Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2141Q3Q
Boehringer Mannheim GmbH
3728/00/
Liponucleotides of seco-nucleosides, their ~roduction as
well as their use as antiviral ~harmaceutical a~ents
The present invention concerns new phospholipid
derivatives of seco-nucleosides that link a lipid moiety
which represents a substituted C3 backbone with a seco-
nucleoside via a phosphate or thiophosphate as well as
their use as antiviral pharmaceutical agents.
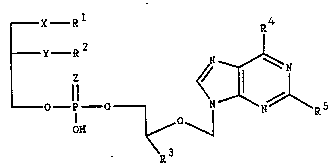
The invention concerns compounds of formula I,
- X Rl ~4
Y--R2
_ O _ I O _ I ~ N ~ RS
OH ~ O ~
\R3
in which
R1 denotes a straight-chained or branched, saturated or
unsaturated aliphatic residue with 1-20 carbon atoms
which can be substituted, if desired, once or
several times by phenyl, halogen, Cl-C6 alkoxy, Cl-C6
alkyl-mercapto, C1-C6 alkoxycarbonyl, C1-C6
alkylsulfinyl or Cl-C6 alkylsulfonyl groups,
2141030
._
-- 2
R2 denotes a straight-chained or branched, saturated or
unsaturated aliphatic residue with 1-20 carbon atoms
which can be substituted, if desired, once or
several times by phenyl, halogen, Cl-C6 alkoxy, Cl-C6
alkyl-mercapto, Cl-C6 alkoxycarbonyl, Cl-C6
alkylsulfinyl or Cl-C6 alkylsulfonyl groups,
R3 denotes hydrogen or a Cl-C6 alkyl group which is
substituted, if desired, by hydroxy
R4 can be hydrogen, hydroxy, amino or an amino group
substituted once or twice by Cl-C6 alkyl,
R5 can be hydrogen, hydroxy, amino or an amino group
substituted once or twice by Cl-C6 alkyl,
X represents a valency dash, oxygen, sulphur, sulfinyl
or sulfonyl,
Y can have the same meaning as X and the two groups X
and Y can be the same or different,
Z can be oxygen or sulphur,
their tautomers and their physiologically tolerated
salts of inorganic and organic acids and bases.
Since the compounds of the general formula I contain
asymmetric carbon atoms, all optically active forms and
racemic mixtures of these compounds are also the subject
matter of the present invention.
21410~0
-- 3
The production and use of liponucleotides as antiviral
pharmaceutical agents are described in J. Biol. Chem.
265, 6112 (1990) and EP 0 350 287. In this case only
dimyristoylphosphatidyl and dipalmitoylphosphatidyl
residues with their fatty acid ester structure coupled
to known nucleosides such as e.g. AZT (azidothymidine)
and ddC (dideoxycytidine) were examined and synthesized.
EP 0 350 287 describes the respective 1,2-diesters of
glycerol.
In J. Med. Chem. 33, 1380 (1990) nucleoside conjugates
of thioether lipids with cytidine diphosphate are
described which exhibit an antitumour action and which
can be used in oncology.
5'-(3-SN-phosphatidyl)nucleosides having an
antileukaemic activity are described in Chem. Pharm.
Bull. 36, 209 (1988), as well as their enzymatic
synthesis from the appropriate nucleosides and
phosphocholines in the presence of phospholipase D
having transferase activity.
Liponucleotides with a cyclic sugar moiety in the
nucleoside which have an antiviral action are described
in the patent application PCT/EP91/01541.
The Acyclovir-phospholipid conjugate from L-a-
dimyristoylphosphatidyl acid and Acyclovir is described
in Acta Chem. Scand., Ser. B. 39, 47 (1985) [cf. also
Organophosphorus Chem. 18, 187 (1987)].
The ether-/thioether lipids (X, Y = O or S) of the
present invention are novel and also exhibit valuable
pharmacological properties. They are particularly
2141030
-- 4
suitable for the therapy and prophylaxis of infections
which are caused by DNA viruses such as e.g. the herpes-
simplex virus, the cytomegaly virus, papilloma viruses,
the varicella-zoster virus or Epstein-Barr virus or RNA
viruses such as toga viruses or retroviruses such as the
oncoviruses HTLV-I and II as well as the lentiviruses
Visna and human immunodeficiency virus HIV-1 and 2.
The compounds of formula I appear to be particularly
suitable for treating clinical manifestations of viral
herpes infection in humans. The compounds of the general
formula I act antivirally without being cytotoxic in
pharmacologically relevant doses.
The compounds are additionally distinguished by a very
good oral tolerance with good bioavailability.
The compounds of the present invention and their
pharmaceutical preparations can also be used in
combination with other pharmaceutical agents for the
treatment and prophylaxis of the above-mentioned
infections. Examples of these agents containing further
pharmaceutical agents which can be used for the
treatment and prophylaxis of HIV infections or diseases
which accompany this illness are 3'-azido-3'-deoxy-
thymidine (AZT), 2',3'-dideoxynucleosides such as e.g.
2'-3-dideoxycytidine (ddC), 2',3'-dideoxyadenosine and
2',3'-dideoxyinosine (ddI) or non-nucleosidic RT
inhibitors such as HEPT, Nevirapin or L-697, 661 and
corresponding derivatives. The compounds of the present
invention and the other pharmaceutical agent can each be
administered individually, simultaneously and optionally
in a single or two separate formulations or at different
times.
2191030
-- 5
Alkali, alkaline-earth and ammonium salts of the
phosphate group come into consideration as possible
salts of compounds of the general formula I. Lithium,
sodium and potassium salts are preferred as alkali
salts. In particular magnesium and calcium salts come
into consideration as the alkaline-earth salts.
According to the invention ammonium salts are understood
as salts which contain the ammonium ion which can be
substituted up to four times by alkyl residues with 1-4
carbon atoms and/or aralkyl residues, preferably benzyl
residues. The substituents can in this case be the same
or different.
The compounds of the general formula I can contain basic
groups, in particular amino groups, which can be
converted into acid addition salts using suitable
organic and inorganic acids. Hydrochloric acid,
hydrobromic acid, sulphuric acid, phosphoric acid,
fumaric acid, succinic acid, tartaric acid, citric acid,
lactic acid, maleic acid or methanesulfonic acid come
for example into consideration as the acid.
In the general formula I R1 preferably denotes a
straight-chained Cg-Cl4 alkyl group which can
additionally be substituted by a C1-C6 alkoxy or a Cl-C6
alkylmercapto group. R1 in particular represents a
decyl, undecyl, dodecyl, tridecyl or tetradecyl group.
Methoxy, ethoxy, butoxy and hexyloxy groups preferably
come into consideration as the C1-C6 alkoxy substituents
of R1. If Rl is substituted by a Cl-C6 alkylmercapto
residue, this is to be understood in particular as a
methylmercapto, ethylmercapto, propylmercapto, butyl-
mercapto and hexylmercapto residue.
2141030
R2 preferably denotes a straight-chained Cg-Cl4 alkyl
group which can in addition be substituted by a C1-C6
alkoxy group or a C1-C6 alkylmercapto group. R2 in
particular represents a decyl, undecyl, dodecyl,
tridecyl or tetradecyl group. The methoxy, ethoxy,
propoxy, butoxy and hexyloxy group preferably come into
consideration as the C1-C6 alkoxy substituents of R2. If
R2 is substituted by a C1-C6 alkylmercapto residue, then
this is understood in particular to be a methylmercapto,
ethylmercapto, butylmercapto and hexylmercapto residue.
In the definition of R3 the alkyl group denotes in
particular a straight-chained or branched alkyl group
preferably having up to four C atoms such as e.g.
methyl, ethyl, n-propyl, isopropyl or n-butyl. These
alkyl groups are preferably substituted by one or two
hydroxy groups such as e.g. hydroxymethyl, 2-hydroxy-
ethyl or 3-hydroxypropyl.
Cl-C6 alkyl groups in general denote straight-chained or
branched alkyl residues preferably having up to four C
atoms such as e.g. methyl, ethyl, n-propyl, isopropyl,
n-butyl or isobutyl.
R4 preferably denotes a hydroxy or amino group.
R5 in particular denotes hydrogen or a hydroxy or amino
group.
X and Y preferably represent an oxygen or sulphur atom.
Z is preferably an oxygen atom.
Especially preferred coupled seco-nucleosides in the
2141030
-
claimed liponucleotides of the general formula I are
Ganciclovir or Acyclovir.
The compounds of formula I can be prepared by reacting
1. a compound of formula II,
X R 1
Y- R
z (II)
Il
O P - OH
OH
in which Rl, R2, X, Y and Z have the stated
meAn;ngs, with a compound of the general formula
III,
. .
</ ~ (III)
HO ~ N ~ R5
~O~
R3
in which R3, R4 and R5 have the above-mentioned
meAn;ng using a condensing agent such as DCC
(dicyclohexylcarbodiimde) in pyridine or in the
presence of 2,4,6-triisopropylbenzenesulfonic acid
chloride and a tert. nitrogen base e.g. pyridine or
lutidine in an inert solvent such as e.g. toluene
or directly in pyridine and, after hydrolysis is
21~1030
- 8 -
completed, the oxygen protecting groups are cleaved
if desired according to conventional methods in
nucleoside chemistry or
2. a compound of formula IV
X R 1
-Y R2 CH3
(IV)
I / N~ CH~
O- O
CH~
in which R1, R2, X, Y and Z have the above-
mentioned meaning is reacted with a compound of
formula III in which R3, R4 and R5 have the stated
meanings in the presence of phospholipase D in an
inert solvent such as e.g. chloroform in the
presence of a buffer and, after the reaction is
completed, the oxygen protecting group is cleaved
if desired according to conventional methods in
nucleoside chemistry.
The production of compounds of formula II and IV is
described in DE 39 29 217.7 and W0 91/05558.
The production of compounds of the general formula III
is described in Progress in Medicinal Chemistry, vol.
23, 187 (1986) and in the literature cited there.
2141030
g
Acyclovir and Ganciclovir are commercially available.
The pharmaceutical agents containing compounds of
formula I for the treatment of viral infections can be
administered enterally or parenterally in a liquid or
solid form. The usual methods of administration come
into consideration in this case such as for example
tablets, capsules, coated tablets, syrups, solutions or
suspensions. Water is preferably used as the injection
medium which contains the usual additives for injection
solutions such as stabilizers, solubilizers and buffers.
Such additives are e.g. tartrate and citrate buffer,
ethanol, complexing agents such as ethylenediamine-
tetraacetic acid and their non-toxic salts, high
molecular polymers such as liquid polyethylene oxide to
regulate viscosity. Liquid carrier materials for
injection solutions have to be sterile and are
preferably dispensed into ampoules. Solid carrier
materials are for example starch, lactose, mannitol,
methylcellulose, talcum, highly dispersed silicic acid,
higher molecular fatty acids such as stearic acid,
gelatin, agar-agar, calcium phosphate, magnesium
stearate, animal and plant fats, solid high molecular
polymers such as polyethylene glycols etc.. Suitable
preparations for oral applications can if desired
contain flavourings or sweeteners.
The dosage can depend on various factors such as mode of
administration, species, age or individual condition.
The compounds according to the invention are usually
administered in amounts of 0.1 - 100 mg, preferably 0.2
- 80 mg per day and per kg body weight. It is preferable
to divide the daily dose into 2-5 administrations, 1-2
tablets being administered at each application with a
content of active substance of 0.5 - 500 mg. The tablets
21 ~1030
-- 10 --
can also be retarded by which means the number of
applications per day can be reduced to 1-3. The content
of active substance of the retarded tablets can be 2 -
1000 mg. The active substance can also be administered
by continuous infusion in which case amounts of 5 -
1000 mg per day are normally sufficient.
The following compounds of formula I come into
consideration within the sense of the present invention
in addition to the compounds mentioned in the examples
and combinations of all the meanings mentioned in the
claims for the substituents:
1. 2'-[9-(ethoxymethyl)guanine]phosphoric acid-(3-
dodecylmercapto-2-decyloxy)-1-propyl ester
2. 2'-(9-{[(1-hydroxymethyl)ethoxy]methyl}guanine)-
phosphoric acid-(3-dodecylsulfonyl-2-decyloxy)-1-
propyl ester
3. 2'-(9-{[(1-hydroxymethyl)ethoxy]methyl}guanine)-
phosphoric acid-(3-dodecylsulfonyl-2-decyloxy)-1-
propyl ester
4. 2'-(9-{[(1-hydroxymethyl)ethoxy]methyl}guanine)-
phosphoric acid-(3-dodecylmercapto-2-decyloxy)-1-
propyl ester
S. 2'-[9-(ethoxymethyl)guanine]phosphoric acid-(3-
undecylmercapto-2-decyloxy)-1-propyl ester
6. 2'-[9-(ethoxymethyl)guanine]phosphoric acid-(3-
dodecyloxy-2-decyloxy)-1-propyl ester
214103~
7. 2'-(9-{[(1-hydroxymethyl)ethoxy]methyl}guanine)-
phosphoric acid-(3-dodecylmercapto-2-nonyloxy)-1-
propyl ester
8. 2'-(9-{[(1-hydroxymethyl)ethoxy]methyl}guanine)-
phosphoric acid-(3-dodecylmercapto-2-decyl-
mercapto)-l-propyl ester
9. 2'-(9-{[(1-hydroxymethyl)ethoxy]methyl}guanine)-
phosphoric acid-(3-undecylmercapto-2-decyloxy)-1-
propyl ester
10. 2'-[9-(ethoxymethyl)guanine]phosphoric acid-(3-
tridecylmercapto-2-decyloxy)-1-propyl ester
11. 2'-(9-{[(1-hydroxymethyl)ethoxy]methyl}guanine)-
phosphoric acid-(3-tridecylmercapto-2-decyloxy)-1-
propyl ester
12. 2'-[9-(ethoxymethyl)guanine]phosphoric acid-(3-
dodecylmercapto-2-dodecyloxy)-1-propyl ester
13. 2'-[9-(ethoxymethyl)guanine]phosphoric acid-(3-
dodecylmercapto-2-undecyloxy)-1-propyl ester
14. 2'-(9-{[(1-hydroxymethyl)ethoxy]methyl}guanine)-
phosphoric acid-(2,3-bis(dodecylmercapto)-1-propyl
ester
15. 2'-(9-{[(1-hydroxymethyl)ethoxy]methyl}guanine)-
phosphoric acid-(3-dodecylmercapto-2-dodecyloxy)-1-
propyl ester
2141030
- 12 -
16. 2'-[9-(ethoxymethyl)guanine]phosphoric acid-(3-
undecyloxy-2-dodecyloxy)-1-propyl ester
17. 2'-[9-(ethoxymethyl)guanine]phosphoric acid-(3-
decylsulfonyl-2-dodecyloxy)-1-propyl ester
18. 2'-[9-(ethoxymethyl)guanine]phosphoric acid-(3-
decyloxy-2-decyloxy)-l-propyl ester
19. 2'-[9-(ethoxymethyl)guanine]phosphoric acid-(3-
dodecylmercapto-2-dodecyloxy)-1-propyl ester
20. 2'-(9-{[(1-hydroxymethyl)ethoxy]methyl}guanine)-
phosphoric acid-(3-tetradecylmercapto-2-decyloxy)-
l-propyl ester
21. 2'-[9-(ethoxymethyl)guanine]phosphoric acid-(3-
pentadecylmercapto-2-decyloxy)-1-propyl ester
22. 2'-(9-{[(1-hydroxymethyl)ethoxy]methyl}guanine)-
phosphoric acid-(3-tridecylmercapto-2-decyloxy)-1-
propyl ester
23. 2'-[9-(ethoxymethyl)guanine]phosphoric acid-(3-
dodecylmercapto-2-octyloxy)-1-propyl ester
21410~0
- 13 -
Example
Phosphoric acid-(3-dodecYlmercapto-2-decyloxy)-1-propyl
ester
A suspension of 4.26 g P4Olo in 60 ml absolute pyridine
was admixed at room temperature with 13 ml
hexamethyldisiloxane and heated to 100C for 1 hour. It
was then slightly cooled, admixed with 25 g 3-
dodecylmercapto-2-decyloxy-1-propanol and heated for a
further 2.5 hours to 100C.
After completely cooling to room temperature and
removing the highly volatile components in a vacuum, the
phosphate could be extracted with ether from the aqueous
suspension of the residue. The evaporation residue of
the ether phase was purified by column chromatography on
silica gel 60 or RP 18. Yield 18.7 g (63 %), Rf = 0.66
(CH2C12/MeOH/H20 6.5/2.5/0.4) on TLC plates, Merck 5715,
silica gel 60.
Example 2
2'-(9-~ r tl-hydroxymethyl)ethoxYlmethyl~guanine)Phos-
phoric acid-(3-dodecylmercapto-2-decyloxy)-1-~ropyl
ester
1.45 g (3 mmol) phosphoric acid-(3-dodecylmercapto-2-
decyloxy)-l-propyl ester and 770 mg (3 mmol) Ganciclovir
were twice admixed with 20 ml absolute pyridine each
time and evaporated. The residue was taken up in 20 ml
absolute pyridine, 2.7 g (8.5 mmol) 2,4,6-triisopropyl-
benzenesulfonic acid chloride was added under nitrogen
2141030
- 14 -
and it was stirred for 24 hours at 40C. Then 10 ml
water was added, the mixture was stirred for a further 2
hours at room temperature and the solvent was removed in
a rotary evaporator.
The oily residue was freed from residual pyridine by
evaporation with toluene and purified by means of column
chromatography on RP 18 with a linear gradient of
methanol/water 7/3 to 9.S/0.5 as the eluant. Yield
0.75 g (34 ~ of theory), oil. Rf = 0.73 (H20/MeOH
0.5/9.5) on RP 8, Rf = O . 30 (CH2C12/MeOH/H20
6.5/2.5/0.4) on TLC plates, Merck 5715, silica gel 60 F.
Example 3
2'- r 9-(ethoxYmethYl)quanine~phosPhoric acid-(3-dodecyl-
mercapto-2-decyloxy)-1-proPYl ester
This compound was produced analogously to example 1 from
Acyclovir in a 47 % yield, oil, Rf = 0.77 (H20/MeOH
0.5/9.5) on RP 8, Rf = O . 35 (CH2C12/MeOH/H20
6.5/2.5/0.4) on TLC plates, Merck 5715, silica gel 60.