 0.7 0.7 0.7
Poly(4-vinylpyridine)-N-oxide 0-1 0-1 0-1
Thermamyl 0.13 0.13 0.13
Carezyme 0.014 0.014 0.014
FN-Base 1.8 1.8 1.8
Lipolase 0.14 0.14 0.14
Endoglucanase A 0.s3 0.53 0.53
Suds supressor (ISOFOLr) 2.'5 2.5 2.5
Minors up to 100
E~AMP~E III ~A/B/C)
A compact granular detergent composition according to the
present invention is prepared, having the following
formulation:
% ~y weight of the total ~etergent composition
B C
Linear alkyl benzene sulphonate 11.40 - -
Alkyl alkoxylated sulfate - 10
SUBSTITUTE SHEET
,
W094/02580 2~ 40~S3 PCT/US93/~6223 ~
Polyhydroxy fatty acid - - 9
Trimethyl ammonium chloride C12-C14 - - 4
Tallow alkyl sulphate 1.80 1.801.80
C45 alkyl sulphate 3.00 3.003.00
C4~ alcohol 7 times ethoxylated 4.00 4.004.00
Tallow alcohol 11 times ethoxylated 1.80 1.801.80
Dispersant 0.07 0.070.07
Silicone fluid 0.80 0.800.80
Trisodium citrate 14.00 14.00 14.Q0
Citric acid - 3.00 3.00 3.00
Zeolite 32.50 32.50 32.50
Maleic acid actylic acid copolymer 5.00 5.005.00
Cellulase (active protein) 0.03 0.030.03
Alkalase/BAN 0.60 0.600.60
Lipase 0.36 0.360.36
Sodium silicate 2.00 2.002.00
Sodium sulphate 3.50 3.503.S0
Poly(4-vinylpyridine)-N-oxide 0-1 0-1 0-1
Minors up to lO0
The above compositions (Example I(A/B/C) and II(A/B/C))
were very good at displaying excellent clay and detergent
performance with outstanding color-care performance on colored t
fabrics and mixed loads of colored and white fabrics.
SUBSTITVTE SHEET