Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
1.
2145509
The invention relates to the object characterized in the
claims, i.e., new porphyrin complex compounds with various
substituents in positions 3, 8, 13 and 17 of the porphyrin
skeleton, pharmaceutical agents containing these compounds, their
use in diagnosis as well as process for the production of these
compounds and agents.
The use of complexing agents or complexes or their salts in
medicine has long been known. As examples can be mentioned:
complexing agents as stabilizers of pharmaceutical preparations,
complexes and their salts as auxiliary agents for administration
of poorly soluble ions (e.g., iron), complexing agents and
complexes (preferably calcium or zinc), optionally as salts with
inorganic and/or organic bases, as antidotes for detoxification
in accidental incorporation of heavy metals or their radioactive
isotopes and complexing agents as. auxiliary agents in nuclear
medicine by using radioactive isotopes such as ~c for
scintigraphy.
Complexes and complex salts have been presented as
diagnostic agents, mainly as NMR diagnostic agents, in patents EP
0 071 564, EP 0 139 934 and DE 34 O1 052. However, they do not
yet optimally meet all requirements which determine the relative
effectiveness of an NMR contrast medium, of which the following
2
2145509
are to be mentioned as examples:
a favorable relaxivity, so that the contrast medium in the
smallest possible concentrations reduces in vivo the relaxation
times of the protons in tissue fluids and other nuclei relevant
for the NMR (such as phosphorus, fluorine, sodium) and makes
possible, for example, the localization of tumors by increasing
the signal intensity of the image obtained with the help of the
nuclear spin tomograph; a concentration and/or retention of the
contrast medium in the target organ as selective as possible;
sufficient water solubility; high effectiveness; good
compatibility; good chemical and biochemical stability.
Thus above all the two first mentioned points are relevant
for the imaging. Since the relaxation times between the tissues
differ mostly only by the factor of 2-3 {T. E. Budinger and P. C.
Lauterbur, Science 226, pp. 288-298, 1984; J. M. S. Hutchinson-
and F. W. Smith in Nuclear Magnetic Resonance Imaging Edit. C. L.
Partain et al., pp. 231-249, Saunders, New York 1983) and the
complexes and. complex salts of the mentioned patents generally
have the drawback that they only spread relatively
nonspecifically in the extracellular space and therefore a
detection of pathologically changed tissues is not always
possible, above all there is a need for selectively binding,
tumor-specific compounds that can be used in diagnosis and .
radiation therapy.
It has been known for some years that porphyrin derivatives
selectively accumulate in human and animal tumors (D. Kessel and
T.-H. Chou, Cancer Res. 43, pp. 1994-1999, 1983,-P. Hambright,
214559 3
Bioinorg. Chem. 5, pp. 87-92, 1975; R. Lipson et al., Cancer 20,
pp. 2250-2257, 1967; D. Sanderson et al., Cancer 30, pp. 1368-
1372, 1972). First attempts to use this class of compound as
diagnostic agents were also described (J. Winkelmann et al.,
Cancer Research 27, pp. 2060-2064, 1967; N. J. Patronas-et al.,
Cancer Treatment Reports 70, pp. 391-395, 1986)..
However, the compounds so far described are far from being
able to meet satisfactorily the above-mentioned criteria; their
deficient concentration in the target organs still requires
special attention. An improvement of this property should at the
same time help reduce the existing problems with toxicity and
compatibility of the previously known compounds.
Substituted hematoporphyrin complex compounds used in
diagnosis and treatment are described in patent application EP 0
355 041.
While these compounds show a good concentration behavior in
various target organs, the described compounds used as NMR
diagnostic agents have a not yet completely satisfactory ratio
between the dose necessary for optimal imaging and the lethal
dose. Hematoporphyrin derivatives also have the drawback that
they can eliminate both pseudobenzylic OH groups in the
hydroxyethyl side chains.
In patent EP 0 071 569 NMR diagnostic agents are described 3
based on DTPA complexes which, while having a favorable safety
margin, are excreted relatively quickly as a result of which the
examination time remaining with optimal enhancement is only
brief .
21455fl9 4
Derivatives of the deuteroporphyrin have been proposed for
tumor imaging with radioisotopes, containing as additional
complexing groups polyaminopolycarboxylic acids bound to the
porphyrin skeleton by ethylene glycol bridges (Photochemistry and
Photobiology Vol. 46, pp. 783-788 (1987)). However, such
porphyrin esters are not very suitab3e for parenteral use in
patients -- especially for NMR diagnosis -- since the injection
solutions obtained from them can neither be heat-sterilized nor
stored for a sufficiently long time.
Therefore, there remains a demand for many purposes for
complex compounds having stable, easily soluble, but also more
compatible, more selectively binding over a greater chemical
variation range of substitutents (which, e.g., make possible the
incorporation of metals other than manganese or several, also
different, metals and thus at the same time also leads to a
control of the properties and uses of the compounds), which,
e.g., are suitable for diagnosis and treatment of tumors.
The object of the invention is thus based on making
available such compounds and pharmaceutical agents containing
these compounds as well as providing processes for their
production.
This object is achieved by the invention.
It was found that porphyrin complex compounds, consisting of !
a porphyrin ligand, at least one ion of an element with atomic
numbers 21-32, 37-39, 42-51 or 57-83 as well as, optionally,
cations of inorganic and/or organic bases, surprisingly are
2145509
excellently suited for the production of NMR and radiodiagnostic
agents as well as radiotherapeutic agents.
The porphyrin complex compounds according to the invention
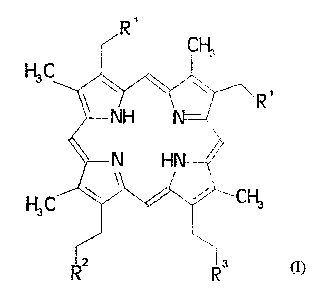
consist of a ligand of general formula I
R'
CH3
R
~ NH \~
N H
w \ i~C~
H3C
1
R R
as well as at least one ion of an element with atomic numbers 21-
32, 37-39, 42-5i or 57-83, in which
R~ stands for a hydrogen atom, for a straight-chain C~-C6
alkyl radical, a CT C~2 aralkyl radical or for a group OR' in
which
R~ is a hydrogen atom or a C~-C3 alkyl radical,
RZ stands for R3, a group -CO-Z or a group -(NH)o-(A)q-NH-D,
in which
Z is a group -OL, with L meaning an inorganic or
organic cation or is a C~-C4 alkyl radical,
'- X145509
A means a phenylenoxy or a C~-C~Z alkylene or
aralkylene group interrupted by one or more oxygen
atoms,
o and q, independently of one another, mean the numbers
0 or 1 and
D means a hydrogen atom or a group -CO-A-(COOL)o-(H)~,
with m equal to 0 or 1 and provided that the sum of
m and o equals 1,
R3 stands for a group - (C=M) (NR4) a- (A) q- (NRS) -K,
in which M stands for an oxygen atom or for two
hydrogen atoms,
R4 means a gxoup -(A)q-H and
K means a complexing agent of general formula (IIa) or
(IIb) and in which R5, if K is a complexing agent of
formula (IIa), has the same meaning as R4, and RS,
if K is a complexing agent of formula (IIb), has the
same meaning as D,
provided that a direct oxygen-nitrogen bond is not
allowed,
- 21455U9 '
and K stands for a complexing agent of general formula
(IIa) or (IIb)
t
'wCO~N/~N~N~---COOL
COOH COOH COOL
. z
COOL .
COOL
~A N
OH < 'NJ
4
COOL (IIb)
with L~ meaning a C~-C6 alkyl radical or an inorganic or
organic cation and in which
L2, L3 and L4, independently of one another, have the meaning
of L~ or a hydrogen atom, provided that at least two free
carboxylic acid groups are present in the complexing agent,
as well as optionally other anions to compensate for the charges
in the metalloporphyrin.
The complex compounds according to the invention comprise a
total of three groups of compounds.
a) Compounds containing a metal ion in the porphyrin,
b) compounds containing at least one metal ion in =.
complexing agent radical K and
c) compounds containing bound metal ions in both the
porphyrin and in complexing agent radical K, in which the metal
ions can be different.
- 2145509
Paramagnetic metal ions must be present in the complex
to use the agents according to the invention in NMR diagnosis.
The latter are especially divalent and trivalent ions of the
elements with atomic numbers 21-29, 42, 44 and 57-70. Suitable
ions are, for example, chromium(III), manganese(II),
manganese(III), iron(III), cobalt(II), cobalt(III), nickel(II),
copper(II), praseodymium(II), neodymium(III), samarium(III) and
ytterbium(III) ions. Because of their high magnetic moment
gadolinium(III), terbium{III), dysprosium(III), holmium(III),
erbium(III) and iron{IIi) ions are especially preferred.
For radiodiagnosis and radiotherapy, complexes are suitable
which contain as central atom a radioisotope of elements 27, 29-
32, 37-39, 42-51, 62, 64, 70, 75, 77, 82 or 83.
If the concentration, e.g., of an yttrium-90 labeled
complex, is to be NMR-diagnostically monitored in use in
radiotherapy, complexes are suitable which contain, in addition
to the radioisotope, a paramagnetic metal ion as well as another
metal ion, preferably a gadolinium ion.
It is possible in this way to combine diagnosis and
treatment with the help of the complex conjugates according to
the invention.
An essential advantage of the metal complexes containing
complexing agent radical K according to the invention is that in
the metal complexes, the diagnostic effect brought about by the
metal ions can be enhanced by the incorporation of other metal
ions.
2145509
Surprisingly, the complexes according to the invention in
comparison with the structurally similar compounds known so far
show a markedly higher relaxivity. Since the relaxivity can be
considered as a measure for the contrast medium effectiveness of
a compound, in using the complexes according to the invention in
the area of.NMR diagnosis a comparable, positive signal
influencing is possible even with a small dose. In this way, the
safety margin increases significantly, for which the product of
relaxivity and compatibility can be considered as recommended
value.
The complex compounds according to the invention also meet
the other requirements, such as, e.g., high selectivity and
concentration, in an outstanding way. With the help of the
complex compounds according to the invention, surprisingly not
only tumor tissues and individual organs can be represented in
vivo such as, for example, liver and kidneys, but also blood
vessels without using special pulse sequences with which they can
be used,.i.a., as perfusion agents.
The metals manganese, iron, cobalt, nickel, copper, zinc and
technetium can be mentioned as examples for the ions bound in the
porphyrin skeleton. Preferred are the metals iron, technetium,
zinc and especially manganese.
If one of the ions bound in the porphyrin is present in a
higher oxidation stage than +2, then the excess charges) is/are
balanced, e.g., by anions of organic or inorganic acids,
preferably by acetate, chloride, oxide or nitride ions.
- 2145509 1~
The transition metals with atomic numbers 21-30, 37, 39 and
43 as well as the elements 57-83 can be mentioned as examples of
the ions bound to complexing agent K. Preferred is the
gadolinium, dysprosium, holmium, erbium and manganese ion.
Optionally the carboxyl groups not necessary for the
complexing of the metal ions can be present as esters, amides or
salts of inorganic or organic bases. Suitable ester radicals are
those with 1 to 6 C atoms, preferably the ethyl esters; suitable
inorganic cations are, for example, the lithium and potassium ion
and, particularly, the sodium ion. Suitable cations of organic
bases are those of primary, secondary or tertiary amines, such
as, for example, ethanolamine, diethanolamine, morpholine,
glucamine, N,N-dimethylglucamine, especially meglumine.
As examples for the group - (NR4) o- (A) q- (NR5) - which, in a
way, serves as "linker" between the porphyrin skeleton and
complexing agent K, there can be mentioned, for example, the
-NHNH-, -NH ( CH2 ) ZNH-, -NH ( CH2 ) 3NH-, -NH ( CHZ ) 4NH-,
-NH ( CH2 ) ZO ( CH2 ) ZNH-, -NH-CH2-CbH4-CH2-NH- and the -CH2-O-C6H4-NH-
group.
As complexing agent radicals K, preferably derivatives of
diethylenetriaminepentaacetic acid and the 1,4,7,10-
tetraazacyclododecane-1,4,7-triacetic acid can be mentioned,
which are bound by a "linker" to the respective porphyrin.
The production of the complex compounds according to the
invention takes place in that by
_ 2145509
a) reduction of a porphyrin of general formula (IIIa)
R'
CH3
H3C
\ \ \ \ R,
NH N-
N HN
\ ~ ~CH3
(CH2)3-CN (CH2)3-CN
(IIIa)
or by
b) reaction of a porphyrin of general formula (IIIb)
C
C \ w ., -~ ,
NH I~ ,, R
N HN-,~
1
tCt-~)3-Br tCl-~)3-Br
with aminophenol, or by
'_ 214550 12
c) reaction of a porphyrin of general formula (IIIc)
R'
H3C
(IBc)
in which R' has the indicated meaning,
V and Y each stand for a hydrogen atom or together for a
multivalent metal ion of an element with atomic numbers 21-32,
37-39, 42-51 or 57-83, preferably for a zinc(II) or a
manganese(III) ion and
X stands for a halogen atom, a group -OR' or for a group -O-
COORS with R' in the mentioned meaning,
with compounds H-NR4-(A)q-NR4-H,
in which A, R~ and q have the indicated meaning,
optionally subsequent reduction of the carbonyl groups or Hofmann
degradation of the amide first yields a porphyrin of general
214509
formula IV
R'
CH3.
w w w
N ~~~ R,
- v Y
N N
~C w ~ C~
~ s'
R
in which R6 stands for a group - (C=M) - (NR4) o- (A) q-NR4-H,
in which M, R4, A, o and q have the indicated meaning and
in which R6~ has the same meaning as R6 or stands for a group -
-OR',
that then
a) is reacted with a complexing agent of general formula V,
~ /~ ~~OR'
N N N
O-
~COOH~COOH
O
(v>
._ 2145509 14
in which R' has the indicated meaning, and optionally the present
ester groups are saponified
or
b) is reacted with a compound of formula VI
O' 'A'~ N N ~
OH
3
(VI)
in which A' is a group A shortened by a carbon atom and M~, M2
and M3, independently of one another, stand for R' or a metal ion
equivalent of the elements with atomic numbers 21-32 , 37-39, 42-
51 or 57-83, under the conditions of a reductive amination, then
the thus obtained product -- optionally after complete or partial
cleavage of the ester groups -- is reacted with (a) metal
oxides) or metal salts) of the elements of the above-mentioned
:atomic numbers, then acylated with a nucleofuge-D' reagent (and
the last-mentioned steps can be interchanged) in which D' has the
meaning indicated under D, with the provision that D' does not
stand for hydrogen and then, optionally, acidic hydrogens
optionally possibly still present are then completely or
partially substituted by cations of inorganic or organic bases.
- 2145509 15
As examples, acetyl chloride, acetic anhydride, succinic
acid anhydride or diglycolic acid anhydride can be mentioned as
nucelofuge-D'.
Thus, the functionalization of the C-13 and C-17 side-chains
to C-13,17-amino- or amidoalkyl-substituted porphyrin derivatives
(serving as feedstocks for the porphyrin complexes according to
the invention containing complexing agent K) takes place either
by
a) reduction of the respective 13,17-bis-(3-cyanopropyl)-
porphyrin to the amine in a way known in the art, for example,
with lithium borohydride/trimethylsilyl chloride in an organic
polar ether, preferably tetrahydrofuran (A. Giannis, K. Sandhoff,
Angew. Chem.; 101 (1989) 220/22) or
b) reaction of the respective 13,17-bis-(3-bromopropyl)-
porphyrin with an aminophenol in a dipolar aprotic solvent such
as, e.g., dimethylformamide or dimethyl suifoxide or
c) reaction of the desired C-13;17-propionic 'acid chain-
carrying porphyrins which optionally can be present in active
form, e.g., as acid chlorides, esters or mixed anhydrides,
optionally containing a metal atom instead of the pyrrolic
hydrogens, with optionally substituted hydrazines or with
terminal alkylenediamines which optionally can be substituted by
a C~-C6 alkyl, aryl or C~ C~2 aralkyl radical and from which an
amino group optionally, e.g., in the form of a carbobenzoxy or
t-butoxycarbonyl radical, is protected. The removal of the
protective groups then takes place according to methods known in
the literature, e.g., by hydrogenation or treatment with
- 21455U9 16
trifluoroacetic acid or with hydrochloric acid/glacial acetic
acid.
If asymmetrically substituted porphyrins are to be produced,
i.e., porphyrins in which the radicals R2 and R3 are not the
same, then this can be controlled by the reaction time and
stoichiometry.
The introduction of complexing agent radical K of general
formula IIa in the thus functionalized C-13, C-17 amino- or
amidoalkyl-substituted porphyrin derivatives takes place in a way
known in the art by reaction with the corresponding acid
anhydrides in liquid phase. Suitable reagents are, for example,
water, dipolar aprotic solvents, such as dimethylformamide,
dimethyl sulfoxide or hexamethylphosphoric acid triamide in the
presence of an inorganic or organic base, such as alkali or
alkaline-earth hydroxides and carbonates and tertiary amines such
as, e.g., trimethylamine, triethylamine, N,N'-
dimethylaminopyridine. Suitable reaction temperatures are
between -10 and 120°C, preferably between 0 and 50°C.
The introduction of radical K of general formula IIb takes
place in a way known in the art, by a corresponding initial
product, that optionally can already be substituted by a metal,
being reactedlby glycol cleavage (e.g., with periodate) first to
an aldehyde of general formula VI and then being reacted with the
respective C-13, C-17 amino- or amidoalkyl-substituted porphyrin
derivative. A reduction, e.g., with sodium cyanoborohydride
follows this reaction step.
2145509 1'
Remaining secondary amines can be acylated by reaction with
activated acid derivatives (nucleofuge-D'):
The introduction of the desired metals (e.g., Mn, Fe, Co,
Ni, Cu, Zn, Tc, Sm, Eu, Gd, Bi) in the porphyrins takes place
according to methods known in the literature (The Porphyries, ed.
D. Dolphin, Academic Press, New York 1980,'Vol. V, p. 459) and
essentially there can be mentioned:
a) the substitution of the pyrrolic NH's (by heating of the
metal-free ligands with the corresponding metal salt, preferably
the acetate, optionally with addition of acid buffering agents,
such as, e.g., sodium acetate, in a polar solvent) or
b) the "recomplexing," in which a metal already complexed by
the ligand is displaced by the desired metal.
As solvents, primarily polar solvents, such as, e.g.,
methanol, glacial acetic acid, dimethylformamide, chloroform and
water, are suitable.
The introduction of the porphyrin metal can take place
before or after joining of complexing agent radical K as well as
before or after chelating of this complexing agent with a metal.
In this way, an especially flexible process for the synthesis of
the compounds according to the invention is made possible so
that, e.g., metal isotopes of little half-life (for example 99m-
technetium), can be introduced only in the final synthesis step
either in the porphyrin ligand or in the complexing agent.
The chelating of radical K takes place in a way known in the
literature (see, e.g., DE 34 O1 052) by the metal oxide or metal
salt (e. g., the nitrate, acetate, carbonate, chloride or sulfate)
- 2145509
of the respectively desired metal being suspended or dissolved in
polar solvents, such as water or aqueous alcohols, and reacted
with the corresponding amount of the complexing ligand. If
desired, any present acidic hydrogen atoms or acid groups can be
substituted by cations of inorganic and/or organic bases or amino
acids.
The neutralization takes place in this case with the help of
inorganic bases, such as, e.g., alkali or alkaline-earth
hydroxides, carbonates or bicarbonates and/or organic bases such
as, i.a., primary, secondary or tertiary amines, such as, e.g.,
ethanolamine, morpholine, glucamine, N-methyl- and N,N-
dimethylglucamine, as well as basic amino acids, such as, e.g.,
lysine, arginine and ornithine or of amides of initially neutral
or acidic amino acids.
For production of the neutral complex compounds, for
example, enough of the desired bases can be added to the acid
complex salts in aqueous solution or suspension that the
neutralization point is reached. The obtained solution can then
be evaporated to dryness in a vacuum. Often, it is advantageous
to precipitate the formed neutral salts by addition of water-
miscible solvents, such as, for example, lower alcohols (e. g.,
methanol, ethanol, isopropanol), lower ketones (e. g., acetone),
polar ethers (e. g., tetrahydrofuran, dioxan, 1,2-dimethoxyethane)
and thus to obtain easy-to-isolate and readily-purified
crystallizates. It has proven especially advantageous to add the
desired base as early as during the complexing of the reaction
mixture and therefore to eliminate a process step.
CA 02145509 2003-06-26
If the acid complex compounds contain several free acidic
groups, it is often advisable try p:rodL~ce nekxtral mixed salts
containing both inorganic and organic rations as counterions.
This can happen, for example, by the complexing ligands in
an aqueous suspension or solution being reamed with the oxide or
salt of the element supplying the <wentral iron and half of the
amount of an organic base necessary for the neutralization, the
formed complex salt being isolated, optionally purified and then
mixed with the necessary amount of inorganic base for complete
neutralization, The sequence of the addition of bases can also
be reversed.
Another possibility of achieving neutral complex compounds
is to convert the remaining acid groups in the complex totally or
partially into esters. This can occur by a later reaction on the
completed complex (e. g,, by exhaustive r~~~ac~.ion of the free
carboxy groups with dimethyl sulfate).
If complex compounds containing radioisotopes are used,
their production can be carried out according to the methods
described in "Radiotracers for Medical Applications," Volume 1,
CRC-Press, Boca Raton, Florida ~ EdiGc~r a»~rimella V.SrRayudu (1983) .
The production of the pharmaceutical agents according to the
invention also takes place in a way known in the art, by the
complex compounds according to the invention -- optionally with
addition of additives usual in galenica:ls -- being suspended or
dissolved in an aqueous medium and then the suspension or
solution optionally being stc~rili.zed. Suitable additions are,
for example, physiologically harmless buffers (such as, e.g.,
2145509
tromethamine), small additions of complexing agents (such as,
e.g., diethylenetriaminepentaacetic acid) or, if necessary,
electrolytes such as, e.g., sodium chloride or, if necessary,
antioxidants, such as, e.g., ascorbic acid.
If, for enteral administration or other purposes,
suspensions or solutions of the agents according ~to the 'invention
in water or in physiological salt solution~are desired, they are
mixed with one or more adjuvant(s) usual in galenicals (e. g.,
methyl cellulose, lactose, mannitol) and/or surfactants) (e. g.,
iecithins, Tween~R~, Myrj~R~ and/or aromatic substances for taste
correction (e. g., essential oils).
In principle, it is also possible to produce the
pharmaceutical agents according to the invention without
isolation of the complex salts. Special care must always be
taken that chelation be carried out so that the salts and salt
solutions according to the invention are practically free of
noncomplexed toxically-acting metal ions.
This can be assured, for example, with the help of color
indicators such as xylenol orange by control titrations during
the production process. The invention also relates therefore to
processes for the production of the complex compounds and their
salts. Purification of the isolated complex salt remains as
final safety measure.
To avoid unwanted photoreactions of the porphyries, the
compounds and agents according to the invention should be stored
and handled as much as possible with exclusion of light.
_ 2145509 21
The pharmaceutical agents according to the invention contain
preferably 20 ~mol/L to 200 mmol/L of the complex salt and are
generally dosed in amounts of 0.01 ~mol to 2 mmol/kg of body
weight. They are intended for enteral and parenteral
administration. -
The complex compounds according to the invention are used
1. in NMR diagnosis in the form of their complexes with the
ions of elements with atomic numbers 21-30, 42, 44 and 57-83;
2. in radiodiagnosis and radiotherapy in the form of their
complexes with the isotopes of elements with atomic numbers 27,
29-32, 37-39, 42-51,'62, 64, 70, 75, 77, 82 or 83.
The agents according to the invention meet the varied
requirements for suitability as contrast media for nuclear spin
tomography. Thus, they are outstandingly suitable after enteral
or parenteral administration to improve the informative value of
the image obtained with the help of the nuclear spin tomograph by
increasing the signal intensity. Further, they show the high
effectiveness necessary to load the.body with the least possible
amounts of foreign substances and the good compatiblity necessary
to maintain the noninvasive nature of the tests.
The good water solubility of the agents according to the
invention allows the production of highly concentrated solutions,
so that the volume load of the circulatory system is kept within .
justifiable limits and the dilution by body fluids is offset.
Further, the agents according to the invention not only show a
high stability in vitro, but also a surprisingly high stability
in vivo, so that a release or an exchange of the ions -- toxic in
-_ 214509 22
themselves -- bound noncovalent in the complexes is insignificant
within the time in which the new contrast media are completely
reexcreted.
Details of the use are discussed, for example, in H. J.
Weinmann et al., Am. J. of Roentgenology 142, 619 (1984).
The~agents according to the invention, because of their
advantageous radioactive properties and the good stability of the
complex compounds contained in them, are also suitable as
radiodiagnostic agents. Details regarding the use and dosage of
the complex compounds according to the invention carrying the
radioactive metal ions in the field of radiodiagnosis are
described, e.g., in "Radiotracers for Medical Applications," CRS-
Press, Boca Raton, Florida.
Another imaging method with radioisotopes is the positron
emission tomography, which uses positron-emitting isotopes, such
as, e.g. , 43Sc, ''BSc, SzFe, 55Co and ~Ga (Heiss, W. D. , Phelps, M.
E., Positron Emission Tomography of Brain, Springer Verlag
Berlin, Heidelberg, New York 1983).
The compounds according to the invention can also be used in
radioimmuno or radiation treatment. The latter is differentiated
from the corresponding diagnosis only by the type and the amount
of the isotope used. The purpose is thus the destruction of
tumor cells by high-energy shortwave radiation with a smallest
possible range of action. Suitable f3-emitting ions are, for
example, 46Sc, 47Sc, 48Sc, 7zGa, ~Ga and 9°Y. Suitable a-emitting
ions having short half-lives are, e.g. , z~~Bi, z~zBi, z~3Bi, z~4Bi
2145509
23
and 2~ZBi is preferred. A suitable photon- and electron-emitting
ion is ~S$Gd, which can be obtained from ~S~Gd by neutron capture.
If the agent according to the invention is intended for use
in the variant of radiation treatment proposed by R. L. Mills et
al. [Nature Vol. 336, (1988), p. 787J, the complexed ions) must
be derived from a Mossbauer isotope, such as, for example, S~Fe
or ~S~Eu.
In the in vivo administration of the therapeutic agents
according to the invention, the agents can be administered
together with suitable vehicles, such as, e.g., serum or
physiological common salt solution and together with another
protein, such as, e.g., human serum albumin. In this case, the
dosage is dependent on the type of cellular disorder, the metal
ion used and the type of method, e.g., brachytherapy.
The therapeutic agents according to the invention are
administered parenterally.
Details of the use of radiotherapeutic agents are discussed,
e.g., in R. W. Kozak et al. TIBTEC, October 1986, 262.
The invention is explained by the following examples:
CA 02145509 2003-06-26
~> t'~
Example 1
a) N,N'-Bis[~-carboxy-2r >--bi.s(c~.arhoxymethyl)-8-
(ethoxycarboxymethyl-2,5,8-triazanonyl°carbamoyl]-mesoporphyrin-
:IX-13,17-diamfide
806.8 mg (2 mmol) of 3-ethoxy-carbonyl.methyl-6-[2-(2,6-
dioxomorpholino)ethyl]-3,6-diazaoctanedioic acid (DTPA-
monoethylester-monoanhydride) i.s su speraded in 250 ml of absolute
dimethylformamide. It is covered with nitrogen, 1.01 g (10 mmol)
of triethylamine and 595 mg ( ~ mnnol.) off:' mesoporphyrin-IX-13, 17-
dihydrazide (produced analogously to ii~ Fischer, E. Haarer and F.
Stadler, Z. Physiol. Chem. 24:1, 203 (lr)::3E~) ) are added and the
resulting reaction mixture is st:~.rred for 3 days at room
temperature. After completion of the reaction, it is ffiltered,
the solvent is removed in a vacuum and t:hEa remaining oil
triturated with 500 ml of diethyl ether. The precipitated solid
is filtered off and washed with diethyl ether and n-hexane. For
purification, it is chromatographed on silica gel RP-18
(Fluent: H20/tetrahydrofurany tetrahydx°ofuran: 0-30%)..
Yield: 1.21 g (86.3% of theory) of reddish brown powder
Analysis(relative tothe ar~rtydroex:~ substance)
C 56.56 H 6,62 N 13.99 O 2:x.83 Cld.
C 56.25 H 6,89 P11:3.70
- 2145509 25
b) N,N'-Bis[9-carboxylato-2,5-bis(carboxylatomethyl)-8-
(ethoxycarboxymethyl-2,5,8-triazanonyl-carbamoyl]-mesoporphyrin-
IX-13,17-diamide, digadolinium complex
1.40 g (1 mmol) of the ligand produced in example la is
dissolved in 400 ml of water. By addition of 2n aqueous sodium
hydroxide solution, it is adjusted to pH 7 and alternately 894.2
mg (2.2 mmol) of gadolinium acetate tetrahydrate and 2n aqueous
sodium hydroxide solution are added in portions, so that the pH
of the reaction mixture always fluctuates between 6.8 and 7.2.
After all gadolinium acetate has been added, it is stirred
overnight at room temperature. For working-up, the solvent is
removed in a vacuum and the residue is chromatographed on silica
gel RP-18 (Eluent: H20/tetrahydrofuran; tetrahydrofuran: 0-30%).
Yield: 1.01 g (59.1% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 46.36 H 5.07 N 11.47 Gd 18.39 O 18.71 Cld.
C 46.08 H 5.29 N 11.27 Gd 18.05
c) N,N'-Bis[9-carboxylato-2,5,8-tris(carboxylatomethyl)-
2,5,8-triazanonyl-carbamoyl]-mesoporphyrin-IX-13,17-diamide,
digadolinium complex, disodium salt
1.4 g (1 mmol) of the ligand produced in example la is .
dissolved in 400 ml of water. By addition of 10 molar aqueous
sodium hydroxide solution, it is adjusted to pH 13 and stirred
for 5 hours at room temperature. After completion of the
saponification of the ester groups, it is adjusted to. pH 7.0 with
m4~~oo 26
concentrated hydrochloric acid and, as described in example lb,
complexed, worked up and purified with 894.2 mg (2.2 mmol) of
gadolinium acetate tetrahydrate.
Yield: 1.19 g (70.1% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 43.88 H 4.51 Gd 18.53 Na 2.71 O 18.85 Cld.
C 43.71 H 4.30 Gd 18.28 Na 2.80
Example 2
a) N,N'-Bis[13-carboxy-4-oxo-6,9-bis-(carboxymethyl)-12-
(ethoxycarboxymethyl)-3,6,9,12-tetraazatridecyl]-mesoporphyrin-
IX-13,17-diamide
806.8 mg (2 mmol) of 3-ethoxy-carbonylmethyl-6-[2-(2,6-
dioxomorpholino)ethyl]-3,6-diazaoctanedioic acid (DTPA-
monoethylester-monoanhydride) is suspended in 250 ml of absolute
dimethylformamide. It is covered with nitrogen, 1.01 g (10 mmol)
of triethylamine and 650.9 mg (1 mmol) of N,N'-bis(2-aminoethyl)-
mesoporphyrin-IX-diamide (produced analogously to H. Fischer, E.
Haarer and F. Stadler, Z. Physiol. Chem. 241, 209 (1936)) are
added and the resulting reaction mixture is stirred for 3 days at
room temperature. After completion of the reaction, it is
filtered, the solvent is removed in a vacuum and the remaining
oil is triturated with 500 ml of diethyl ether. The precipitated
solid is washed with diethyl ether and hexane. For purification,
CA 02145509 2003-06-26
:"? 7
it is chromatographed on silica gel. R.F-1~3 (,Fluent:
H20/tetrahydrofuran; tetrahydrof uran: (:~-°~3G ~) .
Yield: 1.21 g (82.3 of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 57.68 H 6.92 N 13,.4&~ O 21".95 Cld.
C 57.39 H 7.18 N 13.22
b) N,N~-Bis(13-carboxy-4-oxo-6,9-bis-(carboxymethyl)-12-
(ethoxycarboxymethyl)-3,6,9,12-tet.raazatridecyl]-mesoporphyrin-
IX-13,17-diamide, digadolinium complex
1.458 g (1 mmol) of the 1°ic3and produced in example 2a is
dissolved in 400 m1 of water. By addition of 2n aqueous sodium
hydroxide solution, it is adjusted to pF~ 7 and altern<~tE~ly 894.2
mg (2.2 mmol) of gadolinium acetate tetrahydrate and 2n aqueous
sodium hydroxide solution are added in portions, so that: the pH
of the reaction mixture always fluctuates between 6.8 and 7.2.
After all gadolinium acetate has been added, it is stirred
overnight at room temperature. For wox°king-up, it is filtered,
the solvent is removed in a vacuum and the residue is
chromatographed on silica gel 1RP-18~'(~luent: H2o/tetrahydrofuran;
tetrahydrofuran: 0-30~).
Yield: 1.33 g (?3.3% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 47.61 H 5.37 N 11.10 Gd 17.81 O 18.1.2 Cld.
C 47.32 H 5.52 N 10.85 Gd 17.69
- 214~5Q9 28
c) N,N'-Bis[13-carboxylato-4-oxo-6,8,12-tris-
(carboxylatomethyl)-3,6,9,12-tetraazatridecyl]-mesoporphyrin-IX-
3,17-diamide, digadolinium complex, disodium salt
1.458 g (1 mmol) of the ligand produced in example 2a is
dissolved in 400 ml of water. By addition of 10 molar aqueous
sodium hydroxide solution, it is adjusted to pH 13 and stirred
for 5 hours to room temperature. After completion of the
saponification of the ester groups, it is adjusted to pH 7 with
concentrated hydrochloric acid and, as described in example 2b,
complexed, worked up and purified with 894.2 mg (2 mmol) of
gadolinium acetate tetrahydrate.
Yield: 980 mg (55.9% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 45.20 H 4.83 N 11.18 Gd 17.93 Na 2.62 O 18.24 Cld.
C 44.92 H 5.09 N 10.99 Gd 17.71 Na 2.71
Example 3
a) N,N'-Bis-(3-aminopropyl)-mesoporphyrin-IX-13,17-diamide
1 g (1.68 mmol) of mesoporphyrin-IX-dimethyl ester is
suspended in a sealing tube in 300 ml of 1,3-diaminopropane and
200 ml of absolute pyridine. After covering with nitrogen, it is
heated in the sealing tube for 3 days to 150°C. After completion
of the reaction, it is concentrated by evaporation in a vacuum
and the residue repeatedly recrystallized from pyridine/diethyl
ether.
Yield: 930 mg (81.5% of theory) of reddish brown powder
CA 02145509 2003-06-26
29
Analysis (relative to the c~ntjyctrou:~ substance)
C 70.76 H 8.02 N 16.~~C) r) 4.'r'1 Cl.d,
C 70.49 H 8.12 N 16.31
b) N,N~-Bis[14-carboxy-5-oxo-7,10-bis-(carboxymethyl)-13-
{ethoxycarboxymethyl)-4,7,1t),13-tetraazatetradecyl]-
mesoporphyrin-IX-13,1'7-diamide
806.8 mg (2 mmol) of 3-ethoxy-carbonylmethyl-6-[2-(2,6-
dioxomorpholino)ethyl.]-3,6-diazaoctanedioic acid (DTPA-
monoethylester-monoanhydride) .a_s suspended in 250 ml of absolute
dimethylformamide. It is covered with nitrogen, 1.01 g (10 mmol)
of triethylamine arid 679 mg (1 mmol) of N,N~-bis(3-aminopropyl)-
mesoporphyrin-IX-13,17-diarnide (example 3a) are added and the
resulting reaction mixture is stirred for 3 days at room
temperature. After completion of the reaction, it is filtered,
the solvent is removed in a vacuum and the remaining oil is
triturated with 500 ml of diethyl ether. The precipitated solid
is washed with diethyl ether and hexane. For purification, it is
chromatographed on silica gel RP-18~~'(Eluent: Ti20/tetrahydrofuran;
tetrahydrof uran : 0-3 0% ) .
Yield: 1.20 g (80,8% of theory) of reddish brown powder
Analysis {relat:ive to the anhydrous substance):
C 58.21 Hi 7.Ofi N 13.201 U 21.40 Cld.
C 5?.93 H 7.24 N 13.01
CA 02145509 2003-06-26
c) N,N'-Bis[14-carboxylato-5-axo-7,x..0-bis-
(carboxylatomethyl)-13-(ethaxycarboxymethyl)-4,7,10,13-
tetraazatetradecyl]-mesoporphyrin-TX-1.3,17-diamide, digadolinium
complex
1.49 g (1 rnmol) of the ligand produced in example 3b) is
dissolved in 400 ml of water. By additian of 2n aqueous sodium
hydroxide solution, it is adjusted to ply 7 and alternately 894.2
mg (2»2 mmol) of gadolinium acetate= fetrahydrate and 2n aqueous
sodium hydroxide solution are added in partians, sa th<~t the pH
of the reaction mixture always :fluctuates between 6»8 and 7.2.
After all gadolinium acetate has been added, it is :stirred
overnight at room temperature. For working-up, it is filtered,
the solvent is removed in a vacuum and the residue is
chromatographed on silica gel RP-18~'(Eluent: Hzo/tetrahydrofuran;
tetrahydrofuran: 0-30%).
Yield: 1.01 g (56.3% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 48.20 H 5.51 N 10.93 Cd 17.53 O 17.83 Cld.
C 47.95 H 5.71 N 10.7:x. C~d 17.24
d) N,N'-Bis[14-carbaxylata-5-axo-7,10,1::x-tris-
(carboxylatomethyl)-4,7,10,13-tetraazatetradecyl]-mesoporphyrin-
IX-13,17-diamide, digadolinium complex, disodium salt
1.49 g (1 mmol) of the ligand produced in example 3b) is
dissolved in 400 ml of water. By addition of 10 molar aqueous
sodium hydroxide solution, it is adjusted to pH 13 and stirred
X145509 31
for 5 hours at room temperature. After completion of the
saponification of the ester groups, it is adjusted to pH 7 with
concentrated hydrochloric acid and, as described in example 3c,
complexed, worked up and purified with 894.2 mg (2.2 mmol) of
gadolinium acetate tetrahydrate.
Yield: 935 mg (52.5% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 45.83 H 4.9$ N 11.00 Gd 17.65 Na 2.58 O 17.96 Cld.
C 45.58 H 5.13 N 10.79 Gd 17.45 Na 2.72
Example 4
a) N,N'-Bis(4-aminobutyl)-mesoporphyrin-IX-13,17-diamide
1 g (168 mmol) of mesoporphyrin-IX-dimethyl ester is
suspended in a sealing tube in 300 ml of melted 1,4-diaminobutane
and 200 ml of absolute pyridine. After covering with nitrogen,
it is heated in a sealing tube for 3 days to 150°C. It is
concentrated by evaporation in a vacuum, the residue is mixed
with 500 ml of diethyl ether, the precipitated solid is filtered
off and rewashed with plenty of diethyl ether. The crude product
is purified by recrystallization from pyridine/diethyl ether.
Yield: 953 mg (80.21% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 71.35 H 8.27 N 15.85 04.53 Cld.
C 71.08 H 8.10 N 15.57
CA 02145509 2003-06-26
~2
b) N,N'-Bis[15-carboxy-6-oxo-8,1:1-bis-(carboxymethyl)-14-
(ethoxycarboxymethyl)-5,8,11,14-tetraazapentadecylj-
mesoporphyrin-IX-13,1"7-diamide
806.8 mg (2 mmol) of 3-ethoxy-carbonylmethyl-6-[2.-(2,6-
dioxomorpholino) ethyl]-3, 6-diaz~.oc:t~~nedioic acid (DTPA--
monoethylester-monoanhydride) i~ ~usper~c~ed in 250 ml c>f absolute
dimethylformamide. Tt is c:caverec~ with nitrogen, 1.01 g (10 mmol)
of triethylamine and 70? mg (1 mrnol~ of N,N'-bis-(4-aminobutyl)-
mesoporphyrin-IX-13,17-diamide (example 4a) are added and the
resulting reaction mixture is stirred for 3 days at room
temperature. After completion of the reaction, it is filtered,
the solvent is removed in a vacuum and t;he remaining oil is
triturated with 50G ml of diethyl. ether. The precipitated solid
is filtered off and washed with diethyl ether and hexane. For
purification, it is chromatographed on silica gel RP-18~'"(Eluent:
H20/tetrahydrofuran; tetrahydrofuran: 0-30%).
Yield: 1.24 g (81.9% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 58.72 H 7.19 N 12.95 O 21.14 Cld.
C 58.51 H 7.28 N 12.68
- ~145~0~ 33
c) N,N'-Bis[15-carboxylato-6-oxo-8,11-bis-
(carboxylatomethyl)-14-(ethoxycarboxymethyl)-5,8,11,14-
tetraazapentadecyl]-mesoporphyrin-IX-13,17-diamide, digadolinium
complex
1.51 g (1 mmol)-of the ligand produced in example 4b is
dissolved in~400 ml of water. By addition of 2n aqueous sodium
hydroxide solution, it is adjusted to pH 7 and alternately 894.2
mg (2.2 mmol) of gadolinium acetate tetrahydrate and 2n aqueous
sodium hydroxide solution are added in portions, so that the pH
of the reaction mixture always fluctuates between 6.8 and 7.2.
After all gadolinium acetate has been added, it is stirred
overnight at room temperature. For working-up, it is filtered,
the solvent is removed in a vacuum and the residue is
chromatographed on silica gel RP-18 (Fluent: H20/tetrahydrofuran;
tetrahydrofuran: 0-30%). -
Yield: 1.31 g (71.9% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 48.78 H 5.64 N 10.76 Gd 17.26 O 17.56 Cld.
C 48.49 H 5.83 N 10.48 Gd 17.06
d) N,N'-Bis[15-carboxylato-6-oxo-8,11,14-tris-
(carboxylatomethyl)-5,8,11,14-tetraazapentadecyl]-mesoporphyrin-
IX-13,17-diamide, digadolinium complex, disodium salt
1.51 g (1 mmol) of the ligand produced in example 4b is
dissolved in 400 ml of water. By addition of l0 molar aqueous
sodium hydroxide solution, it is adjusted to pH l3 and stirred
'-- 214509 34
for 5 hours at room temperature. After completion of the
saponification of the ester groups, it is adjusted to pH 7 with
concentrated hydrochloric acid and, as described in example 4c,
complexed, worked up and purified with 894.2 mg (2.2 mmol) of
gadolinium acetate tetrahydrate.
Yield.: 1.29 g {71.3% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 46.45 H 5.12 N 10:83 Gd 17.37 Na 2.54 O 17.68 Cld.
C 46.42 H 5.23 N 10.75 Gd 17.27 Na 2.61
Example 5
a) N,N~-Bis(5-aminopentyl)-mesoporphyrin-IX-13,17-diamide
1 g (1.68 mmol) of mesoporphyrin-IX-dimethyl ester is
suspended in a sealing tube in 300 ml of melted 1,5- -
diaminopentane and 200 ml of absolute pyridine. After covering
With nitrogen, it is heated in a sealing tube for 3 days to
150°C. It is concentrated by evaporation in.a medium high
vacuum, the residue is mixed with 500 ml of diethyl ether, the
precipitated solid is filtered off and rewashed with plenty of
diethyl ether. The crude product is purified by
recrystallization from pyridine/diethyl ether.
Yield: 932 mg (75.5% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 71.90 H 8.50 N 15.24 O 4.35 Cld.
C 71.92 H 8.35 N 14.99
2145509
b) N,N~-Bis[16-carboxy-7-oxo-9,12-bis-(carboxymethyl)-15-
(ethoxycarboxymethyl)-6,9,12,15-tetraazahexadecyl]-mesoporphyrin-
IX-13,17-diamide
806.8 mg (2 mmol) of 3-ethoxy-carbonylmethyl-6-[2(2,6-
dioxomorpholino)ethyl]-3,6-diazaoctanedioic acid (DTPA---
monoethyiester-monoanhydride) is suspended in 250 ml of absolute
dimethylformamide. It is covered with nitrogen, 1.01 g (10 mmol)
of triethylamine and 735 mg (1 mmol) of N,N~-bis-(5-aminopentyl)-
mesoporphyrin-IX-13,17-diamide (example 5a.) are added and the
resulting reaction mixture is stirred for 3 days at room
temperature. After completion of the reaction, it is filtered,
the solvent is removed in a vacuum and the remaining oil is
triturated with 500 ml of diethyl ether. The precipitated solid
is filtered off and washed with diethyl ether and hexane. For
purification, it is chromatographed on silica gel RP-18 (Eluenf:
tetrahydrofuran/H20; tetrahydrofuran: 0-30%).
Yield: 1.31 g (85.0% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 59.21 H 7.32 N 12.72 O 20.75 Cld.
C 59.00 H 7.51 N 12.47
CA 02145509 2003-06-26
a ~:>
c) N,N'-BisCl6-carboxylat<~a-7-c>xo-9,:12-~ais-
(car boxylatomethyl) -15-(ethoxy<~:arboxymet~lyl;p -6, 9, 12, 15-
tetraazahexadecyl]-mesoporphyr:in-I~--1,17-d.a..amide, digadolinium
complex
1.54 g (1 mmol) of the aigand produ~ed in example 5b is
dissolved in 400 ml of water» By addition of 2n aqueous sodium
hydroxide solution, it. is ad ju:~ted to ply 7 and alternate:Ly 894 . 2
mg (2.2 mmol) of gadolinium acetate tetrahydrate and 2n <~queous
sodium hydroxide solution are added in portions, so that the pH
of the reaction mixture always flucauates between 6.8 and 7.2.
After all gadolinium acetate has been added, i.t is stirred
overnight at room temperature. F"or working-up, it is filtered,
the solvent is removed in a vacuum and the residue is
chromatographed on silica gel 12P-18~"(Eluent: tetrahydrofuran/H20;
tetrahydrofuran: 0-30%).
Yield: 1.26 g (68.1% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 49. 34 H 5~.7 7 N 10. ti0 Gd 17 . 00 O 1.7. 29 Cld.
C 49.08 H 6.02 N 1Ø41 Gd 16.73
d) N,N'-Bis[1G-carboxylato-7-coxo-9,12,15-tris-
(carboxylatomethyl)-6,9,12,15-t:etraazahe:xadecyl]-mesoporphyrin-
IX-13,17-diamide, digadoi:i.nium complex, disodium salt
1.54 g (1 mmol.) of the ligand produced in examplea 5b is
dissolved in 400 ml. of water. By addition of 10 molar aqueous
sodium hydroxide solution, i.t :~:~ adrjusted to pH 13 and stirred
°
_ 2145~U9 37
for 5 hours at room temperature. After completion of the
saponification of the ester groups, it is adjusted to pH 7 with
concentrated hydrochloric acid and, as described in example 5c,
complexed, worked up and purified with 894.2 mg (2.2 mmol) of
gadolinium acetate tetrahydrate. -
Yield: 1.49 g (81.1% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 47.05 H 5.26 N 10.67 Gd 17.11 Na 2.50 -O 17.11 Cld.
C 39.87 H 5.45 N 10.38 Gd 17.01 Na 2.73
Example 6
a) N,N~-Bis-(4-aminomethylbenzyl)-mesoporphyrin-IX-13,17-
diamide
1 g (1.68 mmol) of mesoporphyrin-IX-dimethyl ester is
suspended in a sealing tube in 300 ml of melted p-xylylenediamine
and 200 ml of absolute pyridine. After covering with nitrogen,
the reaction mixture is stirred for 3 days at 150°C. After
completion of the reaction, the pyridine and the excess of p-
xylylenediamine are distilled off in a medium-high vacuum and the
residue is repeatedly recrystallized from pyridine/diethyl ether.
Yield: 921 mg (68.3% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 74.78 H 7.28 N 13.95 O 3.98 Cld.
C 74.51 H 7.15 N 13.78
CA 02145509 2003-06-26
'3 8
b) N,N'-Bis{4-[12-carboxy_.-~._ono_~~~;..~bis_(carboxymethyl)-11-
(ethoxycarboxymethyl ) -2 , 5 , 8 , 1.1-te~traazad<:~dc~c~:y 1 ] benzy 1 } w
mesoporphyrin-IX-13,17-diamide
806,8 mg (2 mmol) of 3-etrioxy°°carbornylnaethyl-6-[2-(2,6-
dioxomorpholino)ethyl]-3,6-dia~:aoctaned:ics:.ic acid (DTPA-
monoethylester-monoanhydride) i:s s~.aspend~~d ~n 250 ml of absolute
dimethylformamide. It is covered with n~.trcagen, 1.01 c~ (10 mmol)
of triethylamine and 803 mg (1 mmol) of N,N'-bis-(4-
aminomethylbenzyl)-mesoporphyrin-Th:-13,1°~-diarnide (example 6a)
are added and the resulting reaction mixture is stirred for 3
days at room temperature. After completion of the reaction, it
is filtered, the solvent i.s removed in a vacuum and the remaining
oil is triturated with 500 ml of diethyl ether. The precipitated
solid is filtered off and washed with diethyl ether and hexane.
For purification, it is chromatographed pan ~~~ilica gel RP-18TM
(Eluent: tetrahydrofuran/H2~7; tetrahydrofuran: 0-30%).
Yield: 923 mg (57.30 of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 61.18 H 6.76 N 12.18 (7 19.88 old.
C 61.02 H 6.92 N 11.98
c) N,N'-Bis{4-[12-carboxy°-3-oxo-5,8-bis-(carboxylatomethyl)-
11-(ethoxycarboxymethyl)-2,5,8,11-tetraazadodecyl]benzyl.}-
mesoporphyrin-IX-13,17-diamide, da.gadolinium complex
1.61 g (1 mmol) of the :liga~nc~ produced in exampl~a 6b is
dissolved in 400 ml. of water» By addition of 2n aqueous sodium
CA 02145509 2003-06-26
3 ~:3
hydroxide solution, it is adjusted to pH 7 sand alternately 894.2
mg (2.2 mmol) of gadolinium acetate tetrahyc~rate and 2n aqueous
sodium hydroxide solution are ~:~dded i.n port a.ons, so that the pH
of the reaction mixture always fluc"tuate~a bc;~tween 6.8 and 7.2.
After all gadolinium acetate has been added, it is stirred
overnight at room temperature. ~"oz' working~~-up, it is filtered,
the solvent is removed in a vacuum and tree r°esidue is
chromatographed on silica gel 1~P-18~'(Eluent: tetrahydrofuran/H20;
tetrahydrofuran: 0-30%).
Yield: 985 mg (51.3% of theary) of reddish brown powder
Analysis (relative to the anhydrous sul>stance):
C 51.34 H 5.36 N 10.22 Gd 16,":39 (J 16.68 Cld.
C 51.08 H 5.47 I3 9.91 Gd 15.22
d) N,N'-Bis{4-[12-carboxylato°w3-oxo-5,8,11-tris-
(carboxylatomethyl)-2,5,8,11-tetraaxadodecyljbenzyl}-
mesoporphyrin-IX-13,17-diamide, digadolirxium complex, disodium
salt
1.61 g (1 mmol) of the ligand produced in example 6b is
dissolved in 400 ml of water. By addition of to molar aqueous
sodium hydroxide solution, it is adjusted to pH 13 and stirred
for 5 hours at room temperature. After completion of the
saponification of the ester groups, it x.s adjusted to pH 7 with
concentrated hydrochloric acid and, as described in example 6c,
complexed, worked up and purified w.i.th 894.2 mg (2.2 mmol) of
gadolinium acetate tetrahydrate.
40
~1~5~09
Yield: 1.23 g (64.5% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 49.15 H 4.87 N 10.29 Gd 16.50 Na 2.41 O 16.79 Cld.
C 48.98 H 4.95 N 10.12 Gd 16.31 Na 2.53
Example 7
a) N,N'-Bis-butyl-mesoporphyrin-IX-13,17-diamide
1 g (1.68 mmolj of mesoporphyrin-IX-dimethyl ester is
suspended in a sealing tube in 100 ml of absolute pyridine and
300 ml of n-butylamine. It is covered with nitrogen and the
reaction mixture is heated for 3 days to 150°C. After completion
of the reaction, it is concentrated by evaporation in a vacuum,
the residue is taken up in 500 ml of chloroform, washed three
times with 100 ml of 10% aqueous citric acid, three times with-
100 ml of saturated sodium bicarbonate solution, dried on sodium
sulfate and the solvent is removed in a vacuum. The purification
of the amide takes place by chromatography on aluminum oxide
(Eluent: chloroform/methanol 99:1)
Yield: 1.03 g (90.5% of theory) of violet powder
Analysis (relative to the anhydrous substance):
C 74.52 H 8.34 N 12.41 O 4.73 Cld.
C 74.28 H 8.44 N 12.32
- 214~~09 41
b) 3,8-Diethyl-2,7,12,18-tetramethyl-13,17-bis(n-
butylaminopropyl)-porphyrin
677 mg (1 mmol) of N,N~-bis-butyl-mesoporphyrin-IX-13,17-
diamide (example 7a) is dissolved in 150 ml of absolute
tetrahydrofuran under nitrogen atmosphere. It is mixed with 350
mg (16.1 mmol) of lithium borohydride, 2 ml of trimethylsilyl
chloride and the resulting reaction mixture is stirred for 3 days
at room temperature. After completion of the reaction, 20 ml of
methanol and then 200 ml of water are instilled, it is adjusted
to pH 1 with 2 molar hydrochloric acid, stirred for 0.5 hour,
adjusted to pH 13 with 2 molar aqueous sodium hydroxide solution
and stirred again for 0.5 hour. The reaction product is
extracted three times with 100 ml of chloroform and the organic
phase is dried on sodium sulfate. The purification of the amine
takes place by chromatography on silica gel (Eluent:
chloroform/methanol; methanol 0-50%).
Yield: 362 mg (55.8% of theory) of violet powder
Analysis (relative to the anhydrous substance):
C 77.73 H 9.32 N 12.95 Cld.
C 77.48 H 9.30 N 12.76
c) 3,8-Diethyl-2,7,12,18-tetramethyl-13,17-bis[4,7,10,13-
tetraaza-4-butyl-5-oxo-7,10,13-tris(carboxymethyl)-13-
ethoxycarboxymethyl-tridecyl]-porphyrin
806.8 mg (2 mmol) of 3-ethoxy-carbonylmethyl-6-[2-(2,6-
dioxomorpholino)ethyl]-3,6-diazaoctanedioic acid (DTPA-
214~~09 42
monoethylester-monoanhydride) is suspended in 250 ml of absolute
dimethylformamide. .It is covered with nitrogen, 1.01. g (10 mmol)
of triethylamine and 649 mg (1 mmol) of 3,8-diethyl-2,7,12,18-
tetramethyl-13,17-bis(n-butylaminopropyl)-porphyrin (example 7b)
are added and the resulting reaction mixture is stirred for three
days at foom temperature. After completion of the reaction, it
is filtered, the solvent is removed in a vacuum and the remaining
oil is triturated with 500 ml of diethyl ether. The precipitated
solid is filtered off and washed with diethyl ether and n-hexane.
For purification, it is chromatographed on silica gel RP-18
(Eluent: HZO/tetrahydrofuran; tetrahydrofuran: 0-30%).
Yield: 1.05 g (72.1% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 61.05 H 7.62 N 11.55 O 19.78 Cld.
C 60.89 H 7.72 N 11.31
d) 3,8-Diethyl-2,7,12,18-tetramethyl-13,17-bis-[4~,7,10,13-
tetraaza-4-butyl-5-oxo-7,10,13-tris(carboxylatomethyl)-13-
ethoxycarboxymethyl-tridecyl]-porphyrin, digadolinium complex
1.46 g (1 mmol) of the ligand produced in example 7c is
dissolved in 400 ml of water. By addition of two molar sodium
hydroxide solution, it is adjusted to pH 7 and alternately 894.2
mg (2.2 mmol) of gadolinium acetate tetrahydrate and two molar
sodium hydroxide solution are added in portions, so that the pH
of the reaction mixture always fluctuates between 6.8 and 7.2.
After all gadolinium acetate has been added, it is stirred
~~~~ 43
overnight at room temperature. For working-up, the solvent is
removed in a vacuum and the residue is chromatographed on silica
gel RP-18 (Fluent: HZO/tetrahydrofuran; tetrahydrofuran: 0-30%).
Yield: 721 mg (40.9% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 50.38 H 5.94 N 9.53 Gd 17.83 O 16.32 Cld.
C 50.02 H 5.99 N 9.38 Gd 17.73
e) 3,8-Diethyl-2,7,12,18-tetramethyl-13,17-bis[4,7,10,13-
tetraaza-4-butyl-5-oxo-7,10,13,13-tetra(carboxylatomethyl)-
tridecyl]-porphyrin, digadolinium complex, disodium salt
1.46 g (1 mmol) of the ligand produced in example 7c is
dissolved in 400 ml of water. By addition of 10 molar aqueous
sodium hydroxide solution, it is adjusted to pH 13 and stirred
for 5 hours at room temperature. After completion of the
saponification of the ester groups, it is adjusted to pH 7.0 with
concentrated hydrochloric acid and, as described in example 7d,
complexed, worked up and purified with 894.2 mg (2.2 mmol) of
gadolinium acetate tetrahydrate.
Yield: 1.32 g (75.3% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 47.99 H 5.41 N 9.59 Gd 17.95 Na 2.62 O 16.44 Cld.
C 47.76 H 5.63 N 9.31 Gd 17.81 Na 2.79
_ 44
2145509
Example 8
a) N,N'-Bis-benzyl-mesoporphyrin-IX-13,17-diamide
1 g (1.68 mmol) of mesoporphyrin-IX-dimethyl ester is
refluxed in 500 ml of benzylamine under nitrogen atmosphere for
48 hours. After completion of the reaction, it is concentrated
by evaporation in a vacuum, the residue is taken up in 500 ml of
chloroform, the organic phase is washed three times with 150 ml
of 5% aqueous citric acid, dried on sodium sulfate and
concentrated by evaporation in a vacuum. It is crystallized from
chloroform/methanol.
Yield: 920 mg (73.5% of theory) of red violet powder
Analysis (relative to the anhydrous substance):
C 77.39 H 7.04 N 11.28 Cld.
C 77.41 H 6.93 N 11.42
b) 3,8-Diethyl-2,7,12,18-tetramethyl-13,17-
bis(benzylaminopropyl)-porphyrin
745 mg (1 mmol) of N,N'-bis-benzyl-mesoporphyrin-13,17-
diamide is dissolved in 150 ml of tetrahydrofuran under nitrogen
atmosphere. It is mixed with 350 mg (16.1 mmol) of lithium
borohydride, 2 ml of trimethylsilyl chloride and the resulting
reaction mixture is stirred for 3 days at room temperature.
After completion of the reaction, 20 ml of methanol and then 200
ml of water are instilled, it is adjusted to pH 1 with 2 molar
hydrochloric acid, stirred for 0.5 hour, adjusted to pH 13 with 2
molar sodium hydroxide solution and stirred again for 0.5 hour.
- 2145509 45
The reaction product is extracted three times with 100 ml of
chloroform.and the organic phase is dried on sodium sulfate. The
purification of the amine takes place by chromatography on silica
gel {Fluent: chloroform/methanol; methanol 0-50%).
Yield: 425 mg (59.3% of theory) of red violet powder '
Analysis {relative to the anhydrous substance):
C 80.41 H 7.87 N 11.72 Cld.
C 80.20 H 7.92 N 11.45
c) 3,8-Diethyl-2,7,12,18-tetramethyl-13,17-bis[4,7,10,13-
tetraaza-4-benzyl-5-oxo-7,10,13-tris{carboxymethyl)-13-
ethoxycarboxymethyl-tridecyl]-porphyrin
806.8 mg (2 mmol) of 3- ethoxy-carbonylmethyl-6-[2-(2,6-
dioxomorpholino)ethyl]-3,6-diazaoctanedioic acid {DTPA-
monoethylester-monoanhydride) is suspended in 250 ml of absolute
dimethylformamide. It is covered with nitrogen, 1.01 g (10 mmol)
of triethylamine and 717 mg {1 mmol) of 3,8-diethyl-2,7,12,18-
tetramethyl-13,17-bis{benzylaminopropyl)porphyrin {example 8b)
are added and the resulting reaction mixture is stirred for three
days at room temperature. After completion of the reaction, it
is filtered, the solvent is removed in a vacuum and the remaining
oil is triturated with 500 ml of diethyl ether. The precipitated
solid is filtered off and washed with diethyl ether and n-hexane.
For purification, it is chromatographed on silica gel RP-18
(Fluent: HZO/tetrahydrofuran; tetrahydrofuran: 0-30%).
Yield: 1.25 g (82% of theory) of reddish brown powder
2145509 46
Analysis (relative to the anhydrous substance):
C 63.06 H 7.01 N 11.03 O 18.90 Cld.
C 62.89 H 7.14 N 11.18
d) 3,8-Diethyl-2,7,12,18-tetramethyi-13,17-bis[4,7,10,13-
tetraaza-4-benzyl-5-oxo-7,10,13-tris(carboxylatomethyl)-13-
ethoxycarboxymethyl-tridecyl]-porphyrin, digadolinium complex
1.52 g (1 mmol) of the ligand produced in example 8c is
dissolved in 400 m1 of water. By addition of two molar sodium
hydroxide solution, it is adjusted to pH 7 and alternately 894.2
mg (2.2 mmol) of gadolinium acetate tetrahydrate and two molar
sodium hydroxide solution are added in portions, so that the pH
of the reaction mixture always fluctuates between 6.8 and 7.2.
After all gadolinium acetate has been added, it is stirred
overnight at room temperature. For working-up, the solvent is~
removed in a vacuum and the residue is chromatographed on silica
gel RP-18 (Fluent: H20/tetrahydrofuran; tetrahydrofuran: 0-30%).
Yield: 1.01 g (55.1% of theory) of -reddish brown powder
Analysis (relative to the anhydrous substance):
C 52.44 H 5.50 N 9.17 Gd 1'1.16 O 15.72 Cld.
C 52.17 H 5.81 N 9.03 Gd 17.02
- X145509 47
e) 3,8-Diethyl-2,7,12,18-tetramethyl-13,17-bis[4,7,10,13-
tetraaza-4-benzyl-5-oxo-7,10,13,13-tetra(carboxylatomethyl)-
tridecyl]-porphyrin, digadolinium complex, disodium salt
1.52 g (1 mmol) of the ligand produced in example 8c is
dissolved in 400 ml of water. By addition of 10 molar aqueous
sodium hydroxide solution, it is adjusted to pH 13 and stirred
for 5 hours at room temperature. After completion of the
saponification of the ester groups, it is adjusted to pH 7 with
concentrated hydrochloric acid and, as described in example 8d,
complexed, worked up and purified with 894.2 mg (2.2 mmol) of
gadolinium acetate tetrahydrate.
Yield: 1.12 g (61.5% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 50.15 H 4.98 N 9.23 Gd 17.28 Na 2.53 O 15.82 Cld.
C 50.02 H 4.99 N 9.05 Gd 17.03 Na 2.68
Example 9
a) 10-[2,6,7-Trihydroxy-4-oxa-heptyl]-1,4,7-tris-
carboxymethyl-1,4,7,10-tetraazacyclododecane
19.56 g (103.92 mmol) of 2,2-dimethyl-4-(2',3'-epoxy)-
propoxy-methyl-1,3-dioxolane and 10 g (28.86 mmol) of 1,4,7-
triscarboxymethyl-1,4,7,10-tetraazacyclododecane (=D03A) are
dissolved in a mixture of 50 ml of dioxane/80 ml of water, and
the pH is brought to pH l0 with 6N potassium hydroxide solution.
It is stirred for 24 hours at 70°C. It is evaporated to dryness,
the residue is taken up with 200 ml of water/50 ml of methanol
48
2i45~09
and extracted twice with 100 ml of tert.-butyl-methyl ether. The
aqueous solution is adjusted to pH 3 with 5N hydrochloric acid
and evaporated to dryness. The resiude is boiled out (extracted)
with 200 ml of methanol/80 ml of dichloromethane. It is cooled
in an ice bath and filtered from the precipitated potassium
chloride. The filtrate is concentrated by evaporation in a
vacuum, the residue is dissolved in 45 ml of water/20 ml of
ethanol and then put on a column of poly-(4-vinylpyridine). The
product is eluted with a solution of ethanol/water 1:3. After
concentration by evaporation in a vacuum, the residue is
chromatographed on a reversed phase column (RP-18/mobile solvent,
gradient from water/tetrahydrofuran). After concentration by
evaporation of the main fraction, 10.13 g (71% of theory) of a
strongly hygroscopic, vitreous solid is obtained.
Analysis (relative to the anhydrous substance):
C 48.57 H 7.74 N 11.33 Cld.
C 48.46 H 7.81 N 11.24
b) Gd-complex of 10-(2,6,7-trihydroxy-4-oxa-heptyl)-1,4,7-
tris-carboxymethyl-1,4,7,10-tetraazacyclododecane
8.56 g (17.3 mmol) of the title compound from example 9a is
dissolved in 50 ml of deionized water and 3.13 g (8.65 mmol) of
gadolinium oxide is added. It is heated for 3 hours to 90°C.
The cooled solution is stirred for one hour with 3 ml of acidic
ion exchanger (AMB 252c) and 3 ml of weakly basic exchanger (IRA
214509 49
67). It is filtered off from the exchanger and the filtrate
freeze-dried.
Yield: 11.0 g (98% of theory) of a colorless amorphous
powder.
Analysis (relative to the anhydrous substance):
C 37.03 H 5.44 N 8.64 Gd 24.24 Cld.
C 37.00 H 5.51 N 8.57 Gd 24.18
The corresponding yttrium complex of 10-(2,6,7-trihydroxy-4-
oxa-heptyl)-1,4,7-tris-carboxymethyl-1,4,7,10-
tetraazacyclododecane is obtained analogously with yttrium oxide.
c) N,N'-Bis{4,7,10-tris(carboxylatomethyl)-1,4,7,10-
tetraazacyclodecyl}-5-hydroxy-3-oxa-hexylamino}-mesoporphyrin-IX-
13,17-diamide, digadolinium complex
3.89 g (6 mmol) of the Gd complex from example 9b is
dissolved in 40 ml of methanol, mixed with 2.57 g (12 mmol) of
sodium periodate and stirred up for 4 hours with~exclusion of
light. Then, it is filtered from the undissolved and the
filtrate is freeze-dried. The residue is mixed with 500 ml of
absolute dimethylformamide. It is covered with nitrogen, 6.06 g
(60 mmol) of triethylamine and 1.78 g (3 mmol) of mesoporphyrin-
IX-13,17-dihydrazide are added and it is allowed to stir for 3
days at room temperature. It is evaporated to dryness in a
vacuum, the residue is mixed with 75 ml of buffer of pH 9.0
(Riedel de Haen, Borax/HC1), 1.13 g (18 mmol) of sodium
cyanoborohydride is added and allowed to stir for 6 more days
2145509 5~
under nitrogen at room temperature. 2.8 g (52% of theory) of a
reddish brown powder is obtained after chromatography of the
neutral solution on silica gel RP-18.
Analysis (relative to the anhydrous substance):
C 48.20 H 5.73 N 12.49 Gd 17.52 Cld.
C 48.38 H 5.90 N 12.27 Gd 17.63
The di-yttrium complex is obtained analogously from the
yttrium complex (example 9b).
d) N,N'-Bis{4,7,10-tris{carboxylatomethyl)-1,4,7,10-
tetraazacyclododecyl}-5-hydroxy-3-oxahexyl-(1'-oxo-3'-oxa-4'-
carboxylatobutyl)-amino}-mesoporphyrin-IX-13,17-diamide,
digadolinium complex, disodium salt
2.5 g (1.41 mmol) of the title compound from example 9c is
dissolved in 200 ml of N,N-dimethylformamide and 10 ml of
pyridine is added. Then, 3.48 g (30 mmol) of diglycolic acid
anhydride is added and stirred for 4 days at room temperature.
It is evaporated to dryness in a vacuum, the residue is taken up
with 20 ml of 20% aqueous acetic acid and again evaporated to
dryness. The residue is chromatographed on silica gel RP-18.
The main fractions are combined and evaporated to dryness. The
residue is dissolved in 200 ml of water and the pH of the
solution is adjusted to pH 7.2 with 0.1 N sodium hydroxide
solution. The solution is filtered and the filtrate dried.
2.17 g (75% of theory) of a reddish brown powder is obtained.
- 214550 51
Analysis (relative to the anhydrous substance): .
C 45.74 H 5.41 N 10.94 Gd 15.35 Na 2.24 Cld.
C 45.51 H 5.60 N 10.72 Gd 15.14 Na 2.01
Example 10
a) 3,8-Bis-(hydroxymethyl)-deuteroporphyrin-IX-dimethyl
ester
1.00 g (1.67 mmol) of 3,8-diformyl-deuteroporphyrin-IX-
dimethyl ester (H. Fischer, K.O. Deilmann, Hoppe Seyler's Z.
phys.Chem. 280, 186-216 (1944)) is dissolved in a mixture of 700
ml chloroform and 300 ml of methanol. The solution is coo7PC~ t~
0°C and mixed with stirring in portions with 1.00 g (26.43 mmol)
of sodium borohydride. The batch is allowed to warm to room
temperature, neutralized with semiconcentrated acetic acid and
evaporated to dryness in a vacuum. The residue is taken up ink
methylene chloride, shaken out with saturated sodium bicarbonate
solution, dried on sodium sulfate, filtered, concentrated by
evaporation and recrystallized from methylene chloride/diethyl
ether.
Yield: 0.92 g (92% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 68.21 H 6.40 N 9.36 O 16.03 Cld.
C 68.01 H 6.35 N 9.22
'_ X145509 52
b) 3,8-Bis-(methoxymethyl)-deuteroporphyrin-IX-dimethyl
ester
0.90 g (1.44 mmol) of 3,8-bis-(hydroxymethyl)-
deuteroporphyrin-IX-dimethyl ester (example l0a) is refluxed in a
mixture of 40 ml of orthoformic acid-trimethyl ester, 40 m1 of
methanol and 8 ml of sulfuric acid for 2 hours. After cooling,
the solution is neutralized with sodium bicarbonate, taken up in
methylene chloride and washed with water. After drying on sodium
sulfate, filtering and concentration by evaporation, the solid
residue is recrystallized from methylene chioride/diethyl ether.
Yield: 0.78 g (86% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 68.90 H 6.75 N 8.94 O 15.32 Cld.
C 68.81 H 6.71 N 8.85
c) N,N'-Bis-(2-aminoethyi)-3,8-bis-(methoxymethyl)-
deuteroporphyrin-IX-13,17-diamide
0.70 g (1.12 mmol) of 3,8-bis-(methoxymethyl)-
deuteroporphyrin-IX-dimethyl ester (example lOb) is heated in an
autoclave in a mixture of 80 ml of pyridine and 20 ml of 1,2-
diaminoethane after covering with nitrogen for 24 hours to 150°C.
The batch is evaporated to dryness and the solid crude product is
purified by recrystallization from pyridine/diethyl ether.
Yield: 0.63 g (83% of theory) of reddish brown powder
Analysis (relative-to the anhydrous substance):
w X145509 53
C 66.84 H 7.38 N 16.4 1 O 9.37 Cld.
C 66.68 H 7.11 N 16.42
d) N,N'-Bis-[13-carboxy-4-oxo-6,9-bis-(carboxymethyl)-12-
(ethoxycarbonylmethyl)-3,6,9,12-tetraazatridecyl]-3,8-bis-
(methoxymethyl)-deuteroporphyrin-IX-13,17-diamide
0.71 g (1.76 mmol) of 3-ethoxy-carbonylmethyl-6-[2-(2,6-
dioxomorpholino)-ethyl]-3,6-diazaoctanedioic acid (DTPA-
monoanhydride-monoethyl ester) is suspended in l00 m1 of
anhydrous dimethylformamide. It is covered with nitrogen, 0.89 g
(8.80 mmol) of triethylamine and 0.60 g (0.88 mmol) of N,N'-bis-
(2-aminoethyl)-3,8-bis-(methoxymethyl)-deuteroporphyrin-IX-13,17-
diamide (example lOc) are added and stirred for 3 days at room
temperature. After filtering and concentration by evaporation in
a vacuum, the residue is chromatographed on silica gel RP-18 with
water/tetrahydrofuran and evaporated to dryness in a vacuum.
Yield: 1.09 g (83% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 56.44 H 6.77 N 13.16 O 23.63 Cld.
C 56.18 H 6.63 N 13.27
54
. 214509
e) N,N'-Bis-[13-carboxylato-4-oxo-6,9-bis-
(carboxylatomethyl)-12-(ethoxycarbonylmethyl)-3,6,9,12-
tetraazatridecyl]-3,8-bis-(methoxymethyl)-deuteroporphyrin-IX-
13,17-diamide, digadolinium complex
1.00 g (0.67 mmol) of the ligand produced in example lOd) is
dissolved in 250 ml of water. By addition of 2n aqueous sodium
hydroxide solution, it is adjusted to pH 7.0, and 0.60 g (1.47
mmol) of gadolinium acetate tetrahydrate and 2n aqueous sodium
hydroxide solution are added in portions, so that the pH of the
reaction mixture always fluctuates between 6.8 and 7.2. After
all gadolinium acetate has been added, it is stirred overnight at
room temperature. For working-up, it is filtered, the solvent is
removed in a vacuum, the residue is chromatographed on silica gel
RP-18 with water/tetrahydrofuran and evaporated to dryness in a
vacuum.
Yield: 0.87 g (72% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 46.76 H 5.27 Gd 17.49 N 10.91 O 19.58 Cld.
C 46.54 H 5.23 Gd 17.42 N 10.75
f) N,N'-Bis-[13-carboxylato-4-oxo-6,9,12-tris-
(carboxylatomethyl)-3,6,9,12-tetraazatridecyl]-3,8-bis-
(methoxymethyl)-deuteroporphyrin-IX-13,17-diamide, digadolinium
complex, disodium salt
1.00 g (0.67 mmol) of the ligand produced in example lOd is
dissolved in 200 ml of water. By addition of lOn aqueous sodium
- 2145509 55
hydroxide solution, it is adjusted to pH 13 and stirred for 5
hours at room temperature. After completion of the
saponification of the ester, it is adjusted to pH 7 with
concentrated hydrochloric acid and, as described in example 10e,
complexed, worked up and purified with 0.60 g (1.47 mmol) of
gadolinium acetate tetrahydrate.
Yield: 1.01 g (84% of theory)
Analysis(relative to the anhydrous substance):
C 44.39 H 4.74 N 10.98 Gd 17.61 Na 2.57 O 19.71 Cld.
C 44.17 H 4.68 N 10.89 Gd 17.65 Na 2..63
Example 11
a) N-(2-aminoethyl)-3,8-bis-(methoxymethyl)-
deuteroporphyrin-IX-monoamide-monomethyl ester (isomeric mixture)
0.70 g (1.12 mmol) of 3,8-bis-(methoxymethyl)-
deuteroporphyrin-IX-dimethyl ester (example lOb) is heated in an
autoclave for 12 hours to 150°C in a mixture of 90 ml of pyridine
and 10 ml of 1,2-diaminoethane after covering with nitrogen. The
batch is concentrated by evaporation and the solid crude product
is purified by recrystallization from pyridine/diethyl ether.
Yield: 0.56 g (86% of theory) of reddish brown powder
b) N-(2-aminoethyl)-3,8-bis-(methoxymethyl)-
deuteroporphyrin-IX-monoamide-monosodium salt (isomeric mixture)
0.50 g (0.75 mmol) of the compound produced in example ila
is stirred in a mixture of 100 ml of pyridine and 100 ml of In
- 214509 56
sodium hydroxide solution for two hours at 50°C; then it is
concentrated by evaporation in a vacuum. The residue is
suspended in 100 ml of water, the suspension is neutralized with
O.ln hydrochloric acid, the precipitate is suctioned off, washed
with water and dried in a vacuum on phosphorus pentoxide.
Yield: 0.41 g (81% of theory) of reddish brown powder
c) N-[13-carboxy-4-oxo-6,9-bis-(carboxymethyl)-12-
(ethoxycarbonylmethyl)-3,6,9,12-tetraazatridecyl]-3,8-bis-
(methoxymethyl)-deuteroporphyrin-IX-monoamide-sodium salt
(isomeric mixture)
0.24 g (0.60 mmol) of 3-ethoxy-carbonylmethyl-6-[2-(2,6-
dioxomorpholino)-ethyl]-3,6-diazaoctanedioic acid (DTPA-
monoanhydridemonoethyl ester) is suspended in 80 ml of anhydrous
dimethylformamide. It is covered with nitrogen, 0.30 g (3.00
mmol) of triethylamine and 0.40 g (0.60 mmol) of the compound
produced in example 11b are added and stirred for 3 days at room
temperature. After filtering and concentration by evaporation in
a vacuum, the residue is suspended in a little water, dissolved
by addition of 2n sodium hydroxide solution at pH 7.2,
chromatographed on silica gel RP-18 with water/tetrahydrofuran
and the eluate is evaporated to dryness in a vacuum.
Yield: 0.37 g (58% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 58.58 H 6.43 N 11.82 Na 2.16 O 21.01 Cld.
C 58.49 H 6.40 N 11.58 Na 2.35
- 214559 57
d) N-[13-Carboxylato-4-oxo-6,9-bis-(carboxylatomethyl)-12-
(ethoxycarbonylmethyl)-3,6,9,12-tetraazatridecyl]-3,8-bis-
(methoxymethyl)-deuteroporphyrin-IX-monoamide, gadolinium
complex, sodium salt (isomeric mixture)
0.35 g (0.33 mmol) of the ligand produced in example llc is
dissolved in 100 ml of water.. By addition of 2n aqueous sodium
hydroxide solution, it is adjusted to pH 7.0, and 0.15 g (0.36
mmol) of gadolinium acetate tetrahydrate and 2n aqueous sodium
hydroxide solution are added in portions, so that the pH of the
reaction mixture always fluctuates between 6.8 and 7.2. After
all gadolinium acetate has been added, it is stirred overnight at
room temperature. For working-up, it is filtered, the solvent is
removed in a vacuum, the residue is chromatographed on silica gel
RP-18 with water/tetrahydrofuran and the eluate is evaporated to
dryness in a vacuum.
Yield: 0.33 g (81~ of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 51.18 H 5.37 N 10.33 Na 1.88 Gd 12.89 O 18.35 Cld.
C 50.88 H 5.35 N 10.24 Na 1.91 Gd 12.90
e) N-[13-Carboxylato-4-oxo-6,9,12-tris-(carboxylatomethyl)-
3,6,9,12-tetraazatridecyl]-3,8-bis-(methoxymethyl)-
deuteroporphyrin-IX-monoamide, digadolinium complex, disodium
salt (isomeric mixture)
0.35 g (0.33 mmol) of the ligand produced in example 12c is
dissolved in 100 ml of water. By addition of 10 molar aqueous
58
- 2145509
sodium hydroxide solution, it is adjusted to pH 13 and stirred
for 5 hours at room temperature. After completion of the
saponification of the ester, it is adjusted to pH 7.0 with
concentrated hydrochloric acid and, as described in example lid,
complexed, worked up and purified with 0.15 g (0.36 mmoi) of
gadolinium acetate tetrahydrate.
Yield: 0.33 g (83% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 49.46 H 4.98 N 10.38 Na 3.79 Gd 12.95 O 18.45 Cld.
C 49.35 H 5.06 N 10.27 Na 3.85 Gd 12.89
Example 12
a) Manganese(III)-[N,N'-bis-(2-aminoethyl)-3,8-bis-
(methoxymethyl)-deuteroporphyrin-IX-13,17-diamide]-acetate
0.60 g (0.88 mmol) of the compound produced in example lOc
is refluxed with 3.00 g of manganese(II)-acetate for one hour in
120. ml of acetic acid. Then, it is concentrated by evaporation
in a vacuum, the residue is suspended in water, filtered off and
washed with water. The dried crude product is recrystallized
from pyridine/diethyl ether.
Yield: 0.64 g (91% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 60.45 H 6.47 Mn 6.91 N 14.10 O 12.08 Cld.
C 60.18 H 6.51 Mn 6.90 N 13.97
2145509 59
b) Manganese(III)-~N,N'-bis-[13-carboxy-4-oxo-6,9-bis-
{carboxymethyl)-12-{ethoxycarbonylmethyl)-3,6,9,12-
tetraazatridecyl]-3,8-bis-(methoxymethyl)-deuteroporphyrin-IX-
13,17-diamide}-acetate
0.61 g {1.50 mmol) of 3-ethoxy-carbonylmethyl-6-[2-{2,6-
dioxomorpholino)-ethyl]-3,6-diazaoctanedioic acid (DTPA-
monoanhydridemonomethyl ester) is suspended in 100 ml of
anhydrous dimethylformamide. It is covered with nitrogen, 0.76 g
(7.50 mmol) of triethylamine and 0.60 g {0.75 mmol) of the
compound produced in example 12a are added and stirred for 3 days
at room temperature. After filtering and concentration by
evaporation in a vacuum, the residue is chromatographed on silica
gel RP-18 with water/tetrahydrofuran and the eluate is evaporated
to dryness in a vacuum.
Yield: 1.01 g (84% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 54.00 H 6.36 Mn 3.43 N 12.24 O 23:98 Cld.
C 53.79 H 6.31 Mn 3.11 N 12.03
c) Manganese{III)-{N,N'-bis-[13-carboxylato-4-oxo-6,9-bis-
(carboxylatomethyl)-12-(ethoxycarbonylmethyl)-3,6,9,12-
tetraazatridecyl]-3,8-bis-(methoxymethyl)-deuteroporphyrin-IX-
13,17-diamide}-acetate, digadolinium complex
1.00 g (0.62 mmol) of the ligand produced in example 12b is
dissolved in 250 ml of water. By addition of 2n sodium hydroxide
solution, it is ad3usted to pH 7.0, and 0.55 g (1.36 mmol) of
X145509
gadolinium acetate and 2n sodium hydroxide solution are added in
portions, so that the pH of the reaction mixture always
fluctuates between 6.8 and 7.2. After all gadolinium acetate has
been added, it is stirred overnight at room temperature. For
working-up, it is filtered, the solvent is removed in a-vacuum,
the residue is chromatographed on silica gel RP-18 with
water/tetrahydrofuran and the eluate is evaporated to dryness in
a vacuum.
Yield: 0.85 g (72% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 45.28 H 5.01 N 10.27 Gd 16.47 Mn 2.88 O 20.10 Cld.
C 45.07 H 4.88 N 10.16 Gd 16.51 Mn 2.80
d) Manganese(III)-{N,N~-bis-[13-carboxylato-4-oxo-6,9,12-
tris-(carboxylatomethyl)-3,6,9,12-tetraazatridecyl]-3,8-bis-
(methoxymethyl)-deuteroporphyrin-IX-13,17-diamide}-acetate,
digadolinium complex, disodium salt
1.00 g (0.52 mmol) of the ligand produced in example 12b is
dissolved in 250 ml of water. By addition of 10 molar aqueous
sodium hydroxide solution, it is adjusted to pH 13 and stirred
for 5 hours at room temperature. After completion of the
saponification of the ester, it is adjusted to pH 7.0 with
concentrated hydrochloric acid and, as described in example 12c,
complexed and purified with 0.47 g (1.15 mmol) of gadolinium
acetate tetrahydrate.
Yield: 80 g (81% of theory)
' - 2145509 61
Analysis (relative to the anhydrous substance):
C 43.03 H 4.51 N 10.33 Na 2.42 Gd 16.57 Mn 2.89 O 20.23
Cld.
C 42.88 H 4.36 N 10.30 Na 2.24 Gd 16.49 Mn 2.82
Example 13
a) 3,8-Bis-(methoxymethyl)-deuteroporphyrin-IX-13,17-
dihydrazide
1.00 g (1.60 mmol) of the compound produced in example lOb
is dissolved in 80 m1 of anhydrous pyridine under argon and mixed
with 15 ml of hydrazine. After 3 days of stirring at room
temperature, it is concentrated by evaporation in a vacuum, the
residue is stirred in semiconcentrated aqueous hydrochloric acid
and mixed with 6n sodium hydroxide solution to adjust the pH to
7Ø The precipitate is filtered off, washed with water and
recrystallized from pyridine/ether.
Yield: 0.81 g (81% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 65.16 H 6.75 N 17.88 O 10.21 Cld.
C 64.87 H 6.62 N 17.64
b) N,N'-Bis-[11-carboxy-2-oxo-4,7-bis-(carboxymethyl)-10-
{ethoxycarbonylmethyl)-1,4,7,10-tetraazaundecyl]-3,8-bis-
(methoxymethyl)-deuteroporphyrin-IX-13,17-diamide
0.90 g (2.23 mmol) of 3-ethoxy-carbonylmethyl-6;-[2,6-
dioxomorpholino)-ethyl]-3,6-diazaoctanedioic acid (DTPA-
' - 2145509 62
monoanhydridemonoethyl ester) is suspended in 250 ml of anhydrous
dimethylformamide. It is covered with nitrogen, 0.9 g (11.17
mmol) of triethylamine and 0.70 g (1.12 mmol) of the compound
produced in example 13a are added and stirred for 3 days at room
temperature. After filtering and concentration by evaporation in
a vacuum, the residue is chromatographed on silica gel RP-18 with
water/tetrahydrofuran and the eluate is evaporated to dryness.
Yield: 1.35 g (84% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 55.30 H 6.47 N 13.68 O 24.55 Cld.
C 55.01 H 6.42 N 13.36
c) N,N'-Bis-[il-carboxylato-2-oxo-4,7-bis-
(carboxylatomethyl)-10-(ethoxycarbonylmethyl)-1,4,7,10-
tetraazaundecyl]-3,8-bis-(methoxymethyl)-deuteroporphyrin-IX-
13,17-diamide, digadolinium complex
1.30 g (0.91 mmol) of the ligand produced in example 13b is
dissolved in 250 ml of water. The pH is adjusted to 7.0 by
addition of 2 molar aqueous sodium hydroxide solution and 0:80 g
(2.00 mmol) of gadolinium acetate tetrahydrate and 2n aqueous
sodium hydroxide solution are added in portions, so that the pH
of the reaction mixture always fluctuates between 6.8 and 7.2.
After all gadolinium acetate has been added, it is stirred
overnight at room temperature. For working-up, it is filtered,
the solvent is removed in a vacuum, the residue is
2145509 63
chromatographed on silica gel RP-18 with water/tetrahydrofuran
and the eluate is evaporated to dryness.
Yield: 1.27 g (80% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance): -
C 4 5 51 H 4.98 N 11.26 Gd 18.05 O 20.21 Cld.
C 45.43 H 4.86 N 10.97 Gd 17.87
d) N,N'-Bis-[11-carboxylato-2-oxo-4,7,10-tris-_
(carboxylatomethyl)-1,4,7,10-tetraazaundecyl]-3,8-bis-
(methoxymethyl)-deuteroporphyrin-IX-13,17-diamide, digadolinium
complex, disodium salt
1.00 g (0.70 mmol) of the ligand produced in example 13b is
dissolved in 200 ml of water. By addition of 10 molar aqueous
sodium hydroxide solution, it is adjusted to pH 13 and stirred
for 5 hours at room temperature. After completion of the
saponification of the ester, it is adjusted to pH 7.0 with
concentrated hydrochloric acid and,. as described in example 13c,
complexed, worked up and purified with 0.62 g (1.53 mmol) of
gadolinium acetate tetrahydrate.
Yield: 0.86 g (71% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 43.05 H 4.42 N 11.34 Na 2.65 Gd 18.18 O 20.35 Cld.
C 42.88 H 4.31 N 11.12 Na 2.71 Gd 18.09
1 ~ ~ ,~ 64
Example i4
a) Zn-[3,8-bis-(1-propenyl)-deuteroporphyrin-IX-dimethyl
ester]
2.48 g (6.69 mmol) of ethyltriphenylphosphoniumbromide in
600 ml of anhydrous tetrahydrofuran is mixed under argon at room
temperature with a solution of 0.43 g (6.69 mmol) of n-
butyllithium. After completion of the reaction, 2.00 g (3.04
mmol) of Zn-[3,8-diformyl-deuteroporphyrin-IX-dimethyl ester]
(Kevin M. Smith, Eugene M. Fujinari, Keven C. Langry, Daniel W.
Parish and Hani D. Tabba, J. Am. Chem. Soc. 105, 6638-6646 (1983)
is added and stirred for two more hours. After addition of 30 ml
of methanol, the solvent is substantially removed in a vacuum and
the residue shaken out with methylene chloride and
semiconcentrated aqueous sodium bicarbonate solution. The
organic phase is dried on sodium sulfate, concentrated by
evaporation and chromatographed on aluminum oxide (Merck,
activity stages 2-3) with methylene chloride/methanol. The
eluate is evaporated to dryness in a vacuum.
Yield: 1.78 g (86% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 66.91 H 5.91 N 8.21 Zn 9.58 O 9.38 Cld.
C 66.62 H 5.79 N 8.07 Zn 9.55
b) 3,8-Bis-(n-propyl)-deuteroporphyrin-IX-dimethyl ether
1.70 g (2.49 mmol) of the compound produced in example 14a
is catalytically hydrogenated under the conditions.used for the
- ~14~509 65
production of mesoporphyrin-IX-dimethyl ester from
protoporphyrin-IX-dimethyl ester (H. Muir and A. Neuberger,
Biochem. J., 45, 163 (1949)) with simultaneous demetallization
until the W spectrum corresponds to the etio type and is worked
up and recrystallized corresponding to these instructions in the
literature.
Yield: 1.42 g (92% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 73.28 H 7.44 N 9.00 O 10.28 Cld.
C 72.97 H 7.38 N 8.85
c) 3,8-Bis-(n-propyl)-deuteroporphyrin-IX-13,17-dihydrazide
1.40 g (2.25 mmol) of the compound produced in example 14b
is dissolved in 110 m1 of anhydrous pyridine under argon and
mixed with 20 ml of hydrazine. After 3 days of stirring at 20°C,
it is concentrated by evaporation in a vacuum, the residue is
stirred in semiconcentrated aqueous hydrochloric acid and
precipitated with 6n sodium hydroxide solution by adjusting the
pH to 7Ø The precipitate is filtered off, washed with water
and recrystallized from pyridine/ether.
Yield: 1.25 g (89% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 69.43 H 7.44 N 17.99 O 5.38 Cld.
C 69.19 H 7.37 N 17.65
- 214509 66
d) N,N'-Bis-[11-carboxy-2-oxo-4,7-bis-(carboxymethyl)-10-
(ethoxycarbonylmethyl)-1,4,7,10-tetraazaundecyl]-3,8-bis-(n-
propyl)-deuteroporphyrin-IX-13,17-diamide
1.56 g (3.86 mmol) of 3-ethoxy-carbonylmethyl-6-[2-(2,6-
dioxomorpholino)-ethyl]=3,6-diazaoctanedioic acid (DTPA-
monoanhydridemonoethyl ester) is suspended in 250 ml of anhydrous
dimethylformamide. It is covered with nitrogen, 1.95 g (19.30
mmol) of triethylamine and 1.20 g (1.93 mmol) of the compound
produced in example 14c are added and stirred for 3 days at room
temperature. After filtering and concentration by evaporation in
a vacuum, the residue is chromatographed on silica gel RP-18 with
water/tetrahydrofuran and the eluate is evaporated to dryness in
a vacuum.
Yield: 4.75 g (86% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 57.13 H 6.77 N 13.72 O 22.38 Cld.
C 56.86 H 6.69 N 13.65
e) N,N'-Bis-[11-carboxylato-2-oxo-4,7-bis-
(carboxylatomethyl)-10-(ethoxycarbonylmethyl)-1,4,7,10-
tetraazaundecyl]-3,8-bis-(n-propyl)-deuteroporphyrin-IX-13,17-
diamide, digadolinium complex
4.70 g (3.29 mmol) of the ligand produced in example 14d is
dissolved in 1000 mi of water. After addition of 2n aqueous
sodium hydroxide solution, it is adjusted to pH 7.0, 2.94 g (7.24
mmol) of gadolinium acetate tetrahydrate and 2n aqueous sodium
2145~~9 67
hydroxide solution are added in portions, so that the pH of the
reaction mixture always fluctuates between 6.8 and 7.2. After
all gadolinium acetate has been added, it is stirred overnight. at
room temperature. For working-up, it is filtered, the solvent is
removed in a vacuum and the residue is chromatographed on silica
gel RP-18 with water/tetrahydrofuran. The eluate is evaporated
to dryness in a vacuum.
Yield: 4.35 g (76% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 46.99 H 5.22 N 11.28 Gd 18.09 O 18.41 Cld.
C 46.75 H 5.16 N 11.02 Gd 18.02
f) N,N'-Bis-[il-carboxylato-2-oxo-4,7,10-tris-
(carboxylatomethyl)-1,4,7,10-tetraazaundecyl)-3,8-bis-X-(1-
propyl)-deuteroporphyrin-IX-13,17-diamide, digadolinium complex,
disodium salt
2.00 g (1.40 mmol) of the ligand produced in example 14d is
dissolved in 200 ml of water. After addition of 10 molar aqueous
sodium hydroxide solution, it is adjusted to pH 13 and stirred
for 5 hours at room temperature. After completion of the
saponification of the ester, it is adjusted to pH 7.0 with
concentrated hydrochloric acid and, as described in example 14e,
complexed and purified with 1.36 g (3.08 mmol) of gadolinium
acetate tetrahydrate.
Yield: 1.80 g (75% of theory) of reddish brown powder
X145509 6
Analysis (relative to the anhydrous substance):
C 44.54 H 4.67 N 11.36 Gd 18.22 O 18.54 Cld.
C 44.26 H 4.57 N 11.13 Gd 18.13
Example 15
a) Zinc(II)-[3,8-bis-(1-butenyl)-deuteroporphyrin-IX-
dimethyl ester]
2.58 g (6.69 mmol) of propyltriphenylphosphoniumbromide in
600 ml of anhydrous tetrahydrofuran is mixed under argon at room
temperature with a solution of 0.43 g (6.69 mmol) of
butyllithium. After completion of the reaction to the ylide,
2.00 g (3.04 mmol) of Zn-[3,8-diformyl-deuteroporphyrin-IX-
dimethyl ester] (Kevin M. Smith, Eugene M. Fujinari, Kevin C.
Langry, Daniel W. Parish and Hani D. Tabba, J. Am. Chem. Soc.
105, 6638-6646 (1983)) is added and stirred for two more hours:
After addition of 30 ml of methanol, the solvent is substantially
removed in a vacuum and the residue shaken out with methylene
chloride and semiconcentrated aqueous sodium bicarbonate
solution. The organic phase is dried on sodium sulfate,
concentrated by evaporation and chromatographed on aluminum oxide
(Merck, activity stages 2-3) with methylene chloride/methanol.
The eluate is evaporated to dryness in a vacuum.
Yield: 1.75 g (81% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 67.65 H 62.44 N 'x.89 Zn 9.20 O 9.01 Cld.
C 67.41 H 62.27 N 7.63 Zn 9.15
' _ 2145509 69
b) 3,8-Bis-(n-butyl)-deuteroporphyrin-IX-dimethyl ester
~1.6 g (2.25 mmol) of the compound produced in example 15a is
catalytically hydrogenated under the conditions used for the
production of mesoporphyrin-IX-dimethyl ester from
protoporphyrin-IX-dimethyl ester (H. Muir and A. Neuberger,
Biochem. J., 45, 163 (1949)) with simultaneous demetailization,
until the W spectrum correponds to the etio type and is worked
up and recrystallized corresponding~to these instructions in the
literature.
Yield: 1.39 g (95% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 73.82 H 7.74 N 8.61 O 9.83 Cld.
C 73.55 H 7.62 N 8.48
c) 3,8-Bis-(n-butyl)-deuteroporphyrin-IX-13,17-dihydrazide
1.30 g (2.00 mmol) of the compound produced in example 15b
is dissolved in 100 ml of anhydrous pyridine under argon and
mixed with 20 ml of hydrazine. After 3 days of stirring at 20°C,
it is concentrated by evaporation in a vacuum, the residue is
stirred in semiconcentrated hydrochloric acid and precipitated
with 6n sodium hydroxide solution by adjusting the pH to 7Ø
The precipitate is filtered off, washed with water and
recrystallized from pyridine/ether.
Yield: 1.18 g (91% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
- 2145509
C 70.13 H 7.74 N 17.22 O 4.92 Cld.
C 69.85 H 7.68 N 17.01
d) N,N'-Bis-[11-carboxy-2-oxo-4,7-bis-(carboxymethyl)-10-
(ethoxycarbonylmethyl)-1,4,7,10-tetraazaundecyl]-3,8-bis-(n-
butyl)-deuteroporphyrin-IX-13,17-diamide
1.24 g (3.07 mmol) of 3-ethoxy-carbonylmethyl-6-[2-(2,6-
dioxomorpholino)-ethyl]-3,6-diazaoctanedioic acid {DTPA-
monoanhydridemonoethyl ester) is suspended in 200 ml of anhydrous
dimethylformamide. It is covered with nitrogen, 1.55 g {15.36
mmol) of triethylamine and 1.00 g (1.54 mmol) of the compound
produced in example 15c are added and stirred for 3 days at room
temperature. After filtering and concentration by evaporation in
a vacuum, the residue is chromatographed on silica gel RP-18 with
water/tetrahydrofuran. The eluate is evaporated to dryness in~a
vacuum.
Yield: 2.00 g (89% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 57.68 H 6.91 N 13.45 O 21.95 Cld.
C 57.38 H 6.77 N 13.19
_. 214509 71
e) N,N'-Bis-[11-carboxylato-2-oxo-4,7-bis-
{carboxylatomethyl)-10-(ethoxycarbonylmethyl)-1,4,7;10-
tetraazaundecyl]-3,8-bis-{n-butyl)-deuteroporphyrin-IX-13,17-
diamide, digadolinium complex
1.00 g (0.69 mmol) of the ligand produced in example 15d is
dissolved in 200 ml of water. After addition of 2n aqueous
sodium hydroxide solution, it is adjusted to pH 7.0, 0.62 g (1.52
mmol) of gadolinium acetate tetrahydrate and 2n aqueous sodium
hydroxide solution are added in portions, so that the pH of the
reaction mixture always fluctuates between 6.8 and 7.2. After
all gadolinium acetate has been added, it is stirred overnight at
room temperature. For working-up, it is filtered, the solvent is
removed in a vacuum and the residue is chromatographed on silica
gel RP-18 with water/tetrahydrofuran and the eluate is evaporated
to dryness in a vacuum.
Yield: 0.87 g (71% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 47.61 H 5.36 N 11.10 Gd 17.81 O 18.12 Cld.
C 47.65 H 5.51 N 11.23 Gd 17.71
f) N,N'-Bis-[11-carboxylato-2-oxo-4,7-tris-
(carboxylatomethyl)-1,4,7,10-tetraazaundecyl]-3,8-bis-(n-butyl)-
deuteroporphyrin-IX-13,17-diamide, digadolinium complex, disodium
salt
1.00 g (0.69 mmol) of the ligand produced in example 15d is
dissolved in 200 ml of water. After addition-of 10 molar aqueous
s
_ 2145509 72
sodium hydroxide solution, it is adjusted to pH 13 and stirred
for 5 hours at room temperature. After completion of the
saponification of the ester, it is adjusted to pH 7.0 with
concentrated hydrochloric acid and, as described in example 15e,
complexed and purified with 0.62 g (1.52 mmol) of gadolinium
acetate tetrahydrate.
Yield: 0.88 g (73% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 45.20 H 4.83 N 11.18 Na 2.61 Gd 17.93 O 18.25 Cld.
C 44.93 H 4.76 N 11.01 Na 2.52 Gd 17.82
Example 16
a) Zinc(II)-[3,8-bis-(1-heptenyl)-deuteroporphyrin-IX-
dimethyl ester]
2.86 g (6.69 mmol) of hexyltriphenylphosphoniumbromide in
600 ml of anhydrous tetrahydrofuran is mixed under argon at room
temperature with a solution of 0:43 g (6.69 mmol) of
butyllithium. After completion of the reaction to the ylide,
2.00 g (3.04 mmol) of Zn-[3,8-diformyldeuteroporphyrin-IX-
dimethyl ester] (Kevin M. Smith, Eugene M. Fujinari, Kevin C.
Langry, Daniel W. Parish and Hani D. Tabba, J. Am. Chem. Soc.
105, 6638-6646 (1983)) is added and stirred for two more hours.
After addition of 30 ml of methanol, the solvent is substantially
removed in a vacuum and shaken out with methylene chloride and
se~iconcentrated aqueous sodium bicarbonate solution. The
organic phase is dried on-sodium sulfate, concentrated by
s
_ 214509 73
evaporation and chromatographed on aluminum oxide (Merck,
activity stages 2-3) with methylene chloride/methanol. The
eluate is evaporated to dryness in a vacuum.
Yield: 1.91 g (79% of theory) of reddish brown powder
Analysis (relative to. the anhydrous substance):
C 69.56 H 7.11 N 7.05 Zn 8.23 O 8.06 Cld.
C 69.11 H 6.88 N 6.79 Zn 8.16
b) 3,8-Bis-(n-heptyl)-deuteroporphyrin-IX-dimethyl ester
1.50 g (1.89 mmol) of the compound produced in example 16a
is catalytically hydrogenated under the conditions used for the
production of mesoporphyrin-IX-dimethyl ester from
protoporphyrin-IX-dimethyl ester {H. Muir and A. Neuberger,
Biochem. J., 45, 163 (1949)) by simultaneous demetallization
until the W spectrum corresponds to the etio type and is worked
up and recrystallized corresponding to these instructions in the
literature.
Yield: 1.32 g (95% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 75.17 H 8.50 N 7.62 O 8.71 Cld.
C 74.83 H 8.39 N 7.37
c) 3,8-Bis-(1-heptyl)-deuteroporphyrin-IX-13,17-dihydrazide
1.30 g (1.77 mmol) of the compound produced in example 16b
is dissolved in 100 ml of anhydrous pyridine and mixed with 20 ml
2145509 74
of hydrazine. After 3 days of stirring at 20°C, it is
concentrated by evaporation in a vacuum, the residue is stirred
in semiconcentrated aqueous hydrochloric acid and again
precipitated with 6n sodium hydroxide solution by adjusting the
pH to 7Ø The precipitate is filtered off, washed with water
and recrystallized from pyridine/ether. '
Yield: 1.14 g (88% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 71.90 H 8.50 N 15.24 O 4.35 Cld.
C 71.78 H 8.39 N 14.97
d) N,N~-Bis-[11-carboxy-2-oxo-4,7-bis-(carboxymethyl)-10-
(ethoxycarbonylmethyl)-1,4,7,10-tetraazaundecyl]-3,8-bis-(n-
heptyl)-deuteroporphyrin-IX-13,17-diamide
1.21 g (3.00 mmol) of 3-ethoxy-carbonylmethyl-6-[2-(2,6-
dioxomorpholino)-ethyl]-3,6-diazaoctanedioic acid (DTPA-
monoanhydridemonoethyl ester) is suspended in 200 ml of anhydrous
dimethylformamide. It is covered with nitrogen, 1.52 g (15.00
mmol) of triethylamine and 1.10 g (1.50 mmol) of the compound
produced in example 16c are added and stirred for 3 days at room
temperature. After filtering and concentration by evaporation in
a vacuum, the residue is chromatographed-on silica gel RP-18 with
water/tetrahydrofuran and the eluate is evaporated to dryness in
a vacuum.
Yield: 2.06 g (89% of theory) of reddish brown powder
a
2145509
. .
Analysis (relative to the anhydrous substance):
C 59.21 H 7.32 N 12.72 O 20.75 Cld.
C 58.97 H 7.11 N 12.61
e) N,N'-Bis-[11-carboxylato-2-oxo-4,7-bis-
(carboxylatomethyl)-10-ethoxycarbonylmethyl-1,4,7,10- .
tetraazaundecylj-3,8-bis-(n-heptyl)-deuteroporphyrin-IX-13,17-
diamide, digadolinium complex
1.00 g (0.65 mmol) of the ligand produced in-example i6d is
dissolved in 300 ml of water. After addition of 2n aqueous
sodium hydroxide solution, it is adjusted to pH 7.0, and 0.58 g
(1.43 mmol) of gadolinium acetate tetrahydrate and 2n aqueous
sodium hydroxide solution are added in portions, so that the pH
of the reaction mixture always fluctuates between 6.8 and 7.2.
After all gadolinium acetate.has been added, it is stirred
overnight at room temperature. For working-up, it is filtered,
the solvent is removed in a vacuum, the residue is
chromatographed on silica gel RP-18 with water/tetrahydrofuran
and the eluate is evaporated to dryness in a vacuum.
Yield: 0.87 g (72% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 49.34 H 5.77 N 10.60 Gd 17.00 O 17.29 Cld.
C 49.07 H 5.68 N 10.42 Gd 16.89
f) N,N'-Bis-[11-carboxylato-2-oxo-4,7,10-tris-
(carboxylatomethyl)-1,4,7,10-tetraazaundecyl]-3,8-bis-(n-heptyl)-
76
- ~1455~9
deuteroporphyrin-IX-13,17-diamide, digadolinium complex, disodium
salt
1.00 g (0.65 mmol) of the ligand produced in example 16d is
dissolved in 300 ml of water. After addition of 10 molar aqueous
sodium hydroxide solution, it is adjusted to pH 13 and stirred
for 5 hours at room temperature. After completion of the
saponification of the ester, it is adjusted to pH 7.0 with
concentrated hydrochloric acid and, as described in example 16e,
complexed and purified with 0.58 g (1.43 mmol) of gadolinium
acetate tetrahydrate.
Yield: 0.91 g (76% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 47.05 H 5.26 N 10.67 Na 2.49 Gd 17.11 O 17.41 Cld.
C 46.86 H 5.07 N 10.42 Na 2.51 Gd 17.09
Example 17
a) Zinc(II)-[3,8-bis-(2-phenylethenyl)-deuteroporphyrin-IX-
dimethyl ester]
2.60 g (6.69 mmol) of benzyltriphenylphosphoniumchloride in
600 ml of anhydrous tetrahydrofuran is mixed under argon at room
temperature with a solution of 0.43 g (6.69 mmol) of
butyllithium. After completion of the reaction to the ylide,
2.00 g (3.04 mmol) of Zn-[3,8-diformyl-deuteroporphyrin-IX-
dimethyl ester) (Kevin M. Smith, Eugene M. Fujinari, Kevin C.
Langry, Daniel W. Parish and Hani D. Tabba, J. Am. Chem. Soc.
105, 6638-6646 (1983)) is added and stirred for two more hours.
214550 77
After addition of 30 ml of methanol, the solvent is substantially
removed in a vacuum and the residue shaken out with methylene
chloride and semiconcentrated aqueous sodium bicarbonate
solution. The organic phase is dried on sodium sulfate,
concentrated by evaporation, chromatographed on aluminum oxide
(Merck, activity stages 2-3) with methylene chloride/methanol and
the eluate is evaporated to dryness.
Yield: 2.23 g (91% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 71.51 H 5.50 N 6.95 Zn 8.11 O 7.94 Cld.
C 71.24 H 5.39 N 6.68 Zn 8.06
b) Zinc(II)-[3,8-bis-(2-phenylethyl)-deuteroporphyrin-IX-
dihydrazide]
2.10 g (2.60 mmol) of the compound produced in example 17a
is dissolved in 130 ml of pyridine and mixed with 30 ml of
hydrazine. After 3 days.of stirring in an open flask at 20'~C, it
is concentrated by evaporation in a vacuum, the residue suspended
in water, suctioned off, dried in a vacuum and recrystallized
from pyridine/ether.
Yield: 1.96 g (93% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 68.19 H 5.97 N 13.83 Zn 8.07 O 3.95 Cld.
C 67.86 H 5.83 N 13.61 Zn 7.94
' - 214509
c) Manganese(III)-[3,8-bis-(2-phenylethyl)-deuteroporphyrin-
IX-dihydrazide]-acetate
1.90 g (2.34 mmol) of the compound produced in example 17b
is refluxed with 9.50 g of manganese(II)acetate for 1 hour in 500
ml of acetic acid. Then, it is.concentrated by evaporation in a
vacuum, the residue is suspended in-water, filtered off and
washed with water. The dried crude product is recrystallized
from dimethylformamide.
Yield: 1.65 g (82% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 67.12 H 5.98 Mn 6.40 N 13.05 O 7.45 Cld.
C 66.93 H 5.91 Mn 6.32 N 12.89
d) Manganese(III)-~N,N~-bis-[11-carboxy-2-oxo-4,7-bis-
(carboxymethyl)-10-(ethoxycarbonylmethyl)-1,4,7,10-
tetraazaundecyl]-3,8-bis-(2-phenylethyl)-deuteroporphyrin-IX-
13,1.7-diamide}-acetate
1.41 g (3.49 mmol) of 3-ethoxy-carbonylmethyl-6-[2-(2,6-
dioxomorpholino)-ethyl]-3,6-diazaoctanedioic acid (DTPA-
monoanhydridemonoethyl ester) is suspended in 250 ml of anhydrous
dimethylformamide. It is covered with nitrogen, 1.77 g (17.50
mmol) of triethylamine and 1.50 g (1.75 mmol) of the compound
produced in example 17c are added and stirred for 3 days at room
temperature. After filtering and concentration by evaporation in
a vacuum, the residue is chromatographed on silica gel RP-18 with
T
214~50~ 79
water/tetrahydrofuran and the eluate is evaporated to dryness in
a vacuum.
Yield: 2.10 g (72% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 57.69 H 6.11 N 11.77 Mn 3.30 O 21.13 Cld.
C 57.42 H 5.99 N 11.61 Mn 3.24
e) Manganese(III)-{N,N'-bis-[il-carboxylato-2-oxo-4,7,10-
tris-(carboxylatomethyl)-1,4,7,10-tetraazaundecyl]-3,8-bis-(2-
phenylethyl)-deuteroporphyrin-IX-13,17-diamide}-acetate,
digadolinium complex, disodium salt
2.00 g (1.20 mmol) of the ligand produced in example 17d is
suspended in 400 ml of water. After addition of 10 molar aqueous
sodium hydroxide solution, it is adjusted to pH 13 and stirred
for 5 hours at room temperature. After completion of the
saponification of the ester, it is adjusted to pH 7.0, 1.06 g
(2.64 mmol) of gadolinium acetate tetrahydrate is added and by
addition of 2n sodium hydroxide solution, the pH is held at 7Ø
After stirring overnight, it is filtered, the solvent is removed
in a vacuum, the residue is chromatographed on silica gel RP-18
with water/tetrahydrofuran and the eluate is evaporated to
dryness in a vacuum.
Yield: 1.62 g (69% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 46.53 H 4.37 N 10.00 Gd 16.03 Mn 2.80 Na 2.33 O 17.94
214~~09
Cld.
C 46.33 H 4.28 N 9.86 Gd 17.86 Mn 2.67 Na 2.41
Example 18
a) Zinc(II)-[3,8-bis-(2-{naphth-1-yl)-ethenyl)-
deuteroporphyrin-IX-dimethyl ester]
3.23 g {6.69 mmol) of naphth-1-yl-methyl-
triphenylphosphoniumbromide in 600 ml of anhydrous
tetrahydrofuran is mixed under argon at room temperature with a
solution of 0.43 g (6.69 mmol) of butyllithium. After completion
of the reaction to the ylide, 2.00 g (3.04 mmol) of Zn-[3,8-
diformyl-deuteroporphyrin-IX-dimethyl ester] {Kevin M. Smith,
Eugene M. Fujinari, Kevin C. Langry, Daniel W. Parish and Hani D.
Tabba, J. Am. Chem. Soc. 105, 6638-6646 {1983)) is added and
stirred for two more hours. After addition of 30 ml of methanol,
the solvent is substantially removed in a vacuum and the residue
shaken out with methylene chloride and semiconcentrated aqueous
sodium bicarbonate solution. The organic phase is dried on
sodium sulfate, concentrated by evaporation and chromatographed
on aluminum oxide (Merck, activity stages 2-3) with methylene
chloride/methanol and the eluate is evaporated to dryness.
Yield: 2.53 g (92%) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 74.21 H 5.34 N 6.18 Zn 7.21 O 7.06 Cld.
C 74.00 H 5.19 N 5.99 Zn 7.11
~1~5509 81
b) Zinc(II)-[3,8-bis-(2-(naphth-1-yl)-ethyl)-
deuteroporphyrin-IX-dihydrazide]'
2.50 g (2.76 mmol) of the compound produced in example 18a
is dissolved in 130 ml of pyridine and mixed with 30 ml of
hydrazine. After 3 days of stirring at room temperature in an
open flask at 20°C, it is concentrated by evaporation in a
vacuum, the residue suspended in water, suctioned off, dried in a
vacuum and recrystallized from pyridine/ether.
Yield: 2.39 g (95% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 71.24 H 5.76 N 12.31 Zn 7.18 O 3.51 Cld.
C 70.93 H 5.59 N 12.01 Zn 7.09
c) Zinc(II)-{N,N~-bis[11-carboxy-2-oxo-4,7-bis-
(carboxymethyl)-10-(ethoxycarbonylmethyl)-1,4,7,10-
tetraazaundecyl]-3,8-bis-(2-(naphth-1-yl)-ethyl)-
deuteroporphyrin-IX-13,17-diamide}
1.77 g (4.40 mmol) of 3-ethoxy-carbonylmethyl-6-[2-(2,6-
dioxomorpholino)-ethyl]-3,6-diazaoctanedioic acid (DTPA-
monoanhydridemonoethyl ester) is suspended in 300 ml of anhydrous
dimethylformamide. It is covered with nitrogen, 2.20 g (21.75
mmol) of triethylamine and 2.00 g (2.20 mmol) of the compound
produced in example 18b are added and stirred for 3 days at room
temperature. After filtering and concentration by evaporation in
a vacuum, the residue is chromatographed on silica gel RP-18 with
- 2145509 82
water/tetrahydrofuran. The eluate is evaporated to dryness in a
vacuum.
Yield: 2.91 g (77% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 60.15 H 5.99 N 11.42 ~Zn 3.81 O 18.6 Cld.
C 59.89 H 5.81 N 11.26 Zn 3.72
d) Zinc(II)-{N,N'-bis[11-carboxylato-2-oxo-4,7,10-tris-
(carboxylatomethyl)-1,4,7,10-tetraazaundecylJ-3,8-bis-(2-(naphth-
1-yl)-ethyl)-deuteroporphyrin-iX-13,17-diamide}, digadolinium
complex, disodium salt
2.00 g (1.16 mmol) of the ligand produced in example 18c is
suspended in 400 ml of water. After addition of 10 molar aqueous
sodium hydroxide solution, it is adjusted to pH 13 and stirred
for 5 hours at room temperature. After completion of the
saponification of the ester, it is adjusted to pH 7.0, 1.06 g
(2.64 mmol) of gadolinium acetate tetrahydrate is added and by
addition of 2n sodium hydroxide solution, the pH is held at 7Ø
After stirring overnight, it is filtered, the solvent is removed
in a vacuum, the residue is chromatographed on silica gel RP-18
with water/tetrahydrofuran and the eluate is evaporated to
dryness in a vacuum.
Yield: 1.47 g (63% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 48.92 H 4.31 N 9.74 Na 2.27 Gd 15.62 Zn 3.25 O 15.89 Cld.
214509 $3
C 48.66 H 4.21 N 9.48 Na 2.31 Gd 15.46 Zn 3.18
Example 19
a) 13,17-Bis-(4-aminobutyl)-3,8-diethyl-2,7,12,18-
tetramethylporphyrin
2.00 g (3.59 mmol) of 13,17-bis-(3-cyanopropyl)-3,8-
diethyl-2,7,12,18-tetramethylporphyrin is added in portions to
the reducing agent formed from 0.32 g (14.5 mmol) of lithium
borohydride and 3.18 g (29.0 mmol) of trimethylsilyl chloride (A.
Giannis, K. Sandhoff, Angewandte Chemie [Applied Chemistry] 101
No. 2, 220-222 (1989)) in 100 ml of anhydrous tetrahydrofuran and
stirred for 24 hours under argon at 25°C. If by thin-layer
chromatography more feedstock is detectable, a further reducing
agent is added and stirred again. For working-up, 2o ml of
methanol is carefully instilled, it is adjusted to pH 1.0 with
semiconcentrated hydrochloric acid and allowed to stir for one
hour. The batch is then adjusted strongly alkaline by addition
of 20 ml of 30% sodium hydroxide solution, stirred for 30 minutes
and shaken out twice with methylene chloride. The organic phases
are combined, dried on anhydrous sodium sulfate, filtered and
concentrated by evaporation. The reddish brown, solid residue is
reused as crude product.
Yield: 1.80 g (89% of theory) of reddish brown solid
' 2145509 84
b) 13,17-Bis-[4-N-(benzyloxycarbonyl)-aminobutyl]-3,8-
diethyl-2,7,12,18-tetramethyl-porphyrin
50 mg (0.09 mmol) of the compound produced in example 19a is
dissolved in 5 ml of anhydrous pyridine. 34 mg (0.20 mmol) of
benzyloxycarbonyl chloride is instilled in the solution stirred
at -10°C under argon and the batch is'allowed to stir for 12 more
hours at room temperature. Then, the solvent is removed, taken
up in methylene chloride, and shaken out once with 2n citric acid
solution and once with concentrated sodium bicarbonate solution
and the organic phase is dried on sodium sulfate. It is
filtered, concentrated by evaporation, chromatographed on
aluminum oxide with methylene chloride/methanol and evaporated to
dryness.
Yield: 61 mg (81% of theory) of reddish brown solid
Analysis (relative to the anhydrous substance):
C 74.97 H 7.26 N 10.09 O 7.68 Cid.
C 74.76 H 7.19 N 9.88
c) 13,17-Bis-[15-carboxy-6-oxo-8,11-bis-(carboxymethyl)-14-
(ethoxycarbonylmethyl)-5,8,11,14-tetraazapentadecyl]-3,8-diethyl-
2,7,12,18-tetramethylporphyrin
1.21 g (3.00 mmol) of 3-ethoxy-carbonylmethyl-6-[2-(2,6-
dioxomorpholino)-ethyl]-3,6-diazaoctanedioic acid (DTPA-
monoanhydridemonoethyl ester) is suspended in 200 ml of anhydrous
dimethylformamide. It is covered with nitrogen, 1.52 g (15.00
mmol) of triethylamine and 0.85 g (1.50 mmol) of the~compound
2145509 $5
produced in example 19a are added and stirred for 3 days at room
temperature. After filtering and concentration by evaporation in
a vacuum, the residue is chromatographed on silica gel RP-18 with
water/tetrahydrofuran and the eluate is concentrated by
evaporation in a vacuum. -
Yield: 1.50 g (73%.of theory) of reddish brown powder.
Analysis (relative to the anhydrous substance):
C 59.55 H 7.20 N 12.25 O 21.00 Cld.
C 59.41 H 7.14 N 12.13
d) 13,17-Bis-[15-carboxylato-6-oxo-8,11-bis-
(carboxylatomethylj-14-(ethoxycarbonylmethyl)-5,8,11,14-
tetraazapentadecyl]-3,8-diethyl-2,7,12,18-tetramethylporphyrin,
digadolinium complex
1.20 g (0.87 mmol) of the ligand produced in example 19c is
dissolved in 400 ml of water. After addition of 2n aqueous
sodium hydroxide solution, it is adjusted to pH 7.0 and 0.78 g
(1.91 mmol) of gadolinium acetate tetrahydrate and 2n aqueous
sodium hydroxide solution are added in portions, so that the pH
of the reaction mixture always fluctuates between 6.8 and 7.2.
After all gadolinium acetate has been added, it is stirred
overnight at room temperature. For working-up, it is filtered,
the solvent is removed in a vacuum and the residue is
chromatographed on silica gel RP-18 with water/tetrahydrofuran
and the eluate is evaporated to dryness in a vacuum.
Yield: 1.01 g (69% of theory) of reddish brown powder
Y
86
2~.4~~~~
Analysis (relative to the anhydrous substance):
C 48.62 H 5.52 Gd 18.72 N 10.10 O 17.14 Cld.
C 48.46 H 5.47 Gd 18.75 N 9.83
e) 13,17-Bis-[15-carboxylato-6-oxo-8,11,14-tris-
(carboxylatomethyl)-5,8,11,14-tetraazapentadecyl]-3,8-diethyl-
2,7,12,18-tetramethylporphyrin, digadolinium complex, disodium
salt
0.90 g (0.66 mmol) of the ligand produced in example 19c is
dissolved in 300 ml of water. After addition of 10 molar aqueous
sodium hydroxide solution, it is adjusted to pH 13.0 and it is
stirred for 5 hours at room temperature. After completion of the
saponification of the ester, it is adjusted to pH 7.0 with
concentrated hydrochloric acid, and as described in example 19d,
complexed and purified with 0.59 g (1.44 mmol) of gadolinium
acetate tetrahydrate.
Yield: 0.91 g (83% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 46.09 H 4.96 Gd 18.86 N 10.08 Na 2.75 O 17.27 Cld.
C 45.85 H 4.87 Gd 18.72 N 9.84 Na 2.54
Example 20
a) 13,17-Bis-[4-(4-aminophenyl)-4-oxabutyl]-3,8-diethyl-
2,7,12,18-tetramethylporphyrin
3.28 g (30.10 mmol) of 4-aminophenol and 1.69 g (30.10 mmol)
of powdered potassium hydroxide are stirred in 50 ml of anhydrous
__ ~14~509 87
dimethylformamide under argon for 30 minutes at room temperature
and then mixed with 2.00 g (3.01 mmol) of 13,17-bis-(3-
bromopropyl)-3,8-diethyl-2,7,12,18-tetramethylporphyrin (CA RN
112635-99-1). After 30 hours of stirring at room temperature, it
is filtered, substantially concentrated by evaporation in a
vacuum, the residue is taken up in methylene chloride and shaken
out once each with water and with saturated aqueous sodium
bicarbonate solution. The organic phase is dried on sodium
sulfate, filtered and concentrated by evaporation and the residue
is chromatographed to aluminum oxide with methylene
chloride/methanol. After the concentration by evaporation, the
product is obtained as reddish brown solid, which can be
recrystallized from methylene chloride/ether.
Yield: 1.97 g (91% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 76.64 H 7.27 N 11.66 O 4.44 Cld.
C 76.48 H 7.21 N 11.54
b) 13,17-Bis-{4-(4-(11-carboxy-2-oxo-4,7-bis-
(carboxymethyl)-10-(ethoxycarbonylmethyl)-1,4,7,10-
tetraazaundecyl)-phenyl]-4-oxabutyl}-3,8-diethyl-2,7,12,18-
tetramethylporphyrin
1.21 g (3.00 mmol) of 3-ethoxy-carbonylmethyl-6-[2-(2,6-
dioxomorpholino)-ethyl]-3,6-diazaoctanedioic acid (DTPA-
monoanhydridemonoethyl ester) is suspended in 200 ml of anhydrous
dimethylformamide. It is covered with nitrogen, 1.52 g (15.00
- ~m~~o9 88
mmol) of triethylamine and 1.08 g (1.50 mmol) of the compound
produced in example 20a are added and stirred for 3 days at room
temperature. After filtering and concentration by evaporation in
a vacuum, the residue is chromatographed on silica gel RP-18 with
water/tetrahydrofuran and evaporated to dryness in a vacuum.
Yield: 1.89 g (82% of theory) of reddish brown powder
Analysis (relative to~the anhydrous substance):
C 61.32 H 6.73 N 11.00 O 20.95 Cld.
C 61.09 H 6.64 N 10.87
c) 13,17-Bis-~4-(4-(il-carboxylato-2-oxo-4,7-bis-
(carboxylatomethyl)-10-(ethoxycarbonylmethyl)-1,4,7,10-
tetraazaundecyl)-phenyl)-4-oxabutyl}-3,8-diethyl-2,7,12,18-
tetramethylporphyrin, digadolinium complex
1.50 g (0.98 mmol) of the ligand produced in example 20b is
dissolved in 350 ml of water. After addition of 2n aqueous
sodium hydroxide solution, it is adjusted to pH 7.0 and 0.88 g
(2.16 mmol) of gadolinium acetate tetrahydrate and 2n aqueous
sodium hydroxide solution are added in portions, so that the pH
of the reaction mixture always fluctuates between 6.8 and 7.2.
After all gadolinium acetate has been added, it is stirred
overnight at room temperature. For working-up, it is filtered,
the solvent is removed in a vacuum and the residue is
chromatographed on silica gel RP-18 with water/tetrahydrofuran.
The eluate is evaporated to dryness in a vacuum.
Yield: 1.44 g (80% of theory) of reddish brown powder
1 _ 21455D9 $9
s
Analysis (relative to the anhydrous substance):
C 51.02 H 5.27 Gd 17.13 N 9.15 O 17.43 Cld.
C 50.83 H 5.18 Gd 17.11 N 8.94
d) 13,17-Bis-{4-(4-(11-carboxylato-2-oxo-4,7,10-tris-
(carboxylatomethyl)-1,4,7,10-tetraazaundecyl)-phenyl]-4-
oxabutyl}-3,8-diethyl-2,7,12,18-tetramethylporphyrin,
digadolinium complex, disodium salt
1.50 g (0.98 mmol) of the ligand produced in example 2ob is
dissolved in 350 ml of water. After addition of 10 molar aqueous
sodium hydroxide solution, it is adjusted to pH 13.0 and stirred
for 5 hours at room temperature. After completion of the
saponification of the ester, it is adjusted to pH 7.0 with
concentrated hydrochloric acid, and as described in example 20c,
complexed, worked up and purified with 0.88 g (2.16 mmol) of
gadolinium acetate tetrahydrate.
Yield: 1.41 g (79% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 48.73 H 4.75 Gd 17.24 N 9.22 Na 2.51 O 17.54 Cld.
C 48.61 H 4.72 Gd 17.25 N 9.13 Na 2.39
- ~I45509
Example 21
a) Manganese(III)-{N,N'-bis-[11-carboxy-2-oxo-4,7-bis-
(carboxymethyl)-10-(ethoxycarbonylmethyl)-1,4,7,10-
tetraazaundecyl]-3,8-bis-(1-propyl)-deuteroporphyrin-IX-13,17-
diamide}-acetate -
2.00 g (1.40 mmol) of the ligand produced in example 14d and
10.00 g of manganese(II)acetate tetrahydrate were refluxed in 150
ml of acetic acid for one hour. The above is evaporated to
dryness in a vacuum and the residue is chromatographed in a
mixture of dichloromethane, methanol, acetic acid and water. The
product-containing fractions yield a finely crystalline, reddish
brown powder after concentration by evaporation and drying in a
vacuum.
Yield: 2.01 g (93% of theory)
Analysis (relative to the anhydrous substance):
C 54.54 H 6.34 Mn 3.56 N 12.72 O 22.83 Cld.
C 54.63 H 6.41 Mn 3.42 N 12.58
b) Manganese(III)-{N,N'-bis-[11-carboxylato-2-oxo-4,7-bis-
(carboxylatomethyl)-10-(ethoxycarbonyimethyl)-1,4,7,10-
tetraazaundecyl]-3,8-bis-(1-propyl)-deuteroporphyrin-IX-13,17-
diamide}-acetate, digadolinium complex
1.92 g (1.25 mmol) of the ligand produced in example 21a is
dissolved in 300 ml of water. After addition of 2n sodium
hydroxide solution, it is adjusted to pH 7.0 and 1.18 g (2.90
mmol) of gadolinium acetate tetrahydrate and 2n aqueous sodium
- 2145~Q9 91
hydroxide solution are added in portions, so that the pH always
fluctuates between 6.8 and 7.2. After all gadolinium acetate has
been added, it is stirred overnight at room temperature. For
working-up, it is filtered, the solvent is removed in a vacuum
and the residue is chromatographed on silica gel RP-18 with
water/tetrahydrofuran. The fractions contained in the product
are evaporated to dryness in a vacuum.
Yield: 1.89 g (82% of theory) of reddish brown powder
Analysis (relative to the anhydrous substance):
C 45.45 H 4.96 Gd 17.00 Mn 2.97 N 10.60 O 19.03 Cld.
C 45.22 H 4.83 Gd 16.89 Mn 3.05 N 10.50
c) Manganese(III)-{N,N'-bis-[il-carboxylato-2-oxo-4,7-bis-
(carboxylatomethyl)-10-(ethoxycarbonylmethyl)-1,4,7,10-
tetraazaundecyl]-3,8-bis-(1-propyl)-deuteroporphyrin-IX-13,17-
diamide}-acetate, digadolinium complex, disodium salt
1.55 g (1.01 mmol) of the manganese porphyrin produced in
example 21a is dissolved in 150 ml of water. After addition of
molar aqueous sodium hydroxide solution, it is adjusted to pH
13.0 and stirred for 5 hours at room temperature. After
completion of the saponification of the ester, it is adjusted to
pH 7.0 With concentrated hydrochloric acid, and as described in
example 21b, complexed and purified with 0.90 g (2.21 mmol) of
gadolinium acetate tetrahydrate.
Yield: 1.45 g (78% of theory) of reddish brown powder
- ~14~509 92
Analysis (relative to the anhydrous substance):
C 43.13 H 4.44 Gd 17.11 Mn 2.99 N 10.67 Na 2.50 O 19.15
Cld.
C 43.24 H 4.52 Gd 17.03 Mn 2.84 N 10.39 Na 2.81
Example 22
a) 9 nude mice (Balb/c nu/nu) with subcutaneously implanted
colon cancers (HT29, WiDr) were each administered by i.v. 0.1
mmol/kg of the mesoporphyrin derivative of example lb (10 mmol/1
dissolved in bidistilled water, pH 7.2, 37°C). After 0.5, 3 and
24 hours the animals were killed and dissected. The tissue
concentrations were determined after corresponding working-up
(HN03 cone ) by ICP-AES and calculated for % of the administered
dose per gram of tissue net weight.
. ~ 2145509 93
1
Table 1:
% of dose/g organ
over 0.5 over 3 hours over 24hours
MW hour MW S MW S
S
blood 19.34 1.06 6.11 1.13 0.21 0.04
liver 9.26 0.10 8.68 0.53 10.47 0.64
kidneys 42.29 7.05 6.71 0.79 3.93 1.01
spleen 7.02 0.53 3.21 0.46 3.85 0.63
muscle 3.13 0.68 0.85 0.15 0.53 0.04
intestine 3.53 0.07 2.03 + 0.13 0.95 + 0.30
skin 8.63 0.91 2.81 0.30 2.71 0.42
lung 13.83 1.43 4.57 0.67 1.84 0.38
HT29 tumor 4.80 1.23 3.03 0.22 1.95 0.52
WiDr tumor 4.54 3.37 3.23 + 0.95 2.71 + 0.57
rest of body 4.84 0.48 1.57 0.21 1.20 0.45
By 0.5 hour p.i., the concentration in the liver is already
10% of the administered dose per gram of tissue net weight.
While in all other tissues examined, the concentration
drastically decreases within 24 hours, the porphyrin
concentration in the liver tissue remains constant at l0%.
214559 94
f
Table 2:
Average values (enhancement):
Time {min)HT29 WiDr Liver Kidneys Muscle
0 1.00 1.00 1.00 1.00 1.00
.5 1.36 1.16 1.43 2.89 1.41
15 1.49 1.16 1.43 2.70 1.41
30 1.54 1.18 1.40 2.65 1.39
45 1.55 1.25 1.42 2.79 1.40
60 1.59 1.05 1.38 2.56 1.29
75 1.97 1.23 1.51 2.53 1.47
90 1.51 1.28 1.43 2.40 1.50
120 1.64 1.22 1.47 2.15 1.34
24 hours 1.05 1.64 1.19 1.51 1.24
Already 5 minutes p.i., a marked signal intensity increase
in the liver tissue was detected and after 30 minutes in the
tumor examined. The maximum enhancement (liver: +50%, tumor:
+100%) was achieved in 75 minutes p.i. On the other hand, only a
small change was noted in the muscle as reference tissue. A
stronger signal intensity change was noted in the kidneys.
Example 23
On a Bruker Biospec nuclear spin tomograph (2.35 Tesla), the
signal intensity change in various tissues of tumor-bearing (HT29
colon cancer) nude mice (Balb/c nu/nu) was studied after i.v.
administration of 0.05 mmol/kg of the compound according to
-~ - 2145509 95
i
example lc (10 mmol/1, pH 7.2; 37°C in bidistilled water). The
measurement takes place with a multi slice single echo sequence
(MSSE). Imaging parameters: TR: 400 ms, TE: 30 ms, layer
thickness: 4 mm, number of averages: 4/slice, matrix 2562. After
a preliminary image, 0.05 mmol/kg of the substance was
administered and the signal intensity change (relative to an
internal standard) was studied 5, 15, 30, 45, 60, 75, 90 and 120
minutes p.i.
Table 3:
Enhancement after intravenous administration of 0.05 mmol/kg of
body weight
Time (min) liver muscle HT29 Kidneys
0 100 100 100 100
122 31 119 8 116 19 185 34
60 164 22 129 7 128 + 21 240 26
90 202 37 143 21 148 + 22 248 + 18
120 194 + 33 147 + 17 155 + 30 246 + 6
150 197 17 146 8 167 29 225 12
180 212 9 157 8 161 + 22 212 + 16
A marked signal intensity increase in the liver tissue
occurred as early as 5 minutes p.i., while only a slight change
was able to be noted in tumor and muscle as reference tissues.
This effect lasted during the entire observation time. Only in
- ~ 2145509 96
the kidneys is it possible to note an even stronger signal
intensity change up to 3 hours p.i.
b) On a Bruker Biospec nuclear spin tomograph (2.35 Tesla),
the signal intensity change in various tissues of rats with a
DMBA-induced breast cancer was studied after i.v.~ administration
of 0.05 mmol/kg or 0.1 mmoi of the compond of example lc (20
mmol/1, pH 7.2; 37°C in bidistilled water). The measurements
took place with a multi slice variable echo sequence (MSVE).
Imaging parameters: TR: 400 ms, TE: 25 ms, layer thickness: 4 mm,
number of averages: 4/slice, matrix 2562.
After a preliminary image, 0.05 mmol/kg or 0.1 mmol/kg of
the substance was administered and the signal intensity change
(relative to an internal standard) was studied.
The results are compiled in the table below. The ~H-NMR
images of the DMBA-induced breast cancer after administration of
0.1 mmol/kg i.v. after 5 minutes or 3 or 24 hours p.i. are
reproduced in Figure 1.
- 2145509 97
t
Table 4:
Enhancement after intravenous administration of 0.05 mmol/kg
(average values)
Time(min)Tumor Liver Kidneys Muscle
0 1.40 1.00 1.00 1.00
1.88 1.48 2.57 1.34
1.97 1.29 2.81 1.26
30 1.71 1.42 2.94 1.22
45~ 1.71 1.49 2.84 1.12
60 1.50 1.52 2.53 1.10
75 1.71 1.50 2.47 1.04
90 1.62 1.53 2.15 1.11
105 1.75 1.63 2.63 1.07
160 1.69 1.47 2.38 1.07
180 1.67 1.57 2.54 1.02
24 hours 1.31 0.93 1.17 1.07
X145509
- 98
Table 5:
Enhancement after intravenous administration of 0.1 mmol/kg
(average values)
Time(min) Tumor Liver Kidneys Muscle
0 1.00 1.00 1.00 1.00
1 2.48 1.55 1.16 1.46
15 2.40 1.61 2.36 1.30
26 2.41 1.45 2.33 1.37
40 2.34 1.51 2.38 1.23
60 2.34 1.36 1.86 1.23
71 2.36 1.62 1.66 1.17
85 2.39 1.48 1.64 1.32
115 2.38 1.62 2.43 1.23
150 2.31 1.48 2.27 1.17
180 2.38 1.59 2.18 1.22
24 hours 2.02 1.33 0.94 1.15
As early as 5 minutes p.i., a marked signal intensity
increase in the liver tissue and especially in the studied tumors
occurred. On the other hand, only a slight change was noted in
the muscle as reference tissue. This effect lasted during the
entire observation time (up to 24 hours p.i.). Only in the
kidneys is it possible to note a stronger signal intensity change
after administration of 0.05 mmol/kg.
2145509
c) On a Bruker Biospec nuclear spin tomograph (2.35 Tesla),
the signal intensity change in various tissues of tumor-bearing
(Novikoff-hepatoma, i.m.) nude rats (LEW/MoT rnu/rnu) was studied
after a one-time intravenous administration of 0.1 mmol/kg of the
compound of example is (20 mmol/1; pH 7.2-7.4; 37°C in tris/NaCl
buffer). The~~measurements were made with a multi slice single
echo sequence (MSSE). Imaging parameters: TR: 412 ms, TE: 25 ms,
layer thickness: 4 mm, number of averages: 4/slice, matrix 2562.
After a preliminary image, 0.1 mmol/kg each of the substance was
administered in a caudal vein and the signal intensity changes
(relative to an also measured standard) were studied at intervals
of 15 or 30 minutes up to 180 minutes p.i. The relative
intensities relative to the respective initial intensity (=1.00
set) are indicated.
- _ 214509 l00
Table 6:
Average enhancement n = 3:
Time(min) Tumor Liver Kidneys Muscle
MW MW MW MW
S
S S S
0 1.00 0.00 1.00 0.00 1.00 0.00 1.00 0.00
1 1.63.-I-0.24 1.43 0.24 1.67 0.35 1.60 0.01
15 1.71 0.19 1.34 0.22 2.13 0.28 1.54 0.06
30 1.72 0.23 1.31 0.21 2.14 0.38 1.47 0.08
45 1.79 0.21 1.17 0.16 2.10 0.30 1.41 0.10
60 1.78 0.24 1.31 0.09 2.12 0.19 1.33 0.05
75 1.64 0.17 1.37 + 0.05 1.93 0.19 1.37 0.01
90 1.75 0.27 1.22 0.24 1.96 0.08 1.24 0.03
120 1.77 0.23 1.23 0.07 1.92 0.33 1.28 0.08
150 1.73 0.23 1.16 0.22 1.86 0.18 1.20 0.06
180 1.85 0.09 1.07 0.20 1.84 0.24 1.15 0.04
190 1.45 0.00 1.27 0.00 1.73 + 0.00 1.21 0.00
Directly after administration, a marked increase in the
signal intensity (~ 60%) occurred in the studied tissues. But
while in liver and muscles the initial intensity almost returned
after 2-3 hours, the intensity in the tumor remained at an almost
unchanged high level during the observation time. The substance
is obviously eliminated mainly renally. Therefore, the signal
intensity in the kidneys was high above that which could be
determined in the tumors for up to 150 minutes.
2145509 101
d) On a Bruker Biospec nuclear spin tomograph (2.35 Tesla),
the signal intensity change in various tissues of tumor-bearing
(HT29 and WiDr colon cancer) nude mice (Baib/c nu/nu) was studied
after i.v. administration of 0.5 mmol/kg of the compound of
example is (10 mmol/1, pH 7.2; 37°C in bidistilled water). The
measurement takes place with a multi slice single echo sequence
(MS~SE). Imaging parameters: TR: 400 ms, TE: 30 ms, layer
thickness: 4 mm, number of averages: 4/slice, matrix 2562. After
a preliminary image, 0.5 mmol/kg of the substance was
administered and the signal intensity change studied up to 180
minutes p.i.
As early as 5 minutes p.i., a marked signal intensity
increase occurred in the liver tissue and in both studied tumors.
However, a smaller signal increase could be noted in the muscle
as reference tissue. This effect lasted during the entire
observation time. Figure 2 shows the ~H-NMR image of the WiDr
colon cancer after 5, 82 or 180 minutes p.i., Figure 3 that of
the HT29 carcinoma.
e) 9 nude mice (Balb/c nu/nu) with subcutaneously implanted
colon cancers (HT29 and WiDr) were each administered by i.v. 0.1
mmol/kg of the deuteroporphyrin derivative of the compound
according to example lc (10 mmol/1 dissolved in bidistilled
water, pH 7.2, 37°C). After 0.5, 3 and 24 hours, the animals
were killed and dissected. The tissue concentrations were
determined after corresponding working-up (HN03 cone ) by ICP-AES
and calculated-for % of the administered dose per gram of tissue
- _ ~ 2145509 102
net weight. Moreover, tissue relaxation times T1 and T2 were
determined (Bruker minispec pc 120).
Table 7:
% of the administered dose per gram of tissue net weight
0.5 hour ~ 3 hours 24 hours
blood 4.78 0.77 2.60 + 0.91 0.13 0.15
liver 1.87 0.71 2.03 + 0.34 2.05 -~ 0.00
kidneys 4.06 1.01 4.47 1.63 4.20 0.27
spleen 1.31 1.20 0.62 0.19 0.63 0.06
muscle 0.38 + 0.14 0.35 0.02 0. i0 0.02
intestine 0.32 0.09 0.49 0.07 1.56 0.65
skin 2.80 0.71 1.61 0.14 0.65 0.12
lung 2.45 0.14 1.71 0.37 0.16 0.03
HT29 tumor 1.09 0.14 1.46 0.22 0.86 0.14
WiDr tumor 1.51 0.20 1.25 + 0.16 0.58 + 0.02
rest of body 1.13 0.04 0.58 + 0.07 0.26 0.04
Because of the high relaxivity of the compound (17.86 1
mmol'~s'~), markedly shortened T1-tissue relaxation times were
able to be measured (factor 2-3) despite the relatively small
tissue concentrations in both tumors. This shortening of the
relaxation times should be sufficient at least up to 3 hours p.i.
for an enhancement in the NMR imaging experiment (T1-weighted
spin echo sequence).
- 2145509 l03
Table 8:
Tissue relaxation time T~
T~ (ms) 0.5 hour 3 hours 24 hours
liver 220 20 220 30 260 10
kidneys 160 10 ~ 230 30 240 20
muscle 500 40 560 40 660 10
HT29 tumor410 30 440 70 540 20
WiDr tumor320 40 430 50 640 10
T~ (ms) Control
liver 380 + 30
kidneys 500 20
muscle 580 + 10
HT29 tumor840 + 50 -
WiDr tumor 760 + 100
214509 la4
Table 9:
Tissue relaxation
time T2
T2 (ms) 0.5 hour 3 hours 24 hours
liver 34 3 42 2 48 + 7
kidneys 42 1 47 5 54 10
muscle 32 1 36 3 . 31 + 1
HT29 tumor 81 + 4 72 8 83 + 7
WiDr tumor 67 7 72 7 85 + 2
T2 (ms) Control
liver 51 + 2
kidneys 69 ~ 4
muscle 58 + 5
HT29 tumor 101 + 8
WiDr tumor 118 + 19
f) 9 nude mice (Balb/c nu/nu) with subcutaneously implanted
carcinomas (HT29, WiDr and MATLu) were each administered by i.v.
0.1 mmol/kg of the deuteroporphyrin derivative of example lc (10
mmol/L dissolved in bidistilled water, pH 7.2, 37°C). After 2
hours, the animals were killed and dissected. The tissue
concentrations were determined after corresponding working-up
(HN03 cone ) by ICP-AES and calculated for % of the administered
dose per gram of tissue net weight. Moreover, tissue relaxation
times T1 and T2 were determined (Bruker minispec pc 120). To
' ~~~~~ 105
determine the blank readings, control animals were
correspondingly studied without KM-administration.
Table 10:
% of the administered dose per gram of tissue net weight
HT29 WiDr MATLu
blood 2.74 0.90 2.13 0.01 1.14 0.10
liver 1.49 0.17 1.28 0.07 0.97 0.11
kidneys 6.23 1.80 4.23 0.19 2.73 0.33
spleen 0.64 0.21 0.44 0.04 0.31 0.06
muscle 0.24 0.11 0.19 0.03 0.26 0.10
intestine 1.18 0.22 0.73 + 0.34 0.68 0.08
skin 1.34 0.52 0.87 0.13 0.41 0.38
lung 2.09 0.42 1.54 0.09 3.69 3.58
HT29 1.00 + 0.27
WiDr 0.83 + 0.05
MATLu ~ 1.92 + 2.09
rest of body0.54 0.4 0.04 0.27 0.01
In all three tumors, i.a. because of the high relaxivity of
the compound (17.86 1 mmol-~s-~), markedly shortened T1 tissue
relaxation times were able to be measured (factor 2-3). In the
prostate cancer (MATLu), the tissue concentration and the effect
on the tissue relaxation times were accordingly greater than in
the colon cancers (HT29 and WiDr). The shortening of the
relaxation times in all the studied tumors should be sufficient
~1455~9
106
for an enhancement in the NMR imaging experiment (T1-weighted
spin echo sequence).
Table 11:
Tissue relaxation time T~
T~ (ms) HT29 WiDr MATLu
liver 174 8 180 30 191 10
kidneys 150 12 159 30 171 20
muscle 489 ~ 57 508 ~ 40 506 ~ 10
HT29 tumor 375 + 32
WiDr tumor 358 + 70
MATLu 361 + 65
T~ (ms) Control
liver 380 + 30
kidneys 500 20
muscle 580 + 10
HT29 tumor 840 + 50
WiDr tumor 760 + 100
MATLu 1024 + 22
X145509
'- 107
Table 12:
Tissue relaxation time T2
TZ (ms ) HT2 9 WiDr MATLu
liver 49 ~ 2 53 ~ 5 51 + 2
kidneys 59 ~ 3 _ 64 ~ 2 60 + 1
muscle 56 ~ 2 62 ~ 3 54 + 2
HT29 tumor 94 + 7
WiDr tumor 110 + 9
MATLu 102 + 10
T~ (ms) Control
liver 51 + 2
kidneys 69 4
muscle 58 + 5
HT29 tumor101 + 8
WiDr tumor118 + 19
MATLu 142 + 14
214~~09
108
Example 24
On a Bruker Biospec nuclear spin tomograph (2.35 Tesla), the
signal intensity change in various tissues of tumor-bearing (HT29
and WiDr colon cancer) nude mice {Balb/c nu/nu) was studied after
i.v. administration of 0.05 mmol/kg of the compound according to
example 2c (10 mmol/1, pH 7.2; 37°C in bidistilled water). The
measurement takes place with a multi slice single echo sequence
(MSSE). Imaging parameters: TR: 400 ms, TE: 30 ms, layer
thickness: 4 mm, number of averages: 4/slice, matrix 2562. After
a preliminary image, 0.05 mmol/kg of the substance was
administered and the signal intensity change (relative to an
internal standard) was studied 5, 30, 60, 120 and 180 minutes
p.i.
Table 13:
Enhancement after intravenous administration of 0.05 mmol/kg of
body weight
Time liver muscle HT29 WiDr Kidneys
(minutes)
0 100 100 100 100 100
173 31 143 23 171 14 165 39 169 45
+
30 157 30 157 29 169 14 170 32 211 + 33
+ +
60 170 16 120 + 168 12 153 33 207 + 31
8 +
120 179 37 126 + 178 32 153 0 191 + 37
10 + +
180 167 7 112 9 136 23 117 33 136 + 25
+ +
21455U9 l09
As early as 5 minutes p.i., a marked signal intensity
increase in the liver tissue and in both studied tumors occurred.
However, only a small change was noted in the muscle as reference
tissue. This effect lasted during the entire observation time.
The greatest signal intensity difference can be noted at 2 hours
p.i. or 3 hours p.i. Only in the kidneys can an even stronger
signal intensity change be noted, up to 2 hours p.i.
Example 25
9 nude mice (Balb/c nu/nu) with subcutaneously implanted
colon cancers (HT29, WiDr) were each administered by i.v. 0.1
mmol/kg of the mesoporphyrin derivative of example 8e (10 mmol/1
dissolved in bidistilled water, pH 7.2, 37°C). After 0.5, 3 and
24 hours, the animals were killed and dissected. The tissue
concentrations were determined after corresponding working-up
(HN03 cone ) by ICP-AES and calculated for % of the administered
dose per gram of tissue net weight.
214509
- 110
Table 14:
% of the administered dose per gram of tissue net weight
over 0.5 over 3 hours over 24 hours
MW hour MW S MW S
S
blood 23.59 1.54 13.84 1.34' 2.82
liver 8.94 0.24 15.12 0.23 29.73 . '
.
kidneys 18.90 1.65 22.68 1.39 22.69
spleen 6.67 0.60 7.45 0.72 11.34
muscle 2.39 0.18 ~ 1.64 0.01 1.13
intestine 3.08 0.36 3.23 0.26 4.63
skin 5.17 0.68 6.67 1.04 5.26
lung 13.99 0.61 8.46 1.35 4.69
HT29 tumor 2.57 0.31 3.31 0.27 2.84
WiDr tumor 2.90 0.21 4.12 0.32 3.93
rest of body 3.65 + 0.18 3.88 + 0.97 2.48
While in the liver tissue, the increase is from 10% of the
administered dose per gram of tissue net weight (0.5 hour p.i.)
to about 30% of the dose per gram of tissue net weight within 24
hours p.i., the tissue concentrations in all other tissues
decrease within 24 hours or at least remain constant. Thus the
liver has the absolutely highest tissue concentration of all
studied tissues 24 hours p.i.
Example 26
9 nude mice (Balb/c nu/nu) with subcutaneously implanted
colon cancers (HT29, WiDr) were each administered by i.v. 0.1
- ._ 2145~~D9 111
mmol/kg of the mesoporphyrin derivative of example 16f (10 mmol/1
dissolved in.bidistilled water, pH 7.2, 37°C). After 0.5 and 3
hours the animals were killed and dissected. The tissue
concentrations were determined after corresponding working-up
(HN03 cone ) by ICP-AES and calculated for % of the administered
dose per gram of tissue net weight. ~ '.
Table 15:
% of the administered dose per gram of tissue net weight
(% of d. dose/g of organ)
for 0.5 hour for 3 hours
MW + S MW + S
blood 30.68 + 0.85 1:1.63 + 4.46
liver 20.91 +9.96 ~ 27.93 + 2.57
kidneys 8.40 1.58 9.72 1.80
spleen 11.31 1.51 15.50 + 2.96
muscle 3.53 + 0.88 3.31 + 2.90
intestine 2.06 + 0.67 2.75 + 0.30
skin 4.17 + 1.35 7.12 + 1.45
lung 21.54 0.94 15.28 2.68
HT29 tumor 4.47 + 2.06 3.16 + 0.95
WiDr tumor 3.24 + 1.23 6.30 + 2.22
rest of body 3.69 + 0.95 3.91 + 0.43
The liver showed the strongest concentration of all the
studied tissues with about 21 and 28%, respectively, of the
__ ' 2145509 112
administered dose per gram of tissue net weight at 0.5 and 3
hours p.i., respectively.
Example 27
9 nude mice {Balb/c nu/nu) with subcutaneously implanted
colon cancers ~ (HT29, ~ W 'iDr~ c~ere each administered ~by i . v. 0 .1
mmol/kg of the mesoporphyrin derivative of example 18d (10 mmol/1
dissolved in bidistiiled water, pH 7.2, 37°C). After 0.5 and 3
hours, the animals were killed and dissected. The tissue
concentrations were determined after corresponding working-up
(HN03 cone ) by ICP-AES and calculated for % of the administered
dose per gram of tissue net weight.
~'~~~~pg 113
Table 16:
% of the administered dose per gram of tissue net weight
for 0.5 hour for 3 hours
MW S MW S
blood 24.44 2.32 16.95 1.53
3iver 13.28 2.90 . 16:89 1.15 ~ .'
kidneys 11.74 2.38 20.41 1.88
spleen 7.52 ~ 0.40 10.82 ~ 0.87
muscle 0.89 ~ 0.17 1.04 ~ 0.06
intestine 4.93 ~ 0.90 2.31 + 0.28
skin 3.71 0.52 4.46 0.53
lung 17.70 2.50 17.56 . 3.34
HT29 tumor 1.48 0.28 3.53 0.52
WiDr tumor 1.94 0.78 4.50 0.98
rest of body 2.38 0.27 2.43 0.13
The studied substance shows a concentration in the liver
tissue with about 14 and 17%, respectively, of the administered
dose per gram of tissue net weight 0.5 and 2 hours p.i.,
respectively. In other studied tissues, with the exception of
the kidneys, the tissue concentrations in some cases are
considerably lower (factor 5 to 10).
Example 28
9 nude mice (Balb/c nu/nu) with subcutaneously implanted
colon cancers (HT29, WiDr) were each administered by i.v. 0.1
mmol/kg of the mesoporphyrin derivative of 'example 19e (10 mmol/1
21455U9 114
dissolved in bidistilled water, pH 7.2, 37°C). After 0.5, 3 end
24 hours, the animals were killed and dissected. The tissue
concentrations were determined after corresponding working-up
(HN03 cone ) by ICP-AES and calculated for % of the administered
dose per gram of tissue net weight.
Table 17:
% of the administered dose per gram of tissue net weight
over 0.5 over 3 over 24 hours
MW hour MW hours MW S
S ' S
blood 11.68 2.i8 4.13 3.62 0.06 0.01
liver 15.07 1.35 13.75 1.54 10.43 1.56
kidneys 26.52 9.65 9.98 0.50 7.87 0.54
spleen 7.38 2.40 4.41 1.41 4.00 1.71
muscle 1.79 0.34 0.35 + 0.09 0.17 0.07
intestine 2.56 0.50 5.43 + 1.08 0.61 0.43
skin 7.13 + 1.95 2.68 + 0.39 1.26 + 0.12
lung 10.14 1.53 2.51 + 0.36 0.69 0.13
HT29 tumor 3.86 1.06 2.22 0.31 0.99 0.33
WiDr tumor 6.15 2.27 5.45 + 6.63 1.95 0.54
rest of body 3.37 0.51 1.18 3.06 0.62 0.27
Except for the liver, the tissue concentration decreases
quickly within 24 hours (factor 5-20 relative to 0.5 hour p.i.).
The liver had the absolutely highest tissue concentrations of all
the studied tissues with about 14 and 10%, respectively, of the
~145~09 115
administered dose per gram of tissue net weight 3 and 24 hours
p.i., respectively.
Example 29
On a Siemens Magneton~R~ (1.5 Tesla; 64 MHz), the signal
intensity in various.tiSsue's of a tumor-bearing rabbit [hare
rabbit, Wulf, female, approximately 4 kg body weight (n=3), tumor
of VX-2 carcinoma implanted intramuscularly about 3 weeks before
the beginning of the test) was studied after the i.v.
administration of 0.0025 mmol/kg body weight (corresponding to
0.05 mmol/kg of gadolinium) of the compound according to the
invention produced according to Example lc. The measurement took
place with the following imaging parameters: T~-weighted spin
echo sequence: TR: 350 ms, TE: 15 ms, layer orientation:
coronary, layer thickness 3 mm, number of averages: 4 per layer,
field of view:w 150 mm, matrix 2562, 4-6 layers 1 echo per
imaging sequence.
The administration resulted in a good long acting
enhancement, namely both in the implanted tumor and in the
majority of the lymph nodes on the tumor-bearing side. Because
of the pronounced enhancement in these lymph nodes or lymph node
areas, after administration of the compound according to the
invention, a metastasis attack of the lymph nodes could therefore
be diagnosed. The histological study of the lymph nodes
confirmed the results of the MR-tomographic study.
The relative signal intensities in various tissues are
represented in Figure 4. The output signal intensity
2145509
(=SIP~econtrast~ in the various tissues was set equal to 1 in each
case.