Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 94/09762 PCI`/US93/10640
~1~7862
- 1 -
TITLE OF THE rNVENTION
DRUG DELIVERY DEVICE
This case is a contiml~tion-in-part of U.S. Serial No.
08/018/912 which was filed on February 17, 1993; which is a
continuation of U.S. Serial No. 07/802,000 which was filed on
December 3, 1991.
BACKGROUNO OF THE INVENTION
Pharmaceutical scientists are rapidly increasing their
understanding of disease at the molecular level. As a result, many of
the pharmaceutical medicaments which are now available to treat
hllm~n~ and other ~nim~l.c are highly potent and useful only in low
dosages. That is, exposure to the medicament, at levels which have
traditionally been considered the "no effect" level for many drugs, may
lead to very serious side effects, including death. For this reason,
conventional procedures may require extensive modifications if they are
to be used to produce dosage forms cont~ining these highly potent
substances.
Oral dosage forms such as tablets and capsules are
convenient for the patient to transport and offer the best chance for
patient compliance with the prescribed medication regiment. However,
since traditional solid dosage forms utilize powder mixing or dry
gr~n~ tion compressing, these forms represent one of the most difficult
and potentially dangerous medicaments to produce. During processing,
inh~l~tion is considered the primary route of exposure. However,
ingestion by swallowing large inhaled particles which impact on the
mucociliary system of the respiratory tract is likely. Ingestion by
cont~min~tion of hands, dermal contact and transdermal absorption are
also possible routes for unintentional receipt of some medicaments.
In addition to the production employee, there is a further
risk to the patient, health care professionals and others who come in
contact with the product, due to inadvertent contact with the act*e
ingredient.
wo 94/09762 2 1 d~ 7 ~ 6 ~ PCI/US93/10640
Liquid preparations which contain these highly potent
compounds are considerably easier to control. Once the active
ingredient is dissolved in an a~l~ro~liate solvent, the chance for
accidental cont~min~tion, at a level which would cause harm, is
dramatically reduced. However, liquid preparations are not as
convenient to use, may taste harsh and may result in poor patient
compliance.
In order to overcome the problems associated with the
production and use of solid dosage forms cont~inin~ highly potent
compounds, Applicants have created a novel coated dosage form,
wherein the active ingredient is contained within a coating, which is
applied to a core, in a quantitative and reproducible process. Using this
technology, the medicament is contained within a liquid until it has been
applied to the canier core as a film coating. The film coating mixture
both fixes the medicament on the surface of the core and seals the
~urfa~e ~ ~at ~e ~hance ~f à~tive ingredient flaking off the doga~e
form is greatly reduced. When even greater protection is desired, the
film coated core may be further sealed with an overcoat. This
additional coating provides an extra level of protection for those who
subsequently handle the dosage form.
BRIEF DESCRIPTION OF THF INVENTION
This invention concerns a dosage form and a method of
making the dosage form, for the delivery of highly potent medicaments
to humans or other ~nim~ comprising:
(a) a carrier core; and
(b) a coating which comprises a highly potent medicament;
wherein, the coating which comprises the highly potent
medicament and a coating material, adheres to the surface of the
3 carrier core fixing the medicament to the surface of the core and
entraining the medicament.
Optionally, a protective overcoating may be added to the
dosage form to provide further protection.
WO 94/09762 2 1 1 7 8 ~ 2 PCI/US93/10640
The Class m antiarrhythmic drugs: metnanesulfonamide,
N-[ 1 '-(6-cyano- 1,2,3 ,4-tetrahydro-2(R)-naphthalenyl)-3 ,4-dihydro-
4(R)-hydroxyspiro~2H-1-benzopyran-2,4'-piperidin]-6-yl]-, (+)-,
monohydrochloride, (structure I);
methanesulfonamide, N-[ 1 '-(6-cyano- 1,2,3 ,4-tetrahydronaphth-2-yl)-
3,4-dihydro-4-oxo-spiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]-, (+)-,
monohydrochloride, (structure II); and
methanesulfonamide, N-[1'-[2-(5-benzofurazanyl)ethyl]-3,4-dihydro-4-
oxospiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]-, monohydrochloride,
(structure m) are examples of highly potent drugs that can be delivered
using this device.
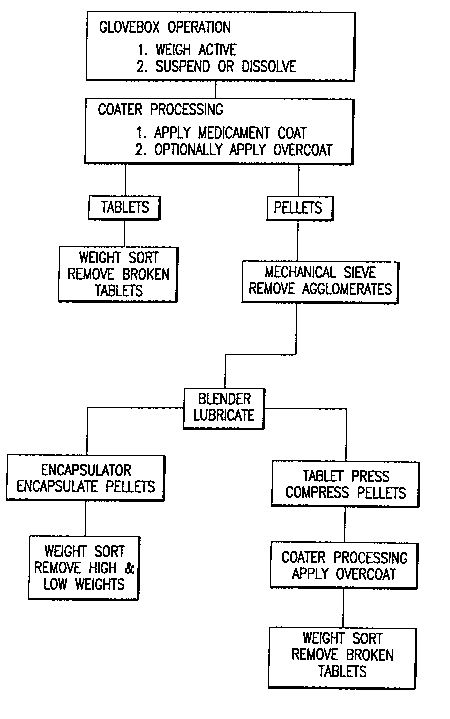
A BRIEF DESCRIPTION OF THE DRAVVINGS
Figure 1 shows a block diagram of the process steps
utilized in the production of the novel dosage form designed to deliver
highly potent drugs.
DETAILED DESCRIPTION OF THE INVENTION
This invention discloses a dosage form and a method of
m~king the dosage form, for the delivery of highly potent drugs to
humans or other ~nim~ comprising:
(a) a carrier core; and
(b) a coating which comprises a highly potent medicament;
wherein, the coating which comprises the highly potent
medicament and a coating material, adheres to the surface of the
carrier core fixing the medicament to the surface of the core and
entraining the medicament.
This invention concerns a method for the safe manufacture
and delivery to h-lm~n~ and other ~nim~l~ in need thereof, of highly
potent medicaments by dispersing the medicament in a coating mixture,
spraying the coating mixture on a carrier core and optionally
overcoating the coated core with a protective over-coating. The entire
coating operation may be conducted within a closed system where the
medicament may be contained.
WO 94/09762 PCI/US93/10640
,~, ., --
o 2147~62
The phrase "dosage form" includes, but is not limited to
tablets, capsules, spheronized particles, coated non-pareil seeds, boluses,
pills, disks, lozenges, controlled delivery devices and any other
regularly shaped solid dosage form.
Although any medicament is considered within the scope of
this invention, this novel dosage form provides for a particularly safe
and effective means to deliver highly potent or highly toxic
medicaments, defined as those medicaments which have an Exposure
Control Limit of about 0.1 mg/m3 or less. These medicaments include
lnorganic and organic compounds without limit~tion, including
medicaments that act on the peripheral nerves, ion channels, nuclear
receptors, adrenergic receptors, cholinergic receptors, nervous system,
skeletal muscles, cardiovascular system, smoo~ muscles, blood
circulatory system, synaptic sites, neuroeffector junctional sites,
endocrine and hormone systems, immlmological system, reproductive
system, skeletal systems, aut~c~id systems, alimentary and excretoly
systems, inhibito~y arld hist~mine systems, and those matenals that act
on the central nervous system such as hypnotics and sedatives.
Examples of beneficial drugs are disclosed in Remington's
Pharmaceutical Sciences~ 16th Ed., 1980, published by Mack Publi.ching
Co., Eaton, Pa.; and in The Pharmacological Basis of Therapeutics, by
Goodman and Gilman, 6th Ed., 1980, published by the MacMillan
Company, London; and in The Merck Index. 11th Edition, 1989,
published by Merck & Co., Rahway, N.J. The drug can be in various
forms, such as charged molecules, charged molecular complexes,
ionizable salts or neutral molecules. Acceptable salts include, but are
not limited to hydrochlorides, hydrobromide, sulfate, laurylate,
p~lmit~te, phosphate, nitrate, borate, acetate, m~le~te, m~l~te,
trometh~mine, tartrate, oleate, salicylate, salts of metals, and amines or
organic cations, ~or example quatemary ammonium.
Derivatives of medicaments such as esters, ethers and
amides without regard to their ionization and solubility characteristics
can be used alone or mixed with other medicaments. Also, a
medicament can be used in a form that upon release from the device, is
WO 94/09762 PCI~/US93/10640
~ ~ 78~
converted by enzymes, hydrolyzed by body pH or other metabolic
processes to the parent form, or to a biologically active form.
Exposure Control Limits are based on ph~rm~cological
considerations and defined as the time-weighted average concentration
5 for a norrnal 8 hour workday and 40 hour workweek to which nearly
all workers may be repeatedly exposed day after day without adverse
effect. To facilitate the derivation of a numerical limit, the equation
shown below has been reported. (See E.V. Sargent and G. D. Kirk,
"Establi~hin~ Airborne Esposure Control Limits in the Pharmaceutical
Industry", Am. Ind. Hyg. Assoc. J., 49(6): 309 - 313, 1988, which is
hereby specifically incorporated by reference.)
ECL (mg/m3) = NOEL (mg/kg/day) x BW (kg)
V (m3/day) x S (days) x a x SF
Where NOEL is the no-observable-effect-level, BW is the
average hl~m~n body weight (70 kg for males; 50 kg for females); V is
the volume of air breathed in an 8 hour day (10 m3/day); S is the time
to achieve a plasma steady state; SF is a safety factor; and a is the
percent of the compound absorbed.
This concept of exposure limits is based on the principle
that exposure to a chemical agent may be permitted up to some
tolerance limit greater than zero. This assumes a nonlinear dose-
response relationship and allows for the estimation of a NOEL.
Generally, the NOEL is based on either the therapeutic effect for which
the drug is intended or on one or more clinically reco~ni7~ble side
effects which can occur at or below the therapeutic level.
Generally, the NOEL is determined in populations where it
is associated with a certain amount of variability. Individual variability
in response to a drug may be due to differences in age, sex, health and
nutritional status or genetic factors. When variability within a species is
known to be large, a safety factor, usually up to 10 fold, can be used. A
10 fold safety factor also can be used when the NOEL has not been
WO 94/09762 PCI/US93/10640
2~47$~2
identified and the lowest observable effect dose is used. Larger safety
factors (100 to 1000 fold) generally are reserved for risk estimated or
inconclusive hllm~n data or ~nim~l data. ~he larger safety factors may
be applied to compounds with carcinogenic or teratogenic potential.
Exposure Control Limits of O.S ug/m3 have been calculated
for the Class m antiarrhythmic drugs:
methanesulfonamide, N-[ 1 '-(6-cyano- 1,2,3 ,4-tetrahydro-2(R)-
naphthalenyl)-3,4-dihydro-4(R)-hydroxyspiro[2H- 1 -benzopyran-2,4'-
piperidin]-6-yl]-, (+)-, monohydrochloride, (structure I);
o methanesulfonamide, N-[ 1 '-(6-cyano- 1 ,2,3,4-tetrahydro-naphth-2-yl)-
3,4-dihydro-4-oxo-spiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]-, (+)-,
monohydrochloride, (structure II); and
methanesulfonamide, N-[1'-[2-(S-benzofurazanyl)ethyl]-3,4-dihydro-4-
oxospiro [2H- 1 -benzopyran-2,4'-piperidin] -6-yl] -, monohydrochloride,
(structure m). This very low exposure control limit makes these
medicaments part;cularly sulted for thls dosage ~orm and process~ng.
/S b~o-- I Cl-
N~,~
\~C --N
Structure I
WO 94/09762 PCI~/US93/106~0
5 21~78~2
S'N~ Cl-
NH~3~c ~N
Structure 11
o H
S~o~ Cl-
NH,~ N~
- ~o
--N
Structure 11
The above list of pharmaceutical products and medicaments
20 is not meant to be exhaustive. Many other compounds will certainly
work in the instant invention and are included within the scope of this
nvention.
The novel dosage form and process of the instant invention
may be used to deliver medicament to hllm~n~ or other 2nim~1~. The
25 term "~nim~l" includes m~mm~lc, hllm~n~ and primates, such as
domestic, household, sport or farm ~nim~lc such as dogs, sheep, goats,
cattle, horses and pigs, laboratory ~nim~l~ such as mice, rats and guinea
pigs, fish, avians, reptiles and zoo ~nim~
By "carrier core" is meant a nucleus upon which a mixture
30 cont~inin~ the medicament may be applied. The carrier core may be,
for example, a non-pareil seed, a compressed tablet, a triturate, a
spheronized particle, an inert bead and slugged material. This list is not
meant to be in any way inclusive of the carrier cores which are included
within the scope of this invention.
WO 94/09762 PCI/US93/10640
214786~ --
When the particle size of the carrier core is considered
important, the carrier cores may be sized by passing them ~rough a
screen which has a pore size at the upper diameter limit and collecting
the carrier cores on a screen which has a pore size at the lower diameter
limit. For example, when non-pareil seeds are used, the seeds are
generally sized by collecting those seeds which pass through a #25 mest
size screen and are collected on a #30 mesh size screen. This results in
a more uniform particle size for the finished product.
The carrier core may be composed of lactose and other
o excipients such as magnesium stearate, microcrystalline cellulose,
starch, stearic acid, calcium phosphate, glycerol monostearate, sucrose,
polyvinylpyrrolid~ne, gelatin, methylcellulose, sodium
carboxymethylcellulose, sorbitol, mannitol, polyethylene glycol and
other ingredients cornrnonly utilized as stabilizing agents or to aid in the
production of tablets, spheronized particles, or other carrier core forms
mentioned a~ove.
The carrier core may also contain, within the core, a
second medicament. For example, cardiovascular agents such as Class I
antiarrhythmic compounds, anti-~ngin~l compounds, vasodilators,
potassium supplements, B-adrenergic receptor blocking agents, sodium
channel blockers, calciurn channel blockers, angiotensin converting
enzyme inhibitors, A II receptor antagonists, and diuretics may be
delivered in combination with the compounds of structures I, ~ or III,
by including them within the carrier core.
The coating cont~ining the highly potent medicament may
also include hydroxypropylmethylyethylene glycols, sodium
carboxymethyl cellulose, carboxymethyl cellulose, methacrylate
hydrogels, cellulose acetate phth~l~te, polyvinyl alcohol, poylacrylic
acid, poly N-vinyl pyrrolidone, polyacrylamide, polyethylene oxide,
methylhydroxyethyl cellulose, ethyl cellulose, povidone, shellac, gelatin,
wax, acacia, methylcellulose, methacrylic acid, methacrylic acid ester
copolymers, titanium dioxide, talc, colorants, plasticizers and other
soluble ingredients commonly used in film coatings of pharmaceutical
dosage forms.
W 0 94/09762 2f 4 7862 PC~r/US93/10640
The medicament coating may be prepared as a solution in
water or an organic solvent. The medicament coating may also be
prepared as a slurry, a suspension, a dispersion and may be partially or
completely solubilized prior to application. The medicament may be
5 mixed with a binder, dispersant, lubricants, emulsifier, diluent, wetting
agent and colorants.
The overcoat may include hydroxypropylmethyl-
cellulose, hydroxypropylcellulose, polyethylene glycols, sodium
carboxymethyl cellulose, carboxymethyl cellulose, methacrylate
hydrogels, cellulose acetate phth~l~te, polyvinyl alcohol, poylacrylic
acid, poly N-vinyl pyrrolidone, polyacrylamide, polyethylene oxide,
methylhydroxyethyl cellulose, ethyl cellulose, povidone, shellac, gelatin,
wax, acacia, methylcellulose, methacrylic acid, methacrylic acid ester
copolymers, tit~nium dioxide, talc, colorants, plasticizers and other
soluble ingredients commonly used in film coatings of pharmaceutical
dosage forms.
The overcoat may be prepared as a solution in water, an
aqueous solution or an organic solvent. The overcoating may also be
prepared as a slurry, suspension, dispersion and may be partially or
completely solubilized prior to application. In general the film coating
mixture is prepared by mixing a solution cont~ining from about 1 mg to
about S00 mg of the highly potent compound and about 140 ml of water
with about 0.6 to about 10 grams of hydroxypropylmethylcellulose in
about 50 ml of water. However, more or less concentrated solutions of
the highly potent compound or the hydroxypropylmethylcellulose are
within the scope of this invention.
The medicament coating and the overcoat may be applied to
the dosage form core using any coating procedure including the use of a
fluidized bed film coating device, including a roto-processor, a pan
coater or a baffled pan coater or any air suspension process. The film
coating mixture may also be manually added to the carrier cores while
they are mixed in the presence of a heated stream of air or inert gas.
In general the medicament coating and the overcoat may be
applied to any thickness desired. However, a coating thickness of from
WO 94/09762 PCI/US93/10640
2~ 78~2
- 10-
about 1 to about 1000 mm is generally applied to the surface of the
dosage form core. In the preferred embodiment using a non-pareil seed
as a carrier core, a coating thickness of from about 5 to about 100 mrn
is applied depending upon the concentration of medicament to be
5 included on each carrier core. When tablets are used as the carrier
core, the film coating thickness generally ranges from about 25 to about
500 mm, depending upon the concentration of medicament to be
included on each carrier core.
EXAMPLE 1
Capsllles cont~inin~ non-pareil seed coated with a solution
contzlining the Class III antialThyffimic drug methanesulfonamide, N-
[ 1 '-(6-cyano- 1,2,3 ,4-tetrahydro-2(R)-naphthalenyl)-3 ,4-dihydro-4(R)-
5 hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]-, (+)-,
monohydrochloride were prepared using the following procedure.
Preparation of the Binder Solution
In a suitable tared, clean, dry, glass beaker, 100 g of
20 purified water was heated to 80C. To this heated solvent, 200 g of
hydroxypropylmethylcellulose was added slowly with vigorous stirring.
The container was removed from the heat source and while stirring at
low speed, 100 g of unheated purified water was added.
25 Overcoat Preparation
A portion, 44 g, of ~e binder solution was weighed into a
clean, tared 250 ml erlenmeyer flask. This solution was diluted with
110 g of purified water with mixing. The resulting diluted solution was
covered and stored at room temperature until needed.
Raw Material Preparation
A quantity of non-pareil seeds were sized between #25 and
#30 mesh sieves. From the sized non-pareil seeds, 452.8 g were
transferred to a holding container.
WO 94/09762 PCI/US93/10640
o ~?1~78~
Magnesium stearate was bolted through a #60 screen. One
gram was transferred to a suitable storage container until needed.
About 100 g of empty capsules (H.G. #3, white opaque
999) were weighed and kansferred to a suitable storage device.
Manufacturing Conditions
In a class m glovebox, 120.75 mg of methanesulfonamide,
N-[ 1 '-(6-cyano- 1,2,3 ,4-tetrahydro-2(R)-naphthalenyl)-3 ,4-dihydro-
4(R)-hydroxyspiro [2H- 1 -benzopyran-2,4'-piperidin] -6-yl] -, (+)-,
monohydrochloride was transferred to a vessel with 140 ml of water
and mixed for one hour with sh~king. To this solution was added with
stirring, 66 g of the binder solution which had been previously
prepared. This mixture was transferred to a 4 inch in diameter Wurster
Coating Column. The coating column conditions were as follows:
Preheat column for about 10 minutes
Atomization Pressure set to about 1.1 Bar
Inlet Temperature set to about 70C
Outlet Temperatue set to about 34C
Air Flow set to about 80 m3/hour
Inner Partition Height set to about 0.5 inches
Application Rate set to about 5.0 g/min.
Next, 452.8 g of the sized non-pareil seeds were loaded into
the coating column and the polymeric/drug solution was applied. The
pellets were dryed for 10 mimltes in the column.
Once the coating operation was completed, the overcoat
was applied. The binder solution was sprayed onto the coated pellets
using the same coating conditions used to apply the drug cont~ining
coat. Following application of the overcoat, the pellets were dried for
15 mimltes in the column.
The dried coated non-pareils were transferred into a double
plastic bag cont~ining 1 g of the bolted magnesium stearate. The bag
was sealed after allowing a head space of air and then vigorously shaken
to suf~1ciently lubricate the coated pellets.
WO 94/09762 PCI/US93/10640
21 ~78bZ
The lubricated pellets were then encapsulated using a
Bonapace Hand Fill Encapsulator. The weight of pellets in each capsule
was based on a laboratory analysis of the pellets after coating.
EXAMPLE 2
Placebo tablet can be used as carrier cores, in place of non-
pareil seeds. Tablets can be prepared from dry blending a mixture of
about 35% microcrystalline cellulose, about 50% lactose and about 14%
pregel~tini7ed starch. This mixture is then lubricated with magnesium
stearate (about 1% of final tablet weight) and directly compressed into
the core tablet usirlg a standard tableting machine.
These tablets may then be transferred to a film coating
processor where the medicament coating and if desired the overcoat is
added.
EXAMPLE 3
Exemplary of the many forrn~ tions that are included
within the scope of this invention are the following:
WO 94/09762 PCI/US93/10640
~ 21~7~2
EXAMPLE 3(a)
Ingredient . Amount/
Unit
L-706,000-001 -T-012 2.530 mg
(Incorporate Coating Loss)
L-706,000-001 -T-012 2.30 mg
(Equivalent to 2.0 mg Base)
Non-Pareil Seeds White 224.2 mg
(25-30 mesh)
Hydroxypropylmethylcellulose 6cps 5.0 mg
Water Purified (grn)
Magnesium Stearate 0.5 mg
TARGET FILL WEIGHT 232.0 mg
Capsule Hard Gelatin # 3 White 46.5 mg
Opaque 999
TARGET CAPSULE WEIGHT 278.5 mg
WO 94/09762 21 4 7 8 6 2 Pcr/uss3/ln640
- 14 -
EXAMPLE 3(b)
Ingredient . Amount/
Unit
L-706,000-001 -T-012 2.415 mg
~Incorporate Coating Loss)
L-706,000-001-T-012 2.300 mg
(Equivalent to 2.0 mg Base)
Non-Pareil Seeds White 224.2 mg
(25-30 mesh)
Hydroxypropylmethylcellulose 6cps 2.5 mg
Hydroxypropylcellulose LF Grade 2.5 mg
Water Purified (gm)
Acetone NF (gm)
Magnesium Stearate 0.5 mg
TARGET FILL WEIGHT 232.0 mg
Capsule Hard Gelatin # 3 White 46.5 mg
Opaque 999
TARGET CAPSULE WEIGHT 278.5 mg
W O 94/09762 PC~r/US93/10640
5 2~ ~ 7862
- 15 -
EXAMPLE 3(c)
Ingredient Amount/
Unit
L-706,000-001 -T-012 2.5715 mg
(Includes Coating Loss)
L-706,000-001-T-012 2.30 mg
(Equivalent to 2.0 mg Base)
Lactose NF Anhydrous 117.0 mg
Cellulose Microcrystalline NF 80.0 mg
AVICEL 102
Magnesium Stearate NF 1.0 mg
Crosscarmellose Sodium NF 2.0 mg
Type A
Hydroxypropylrnethylcellulose 6cps 2.30 mg
Hydroxypropylcellulose LF Grade 2.30 mg
Water Purified (gm)
Acetone NF (gm)
Hydroxypropylmethylcellulose 6cps 3.25 mg
Hydroxypropylcellulose LF Grade 3.25 mg
Tit~nillm Dioxide USP 0.280 mg
Talc USP Purified 0.100 mg
Blue FD&C # 2 Alllminllm Lake 0.025 mg
(14 % Dye)
Water Purified (gm)
Theoretical Tablet Weight 213.81 mg
wo 94/09762 Pcr/uss3/l0640
21~8~2
- 16 -
EXAMPLE 3(d)
Ingredient . Amoun~/
Unit
L-706,000-001 -T-012 2.415 mg
(Incorporate Coating Loss)
L-706,000-001 -T-012 2.300 mg
(Equivalent to 2.0 mg Base)
NonPareil Seeds White (25-30 mesh)224.2 mg
Hydroxypropylmethylcellulose 6cps2.5 mg
Hydroxypropylcellulose LF Grade 2.5 mg
Water Purified (gm)
Acetone NF (grn)
Sodium Lauryl Sulfate 0.375 mg
Magnesium Stearate 0.125 mg
TARGET FILL WEIGHT 232.0 mg
Capsule Hard Gelatin # 3 White 46.5 mg
Opaque 999
TARGET CAPSULE WEIGHT 278.5 mg
WO 94/09762 PCr/US93/10640
~ 7862
EXAMPLE 3(e)
Ingredient Amount/
Unit
L-706,000-001-T-012 0.06038 mg
(Incorporate Coating Loss)
L-706,000-001-T-012 0.0575 mg
(Equivalent to 2.0 mg Base)
Non-Pareil Seeds White (25-30 226.4 mg
mesh)
Hydro~ypropylmethylcellulose 6cps 2.5 mg
Hydroxypropylcellulose LF Grade 2.5 mg
Water Purified (gm)
Acetone NF (gm)
Sodium Lauryl Sulfate 0.375 mg
Magnesium Stearate 0.125 mg
TARGET FILL WEIGHT 232.0 mg
Capsule Hard Gelatin # 3 White 46.5 mg
Opaque 999
TARGET CAPSULE WEIGHT 278.5 mg
WO 94/09762 2 ~ ~ 7 8 6 2 PCI/U593/10640
- 18 -
EXAMPLE 3(fl
Ingredient . Amount/
Unit
L-706,000-OOl -T-012 1 1.5 mg
(Equivalent to 2.0 mg Base)
Lactose NF Anhydrous 117.0 mg
Cellulose Microcrystalline NF 80.0 mg
AVICEL 102
Magnesium Stearate NF 1.0 mg
Crosscarmellose Sodium NF 2.0 mg
Type A
Hydroxypropylmethylcellulose 6cps 6.00 mg
Hydroxypropylcellulose LF Grade 6.00 mg
Water Purified (gm)
Acetone NF (gm)
Hydroxypropylmethylcellulose 6cps 3.25 mg
Hydroxypropylcellulose LF Grade 3.25 mg
Titanium Dioxide USP 0.280 mg
Talc USP Purified 0.100 mg
Blue FD&C # 2 Aluminum Lake 0.025 mg
(14 % Dye)
Water Purified (gm)
Theoretical Tablet Weight 230.41 mg