Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
i~ ~ S7~ , ~
PROC13SS E'O~ DISPOSING OF SODI~ SULF~7R STORAGE: CEI~I~S .
The invention relates to a ~roces~ for di~osing of sodium-
sulfur ~tora~e cells.
Increasing coneum~tion o~ sodium-~ulfur storage cells crea- ~ ~ ;
tes the ~eed to re~rocess these storage cells and to
reproce~s or recycle the com~onents o~ the cell.
It is kn~wn, accordi~g to the German unexamined }aid-o~en ~ ;
~aten~ application 39 27 225, to embed ~torage cells in
wax-like materials such as ~araffin, remove the sodium by
way of fusing, and ~e~erately remove and recycle the sulfur
electrodes and the casing materials.
Further, it is known from ~uropean patent a~licat~on ~ ~
0 433 654 to shred the cells and, by adding sodium cyaniae ;~`
and water, produce sodium thiocyanide.
',` '
It i~ an ob~ect of the invention to ~rocess sodium-sulfur
storage cells by em~loying simple mQthods and means to .
yield maximum ~ro~ortions of useful and recyclable substan~
ces .
The object is achieved according to the features of Claim 1
hereinbelow.
The followin~ variants ~resent themselves in accordance
with this invention~
Variant 1: `
The battery or individual cells are mechanically crushed or
disinte~rated by shredding or similar techni~ues. In order
to ~revent oxidation from occurring, this process may be
carried out under inert qas or vacuum, or, preferably, --~
under water. ~he use of water ~resents the ad~antage that -~
- 2 - ~-
'`' '.'~'"' `,'`''','
it is ~ossible to intercept the exothermic reaction o~ any
sodium metal which may possibly still be present, thus ~ut- ~`
ting less stre~s on the comminutins~ device, e.~. the shred-
der. In addition, the dissol~ing ~roce8~ i~ already set off
while the disintegrat~on takes ~la~:e, thereby ~aving time.
Hydrogen resulting from the above ~rocess may be em~loyed
~or heating ~urpo~es. The disintegration should result in
pieces of 0.1 cm to 5 cm, ~referably about 1 cm.
The dissolution reaction of the ~olysulfide and the solid
sulfur may take ~lace within a temperature inter~al from ~ ~-
room tem~erature to 200 C, ~referably the reaction i8 car-
ried out at 120 C. De~ending on the ty~e of battery, the
dissolution réaction does generally not require the
addition of chemicals, such as a lye. By adding an oxidant ` ;~
subsequent to dissolution, the entire amount of ~oly-
~ulfide, which has been formed from the solid sulfur, can
generally be oxidated to sulfate without transferring the
solution to another reactor. Pre~exably, hydrogen ~eroxide
is u~ed for the oxidat~on proce~s. To achieve complete con-
~er~ion, the ~H must always be maintained above ~H 8. To
this end a lye i~ u~ed, preferably sodium hydroxide. After
~e~arating the liquid, the residue is washed with water.
The re~ulting washings are used in the dissol~ing ~roces~
Th~ cleaned solids may be se~arated into metals and non~
metals if recycling the components is economically feas-
ible. Otherwise, un~roblematic waste dis~osal i~ possible,
~or example, on a dum~ing ground. The salt ~olution may be
dis~o~ed o~ or reused either immediately or after eva~orat-
ing, the resulting water being reintroduced into the wash-
ing or dissol~ing ~roce~s.
Variant`2:
The disintegration, dissolution and se~aration ~rocesses, - `~
like the treatment of the solid re~idue, are carried out as
described under 1.
4 ~. 5 7 j' ' ' ;, ~
- 3 -
.: ~ ..'':
The solution i~ reacted with acid, ~referably sulfuric
acid, and oxidant, ~referably hydrogen ~eroxide. The reac- f~
t~on tem~exature may be between room temperature and
150 C, and, ~refereably, at the end of the exothermal
reaction, a~roximately 120 C. This yields elementary sul-
~ur. The hydro~en sulfide whlch ha8 been formed i~ o~idize
in situ to ~ul~ata. If the tem~erature i8 maintained above
the meltin~ ~oint of sulfur, a liquid sulfur phase quic~ly
form~, which can be easily decanted as a liquid, purified ~
and recycled. The disposal of the solution and solids iB - `. ,~ ~,
carried out a~ describea under 1. ;
variant 3:
~he size-reduction, dissolution an~ se~aration proces~es,
like the treatment o~ the solid residue, are carried out as
described under 1.
The solution is treated with acid, ~referably sulfuric
acid. This ~rocess yields sulfur, which is decanted as
in 2. ~ydrogen sulfide which has formed simultaneously 18
withdrawn, decocted or stripped with gas, preferably
nitro~en. It is used as a chemical or it is con~erted to
further sul~ur in a Claus process plant. Optionally, the
purification of this sulfur takes place together with the
decanted sulfur.
The salt solution and the solids are dis~osea of as in 1.
, - . ~
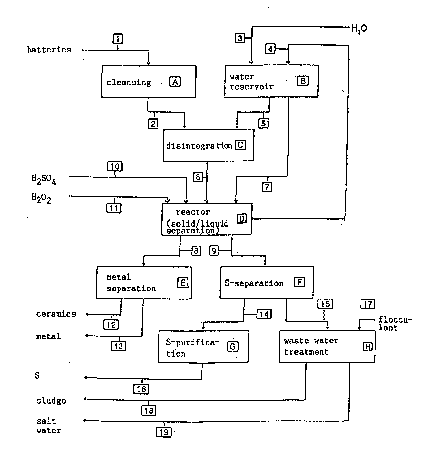
The variants are shown in the form of diagrammes in Figures
1 to 3.
- . -
The ~roce~s variants have in common that initially the
storage cells are ~isinte~rated in the ~resence of water
and the soluble com~onents of the electric element are di~
solved to form a sodium-~olysulfide solution. Either simul~
taneously or subsequent thereto, ~referably sodium hydrox-
ide solution or sulfuric acid is added, which leads to the
7 ~ ~:
- 4 - ~
.
formation, either com~letely or ~artially, of sodium 8ul-
fate. The ~tructural ~arts o~ the cell, i.e. the cell wall,
electrode~, ceramicR, etc. can be se~arated at a~y time a~
soon a~ the Na-~olysulfide has been d~ssolvea.
The amount of sodium sulfate formed can be increased by
adding aqueou~ h~dro~en peroxide ~olutio~.
Hydroge~ sul~ide i8 reacted with ~I202-solution to form sul-
fur and~or ~ulfate. This can yield ~odium salt as a uni~orm
~roduct, which may be eva~orated, o~tionally ~urified and
crystalliz~d in a known manner.
Sulfuric acid together with H202-solution re~uir~s con- ` ~-
siderably less ~eroxide as compared to alkalina oxidation
and leads to the for~3tion of sodium sulfatQ a~art from
elementary sulfur, whereby the sulfur may be se~arated.
Sodium sulfate and sulfur are to be ~urified in a known
manner.
....
Sulfuri¢ acid without the addition of ~eroxide rQsults in
the formation o~ Na2S0~, sulfur and hydrogen sulfide, the ~ ~
hydrogen sulfide being expulsed and oxidized to elementary ~ ~ -
sulfur, for example, in a Claus ~lant. Na2S0~ and the
~rimar~ly formed sulfur may be se~arated as described
hereinabove.
Embodiments of the process are ~rovided according to the ~ ~-
~ubclaims.
~'
The disintegxation o~ batterie~ and inaividual cell~, re-
~ectively, often with a content of 40 g ~er individual
cell, i~ carried out using breaking or cutting ~lants,
advantageously by shredding under water or un~er irrigation
with water if ~rovision is made for the inertization of the
gas volume. The resulting ~ieces are to have a diameter of
0.1 to 5 cmr ~referably 0.2 to 2 cm. Residues of sodium
from cells which have not been com~letely di~char~ed are
.. , . ~ . ~ . ........ ...... .
1 5 7 ~ !
~ .
- 5 -
. ' ':'~
rendered harmle~ through water. iHydro~en ~roduced in this
~roce~s can be withdrawn. During comminution the dissolu~
tion reaction o~ the Na-~olysul~ e be~ins and is continued
after having been trans~erred to a reactor at temperature~
between room tem~erature and 200C, ~re~erably about 80 to ~;
about 120C, while the liquid is being moved. The dis~olu-
tion rate o~ Na-~olysulfide is increasea by elevated tem- ^~
~eratures and in one exam~le is 10 min at 80C. Saturated
solutions o~ about 260 g/l with a mean com~osition o~
Na~S2 7 are obtained using water. The dissolution reaction
and the sub6equent reactions for recycling the aative sub-
stances sod~um/sul~ur are preferably carried out in a reac-
tor.
It is ~referred that the reactor be o~ a closed ty~e and
~rovided with a feeding device, gas discharge, bottom
drain, heating and stirrer. It is ex~edient to desi~n the
reacto~ ~or increased ~ressure of up to 20 bar and for
lower ~ressure of. The reaction i~ su~ervised ~refexably by
conducti~ity measurements. `~
There are se~eral ~ar~ants for ~he re~rocessing of the Na~
polysulfide solution.
Sodium hydroxide solution with added H202-solution, - ~-
advantageously u~ to 30% ~m/m), yields only sodium sulfate.
At temperatures of 80 to 100C the reaction terminates
within less than 1 minute. In order to oxidize the sulfur
com~letely, 9.1 mol H2O2 ~er mole Na2S27 are necessary, i.e.
a comparatively large amount. This re~rocessing method is ~ -~
thus es~ecially suitable ~or smaller ~lants, all the more ;~ -
80 since a~art *rom the reactor no other ~lants are re~
quixed.
The further ~rocessing and recycling, res~ectively, o~ the
Na-~olysulfide solution may also be carried out under
acidic conditions, pre~erably adding sulfuria acid or, op- -~
tionally, a~ueous hydrochloric acid.
H202 may or may not be added. ~ ;
~ l 4 l ~ ~7 ;~
- 6
. ' ~
The acidic oxidation with sulfurlc acid and ~202-solu~ion,
consumes only one mol~ H2SO~ ~er mole Na2S27, and one mole
~22 under formation of sodi~m sul~ate and sulfur. Tem~era~
tures about of 120C are usable. ~t 120C the sul~ur is
able to accumulate at the bottom of t~e reactor and can be
arained in liquid form and re~roce~ssed. It i8 of advanta~e
that the H202 consum~tion i8 low.
A ~articularly ad~a~ta~eous embodiment with res~ect to
minimizing the ~roducts to be withdrawn from the ~rocess,
~ro~ides for the sodium salt to be di~integrated by way of ~;
electrodialysis to form 60dium hydroxido solution ana acid, `~
and, ~referably, to reuse the acid in the ~rocess. ~f
Na2SO~ i8 formed it i8 advantageous to carry out a ~reci~
tation with CaO; the resulting gypsum and NaOH can be sup-
~lied to a recyclin~ ~rocess or marketed.
~he ac~dic reaction of Na-~oly~ulfide solution may also be `~
effected wlthout the addition of H202 or other oxidants. In
this case sodium sulfate, hydrogen sulfide and elementary
sulfur i8 ~ormed stoichiometrically. Optionally, hydrogen - ,~
sul~ido may be expulsed at elevated tem~erature with inert
gases such as nitrogen, and it may be oxidized in a conven-
tional manner with atmos~heric oxygen in a sulfur recovery
~lant to yield sulfur. This variant is suitable for sites
with a ~lau~ ~lant and for dis~osing large ~uantities of
stora~e cell~. Only one mole H2SO, is consumed ~er mole of
Na~S2,7-
~xamDle 1:
Alkaline Oxidation
20 g sodium ~olysulfide (0.15 mol calculated as Na2S27) in
a~ueous solution is ~laced in a ~res~ure-~roof reactor of
: :
~ ~141~7~
.~
- 7 - ~
0.5 1 ca~acity. A~ter addi~g 21.8 g (0.54 mol) sodium hy- ;
droxide, 46.2 g (1.36 mol) hydro~en ~eroxide i8 added as
30 ~ (m/m) 801ution while ~tirrinsr. ~he measured conducti- ~`
vity shows that at room term~eratur the reaction terminates ;`~
wit~in 10 to 12 minute~. The react:ion i8 quantitati~e and
53 g (0.4 mol) 80dium sulfate i8 obtained as aqueous 801u-
tion.
~:. :,".
~xamDle 2
Acldic Oxidation , ~` ~
,~ ` , ` . ..:
In a reactor a solution of 20 g (0.15 mol) sodium ~oly8ul- ,:
fide i8 ~roduced ~rom used Na-S storage cells by addin~
water and the solid com~one~ts of the cell are removed. `~
While stirrin~ a mixture of 14.7 g (0,15 mol) sulfuric acid ` ``
in 5.1 g (0.15 mol) hydrogen ~eroxide is added as 30 ~
(m/m) solution, Then the closed reactor is heated to 120C.
In this ~rocess the precipitated ~ulfur i8 melted and forms
~ro~let~ which collec~ at the bottom of the reactor. The
sul~ur can be removed. 21.5 g (0.15 mol) sodium sulfate and `~
13 g (0.4 mol) sulfur are obtained.
~xam~le 3: -`
Ac~difying and Strip~ing `-
A like solution o~ 2Q g sodium ~olysulfide is ~laced in the
reactor. 14.7 g (0.15 mol) sulfuric acid is added while
stirring. The nascent hydrogen sulfide ga~ is stri~ed ~rom ;~
the reactor with nitrogen and is absorbed in a washing
bottle with sodium hydroxide solution. After the reaction
has come to an end, the rsactor is heated to 120C internal
temperature in order to obtain larger dro~lets. After coo-
ling, 8.2 g (0.25 mol) solid sulfur and 21.4 g (0.15 mol)
sodium sul~ate is obtained.
The hydxogen sulfide yield i~ 5.1g (0.15 mol).