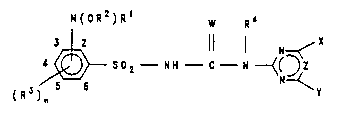Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
-- ~ILE. P~N ~H~S 4~ r~r~ 1 4 ~7 6
~ T T~ANSLA~
WO 94/06778 PCT/EP93/02469
Description
~ydroxylaminophenylsulfonylureas, their synthesis, and
their use as herbicides and plant growth regulators
The invention relates to the technical field of
herbicides and plant growth regulators, in particular the
herbicides for selectively controlling broad-leaved weeds
and grass weeds in crops of useful plants.
It has been disclosed that heterocyclically substituted
phenylsulfonylureas which have an amino or a functional-
ized amino group on the phenyl ring have herbicidal and
plant-growth-regulating properties (US-A-4,892,946;
US-A-4,981,509; EP-A-169,815).
Furthermore, WO-89/10921 discloses phenylsulfonylurea
herbicides in which the phenyl ring is 2,4-disubstituted
or 2,4,6-trisubstituted and can have, inter alia, an
alkylamino or (substituted) hydroxylamino group in the
4-position.
Surprisingly, it has now been found that certain hetero-
cyclically substituted phenylsulfonylureas which have an
unsubstituted or a substituted hydroxyl~;no function are
particularly suitable as herbicides or plant growth
regulators.
The present invention relates to compounds of the
formula (I) or salts thereof
- 2 - ~1447
N(0R2)Rl W R4
~ S02- N~ - C - N ~ ( ~ (I)
in which
Rl is Co-R5, CO-OR6, CO-NR'R8, CS-R9, CS-OR', CS-NR'R8,
SO2Rll, SOR", So2NR7R8 or CO-COR'2,
R2 is hydrogen, C,-C6-alkyl, C2-C6-alkenyl or C2-C6-
alkynyl, the last-mentioned 3 radicals independently
of one another being unsubstituted or substituted by
one or more radicals selected from the group com-
prising halogen, C,-C4-alkoxy and C,-C4-alkylthio,
R3 is halogen, C,-C6-alkyl, C2-C6-alkenyl, C2-C6-alkynyl,
C,-C6-alkoxy, C2-C6-alkenyloxy or C2-C6-alkynyloxy,
the last-mentioned 6 radicals independently of one
another being unsubstituted or substituted by one or
more radicals selected from the group comprising
halogen, C,-C4-alkoxy and alkylthio, or is CO-CO-R'6,
CS-R'6, CO-CO-OR", CS-OR'7, CS-SR'7, CO-NR'8R'9,
CS-NRl8Rl9, NR'8R'9, SO2NR'8R'9, cyano, S(O)o~R20, CO-R'6,
CO-OR", NO2 or C(R21)=N-R22
or
a 5- or 6-membered saturated, unsaturated or hetero-
aromatic heterocycle having up to 4 hetero atoms
selected from the group comprising N, O and S, which
is unsubstituted or substituted by one or more
radicals selected from the group comprising halogen,
C,-C4-alkyl, C,-C4-alkoxy, C1-C4-alkylthio, C,-C4-
haloalkyl, CN, NO2, C~O, (C,-C4-alkyl)-carbonyl and
(C,-C4-alkoxy)-carbonyl and in which up to 2 carbon
ring positions can be replaced by CO and up to two
sulfur atoms in the hetero ring can be replaced by
_ 3 _ 2~ ~ ~769
so2l
R4 is hydrogen or C,-C,-alkyl,
R5 is hydrogen or C,-C6-alkyl, C2-C6-alkenyl or C2-C6-
alkynyl, the last-mentioned 3 radicals being unsub-
stituted or substituted by one or more radicals
selected from the group comprising halogen, Cl-C4-
alkoxy, C,-C4-alkylthio, NR13R'4 and NR'52, or is C3-C,-
cycloalkyl which is unsubstituted or substituted by
one or more radicals selected from the group com-
prising halogen, C,-C4-alkyl, C,-C4-alkoxy,
C,-C4-alkylthio, NR'3R'4 and NR'52, or is benzyl,
unsubstituted or substituted aryl or an
unsubstituted or substituted heterocyclic radical,
R6 is C,-C6-alkyl, C2-C6-alkenyl or C2-C6-alkynyl, the
last-mentioned 3 radicals being unsubstituted or
substituted by one or more radicals selected from
the group comprising halogen, C,-C4-alkoxy, C,-C4-
alkylthio, NR'3Rl4 and NR'52,
R' is hydrogen or C,-C6-alkyl, C2-C6-alkenyl, C2-C6-
alkynyl or C,-C6-alkoxy, the last-mentioned 4 radi-
cals being unsubstituted or substituted by one or
more radicals selected from the group comprising
halogen, C,-C4-alkoxy, Cl-C4-alkylthio and NR'3R'4,
R8 is hydrogen or C,-C6-alkyl, C2-C6-alkenyl or C2-C6-
alkynyl, the last-mentioned 3 radicals being unsub-
stituted or substituted by one or more rA~;cAls
selected from the group comprising halogen, Cl-C4-
alkoxy and C1-C4-alkylthio, or is CoR23, Co2R24,
CoNR'3R'4 or SO2R'l,
0 R9 is analogous to R5, preferably hydrogen, C,-C6-alkyl
or C3-C6-alkenyl, the last-mentioned 2 radicals being
unsubstituted or substituted by one or more halogen
atoms or by one or two radicals selected from the
_ 4 _ 2 1 4 4~ 6~
group comprising C,-C4-alkoxy, alkylthio and NRl3R~4,
Rl is analogous to ~6, preferably C,-C6-alkyl or C3-C6-
alkenyl, the last-mentioned 2 radicals being unsub-
stituted or substituted by one or more halogen atoms
or by one or two radicals selected from the group
comprising Cl-C4-alkoxy and Cl-C4-alkylthio,
Rll is Cl-C6-alkyl, C2-C6-alkenyl or C2-C6-alkynyl, the
last-mentioned 3 radicals being unsubstituted or
substituted by one or more radicals selected from
the group comprising halogen, Cl-C4-alkoxy and Cl-C4-
- alkylthio,
Rl2 is hydrogen, OH, NH2, mono- or di(C,-C4-alkyl)amino,
C,-C4-alkyl or Cl-C4-alkoxy, the last-mentioned 2
radicals being unsubstituted or substituted by one
or more radicals selected from the group comprising
halogen, Cl-C4-alkoxy and Cl-C4-alkylthio,
the Rl3 radicals independently of one another are hydrogen
or Cl-C6-alkyl, C3-C6-alkenyl or C3-C6-alkynyl, the
last-mentioned 3 radicals being unsubstituted or
substituted by one or more radicals selected from
the group comprising halogen, Cl-C4-alkoxy and Cl-C4-
alkylthio, or are COR2s or CO2R26,
the Rl4 radicals independently of one another are hydrogen
or Cl-C6-alkyl, C3-C6-alkenyl or C3-C6-alkynyl, the
last-mentioned 3 radicals being unsubstituted or
substituted by one or more radicals selected from
the group comprising halogen, Cl-C4-alkoxy and Cl-C4-
alkylthio,
NRls2 radicals independently of one another are a hetero-
cyclic 3- to 7-membered ring which can contain,
besides the nitrogen atom, one or two further hetero
ring atoms selected from the group comprising N, O
and S and which is unsubstituted or substituted,
2i~7~3
- 5 -
Rl6 is a rA~;c~l analogous to R5,
Rl' is hydrogen, Cl-C6-alkyl, C2-C6-alkyl or
C2-C6-alkynyl, the last-mentioned 3 radicals being
unsubstituted or substituted by one or more radicals
selected from the group comprising halogen, Cl-C4-
alkoxy, Cl-C4-alkylthio, NRl3Rl4 and NRl52,
Rl8 is a radical analogous to R',
Rl9 is a radical analogous to R8,
R2C is a radical analogous to Rll,
R2l is hydrogen or Cl-C6-alkyl, C2-C6-alkenyl or C2-C6-
alkynyl, the last-mentioned 3 rA~irAls being unsub-
stituted or substituted by one-or more radicals
selected from the group comprising halogen, Cl-C4-
alkoxy and Cl-C4-alkylthio, or is C,-C4-alkoxy, O~,
N~2, mono- or di(Cl-C4-alkyl)amino, CO-Rl6, CO2-Rl' or
SO2 -R20,
R22 is hydrogen, OH, N~2, mono- or di(Cl-C4-alkyl)amino,
Cl-C4-alkyl or Cl-C4-alkoxy, the last-mentioned
2 radicals being unsubstituted or substituted by one
or more radicals selected from the group comprising
halogen, Cl-C4-alkoxy and C,-C4-alkylthio,
R23 is a radical analogous to R9,
R24 is a radical analogous to Rl,
R2s is a radical analogous to R9,
.
R26 is a radical analogous to Rl,
X and Y independently o~ one another are hydrogen,
halogen, Cl-C6-alkyl, Cl-C6-alkoxy or Cl-C6-alkylthio,
the last-mentioned 3 radicals being unsubstituted or
- 21~76~
-- 6 --
substituted by one or more radicals selected from
the group comprising halogen, Cl-C4-alkoxy and C1-C4-
alkylthio, or are mono- or di ( C,-C4-alkyl ) amino,
C3-C6-cyClOalkyl, C3-c5-alkenyl ~ C3-C6-alkynyl ~ C3-C5-
alkenyloxy or C3-C5-alkynyloxy,
Z is CH or N,
W is O or S,
n is 0, l, 2 or 3 and
o is 0, l, 2 or 3.
lO In formula (I) and hereinafter, the radicals alkyl,
alkoxy, haloalkyl, haloalkoxy, alkylamino and alkylthio
and the corresponding unsaturated and/or substituted
radicals can in each case be straight-chain or branched
in the carbon skeleton. Unless specifically indicated,
15 preferred among these radicals are carbon skeletons
having l to 4 carbon atoms or, in the case of unsaturated
groups, 2 to 4 carbon atoms. Alkyl radicals, also in
composite lneA~; ngs, such as alkoxy, haloalkyl and the
like, are, f or example, methyl, ethyl, n- or i-propyl,
20 n-, i-, t- or 2-butyl, pentyl radicals, hexyl radicals,
such as n-hexyl, i-hexyl and l, 3-dimethylbutyl, heptyl
radicals, such as n-heptyl, l-methylhexyl and l, 4-di-
methylpentyl; alkenyl and alkynyl radicals have the
meaning of the unsaturated radicals which are possible,
25 corresponding to the alkyl radicals; alkenyl is, for
example, allyl, l-methylprop-2-en- l-yl, 2 -methyl-prop-2 -
en-l-yl, but-2-en-l-yl, but-3-en-l-yl, l-methyl-but-3-en-
l-yl and l-methyl-but-2-en-l-yl; alkynyl is, for example,
propargyl, but-2 -yn- l-yl, but- 3 -yn- l -yl,
3 0 l-methyl-but- 3 -yn- l -yl .
Halogen is f luorine, chlorine, bromine or iodine . Halo-
alkyl, haloalkenyl and haloalkynyl are alkyl, alkenyl or
alkynyl, each of which is partially or fully substituted
- 7 - 2~4 f~7 ~
by halogen, preferably by fluorine, chlorine and/or
bromine, in particular by fluorine or chlorine, for
example CF3, C~F2, C~2F, CF3CF2, CH2FCHCl, CCl3, CHCl2,
C~2C~2Cl; halo~lkoYy is, for example, OCF3, OCHF2, OCH2F,
CF3CF20, OC~2CF3 and OC~2C~2Cl. The same applies
analogously for haloalkenyl and other halogen-substituted
radicals.
Aryl is a mono-, bi- or polycyclic aromatic system, for
example phenyl, naphthyl, tetrahydronaphthyl, indenyl,
indanyl, pentalenyl, fluorenyl and the like, preferably
phenyl; aryloxy is preferably the oxy radicals which
correspond to the abovementioned aryl radicals, in
particular phenoxy.
Heteroaryl or a heteroaromatic radical is a mono-, bi- or
polycyclic aromatic system in which at least 1 ring has
one or more hetero atoms, for example pyridyl,
pyrimidinyl, pyridazinyl, pyrazinyl, thienyl, thiazolyl,
oxazolyl, furyl, pyrrolyl, pyrazolyl and imidazolyl, but
also bicyclic or polycyclic aromatic or araliphatic
compounds, for example quinolinyl, benzoxazolyl and the
like.
Substituted aryl, heteroaryl, phenyl, benzyl, or substi-
tuted bicyclic radicals having aromatic moieties are, for
example, a substituted radical derived from an unsubsti-
tuted basic structure, the substituents being, forexample, one or more, preferably 1, 2 or 3, radicals
selected from the group comprising halogen, alkyl,
haloalkyl, alkoxy, haloalkoxy, alkylthio, hydroxyl,
amino, nitro, cyano, alkoxycarbonyl, alkylcarbonyl,
formyl, carbamoyl, mono- and dialkylaminocarbonyl, mono-
and dialkyl~;no, alkylsulfinyl and alkylsulfonyl,~lhere,
in the case of radicals which have carbon atoms, those
having 1 to 4 carbon atoms, in particular 1 or 2 carbon
. . .
atoms, are preferred. As a~rule, preferred substituents
are those selected from the group comprising halogen, for
example fluorine and chlorine, C1-C4-alkyl, preferably
8 21~69
methyl or ethyl, Cl-C4-haloalkyl, preferably trifluoro-
methyl, Cl-C4-alkoxy, preferably methoxy or ethoxy,
Cl-C4-halo~lko~y, nitro and cyano. Particularly preferred
are the substituents methyl, methoxy and chlorine.
Optionally substituted phenyl is, for example, phenyl
which is unsubstituted or monosubstituted or
polysubstituted, preferably up to trisubstituted, by
identical or different radicals selected from the group
comprising halogen, C~-C4-alkyl, Cl-C4-alkoxy, Cl-C4-halo-
alkyl, Cl-C4-haloalkoxy and nitro, for example o-, m- and
p-tolyl, dimethylphenyl radicals, 2-, 3- and 4-chloro-
phenyl, 2-, 3- and 4-trifluoromethyl- and -trichlorometh-
ylphenyl, 2,4-, 3,5-, 2,5- and 2,3-dichlorophenyl and o-,
m- and p-methoxyphenyl.
A heterocyclic radical is preferably 5- or 6-membered and
heteroaromatic, saturated or unsaturated and contains 1,
2 or 3 hetero atoms, preferably selected from the group
comprising N, O and S. The radical can be benzo-fused.
Radicals such as oxiranyl, pyrrolidyl, dioxolanyl,
pyrazolyl, morpholyl, furyl, tetrahydrofuryl, indolyl,
quinolinyl, pyrimidyl, azepinyl, imidazolyl, triazolyl,
thienyl and oxazolyl are suitable.
The invention also relates to all stereoisomers which are
embraced by the formula (I) and to mixtures of these.
Such compounds of the formula (I) contain one or more
asymmetric carbon atoms or else double bonds which are
not indicated separately in the formulae (I). Formula (I)
embraces all the possible stereoisomers which are defined
by their specific spatial shape, such as enantiomers,
diastereomers and Z- and E-isomers, and these can be
obtained from the stereoisomer mixtures by conventional
methods or else by stereoselective reactions in combina-
tion with the use of stereoch~ lly pure starting
.
- materials.
The compounds of the formula (I) can form salts in which
2~4~7fi~
_ 9 _
the hydrogen of the -SO2-N~- group or else other acidic
hydrogen atoms (for example from, inter alia, COOH) is
replaced by an agriculturally suitable cation. Examples
of these salts are metal salts, in particular alkali
metal salts (for example sodium or potassium salts) or
alkaline earth salts, or else ammonium salts or salts
with organic ~m; nes. Salt formation can also be effected
by an addition reaction of an acid with basic groups, for
example amino and alkyl~;no. Acids which are suitable
for this purpose are strong inorganic and organic acids,
for example HCl, HBr, ~2SO4 or HNO3.
Compounds of the formula (I) or salts thereof which are
of particular interest, mainly because they have a better
herbicidal activity and selectivity and/or can be pre-
pared more readily are those in which
Rl is Co-R5, CO-OR6, CO-NR'R8, CS-R9, CS-NR'R8, SO2Rll,
So2NR7R~ or CO-COR~2 or
R2 is hydrogen, Cl-C6-alkyl, C3-Cs-alkenyl or C3-Cs-
alkynyl, the last-mentioned 3 radicals independently
of one another being unsubstituted or substituted by
one or more halogen atoms or by one or two radicals
selected from the group comprising Cl-C4-alkoxy and
Cl-C4-alkylthio, or
R3 is halogen, Cl-C4-alkyl, C2-Cs-alkenyl, C2-Cs-alkynyl,
Cl-C4-alkoxy, C3-Cs-alkenyloxy or C3-Cs-alkynyloxy,
the last-mentioned 6 radicals independently of one
another being unsubstituted or substituted by one or
more halogen atoms or by one or two radicals
selected from the group comprising Cl-C4-alkoxy and
alkylthio, or is CO-CO-Rl6, CO-CO-ORl', CO-NRl~Rl9,
NRl~Rl9, SO2NRl~Rl9, cyano, S(O)o~R20, CO-Rl6, CO-ORl', NO2
or C(R2l)=N_R22
or
is a 5- or 6-membered saturated, unsaturated or
heteroaromatic heterocycle having up to 3 hetero
atoms selected from the group comprising N, O and S,
which is unsubstituted or substituted by one or more
- 10 - 2 t IL ~17 6 ~1
radicals selected from the group comprising halogen,
Cl-C4-alkyl, such as methyl, Cl-C4-alkoxy, such as
methoxy, Cl-C4-alkylthio, Cl-C4-haloalkyl, such as
CF3, CN, NO2, C~O, (Cl-C4-alkyl)carbonyl, such as
acetyl, and (C1-C4-alkoxy)carbonyl, such as CO2C~3,
and in which up to 2 carbon ring positions can be
replaced by CO and up to two sulfur atoms in the
hetero ring can be replaced by SO2,
R4 is hydrogen or methyl,
R5 is hydrogen or Cl-C6-alkyl which is unsubstituted or
substituted by one or more halogen atoms or by one
or two radicals selected from the group comprising
Cl-C4-alkoxy, Cl-C4-alkylthio, NRl3Rl4 and NRl52, or is
C3-C,-cycloalkyl, benzyl, unsubstituted or
substituted aryl or unsubstituted or substituted
heteroaryl, preferably H, Cl-C6-alkyl, Cl-C6-
haloalkyl, C3-C6-cycloalkyl, benzyl, phenyl which is
unsubstituted or substituted by one or more,
preferably up to 3, radicals selected from the group
comprising halogen, methoxy or methyl, or is a 5- or
6-membered heteroaromatic radical which has up to
4 hetero atoms selected from the group comprising N,
O and S and which is unsubstituted or substituted by
one or more radicals selected from the group
comprising halogen, NO2, CN, CHO, COCH3, CO2CH3, OCH3
and SCH3, or
R6 is Cl-C6-alkyl, C3-C6-alkenyl or C3-C6-alkynyl, the
last-mentioned 3 radicals being unsubstituted or
substituted by one or more halogen atoms or by one
or two radicals selected from the group comprising
Cl-C4-alkoxy, Cl-C4-alkylthio, NRl3Rl4 and NRls2, pre-
ferably Cl-C6-alkyl, Cl-C6-haloalkyl, C3-C6-alkenyl or
C3-C6-haloalkenyl, or
R' is hydrogen or Cl-C6-alkyl which is unsubstituted or
substituted by one or~more halogen atoms or by one
or two rA~icAls selected from the group comprising
C1-C,-alkoxy, Cl-C4-alkylthio and NRl3Rl4, preferably
- 21~76g
H, cl-c4-alkyl or Cl-C4-haloalkyl, or
Rs is hydrogen or Cl-C4-alkyl which is unsubstituted or
substituted by one or more halogen atoms or by one
or two radicals selected from the group comprising
C1-C4-alkoxy and Cl-C4-alkylthio, or is CoR23, Co2R24-
or SO2Rll, preferably H, CH3, C2H5, CHO, COCH3 or
CO2CH3, or
R9 is analogous to R5, preferably hydrogen, Cl-C6-alkyl
or Cl-C6-haloalkyl, orO Rl is analogous to R6, preferably Cl-C4-alkyl or Cl-C4-
haloalkyl, or
Rll is Cl-C4-alkyl or Cl-C4-haloalkyl, or
Rl2 is hydrogen, OH, cl-c4-alkyl, cl-c4-alkoxy or Cl-C4-
haloalkyl, preferably ~ or Cl-C4-alkyl, or5 the Rl3 radicals independently of one another are hydrogen
or Cl-C4-alkyl, CoR25 or CO2R26, preferably H, C~3,
C2E5, CHO, COC~3, CO-OCH3 or CO-OC2~5,
the Rl4 radicals independently of one another are hydrogen
or Cl-C4-alkyl, preferably H, CH3 or C2H5,
NRl52 radicals independently of one another are a hetero-
cyclic 3- to 7-membered ring which can contain,
besides the nitrogen atom, a further hetero ring
atom selected from the group comprising N, O and S
and which is unsubstituted or substituted by one or
more radicals selected from the group comprising
Cl-C4-alkyl and oxo, for example heterocyclic radi-
cals of the formulae Al to A5
o
--N3 --N~ _ N~ - N,~
A ~ Az A3 A~
7 6 9
-- 12 --
N~ N~ N,~
o O O
A5 A6 A~ 9
or
R16 is a radical analogous to R5,
Rl' is hydrogen, C,-C6-alkyl, C3-C6-alkenyl,
C3-C6-alkynyl, the last-mentioned 3 radicals being
unsubstituted or substituted by one or more halogen
atoms or by one or two rA~;c~ls selected from the
group comprising C,-C4-alkoxy, C,-C,-alkylthio, NR'3R'4
and NR'52, preferably H, C,-C6-alkyl, C,-C6-haloalkyl,
C3-C6-alkenyl or C3-C6-haloalkenyl,
Rl8 is a radical analogous to R',
R15 is a radical analogous to R8,
R20 is a radical analogous to R",
R2l is hydrogen, C,-C4-alkyl, Cl-C4-haloalkyl,C,-C4-
alkoxy, OH, NH2, mono- or di-(C,-C4-alkyl)-amino,
preferably H, methyl, ethyl, n- or i-propyl, meth-
oxy, ethoxy, hydroxyl, amino, methylamino or
dimethylamino,
R22 is hydrogen, OH, NH2, mono- or di(C,-C4-alkyl)-amino,
Cl-C4-alkyl or Cl-C4-alkoxy, preferably H, OH, NH2,
NHCH3, N(CH3) 2 ~ CH3 or OCH3,
R23 is a radical analogous to R5,
R24 is a radical analogous to R',
R25 is a radical analogous to R9,
R26 is a radical analogous to Rl,
X and Y independently of one another are hydrogen,
~ - 13 - 2~1~7~
halogen, Cl-C4-alkyl, Cl-C4-alkoxy or Cl-C,-alkylthio,
the last-mentioned 3 rA~icAls being unsubstituted or
substituted by one or more halogen atoms, or are
mono- or di(Cl-C2-alkyl)~m; no, one of the radicals X
and Y preferably being CH3, C2Hs, OCH3 or OC2Hs and
the other radical preferably being CH3, C2H5, OCH3,
OC2H5 ~ Cl ~ CF3 ~ OCF3C ~ OCF2H ~ N ( CH3 ) 2 1 NHCH3 or
N(C2Hs)2/ or
Z is CH or N,
W is O or S, preferably O,
n is 0, 1 or 2 or
o is 0, 1 or 2,
or preferably those compounds of the formula (I) or salts
thereof in which two or more of the abovementioned
particular or preferred meanings of the general radicals
or indices Rl to o in formula (I) are combined.
Preferred compounds of the abovementioned formula (I) or
salts thereof are those in which
R1 is CoR5, CO2R6, Co-NR7R8, CO-CO-Rl2, CS-NR'R~, SO2R11 or
SO2-NR'R8 and/or
R2 is hydrogen or C1-C4-alkyl, preferably H~ CH3~ C2H5 or
n- or i-C3H7, in particular H.
Other preferred compounds of the formula (I) according to
the invention or salts thereof are those in which
Rs is hydrogen, C1-C4-alkyl, C1-C4-haloalkyl, such as
CF3, cyclopropyl, cyclopentyl, cyclohexyl, benzyl,
phenyl which is unsubstituted or substituted by 1 to
3 radicals selected from the group comprising
halogen, preferably fluorine or chlorine, methyl and
methoxy, or is thienyl, furyl or pyridyl, the above
heteroaromatic radicals being unsubstituted or
substituted by one or more rA~icAls selected from
the group comprising halogen, C,-C4-alkyl and Cl-C4-
alkoxy, or
R6 is Cl-C4-alkyl, C,-C4-haloalkyl, C3-C4-alkenyl or
- 14 - ~g ~ 6
C3-C4-alkynyl,
R' is H, C1-C4-alkyl or Cl-C4-haloalkyl or
R3 is ~, Cl-C4-alkyl, COCH3, CO2C~3 or CO2C2~s~
or which contain a combination of the abovementioned
preferred radicals.
- Other preferred compounds are compounds of the
formula (I) according to the invention or salts thereof
in which the group of the formula N(OR2)Rl is in the 2-,
3-, 5- or 6-position on the phenyl ring and
n is 0 or 1.
Particularly preferably, the group of the formula N(OR2)R1
is in the 2-position on the phenyl ring and n is 0 or 1
or
the group of the formula N(OR2)Rl is in the 5-position (or
3-position) on the phenyl ring, n is 0 or 1 and the
radical
R3 is in the 2-position (or 6-position) on the phenyl
ring .
Particularly preferably, R3 is a group of the formula
CO-ORl' in the 2- or 6-position on the phenyl radical and
n is 1.
The present invention furthermore relates to processes
for the preparation of the compounds of the formula (I)
according to the invention or of the salts thereof, which
comprises
a) reacting a compound of the formula (II)
N(oR2~Rl
~ S2-NH2 ( I I )
(R ) n
with a heterocyclic carbamate of the formula (III)
7 ~ 3
- 15 -
International Patent Application
No. PCT/EP93/02469 (Our Ref: HOE 92/F300 WO)
Annex to the submission of 19.9. 94
I~ _
I
c) reacting a compound of the formula (II) with a
(thio)isocyanate of the formula (X)
X
W ~ C ~ N ~ (X)
in the presence of a base,
where in formulae (II) - (V) and (X) the radicals Rl
to R4 and n are as defined in formula (I) and in
process variants a) and b) compounds of the
formula (I) are initially obtained in which W is O.
AMENDED SHEET
~14~76~
- 16 -
The compounds of the formulae (II) and (III) are prefer-
ably reacted in an inert organic solvent, such as, for
example, dichloromethane, acetonitrile, dioxane or THF,
at temperatures between 0C, preferably 20C, and the
boiling point of the solvent, with base catalysis.
Examples of bases which are used are organic ~m;ne bases,
such as 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), in
particular when R* is (substituted) phenyl (cf.
EP-A-44,807), or trimethylaluminum or triethylaluminum,
the latter substances in particular when R* is alkyl (cf.
EP-A-166,516).
Two sulfonamides of the formula (II) are known from Chem.
Abstr. 99:37857e (1983), that is 4-(N-acetyl-N-hydroxy-
or -N-methoxy-amino)-benzenesulfon~ide. New compounds
(II) and their preparation are also provided by the
invention.
The compounds of the formula (II) are obtained, for
example, starting from compounds of the formula (VI)
N(OR2)R1
(R )n~ (Yl)
S02NH- t -C4H9
by reacting them with a strong acid.
AMENDED S~EET
-
C2l~7~g
- 17 -
presence of a base, such as, for example, potassium
carbonate or sodium hydride, in an inert solvent, such
as, for example, DMF (dimethylformamide) or DMSO (dimeth-
yl sulfoxide), TH~ (tetrahydrofuran) or
1,2-dimethoxyethane. Suitable alkylating agents are
dialkyl sulfates or corresponding alkyl halides (cf.
Houben-Weyl-Klamann, "Methoden der organischen Chemie"
[Methods of Organic Chemistry], 4th Ed., Vol. E XVI/a,
p. 271, Thieme Verlag Stuttgart, 1990).
The substituent R' can be introduced by reacting the
hydroxylamine aromatics (VI) in which R1 = R2 = H with
suitable electrophilic substances, such as, for example,
acid chlorides, acid anhydrides, isocyanates and thioiso-
cyanates (cf. Houben-Weyl-~l A~ nn, "Methoden der
organischen Chemie" [Methods of Organic Chemistry],
4th Ed., Vol. E XVIa/1, p. 199 et seq., Thieme Verlag
Stuttgart, 1990).
The hydroxylamine derivative of the formula (VI) in which
Rl = R2 = H is accessible from the corresponding nitro
compound (VII) by reduction, for example using zinc
powder in ethanol buffered with ammonium chloride. Other
examples of suitable reducing agents for this transforma-
tion are: platinum/tributyl phosphite/4-dimethylamino-
pyridine, hydrazine hydrate or hydrogen/Raney nickel-
/ethanol, hydra~ine hydrate/rhodium, sodium dihydrogenphosphate/palladium, and the like (cf., in this context:
Houben-Weyl-Kl ~nn ~ "Methoden der organischen Chemie"
~Methods of Organic Chemistry], 4th Ed., Vol. E XVIa/l,
p. 49 et seq., Thieme Verlag Stuttgart, 1990).
The abovementioned nitro compound of the formula (VII)
can be prepared from the corresponding nitrobenzenesul-
fonic acid (VIII)
18 2~ 7~
N02 N02
~,~ S03H ~ SO2NH-t-C4H9
(R )n ~R3)
( V I I I ) ( V I I )
First, the sulfo group of the compounds (VIII) is con-
verted into the sulfonyl chlorides, for example by
standard methods, such as reaction of phosphorus oxy-
chloride or thionyl chloride with potassium salts of the
corresponding sulfonic acids in inert solvents, such as
acetonitrile and/or sulfolane or in substance by reflux-
ing (cf. Houben-Weyl-Klamann, "Metho~e~ der organischen
Chemie" ~Methods of Organic Chemistry], 4th Ed.,
Vol. E XI/2, p. 1067-1073, Thieme Verlag Stuttgart,
1985).
The formation of sulfonamide from the sulfonyl chlorides
with tert.-butylamine in ethanol gives the com-
pounds (VII) in good yields (cf. analogous reactions in
WO 89/10921).
Alternatively, the sulfo~A~i~es of the for~ll A II in
which Rl and R2 are other than ~ can be obtained by
A~;nolysis of the corresponding sulfonyl chlorides of the
fo~mula (IXb)
N ( O R 2 j R 1
( IXa, X' OH)
S02X' ( IXb, X' Cl )
( R3 )
which, in turn, are readily obtAinAhle from the sulfonic
acids ~IXa) by the abovementioned stAn~Ard methods (see
reactions of (VIII) to give (VII)).
The compounds (IXa), in turn, can be obtained from the
- 21~4~S9
-- 19 --
nitrobenzenesulfonic acid (VIII) by the reaction sequence
(1) reduction analogous to the reduction of (VII),
(2) N-acylation and
(3) O-acylation,
the latter as described in the preparation of (VI).
The abovementioned starting compounds of the
formula (VIII) or the sulfonyl chlorides thereof are
either known or commercially available compounds or they
can be prepared in a simple manner, for example by
10 - nitration (cf. ~ouben-Weyl-Muller, ~Methoden der
organischen Chemie" [Methods of Organic Chemistry~,
4th Ed., Vol. X/1, p. 463 et seq., Thieme Verlag
Stuttgart, 1971),
- sulfonation (cf. Houben-Weyl-Klamann, "Methoden der
organischen Chemie" [Methods of Organic Chemistry],
4th Ed., Vol. E XI/2, p. 1055 et seq., Thieme Verlag
Stuttgart, 1985); ~ouben-Weyl-Muller, "Methoden der
organischen Chemie" [Methods of Organic Chemistry],
4th Ed., Vol. IX, p. 435 et seq., Thieme Verlag
Stuttgart, 1955),
- sulfochlorination (Houben-Weyl Rlamann, "Methoden
der organischen Chemie" tMethods of Organic
Chemistry], 4th Ed., Vol. E XI/2, p. 1067 et seq.,
Thieme Verlag Stuttgart, 1985; ~ouben-Weyl-Muller,
"Methoden der organischen Chemie~ ~Methods of
Organic Chemistry], 4th Ed., Vol. IX, p. 563 et
seq., Thieme Verlag Stuttgart, 1955),
- introduction of a radical R3, for example by halo-
genation (~ouben-Weyl-Muller: "Methoden der organis-
chen Chemie" [Methods of Organic Chemistry],
4th Ed., Vol. V/3, p. 213 et seq., Thieme Verlag
Stuttgart, 1962; Houben-Weyl-Muller, "Methoden der
organischen Chemie" [Methods of Organic Chemistry],
4th Ed., Vol. V/3, p. 556-561 and 845-853, Thieme
Verlag Stuttgart, 1962; Houben-Weyl-Muller,
"Methoden der organischen Chemie" [Methods of
Organic Chemistry], 4th Ed., Vol. V/4, p. 233-299
and 437-447, Thieme Verlag Stuttgart, 1960;
~i~476
- 20 --
Rouben-Weyl-Muller, "Methoden der organischen
Chemie" [Methods of Organic Chemistry], 4th Ed.,
Vol. V/4, p. 557-593 and 639-647, Thieme Verlag
Stuttgart, 1960) or
alkylation (G.A. Olah, "Friedel-Crafts-Chemistry",
Wiley-Interscience, New York, 1973),
- or by derivatization of an already existing third
substituent, for example by oxidation of an alkyl
group (R3) to give a carboxylic acid, followed by an
esterification (R3 s CO2-alkyl) (Houben-Weyl-Falbe:
"Methoden der organischen Chemie~' [Methods of
Organic Chemistry], 4th Ed. Vol. EV/l, Thieme Verlag
Stuttgart, 1985, p. 199 - 202 and references cited
therein).
The carbamates of the formula (III) required for the
reaction of the compounds (II) by variant a) are known
from the literature or can be prepared analogously to
known processes (cf. EP-A-70804 or US-A-4,480,101).
The compounds of the formulae (IV) and (V), which can be
employed in process variant b), can also be prepared from
the abovementioned compounds of the formulae (III)
and (VIII) and from their precursors by, or analogous to,
generally ~nown methods.
The phenylsulfonyl isocyanates of the formula (IV) where
R2 is other than H can be prepared, for example, analo-
gously to the processes in EP-A-184,385 from com-
pounds (II), for example using phosgene.
The reaction of the compounds (IV) with the amino hetero-
cycles (V) is preferably carried out in inert, aprotic
solvents, such as, for example, dioxane, acetonitrile or
tetrahydrofuran, at temperatures between 0C and the
boiling point of the solvent.
The (thio)isocyanates of the formula (X) can be synthe-
sized by processes known from the literature (cf.
- 21 _ 2 1 ~ ~7 6 9
EP-A-232,067A, EP-A-166,516).
The reaction of compounds of the formula (X) with sulfon-
amides of the formula (II) is carried out at temperatures
between -10C and 100C, preferably between 20 and 80C,
in an inert aprotic æolvent, such as, for example,
acetone or acetonitrile, in the presence of a suitable
base, such as, for example, triethylamine or potassium
carbonate.
The salts of the compounds of the formula (I) are prefer-
ably prepared in inert solvents, such as, for example,
water, methanol, acetone, dichloromethane, tetrahydro-
furan, toluene or heptane, at temperatures from 0 to
100C. Suitable bases for the preparation of the salts
according to the invention are, for example, alkali metal
carbonates, such as potassium carbonate, alkali metal
hydroxides and alkaline earth metal hydroxides, ammonia
or a suitable amine base, such as triethyl~m;ne or
ethanolamine. Acids which are suitable for salt formation
are, for example, ~Cl, ~Br, ~2SO4 or HN03 -
The solvents termed "inert solvents~ in the above processvariants are understood as meaning in each case solvents
which are inert under the reaction conditions in ques-
tion, but which need not be inert under any reaction
conditions.
The compounds of the for~lllA (I) according to the inven-
tion or salts thereof have an outst~n~;ng herbicidal
activity against a broad range of economically important
monocotyledon and dicotyledon harmful plants. The active
substances also act efficiently on pere~ni Al weeds which
produce shoots from rhizomes, rootstocks or other
perennial organs and which are difficult to control. In
this context, it does not matter whether the substances
are applied by the pre-plant, pre-emergence or post-
emergence method. Specifically, examples may be mentioned
of some representatives of the monocotyledon and dicotyl-
edon weed flora which can be controlled by the ccmpounds
2 1 9L .1 7 ~a ~
- 22 -
according to the invention, without the enumeration being
a restriction to certain species.
Examples of weed species on which the active substances
act efficiently are, from amongst the monocotyledons,
5 - Avena, Lolium, Alopecurus, Phalaris, Echinochloa,
Digitaria, Setaria and Cyperus species from the annual
sector and, from amongst the perenn;~l species,
Agropyron, Cynodon, Imperata and Sorghum, and also
perennial Cyperus species.
In the case of the dicotyledon weed species, the range of
action extends to species such as, for example, Galium,
Viola, Veronica, Lamium, Stellaria, Amaranthus, Sinapis,
Ipomoea, Matricaria, Abutilon and Sida from amongst the
annuals, and Convolvulus, Cirsium, Rumex and Artemisia in
the case of the perenn;~l weeds.
The active substances according to the invention also
effect an outst~n~;ng control of weeds which occur under
the specific conditions of rice growing, such as, for
example, Sagittaria, Alisma, Eleocharis, Scirpus and
Cyperus.
If the compounds according to the invention are applied
to the surface ~efore ger~;n~tion, then the weed
seedlings are either prevented completely from emerging,
or the weeds grow until they have re~ched the cotyledon
stage but then their growth stops, and, eventualIy, after
three to four weeks have elapsed, they die completely.
If the active substances are applied post-emergence to
the green parts of the plants, growth also stops drastic-
211y a very short time after the treatment and the weed
plants remain at the growth stage of the point of time of
application, or they die completely after a certain time,
so that, in this manner, competition by the weeds, which
is harmful to the crop plants, is eliminated at a very
early point in time and in a sustained manner.
21447~
- 23 -
Even though the compounds according to the invention have
an excellent herbicidal activity against monocotyledon
and dicotyledon weeds, crop plants of economically
important crops, such as, for example, wheat, barley,
rye, rice, maize, sugar beet, cotton and soya, are not-
damaged at all, or only to a negligible extent. For these
reasons, the present compounds are highly suitable for
selectively controlling undesired vegetation in plantings
for agricultural use.
Moreover, the substances according to the invention have
excellent growth-regulating properties in crop plants.
They engage in the plant metabolism in a regulating
manner and can thus be employed for the targeted control
of plant constituents and for facilitating harvesting,
for ex2mple by provoking desiccation and stunted growth.
Furthermore, they are also suitable for generally regul-
ating and inhibiting undesired vegetative growth without
simultaneously destroying the plants. Inhibition of
vegetative growth plays an important role in many mono-
cotyledon and dicotyledon crops since lodging can bereduced hereby, or prevented completely.
The compounds according to the invention can be employed
in the conventional preparations as wettable powders,
emulsifiable concentrates, sprayable solutions, dusts or
granules. The invention therefore also relates to herbi-
cidal and plant-growth-regulating composit~ons which
comprise compounds of the formula (I) or salts thereof.
The compounds of the formula (I) or the salts thereof can
be formulated in various ways, depe~;ng on the prevail-
ing biological and/or chemico-physical parameters.
Examples of possible formulations which are suitable are:
wettable powders (WP), water-soluble powders (SP),
water-soluble concentrates, emulsifiable concen-
trates (EC), emulsions (E~), such as oil-in-water and
water-in-oil emulsions, sprayable solutions, suspension
concentrates (SC), dispersions on an oil or water basis,
2~7~9
- 24 -
oil-miscible solutions, capsule suspensions (CS),
dusts (DP), seed-dressing products, granules for broad-
casting and soil application, granules (GR) in the form
of microgranules, spray granules, coated granules and
adsorption granules, water-dispersible granules (WG),
water-soluble granules (SG), ULV formulations, microcap-
sules and waxes.
These individual formulation types are known in principle
and described, for example, in:
Winnacker-Kuchler, "Chemische Technologie" [Chemical
Technology], Volume 7, C. Hauser Verlag, Munich, 4th
Edition 1986; Wade van Valkenburg, "Pesticide
Formulations" Marcel Dekker, N.Y., 1973; R. Martens,
-Spray Drying ~andbook", 3rd Ed. 1979, G. Goodwin Ltd.
London.
The necessary formulation a~xil;~ries such as inert
materials, surfactants, solvents and other additives are
also known and described, for example, in: Watkins,
"Handbook of Insecticide Dust Diluents and Carriers", 2nd
Ed., Darland Books, Caldwell N.J., H.v. Olphen,
"Introduction to Clay Colloid Chemistry"; 2nd Ed., J.
Wiley & Sons, N.Y.; C. Marsden, "Solvents Guide", 2nd
Ed., Interscience, N.Y. 1963; McCutcheon's "Detergents
and Emulsifiers Annual", MC Publ. Corp., Ridgewood N.J.;
Sisley and Wood, "Encyclopedia of Surface Active Agents",
Chem. Publ. Co. Inc., N.Y. 1964; Schonfeldt,
"Grenzflachenaktive Athylenoxi~A~-lkte" tSurface-active
Ethylene Oxide Adducts], Wiss. Verlagsgesell., Stuttgart
1976; Winnacker-Kuchler, ~'Chemische Technologie" [Chem-
ical Technology], Volume 7, C. ~auser Verlag, Munich, 4thEd. 1986.
Based on these formulations, it is also possible to
prepare combinations with other pesticidally active
substances, such as, for èxzmple, insecticides, acari-
cides, herbicides, fungicides, safeners, fertilizersand/or growth regulators, for example in the form of a
- 21~476~
- 25 -
readymix or a tank mix.
Wettable powders are preparations which are uniformly
dispersible in water and which, besides the active
substance, also contain ionic and/or nonionic surfactants
(wetting agents, dispersants), for example polyoxy-
ethylated alkylphenols, polyoxyethylated fatty alcohols
and, polyoxyethylated fatty amines, fatty alcohol poly-
glycol ether sulfates, alkanesulfonates, alkyl-
benzenesulfonates, sodium ligninsulfonate, sodium 2,2'-
dinaphthylmethane-6,6~-disulfonate, sodium dibutylnaph-
thalenesulfonate or else sodium oleoylmethyltaurinate, in
addition to a diluent or inert substance. To prepare the
wettable powders, the herbicidal active substances are
ground finely, for example in conventional apparatuses,
such as hammer mills, blowing mills and air-jet mills and
mixed with the formulation auxiliaries, either simul-
taneously or subsequently.
Emulsifiable concentrates are prepared by dissolving the
active substance in an organic solvent, for example
butanol, cyclohexanone, dimethylforr-m;~e, xylene or else
higher-boiling aromatics or hydrocarbons or mixtures of
the organic solvents, with an addition of one or more
ionic and/or non-ionic surfactants (emulsifiers). Emulsi-
fiers which can be used are, for example: calcium salts
of alkylarylsulfonic acids, such as calcium dodecyl-
benzenesulfonate, or non-ionic emulsifiers, such as fatty
acid polyglycol esters, alkylaryl polyglycol ethers,
fatty alcohol polyglycol ethers, propylene oxide/ethylene
oxide condensation products, alkyl polyethers, sorbitan
esters, such as, for example, sorbitan fatty acid esters
or polyoxethylene sorbitan esters, such as, for example,
polyoxyethylene sorbitan fatty acid esters.
Dusts are obtained by grinting the active substance with
finely divided solid substances, for example talc,
natural clays, such as kaolin, bentonite and pyrophyl-
lite, or diatomaceous earth.
`- 21~76~
- 26 -
Suspension concentrates can be oil- or water-based. They
can be prepared, for example, by wet grinding using
commercially available bead mills, optionally with an
addition of surfactants, such as those already listed
above for the other types of formulation.
Emulsions, for example oil-in-water emulsions tEW), can
be prepared, for example, using aqueous organic solvents,
optionally together with surfactants, such as those
already listed above for the other types of formulation
by means of stirrers, colloid mills and/or static mixers.
Granules can be produced either by spraying the active
substance onto adsorptive, granulated inert material or
by applying active substance concentrates onto the
surface of carriers such as sand, kaolinites or of
granulated inert material, by means of binders, for
example polyvinyl alcohol, sodium polyacrylate or alter-
natively mineral oils. Suitable active substances can
also be granulated in the manner which is conventional
for the production of fertilizer granules, if desired in
a mixture with fertilizers.
Water-dispersible granules are prepared, as a rule, by
the customary methods such as spray drying, fluidized-bed
granulation, disk granulation, mixing using high-speed
mixers and extrusion without solid inert material.
The agrochemical preparations comprise, as a rule, 0.1 to
99 % by weight, in particular 0.1 to 95 % by weight, of
active substance of the formula (I) or salts thereof.
The active substance concentration in wettable powders
i~, for example, about 10 to 90 % by weight; the remain-
der to 100 % by weight is composed of conventionalformulation components. In the case of emulsifiable
concentrates, the active sùbstance concentration can be
about 1 to 90, preferably 5 to 80 % by weight. Formula-
tions in the form of dusts usually contain 1 to 30,
- 27 - 2 1 4 1 7~ 3
usually preferably 5 to 20 % by weight of active sub-
stance, sprayable solutions about 0.05 to 80, preferably
2 to 50 % by weight. In the case of water-dispersible
granules, the active substance content depends partly on
whether the active compound is liquid or solid and on
which granulation all~il;Aries, fillers and the like are
used. It is for example between 1 and 95 % by weight,
preferably between 10 and 80 % by weight in the case of
the water-dispersible granules.
In addition, the active substance formulations mentioned
comprise, if appropriate, the adhesives, wetting agents,
dispersants, emulsifiers, penetrants, preservatives,
antifreeze agents, solvents, fillers, carriers,
colorants, defoamers, evaporation inhibitors and pH and
viscosity regulators which are conventional in each case.
Active substances which can be employed in combination
with the active substances according to the invention in
formulation mixtures or in a tank mix are, for example,
known active substances as they are described, for
example, in Weed Research 26, 441-445 (1986), or "The
Pesticide Manual", 9th Edition, The British Crop
Protection Council, 1990/91, Bracknell, England, and in
the literature cited therein. Active substances which may
be mentioned as examples of herbicides which are known
from the literature and which can be combined with the
compounds of the formula (I) are the following (note: the
compounds are either given by their common name, using
the terminology of the International Organization for
StA~Ardization (ISO), or by their chemical name, if
appropriate together with a conventional code number):
acetochlor; acifluorfen; aclonifen; AR~ 7088, i.e. [[[1-
[5-[2-chloxo-4-(trifluoromethyl)phenoxy]-2-nitrophenyll-
2-methoxyethylidene]amino]oxylacetic acid and its methyl
ester; alachlor; alloxydim; ametryn; amidosulfuron;
amitrol; AMS, i.e. ammonium sulfamate; anilofos; asulam;
atrazine; aziprotryn; barban; BAS 516 ~, i.e. 5-fluoro-2-
phenyl-4~-3,1-benzoxazin-4-one; benazolin; benfluralin;
- 28 - ~ 1769
benfuresate; bensulfuron-methyl; bensulide; bentazone;
benzofenap; benzofluor; benzoylprop-ethyl; benzthiazuron;
bialaphos; bifenox; bromacil; bromobutide; bromofenoxim;
bromoxynil; bromuron; buminafos; busoxinone; butachlor;
butamifos; butenachlor; buthi~zole; butralin; butylate;
carbetamide; CDAA, i.e. 2-chloro-N,N-di-2-propenylacet-
A~; de; CDECl i.e. 2-chloroallyl diethyldithiocarbamate;
CGA 184927, i.e. 2-~4-t(5-chloro-3-fluoro-2-pyridinyl)-
oxy]phenoxy]propanoic acid and its 2-propynyl ester;
chlomethoxyfen; chloramben; chlorazifop-butyl,pirifenop-
butyl; chlorbromuron; chlorbufam; chlorfenac; chlor-
flurecol-methyl; chloridazon; chlorimuron-ethyl; chlor-
nitrofen; chlorotoluron; chloroxuron; chlorpropham;
chlorsulfuron; chlorthal-dimethyl; chlorthi~;d; cin-
methylin; cinosulfuron; clethodim; clomazone; clomeprop;cloproxydim; clopyralid; cyanazine; cycloate; cycloxydim;
cycluron; cyperquat; cyprazine; cyprazole; 2-4-DB;
dalapon; desmediphan; desmetryn; di-allate; dicamba;
dichlobenil; dichlorprop; diclofop-methyl; diethatyl;
difenoxuron; difenzoquat; diflufenican; dimefuron;
dimethachlor; dimethametryn; dimethazone, clomazon;
dimethipin; dimetrasulfuron, cinosulfuron; dinitramine;
dinoseb; dinoterb; diphenamid; dipropetryn; diquat;
dithiopyr; diuron; DNOC; eglinazine-ethyl; EL 177, i.e.
5-cyano-1-(1,1-dimethylethyl)-N-methyl-3H-pyrazole-4-
carboxamide; endothal; EPTC; esprocarb; ethalfluralin;
ethametsulfuron-methyl; ethidimuron; ethiozin; ethofume-
sate; F5231, i.e. N-[2-chloro-4-fluoro-5-[4-(3-fluoro-
propyl)-4,5-dihydro-5-oxo-lH-tetrazol-1-yl]phenyl]ethane-
sulfonamide, F6285, i.e. 1-[5-(N-methylsulfonyl)-amino-
2,4-dichlorophenyl]-3-methyl-4-difluoromethyl-1,2,4-
triazol-5-one, fenoprop; fenoxan, clomazon;
fenoxaprop-ethyl; fenuron; flamprop-methyl; flaza-
sulfuron; fluazifop and its ester derivatives;
fluchloralin; flumetsulam; N-[2,6-difluorophenyl]-5-
methyl-(1,2,4)-triazolo[1,5a]pyrimidine-2-sulfonamide;
flumeturon; flumipropyn; fluorodifen; fluoroglycofen-
ethyl; fluridone; flurochloridone; fluroxypyr; flurt-
amone; fomesafen; fos~;ne; furyloxyfen; glufosinate;
- 214 17~9
- - 29 - -
glyphosate; halosafen; haloxyfop and its ester
derivatives; hexazinone; Hw 52, i.e. N-(2,3-dichloro-
phenyl)-4-(ethoxymethoxy)benzamide; imdazamethAhçnz-
methyl; imazapyr; imazaquin; imazethamethapyr; imaze-
thapyr; imazosulfuron; ioxynil; isocarbamid; isopropalin;isoproturon; isouron; isoxaben; isoxapyrifop; karbutil-
ate; lactofen; lenacil; linuron; MCPA; MCPB; mecoprop;
mefenacet; mefluidid; metAm;tron; metazachlor; meth~hen
thiazuron; metham; methazole; methoxyphenone; methyldym-
ron; metobromuron; metolachlor; metoxuron; metribuzin;
metsulfuron-methyl; M~; molinate; monalide; monocarbamide
dihydrogensulfate; monolinuron; monuron; MT 128, i.e. 6-
chloro-N-(3-chloro-2-propenyl)-5-methyl-N-phenyl-3-
pyridaz;n~m;ne; MT 5950, i.e. N-[3-chloro-4-(1-methyl-
ethyl)phenyl]-2-methylpentAnAm;~e; naproAn;l;~e; naprop-
amide; naptalam; NC 310, i.e. 4-(2,4-dichlorobenzoyl)-1-
methyl-5-benzyloxypyrazole; neburon; nicosulfuron;
nipyraclophen; nitralin; nitrofen; nitrofluorfen; nor-
flurazon; orbencarb; oryzalin; o~;Azon; oxyfluorfen;
paraquat; pebulate; pendimethalin; perfluidone; phen-
medipham; phenisopham; phe~ ;pham; picloram; pipero-
phos; piributicarb; pirifenop-butyl; pretilachlor;
primisulfuron-methyl; procyazine; pro~;A~;ne; proflura-
lin; proglinazine-ethyl; prometon; prometryn; propachlor;
propanil; propaquizafop and its ester derivatives;
propazine; propham; propyzamide; prosulfalin; prosulfo-
carb; prynachlor; pyrazolinate; pyrazon; pyrazosulfuron-
ethyl; pyrazoxyfen; pyridate; quinclorac; quinmerac;
quinofop and its ester derivatives, quizalofop and its
ester derivatives, quizalofop-ethyl; quizalofop-p-tefur-
yl; renriduron; dymron; S 275, i.e. 2-[4-chloro-2-fluoro-
5-(2-p-o~yll~loxy)phenyl]-4,5,6,7-tetrahydro-2~-indazole;
S 482,i.e.2-[7-fluoro-3,4-dihydro-3-oxo-4-(2-propynyl)-
2~-1,4-benzoxazin-6-yl]-4,5,6,7-tetrahydro-lH-isoindole-
1,3(2~)-dione, secbumeton; sethoxydim; siduron; simazine;
simetryn; SN 106279, i.e. 2~[[7-[2-chloro-4-(trifluoro-
methyl)phenoxy]-2-naphthalenyl]oxy]propanoic acid and its
methyl ester; sulfometuron-methyl; sulfazuron; flazasul-
furon; TCA; tebutam; tebuthiuron; terbacil; terbucarb;
214~`763
- 30 -
terbuchlor; terbumeton; terbuthylazine; terbutryn;
TF~ 450,i.e.N,N-diethyl-3-[(2-ethyl-6-methylphenyl)sul-
fonyl]-l~-1,2,4-triazole-1-carboxamide, thiazafluron;
thifensulfuron-methyl; thiobencarb; tiocarbazil; tral-
koxydim; tri-allate; triasulfuron; triazofenamide;
tribenuron-methyl; triclopyr; tridiphane; trietazine;
trifluralin; trimeturon; vernolate; WL 110547, i.e.
5-phenoxy-1-t3-(trifluoromethyl)phenyl]-lH-tetrazole.
For use, the formulations which are present in commer-
cially available form are, if appropriate, diluted in the
customary manner, for example using water in the case of
wettable powders, emulsifiable concentrates, dispersions
and water-dispersible granules. Preparations in the form
of dusts, granules for cell application and for broad-
casting, and sprayable solutions are conventionally notdiluted further with other inert substances prior to use.
The application rate required of the compounds of the
formula (I) varies with the external conditions, such as,
inter alia, temperature, humidity and the type of herbi-
cide used. It can vary within wide limits, for examplebetween 0.001 and 10.0 kg/ha or more of active ingredi-
ent, but it is preferably between 0.005 and 5 kg/ha.
A. Chemical Examples
a) 2-Carboxy-5-nitrobenzenesulfonic acid
25 400.0 g (2.53 mol) of potassium permanganate are added in
portions in the course of 2 hours at 80C to a solution
of 106.0 g (0.40 mol) of 2-methyl-5-nitrobenzenesulfonic
acid and 80.0 g (0.58 mol) of potassium carbonate in
1300 ml of water. The reaction temperature is maint~;ne~
between 80 and 95C. After stirring has been continued
for a further 4 hours, the solid which has formed is
removed by filtration. The filtrate is acidified using
concentrated hydrochloric -acid (p~ = 1). The colorless
2-carboxy-5-nitrobenzenesulfonic acid which has
precipitated is filtered off with suction using a Buchner
- 214~769
- 31 -
funnel and dried at approximately 50C/100 torr (82.0 g;
83.7 % of theory). m.p. >300C.
b) 2-Methoxycarbonyl-5-nitrobenzenesulfonic acid
A suspension of 190.0 g (0.77 mol) of 2-hydroxycarbonyl-
5-nitrobenzenesulfonic acid, 10 ml of dimethylforr-miAe
and 250 ml (3.43 mol) of thionyl chloride is heated at
the boil for 3 hours. After the insoluble components have
been removed by means of filtration, the filtrate is
concentrated. 200 ml (4.94 mol) of methanol are added
dropwise to the resulting residue. After the addition has
ended, the reaction mixture is cooled to 0C. The solid
which has precipitated is filtered off and dried. This
gives 70.9 g (35.3 % of theory) of colorless, crystalline
2-methoxycarbonyl-5-nitrobenzenesulfonic acid
(m.p.: 92-94C).
A second fraction (62.5 g, 31.1 % of theory) is obtained
by removing the volatile components of the mother liquor
by distillation on a rotary evaporator.
c) 2-Methoxycarbonyl-5-nitrobenzenesulfonyl chloride
A solution of 70.9 g (0.27 mol) of 2-methoxycarbonyl-5-
nitrobenzenesulfonic acid in 300 ml of methanol is
treated carefully with a solution of 17.3 g (0.27 mol) of
potassium hydroxide (88 % strength) and 100 ml of
methanol, with vigorous stirring. After the mixture has
been cooled to 0C, the salt which has formed is filtered
off, dried and subsequently suspended in 150 ml of
sulfolane, 150 ml of acetonitrile and 10 ml of dimethyl-
formamide. After 100 ml (1.07 mol~ of phosphorus
oxychloride have been added, the mixture is heated at the
boil for 2.5 hours and subsequently poured into
ice-water. The 2-methoxycarbonyl-5-nitro-benzenesulfonyl
chloride which has precipitated (60.1 g, 70 ~ of theory)
is filtered off with suction using a Buchner funnel and
freed from traces of solvent under reduced pressure.
21447~9
- 32 -
M.p.: 86-88C.
d) N-tert.-Butyl-2-methoxycarbonyl-5-nitrobenzene-
sulfon~m;de
50 ml (0.48 mol) of tert.-butylamine are slowly added
dropwise at 0C to a solution of 34.4 g (0.12 mol) of
2-methoxycarbonyl-5-nitro-benzenesulfonyl chloride in
200 ml of ethyl acetate and 250 ml of ethanol. Stirring
of the reaction solution is subsequently continued for
10 minutes at room temperature. After 500 ml of water
have been added, a colorless solid crystallizes out.
After filtration and drying, 28.1 g (89 %) of N-tert.-
butyl-2-methoxycar~onyl-5-nitrobenzenesulfonamide are
obtained. M.p.: 121-124C.
e) N-tert.-Butyl-5-hydroxylamino-2-methoxycarbonyl-
benzenesulfonamide
23.4 g tO.08 mol) of N-tert.-butyl-2-methoxycarbonyl-5-
nitrobenzenesulfonamide are introduced into 600 ml of
ethanol at 40C. After the mixture has been buffered with
42.1 g (0.79 mol) of ammonium chloride and 170 ml of
water, 32.0 g (0.49 mol) of zinc powder are added in
- portions. Stirring of the mixture is subsequently
continued for 4 hours at approximately 55C. The solid is
removed by filtration, and the filtrate is evaporated on
a rotary evaporator. The residue is taken up in 800 ml of
ethyl acetate, and the mixture is washed with 300 ml of
water. The aqueous phase is re-extracted three times
using 300 ml of dichloromethane. The combined organic
phases are dried over magnesium sulfate. Volatile
components are subsequently removed by distillation on a
rotary evaporator. The crystalline residue is extracted
by stirring with a little ethyl acetate: heptane (1:1).
In this manner, 17.2 g (77 %) of N-tert.-butyl-S-
hydroxylamino-2-methoxycarbonyl-benzenesulfonamide are
ob~A;ne~. M.p.: 190-195C.
- 21447~9
- 33 -
f) N-tert.-Butyl-5-(N-acetylhydroxylamino)-2-methoxy-
carbonylbenzenesulfo~ATni~le
3.31 g (0.01 mol) of N-tert.-butyl-5-hydroxylamino-2-
methoxycarbonylbenzenesulfonamide are briefly heated in
20 ml of acetonitrile to obtain a homogeneous solution.
0.94 g (0.01 mol) of acetyl chloride and 1.30 g
(0.01 mol) of triethylamine are then added, with ice-bath
cooling. After 2 hours, the mixture is taken up in ethyl
acetate and washed in succession with 1 N hydrochloric
acid and saturated sodium chloride solution. After the
organic phase has been dried over magnesium sulfate, the
solvent is removed by distillation on a rotary
evaporator. After recrystallization o~ the residue from
ethyl acetate/heptane, 3.12 g (82 %) of N-tert.-butyl-5-
(N-acetylhydroxylamino)-2-methoxycarbonylbenzenesulfon-
amide are obtained. M.p.: 168-169C.
g) 5-(N-Acetylhydroxylamino)-2-methoxycarbonyl-
benzenesulfonamide
1.70 g (4.9 mmol) of N-tert.-butyl-5-(N-acetylhydroxyl-
amino)-2-methoxycarbonylbenzenesulfonamide are dissolved
in 20 ml of trifluoroacetic acid. After 18 hours at 25C,
the volatile components are removed by distillation under
reduced pressure. This gives 1.48 g of 5-N-(acetylhydrox-
ylamino)-2-methoxycarbonylbenzenesulfonamide.
M.p.: 246-247C.
h) 5-(N-Acetylhydroxylamino)-N-[(4,6-dimethoxypyrimid-
in-2-yl)aminocarbonyl]-2-methoxycarbonylbenzene-
sulfonamide (corresponds to Example 192 in Table 1)
0.8 ml of DBU are added to a mixture of 1.00 g (4.4 mmol)
of 5-(N-acetylhydroxylamino)-2-methoxycarbonylbenzene-
sulfonamide and 0.65 g (2.4 mmol) of 4,6-dimethoxy-2-
(phenoxycarbonyl~;no)pyrimidine in 50 ml of acetonit-
rile. After the mixture has been stirred for 18 hours,
the solvent is removed by distillation on a rotary
21~476~
- 34 -
evaporator. The residue is taken up in water and washed
with diethyl ether. The aqueous phase is acidified using
concentrated hydrochloric acid (pH = 1). The colorless
solid is removed by filtration and extracted by stirring
with a little methanol. The resulting 5-(N-acetylhydrox-
ylamino)-N-[(dimethoxypyrimidin-2-yl)aminocar~onyl]-2-
methoxycarbonylbenzenesulfonamide (0.81 g, 79 % of
theory) has a melting point of 205-208C.
i) N-[(4,6-Dimethoxypyrimidin-2-yl)aminocarbonyl]-2-
methoxycarbonyl-5-(N-methoxycarbonylhydroxylamino)-
benzenesulfonamide (Ex. 238, Table 1)
0.75 ml of DBU are added to a mixture of 0.74 g
(2.43 mmol) of 2-methoxycarbonyl-5-(N-methoxycarbonyl-
hydroxylamino)benzenesulfonamide and 0.67 g (2.43 mmol)
of 4,6-dimethoxy-2-(phenoxycarbonylamino)pyrimidine in
50 ml of CH3CN. After the mixture has been stirred for
18 hours, the solvent is removed by distillation on a
rotary evaporator. The residue is taken up in water and
washed with diethyl ether. After the aqueous phase has
been acidified with concentrated hydrochloric acid
(p~ = 1) a colorless solid precipitates. After filtration
with suction and washing with methanol, 0.66 g of the
above-designated sulfonylurea is obtained; colorless
crystals of m.p. 192-193C.
j) N-[(4,6-Dimethoxypyrimidin-2-yl)aminocarbonyl]-2-
methoxycarbonyl-4-(N-methoxycarbonylhydroxyl~;no)-
benzenesulfonamide (Examples 3-9, Table 3)
1.2 ml of DBU are added dropwise to a suspension of 1.2 g
of 2-methoxycarbonyl-4-(N-methoxycarbonylhydroxylamino)-
benzenesulfon~;de and 1.1 g of 4,6-dimethoxy-2-(phenoxy-
carbonylamino)pyr;mi~;ne in 30 ml of C~3CN. After a
reaction time of 4 hours, the mixture is worked up
analogously to Example i). 1.09 g of the above-designated
sulfonylurea are obtained; colorless crystals of m.p.
171-174C.
21~il7~9
- 35 - -
k) Sodium salt of N-[(4,6-dimethoxypyrimidin-2-yl)-
aminocarbonyl]-2-methoxycarbonyl-5-(N-methoxy-
carbonylhydroxylamino)benzenesulfonamide
(Examples 4-10, $able 4)
1.2 ml of 1 N sodium hydroxide solution are added to a
solution of 0.57 g of N-~(4,6-dimethoxypyri~i~in-2
yl)aminocarbonyl]-2-methoxycarbonyl-5-(N-methoxycarbonyl-
hydroxylamino)benzenesulfonamide in 20 ml of CH2Cl2 and
20 ml of CH3CN. After the reaction mixture has been
stirred for 2 hours, it is evaporated in vacuo. The
resulting product (0.61 g) melts at 175-180C and is the
above-designated sulfo~ylurea.
The rP~ining compounds described in Tables 1 - 4 are
obtained analogously to Examples a) to k).
The following abbreviations are used in the tables:
M.p. = melting point in C
No. = Example No.
Et = ethyl (C2Hs)
Me = methyl (CH3)
Ph = phenyl
c-Pr = cyclopropyl
n-Pr = n-propyl
i-Pr = i-propyl
- 36 _ 21~4763
Table 1
R~ - N~2 ~ 5O2NHCONR4lN1X
No Rl R2 R3 R~ X Y Z M.p.
CHO H H H OMe OMe CH 150
2 CHO M~ H H OMe OMe CH
3 COCH3 H H H OMc OM~ CH 154
4 H H H OMc OMc N
H H H OMe Me CH
6 H H H Me Me CH
7 H H H OMe Cl CH
8 H H H OMe Me N
9 COCH3 H H H OMo OMe N
10 ~ H H H NHMc OCH2CF3 N
11 H H H NMc2 SMo N
12 H H Me OMe OMe CH
13 M~ H H OMc OMc CH
14 Mc H Mc OMe OMe CH
15 COCH2CH3 H H H OMe OMe CH 158-159
16 H H H OMe OM~ N
17 COCH2CH3 H H H OMe Cl CH
18 H H H OM~ Me N
19 Mc H H OMc OMe CH
20 COCF3 H H H OMII OM~ CH
21 COCF3 H H M~ OMe OM~ CH
22 COCF3 H H H OMc M~ N
23 COCH2Br H H H OMe OM~ CH
24 COCHCI2 H H H OMe OMe CH
25 COCH~CH312 H H H OMe OMI~ CH
26 COPh H H H OMc OMe CH
27 -t~O-c-Pr H H ~ H OMc OMe CH
-
37 ~14~76~
No. Rl R2 R3 R~ X Y ZM.p.
28 CO c~Pr Mo H H OMo OMo CH
2g ~ Me H H OMe Mo N
30 H H H OMo M~ N
31 eo ~ H H H OM~ OMo CH
32 CO CH-CH2 H H H Mo OM- N
33 CO-CH -CH2 H H H OMo OMo CH
34 CO2CH3 H H H OMo OMo CH210
35 H H H OM- nM~ N
36 CO2CH3 Mo H H OMe OMo CH
37 CO2-i-Pr H H H OM~ OMe CH
38 CO2-i-Pr Mo H H OMo OMe CH
39 CS-NHEt H H H OMo OMo CH
40 CS-NHEt H H H Cl OMo CH
41 CS NHEt H H M~ OMo OMo CH
42 CS NH-CO2Et H H H OMe OMo CH
43 CS-NH-CO2Et Mo H H OMo OMo CH
44 H H H H OMo OMo CH
45 502CH2CI H H H OMo Me N
46 Mo H H OMe OMo CH
47 ' Mo H H Mo MoCH
48 SO2Me Me H H OMo OMe CH
49 SO2Me H H H OMe OMo CH
50 SO2NHMe H H H OMo OMe CH
51 502NHMo H H H SMo NM~2 N
52 SO2NMo H H H OMo OM~ CH
53 502NMo Mo H H OM- OMo CH
54 CHO H 6~Me H OMo OMo CH
55 H ' H OEt OEt CH
56 CHO H 6 Mo H NMe2 SMo N
57 ' H ' H OMo Mo N
2~4763
-- 38 ~
No, Rl R2 R3 R~ X Y Z M.p.
58 CHO Me H OMe OMe N
59 CHO H H OMo Cl CH
60 ~ H H OCF2H OCF2H CH
61 CO-CH3 H ~Me H OMe OMs CH 178-18Z
62 ' H ' H Me Me CH
63 ' H H NMo2 OCH2CF~ N
64 ' H H OMo Mo N
65 ' Mo 6 Mo H OMe OMe N
66 ' H Me OMo OMe N
67 CO CH~CH2 H ' H OMe OMe CH 178-180
68 CO-CH2CH~ H 6 M- H OMo OMo CH 180
69 CO CH2CH3 Me ' H OMe OMe CH
70 CO i Pr H H OMo OMo CH
71 CO-CH - CH2 H H ~ . CH
72 COCF3 H ' H ' ' CH
73 COCF3 H ' Me OMe Me N
74 CO-c Pr H 6 Mo H OMo Mo N
75 CO c-Pr H H ' OMe CH 127 130
76 ' H ' H Me Mo CH
77 COCsHll H 6-Me H OMe OMc CH 179
78 CO2Mo H 6-Me H OMc OMe CH 198~201
79 ' H ' H Mo Me CH
80 CO2Me H 6~Me H NEt2 SMe N
81 ~ Me ' H OM~ OMe CH
82 CO2CH2CH2CI H ' H OMo OM~ CH
83 CO2CH2- H ' H
CH - CH2
84 C02CH2CCI~ H ' H
CO2CH2CH2OMe H ' H
86 CONHEt H ' H
87 CONHEt H 6 M- H OMo Mo N
88 CONHEt Me ' H
89 CONHPr H H OMe CH 204
21~69
-- 39 ~
NO. Rl R2 R3 R~ X Y Z M.p.
90 CONHPr H ' H OcH2cF3 NMe2 N
91 CSNHEt H 6-Me H OMe OMo CH 108
92 CSNHEt H 6~Me Me OMe Me N
93 CSNHCH2CH-CH2H 6-Me H OMc OMeCH 134-137
94 CSNHCO2Et H 6-Mo H OMe OM-CH 130-132
5O2Me H H
96 Me H
97 H H Cl OMe
98 SO~NHMq H 6-Me H OMe OM~ CH
99 H ' Me OMe M- N
100 SO2NMei H 6-Me H OMo OMe CH
101 SO2CH2CI Me 6~Me H OMe OMe CH
102 H H CH
103 CHO H 6 CI H OMe OM- CH 203
104 CHO H 6-CI H SMe OM- N
105 H H OCH2CF~ NM~2 N
106 H H OMe a CH
1 Q7 COCH3 H 6-CI H OMe OMe CH 193
108 Me H
109 H H ' Me N
110 COCH2CH3 H H OMe N
111 H H CH 195
1t2 CO-c-Pr H 6 CI H . CH 145
113 CO-c Pr Me ~CI H OMe OMe CH
114 COCFI H H
115 COCF3 H M- OMe M~ N
116 COPh H 6-CI H OMe OM- CH
117 CO-C6H4-4-CI H H . 192
118 CO-C~sH3-2,4 C12 H ' H . 230
119 ~53_ H 6-CI H OM~ OMe CH 202
~o~ tl
120 c~ ' 203
121 CO-C5Hll H H 158
122 CO-CMe3 H H 225
123 CO CHBr-CH2CH3 H 6-CI H OM~ OMe CH 194
21 4 ~7~
~ 40 ~
No. R1 R2 R3 R~ X Y Z M.p.
124 CO-CMs2-CH2CI H H . 195
125 COCICII - CCI2 H 6 CI H OM~OMo CH 203
126 COCH20Me H ' H
127 CO2Me H H 212
128 CO2Me Mo H
129 Co2cH2ccl3 H H
130 CSNHEt H 6-CI H OMe OMe CH
131 CSNHPr H H CH
132 CS NH- H 6-CI H OMe OM- CH
CH2CH - CH2
133 CSNHCO2Et H H 190 191
134 S02Mel H H
135 S02Me H 6 CI H NMs2 OCH2CF3 N
136 S02 i-Pr H H OMe OMe CH
137 SO~Nl lMe H 6-CI H . 140
138 ' H H Me M~ CH
139 ' OM~ M~ N
140 Cl OMe CH
141 SO~Nr~le2 OMe OM~ CH
142 Me H
143 SO2CH2CH3 Ms
144 CHO H 6 F H OMo OM- CH
145 COCH3 H 6-F H OM~ OM- CH
146 COCH3 Me 6-F H OMe OM~ CH
147 COCH3 H 6-F H OMe Me N
14~ C02Me H 6-F H OMe OMe CH
149 COCCI3 H 6-F H OMo OMe CH
150 CONHPr H 6-F H OMe OM~ CH
151 502Me H 6-F H OMe OM~ CH
152 502NI'M- H 6-F H OMe OMe CH
153 COCH3 H 6 Br H OMe OM~ CH
154 COCH3 H 6-Br H OMe Me N
155 S0~!Nl'r1e H 8 Br H 0Me OMe CH
156 SO~N MP2 H 6~6r H OMe OM~ CH
157 SO~N'Me~ M~ 6 Br H OM- OM~ CH
214~769
_
-- 41 ~
No Rl RZ R3 R~ X Y Z M.p.
158 C02Mo H 6-Bt H OMe OMe CH
159 CO2Me Me B-8r H OMe OMe CH
160 CHO H 6-OM~ H OMe OMe CH
161 COCH3 H ~OMo H OMe OMe CH
162 COCHMe2 H B-OM- H OMe OM~ CH
163 COCH3 Me 6-OMe H OMrt OMe CH
164 COCH3 H 6 OMe H OM- Me N
165 COCH3 H 6-OMe H Mo Me CH
166 CO2Me H 6-OMe H OMe OMe CH
167 C02M~ Me B-OMe H OMe OMe CH
168 C02Me H 6-OMe H NMe2 OCH2CF3 CH
169 so~rJ~ H 6-OMe H OM- OMo CH
170 502Me H 6-OMs H OMo OMe CH
171 COCH3 H 6-OEt H OMo OMe CH
172 COCH3 M- 6-Ok H OM~ OMe CH
173 C02Me H 6-OEt H OMe OMe CH
174 C02Mo H 6 0 i-Pr H OMe OM~ CH
175 C02M~ H 6-0-i-Pr H NMe2 OCHzCF3 N
176 CO ~ H 6-OEt H OMe OMe CH
177 C o ~ H B-OEt H OMe OMe CH
178 CHO H ~CO2Me H OMe OMe CH 161
t79 H H M~ Me CH
180 CHO H 6-C02Me H OMe Cl CH
181 H H OCH2CF3 NMe2 N
182 ~ H H OMe Me N
183 H ' M~ OMe Me N
184 CHO H 6-C02Me H NEt2 OMe N
185 CHO H 6~COzM- H SMe OMe N
186 CHO H 6 C02Ms H OMs OMe N
187 H H OEt OEt N
188 H H OEt OEt CH
189 Ms H OMe OMe CH
190 Me H OMe Me N
- 214~76~
- 42 ~
No. Rl R2 R3 R~ X Y Z M.p.
191 ' Mo H Mo Mo CH
192 COCH~ H 6-CO2Mo H OMo OMe CH 205-208
193 H H Ok OEt CH
194 H H NEt2 SM~ N
195 H H Cl OMo N
196 COCH3 M~ 6CO2Me H Cl OM~ N
197 H H OMe Mo N
198 H Mo OMo Me N
199 COCH3 H 6-CO2M- H Mo Mo CH 122
200 H H OCH2CF3 NMe2 N
201 H H OCF2H OCF2H CH
202 COEt H 6-CO2Me H OMo OMs CH 117
203 Me H OMe OM- CH
204 Et H OMe OM- CH
205 COEt H 6-CO2Mo H OMo Mo N
206 COEt H ~CO2M~ H NMo2 OCH2CF3 N
207 C0 ~ H 6-CO2M- H OMo OMo CH 125-127
C0 ~ Et 6-CO2M~ H OM~ OM~ CH
209 C0 ~ Mo H OMe OMe CH
210 C0 ~ H H Mo Mo CH
211 C0 ~ H 6-CO2Me Me OMe OMe CH
212 C0 ~ H H OMo Me N
.213 C0 ~ H H OMo SM~ N
214 C0 ~ H H Cl OMe CH
215 C0 ~ H H NM~2 OCH2CF3 N
216 C0 ~ H H NHM~ SMo N
2tg.~7i~
-- 43 --
No. Rl R2 R3 R~ X Y Z M.p.
217 C0 "~ H ~C02Me H OMe OMe CH 213
218 C0 "c~ H ' M~ OMe N
219 Co "c~ Me H OMe OM~ CH
220 CO ~ ~ Me H M~ Me CH
221 C0Ph H ' OMe OMe CW 218-220
222 eo ~ H 6-CO2Mc H OMe OMe CH
223 COCF3 H . OMe OMo CH 186-188
224 Me OM~ OMe CH
226 Me OM~ Me N
226 Mo . U OM~ CH
227 H ' Nk2 OM- CH
228 COCF~ H 6-CO2M- H NEt2 OMe N
229 ' Me OMe OMe N
230 ' ~ Me SMe OMe N
231 COCH2~ H 6-CO2M- H SM- OMe CH 110
232 COCH20Me H H
233 COCH2Br H H
234 COCHCI2 H 6-CO2Me H SMe OM~ CW
235 COCHCI2 H H OMo Mo CH
236 COCH2NM~2 H H OMe OMe CH
237 COCH2NHCO2Me H H
238 CO2Me H H 192-193
239 H H OMe OMo N
240 CO2Me H 6CO2M3 H Me M~ CH
241 Me H OMe OMe N
242 H H NEt2 SM- N
243 H H OCH2CF3 NHMe N
244 Me H OMe OM~ CW
245 C02CH2CH2CI H H . 182
246 CO2CHM~2 H H . 96-98
247 C02CHM-2 H 6CO2Me M~ OMe M~ N
. , ,
214~76~
~ 44 --
NO. R 1 R2 R3 R~ X Y ZM.p.
248 CO2CH2CH~ H H OMe OMe CH
249 CO2CH2CCI3 H H OMo OMe CH
250 CO2CH2CH2OM- H H OMe OMe CH
251 CO2CH2CHCH2 H H . CH
252 CO2CH2C-CH H 6-CO2M~ H OMe OMe CH
253 CONM-2
254 CONHPr 149-151
255 CONHEt 149-150
Z56 CONHSO2- H 6-CO2Me H OMe OMe CH 180-182
CH2CHC12
257 CONHSO2- H H OMe Me N
CH2CHCl2
258 CSNHEt H H OMe OMe N
259 Me CH
260 CSNHEt H 6 CO2Me H OMo OM~ CH
261 CSNHCH2CH - CH2 H ~ 115-117
262 CSNHCO2Et H . 190-191
263 CSNHCO2Et H Me N
264 SO2M~ H 6-CO2Mo H OMe OMe CH
265 5O2-i-Pr H
266 SO2NHM~ t43
267 Me M~
268 OMe OMe N
269 Cl OM~ CH
270 SO2NH2 ! . OMr~ OM- N
221 M~ NMQ2 OCH2CF~ N
272 H M- OMe Me N
2?3 COCH3 H 6-CO2 i-Pr H OMe OMe CH
274 H H M- M- CH
275 H H OMe M~ N
276 CHO H 6-CO2-i-Pr H OMe OMe N
277 CHO H 6-CO2Et H
278 CHO H H OM- M2 N
279 CO2Et H OM~ OM~ CH
280 CO2M~ . .
-
~14~7~9
-- 45 --
No. R 1 R2 R3 R~ X Y Z M.p.
281 COCH3 H 6-CN H OM~ OMa CH
282 CO2M~ H H OMe OM~ CH
283 CHO
284 CO2Mo H 6-l H
285 CO2M~ H 6-l H Mo OMo N
286 CHO H 6-l H Mo OMe N
287 CHO H OMo
288 COCH~ H 6-NIOHIC0CH3 H OM~ OMe CH
289 M- Me
290 OMo Me N
291 Me 6-NlOMe)COCH3 ~
292 ~ OMe CH
293 CHO H 6-NIOH)COCH
294 CHO Me ~N~OMe)COCH
295 CO2Me H 6-N~OH~CO2M~
296 CHO H 6 NHCHO
297 COCH3
298 COCH3 H 6-NH-COCH3
299 COCH3 Mo 6-NM~-COCH3 H OMe OMe CH
300 CO2CHCI2 H 6-NMe-COCF3 H OMs OM~ CH
301 CO2CHCI2 H 6-NHCOCF3 H OM~ OMe CH
302 COCH3 H 2 CO2Me H OMe OMe CH
303 OMo Me N
304 CO-i-Pr OMe CH
305 CO2Mo
306 ' ' OMs Me N
307 ~ ~ Cl OMe CH
308 COCH3 ~ 2-CI OMo OMe CH
309 5O2NHMe ' OMe OMe CH
310 5O2NMo2
311 SO2Me
312 CHO H H H OMe OMo N
313 ' OMo Mc N
314 Me Me CH
315~' OMe CH
316 ' Cl CH
- 46 - 21~S9
-
No. Rl R2 R3 R4 X Y Z M.p
317 Me ' ' OMe OMo N
318 ' ' ' OMo Mo N
319 Cl OMe N
320 Me Me N
321 Et ' OMe OMe CH
322 ' ' ' Me N
323 Me Mo CH
324 CHO Et H H Cl OMo CH
325 Pr OMe OMe CH
326 CO-CH3 Mo OM~ Mo N
327 Et OMe OMe CH
328 CO-CH2CH3 H OMe Mo N
329 Mo ' OMe OMo CH
330 ' Et
331 OM- OMo N
332 Mo Mo CH
333 ~ ' Cl OMo CH
334 COCF3 H H H Mo Mo CH
335 ' ' Cl OMo CH
336 COCF3 Me H H OMe OMe CH
337 Et ' ~ ' CH
338 Pr
339 COCH2Br H OMe Me N
340 ' Me Me CH
341 Me ' OMe OMo CH
342 Et
343 COCHCI2 H H H OMe OMe N
- 21447~9
- 47 -
No. Rl R2 R3 R~ X Y Z M.p.
344 Me N
345 ' Me ~ OMe CH
346 ' Et
347 COCH2CI H
348 COCH2CI Me H H OMe OMe CH
349 H ~ OMe Me N
350 Me Mo CH
351 COCCI3 -
352 ' OMe OMe CH
353 Me ' OMe OMe CH
354 ' Cl OMe CH
355 CO-CH(CH3)2 H H H OMe Me N
356 Mo Me N
357 Cl OM~ CH
358 ' Me OMe OMe CH
359 ~ ' Cl OMe CH
360 CO CH~CH3)2 Me H H OMe Me N
361 Et OMe CH
362 CO-c-Pr H H H OMe MQ N
362 ' OMe OMe N
363 Ct Ms CH
364 Me Me CH
365 Me OMo OMe N
366 OMe M~ N
367 Et ' ' ' OMe CH
368 ~ Me H H OMe OMe CH
. .
- 48 - 214476~
No. Rl R2 R3 R~ X Y Z M.p.
369 Et
370 CO2Me Et H H OMe OMe CH
371 ' Me N
372 ' Cl CH
373 Me Me CH
374 Me Me Me CH
375 Me OMe N
376 Et ~ OMe OMe CH
377 CO2Et H H H OMe OMe CH
378 OMe Me N
379 ~ M~ Me CH
380 Me OMe OMe CH
381 Et
382 CO2-n-Pr H H H OMo OMo CH
383 Me N
384 Mo OMe CH
385 Et
386 COCCI2CH3 H H H
387 Me
388 Et -
389 CHO H 6-CI Me N
390 M~ -
391 OMe CH
392 Et
393 CH2CH
-CH2
3g4 COCH3 H 6-CI H Me Me CH
- 21~4769
-- 49 --
NO. R1 R2 R3 R~ X Y Z M.p.
395 ' M0
396 OMe N
397 ' Cl OMe CH
398 Et OMe OMe CH
399 n-Pr
400 COCH2CH3 H ~CI H OM0 MO N
401 OMO Cl CH
402 M0 MB CH
403 MO OM0 OM~ CH
404 ~ Et Me N
405 COCH2CH3 n-Pr 6-CI H OMO OMe CH
406 CO C-Pr H B-CI H OMe Mo N
407 H ~ Me M0 CH
408 Cl OMO CH
409 ~ MO MO OM0 N
410 ~ OMo OMe CH
411 ~ Et
412 ~ Et ~ OMe Me N
413 n-Pr ~ OM6 OM0 CH
414 CO-CF3 H 6 CI H OMe Cl CH
415 ~ Me MO CH
416 ~ M0 OMo OMe CH
417 CO-CF3 Me 6-CI H OMe Mo N
418 ' MO Me CH
419 Et
420 OMe OMO CH
421 ~ OM0 MO N
- 214~76~
-- 50 --
No. R ~ R2 R3 R~ X Y Z M.p.
422 ' Cl OMe CH
423 ~_ Me 6 CI H OM~ Me N
co e,
424 ~3 H OMe CH
425 Me
426 CO~C5H11 Me
427 CO-CsH l l Me 6-CI H OMe Me N
42a CO-CMe3 Me ~ OMe CH
429 CO-CH2 0Me Me
Me N
431 ~ Et OMe CH
432 CO2Me H Me N
433 ~ Me M- CH
434 ~ Cl OMe CH
435 Me ~ ~ -
436 ~ Me N
437 ~ Et OMe OMe CH
438 CO2Me Et 6-CI H OMo Me N
439 n Pr OMe OMe CH
440 CO2Et H 6-CI H '
441 ~ OMe M~l N
442 ~ ' Me Me CH
443 ' ~ Cl OMe CH
444 ~ Me
OMe
446 ~ OMe Me N
214~769
-- 51 --
No. Rl R2 R3 R~ X Y Z M.p.
447 Et OMe OMe CH
448 C02-i Pr H
449 ~ Me N
450 Mo ' ~ -
451 C02-i-Pr Me 6-CI H . OMe OMe CH
452 Et
453 . . Me N
454 CHO H B-OMe H OMe Me N
455 ' OMe Cl CH
456 ' ' OCH2CF3 NMe2 N
457 Me ' OMe OMo CH
458 Et
459 ' n-Pr -
460 Me Me N
461 Et
462 n Pr
463 CO CH3 H 6-OMe H Cl OMe CH
464 OCHzCF3 NMe2 N
465 Me
466 OMe Me N
467 Et
468 OMo OMe CH
469 CO-CHICH3~2 H 8-OMe OMe Me N
470 Mo
471 OMo OMe CH
472 C02Me H 8 0Me H OMe Me N
473 Me ~ ~ -
474 Cl OMe CH
.
~lA~7~3
-- 52 --
No. R ~ R2 R3 R~ X Y Z M.p.
475 CO2Me Et 6-OMa H Cl OMe CH
476 ' ~ OMa C!Me CH
477 " MB N
478 CO2Et H 6-OM~ H OMe OMe CH
479 Me N
480 ' Me
481 Et -
482 OMe CH
483 CO2-i-Pr H . . CH
484 Me
485 CHO H 6-OEt H OMe OMe CH
486 Me
487 CHO Et 6-OEt H OMe OMe CH
488 CO-CH3 H OMe CH3 N
489 Cl OMe CH
490 Ms
491 CH3 CH3 CH
492 Et OCH3 OCH3 CH
_ ~ ,~ .. . . . . .
493 ~,v-CH2vH3 n
494 Me
495 Et
496 CO-CHICH3)2 H
497 Me
498 CO2Me Me ~ ~ ~
499 CHO Me 6-CO2Me H Cl OMe CH
500 ' Et
- 21~47`69
-- 53 --
No. Rl Rl R3 R~ X Y z M.p.
501 ' ' ' OMs OMe CH
502 ~ Mo M~ CH
OMg Me N
504 ' nPr ' ' '
505 ' ' OMs CH
506 CO-CH3 MB 6-CO2Mo H OM~ OMe CH
507 ' ' ' OMs Ms N
508 ' Ms Ms CH
509 ' Et ' ' OMs OMe CH
510 ' ' OMo Me N
511 CO-CH3 Et 6-CO2M8 H Ms Me CH
512 ' ' ' ' OMe Cl CH
513 Pr ~ ' OMs OMo CH
514 CO-CH2CH3 ~ MB Me CH
515 ' OMo Cl CH
516 ' Me ' OMe Me N
517 ~ ' Cl OMs CH
518 ' Me Me CH
519 Et
520 ' ' OMe Me N
521 n-Pr OMe OMs CH
522 CO-c-Pr MB 6-CO2Ms H OMs Me N
523 CO-c-Pr MB 6CO2Me H OMe Cl CH
524 ' Me Me CH
525 Et
526 ' ' ' OMo Cl
527 ' ' ' OMo Ms N
528 COCF3 H 6-CO2Me H OMe Me CH
529 ' ' ' Cl OMe CH
- 214~76~
~ 54 --
No. pl R2 R3 R~ X Y Z M.p.
530 Et
531 OMe OMe
532 ' Me N
S33 ' ' Mo Me CH
534 CO-CH2 0Me H 6-CO2Me H OMe OMe CH
535 CO-CH2-OMe H 6-CO2Me H OMe M~ N
536 ' ' OMe Cl CH
537 ~ Mo Me CH
538 Me ' '
539 OMe OMe
540 . . OMc M- N
541 ' Et
542 ~ OMo OMe CH
543 CO-CH2Br H 6 CO2M~
544 M-
545 Et
546 CO-CHCI2 H 6-CO2Me 90-93
547 CO-CHCI2 Me 6-CO2Me H OMo OMe CH
548 Et
549 CO-CCI3 H ~ .
550 Me
551 Et ' -
552 CO2M~ H 6-CO2Me H OMe Me N
553 Cl OM- CH
554 M~
555 ' OM~ Me N
556 Me Me CH
557 Et H OMe OMe CH
558 Pr
559 CO2CH2CH2CI H 6 CO2Me H OMe Me N
560 ' Me Me CH
561 Cl OMe CH
562 ' Me OMe OMe CH
563 OM~ M~ N
564 ' OM- Cl CH
565 Et
21~4~
- 55 -
No. R ~ R2 R3 R~ X Y Z M.p.
566 OM8 OMe CH
567 CO2CH2CH3 H ~ . . N
568 OMe M~ N
569 M~ -
570 Cl CH
571 CO2CH2CH3 Mo 6-C02M~ H OMe OMe CH
572 Et
573 . . . Me N
674 CO2CHICH3)2 H Cl OMe CH
575 M. M~ CH
576 M8
577 ~ OMe OM~ -
578 OM. M~ N
579 Et OM. CH
580 CO2CH2CCI3 H OM. Me N
581 ~ Me
582 ' M~ OMe OM8 CH
5E3 CO2CH2Ccl3 Et 6-CO2M8 H OMe OM8 CH
584 Et MO N
585 CO2CH2CH2OMc Me OM~ N
586 CONHEt H ' M8 N
587 ~ M8 MB CH
5a8 CONHEt Me ~ OMe OMe CH
589 CO-NHPr H ' OM~ M8 N
590 CSNHMe H ~ ' OMo OM8 CH 132-135
591 ' ' ' ' ' Me N
592 ' ' ' ' M~ M- CH
593 ' ' a OMe CH
594 CSNMe2 Me ' ' OMe OMe CH
595 CSNMs2 Me 6CO2Me H OMe M~ N
596 CHO H 6-C02Et H OMe OMe CH
597 ~ OM8 Cl CH
598 ' ' ' ' M8 M- CH
599 ' M.
600 ' ' M- OMe CH
~ 56 _ 2 14 4~ 6 3
No. R ~ R2 R3 R~ X Y Z M.p.
601 ~ OMe OMe CH
602 OMI~ Me N
603 Es OM~ OM~ CH
604 OMe Me N
605 CO CH3 H -
606 OMe CH
607 CO-CH~ H 6 CO2Et H OM~ Cl CH
608 Me M~ . CH
609 Me OMe OMe CH
610 ' Et
611 CO2Me Me
612 Et
813 CO2Et Me
614 Et '
615 CO2-CH(CH3)2 Me
616 CHO Me 6-l W OMe OM~ CH
617 Et -
618 Mo OMe Me N
619 CHO Et 6-l H OMe Me N
620 H Me Me CH
621 Me ~
622 Et
623 H Cl OMe CH
624 Me
625 Et
626 CO-CH3 H OMe OMe CH
627 ~ ~ OMe Me N
628 Me
629 OMe OMe CH
630 Et
631 C02Mo Me 6-l H OMe OMe CH
632 Et -
633 CS-NHMe H H H OMe OMe CH
634 Me
635 Et ~
636 CS-NHEt Me
- 21~6~
-- 57 --
No. Rl R2 R3 R~ X Y ZM.p.
- 637 Et '
638 CO-NHEt H H H
639 OMe Me N
640 Mo
641 OMs OMe CH
642 Et -
643 CO NH-n Pr H H H OMe OMe CH
644 Me
645 Et ~ -
646 CS-NHMe H 6-CI H OMe OMe CH
647 Me
648 Et
649 CS-NHEt Me
650 C0-NHEt H 6-CI H
651 H H OMe Me N
652 Me
653 Me OMe CH
6S4 Et
655 Co NH n-Pr H 6-CI H OMe OMe CH
6S6 Me
657 CS-NHMe H 6-OMe
658 ' Me
659 Me OMe N
660 CS-NHEt H 6-OMe - OMrs OMe CH
661 ~ Me
662 C0-NHEt H
663 Me
58 21g~769
Table 2
R3
~ 5 2 R H--C O --N ~Z
R1 ~oR2 )~
No. Rl R2 R3 R~ X Y Z M.p.
2-l COCH3 H H H 0Me OMe CH
2-2 ' Me OMe N
2-3 Me M~ aH
24 M~ OMe OMe CH
2-S N
2-6 CH0 H CH
2-7 OMe Me N
2-8 CHO Me H H OMe Me N
2-9 ' H Me OMe Me N
2-10 COCH2CH3 H ' H OMe OMe CH 161
2-11 CO c-Pr H ' ' ' ' 154
2-12 COCF~ ' ' ' ' ' ' 158
2-13 ' M- M- OMe N
2-1~ ' H M- CH
215 OM- a
2-16 COCF3 H H H OMe Me N
2-17 CO2Me OM- OMo CH
2-18 OM- M- N
2-19 CONHPr OMe CH
REPT ~ T SHEET
- 214~7~3
~ 59 ~
No. Rl R2 R3 R~ X Y Z M.p.
2-20 CONHEt -
2 21 CO2Me H ~CI H OMe OMe CH
2-22 Me
2-23 Me OMe OMe N
2-24 CHO Me CH
2-25 CHO H
2-26 SO2Me H H H OMe OMo CH
2-27 SO2NHMIl H H H
2-28 NM~2 OCH2CF, N
2-2g 502NMo2 H . OM- OMe CH
2-30 Mc
2 31 CO2Me H 5-COIMe H OM- OM- CH
2-32 COCH~ H
2-33 CocH~ H 6-CF3
2-34 COCH~ M~ 5-CF~ H OMo OMe CH
2 35 H 5-CN
2-36 Me
2 37 COCH3 H 5-F H OMe OM~ CH
2-38 COCH3 . Me
2-39 COCOOMe H H H 169-170
2-40 COCH~ Mc H H OM- Me N
2-41 a OMc CH
2-42 Et H H OM- OMc CH
2-43 ' OMe Mc N
2-44 CHO M- H H OMe OMc CH
REPLACEMENT SEIEET
~ 60 _ 21~7~3
No. Rl R2 R3 R~ x Y Z M.p.
2~5 ' Me Mo CH
246 '' Cl OMo CH
2-47 Et OMe OMe CH
2~8 '' Me N
2~9 CO-CH2CH3 H . OMe Mo N
2-50 Mo Me CH
2-51 Me -
2-52 ~ ~ . OMe OMe CH
2-53 OMe Me N
2-54 ~ Cl OMe CH
2-55 Et OMe OMe CH
2-56 CO-CH2CH3 Et H H OMo Me N
2-57 COCF3 Me H
2-58 ' ' ' OMe OMe CH
2-59 Et
2-60 CO ~ H OMe Me N
2-61 Me
2-62 Me OMo CH
2-63 Et
2-64 CO2Me Me
2-65 Mo N
2-66 Et
2-67 CO2Me Et H H OMa OM~ CH
REPT ~r~M~NT SHEET
~ 61 - 214~763
No. Rl R2 R~ R~ X Y Z M.p
2~8 CONHEt H H H OMe OMo CH
2-69 ~ M- N
2-70 CONHEt Me
2 71 OMo OMo CH
2-72 CSNHMo H
2-73 ' ' ' ' OMo Me N
2-74 Me
2-75 ' ~ ' OMe OMo CH
2-78 CSNMe2
2-77 CSNHEt H
2-78 ~ Mo N
2-79 CSNHEt Me H H OMe OMe CH
2 80 CSNEt2 Et
2 81 COOCH2CH2CI H H OMo OMe CH
2-82 ' ' ' ' Cl CH
2-83 ' ' ' Me Me CH
2-84 ' Me ' OMe OMe CH
2-85 COOEt H H OMe OMe CH
2-86 ' ' ' Cl CH
2-87 COOiPr ' '
2-88 ' OMe OMe
2~89 COOCH2CCI3
2-90 COOCH2CH2CI Mo H OMo Mo N
REPT.~r~M~NT SHEET
214~1763
-- 62 --
Table 3
oR2
~ 5 o ~ N H C O ~ R --~N~(
No. Rl R2 R3 R~' X Y Z M.p.
3-1 CO-H H 2-CI H OMe Me N
3-2 COCH3 H 2-CI H OMo OMo CH
3-3 COCH3 CH3 2-CI H
3-4 COCH2CH3 H 2-CI H
3-5 CO-c-Pr H 2-CI H
3-6 COCF3 H 2-CI H ~
3-7 CO2Me H 2-CI H ~ ~
3-8 Co2MB Me 2-CI H OMo OMo CH
3-9 CO2Me H 2-CO2Me H ~ -
3-10 CO2Me H H Mo N
3-11 CO~-i-Pr H H ~ OM~ CH
3-12 CONHEt H ' H OMo CH
3-13 CONHPr H
3 14 CSNHEt ~
3-15 COCH3 H H H
3-16 COCH2CH3 H H H
3-17 CO2Me Me 2-CO2Me H OMe OMo CH
31a ~ Me N
REPLACEMENT SHEET
-
- 63 _ ~ 21 ~ 4 76 9
No. R~ R2 R3 R~ x Y Z M.p.
3 19 CO2Me Et 2-CO2Me H OM- Me N
3 20 ' ' ~ ~ ' OMs CH
3-21 CO2Et
3-22 ' Me
3-23 CHO H ' '
3-24 ' Me ' ' '
3-25 ' Et
3-26 ' H ' ' ' Me N
3-27 Me
3 28 ~ Et
3-29 CO-CH3 H
3-30 ~ Me
3-31 CO CH3 Me 2CO2Me H OMe Me N
3-32 ~ H ~ ' OMe CH
3 33 ~ Me
3-34 ~ E~ ' ' '
3-35 CO-OMe H 2-COOMe H Ma Me CH
3 36 COOMe H 2-COOMe H OMe Cl CH
3-37 COOMe Ms 2-COOMe H Me Me CH
3-38 COOMe Me 2-COOMe H OMe Cl CH
.
REPT ~CFMF NT SHEET
21447~9
-- 64 ~
No. Rl R2 R3 R~ x Y Z M.p.
3-39 COOEt H ' OMo OMe
3,40 ~ OMe Cl
341 ~ Me N
3-42 Me ' OMe M~ N
3-43 CHO Me ' OMe Cl CH
Ms M~ CH
3 45 COCH3 H . Cl OMs CH
3-46 ' M~ Me CH
REPLACEMENT SEIEET
21447~
-- 65 --
Ta}: le 4
oR2 R3
R 1 _ N ~[~( S2N - CO - NR 4 ~
N
~ Y
No. Rl R2 R3 ~ M X Y Z M.p.
4-1 CHO H 2-COOMIl H N~ OMe OM~ CH
4-2 CHO H ' K ' '
4-3 ' ~ ' N~ OM~ M~ N
4 4 ' M~ ' N~
4-5 OM~I CH
4 6 COCHJ H 192196
M~ N
4-8 ' M~
4-9 ' ' ' ' OM- CH
410 COOM- H ' 175 180
4-11 M~ N
4-12 M~ ' ~ -
4-13 ' ' ' CH
REPr ~ NT S~EET
- 21~769 - 66 -
B. Formulation Examples
a) A dust is obtained by mixing 10 parts by weight of
a compound of the formula (I) and 90 parts by weight
of talc as inert substance and comminuting the
mixture in a hammer mill.
b) A wettable powder which is readily dispersible in
water is obtained by mixing 25 parts by weight of a
compound of the formula (I), 64 parts by weight of
kaolin-contAin;ng quartz as inert substance,
10 parts by weight of potassium ligninsulfonate and
1 part by weight of sodium oleoylmethyltaurinate as
wetting agent and dispersant, and grinding the
mixture in a pinned-disk mill.
c) A dispersion concentrate which is readily dispers-
ible in water is obtained by mixing 20 parts by
weight of a compound of the formula (I) with 6 parts
by weight of alkylphenol polyglycol ether
(-Triton X 207), 3 parts by weight of isotridecanol
polyglycol ether (8 EO) and 71 parts by weight of
paraffinic mineral oil (boiling range for example
approximately 255 to above 277C), and grinding the
mixture in a ball mill to a fineness of below
5 microns.
d) An emulsifiable concentrate is obtained from
15 parts by weight of a compound of the formula (I),
75 parts by weight of cyclohexanone as solvent and
10 parts by weight of ethoxylated nonylphenol as
emulsifier.
e) Water-dispersible granules are obtained by mixing
75 parts by weight of a compound of the formula (I),
10 " of ~calcium ligninsulfonate,
5 ~ of sodium lauryl sulfate,
3 ~ of polyvinyl alcohol and
- 21~4769
- 67 -
7 " of kaolin,
grinding the mixture on a pinned-disk mill, and
granulating the powder in a fluidized bed by spray-
ing on water as granulation liquid.
f) Water-dispersible granules are also obtained by
homogenizing and precomminuting
25 parts by weight of a compound of the formula (I),
" of sodium 2,2'-dinaphthyl-
methane-6,6'-disulfonate,
2 " of sodium oleoylmethyltaurinate,
1 part by weight of polyvinyl alcohol,
17 parts by weight of calcium carbonate and
50 " of water
on a colloid mill, subsequently grinding the mixture
on a bead mill, and atomizing and drying the result-
ing suspension in a spray tower by means of a
single-substance nozzle.
C. Biological Examples
1. Pre-emergence effect on weeds
Seeds or rhizome pieces of monocotyledon and dicotyledon
weed plants were placed in sandy loam soil in plastic
pots and covered with soil. The compounds according to
the invention which were formulated in the form of
wettable powders or emulsion concentrates were then
applied to the surface of the soil, in the form of
aqueous suspensions or emulsions at an application rate
of 600 to 800 l/ha (converted), in various dosages.
After the treatment, the pots were placed in a greenhouse
and kept under good growth conditions for the weeds.
After the test plants had emerged, the damage to the
plants or the negative effect on the emergence was scored
- 21~7~
- 68 -
visually after a test period of 3 to 4 weeks by compari-
son with untreated controls. A~ shown by the test
results, the compounds according to the invention have a
good herbicidal pre-emergence activity against a broad
range of grass weeds and broad-leaved weeds. For example,
the compounds of Examples 61, 192, 217, 238, 256 and 262
of Table 1, 3-9 and 3-29 of Table 3 and 4-9 of Table 4
have a very good herbicidal activity against noxious
plants, such as Sinapis alba, Chrysanthemum segetum,
Avena sativa, Stellaria media, Echinochloa crus-galli and
Lolium multiflorum when applied pre-emergence at an
application rate of 0.3 kg and less of active ingredient
per hectare.
2. Post-emergence effect on weeds
Seeds or rhizome pieces of monocotyledon and dicotyledon
weeds were placed in sandy loam soil in plastic pots,
covered with soil and grown in a greenhouse under good
growth conditions. Three weeks after sowing, the test
plants were treated in the three-leaf stage.
The compounds according to the invention which were
formulated as wettable powders or as emulsion concen-
trates were sprayed in various dosages onto the green
parts of the plants at an application rate of 600 to
800 l/ha (converted) and, after the test plants had
remained in the greenhouse for approx;~ately 3 to 4 weeks
under ideal growth conditions, the effect of the prepara-
tions was scored visually by comparison with untreated
controls. The compositions according to the invention
also have a good herbicidal post-emergence activity
against a broad range of economically important grass
weeds and broacl-leaved weeds. For example, the compounds
of Examples 61, 192, 217, 238, 256 and 262 of Table 1,
3-9 and 3-29 of Table 3 and 4-9 of Table 4 have a very
. .
good herbicidal activity against noxious plants such as
Sinapis alba, Stellaria media, Echinochloa crus-galli,
Lolium multlflorum, ChrysanthPmllm segetum and Avena
21~ 76~
- 69 -
sativa when applied post-emergence at an application rate
of 0.3 kg and less of active ingredient per hectare.
- 3. Tolerance by crop plants
In further greenhouse experiments, seeds of a substantial
number of crop plants and weeds were placed in sandy loam
soil and covered with soil. Some of the pots were treated
immediately as described in Section 1, and the remaining
pots were placed in a greenhouse until the plants had
developed two to three true leaves and were then sprayed
with various dosages of the substances of the formula (I)
according to the invention as described in Section 2.
Visual scoring four to five weeks after the application
and after the plant had been in the greenhouse revealed
that the compounds according to the invention did not
inflict any damage to dicotyledon crops such as, for
example, soya, cotton, oilseed rape, sugar beet and
potatoes when used pre- and post-emergence, even when
high dosages of active substance were used. Some of the
substances also left Gramineae crops such as, for
example, barley, wheat, rye, sorghum species, maize or
rice particularly unharmed.
The compounds of the formula (I) therefore have a high
selectivity when used for controlling undesired vegeta-
tion in agricultural crops.